Significance
The unique host specificity and antimicrobial activity of bacterial viruses have inspired many diagnostic and antibacterial applications in industry, agriculture, and medicine. Because of the rise in antibiotic-resistant infections, phage therapy is a reemerging field of interest. Many restrictions that are associated with the use of native, isolated phages can be overcome by genetic engineering. Thus, efficient genome-editing tools are needed to unleash the full potential of phage therapy and biotechnology. In vitro assembly of synthetic virus genomes and the rapid isolation of corresponding phages is an important step in this direction. By using L-form bacteria as rebooting compartments of synthetic genomes, we report a simple, highly efficient, and broadly applicable technology that enables engineering of diverse phage families.
Keywords: bacteriophage engineering, synthetic biology, L-form bacteria, Listeria monocytogenes, biotechnology
Abstract
Engineered bacteriophages provide powerful tools for biotechnology, diagnostics, pathogen control, and therapy. However, current techniques for phage editing are experimentally challenging and limited to few phages and host organisms. Viruses that target Gram-positive bacteria are particularly difficult to modify. Here, we present a platform technology that enables rapid, accurate, and selection-free construction of synthetic, tailor-made phages that infect Gram-positive bacteria. To this end, custom-designed, synthetic phage genomes were assembled in vitro from smaller DNA fragments. We show that replicating, cell wall-deficient Listeria monocytogenes L-form bacteria can reboot synthetic phage genomes upon transfection, i.e., produce virus particles from naked, synthetic DNA. Surprisingly, Listeria L-form cells not only support rebooting of native and synthetic Listeria phage genomes but also enable cross-genus reactivation of Bacillus and Staphylococcus phages from their DNA, thereby broadening the approach to phages that infect other important Gram-positive pathogens. We then used this platform to generate virulent phages by targeted modification of temperate phage genomes and demonstrated their superior killing efficacy. These synthetic, virulent phages were further armed by incorporation of enzybiotics into their genomes as a genetic payload, which allowed targeting of phage-resistant bystander cells. In conclusion, this straightforward and robust synthetic biology approach redefines the possibilities for the development of improved and completely new phage applications, including phage therapy.
Bacteriophages are viruses that exclusively infect bacteria and constitute their natural enemies. Based on their extraordinary host specificity and bacteriolytic potential, phages are considered for various medical and technological applications and are used as diagnostic tools for rapid and sensitive detection of viable bacterial cells (1). Virulent/“strictly lytic” phages are especially useful for biocontrol, targeting potential pathogens in agriculture and food production (2). In addition, the antibiotic resistance crisis prompted reevaluation of phages as alternative antimicrobials, and phage therapy approaches are beginning to show promising results (3–5). Despite their high genus- and species-specificity, self-replicating nature, and low production cost, wide-spread antibacterial and medical application of phages is hindered by several challenges (6): Due to restricted host ranges of individual phages, mixtures of phages (cocktails) are often required to cover all relevant strains of a pathogen, and the regulatory framework for cocktail approval is unclear (7, 8). In addition, temperate phages can integrate into the host genome without inducing cell lysis, may contribute to the spread of antibiotic resistance by transduction (9), or may increase bacterial virulence through lysogenic conversion, effectively excluding their use as biocontrol agents (10). Also, target cells may possess several phage-resistance mechanisms, including receptor diversification, biofilm formation, restriction-modification systems, and CRISPR interference (11). Such limitations can potentially be overcome by tailored modification of phage genomes [reviewed by Pires et al. (12)], which would also allow the introduction of additional genetic traits for diagnostics, biocontrol, and other applications (12, 13). For example, phages have been engineered as sequence-specific antimicrobials that selectively remove antibiotic-resistant bacteria from mixed populations (14, 15).
However, targeted genome engineering of virulent phages is, at best, a difficult and labor-intensive process (12). Currently, the most broadly applicable approach is based on modification of phage genomes during infection by homologous recombination. Because recombination rates are relatively low (10−4 to 10−10), screening for recombinant phages is very time consuming and often requires the coincorporation of selectable marker genes into virus genomes (12). To accelerate the isolation of modified phages, recombinants can alternatively be enriched by negative selection using CRISPR-Cas systems. To this end, sequence-specific RNA-guided nucleases are designed to cleave the WT allele while leaving recombinant genomes intact. So far, this approach was used to modify virulent phages of Escherichia coli, Lactococcus, and Streptococcus (16–19). CRISPR-Cas allows marker-free phage engineering but is limited to host strains for which such a system is available. For negative selection to work, recombination rates need to exceed the frequency of naturally occurring CRISPR escape mutants (typically 10−6 to 10−5) (16, 18). In addition, editing templates and CRISPR-RNA vectors need to be constructed and transformed into the host bacterium for each planned modification. Thus, CRISPR-based phage engineering remains relatively time-consuming and is currently limited to a few bacterial hosts and phages. Multiple modifications can only be introduced sequentially, hampering the construction of more complex engineered viruses. Efficient, faster, and more broadly applicable methods are required to fully develop the potential of phage engineering. One intriguing option is the reactivation of synthetic bacteriophage DNA, a process also known as “genome rebooting.” Some phage genomes can be rebooted either using cell-free systems (in vitro transcription-translation) (20, 21) or in E. coli cells transfected with full-length phage genomic DNA (gDNA) (22, 23). Based on the latter approach, Ando et al. (22) have presented an elegant platform technology to genetically modify phages, which is based on assembly and capture of synthetic genomes into yeast artificial chromosomes (YAC) and subsequent rebooting of YAC-phage DNA in E. coli recipient cells. While in vitro- and yeast-based approaches allow rapid phage genome engineering, their use with phages of Gram-positive bacteria has not been demonstrated.
So far, phages that target Gram-positive bacteria could not be modified by any synthetic approach, because virus genomes could not be transferred back into the thick-walled Gram-positive recipient cells for rebooting. Here, we overcome this limitation by employing L-form bacteria as genome recipients and rebooting compartments. We present a robust and broadly applicable platform technology for rapid phage engineering and demonstrate that fully tailor-made phage genomes can be designed on the drawing board, synthesized, and rebooted within as few as 6 days. As proof of concept, we created synthetic, virulent phages with specifically tailored antimicrobial properties.
Results
Rebooting Genomic Bacteriophage DNA in Listeria monocytogenes L-Form Cells.
L-form bacteria are pleomorphic, wall-deficient cells that undergo division and feature metabolic activity (24). L-forms have the ability to take up large molecules of DNA (25, 26) and should be able to replicate, transcribe, and translate newly introduced genetic information. Based on these assumptions, we evaluated L-form cells of Listeria monocytogenes as recipients and rebooting compartments for native, purified phage gDNA. Listeria L-form cells can be generated by prolonged subcultivation in the presence of cell wall-active antibiotics in an osmoprotective environment (24, 27). We used a L. monocytogenes EGDe strain variant designated “Rev2,” which can rapidly be induced to grow as an L-form (designated “Rev2L”) (27). To provide a proof of concept for the rebooting approach, we used gDNA of the virulent L. monocytogenes phage P35. Its 35,822-bp linear gDNA (28) is too large for electroporation into walled Listeria cells, which only accept supercoiled plasmids of up to 10 kb with low efficiency (29). We devised a workflow for PEG-mediated transfection of Rev2L bacteria with full-length phage DNA. To this end, purified gDNA was mixed with cells from a growing penicillin G (PenG)-induced Rev2L culture, and gDNA uptake was mediated by addition of PEG-8000. Following dilution in osmotically stabilized L-form medium (modified DM3), the transfection reaction was incubated to allow rebooting, i.e., DNA uptake, phage gene expression, and virus assembly. Subsequently, we assayed the transfection reaction for the presence of reactivated phages using a suitable phage propagation strain as an indicator. A workflow is shown in Fig. 1A. We found that P35 could be rebooted in a gDNA-, L-form-, and PEG-dependent process (Fig. 1B). To maximize phage yield, each step of the rebooting protocol was optimized (Fig. S1 A–F). We found a linear correlation between input DNA quantity and phage production (Fig. 1C), with a lower detection limit of 2.6 pg of P35 gDNA. This corresponds to about 66,000 genomes, assuming that the DNA used consisted solely of intact, infectious gDNA (the quality of phage DNA was assessed by pulsed-field gel electrophoresis) (Fig. S1G). Besides P35, the L-form transfection protocol allowed rebooting of seven other Listeria phages (Fig. 1D and Fig. S2) (30). These included both temperate (B025, B035, B056, PSA) and virulent (P70, P100, A511) phages, some with large genomes (>130 kb; P100, A511), and morphologically diverse phages featuring either contractile (Myoviruses; P100, A511) or noncontractile (Siphoviruses; all other) tails. The phage genomes used featured either single-stranded overlapping “cohesive” ends (hereafter, “cos” phages; B025, PSA) or terminally redundant DNA molecules, with or without circular permutation (A511, P100, P70, P35). This suggests that rebooting is largely independent of virus morphology, genome structure, DNA packaging mode, replication strategy, and genome size. Moreover, we were able to produce Listeria phages that would normally not infect the walled Rev2 strain (P70, B025, B035, B056, and PSA) (Fig. S1H). These findings indicate that virus attachment and/or genome translocation are the major barriers restricting the infectious range of these phages among the different serovars of Listeria. The diverse features of the reactivated Listeria phages (summarized in Table S1) indicate that Rev2L represents a suitable rebooting compartment for all Listeria phage genomes. The rebooting kinetics of phages P35, P70, and A511 (Fig. 1E) revealed that virus production peaked at 24 h posttransfection. The large volume of the L-form cells and their attenuated metabolic activity (24, 27) may explain why rebooting is a slow process compared with infection of walled cells. Because L-forms are devoid of a cell wall, progeny phages are released by osmotic disruption when transfected Rev2L cells are diluted with nonstabilized medium. Disruption releases any viral particles produced from heterologous DNA, including phages that cannot bind or infect Rev2, and/or whose cell wall lytic enzymes (endolysins) are unable to degrade Listeria peptidoglycan.
Fig. 1.
Rebooting genomic bacteriophage DNA in L. monocytogenes L-form cells. (A) The workflow for rebooting of phage genomes in Listeria L-form strain Rev2L is shown (see SI Materials and Methods and Fig. S1A for more details). (B) Rebooting was initially assessed using purified gDNA of Listeria phage P35. L-form transfection reactions were prepared as indicated, incubated at 32 °C, and tested for plaque formation on the indicator strain at 24 h posttransfection. Soft-agar overlays of the rebooting reactions are shown. DNaseI indicates a 30-min predigestion of P35 gDNA with DNaseI. (C) Efficiency of transfection and rebooting was determined using a dilution series of P35 gDNA. (D) Listeria phages P70, A511, and B035 were rebooted in Rev2L using 500–1,000 ng gDNA and were detected using L. ivanovii WSLC 3009 as an indicator. (E) Rebooting kinetics in Rev2L were assessed for phages P35, P70, and A511 over a period of 96 h. Data are mean ± SD (n = 3). Φ, phage.
Listeria L-Forms Reboot Phage Genomes from Other Gram-Positive Bacteria.
By transfecting L-form bacteria, we were able to bypass the first and last steps of the phage infection cycle but not virus genome replication, gene expression, and virion morphogenesis. This system allowed us to explore whether Listeria L-forms could possibly reactivate genomes of phages specific for other Gram-positive pathogens (but which cannot infect Listeria). We show that the 37.46-kb Siphovirus TP21-L (31) of Bacillus cereus (Fig. 2A) as well as the large, 153.96-kb Myovirus Bastille (32) of Bacillus thuringiensis (Fig. S3A) can be rebooted in Rev2L and subsequently infect their original Bacillus host cells. In addition, Listeria L-forms also supported rebooting of Staphylococcus aureus phage 2638A (33), a 41.32-kb Siphovirus (Fig. S3B), and phage K (34), a large 127.40-kb Myovirus (Fig. 2B). We de novo sequenced two reactivated L. monocytogenes phage P35 clones as well as two S. aureus phage 2638A clones and compared their sequence to the transfected DNA. Only one noncoding point mutation in one 2638A clone was found, suggesting that facilitating mutations are not required for rebooting. Our data show that Rev2L represents a highly versatile system for uptake and cross-genus rebooting of phages from linear gDNA.
Fig. 2.
Listeria L-forms reboot phage genomes from other Gram-positive bacteria. To test for cross-genus rebooting of Bacillus (A) and Staphylococcus (B) phage genomes in Listeria L-forms, Rev2L cells were transfected with 500–1,000 ng gDNA, and rebooted phages were detected on suitable indicator strains. Reactions lacking either L-forms or phage gDNA served as controls. Soft-agar overlays of the rebooting reactions are shown.
Efficient Rebooting of Synthetic, in Vitro-Assembled Phage Genomes.
To enable phage engineering, we tested rebooting of fully synthetic phage genomes (workflow in Fig. 3A). For this, we amplified overlapping segments of phage DNA and reassembled them using the Gibson method (35). Because most phages require circular genome replication intermediates (36), overlaps between fragments were designed to allow end-joining (circular closure). First, we used six fragments of about 6 kb to assemble synthetic genomes of Listeria phage P35 (Fig. 3B). An incomplete assembly lacking fragment three (−f3) served as control. We found that Rev2L efficiently reboots phage P35 from in vitro-assembled DNA (Fig. 3C). Surprisingly, synthetic DNA yielded a higher rebooting efficiency than native phage gDNA (detection limit, 1.1 pg DNA) (Fig. S4). This may be explained by the fact that synthetic gDNA is already circularized, whereas native genomes need to be closed inside the L-form cell after transfection. Alternatively, the fraction of infectious genomes in native phage DNA preparations may have been low. In addition to P35, we assembled and rebooted synthetic genomes of temperate Listeria phages PSA (37) and B025 (28) (Fig. S5). To demonstrate reactivation of synthetic phages from other genera, assembly and rebooting of B. cereus phage TP21-L was also established (Fig. 3 D and E).
Fig. 3.
A synthetic platform for rebooting of in vitro-assembled bacteriophage genomes in Listeria L-forms. (A) The workflow for assembly and rebooting of synthetic genomes is depicted. Genome fragments can be generated by either PCR or gene synthesis. Red arrows indicate PCR primers. (B–E) To create synthetic phages with nonmodified sequences, phage DNA was extracted from Listeria phage P35 and Bacillus phage TP21-L. (B and D) Overlapping PCR fragments covering the full P35 and TP21-L genomes were amplified by PCR and assembled in vitro to generate circular DNA. (C and E) Rev2L cells were transfected with assembled DNA and rebooting reactions plated on suitable indicator strains (labeled on the right) to probe for infectious phage formation. Incomplete assemblies served as controls. circ, circular; f, genome fragment; m, molecular weight standard. (Scale bar in C: 35 mm.)
Rebooting synthetic phage genomes in L-form bacteria offers a broadly applicable platform technology for phage engineering that enables full control of sequence composition and organization. By modification, removal, addition, or reorganization of individual fragments in the assembly, any genome configuration can be realized in a single step. Since the input DNA is defined, all emerging phages have the desired sequence, which eliminates lengthy screening procedures. Because Rev2L cells also reboot phage genomes across otherwise conserved genus boundaries, the technology is applicable to other relevant Gram-positive pathogens, such as bacilli and staphylococci. Overall, engineering is fast, requiring only 6 d from in silico phage design to isolation of the synthetic virus (Fig. S6).
Modifying Temperate Phage Genomes to Create Synthetic, Virulent Phages.
Next, we applied the synthetic platform to create tailor-made phages. Any antibacterial phage application, including phage therapy, requires strictly lytic viruses. To date, virulent candidate phages that feature the desired host range, killing efficiency, and physical properties must be identified from natural isolates. Here, we applied the rebooting platform to rapidly create virulent phages using temperate phages as genetic backbones, a process we call “virulent conversion.” To this end, we de novo assembled the genome of temperate Listeria phage B025 (28) but removed the gene cluster encoding the lysogeny control functions that mediate genome integration and prophage maintenance. In B025, these genes are located on a 2.7-kb genetic module (28), the lysogeny control region (LCR). We designed synthetic genomes to lack either just the repressor of the lytic cycle (B025 Δrep) or the entire LCR, including the integrase (B025 ΔLCR). The assembly strategy is shown in Fig. 4A, and fragments for the assembly of mutant phage genomes are shown in Fig. 4B. Engineered genomes were then rebooted, plaque phenotypes visualized (Fig. 4C), and phage genomes validated by PCR (LCR-PCR) (Fig. S7) and sequencing of the LCR. We used the soft-agar overlay method to visualize multiplicity of infection (MOI)-dependent host-cell killing by the engineered B025 phages (Fig. 4D). As expected, synthetic B025 WT phages integrated readily, and lysogenization effectively prevented subsequent killing of the host, especially at higher MOIs. As a result, a seemingly unperturbed bacterial lawn, which consisted of homoimmune WSLC 3009::B025 lysogens (Fig. S8), was obtained after overnight incubation (B025 WT 105/106 pfu) (Fig. 4D). In contrast, the virulent B025 derivatives B025 Δrep and B025 ΔLCR displayed strongly enhanced killing efficacy and lysed almost all host cells at an identical MOI (105/106 pfu) (Fig. 4D). Only a few survivors were able to regrow upon infection with the mutants after 48 h (Fig. 4D, 3-fold magnified areas). These survivors were not genetically resistant to B025 but regained sensitivity within one passage. We found that they featured an empty integration site (Fig. S8), confirming the lack of lysogenization by LCR-mutant phages. A similar approach was applied to B. cereus phage TP21-L, from which we also removed either the repressor or the complete LCR (Fig. S9). Taken together, these results show that virulent conversion represents a fast and broadly applicable method to increase the arsenal of phages with strong lytic activity, an important prerequisite for applications in biocontrol and especially for phage therapy.
Fig. 4.
Engineering of a strictly lytic virus by converting the life-style of temperate phage B025. Genome assembly and rebooting were used to produce mutants of the temperate Listeria phage B025 lacking either the repressor of the lytic genes (Δrep) or the complete lysogeny control region (ΔLCR). (A) The phage engineering strategy is shown. Arrows indicate primers for fragment amplification. Genome fragments f3 and f4 were designed to exclude the repressor or the complete LCR, yielding (B) five PCR fragments for genome assembly. Synthetic genomes were rebooted in Rev2L and tested for plaque formation on L. ivanovii WSLC 3009. (C) Plaque phenotypes of synthetic WT and mutant phages are shown. (D) To visualize MOI-dependent host cell infection and killing, 200 μL of a WSLC 3009 culture was infected with increasing MOIs of B025 WT, Δrep, and ΔLCR phages using the soft-agar overlay technique and plates were incubated for 24 h, unless indicated otherwise. int, integrase; rep, repressor of the lytic cycle.
Genetic Arming Enables Control of Phage-Resistant Cells.
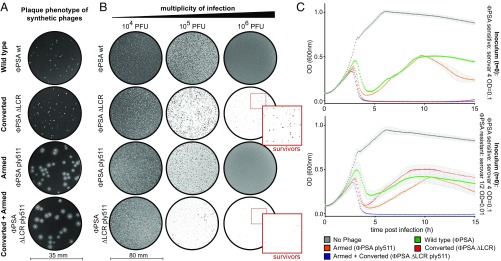
The occurrence and selection of phage-resistant cells within a bacterial target population can hamper the potential application of bacteriophages. As proof of concept, we addressed this limitation using phage PSA. This temperate virus specifically infects L. monocytogenes serovar 4b cells (38). Using the same strategy as for B025, we first removed the LCR of PSA to yield a virulent derivative that can no longer integrate into the tRNA attB site (PSA ΔLCR, virulent conversion) (37, 39). To target phage-resistant cells, we introduced an additional effector gene into synthetic PSA WT and PSA ΔLCR phage genomes as genetic payload, a strategy we refer to as “arming.” The effector is synthesized during the lytic cycle, coreleased upon host cell lysis, and subsequently acts on neighboring cells. Here, PSA WT and PSA ΔLCR phages were armed with an additional, heterologous endolysin gene derived from Listeria phage A511 (ply511). In contrast to PSA’s endogenous endolysin (PlyPSA), Ply511 efficiently degrades cells of all Listeria serovars, including serovar 1/2 (40). The strategy for LCR deletion and incorporation of ply511 into the late gene cluster of PSA and PSA ΔLCR and the validation of recombinants are shown in Fig. S10. We found that expression of ply511 from modified PSA genomes results in the formation of very large plaques with a characteristic halo (Fig. 5A) caused by the diffusion and enzymatic activity of the A511 endolysin effector. As for phage B025, deletion of the PSA LCR induced a strong lytic phenotype in plate culture (Fig. 5B, 106 pfu). Compared with PSA ΔLCR, the combination of LCR deletion with ply511 expression (PSA ΔLCR ply511) significantly reduced the occurrence of survivors (Fig. 5B, magnified areas). Next, we tested the ability of all PSA derivatives to kill target bacteria in liquid culture. A PSA-sensitive culture of L. monocytogenes serovar 4b cells was infected at an MOI of 0.1, and OD was monitored over time (Fig. 5C, Upper). Alternatively, to determine the control of Listeria cells that are resistant to phage PSA infection, the sensitive host strain was mixed with a PSA-resistant strain (serovar 1/2) at a sensitive:resistant ratio of 10:1 (Fig. 5C, Lower). Because of rapid lysogenization and subsequent growth of homoimmune cells, both PSA WT and the armed but nonvirulent PSA derivative (PSA ply511) were unable to control bacterial growth in both assays. While virulent conversion was sufficient to control phage-sensitive cells, the sensitive:resistant host cell mixture could not be cleared by the virulent PSA derivative (Fig. 5C, PSA ΔLCR). However, by targeting via the released cell wall hydrolase payload, the combination of virulent conversion and enzymatic arming (Fig. 5C, PSA ΔLCR ply511) equipped the synthetic phage with the ability to efficiently control native host cells and PSA-resistant serovar 1/2 cells in mixed culture. In conclusion, our results demonstrate that rational design of synthetic phages can provide antimicrobial agents with specifically tailored killing functions.
Fig. 5.
Control of phage-resistant cells using a virulent and genetically armed PSA derivative. (A) Plaque phenotypes of PSA WT, PSA ΔLCR, PSA ply511, and PSA ΔLCR ply511 at 48 h postinfection. (B) To visualize host lysis in plate culture, 200 μL of a L. monocytogenes WSLC 1042 overnight culture was infected with increasing MOIs of WT, ΔLCR, and ply511-armed phages using the soft-agar overlay technique. Magnifications (2.6-fold) show surviving colonies that grew upon infection with ΦPSA ΔLCR and ΦPSA ΔLCR ply511. (C, Upper) To monitor host killing by PSA-derived phages in liquid medium infections, WSLC 1042 cells (Listeria serovar 4b) were infected with the indicated phages at an MOI of 0.1, and turbidity was monitored for 15 h. (Lower) To assess indirect targeting of phage-resistant cells by the released Ply511 effector, a mixture of PSA-sensitive (serovar 4) and PSA-insensitive (serovar 1/2, WSLC 1001) cells was exposed to PSA-derived phages in the same assay. Data are mean ± SD (n = 3). ply, phage endolysin.
Discussion
Synthetic genome rebooting allows rapid, selection-free engineering and production of genetically modified phages. It should be noted that, unlike CRISPR-based editing and other recombination-based strategies, this approach offers complete and unrestricted freedom of genome design and editing, including major rearrangements, hybrid phages, and even fully tailor-made genomes. Multiple modifications can be incorporated at once, in a single assembly. The method is fully independent of transformation and recombination efficiencies of the host organism and circumvents cloning of genes potentially toxic to an intermediate host cell. Moreover, Rev2L L-forms can be employed not only for rebooting of Listeria phage genomes but also for reactivation of viruses infecting Bacillus, Staphylococcus and likely other Gram-positive hosts. Thus, the synthetic platform is broadly applicable and does not require any additional specialized strains for recombinant phage construction.
The virulent conversion and arming approach demonstrates the utility of rebooting for the construction of phages with customized antimicrobial properties. This technology will facilitate modifications that have previously been very difficult or impossible, such as incorporation of reporter genes for pathogen detection or host-range alterations by modification of receptor-binding proteins. It will also allow the incorporation and phage-mediated production of any desired genetic payload. This may be of particular interest for the delivery of therapeutic genes to specific bacterial populations within complex environments, such as the gut microbiome. Because rebooting of synthetic genomes is highly efficient (Fig. S4), this platform technology will also permit screening of DNA fragment libraries in a directed evolution approach, for example using error-prone PCR. Potentially, directed evolution of defined genome fragments may allow the generation of phages with altered host range or increased stability and circulation half-life. Finally, many aspects of basic phage biology are still poorly understood, mostly due to the lack of efficient genetic tools for mutation, deletion, and molecular tagging of phage genes. We are convinced that the approach presented here will contribute substantially to an enhanced understanding of the biology of these versatile self-replicating units and will pave the way for novel phage-based applications beyond their use as biocontrol and detection agents.
Materials and Methods
L. monocytogenes strains WSLC 1042, WSLC 1001, and Mack, Listeria ivanovii WSLC 3009, B. thuringiensis HER 1211, and B. cereus HER 1399 were grown at 30 °C in 0.5× Brain Heart Infusion (BHI, Biolife Italiana) medium. S. aureus strains ATCC 19685 and 2638A were grown at 37 °C in 0.5× BHI medium. L. monocytogenes L-form strain Rev2L was grown at 32 °C in modified DM3 medium (5 g/L tryptone, 5 g/L yeast extract, 0.01% BSA, 500 mM succinic acid, 5 g/L sucrose, 20 mM K2HPO4, 11 mM KH2PO4, 20 mM MgCl2, pH 7.3) (41). Detailed methods for bacteriophage propagation and DNA extraction, L-form transfection and rebooting, in vitro assembly of synthetic genomes, time-course turbidity assay, and de novo phage sequencing are described in SI Materials and Methods. All primers used in this study are listed in Table S2.
Supplementary Material
Footnotes
Conflict of interest statement: The technology described in this manuscript is a pending patent.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714658115/-/DCSupplemental.
References
- 1.Schmelcher M, Loessner MJ. Application of bacteriophages for detection of foodborne pathogens. Bacteriophage. 2014;4:e28137. doi: 10.4161/bact.28137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endersen L, et al. Phage therapy in the food industry. Annu Rev Food Sci Technol. 2014;5:327–349. doi: 10.1146/annurev-food-030713-092415. [DOI] [PubMed] [Google Scholar]
- 3.Knoll BM, Mylonakis E. Antibacterial bioagents based on principles of bacteriophage biology: An overview. Clin Infect Dis. 2014;58:528–534. doi: 10.1093/cid/cit771. [DOI] [PubMed] [Google Scholar]
- 4.Reardon S. Phage therapy gets revitalized. Nature. 2014;510:15–16. doi: 10.1038/510015a. [DOI] [PubMed] [Google Scholar]
- 5.Wright A, Hawkins CH, Anggård EE, Harper DR. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol. 2009;34:349–357. doi: 10.1111/j.1749-4486.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 6.Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage. 2011;1:111–114. doi: 10.4161/bact.1.2.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper CJ, Khan Mirzaei M, Nilsson AS. Adapting drug approval pathways for bacteriophage-based therapeutics. Front Microbiol. 2016;7:1209. doi: 10.3389/fmicb.2016.01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nobrega FL, Costa AR, Kluskens LD, Azeredo J. Revisiting phage therapy: New applications for old resources. Trends Microbiol. 2015;23:185–191. doi: 10.1016/j.tim.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Shousha A, et al. Bacteriophages isolated from chicken meat and the horizontal transfer of antimicrobial resistance genes. Appl Environ Microbiol. 2015;81:4600–4606. doi: 10.1128/AEM.00872-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. Phage treatment of human infections. Bacteriophage. 2011;1:66–85. doi: 10.4161/bact.1.2.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labrie SJ, Samson JE, Moineau S. Bacteriophage resistance mechanisms. Nat Rev Microbiol. 2010;8:317–327. doi: 10.1038/nrmicro2315. [DOI] [PubMed] [Google Scholar]
- 12.Pires DP, Cleto S, Sillankorva S, Azeredo J, Lu TK. Genetically engineered phages: A review of advances over the last decade. Microbiol Mol Biol Rev. 2016;80:523–543. doi: 10.1128/MMBR.00069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu TK, Collins JJ. Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci USA. 2007;104:11197–11202. doi: 10.1073/pnas.0704624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bikard D, et al. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat Biotechnol. 2014;32:1146–1150. doi: 10.1038/nbt.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Citorik RJ, Mimee M, Lu TK. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat Biotechnol. 2014;32:1141–1145. doi: 10.1038/nbt.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiro R, Shitrit D, Qimron U. Efficient engineering of a bacteriophage genome using the type I-E CRISPR-Cas system. RNA Biol. 2014;11:42–44. doi: 10.4161/rna.27766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemay ML, Tremblay DM, Moineau S. Genome engineering of virulent lactococcal phages using CRISPR-Cas9. ACS Synth Biol. 2017;6:1351–1358. doi: 10.1021/acssynbio.6b00388. [DOI] [PubMed] [Google Scholar]
- 18.Martel B, Moineau S. CRISPR-Cas: An efficient tool for genome engineering of virulent bacteriophages. Nucleic Acids Res. 2014;42:9504–9513. doi: 10.1093/nar/gku628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao P, Wu X, Tang WC, Zhu J, Rao V. Engineering of bacteriophage T4 genome using CRISPR-Cas9. ACS Synth Biol. 2017;6:1952–1961. doi: 10.1021/acssynbio.7b00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bundy BC, Franciszkowicz MJ, Swartz JR. Escherichia coli-based cell-free synthesis of virus-like particles. Biotechnol Bioeng. 2008;100:28–37. doi: 10.1002/bit.21716. [DOI] [PubMed] [Google Scholar]
- 21.Shin J, Jardine P, Noireaux V. Genome replication, synthesis, and assembly of the bacteriophage T7 in a single cell-free reaction. ACS Synth Biol. 2012;1:408–413. doi: 10.1021/sb300049p. [DOI] [PubMed] [Google Scholar]
- 22.Ando H, Lemire S, Pires DP, Lu TK. Engineering modular viral scaffolds for targeted bacterial population editing. Cell Syst. 2015;1:187–196. doi: 10.1016/j.cels.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaschke PR, Lieberman EK, Rodriguez J, Sierra A, Endy D. A fully decompressed synthetic bacteriophage øX174 genome assembled and archived in yeast. Virology. 2012;434:278–284. doi: 10.1016/j.virol.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Dell’Era S, et al. Listeria monocytogenes L-forms respond to cell wall deficiency by modifying gene expression and the mode of division. Mol Microbiol. 2009;73:306–322. doi: 10.1111/j.1365-2958.2009.06774.x. [DOI] [PubMed] [Google Scholar]
- 25.Allan EJ, Hoischen C, Gumpert J. Bacterial L-forms. Adv Appl Microbiol. 2009;68:1–39. doi: 10.1016/S0065-2164(09)01201-5. [DOI] [PubMed] [Google Scholar]
- 26.White TB, Doyle RJ, Streips UN. Transformation of a Bacillus subtilis L-form with bacteriophage deoxyribonucleic acid. J Bacteriol. 1981;145:878–883. doi: 10.1128/jb.145.2.878-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Studer P, et al. Proliferation of Listeria monocytogenes L-form cells by formation of internal and external vesicles. Nat Commun. 2016;7:13631. doi: 10.1038/ncomms13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorscht J, et al. Comparative genome analysis of Listeria bacteriophages reveals extensive mosaicism, programmed translational frameshifting, and a novel prophage insertion site. J Bacteriol. 2009;191:7206–7215. doi: 10.1128/JB.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monk IR, Gahan CG, Hill C. Tools for functional postgenomic analysis of listeria monocytogenes. Appl Environ Microbiol. 2008;74:3921–3934. doi: 10.1128/AEM.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klumpp J, Loessner MJ. Listeria phages: Genomes, evolution, and application. Bacteriophage. 2013;3:e26861. doi: 10.4161/bact.26861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klumpp J, Calendar R, Loessner MJ. Complete nucleotide sequence and molecular characterization of Bacillus phage TP21 and its relatedness to other phages with the same name. Viruses. 2010;2:961–971. doi: 10.3390/v2040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klumpp J, et al. The odd one out: Bacillus ACT bacteriophage CP-51 exhibits unusual properties compared to related Spounavirinae W.Ph. and Bastille. Virology. 2014;462-463:299–308. doi: 10.1016/j.virol.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Kwan T, Liu J, DuBow M, Gros P, Pelletier J. The complete genomes and proteomes of 27 Staphylococcus aureus bacteriophages. Proc Natl Acad Sci USA. 2005;102:5174–5179. doi: 10.1073/pnas.0501140102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill JJ. Revised genome sequence of Staphylococcus aureus bacteriophage K. Genome Announc. 2014;2:e01173-13. doi: 10.1128/genomeA.01173-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson DG, et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 36.Weigel C, Seitz H. Bacteriophage replication modules. FEMS Microbiol Rev. 2006;30:321–381. doi: 10.1111/j.1574-6976.2006.00015.x. [DOI] [PubMed] [Google Scholar]
- 37.Zimmer M, Sattelberger E, Inman RB, Calendar R, Loessner MJ. Genome and proteome of Listeria monocytogenes phage PSA: An unusual case for programmed + 1 translational frameshifting in structural protein synthesis. Mol Microbiol. 2003;50:303–317. doi: 10.1046/j.1365-2958.2003.03684.x. [DOI] [PubMed] [Google Scholar]
- 38.Farber JM, Peterkin PI. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lauer P, Chow MY, Loessner MJ, Portnoy DA, Calendar R. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J Bacteriol. 2002;184:4177–4186. doi: 10.1128/JB.184.15.4177-4186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loessner MJ, Wendlinger G, Scherer S. Heterogeneous endolysins in Listeria monocytogenes bacteriophages: A new class of enzymes and evidence for conserved holin genes within the siphoviral lysis cassettes. Mol Microbiol. 1995;16:1231–1241. doi: 10.1111/j.1365-2958.1995.tb02345.x. [DOI] [PubMed] [Google Scholar]
- 41.Chang S, Cohen SN. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979;168:111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.