Significance
We demonstrate that STAT3 is a critical transcriptional regulator of the activated B cell–like subtype of diffuse large B cell lymphoma (ABC DLBCL), the most common, aggressive, non-Hodgkin lymphoma. By genome-wide assessment, we have identified target genes of STAT3. Gene regulation by STAT3 in ABC DLBCL accentuates survival signaling pathways while dampening the lethal type I interferon pathway. Knowledge of these STAT3-regulated genes has led to our demonstration that a small-molecule inhibitor in the JAK1-STAT3 signaling pathway synergizes with the type I interferon inducer lenalidomide, suggesting a new therapeutic strategy for ABC DLBCL, a subtype that is particularly difficult to treat and has poor prognosis.
Keywords: STAT3, interferon, diffuse large B cell lymphoma
Abstract
STAT3 is constitutively activated in many cancers and regulates gene expression to promote cancer cell survival, proliferation, invasion, and migration. In diffuse large B cell lymphoma (DLBCL), activation of STAT3 and its kinase JAK1 is caused by autocrine production of IL-6 and IL-10 in the activated B cell–like subtype (ABC). However, the gene regulatory mechanisms underlying the pathogenesis of this aggressive lymphoma by STAT3 are not well characterized. Here we performed genome-wide analysis and identified 2,251 STAT3 direct target genes, which involve B cell activation, survival, proliferation, differentiation, and migration. Whole-transcriptome profiling revealed that STAT3 acts as both a transcriptional activator and a suppressor, with a comparable number of up- and down-regulated genes. STAT3 regulates multiple oncogenic signaling pathways, including NF-κB, a cell-cycle checkpoint, PI3K/AKT/mTORC1, and STAT3 itself. In addition, STAT3 negatively regulates the lethal type I IFN signaling pathway by inhibiting expression of IRF7, IRF9, STAT1, and STAT2. Inhibition of STAT3 activity by ruxolitinib synergizes with the type I IFN inducer lenalidomide in growth inhibition of ABC DLBCL cells in vitro and in a xenograft mouse model. Therefore, this study provides a mechanistic rationale for clinical trials to evaluate ruxolitinib or a specific JAK1 inhibitor combined with lenalidomide in ABC DLBCL.
Diffuse large B cell lymphoma (DLBCL), the most common non-Hodgkin lymphoma, includes two main molecular subtypes: an activated B cell–like (ABC) and a germinal center B cell–like (GCB) (1, 2). ABC DLBCL is more aggressive, with autocrine signaling from the cytokines IL-6 and IL-10 that constitutively activates JAK1 (3) and STAT3 (4–7) to promote cell survival. ABC DLBCL cells have high NF-κB activity (8) due to genetic alterations in the Toll-like receptor (TLR) and B cell receptor signaling pathways (9–11). Somatic mutations of MYD88 (mainly L265P), a key signaling adaptor in TLR signaling, engage the NF-κB pathway and induce production of IL-6, IL-10, and IFNβ (11). In contrast to IL-6 and IL-10, IFNβ is proapoptotic, and its basal level is low to undetectable in ABC DLBCL cells (12). IFNβ production can be prevented by the transcription factors IRF4 and SPIB through repression of IRF7, a transcription factor for IFNβ expression (12).
Recently, we revealed that, in addition to STAT3 activation, JAK1 is present in the nucleus and directly phosphorylates histone H3 on tyrosine 41 (H3Y41) to induce expression of nearly 3,000 genes, including the NF-κB pathway genes MYD88 and IRF4 (3, 13). These findings suggest a positive feedback loop between the cytokine and NF-κB signaling pathways that promotes the malignant phenotype of ABC DLBCL cells. This epigenetic gene regulatory mechanism by JAK1 is distinct from the canonical JAK-STAT signaling pathway, given that more than 90% of these genes do not bear a STAT motif in their promoter region (3). Using STAT3 chromatin immunoprecipitation followed by DNA sequencing (ChIP-seq) and whole-genome transcriptome (RNA-seq) analysis in ABC DLBCL cell lines and control GCB DLBCL cell lines that lack STAT3 activation, a recent study has identified a total of 10,337 STAT3-binding regions corresponding to 8,531 genes, of which 1,545 genes are differently expressed between the two subtypes and largely associated with ABC DLBCL biology (14). However, biological significance and functional association of these STAT3 targets in ABC DLBCL cells remain to be studied.
Here, we use genetic and pharmacological inhibition of STAT3 in ABC DLBCL cells and identify genes that are directly regulated by STAT3. These STAT3 target genes are involved in multiple signaling pathways, which promote cancer cell survival and proliferation. In addition, STAT3 negatively regulates lethal type I signaling by inhibiting expression of IRF7, IRF9, STAT1, and STAT2, key transcription factors for IFNβ production and signaling.
Results
Genome-Wide Analysis Identifies STAT3 Transcriptional Target Genes in ABC DLBCL Cells.
To identify STAT3 target genes genome-wide, we performed STAT3 ChIP-seq in the ABC DLBCL cell lines TMD8 and OCI-Ly10, in which high levels of STAT3 phosphorylation were detected by immunoblot analysis (Fig. 1A). Since STAT3 activity was sufficiently inhibited by the JAK1/JAK2 inhibitor AZD1480 (Fig. 1A) and this inhibitor was used for H3Y41-P ChIP-seq analysis (3), AZD1480-treated cells served as a control for STAT3 ChIP-seq experiments. Using the model-based analysis of ChIP-seq (MACS) for peak calling (16), we identified a total of 11,487 STAT3-binding sites (peaks) in TMD8 cells and 22,856 in OCI-Ly10 cells compared with the AZD1480-treated control sample (Fig. 1B and Dataset S1). Specificity of these STAT3-binding sites was confirmed by the MEME motif enrichment analysis (15), with a similar distribution pattern of STAT3 motifs between the two cell lines (Fig. 1C).
Fig. 1.
Genome-wide analysis reveals STAT3 transcriptional targets in ABC DLBCL. (A) Immunoblot analysis of phospho-STAT3 and total STAT3 protein in TMD8 and OCI-Ly10 cells treated with 2 μM AZD1480. (B) Heat maps of STAT3 ChIP-seq in TMD8 and OCI-Ly10 cells after 4 h of treatment with either DMSO or 2 μM AZD1480. STAT3 peak summits were centered within 5 kb of the flanking sequence on either side. Darker color indicates a higher density of reads. STAT3 peaks were ranked by signal intensity at the peak center, and the same order is used to display the AZD1480-treated sample. (C) The CentriMo plots show the distribution of known the STAT3 motif in the ChIP-seq peak summit regions (P < 0.001). De novo motif discovery from STAT3 ChIP-seq peaks shows identical sequence logs to the known STAT consensus motif (15). (D) Venn diagram shows 2,251 STAT3-binding genes that are shared in TMD8 and OCI-Ly10 cells. (E) Gene ontology analysis of the 2,251 STAT3-binding genes (P < 0.05).
Based on genomic loci of these peaks, we mapped near a protein-coding gene within a window extending from −15 kb 5′ of the transcriptional start site to the 3′ end of any annotated transcript associated with the gene, as for our previous study (3). We identified 4,746 potential STAT3 target genes in TMD8 cells and 6,058 in OCI-Ly10 cells, with an overlap of 2,251 genes between the two cell lines (Fig. 1D and Dataset S1). Considering these overlapped genes as common STAT3 targets in ABC DLBCL, we performed PANTHER gene ontology analysis (17). The results revealed that these common target genes were enriched for biological processes that include B cell activation, proliferation, differentiation, cell-cycle progression, stress response, cell migration, and metabolism (Fig. 1E), suggesting an important role for STAT3 in the pathogenesis of ABC DLBCL.
Whole-Transcriptome Profiling Reveals That STAT3 Acts as both a Transcriptional Activator and a Repressor in ABC DLBCL.
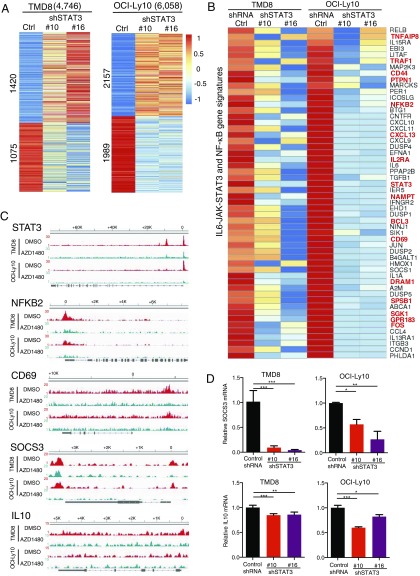
To determine genes that are directly regulated by STAT3, we performed the whole-transcriptome analysis by RNA-seq in the above TMD8 and OCI-Ly10 cell lines. We knocked down STAT3 by two shRNAs from our previous study (7). As shown in Fig. 2A, 53% (2,495/4,746) of the STAT3 target genes in TMD8 cells and 68% (4,146/6,058) in OCI-Ly10 cells changed their expression when STAT3 was knocked down. Of note, the number of down-regulated genes was comparable to that of up-regulated genes, suggesting that STAT3 functions as both a transcriptional activator and a repressor in ABC DLBCL cells.
Fig. 2.
Whole-transcriptome profiling reveals signaling pathways regulated by STAT3 in ABC DLBCL. (A) Heat maps show expression changes of STAT3-binding genes in TMD8 and OCI-Ly10 cells after 2 d of knockdown of STAT3 by two shRNAs. (B) Heat maps show down-regulation of IL6-JAK-STAT3 and NF-κB pathway genes by two STAT3 shRNAs. STAT3-binding genes are shown in red. (C) STAT3 is recruited to regulatory regions of indicated genes, as shown by read density tracks (DMSO controls in red, AZD1480-treated samples in green). (D) Quantitative PCR analysis of SOCS3 and IL-10 mRNA levels (normalized to GAPDH mRNA levels) in TMD8 and OCI-LY10 cells after 1 d (only for SOCS3 in TMD8) or 2 d of knockdown of STAT3 by shRNAs. Error bars represent mean ± SD of triplicates (*P < 0.05, **P < 0.01, ***P < 0.001).
Next, we performed gene set enrichment analysis (GSEA) to identify the signaling pathways in which these up-regulated and down-regulated genes are involved. The results revealed significant enrichment in gene signature of multiple signaling pathways (Fig. S1), including the PI3K/AKT/mTORC1 (Fig. S2), E2F/G2M cell-cycle checkpoint (Fig. S3), IL6-JAK-STAT3, NF-κB, and type I IFN signaling pathways. Consistent with previous studies (4, 5, 14), STAT3 is involved in the positive feedback regulation of the IL-6/IL-10 signaling and shows crosstalk with NF-κB signaling pathways in ABC DLBCL cells (Fig. 2 B–D). We verified SOCS3, a negative regulator of JAK-STAT signaling (18), as a target gene of STAT3 (Fig. 2D). More significantly, 16 of the genes down-regulated by STAT3 shRNAs are direct STAT3 targets, including TNFAIP8, TRAF1, CD44, CD69, BCL3, FOS, SGK1, NF-κB2, and STAT3 itself (Fig. 2 B and C).
STAT3 Suppresses Type I IFN Signaling in ABC DLBCL.
In ABC DLBCL, production of the proapoptotic cytokine IFNβ can result from oncogenic MYD88 mutations (11). This type I IFN signaling is suppressed by the transcription factors IRF4 and SPIB, which repress IRF7 expression to prevent IFNβ transcription and TYK2 activation (12). Our recent work demonstrated that IRF4 and SPIB are epigenetic targets of JAK1 due to H3Y41 phosphorylation, but IRF4 expression is not regulated by STAT3 (3). These findings along with the above RNA-seq analysis prompted us to investigate whether STAT3 directly targets critical genes in the IFNβ signaling pathway. It is known that, in response to IFNβ, STAT1 and STAT2 are phosphorylated, together with IRF9, to form the tripartite transcription factor IFN-stimulated factor gene 3 (ISGF3), which binds to distinct IFN-stimulated elements of genomic DNA for gene transcription (19, 20).
Indeed, STAT3 ChIP-seq data displayed peaks in the promotor region, near the transcription start sites of STAT2, IRF7, and IRF9, and a peak in the enhancer region of STAT1. These peaks were significantly reduced after AZD1480 treatment, suggesting that they are direct targets of STAT3 (Fig. 3A). Increased expression of STAT1, STAT2, and IRF9 was observed when STAT3 was knocked down by two different shRNAs in both TMD8 and OCI-Ly10 cell lines (Fig. 3B). Immunoblot analysis confirmed that protein levels of STAT1, STAT2, IRF7, and IRF9 were all increased by STAT3 shRNA in these two ABC DLBCL cell lines but not in the control GCB cell line SUDHL7 (Fig. 3C). In addition, phosphorylation of STAT1 and STAT2 was remarkably increased in the STAT3 shRNA expressing ABC DLBCL cells (Fig. 3C). To further validate these results, we used the constitutively activated form of STAT3 (STAT3-C) with activating mutations (A661C and N663C) in the SH2 domain (21). Retroviral expression of STAT3-C in OCI-Ly10 and HBL1 ABC DLBCL cells reduced IRF7 and IRF9 expression and completely removed STAT1 phosphorylation (Fig. 3D). Taken together, these data suggest that STAT3 activity blocks the type I IFN signaling pathway by inhibiting expression of multiple essential signaling components, including STAT1, STAT2, IRF7, and IRF9.
Fig. 3.
STAT3 suppresses type I IFN signaling in ABC DLBCL. (A) STAT3 is recruited to regulatory regions of STAT1, STAT2, IRF7, and IRF9, as shown by read density tracks (DMSO controls in red, AZD1480-treated samples in green). (B) Heat maps show expression changes of type I IFN pathway genes in TMD8 and OCI-Ly10 cells after 2 d of knockdown of STAT3 by two shRNAs. STAT3-binding genes are shown in red. (C) Immunoblot analysis of the indicated proteins in TMD8, OCI-Ly10, and the control cell line SUDHL7 after 2 d of knockdown of STAT3 by shRNA. (D) Immunoblot analysis of the indicated proteins in TMD8 and HBL1 cells after 2 or 4 d of retroviral expression of constitutively activated STAT3 (STAT3-C).
Synergism Between STAT3 Inhibition and Lenalidomide in Growth Inhibition of ABC DLBCL.
Lenalidomide, an active agent in ABC DLBCL, induces type I IFN response by down-regulation of IRF4 and SPIB, which otherwise inhibit IRF7 expression (12, 22). Given partial inhibition of IRF4 expression by lenalidomide (12) and that IRF4 is not a direct target of STAT3, we hypothesized that inhibition of STAT3 activity augments IFNβ production and synergizes with lenalidomide in killing ABC DLBCL cells. To test this hypothesis, we performed an in vitro survival assay in TMD8 and OCI-Ly10 cells when STAT3 shRNA was induced for expression in the presence of lenalidomide. We used the GCB DLBCL cell line SUDHL7 as a control. As shown in Fig. 4A, after 3 d of culture, both STAT3 shRNA and lenalidomide significantly reduced cell viability in the two ABC DLBCL cell lines but not in the control. Of note, expression of STAT3 shRNA increased lenalidomide-mediated cytotoxicity in these ABC DLBCL cultures (Fig. 4A). A reduction in viable cells was mainly due to inhibition of cell proliferation (Fig. S3B) although a slight increase in apoptosis was observed (Fig. S4). Quantitative PCR analysis confirmed that IFNβ expression was significantly increased in cells that expressed STAT3 shRNA and were treated with lenalidomide, consistent with the above survival assay demonstrating a cytotoxic synergism between STAT3 shRNA and lenalidomide (Fig. 4B).
Fig. 4.
Synergism between STAT3 inhibition and lenalidomide in growth inhibition of ABC DLBCL. (A) STAT3 shRNA enhanced lenalidomide-mediated toxicity in TMD8 and OCI-Ly10 cells but not in control SUDHL7 cells. The 4,000 cells per well from each cell line were plated on a 96-well plate and induced with doxycycline for expression of STAT3 shRNA. No doxycycline cultures served as a control. Total live cells were counted with trypan blue exclusion assay after 3 d of shRNA induction in the presence of 4 μM lenalidomide or DMSO. Data are presented as mean ± SD of triplicates (*P < 0.05, **P < 0.01). (B) Quantitative PCR analysis of IFNβ mRNA levels (normalized to GAPDH mRNA levels) in TMD8 and OCI-Ly10 cells after 3 d of knockdown of STAT3 by shRNAs in the presence of 4 μM lenalidomide or DMSO. Error bars represent mean ± SD of triplicates (*P < 0.05, **P < 0.01, ***P < 0.001). (C) Immunoblot analysis of phospho-STAT3 and β-actin loading control in TMD8 and OCI-Ly10 cells after 30 min of treatment with the indicated concentrations of ruxolitinib. (D) Synergism between ruxolitinib and lenalidomide in cell killing in TMD8 and OCI-Ly10 cells. Cells were treated with ruxolitinib and lenalidomide for 5 d before trypan blue dye exclusion viability assay. Combination index (CI) was calculated with CompuSyn software. (E) Cell-cycle analysis of TMD8 and OCI-Ly10 cells after 5 d of ruxolitinib and lenalidomide. Cells were pulsed with 10 µM BrdU for 3 h before flow cytometric analysis. (F) The combination therapy of lenalidomide with ruxolitinib significantly inhibits OCI-Ly10 tumor growth in vivo. Female NOD/SCID mice were injected s.c. with 1 × 107 OCI-Ly10 cells. Ten days later, lenalidomide was given i.p. at a dose of 15 mg/kg/d for 14 d, and ruxolitinib was continuously administered by an s.c. miniosmotic pump at a dose of 60 mg/kg/d for 14 d. Shown is a photograph of tumors from each group at day 15 (Top) and average tumor volumes during the therapeutic course for each group (Bottom). Error bars show the SD of eight mice/group (*P < 0.05, **P < 0.01, lenalidomide or ruxolitinib group compared with vehicle control group; ***P < 0.001, combination group compared with lenalidomide or ruxolitinib group).
To further examine the above synergistic effect, we used ruxolitinib, a clinically used JAK1 and JAK2 inhibitor (23), to inhibit STAT3 activity. Immunoblot analysis confirmed dose-dependent inhibition of STAT3 phosphorylation in both TMD8 and OCI-Ly10 cell lines (Fig. 4C). As expected, we observed a synergism between ruxolitinib and lenalidomide in killing these cells (Fig. 4D). Cell-cycle analysis revealed that a combination of the two drugs increased G1 phase population (Fig. 4E), suggesting inhibition of cell proliferation. More importantly, our xenograft analysis in the OCI-Ly10 cell line demonstrated that cotreatment of ruxolitinib and lenalidomide caused nearly complete tumor growth inhibition during the period of treatment, but the single drug treatment achieved only partial inhibition (Fig. 4F). Thus, these data suggest that the ruxolitinib and lenalidomide combination is a potential therapeutic strategy for ABC DLBCL cases.
Discussion
Deregulation of the JAK-STAT signaling pathway, such as constitutive activation of STAT3, plays a pathogenic role in many hematologic malignancies (24–26). In DLBCL, STAT3 is activated in the ABC subtype by IL-6/IL-10 and JAK1 to promote cancer cell survival (3–7). STAT3 activity is also associated with a poor prognosis in DLBCL (27, 28). Here, we conducted genome-wide assessment and established a working model of STAT3 in the pathogenesis of ABC DLBCL (Fig. 5). STAT3 acts as both a transcriptional activator and a suppressor. Gene set enrichment analysis revealed that genes regulated by STAT3 are involved in several oncogenic signaling pathways, including NF-κB, PI3K/AKT/mTORC1, cell-cycle checkpoint, and STAT3 itself. Notably, STAT3 suppresses expression of STAT1, STAT2, IRF7, and IRF9, all of which are critical transcription factors in the type I IFN pathway. Thus, gene regulation by STAT3 in ABC DLBCL accentuates the survival signaling pathways while dampening the lethal type I IFN pathway.
Fig. 5.
Schematic illustration of gene regulation by STAT3 in ABC DLBCL. JAK1 and STAT3 are activated by autocrine IL-6/IL-10 due to the MYD88 L265P mutation. STAT3 regulates expression of genes that involve many oncogenic pathways, including NF-κB, PI3K, cell cycle, and itself, signaling to promote cancer cell survival and proliferation while suppressing proapoptotic type I IFN signaling.
Crosstalk between the NF-κB and IL-6/IL-10/JAK1/STAT3 signaling pathways is an oncogenic process in ABC DLBCL (8, 29). Through histone H3 phosphorylation but independent of STAT3, JAK1 up-regulates expression of IRF4 and MYD88, which is required for cancer cell survival (3). The present study revealed that many other genes in the NF-κB pathway are STAT3 targets, including NF-κB2 and TRAF1. Interestingly, NF-κB2 signaling is associated with MYD88 mutations and promotes development of the ABC subtype (30). In a transgenic mouse model, TRAF1 is involved in lymphomagenesis mediated by constitutively activated NF-κB2 (31). These findings suggest a role for the noncanonical NF-κB pathway in the pathogenesis of ABC DLBCL. Disruption of oncogenic loops between the NF-κB and JAK1/STAT3 signaling pathways by their small-molecule inhibitors produces the synergistic cytotoxicity in ABC DLBCL (3, 4, 32).
Several biochemical studies have found a phenomenon of STAT3-mediated suppression of IFN antiviral responses in immune cells (33–36). In ABC DLBCL, the type I IFN signaling pathway, which can be activated by the MYD88 L265P mutation, is proapoptotic to the cancer cells (11) (Fig. 5). Our integrated genomic analysis elucidates the molecular mechanisms of STAT3 in suppression of this lethal pathway in ABC DLBCL: active STAT3 prevents the cancer cells from producing IFNβ through inhibition of IRF7 expression and also suppresses transcription of STAT1, STAT2, and IRF9 (ISGF3 complex) to block IFNβ signaling. The multilayer suppression of IFNβ signaling by STAT3 is one of the major mechanisms by which autocrine IL-6/IL-10 signaling prevents cancer cell death. In addition, this cytokine signaling inhibits IFNβ production through the JAK1-mediated epigenetic mechanism; that is, JAK1 targets histone H3 to induce expression of IRF4 and SPIB, which form a transcription complex to inhibit IRF7 expression (3, 12).
The type I IFN signaling pathway has emerged as an effective therapeutic target in DLBCL. Recent clinical trials have revealed that lenalidomide treatment alone or combined with immunochemotherapy achieved promising efficacy in DLBCL (37–39). However, remission after a single lenalidomide treatment lasted for only 6 mo (37), suggesting that combinations of targeted agents that inhibit distinct survival pathways will be necessary. Our findings demonstrated that STAT3 regulates expression of genes that involve multiple oncogenic pathways and suppresses genes in the type I IFN signaling pathway. Inhibition of STAT3 activity by ruxolitinib synergizes with lenalidomide in growth inhibition of ABC DLBCL cells in vitro and in a xenograft mouse model. Therefore, this study provides a mechanistic rationale for clinical trials of ruxolitinib or a specific JAK1 inhibitor and lenalidomide in ABC DLBCL.
Materials and Methods
Full details of the methods used and data analysis are presented in SI Materials and Methods.
Cell Lines and Culture.
All doxycycline-inducible human DLBCL cell lines that express the bacterial tetracycline repressor were engineered as described previously (40). Doxycycline (20 ng/mL) was used to induce the expression of genes or shRNAs of interest. All cultures were routinely tested for mycoplasma contamination.
ChIP-Seq Analysis.
ChIP-enriched DNA samples were used to create adapter-ligated libraries for massively parallel sequencing with the Ovation Ultralow Library System V2 (NuGen Technologies) following the manufacturer’s protocol. ChIP-Seq data are available at https://www.ncbi.nlm.nih.gov/geo/ (accession no. GSE106844).
RNA-Seq Analysis.
Total RNA was extracted using RNeasy plus mini kit (Qiagen) according to the manufacturer’s protocol. RNA-seq libraries were prepared by using the Illumina TruSeq stranded mRNA LT sample preparation kit (Illumina). Sequencing was performed on Illumina Hiseq 2500 at 50-bp length. RNA-seq data are available at https://www.ncbi.nlm.nih.gov/geo/ (accession no. GSE106844).
Xenografts.
The xenograft tumor model of human ABC DLBCL lymphoma was established by s.c. injection of OCI-Ly10 cells into nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice (Jackson Labs). The tumor growth was monitored by measuring tumor size in two orthogonal dimensions. All animal experiments were approved by the National Cancer Institute Animal Care and Use Committee (NCI ACUC) and were performed in accordance with NCI ACUC guidelines.
Supplementary Material
Acknowledgments
This research was supported by the University of Wisconsin, Madison, Start-up Funds and KL2 Scholar Award (UL1TR0000427 and KL2TR000428); the National Cancer Institute (Grant 1R01 CA187299) (to L.R.); the University of Wisconsin, Madison, T32 Hematology Training Award (T32 HL07899) (to A.C.D.); the University of Wisconsin Forward Lymphoma Fund (L.L. and S.K.); and the Intramural Research Program of the National Cancer Institute (T.A.W.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1715118115/-/DCSupplemental.
References
- 1.Alizadeh AA, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 2.Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med. 2010;362:1417–1429. doi: 10.1056/NEJMra0807082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rui L, et al. Epigenetic gene regulation by Janus kinase 1 in diffuse large B-cell lymphoma. Proc Natl Acad Sci USA. 2016;113:E7260–E7267. doi: 10.1073/pnas.1610970113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam LT, et al. Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-kappaB pathways in subtypes of diffuse large B-cell lymphoma. Blood. 2008;111:3701–3713. doi: 10.1182/blood-2007-09-111948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding BB, et al. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood. 2008;111:1515–1523. doi: 10.1182/blood-2007-04-087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scuto A, et al. STAT3 inhibition is a therapeutic strategy for ABC-like diffuse large B-cell lymphoma. Cancer Res. 2011;71:3182–3188. doi: 10.1158/0008-5472.CAN-10-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng M, et al. A mix of S and ΔS variants of STAT3 enable survival of activated B-cell-like diffuse large B-cell lymphoma cells in culture. Oncogenesis. 2016;4:e184. doi: 10.1038/oncsis.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staudt LM. Oncogenic activation of NF-kappaB. Cold Spring Harb Perspect Biol. 2010;2:a000109. doi: 10.1101/cshperspect.a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis RE, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Compagno M, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717–721. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngo VN, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell. 2012;21:723–737. doi: 10.1016/j.ccr.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu F, Hwang B, Miyamoto S, Rui L. Nuclear import of JAK1 is mediated by a classical NLS and is required for survival of diffuse large B-cell lymphoma. Mol Cancer Res. 2017;15:348–357. doi: 10.1158/1541-7786.MCR-16-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardee J, et al. STAT3 targets suggest mechanisms of aggressive tumorigenesis in diffuse large B-cell lymphoma. G3 (Bethesda) 2013;3:2173–2185. doi: 10.1534/g3.113.007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey TL, et al. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, et al. Model-based analysis of ChIP-seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8:1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krebs DL, Hilton DJ. SOCS: Physiological suppressors of cytokine signaling. J Cell Sci. 2000;113:2813–2819. doi: 10.1242/jcs.113.16.2813. [DOI] [PubMed] [Google Scholar]
- 19.Borden EC, et al. Interferons at age 50: Past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheon H, et al. IFNβ-dependent increases in STAT1, STAT2, and IRF9 mediate resistance to viruses and DNA damage. EMBO J. 2013;32:2751–2763. doi: 10.1038/emboj.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bromberg JF, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 22.Gribben JG, Fowler N, Morschhauser F. Mechanisms of action of lenalidomide in B-cell non-Hodgkin lymphoma. J Clin Oncol. 2015;33:2803–2811. doi: 10.1200/JCO.2014.59.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mesa RA, Yasothan U, Kirkpatrick P. Ruxolitinib. Nat Rev Drug Discov. 2012;11:103–104. doi: 10.1038/nrd3652. [DOI] [PubMed] [Google Scholar]
- 24.O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen E, Staudt LM, Green AR. Janus kinase deregulation in leukemia and lymphoma. Immunity. 2012;36:529–541. doi: 10.1016/j.immuni.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldmann TA, Chen J. Disorders of the JAK/STAT pathway in T cell lymphoma pathogenesis: Implications for immunotherapy. Annu Rev Immunol. 2017;35:533–550. doi: 10.1146/annurev-immunol-110416-120628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang X, et al. Activation of the STAT3 signaling pathway is associated with poor survival in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2013;31:4520–4528. doi: 10.1200/JCO.2012.45.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ok CY, et al. Clinical implications of phosphorylated STAT3 expression in de novo diffuse large B-cell lymphoma. Clin Cancer Res. 2014;20:5113–5123. doi: 10.1158/1078-0432.CCR-14-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rui L, Schmitz R, Ceribelli M, Staudt LM. Malignant pirates of the immune system. Nat Immunol. 2011;12:933–940. doi: 10.1038/ni.2094. [DOI] [PubMed] [Google Scholar]
- 30.Guo X, et al. Molecular impact of selective NFKB1 and NFKB2 signaling on DLBCL phenotype. Oncogene. 2017;36:4224–4232. doi: 10.1038/onc.2017.90. [DOI] [PubMed] [Google Scholar]
- 31.Zhang B, Wang Z, Li T, Tsitsikov EN, Ding HF. NF-kappaB2 mutation targets TRAF1 to induce lymphomagenesis. Blood. 2007;110:743–751. doi: 10.1182/blood-2006-11-058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly PN, et al. Selective interleukin-1 receptor-associated kinase 4 inhibitors for the treatment of autoimmune disorders and lymphoid malignancy. J Exp Med. 2015;212:2189–2201. doi: 10.1084/jem.20151074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qing Y, Stark GR. Alternative activation of STAT1 and STAT3 in response to interferon-gamma. J Biol Chem. 2004;279:41679–41685. doi: 10.1074/jbc.M406413200. [DOI] [PubMed] [Google Scholar]
- 34.Costa-Pereira AP, et al. Mutational switch of an IL-6 response to an interferon-gamma-like response. Proc Natl Acad Sci USA. 2002;99:8043–8047. doi: 10.1073/pnas.122236099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahony R, et al. A novel anti-viral role for STAT3 in IFN-α signalling responses. Cell Mol Life Sci. 2017;74:1755–1764. doi: 10.1007/s00018-016-2435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho HH, Ivashkiv LB. Role of STAT3 in type I interferon responses. Negative regulation of STAT1-dependent inflammatory gene activation. J Biol Chem. 2006;281:14111–14118. doi: 10.1074/jbc.M511797200. [DOI] [PubMed] [Google Scholar]
- 37.Wiernik PH, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:4952–4957. doi: 10.1200/JCO.2007.15.3429. [DOI] [PubMed] [Google Scholar]
- 38.Vitolo U, et al. Fondazione Italiana Linfomi Lenalidomide plus R-CHOP21 in elderly patients with untreated diffuse large B-cell lymphoma: Results of the REAL07 open-label, multicentre, phase 2 trial. Lancet Oncol. 2014;15:730–737. doi: 10.1016/S1470-2045(14)70191-3. [DOI] [PubMed] [Google Scholar]
- 39.Czuczman MS, et al. A phase 2/3 multicenter, randomized, open-label study of lenalidomide vs investigator’s choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res. 2017;23:4127–4137. doi: 10.1158/1078-0432.CCR-16-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ngo VN, et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 2006;441:106–110. doi: 10.1038/nature04687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.