Significance
Simple voluntary movements (e.g., reaching or gripping) deteriorate with distraction, suggesting that the attention-control system—which suppresses distraction—influences motor control. Here, we tested the causal dependency of simple movements on attention control, and its neuroanatomical basis, in healthy elderly and patients with focal brain lesions. Not only did we find that attention control correlates with motor performance, correcting for lesion size, fatigue, etc., but we found a revealing pattern of dissociations: Severe motor impairment could occur with normal attention control whereas impaired attention control never occurred with disproportionately milder motor impairment—suggesting that attention control is required for normal motor performance. One implication is that a component of stroke paralysis arises from poor attentional control, which could itself be a therapeutic target.
Keywords: motor, attention, cognitive control, stroke, fMRI
Abstract
Attention control (or executive control) is a higher cognitive function involved in response selection and inhibition, through close interactions with the motor system. Here, we tested whether influences of attention control are also seen on lower level motor functions of dexterity and strength—by examining relationships between attention control and motor performance in healthy-aged and hemiparetic-stroke subjects (n = 93 and 167, respectively). Subjects undertook simple-tracking, precision-hold, and maximum force-generation tasks, with each hand. Performance across all tasks correlated strongly with attention control (measured as distractor resistance), independently of factors such as baseline performance, hand use, lesion size, mood, fatigue, or whether distraction was tested during motor or nonmotor cognitive tasks. Critically, asymmetric dissociations occurred in all tasks, in that severe motor impairment coexisted with normal (or impaired) attention control whereas normal motor performance was never associated with impaired attention control (below a task-dependent threshold). This implies that dexterity and force generation require intact attention control. Subsequently, we examined how motor and attention-control performance mapped to lesion location and cerebral functional connectivity. One component of motor performance (common to both arms), as well as attention control, correlated with the anatomical and functional integrity of a cingulo-opercular “salience” network. Independently of this, motor performance difference between arms correlated negatively with the integrity of the primary sensorimotor network and corticospinal tract. These results suggest that the salience network, and its attention-control function, are necessary for virtually all volitional motor acts while its damage contributes significantly to the cardinal motor deficits of stroke.
Stroke is one of the commonest causes of adult disability, resulting in impairments of both physical (e.g., hemiparesis) and cognitive (e.g., aphasia and neglect) function (1). These two broad types of impairment are commonly regarded as having distinct neuroanatomical bases, entailing different therapeutic strategies, whereas, in fact, physical and cognitive functions may share mechanisms, and their measures interdepend. A well-known example of this is motor neglect (2), in which unilateral motor dysfunction arises from a lateralized attentional bias, rather than because of primary motor, or corticospinal tract, disruption. However, focal brain lesions also impair nonspatial forms of attention, such as alertness (1), that are associated with motor function (3, 4) although the nature of this relationship is unclear.
Associations could occur because lesions tend to overlap anatomically adjacent, yet functionally independent, motor and attention pathways (5) or because of general illness effects on mood or fatigue. Alternatively, motor disability or fatigue demands extra attentional resources, which may secondarily impair performance on tests of attention (6, 7). In the current study, we sought to distinguish these possibilities from the third reason for an association: That nonspatial attentional deficits are a cause of motor impairment.
The type of attention focused on here is attention control (also termed “executive control” or “cognitive control”), which refers to the ability to maintain performance as challenges increase, be they competitive choices or distraction (8, 9). It is an everyday observation that distraction worsens motor performance (e.g., on strength (10) or tracking (11) tasks), indicating that the motor system competes for finite attentional resources. Increasing evidence suggests that a distinct control system manages such resource allocation and is recruited during motor acts, even in the absence of experimental challenges, because purposeful movements entail inhibition of irrelevant or competing motor plans (12, 13), distractor suppression (14), rule following (15), error monitoring, and correction (16). In keeping with this, functional–anatomical associations have been found between basic motor functions (e.g., strength and dexterity) and cerebral regions involved in attention control, particularly cingulate, inferior frontal, and temporoparietal cortices (17, 18), that become more apparent with aging or brain injury (19–22). Such findings extend studies showing that higher motor functions (e.g., drawing, speech, object use, and walking) commonly engage executive systems (23–26). However, while imaging studies suggest anatomical overlap of motor with attention-control functions, they have not to date shown whether attention control is a requisite for basic motor functions—as is the objective here.
Given evidence for motor and attention-control interactions, and noting that stroke-induced impairments in these two functions are associated behaviorally (3, 4), and anatomically (5), we hypothesized that one component of stroke hemiparesis arises from damage to a domain-general, attention-control system, thereby implying that normal motor performance requires intact attention control. To test this, we observed the pattern of dissociations between motor and attention-control performance in a large cohort of hemiparetic stroke patients that, by random distribution, should include lesions with variable differential overlap of motor versus attention-control systems. We reasoned that, if attention control is necessary for motor function, then attention-control impairments would always be associated with motor deficits that are proportionate or worse (i.e., we would not find dissociations where severe attentional impairment coexists with relatively mild motor impairment). Conversely, if poor motor performance increases distractibility (6, 7), then severe motor impairment would always be associated with impaired attention control (i.e., we would not find dissociations of severe motor impairment and preserved attention control). A third possibility—that associations occur because of anatomical proximity, but not functional interaction, between attention-control and motor systems (5)—allows for both types of dissociation because, by chance, some lesions are likely to target either system solely or preferentially. To increase the likelihood of discovering such dissociations, we focused on subjects with mild-moderate deficits who had small lesions that are likely to result in variable relative overlap of two adjacent networks and without confounding cognitive deficits: e.g., gross executive impairments, neglect, or apraxia.
In the following experiments we (i) characterize motor performance in healthy subjects and hemiparetic stroke patients in terms of separable bilateral and unilateral components; (ii) show how these motor components relate to distractibility during motor and nonmotor cognitive tasks, correcting for lesion size and other potential confounds; (iii) characterize the profile of motor/attention-control dissociations; and (iv) ascertain how the two motor components and attention control relate to lesion overlap and functional connectivity, of a putative attention-control (“salience”) network (9), as opposed to other cognitive or motor networks.
Results
Undistracted Motor Performance: Bilateral Versus Unilateral Impairments.
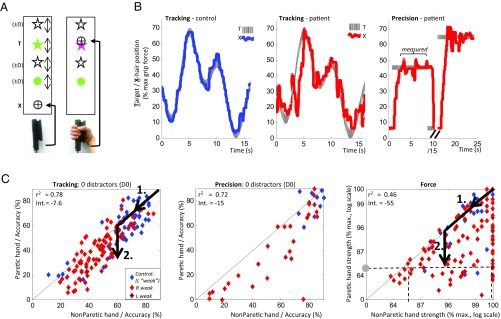
We tested hemiparetic stroke patients and age-matched controls on visuomotor tracking, precision, and force grip tasks (Fig. 1 A and B and Materials and Methods) (n = 176, 37, and 213 for the three tasks, respectively) (SI Appendix, Tables S1–S4 provide subject details). In all three tasks, in the absence of distractors, patients’ paretic-hand performance was worse than with their nonparetic hand while controls were worse using left relative to right hand with tracking only (SI Appendix, Table S5). Additionally, the two hands correlated strongly with each other (Fig. 1C) although correlation strength (r2) decreased and offset (y intercept) increased progressively between tracking, precision, and force tasks, reflecting increasing disparity between hands. Performance of the paretic hand was thus resolvable into bilateral and unilateral components—the former proportionate to nonparetic hand performance, the latter reflecting the difference between hands (Fig. 1C, arrows). Relative to controls, patients performed worse not only in their paretic, but also nonparetic, hands, thus revealing ipsilesional deficits (27, 28) in all tasks (hence the term “nonparetic hand” in fact refers to the clinically unaffected hand). There was no significant difference comparing second versus first block performance for all tasks in controls or patients with either hand (P > 0.05, sign test).
Fig. 1.
(A) Tracking and precision tasks required subjects to vary grip force, thereby moving a crosshair (X) onto a target (T) that moved vertically (tracking) or was stationary (precision), in presence of 0, 1, or 3 distractors (D). The force task required subjects to apply a maximal, brief grip, with only the peak force recorded. (B) Examples of X and T vertical positions in single trials of tracking and precision, in control (blue) and patient (red). (C) Scatterplots of accuracy in paretic versus nonparetic hands, in patients with R-hemiparesis (red), L-hemiparesis (crossed red), and controls (blue), for undistracted trials of tracking, precision, and force tasks. Diagonal line reflects equal performance between hands. Arrows show how performance can be resolved into bilateral (1.) and unilateral (2.) components, where the former is directly proportional to absolute nonparetic-hand performance (horizontal axis), and the latter represents performance difference between hands. For example, a patient with paretic-hand strength of 66% (gray circle) may have a nonparetic hand strength ranging between 66% and 100% (range of data points along dashed line).: the former extreme representing 100% bilateral and 0% unilateral components, the latter representing 0% bilateral and 100% unilateral components.
Relationship Between Distractibility and Motor Performance.
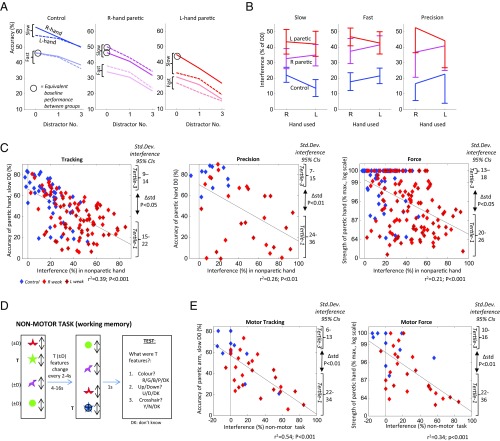
When distractors were introduced, performance deteriorated proportionately to the number of distractors, with the degree of impairment (“interference”) greater for patients than controls (Fig. 2 A and B and SI Appendix, Table S6A). One explanation for this is that effortful use of a disabled arm results in less attention available to resist distraction (6, 7). However, interference increases in patients occurred similarly in paretic and nonparetic hands (20% vs. 23% absolute ∆ relative to controls; P > 0.1) or were even greater in patients’ nonparetic than paretic hands, using unnormalized data (16% vs. 13%; P < 0.05). Furthermore, by matching undistracted performance (comparing fast tracking in controls, with slow tracking in patients’ nonparetic hands; P > 0.1 comparing baselines), interference was still greater in patients (P < 0.01)—suggesting that poor performance per se did not drive increased distractibility. Interference increases in patients, relative to controls, were also similar comparing slow and fast versions of tracking (19% vs. 23%) (Fig. 2B) and comparing precision, in which the target was stationary, versus tracking (24% vs. 21%; all comparisons: P > 0.1). These findings suggest that attention-control impairments in patients are not specific to target tracking but rather relate to motor dexterity in general.
Fig. 2.
(A) Accuracy as a function of distractor number, in slow and fast versions of tracking, in right and left hands. Line gradients—i.e., distractibility—are steeper in patients in both hands, relative to controls, even when matching groups for baseline accuracy (circled), indicating that higher interference is not secondary to poorer performance. L, left; R, right. (B) Normalized interference values (i.e., performance decrement with 3 vs. 0 distractors), with slow and fast tracking, and precision. The interference increases in patients relative to controls are similar comparing hands and tasks (P > 0.05). (C) Scatterplots of paretic-hand motor performance versus normalized interference measured in nonparetic hand, in tracking and precision [both without distractors (D0)], and force. As well as showing negative correlations for all three tasks (P < 0.001), asymmetric dissociations are also observed for all three (seen as outliers in bottom-left but not top-right of each graph). This is also demonstrated as significantly higher variance of interference values in lowest versus highest performance tertiles (P < 0.05), or by the residuals of the regression lines shown getting progressively smaller with better paretic-arm performance (r = −0.21, −0.24; P < 0.05; tracking and force). Regression lines shown (also in E). (D) Nonmotor version of tracking required subjects to watch a moving target T, whose features changed every 2–4 s, before being probed on T’s last features. On half of trials, they ignored three distractors (D). Interference is quantified as the recall accuracy without distractors, minus accuracy with distractors. B, blue; D, down; G, green; N, no; P, pink; R, red; U, up; Y, yes. (E) Scatterplots of normalized interference on nonmotor task versus motor-tracking or force performance. Asymmetric dissociations are observed once again. Std. Dev., standard deviation.
An association of high interference with poor undistracted motor performance was seen not only comparing patients versus controls, but also as between-subject negative correlations for all three tasks (P < 0.001) (Fig. 2C and SI Appendix, Table S6B), correcting for lesion volume, mood, fatigue, or pain. In the force task, similar correlations with interference were seen, whether measuring the average or best-out-of-four readings, the latter of which resemble conventional clinical power scores [e.g., Medical Research Council (MRC)] that typically score maximum effort. Correlation strengths with interference were similar for motor performance in paretic and nonparetic arms (bilateral component), but significantly less for motor performance difference between arms (unilateral component; ∆r: P < 0.01).
Dissociations Between Distractibility and Motor Performance.
To understand the directionality of these associations, it is notable that, for all tasks, dissociations between motor performance and distractor resistance occurred asymmetrically: Poor motor performance could coexist with low interference values (i.e., intact attention control) whereas good motor performance did not occur with high interference values, but rather was strictly associated with a narrow band of low-interference values (seen as a relative absence of cases in upper-right, relative to lower-left, wedges of Fig. 2C graphs, the range of low-interference values associated with normal performance being in the following order: tracking < precision < force). More formally, this is demonstrated as less interference variability comparing upper- versus lower-tertile paretic-arm motor performances (P < 0.05, for all three tasks) or as a negative correlation between absolute residuals (from linear models shown in Fig. 2C) and paretic-arm performance (r = −0.21, −0.14; P < 0.05, for tracking and force: i.e., as performance worsens, the association with interference weakens). This suggests that normal motor performance in all three tasks (without distraction) requires intact attention control whereas impaired motor performance may occur due to impaired attention control or other factors. Since the absence of good-performance/poor-attention-control dissociations was observed for both paretic and nonparetic arm performance, the performance component dependent upon attention control appears to be bilateral. By contrast, poor-performance/good-attention-control dissociations were largely selective for paretic arm (comparisons of interference variability between upper- versus lower-tertiles of nonparetic arm tracking or force were not significant; P > 0.05)—indicating that the performance component independent of attention control is more unilateral (i.e., measured as a difference between arms).
Distractibility During a Nonmotor Memory Task.
An alternative explanation for correlated interference and motor performance, measured in opposite arms, is that unilateral lesions disrupt bilateral motor systems (27, 28). Consequently, increased interference, measured in the “nonparetic’ arm, may be secondary to performance difficulties in this arm, causing attentional reallocation (6, 7), rather than vice versa. Against this is our earlier observation that patients’ interference level (taking unnormalized data) was higher using the nonparetic than the paretic arm. Additionally, we measured interference during a nonmotor, working-memory (WM) task (n = 36) (Fig. 2D) and compared this to undistracted motor performance on tracking and force tasks. Patients showed greater interference with WM performance than controls (25% vs. 0%; P < 0.01). Furthermore, negative correlations occurred between tracking and force performance and WM interference, at least as strong as with motor interference (corrected for lesion volume and mood) (Fig. 2E and SI Appendix, Table S6C) (e.g., correlation of paretic arm tracking performance against WM interference: r2 = 0.54; and against motor interference: r2 = 0.39; or for paretic arm force, correlations were r2 = 0.34 and 0.23 respectively; all P < 0.001). Once again, poor motor performance was associated with a wide range of WM interference values whereas good motor performance was strictly associated with low WM interference (interference variance of upper- versus lower-tertile performance: P < 0.01)—indicating that not only is attention control necessary for normal motor performance, but this is a domain-general capacity, engaged also in nonmotor, cognitive tasks.
Dependency of Motor and Attention-Control Components on Lesion Anatomy.
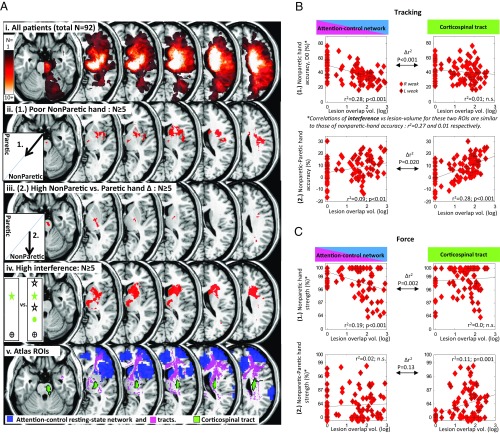
We next determined how lesion-anatomical correlates of bilateral and unilateral motor components [defined, respectively, as absolute nonparetic hand performance and (nonparetic–paretic) hand difference] compared with that for attention control: i.e., resistance to distraction (Fig. 3A). First, we mapped lesion locations associated with the highest 50% values for either motor deficit, while excluding locations seen with the lowest 50% deficit values (for tracking and force tasks). This showed an anatomical separation of the two deficits: Bilateral motor impairments were associated with (unilateral) lesions to the anterior striatum, insula, medial thalamus, and anterior corona radiata whereas unilateral motor impairments were associated with lesions to the posterior internal capsule and pons [i.e., along the corticospinal tract (CST)] (Fig. 3 A, ii and iii) (maps show lesion locations common to both tracking and force analysis). Importantly, the anatomical pattern of attention-control impairments (defined as interference values greater than those of controls, P < 0.05) matches more closely the lesion pattern associated with bilateral-motor, rather than unilateral-motor, deficits (Fig. 3 A, iv) (Dice similarity index = 18.2 vs. 4.4, respectively; comparison with randomly permuted lesion maps’ Dice scores: P < 0.001 and P = 0.843, respectively; spatial correlation r: 0.309 vs. 0.107; ∆r: Z = 380, P < 0.001).
Fig. 3.
(A) Lesion overlay maps demonstrating selective lesion associations for bilateral and unilateral motor components. (i) Frequency of lesion locations (flipped onto a common side). (ii) Poor nonparetic-hand accuracy: i.e., bilateral component (1.) (median split, low-minus-high, n ≥ 5), common to both tracking and force. (iii) High [nonparetic–paretic] hand difference tracking accuracy: i.e., unilateral component (2.) (median split, high-minus-low, n ≥ 5), common to both tracking and force. (iv) High interference effect: i.e., tracking performance impairment with distractors relative to no distractors (P < 0.05 compared with controls, n ≥ 5). Dice similarity index comparing lesion pattern of ii vs. iv = 18.2 whereas iii vs. iv = 4.4; ∆r between comparisons: Z = 380, P < 0.001. (v) A priori regions of interest: attention control (salience) network (RSN from control fMRI data, and tracts) and corticospinal tract. (B) Scatterplots of bilateral (1.) and unilateral (2.) motor components for tracking task relative to lesion overlap of attention-control (salience) network and corticospinal tract, showing selective anatomical relationship for each component. Equivalent scatterplots of interference versus each of these two ROIs show similar anatomical relationships as those of bilateral motor component. Correlations shown are significant, corrected for lesion volume. Regression lines shown (also in C). D0, without distractors. (C) As for B, only for force task. Correlations of either motor component with lesion overlap of right frontoparietal, left frontotemporoparietal, or callosal ROIs were not significant. L, left; R, right; vol., volume.
We next determined lesion locations significantly associated with each type of performance impairment using a voxelwise Brunner–Munzel statistical analysis. This showed that bilateral-motor and attention-control deficits were associated with lesions to the anterior corona radiata, anterior internal capsule, and anterior striatum whereas unilateral-motor deficits were associated with damage to the posterior internal capsule (P < 0.05, corrected) (SI Appendix, Fig. S7).
A more focused approach for investigating the anatomical bases of motor and attention interactions was conducted by interrogating how each function relates to lesion overlap of a priori attention and motor networks (Fig. 3 A, v and SI Appendix, Table S8). Bilateral-motor and attention-control deficits both correlated with lesion load to the cingulo-opercular (attention-control or salience) network (9, 29) (r2 = 0.10–26; P < 0.001, corrected for lesion volume), but not with lesion volume of CST [∆r between regions of interest (ROIs): P < 0.001] (Fig. 3 B and C). Conversely, unilateral motor deficits correlated with CST lesion load (r2 = 0.10–25; P ≤ 0.01, corrected), but significantly less strongly with attention-control network overlap (∆r between ROIs: P = 0.020 and 0.13, for tracking and force, respectively). Lesion overlap with left frontoparietotemporal (30) (“praxis”) or right dorsal frontoparietal (“visuospatial”) networks or corpus callosum correlated with neither motor nor attention-control deficit (r2 = 0.00–0.01; P > 0.05 corrected).
Relationship of Motor and Attention-Control Components with Functional Connectivity.
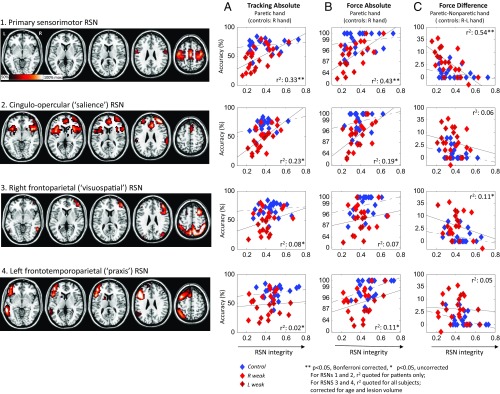
Undistracted motor performance in patients’ paretic arm (or right hand of controls) was related to the functional integrity of eight robust cerebral and cerebellar resting-state networks (RSNs) (31) (n = 46). The only RSNs whose integrity correlated with tracking or force performance, after correction for lesion volume and age, were primary sensorimotor and cingulo-opercular RSNs (Fig. 4 A and B and SI Appendix, Table S9) (r2: 0.16–0.43; P < 0.05 corrected). On partialing out force hand difference, correlations of absolute motor performance with sensorimotor RSN integrity became insignificant (P = 0.243) whereas partial correlations remained significant for cingulo-opercular RSN (P < 0.01). This can be explained by a negative correlation between force hand difference and sensorimotor RSN (r2 = 0.54; P < 0.01 corrected, patients only) but not cingulo-opercular RSN integrity (r2 = 0.06; P = 0.28) (Fig. 4C). This indicates that sensorimotor and cingulo-opercular RSNs correspond, respectively, to unilateral and bilateral motor performance components.
Fig. 4.
(1–4) Maps of resting-state networks (RSNs) of interest obtained by independent-components analysis of resting-fMRI time-series data from a separate cohort of healthy controls (voxel intensity represents contribution to each component). (A–C) Scatterplots of three performance measures against RSN integrity for each of the RSNs 1–4. Of all RSNs, only primary sensorimotor and attention-control (salience) RSN integrity showed significant, corrected correlations with absolute tracking and force performance in patients (using paretic arm) and controls (using right hand) (A and B) while only primary sensorimotor RSN showed correlation with difference in performance during force task (C). This indicates that these two RSNs are specific for the bilateral and unilateral motor performance components, respectively. R, right; R-L, right-left.
Since correlations between absolute, or hand-difference, performance measures versus RSN integrity appear to mirror the functional-network lesion-anatomy correlations presented earlier, we tested whether RSN integrity related to lesion anatomy profiles. A cross-correlation analysis of the eight principle RSNs versus our earlier anatomical ROIs demonstrated significant negative correlations between primary sensorimotor RSN integrity and CST-M1 (and, to a weaker extent, callosal) lesion load and between cingulo-opercular RSN integrity and attention-control ROI lesion load (SI Appendix, Table S10).
Head movement did not differ significantly between controls versus patients, or between high versus low values of tracking accuracy, force grip, or hand-difference force grip [t (44) ≤ 1.92, P > 0.05].
Discussion
Movement selection and execution require inhibition of irrelevant or competing motor plans (12), distractor suppression (14), and error monitoring (16)—all core functions of the attention-control system (8). We therefore hypothesized that lesions within the attention-control network would have consequences, not only on cognitive performance, but also on basic motor abilities. Supporting this, not only did we find consistent and strong correlations between attention control and motor performance, correcting for lesion size, fatigue, mood, baseline performance, and task factors, but three key findings here suggest a causal relationship.
First, we observed asymmetric behavioral dissociations across all four behavioral experiments, showing that, while severe motor impairment coexists with a wide range of attention control, there are no cases where normal or mildly impaired motor function coexists with impaired attention control (the cutoff for attention impairment being stricter for dexterity than strength tasks). Were associations between motor performance and attention control caused by mere anatomical proximity of functionally separate systems, then we might expect double dissociations, in which mild motor impairment associates with severe attention-control deficit. Although our patient sample was biased toward subjects with clinical motor deficits (arm paresis was an inclusion criterion), it is notable that, even among healthy aged controls, all subjects with greater attention-control impairment had proportionate (subclinical) bilateral motor impairments [parallel impairments in cognition and physical strength with aging have been observed previously (32)]. Furthermore, we tested 167 patients, many of whom performed grip tasks within normal limits despite having paresis in the same arm. As motor performance verged toward normality, so too did the range of attention control converge strictly upon normality. Lesion size tended to be small (volume interquartile range: 3–15 cc), with 18 cases in whom attention-control regions were damaged, while sparing primary motor/CST regions; and a further 26 cases occurred in whom attention-control regions were damaged in greater volume than motor regions—together representing 38% of the patient sample. Such lesions, in targeting executive regions exclusively or predominantly, might be expected to result in proportionately worse impairments in attention control than motor performance, if motor and attention-control functions were independent—but this was not found.
Secondly, we confirmed that the relationship between attention control and dexterity or strength, as well as asymmetric dissociations of the kind just described, were seen equally with distractibility during a nonmotor cognitive task. This excludes the possibility that poor or effortful motor performance was the cause of poor attention control (6, 7). Furthermore, this indicates that the attention-control function underpinning low-level motor actions is a domain-general function, explaining why strength or dexterity is impaired by concurrent cognitive tests (7, 10, 11).
Finally, we found that one component of motor performance (common to both arms), as well as attention control, was associated with the anatomical and functional integrity of the cingulo-opercular network [previously implicated in higher cognitive control (9)]. This association existed after correction for lesion size and was specific, not being found with integrity of primary sensorimotor/CST, dorsal-attention, and praxis-associated networks. The fact that attention-control dysfunction was associated with the bilateral motor component distinguishes our findings from that seen in motor neglect (2), in which a unilateral (contralesional) motor impairment is seen secondary to impairment of the spatial-orienting system.
Relevance to Associations Between Cognitive and Motor Functions.
Our results help interpret a diverse set of studies showing that domain-general cognition, especially executive or attention control, is closely related to motor ability. Studies on standing or walking efficiency, mostly in elderly subjects, show associations with executive functions and divided attention, independently of general reaction time (25). Tests of manual dexterity correlate with the ability to resist distraction and alertness (33) while impairments in handgrip strength are preceded by, and proportionate to, reductions in general cognition (34). In childhood development, motor dexterity predicts subsequent cognitive attainment (35) while both attentional and motor impairments are reversible with methylphenidate (36). However, various reasons may account for associations between cognition and motor performance that do not imply direct causation. For example, genes, aging, disease, and drugs may affect motor and attention systems conjointly, rather than acting via attention alone. Furthermore, impairments in motor performance due to disability or exhaustion can have secondary effects on attention control, rather than vice versa (6, 7). To this end, patients with focal brain injury can provide an explanatory advantage since attention and motor performance potentially may dissociate and functional impairments can be related to lesion neuroanatomy.
Executive deficits are common following stroke (1) and correlate with motor performance and recovery (3). Lesion locations associated with executive impairments occur not just in higher frontoparietal regions (37), but also in premotor, striatal, and thalamic regions (5), that run close to corticospinal tract. Thus, relationships between attention control and motor performance could arise because lesions frequently overlap spatially contiguous, but functionally independent, neural systems. Our results argue otherwise. Since high distractibility was found to be incompatible with normal, or mildly impaired, motor performance on formal tests of dexterity and strength, this suggests that intact attention control is necessary for normal performance on clinical measures of motor function. It will be beneficial to address the question of whether naturalistic movements (e.g., measured by wearable sensors) are as susceptible to attention-control impairments.
Relevance to Ipsilesional Motor Impairment and Functional-Imaging Patterns Poststroke.
Ipsilesional motor impairments are well-described after stroke (27), and have been interpreted in terms of bilaterally represented sensorimotor circuits, including uncrossed corticospinal tracts (38), praxis (28), and visuospatial systems (39). Our anatomical findings argue against ipsilesional deficits being caused by lesions to the uncrossed corticospinal tract because ipsilesional impairments (i.e., bilateral motor component) correlated selectively with lesion load of the attention network, but not the predecussation corticospinal tract (Fig. 3A). Furthermore the bilateral, relative to the unilateral, component increased as task complexity increased (Fig. 1C), despite movement type (grip) remaining constant, indicating that ipsilesional motor impairment relates to a nonmotor, cognitive, rather than praxis, function. Potential confounds of apraxia and visuospatial neglect were also minimized in our study by excluding patients who manifested such deficits using formal testing, and by minimizing praxis requirements (the grip was already placed in the hand by the researcher). The fact that ipsilesional impairments were of a similar magnitude between right and left hemiplegics also indicates involvement of a broadly symmetric network (as for attention control), rather than strongly lateralized networks (such as those for visuospatial processing or praxis) (Fig. 4). Attentional impairment as an interpretation for ipsilesional deficits has previously been discounted (28) on the basis of normal bedside tests of inattentiveness or cognition. However, our patients also passed similar cognitive tests, suggesting that the relevant test of attention control may only be detected by computerized tests: e.g., distractor resistance.
A further implication of our findings relates to interpreting functional-imaging patterns after stroke, particularly the observation that hemiplegic arm use consistently activates more bilateral and anterior regions, than healthy controls (40). While these are interpreted in terms of motor-network reorganization, an alternative account is that such additional activations reflect attention-control network dysregulation or compensatory recruitment of attentional areas. Recruitment of frontoparietal areas occurs in healthy subjects when they attend to action or during movements without explicit attention in elderly or disabled subjects (19, 41, 42). Moreover, the dorsal premotor cortex—a common site for movement-associated hyperactivation after stroke—forms part of an executive-control network (43). Movement-associated hyperactivation in hemiparetic stroke patients has also been observed in anterior cingulate, lateral/inferior prefrontal regions, and insulae (20, 21), which might reflect compensatory up-regulation of the attention-control system or increased effort, since such studies focus on chronic stroke, when early disability has partly recovered. Future studies should ascertain whether movement-related activation patterns and connectivity [including reciprocal inhibition (44)] are consistent with the resting-state connectivity profiles of cingulo-opercular and sensorimotor networks observed here.
Relevance to Stroke Hemiparesis More Generally.
To increase our chance of discovering attention–motor dissociations, it was advantageous to use a patient sample with relatively small lesions and to exclude subjects with additional cognitive impairments. However, this raises the question of whether our findings generalize to subjects with larger lesions and more severe hemiplegia. In this regard, it is first important to realize that the motor impairment we recorded in our experiment appears milder than the same patients’ arm-function/clinical scores because our tasks tested power grip—that is relatively spared in stroke arm paresis (45, 46). Secondly, 17% of our tested patients (n = 28) had moderate-severe weakness (grip-force or arm Fugl–Meyer score <50%), whose behavioral and anatomical–functional imaging profiles extend the pattern observed in subjects with milder weakness. Thus, as hemiplegia severity increases, so too does the size of the ipsilesional motor impairment (Fig. 1C) (see also ref. 28), that we have found is proportionate to both attention-control impairment (Fig. 2C) and relative damage of the cingulo-opercular network (Figs. 3 and 4). For example, patients with contralateral grip weakness <65% normal (data points below the horizontal dashed line in Fig. 1C) showed a range of ipsilesional grip impairment (range: ∼60–100%; median ∼90%) that our results indicate was due to an attention-control impairment acting bilaterally. Finally, we note that the median disability of our sample [National Institute of Health Stroke Scale (NIHSS): 4] is similar to that of large unselected samples of incident stroke (e.g., ref. 47; NIHSS: 3) yet even mild motor deficits often result in significant handicap (48).
Conclusion
Our study provides converging behavioral, lesion–anatomical, and functional–anatomical evidence for simple motor performance (dexterity or force) being dependent, not only upon primary motor structures, but also an intact attention-control system. Given that attention-control deficits are common after stroke (1), our finding suggests that one component of the cardinal hemiplegic syndrome after focal brain injury, measurable in the ipsilesional limb, reflects a disorder of nonspatial attention. Furthermore, motor assessments can be usefully parameterized in terms of the degree to which they engage attention control, as opposed to the unilateral primary motor system. One implication is that executive-boosting therapies, such as “brain training” (49) or magnetic stimulation (50), may confer secondary benefits on physical rehabilitation.
Materials and Methods
Subjects.
Right-handed patients with mild-to-moderate unilateral arm weakness due to recent unilateral stroke (<4 wk), as well as age-matched controls, were screened. To reduce confounding explanations for impaired motor performance, we excluded subjects with clinically manifest cognitive impairments, as judged from history or bedside testing, including frontal dysfunction (inability to complete the Trail-Making Part B test), apraxia (ideational and ideomotor) (51), and neglect (Behavioral Inattention Test: star cancellation, line bisection, figure copying, and visual inattention) (52). Other exclusion criteria were as follows: (i) severe arm weakness (finger-flexion MRC power grade <2), (ii) preexisting weakness in either arm, or bilateral arm weakness or ataxia, (iii) comprehension difficulty (inability to signal understanding of study purpose or task instructions), (iv) psychiatric disorder, and (v) severe cerebral white-matter disease [graded using the Fazekas score (53)] or other brain lesions.
Age-matched controls were healthy volunteers or nonstroke patients (e.g., migraine) who had no limb impairment or neuroimaging lesions but otherwise fitted the above criteria. Subjects were excluded who were unable to perform the tracking task with either hand with ≥10% accuracy (i.e., >95% upper confidence interval for chance performance).
For precision and resting-state fMRI studies, we extended recruitment to patients with severe paresis (MRC power grade of >0) to see if our earlier results in more mildly affected subjects extended to more severely affected subjects.
Subject characteristics for the four experiments are shown in SI Appendix, Tables S1–S4. There were no significant differences between right-arm–weak patients, left-arm–weak patients, and controls in terms of age, gender, handedness, background cerebral white-matter disease, and anxiety, but patients were slightly more depressed (difference: 1/15 points; P < 0.002). Comparing left-arm–weak with right-arm–weak patients, there were no significant differences in lesion volume or function and strength of affected arms.
Tasks.
The study was approved by the United Kingdom South East Coast Research Ethics Committee. Patients underwent informed consent.
Subjects were tested for dexterity and strength using visuomotor force tracking and precision and maximum-force grip tasks, in each hand. Power grip has the advantage of being a relatively less affected movement in stroke hemiplegia and has minimal praxis requirements (the device is prepositioned in the subject’s hand by the experimenter), and grip tracking can be achieved relatively accurately even in the presence of significant hand or upper-arm weakness (45, 46). This accounts for why subjects’ mean performance difference was relatively small (1–12%), yet the mean clinical score suggested a more profound functional impairment (NIHSS motor-arm component = 2). Tasks were run on E-Prime software, using a 15-inch laptop screen and a digital handgrip (<100 N force; Current Designs).
The tracking task consisted of a green star (1.2°) moving up and down, according to a polynomial sine–cosine function time course, with random parameters, varying trial-to-trial, but fixed between subjects (Fig. 1A). Subjects varied hand-grip force, that proportionately moved a crosshair, which, when overlying the star, turned it pink. Performance was calculated as the percentage of time that the crosshair overlay the target star, across a 16-s trial, with 16 trials per hand, excluding the first 2 s and with 6-s rests between trials. Hand use alternated twice across a session (right-left-right-left/left-right-left-right), order counterbalanced across subjects. Subjects practiced for eight trials before the test session. The software was calibrated so that the maximum required grip force was ≤70% of each subject’s best.
Attention control was manipulated by varying the number of visual distractor stimuli (0,1,3), that moved according to the same pattern as the target star in the tracking task, but asynchronously. The task also varied speed (number of target direction changes per trial: 4–6 versus 8–12). All results reflect slow trials, except that fast trials (of controls) were used to match group baselines by comparison with slow trials (of patients) and to test whether attention-control effects depend upon tracking speed.
Attention control was analyzed in two ways. First, performance accuracy was entered as a dependent variable into an ANOVA with factors: distractor number, hand use, speed, and group (between-subject factor). Second, we calculated normalized interference as the difference in performance between 0 and 3 distractors, divided by baseline (0 distractor) performance. The latter value was used as the dependent variable in an ANOVA with factors hand use, speed, and group. One advantage of analyzing raw scores was to allow for selection of conditions that equated baseline performance between groups (namely, fast tracking in controls versus slow tracking in patients).
The precision task was similar to that of tracking, except that the target was set at a random vertical level and remained stationary for 8 s (Fig. 1B). Tracking accuracy was calculated from 2 s after target presentation to the end of the trial. This was performed by a separate, smaller cohort of subjects to tracking. The purpose of this was to see if results seen during tracking generalized to a dexterity task without the visuomotor demands of a continuously moving target.
The force task required subjects to exert a power grip maximally at any time over a 7-s measurement window, while recording peak force relative to the device’s maximum range. Visual feedback was provided as a vertical bar proportionate to force. Subjects were instructed as follows: “Squeeze as hard as you can for a split second. In so doing, try to make the red bar hit the top of the screen.” The maximum was achievable in both hands, in 19 out of 20 subjects aged <40 y (from a preliminary pilot study). Four trials per hand were performed, split either side of the tracking task. There was no significant difference between pretracking versus posttracking values. Subjects who undertook the tracking or precision tasks (separate cohorts) both contributed data to the force task. Performance on this task was compared with interference measured during the tracking or precision task.
The nonmotor task was similar to the slow tracking task, only subjects paid attention to the star’s color (red/green/blue/pink), movement direction (up/down), and whether a crosshair, no longer controlled by the subject, overlay it (yes/no) (Fig. 2D). Characteristics changed every 2–4 s. Trials varied 4–16 s in duration, and 1 s afterward, subjects were probed for the star characteristics just before trial termination, via three questions (subjects were told that only response choice was important and not response time; subjects could reply “don’t know”). Accuracy reflects the proportion of trials in which all three characteristics were correctly remembered. Attention control was manipulated by adding, in half of trials, three distractors. Interference was measured as the proportionate decrease in accuracy, comparing trials with distractors to trials without. This was compared with the same subjects’ performance on the undistracted version of the tracking task and force task.
In 46 subjects (23 patients), we also provided subjects with two visual analog scales (1–6), rating their experience of fatigue and pain, respectively, before and after performance of tracking and force tasks. These scores did not correlate with motor performance or interference scores.
Statistical tests were nonparametric throughout, including permutation ANOVAs, and were performed in MATLAB (v2012b).
Voxel–Lesion Function Mapping.
Lesion analysis was performed on patients who undertook both tracking and force tasks, as well as those undergoing fMRI (see later) (SI Appendix, Fig. S12 and Table S11). Lesions were manually delineated in MRICroN and spatially normalized in SPM software, as previously described (54). MRI diffusion-weighted imaging (and/or fluid-attenuated inversion recovery) images were used in all cases, except six patients in whom the lesion was clearly discernible on computerized tomography. Imaging resolution for each image type was as follows: DWI: 1.4 × 1.4 × 6.5 mm; FLAIR: 0.6 × 0.6 × 6 mm; CT: 0.4 × 0.4 × 2.4 (brainstem) or 7.2 mm (cerebrum). Right- and left-sided lesions were flipped onto a single side given nonsignificant differences in standard motor measures of right- and left-hemiparetic patients and given approximately symmetric a priori regions of interest (see below). Although all cases had essentially unilateral lesions contralateral to hemiparesis, in nine cases, a tiny part of the overall acute lesion (<5%) was seen on the opposite side. This was included within the unilateral composite although deleting these portions was not found to be influential to the results.
A primary question was whether the anatomical basis for one component of motor performance related to the anatomical basis of attention control. We first derived lesion maps specific to the bilateral and unilateral motor components defined as absolute nonparetic-hand performance and (nonparetic–paretic) hand difference, respectively. These maps were created by performing median splits of patients’ performance for each component and plotting lesion distributions for subjects in the top half (i.e., highest impairment), masking for locations found in >10% of bottom-half (low-impairment) subjects. These two motor component lesion maps were then compared with the lesion map for highly impaired attention-control patients, defined as subjects with interference values of >0.95 percentile relative to controls. Spatial similarities (Dice scores) between each motor component lesion map versus attention control were calculated, and these were compared with Dice scores of 1,000 randomly permuted “motor component” lesion maps (of the same size as equivalent motor lesion maps, limited to voxels containing at least the minimum number of lesions seen in the highly impaired attention-control contrast (i.e., n > 6). We also calculated the difference between the two spatial correlations using nonparametric Fisher Z transformation.
A secondary question was to determine the anatomical basis for each performance measure—bilateral, unilateral motor components, and interference—and, specifically, to determine how each related to a priori functional motor and attentional networks. First, we performed voxelwise statistical associations for each measure, using the rank-order Brunner–Munzel test (55) and impairment cutoff thresholds as defined above. Statistical significance was set at P < 0.05 corrected and was determined from Z thresholds using lesion permutation testing (restricted to lesion n ≥ 10) (56) implemented in MRICroN (people.cas.sc.edu/rorden/mricron/). Secondly, we determined correlations between each of the two motor, and one attentional, measures, with lesion overlap of a priori functionally defined regions: namely, (i) the cingulo-opercular, attention-control (or salience) network (9), equivalent to the executive-control network described by ref. 31, (ii) the corticospinal tract, (iii) the right dorsal frontoparietal (visuospatial attention) (57), (iv) the left frontotemporoparietal (praxis) (30), and (v) the callosal tracts. For ROIs iii and iv, unflipped lesion maps were used while, for ROI ii, we assessed correlations using flipped and unflipped lesions separately.
The corticospinal tract and callosal ROIs were derived from a probabilistic white-matter atlas, thresholded at 0.10 (58). For the attention-control network ROI, we used a mask of anatomical regions identified by resting-state network (RSN) independent-component analysis of resting-state fMRI data (31) (n = 54 healthy controls; component thresholded at >50% peak component weight; see below for resting-state fMRI analysis). The component chosen was that which was most similar to a cingulo-opercular, attention-control (or salience) RSN, based upon spatial correlation with a previously published RSN (31) (referred to as the “executive network” within that publication) (Fig. 4, image 2), that is associated with error detection, distraction suppression, and conflict resolution (9). The executive-control RSN was combined with a coregistered probabilistic atlas of cerebral white-matter tracts that connect executive-control network RSN nodes (29) (Fig. 3 A, v).
Right frontoparietal and left frontotemporoparietal anatomical ROIs were identified by additional resting-state analysis components (Fig. 4, images 3 and 4), that were most spatially similar to those previously associated with visuospatial attention (right-sided) (59), and praxis (left-sided network), respectively (31). These cortical ROIs were combined with atlases of the superior longitudinal fasciculus on the corresponding side.
Normalized lesions were superimposed upon each ROI, allowing determination of the number of lesioned voxels within each ROI. This value was correlated with either bilateral or unilateral components of tracking or force. Correlations were corrected for lesion volume. Differences between correlation coefficients comparing the two ROIs [i.e., (r for correlation of performance with ROI1 lesion volume) − (r for correlation of performance with ROI2 lesion volume)] were performed by permutation testing, in which the ROI label (attention-control network or CST) was randomized with respect to the lesion volumes for each ROI, and the r difference for each of 2,000 such samples was compared with the correct labeling.
Resting-State Functional MRI.
A group of hemiparetic stroke patients and controls, different from those participating in the behavioral experiments, underwent a 6.5-min MRI scan measuring blood-oxygen level-dependent (BOLD) functional activity at rest. Resting-state functional MRI involved the following imaging parameters: scanner type: Siemens 3T; duration: 6.5 min; volumes: 192; repetition time (TR): 2 s; echo time (TE): 25 ms; voxel size: 3.4 × 3.4 × 4 mm; 32 slices; flip angle (FA): 90°; field of view (FOV): 220 mm. For spatial normalization and lesion masking of BOLD time-series data, a high-resolution T1 structural image was also obtained: TR: 1,900 ms; TE: 2.5 ms; FA: 90°; voxel-size: 1 × 1 × 1 mm; FOV: 250 mm. Subjects were instructed to keep their eyes open and maintain fixation on a cross displayed in the center of their visual field.
Preprocessing of resting-state BOLD time-series data using FSL software (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki, v 3.10) (60) consisted of the following: removal of nonbrain tissue, and first 6 volumes per scan; bandpass filtering (0.01–0.2 Hz); motion correction; grand-mean intensity normalization; spatial smoothing (Gaussian kernel FWHM 5 mm); registration to standard Montreal Neurological Institute space; and exclusive masking with a composite of all patients’ acute-stroke lesions (from coregistered FLAIR or DWI). To identify resting-state networks (RSNs) of interest, we performed independent component analysis (ICA) using MELODIC-FSL (31) on the concatenated resting-state BOLD time series of 54 healthy subjects (median age = 51, 95% confidence intervals: 28–76 y; males: 52%; low-pass filter: <0.1 Hz). ICA was set to generate 20 components, which was the smallest number that enabled the following eight well-characterized and robust RSNs (31) to be distinguished: primary sensorimotor, attention control (salience, cingulo-opercular, or executive control), right dorsal frontoparietal (visuospatial), left frontotemporoparietal (praxis), default mode, visual and auditory, and a cerebellar-brainstem RSN. The top 0.1% component-weighted voxels—representing the anatomical peaks of each healthy RSN—were used as masks to sample BOLD time series from each of our test subjects. Sampled peaks did not overlap any patients’ acute lesions (Fig. 4 and SI Appendix, Fig. S12). Within these masks, the mean correlation coefficient across every voxel-pair time series was computed to obtain a measure of functional network integrity, per subject, per RSN. Head movement was quantified as rms error and framewise displacement of six translational and rotational movement parameters.
Post-fMRI Tracking Task.
Immediately after the resting-state FMRI block, subjects performed tracking and force tasks in the scanner, similar to that described in Tasks above. In this tracking version, only the paretic (or right hand in controls) was tested, and only the slow, undistracted condition was used. Combining subjects using different hands is justified from behavioral results of the main study, showing that intersubject variation of tracking accuracy is roughly sixfold larger than intrasubject between-hand accuracy variation (Fig. 1C). Active trials varied in length between 6 and 15 s, with rest intervals of 6–15 s, over a 6.5-min epoch. Accuracy was calculated as the proportion of time the crosshair overlay the target, excluding the first 2 s. Subjects were introduced to, and practiced, the task for 2 min before the test session.
Functional integrity of the eight principle resting-state networks was correlated against tracking and force accuracy, correcting for lesion volume and age. Between-hand force differences were used to partial out the unilateral motor component from correlations of RSN integrity with absolute performance in both tasks, given strong correlations between force and tracking tasks in hand difference (r = 0.63; P < 0.001). To appreciate correspondences between lesion anatomy and RSNs, we profiled patients’ lesions in terms of volumes overlapping with anatomical ROIs (described in Voxel–Lesion Function Mapping), and these values were cross-correlated with integrity of each of the principle RSNs. Correlations of RSN integrity with performance, or anatomical-network overlap, were thresholded at P < 0.05, corrected for multiple comparisons.
Supplementary Material
Acknowledgments
We thank Lesley Honeyfield and Rebecca Quest (Imperial College National Health Service Healthcare Trust) for assistance with the functional imaging substudy. The project was funded by an National Institute for Health Research-Imperial College London Biomedical Research Centre Project Award (to P.B.). D.S. is supported by the “Severo Ochoa” Programme for Units of Excellence in Research & Development (SEV-2015-490, Spain) and Project Grant PSI2016-76443-P from the Spanish Ministry of Economy, Industry and Competitiveness and by Project PI-2017-25 from the Basque Government.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1715617115/-/DCSupplemental.
References
- 1.Leśniak M, Bak T, Czepiel W, Seniów J, Członkowska A. Frequency and prognostic value of cognitive disorders in stroke patients. Dement Geriatr Cogn Disord. 2008;26:356–363. doi: 10.1159/000162262. [DOI] [PubMed] [Google Scholar]
- 2.Laplane D, Degos JD. Motor neglect. J Neurol Neurosurg Psychiatry. 1983;46:152–158. doi: 10.1136/jnnp.46.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson IH, Ridgeway V, Greenfield E, Parr A. Motor recovery after stroke depends on intact sustained attention: A 2-year follow-up study. Neuropsychology. 1997;11:290–295. doi: 10.1037//0894-4105.11.2.290. [DOI] [PubMed] [Google Scholar]
- 4.Arsic S, et al. Correlation between the quality of attention and cognitive competence with motor action in stroke patients. BioMed Res Int. 2015;2015:823136. doi: 10.1155/2015/823136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinne P, et al. Triple dissociation of attention networks in stroke according to lesion location. Neurology. 2013;81:812–820. doi: 10.1212/WNL.0b013e3182a2ca34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houwink A, Steenbergen B, Prange GB, Buurke JH, Geurts AC. Upper-limb motor control in patients after stroke: Attentional demands and the potential beneficial effects of arm support. Hum Mov Sci. 2013;32:377–387. doi: 10.1016/j.humov.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Brown DM, Bray SR. Isometric exercise and cognitive function: An investigation of acute dose-response effects during submaximal fatiguing contractions. J Sports Sci. 2015;33:487–497. doi: 10.1080/02640414.2014.947524. [DOI] [PubMed] [Google Scholar]
- 8.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welch JC. On the measurement of mental activity through muscular activity and the determination of a constant of attention. Am J Physiol. 1898;1:283–306. [Google Scholar]
- 11.Wickens CD. The effects of divided attention on information processing in manual tracking. J Exp Psychol Hum Percept Perform. 1976;2:1–13. doi: 10.1037//0096-1523.2.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Mostofsky SH, Simmonds DJ. Response inhibition and response selection: Two sides of the same coin. J Cogn Neurosci. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- 13.Rounis E, Humphreys G. Limb apraxia and the “affordance competition hypothesis”. Front Hum Neurosci. 2015;9:429. doi: 10.3389/fnhum.2015.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baylis GC, Tipper SP, Houghton G. Externally cued and internally generated selection: Differences in distractor analysis and inhibition. J Exp Psychol Hum Percept Perform. 1997;23:1617–1630. doi: 10.1037//0096-1523.23.6.1617. [DOI] [PubMed] [Google Scholar]
- 15.Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharika KM, Ray S, Murthy A. Attention for action during error correction. Prog Brain Res. 2009;176:227–244. doi: 10.1016/S0079-6123(09)17613-6. [DOI] [PubMed] [Google Scholar]
- 17.Kuhtz-Buschbeck JP, Ehrsson HH, Forssberg H. Human brain activity in the control of fine static precision grip forces: An fMRI study. Eur J Neurosci. 2001;14:382–390. doi: 10.1046/j.0953-816x.2001.01639.x. [DOI] [PubMed] [Google Scholar]
- 18.Hoffstaedter F, et al. The role of anterior midcingulate cortex in cognitive motor control: Evidence from functional connectivity analyses. Hum Brain Mapp. 2014;35:2741–2753. doi: 10.1002/hbm.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noble JW, Eng JJ, Kokotilo KJ, Boyd LA. Aging effects on the control of grip force magnitude: An fMRI study. Exp Gerontol. 2011;46:453–461. doi: 10.1016/j.exger.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992;31:463–472. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- 21.Martin M, et al. Brain activity underlying tool-related and imitative skills after major left hemisphere stroke. Brain. 2016;139:1497–1516. doi: 10.1093/brain/aww035. [DOI] [PubMed] [Google Scholar]
- 22.Corbetta M, et al. Common behavioral clusters and subcortical anatomy in stroke. Neuron. 2015;85:927–941. doi: 10.1016/j.neuron.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massa MS, et al. On the importance of cognitive profiling: A graphical modelling analysis of domain-specific and domain-general deficits after stroke. Cortex. 2015;71:190–204. doi: 10.1016/j.cortex.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Brownsett SL, et al. Cognitive control and its impact on recovery from aphasic stroke. Brain. 2014;137:242–254. doi: 10.1093/brain/awt289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342, quiz 472. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camilleri JA, et al. Multi-modal imaging of neural correlates of motor speed performance in the trail making test. Front Neurol. 2015;6:219. doi: 10.3389/fneur.2015.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pohl PS, Winstein CJ, Onla-Or S. Sensory-motor control in the ipsilesional upper extremity after stroke. NeuroRehabilitation. 1997;9:57–69. doi: 10.3233/NRE-1997-9106. [DOI] [PubMed] [Google Scholar]
- 28.Noskin O, et al. Ipsilateral motor dysfunction from unilateral stroke: Implications for the functional neuroanatomy of hemiparesis. J Neurol Neurosurg Psychiatry. 2008;79:401–406. doi: 10.1136/jnnp.2007.118463. [DOI] [PubMed] [Google Scholar]
- 29.Figley TD, Bhullar N, Courtney SM, Figley CR. Probabilistic atlases of default mode, executive control and salience network white matter tracts: An fMRI-guided diffusion tensor imaging and tractography study. Front Hum Neurosci. 2015;9:585. doi: 10.3389/fnhum.2015.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vry MS, et al. The ventral fiber pathway for pantomime of object use. Neuroimage. 2015;106:252–263. doi: 10.1016/j.neuroimage.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christensen H, Mackinnon AJ, Korten A, Jorm AF. The “common cause hypothesis” of cognitive aging: Evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychol Aging. 2001;16:588–599. doi: 10.1037//0882-7974.16.4.588. [DOI] [PubMed] [Google Scholar]
- 33.Strenge H, Niederberger U. Unidirectional interference in use of nondominant hand during concurrent Grooved Pegboard and random number generation tasks. Percept Mot Skills. 2008;106:763–774. doi: 10.2466/pms.106.3.763-774. [DOI] [PubMed] [Google Scholar]
- 34.Taekema DG, et al. Temporal relationship between handgrip strength and cognitive performance in oldest old people. Age Ageing. 2012;41:506–512. doi: 10.1093/ageing/afs013. [DOI] [PubMed] [Google Scholar]
- 35.Piek JP, Dawson L, Smith LM, Gasson N. The role of early fine and gross motor development on later motor and cognitive ability. Hum Mov Sci. 2008;27:668–681. doi: 10.1016/j.humov.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Flapper BC, Houwen S, Schoemaker MM. Fine motor skills and effects of methylphenidate in children with attention-deficit-hyperactivity disorder and developmental coordination disorder. Dev Med Child Neurol. 2006;48:165–169. doi: 10.1017/S0012162206000375. [DOI] [PubMed] [Google Scholar]
- 37.Muir RT, et al. Trail making test elucidates neural substrates of specific poststroke executive dysfunctions. Stroke. 2015;46:2755–2761. doi: 10.1161/STROKEAHA.115.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jankowska E, Edgley SA. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist. 2006;12:67–79. doi: 10.1177/1073858405283392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sunderland A, Bowers MP, Sluman SM, Wilcock DJ, Ardron ME. Impaired dexterity of the ipsilateral hand after stroke and the relationship to cognitive deficit. Stroke. 1999;30:949–955. doi: 10.1161/01.str.30.5.949. [DOI] [PubMed] [Google Scholar]
- 40.Rehme AK, Eickhoff SB, Rottschy C, Fink GR, Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. Neuroimage. 2012;59:2771–2782. doi: 10.1016/j.neuroimage.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 41.Rowe J, et al. Attention to action in Parkinson’s disease: Impaired effective connectivity among frontal cortical regions. Brain. 2002;125:276–289. doi: 10.1093/brain/awf036. [DOI] [PubMed] [Google Scholar]
- 42.Abela E, et al. A thalamic-fronto-parietal structural covariance network emerging in the course of recovery from hand paresis after ischemic stroke. Front Neurol. 2015;6:211. doi: 10.3389/fneur.2015.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Picton TW, et al. Effects of focal frontal lesions on response inhibition. Cereb Cortex. 2007;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- 44.Grefkes C, Fink GR. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 2014;13:206–216. doi: 10.1016/S1474-4422(13)70264-3. [DOI] [PubMed] [Google Scholar]
- 45.Rinne P, et al. Democratizing neurorehabilitation: How accessible are low-cost mobile-gaming technologies for self-rehabilitation of arm disability in stroke? PLoS One. 2016;11:e0163413. doi: 10.1371/journal.pone.0163413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindberg PG, et al. Affected and unaffected quantitative aspects of grip force control in hemiparetic patients after stroke. Brain Res. 2012;1452:96–107. doi: 10.1016/j.brainres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Reeves M, et al. Distribution of National Institutes of Health Stroke Scale in the Cincinnati/Northern Kentucky Stroke Study. Stroke. 2013;44:3211–3213. doi: 10.1161/STROKEAHA.113.002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hand B, Page SJ, White S. Stroke survivors scoring zero on the NIH Stroke Scale score still exhibit significant motor impairment and functional limitation. Stroke Res Treat. 2014;2014:462681. doi: 10.1155/2014/462681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Björkdahl A, Akerlund E, Svensson S, Esbjörnsson E. A randomized study of computerized working memory training and effects on functioning in everyday life for patients with brain injury. Brain Inj. 2013;27:1658–1665. doi: 10.3109/02699052.2013.830196. [DOI] [PubMed] [Google Scholar]
- 50.Au-Yeung SS, Wang J, Chen Y, Chua E. Transcranial direct current stimulation to primary motor area improves hand dexterity and selective attention in chronic stroke. Am J Phys Med Rehabil. 2014;93:1057–1064. doi: 10.1097/PHM.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 51.van Heugten CM, Dekker J, Deelman BG, Stehmann-Saris FC, Kinebanian A. A diagnostic test for apraxia in stroke patients: Internal consistency and diagnostic value. Clin Neuropsychol. 1999;13:182–192. doi: 10.1076/clin.13.2.182.1966. [DOI] [PubMed] [Google Scholar]
- 52.Wilson B, Cockburn J, Halligan P. Development of a behavioral test of visuospatial neglect. Arch Phys Med Rehabil. 1987;68:98–102. [PubMed] [Google Scholar]
- 53.Wahlund LO, et al. European Task Force on Age-Related White Matter Changes A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 54.Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61:957–965. doi: 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- 56.Medina J, Kimberg DY, Chatterjee A, Coslett HB. Inappropriate usage of the Brunner-Munzel test in recent voxel-based lesion-symptom mapping studies. Neuropsychologia. 2010;48:341–343. doi: 10.1016/j.neuropsychologia.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. 2011;34:569–599. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, et al. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage. 2010;52:1289–1301. doi: 10.1016/j.neuroimage.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.