Significance
This study provides molecular evidence indicating that female hormone/estrogen enables inhibition of vascular soluble epoxide hydrolase (sEH) expression in physiological conditions via an epigenetic-based regulatory mechanism, leading to an increase in the vascular level of epoxyeicosatrienoic acids (EETs) that: (i) possess cardio-protective properties, such as vasodilation, resulting in better blood supply to tissues and lower blood pressure, and (ii) are degraded/inactivated by sEH. To this end, increases in tissue EETs, as a consequence of estrogen suppression of sEH to reduce EET degradation, can serve as an explanation, at least in part, of why women in general perform better cardiovascular functions, associated with lower incidence of ischemic cardiovascular diseases, compared with men.
Keywords: estrogen, Ephx2 gene, soluble epoxide hydrolase, methylation, transcription factors
Abstract
To elucidate molecular mechanisms responsible for the sexually dimorphic phenotype of soluble epoxide hydrolase (sEH) expression, we tested the hypothesis that female-specific down-regulation of sEH expression is driven by estrogen-dependent methylation of the Ephx2 gene. Mesenteric arteries isolated from male, female, ovariectomized female (OV), and OV with estrogen replacement (OVE) mice, as well as the human cell line (HEK293T) were used. Methylation-specific PCR and bisulfite genomic sequencing analysis indicate significant increases in DNA/CG methylation in vessels of female and OVE compared with those of male and OV mice. The same increase in CG methylation was also observed in male vessels incubated with a physiological concentration of 17β-estradiol (17β-E2) for 48 hours. All vessels that displayed increases in CG methylation were concomitantly associated with decreases in their Ephx2 mRNA and protein, suggesting a methylation-induced gene silencing. Transient transfection assays indicate that the activity of Ephx2 promoter-coding luciferase was significantly attenuated in HEK293T cells treated with 17β-E2, which was prevented by additional treatment with an estrogen receptor antagonist (ICI). ChIP analysis indicates significantly reduced binding activities of transcription factors (including SP1, AP-1, and NF-κB with their binding elements located in the Ephx2 promoter) in vessels of female mice and human cells treated with 17β-E2, responses that were prevented by ICI and Decitabine (DNA methyltransferase inhibitor), respectively. In conclusion, estrogen/estrogen receptor-dependent methylation of the promoter of Ephx2 gene silences sEH expression, which is involved in specific transcription factor-directed regulatory pathways.
Sex hormones have the property to operate as transcriptional regulators during the process of gene expression by serving as ligands for nuclear hormone receptors, therefore yielding the phenotype of sex-different gene expression. One potential gene susceptible to the influence of sex hormones is the Ephx2 gene, which encodes the soluble epoxide hydrolase (sEH) protein, an enzyme responsible for converting cardioprotective epoxyeicosatrienoic acids (EETs), metabolites of arachidonic acids by cytochrome P450 (CYP), to their corresponding non- or less-bioactive dihydroxyeicosatrienoic acids. The identification of sexual dimorphism of sEH activity, followed by cDNA cloning and expression of sEH from human and murine livers (1, 2), was originally published around the 1980s, when sEH activity was demonstrated to be remarkably higher in the liver and kidneys of male mice in comparison with female littermates (3, 4), although little functional relevance was reported in these pioneer studies. Only recently has the sexually dimorphic regulation of sEH come to the forefront of research, with aims to specifically evaluate the pathophysiological significance of sEH, particularly in females. The rationale for the present study was inspired by a phenomenon delineated as a “male-specific hypotensive phenotype” that occurs in response to the deletion of the Ephx2 gene (sEH-KO) or the use of sEH inhibitors (sEHIs) and is characterized by significantly lower blood pressure in male mice deficient in the Ephx2 gene/or sEH activity, a response that failed to be initiated in their female counterparts (5, 6). In this regard, we proposed that in vivo presence of female hormones/estrogens heritably possesses an action that imitates the effect caused by sEH-KO in males, which makes females insensitive to additional disruption of the Ephx2 gene. This hypothesis was verified by our, as well as by others’ studies, in both physiological and pathological conditions. Indeed, findings of estrogen-dependent suppression of sEH to increase tissue EETs in the cerebral circulation provide mechanistically based evidence for a female-favorable protection from cerebral ischemic damages (7, 8). We have also provided evidence indicating that female-specific down-regulation of sEH protein expression in cardiac, pulmonary, and vascular tissues favors a greater contribution of EETs in females than males and initiates the same cardiovascular responses as those observed in male sEH-KO mice, or male mice treated with sEHIs (9–12). Particularly in the microcirculation, a genetic deficiency in the Ephx2 gene or down-regulation of sEH expression elicits significantly enhanced flow/shear stress-induced vasodilation and attenuated pressure-induced vasoconstriction via an EET-dependent mechanism in the mesenteric, coronary, and skeletal muscle arterioles (9, 11, 13). To date, however, the responsible signaling and specifics regarding which female hormones and receptors are involved have been left unaddressed. To this end, we hypothesized that the sex-specific phenotype, in terms of female-led down-regulation of sEH, is driven by estrogen-dependent methylation of the Ephx2 gene, a response that is categorized as an epigenetic regulation.
Epigenetics is defined as “the interactions of genes with their environment that bring the phenotype into being.” Epigenetics is characterized as inheritable changes in the expression pattern of a gene, which is not caused by alterations in the nucleotide sequence of the genetic code per se, but rather triggered by modifications in DNA-associated molecules, such as small noncoding RNAs, or by chemical modifications of DNA, such as DNA methylation (14). DNA methylation is a typical epigenetic event that involves attachment of a methyl group (CH3) on the 5′ carbon position of the cytosine ring, and occurs basically at the site of a CG dinucleotide (15), oligoes that are present mostly in the promoter region of genes. Thus, the presence of putative CpG sites and specific transcription factor (TF) binding elements in the promoter region of the human Ephx2 gene (16, 17) provide the molecular basis for the methylation-dependent silencing of Ephx2 expression. The present study was therefore, conducted on both freshly isolated mouse arteries and a human cell line to verify the estrogen-induced down-regulation of Ephx2 gene expression as a result of CG methylation.
Results
Estrogen-Dependent Transcriptional Down-Regulation of sEH.
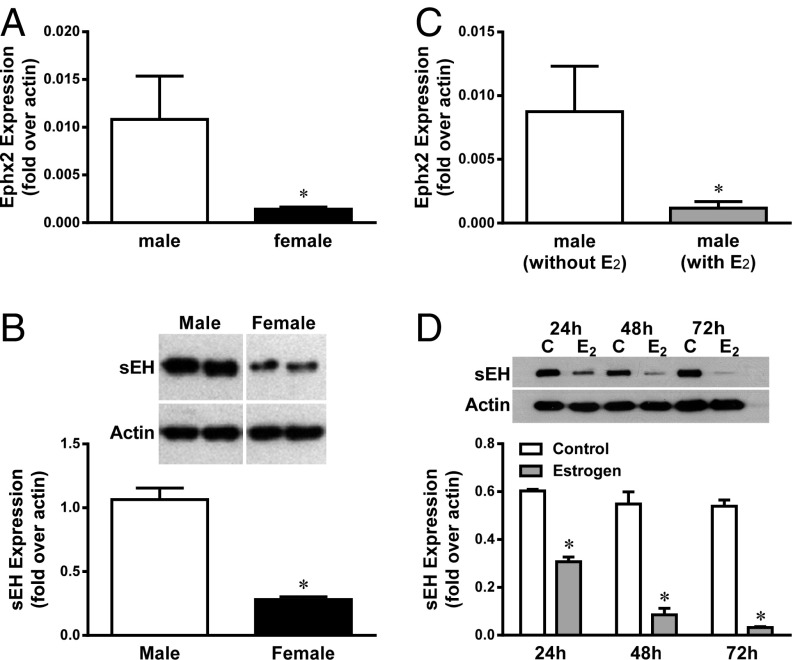
To verify that female-specific down-regulation of sEH expression occurs at the transcriptional level, as a function of estrogen, Ephx2 mRNA expression in mesenteric arteries isolated from male and female mice, and male vessels incubated with a physiological concentration of 17β-estradiol (17β-E2, 10 nM) for 48 h, were assessed. Real-time RT-PCR analysis indicates that female vessels exhibit a significantly reduced Ephx2 mRNA level by ∼80% (Fig. 1A), concomitantly with the same reduction in the protein expression (Fig. 1B) compared with male vessels. Similarly, male vessels treated with 17β-E2 also displayed the same suppression (∼80%) of Ephx2 mRNA (Fig. 1C) and protein content in a time-dependent manner (Fig. 1D), compared with untreated controls, suggesting that the down-regulation of vascular sEH in females is mediated via an estrogen-dependent transcriptionally regulated pathway.
Fig. 1.
Changes in sEH mRNA (A and C) and protein expression (B and D) in mesenteric arteries isolated from male and female mice (A and B) and male arteries with and without treatment with 17β-estradiol (E2, 10 nM) for 48 h (C). (D) Time-course of sEH protein expression in male vessels incubated without (as control) and with E2 for 24, 48, and 72 h, respectively. *Significant difference from controls (n = 5–6).
The Promoter Region of the Ephx2 Gene.
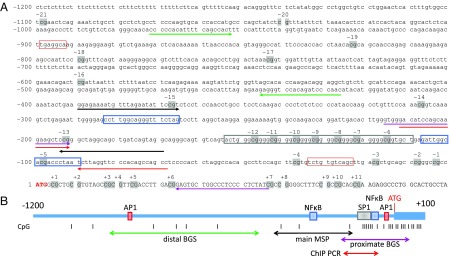
Fig. 2A presents the mouse Ephx2 promoter region (GenBank accession no. AC126272.3). As indicated, there is ∼1,200-bp sequence upstream of the Ephx2 coding region, containing 21 CpG sites, numbered from −1 to −21, that is located upstream of the start codon (ATG in red). The location of primers for both proximal (Fig. 2A, magenta underline arrows) and distal sequencing (Fig. 2A, green underline arrows) are marked. Specific binding elements for the TFs SP1 (Fig. 2A, gray box), AP-1 (Fig. 2A, red box), and NF-κB (Fig. 2A, blue box) are also indicated. In the present study, we focused on the methylation of CpG −13 and −15 using methylation specific PCR (MSP) to determine the methylated/unmethylated ratio. The primers for MSP (Fig. 2A, black underline arrows) were picked up by the software MethPrimer 2.0 (Li Laboratory, www.urogene.org/methprimer/). Fig. 2B is a diagrammatic sketch of the promoter region of Ephx2 gene.
Fig. 2.
(A) A mouse Ephx2 promoter region containing an ∼1,200-bp sequence upstream of the start codon (ATG in red), with 21 CpG sites numbered as −1 ∼ −21 (gray shadow). The primers for MSP are shown with black underline arrows. The primers for both proximal (magenta underline arrows) and distal sequence (green underline arrows), ChIP PCR primers (red underline arrows), and specific binding elements for transcription factors of SP1 (gray box), AP-1 (red box), and NF-κB (blue box) are indicated. (B) Within the diagram of the mouse Ephx2 promoter region, each designated color or font pattern corresponds to the region on the nucleotide sequence in A. Additionally, the position of each GpG site is demarcated by gray bars.
Estrogen-Dependent Increase in Methylation of the Mouse Ephx2 Gene Promoter.
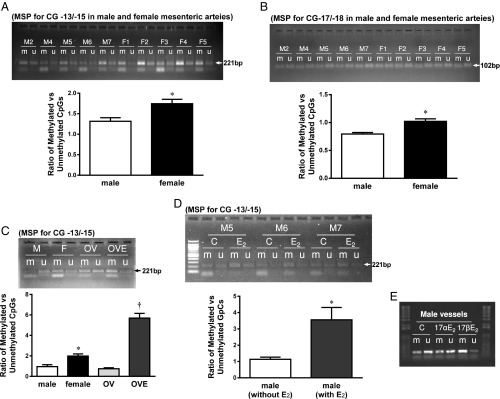
The original tracing and summarized results from MSP are depicted in Fig. 3. Fig. 3A demonstrates that DNA methylation of CpG −13/−15 was significantly increased in vessels of female compared with male mice, as indicated by a greater ratio of methylated to unmethylated DNA (M/U) in female vessels, thereby yielding a sex-different CG methylation. A similar increase in methylation at CpG positions of −17/−18 of female vessels was also observed (Fig. 3B). To clarify if estrogen or other female hormones, such as progesterone, are responsible for the sex-bias in methylation of the Ephx2 promoter, MSP was performed on mesenteric arteries that were isolated from ovariectomized (OV) female mice, OV mice with replacement of estrogen (OVE), and male arteries incubated with 17β-E2 (10 nM) or 17α-E2 (10 nM, physiologically inactive stereoisomer of E2) for 48 h. As shown in Fig. 3C, vessels isolated from OV mice exhibited a significant decrease in the methylation of CpG −13/−15 compared with vessels from intact females, which was comparable to vessels of male mice. Estrogen replacement (OVE) fully prevented the OV-induced reduction in DNA methylation. The functional consequence of ovariectomy was validated by the significant decreases in plasma 17β-E2 and uterus weight in an estrogen replacement-reversible manner. Moreover, treatment of male vessels with 17β-E2, but not 17α-E2, significantly increased DNA methylation (Fig. 3 D and E). Collectively, results from both in vivo (females and OVE) and in vitro (male vessels treated with 17β-E2) treatment with estrogens provide solid evidence indicating that the sex-different methylation of the mouse Ephx2 promoter is purely an estrogen-mediated response.
Fig. 3.
Original tracing and summarized data from MSP showing the ratio of methylated (m) to unmethylated (u) CG −13/−15 (A and C–E) and CG −17/−18 (B) in mesenteric arteries isolated from male (M) and female (F) mice (A and B), female mice with OV, and OVE (C), and male vessels treated with and without 10 nM 17β-E2 (D and E) or 17α-E2 (E). *Significant difference from male and OV. †Significant difference from male, female and OV (n = 4–6 for each group, except for E).
Predominant Methylation of the Female Ephx2 Promoter.
Bisulfite genomic sequencing (BGS) analysis was performed to investigate the CpG methylation status in flanked sequences of the major MSP amplicon and to provide a general profile of sex-differential methylation of the promoter. As shown in Fig. 2, there were 12 CpGs in the approximate sequencing region. The majority of these CpGs were unmethylated, but associated with more methylated mixture in females compared with males. On the other hand, Table 1 summarizes the results from the distal sequencing region (CpG sites −16 to −19) showing that both males and females exhibited decreases in methylation from upstream promoter toward the coding region (from CG −19 to −16), but females presented a greater potential for methylation of these CpG sites than males.
Table 1.
Ratio of C/T (%) in CpG sites of upstream end of Ephx2 promoter
| CG position | −16, % | −17, % | −18, % | −19, % |
| Male (n = 2) | 32.5 | 35 | 52.5 | 67.5 |
| Female (n = 2) | 45 | 47.5 | 65 | 87.5 |
The percentage was calculated by estimating peak areas from each mouse using a Finch TV image.
Specific Target Region for Estrogen-Mediated Methylation in the Ephx2 Promoter.
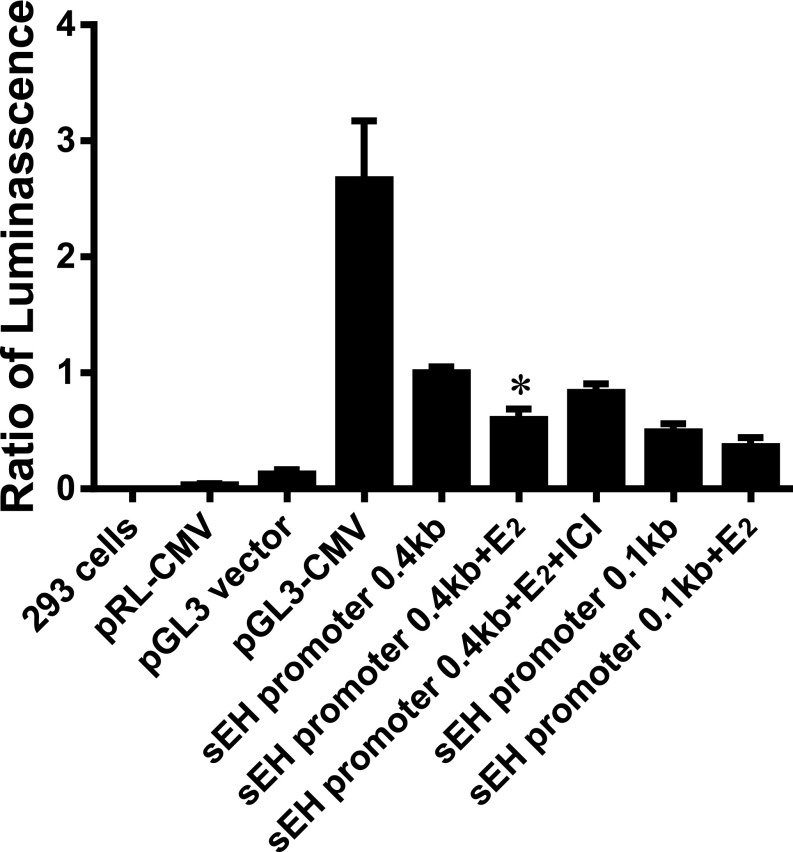
To identify the specific target region of the Ephx2 promoter responsible for the estrogen-dependent methylation, as well as possible roles of estrogen receptors (ERs) in the mediation of estrogen responses, a luciferase-based reporter assay was conducted on a human cell line (293 cells). Fig. 4 shows that in comparison with pGL3-CMV, transfection with the pGL3-sEH promoter (0.4-kb fragment containing two groups of multiple SP1 binding sites, and one AP-1 and one NF-κB binding site, respectively) elicited an ∼40% of total activation induced by the positive control. This activation was significantly attenuated by 17β-E2 (sEH promoter + 17β-E2), and completely reversed by the addition of an ER blocker (sEH promoter + 17β-E2 + ICI 182,780). These results indicate that 17β-E2 directly methylates the Ephx2 promoter region to inhibit sEH transcription via an ER-dependent mechanism. Alternatively, a shorter form of the Ephx2 promoter, which includes a deletion of a 0.4-kb promoter with a 0.12-kb fraction (upstream from five-flanking regions of putative transcriptional start codon of Ephx2) and contains only one group of SP1 binding sites, was also used. In response to transfection with the shorter promoter, treatment with 17β-E2 failed to significantly affect luciferase activity, negating the importance of the proximate end of the human Ephx2 promoter as a major target area for estrogen. Noteworthy, both human (Fig. 4) and mouse Ephx2 promoters (Figs. 1–3) exist with an identical responsiveness to 17β-E2, pointing to estrogen-dependent epigenetic regulation of the Ephx2 gene as being universally applicable.
Fig. 4.
Luciferase activity on human 293 cells transfected with different plasmids of pRL or pGL3 under the control of different promoters, including vector, CMV, and sEH promoters, respectively, in the absence and presence of 17β-E2 (E2, 10 nM) or E2 pus ICI 182,780 (ICI, 100 nM). *Significant difference from transfection with sEH promoter (0.4 kb) alone, and from the 0.4-kb sEH promoter plus E2 and ICI, respectively (n = 3–6).
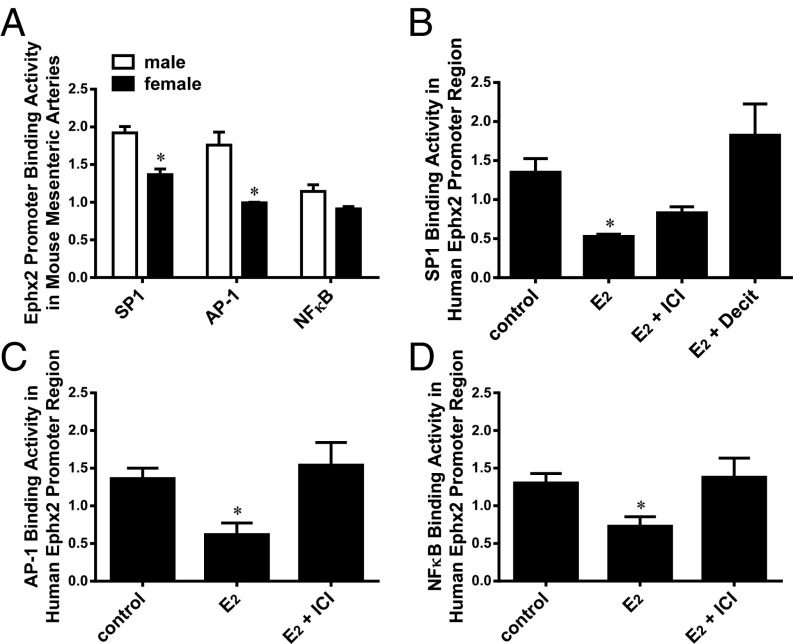
Estrogen/ER Attenuation of TF Binding Activity.
TFs play crucial roles in the regulation of gene expression, and both human and mouse Ephx2 promoter regions contain SP1, AP-1, and NF-κB binding elements (18) (Fig. 2). We therefore performed a ChIP assay to test whether a sex-bias exists in TF binding activities, and if so, whether the change in TF binding is attributed to the estrogen-dependent methylation of the Ephx2 promoter (Figs. 3 and 4). Fig. 5A shows the significant attenuation of SP1 and AP-1 binding activities in female vessels compared with those of males, whereas the reduction in NF-κB binding activity of females was not statistically significant (P = 0.065) from male littermates. A ChIP assay was also performed in human cells, and similar results were observed. As indicated, the treatment of 293T cells with a physiological dose of 17β-E2 elicited significant decreases in SP1 (Fig. 5B), AP-1 (Fig. 5C), and NF-κB (Fig. 5D) binding activities, revealing a functionally identical responsiveness with mouse vessels (Fig. 5A). At CG-rich promoter elements, the reduced SP1 binding activity was prevented by ER antagonist and a DNA methyltransferase (DNMT) inhibitor (5-Aza-CdR), respectively, implying an estrogen/ER-dependent DNA methylation of the binding sequences. However, alternative pathways that are capable of being activated by estrogen/ERs may be involved in the mediation of reductions in AP-1 and NF-κB binding activities.
Fig. 5.
Ephx2 promoter binding activity of transcription factors including SP1 (A and B), AP-1 (A and C), and NF-κB (A and D) in mouse arteries (A) and human 293 cells (B–D) in control conditions and in the presence of E2, E2 pus ICI, and E2 plus Decitabine (Decit), respectively. *Significant difference from males (A); and from their controls and E2 with ICI, respectively (B–D) (n = 5–8 for each group).
Taken together, our results illustrate an integrated network that operates in concert with estrogen, ERs, DNA methylation, and TF binding activities to process the modulation of Ephx2 gene expression.
Discussion
We provide direct evidence that the molecular nature of the sexually dimorphic phenotype of sEH expression is an epigenetic-based event, by which estrogen, through ERs, methylates the Ephx2 gene promoter to silence the gene expression. In particular, specific TF binding motifs act as targets for estrogen to initiate DNA methylation (mainly in the CG-rich SP1 motif), or as a consequence of methylation in compromising access of their ligands (such as binding of c-jun or c-Fos with the AP-1 motif). While a previous study has reported methylation-induced Ephx2 gene silencing involved in SP1-binding activity in carcinoma HEPG2 cells (17), our studies identify CG methylation at the Ephx2 gene promoter as being triggered by estrogen. During this process, specific TFs interface with both methylation-direct and -indirect procedures to collectively constitute the main component of estrogen-mediated signaling through combinatorial and coordinated actions, leading to estrogen-specific physiological down-regulation of Ephx2 gene/sEH expression. Our findings uncover the molecular mechanism that provides explanations for the sexual dimorphism of sEH expression (3, 19) and all of the consequences arising there, defined as a female-favorable protection against cardiac and cerebral ischemia (7–12).
To unravel the mystery of a sexual dimorphism of Ephx2 gene expression, an epigenetic event emerged as our first consideration, since the definition of epigenetics states that heritable changes in the gene phenotype depend on the surrounding environment (15). In this context, an inherent cellular atmosphere that differs from one sex to the other can be primarily attributed to sex hormones. Indeed, the differential expression of certain genes that are selective targets of sex hormones, or are capable of being regulated by sex hormones, have been believed to be responsible for predisposing or protecting either of the sexes to or from cardiovascular diseases. In general, roles of female hormones/estrogens in the genetic regulation of target genes have been extensively studied (20); however, little is known regarding the estrogen-dependent epigenetic regulation of genes, in particular, via methylation-mediated signaling, which indeed, plays a fundamental role in the regulation of physiological and pathological processes. Given the presence of an unaddressed research subject for the physiological significance of epigenetic regulation of sEH by female hormones, we designed the studies aimed to answer the questions as to whether the female-specific phenotype of sEH expression is estrogen-dependent and is mediated by ERs. If so, whether the regulation is driven by DNA methylation and what transcription factors are involved?
Estrogen/ER Mediates Methylation of the Ephx2 Promoter.
Exposure of male vessels to exogenous estrogen significantly decreased Ephx2 mRNA (Fig. 1C) to the level induced by endogenous estrogens in females (Fig. 1A), indicating that the nature of female-specific down-regulation of sEH is an estrogen-dependent response. Consequentially, the suppression of Ephx2 mRNA is concomitantly associated with the same reduction in sEH protein levels (Fig. 1 B and D), as we reported previously (9–13, 20), demonstrating that regulation of sEH by estrogen is at the transcriptional level. We then indicated that DNA methylation is a crucial step in the estrogen/ER-governed regulatory process for Ephx2 expression. There is a similar outline of DNA methylation pattern in Ephx2 promoter of both sexes and associated with a remarkably increased density of unmethylated CpGs from 5′- to 3′- of the promoter. This conclusion was drawn based on our sequencing results: CpG −1 to −12 (3′-end of the promoter) were basically unmethylated, and CpG −19 to −16 (5′-end of the promoter) showed a transition from methylated to the unmethylated (Table 1). On the other hand, although the Ephx2 gene consisted of major unmethylated CpGs in the proximate promoter region, a sex-bias, as indicated by a female-facilitated methylation presented in the distal promoter region (Table 1), could be discerned. Furthermore, the estrogen-dependent nature is verified by Fig. 3, which shows that both in vivo (Fig. 3 A–C) and in vitro (Fig. 3 D and E) presence of estrogen significantly enhanced vascular methylation of the Ephx2 promoter at CG −13/−15 and CG −17/−18. The increase in methylation of CpGs in the Ephx2 promoter is inversely proportional to its gene expression (Fig. 1A), supporting the general concept of negative correlation between DNA/CG methylation and transcriptional activity. As such, our data strongly confirm that the increase in CG methylation of the Ephx2 promoter, as a function of in vivo (intact female and OVE vessels) and in vitro (17β-E2–treated male vessels) stimulation with estrogen, is causative of silencing the Ephx2 gene. This conclusion was further verified by the result that blockage of ERs completely prevented the 17β-E2–induced reduction in the activity of Ephx2 promoter-coding luciferase (Fig. 4). Additionally, DNA binding motifs in the Ephx2 promoter that appear to be most applicable to evoking estrogen/ER-dependent responses were implicated, as indicated by the fact that in response to 17β-E2, transfection of cells with a 0.4-kb promoter, but not a 0.12-kb fragment, initiates a significant attenuation of luciferase activity (Fig. 4). Thus, the DNA binding elements located in the Ephx2 promoter region are important contributors to estrogen-evoked methylation-mediated regulatory response.
In regards to whether estrogen regulates methylation specifically at a certain region of the genome (such as Ephx2 promoter) or plays a broader role in DNA methylation, currently remains unknown. In general, DNMTs determine the DNA methylation pattern of the genome. Alterations in the expression or epigenetic regulation of DNMTs will elicit methylation changes at both regional and global genomic levels (21, 22). On the other hand, besides the classic interaction of ligand-bound ER dimers with estrogen-responsive elements in target gene promoters, estrogen also regulates gene expression via multiple nonclassic pathways. For example, the ER interacts with other DNA-bound transcription factors (such as SP-1 and AP-1) via protein–protein interactions, where it does not directly bind the promoter DNA (23). Moreover, the promoter region of the mouse DNMT indeed contains putative AP-1 binding sites (24). To this end, elucidation of the signaling that specifically links estrogen/ERs, transcription factors, DNMTs, and the Ephx2 promoter should be the topic of future studies.
Estrogen Methylation of Ephx2 Promoter in a TF Binding-Relevant Manner.
Methylated DNA can prevent some transcription factors from binding to their targets, or recruit other factors necessary for modulating gene expression (14, 16, 17). Binding at the SP1 site located upstream of the transcription start site is deemed necessary for suppressing Ephx2 promoter expression (25). Thus, the CG-rich SP1 binding element (Fig. 2) may serve as an indicator of functional coupling between estrogen and SP1 methylation by means of direct methylation with estrogen in an ER-dependent manner. As we have found, an increase in Ephx2 promoter methylation by estrogen (Fig. 3) is accompanied with compromised SP1 binding activity in a Decitabine-reversible manner (Fig. 5B), confirming the crucial role of SP1 binding in the estrogen-dependent methylation of Ephx2 expression. Particularly, in terms of estrogen indirect actions, the estrogen/ER complex can become tethered to DNA by interacting with TFs (26–28) instead of directly binding to estrogen-responsive elements that are not found in the promoter of Ephx2 gene. We believed that an interaction between SP1 and ERs, an event that has been demonstrated to be necessary for estrogen-indirect responses (27), contributes significantly to the methylation of SP1 binding sites with estrogen.
Alternatively, a lesser presence of CpGs in the NF-κB and AP-1 binding areas have led to the speculation that binding reductions in both of them can be, at least in part, mediated by alternative events that are either methylation-independent, or more likely, an indirect consequence of DNA methylation, which recruits or prevents other actions necessary for silencing or enhancing Ephx2 gene expression, respectively. AP-1 belongs to the leucine zipper family of eukaryotic transcription factors (such as Jun and Fos, and so forth), which work as gene modulators. An up-regulation of the Ephx2 gene was reported to be mediated by an angiotensin II-dependent stimulation of c-jun binding to putative AP-1 (18). Furthermore, the cross-talk between ERα and AP-1 was demonstrated to be important in the etiology and progression of certain cancers that are sensitive to ER blockers (29). Thus, it is plausible to assume that estrogen/ER-dependent methylation of the Ephx2 promoter, followed by preventative access of other AP-1 binding ligands, participates reciprocally in prohibiting the binding activity. Moreover, accumulating evidence has confirmed interactions between NF-κB and estrogen/ERs in affecting one another (28), the reduction in NF-κB binding could be dependent of estrogen-induced (i) direct down-regulation of NF-κB gene and (ii) methylation of NF-κB binding sites to compromise binding affinities of NF-κB and other ligands.
In conclusion, the present study demonstrates an estrogen-dependent epigenetic regulation of the Ephx2 gene via DNA methylation-direct and -indirect pathways, both of which converge to form a highly interconnected network that regulates the Ephx2 gene expression through multiple TF-dependent signaling. Our findings are of importance because of the beneficial effects of having increased levels of vascular EETs via silencing of the Ephx2 gene. More intriguingly, identification of estrogen-induced physiological down-regulation of sEH expression highlights a clinical potential for using sEH inhibitors that share the same target with estrogen, as substitutes at least in part, for estrogen-replacement therapy. This is based on the fact that there is a weak correlation between estrogen-replacement therapy and improvement of cardiovascular function and, therefore, the America Heart Association has made recommendation of avoiding estrogen-replacement therapy as a possible means of preventing cardiovascular disease in postmenopausal women (30), who may exist with an up-regulation of sEH as a consequence of estrogen deficiency.
Materials and Methods
Animals and Vessels.
Mesenteric arteries isolated from 12- to 15-wk-old male, female, OV female, and OVE (31) were used. All protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College and conformed to the guidelines of the National Institutes of Health and the American Physiological Society for the use and care of laboratory animals.
Vessel and Cell Cultures.
Isolated mouse vessels and the human cell line HEK293T were used and incubated with or without 17β-estradiol (17β-E2, 10 nM) for 48 h.
qRT-PCR.
The primers were designed in-house and synthesized by Fisher Scientific customer services (Table S1).
BGS and MSP.
The primers for MSP and BGS amplification are shown in Table S1. Bisulfite modification was performed as described previously (32). The BGS assay involved two PCR amplificons in the Ephx2 promoter region: the proximate one from −217 to +57 bp with 21 CpG sites and the distal one from −970 to −427 bp with 4 CpG sites. MSP-detected methylation was expression as the ratio of methylated/unmethylated CpG sites (CpG/TpG × 100).
Luciferase Activity Assay.
Luciferase assay was assessed on human cells (293T) (16) with or without 17β-E2 treatment.
ChIP Assay.
ChIP PCR primers were shown in Table S1. ChIP assay was performed based on the protocol of cross-linking ChIP (Abcam). Mouse vessels from male and female, as well as human cells (293T) with or without 17β-E2 treatment were used as a chromatin source.
See SI Materials and Methods for more details.
Supplementary Material
Acknowledgments
This work was supported by Grants NIH HL070653 and HL129797, and also partially supported by National Institute on Environmental Health Sciences Grant R01 ES02710 and the National Institute on Environmental Health Sciences Superfund Research Program P42 ES04699.
Footnotes
Conflict of interest statement: B.D.H. is the author of University of California patents on the synthesis and use of soluble epoxide hydrolase inhibitors. These patents are licensed to EicOsis LLC.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716016115/-/DCSupplemental.
References
- 1.Beetham JK, Tian T, Hammock BD. cDNA cloning and expression of a soluble epoxide hydrolase from human liver. Arch Biochem Biophys. 1993;305:197–201. doi: 10.1006/abbi.1993.1411. [DOI] [PubMed] [Google Scholar]
- 2.Grant DF, Storms DH, Hammock BD. Molecular cloning and expression of murine liver soluble epoxide hydrolase. J Biol Chem. 1993;268:17628–17633. [PubMed] [Google Scholar]
- 3.Denlinger CL, Vesell ES. Hormonal regulation of the developmental pattern of epoxide hydrolases. Studies in rat liver. Biochem Pharmacol. 1989;38:603–610. doi: 10.1016/0006-2952(89)90205-0. [DOI] [PubMed] [Google Scholar]
- 4.Gill SS, Hammock BD. Distribution and properties of a mammalian soluble epoxide hydrase. Biochem Pharmacol. 1980;29:389–395. doi: 10.1016/0006-2952(80)90518-3. [DOI] [PubMed] [Google Scholar]
- 5.EnayetAllah AE, et al. Opposite regulation of cholesterol levels by the phosphatase and hydrolase domains of soluble epoxide hydrolase. J Biol Chem. 2008;283:36592–36598. doi: 10.1074/jbc.M806315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinal CJ, et al. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275:40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- 7.Fairbanks SL, et al. Mechanism of the sex difference in neuronal ischemic cell death. Neuroscience. 2012;219:183–191. doi: 10.1016/j.neuroscience.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, et al. Role of soluble epoxide hydrolase in the sex-specific vascular response to cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:1475–1481. doi: 10.1038/jcbfm.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Froogh G, et al. Female-favorable attenuation of coronary myogenic constriction via reciprocal activations of epoxyeicosatrienoic acids and nitric oxide. Am J Physiol Heart Circ Physiol. 2016;310:H1448–H1454. doi: 10.1152/ajpheart.00906.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kandhi S, et al. EET-dependent potentiation of pulmonary arterial pressure: Sex-different regulation of soluble epoxide hydrolase. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1478–L1486. doi: 10.1152/ajplung.00208.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin J, et al. Sexually dimorphic phenotype of arteriolar responsiveness to shear stress in soluble epoxide hydrolase-knockout mice. Am J Physiol Heart Circ Physiol. 2015;309:H1860–H1866. doi: 10.1152/ajpheart.00568.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin J, et al. Sexually dimorphic adaptation of cardiac function: Roles of epoxyeicosatrienoic acid and peroxisome proliferator-activated receptors. Physiol Rep. 2016;4:e12838. doi: 10.14814/phy2.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun D, et al. Soluble epoxide hydrolase-dependent regulation of myogenic response and blood pressure. Am J Physiol Heart Circ Physiol. 2014;306:H1146–H1153. doi: 10.1152/ajpheart.00920.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatterjee R, Vinson C. CpG methylation recruits sequence specific transcription factors essential for tissue specific gene expression. Biochim Biophys Acta. 2012;1819:763–770. doi: 10.1016/j.bbagrm.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lister R, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka H, et al. Transcriptional regulation of the human soluble epoxide hydrolase gene EPHX2. Biochim Biophys Acta. 2008;1779:17–27. doi: 10.1016/j.bbagrm.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D, Ai D, Tanaka H, Hammock BD, Zhu Y. DNA methylation of the promoter of soluble epoxide hydrolase silences its expression by an SP-1-dependent mechanism. Biochim Biophys Acta. 2010;1799:659–667. doi: 10.1016/j.bbagrm.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Ai D, et al. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci USA. 2007;104:9018–9023. doi: 10.1073/pnas.0703229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meijer J, Lundqvist G, DePierre JW. Comparison of the sex and subcellular distributions, catalytic and immunochemical reactivities of hepatic epoxide hydrolases in seven mammalian species. Eur J Biochem. 1987;167:269–279. doi: 10.1111/j.1432-1033.1987.tb13333.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang A, Kaley G. Gender-specific regulation of cardiovascular function: Estrogen as key player. Microcirculation. 2004;11:9–38. doi: 10.1080/10739680490266162. [DOI] [PubMed] [Google Scholar]
- 21.Denis H, Ndlovu MN, Fuks F. Regulation of mammalian DNA methyltransferases: A route to new mechanisms. EMBO Rep. 2011;12:647–656. doi: 10.1038/embor.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarabi MM, Naghibalhossaini F. Association of DNA methyltransferases expression with global and gene-specific DNA methylation in colorectal cancer cells. Cell Biochem Funct. 2015;33:427–433. doi: 10.1002/cbf.3126. [DOI] [PubMed] [Google Scholar]
- 23.Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol. 2008;41:263–275, and erratum (2009) 42:359. doi: 10.1677/JME-08-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouleau J, Tanigawa G, Szyf M. The mouse DNA methyltransferase 5′-region. A unique housekeeping gene promoter. J Biol Chem. 1992;267:7368–7377. [PubMed] [Google Scholar]
- 25.Harris TR, Hammock BD. Soluble epoxide hydrolase: Gene structure, expression and deletion. Gene. 2013;526:61–74. doi: 10.1016/j.gene.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, et al. Inhibition of AP-1 transcription factor causes blockade of multiple signal transduction pathways and inhibits breast cancer growth. Oncogene. 2002;21:7680–7689. doi: 10.1038/sj.onc.1205883. [DOI] [PubMed] [Google Scholar]
- 27.Saville B, et al. Ligand-, cell-, and estrogen receptor subtype (alpha/beta)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem. 2000;275:5379–5387. doi: 10.1074/jbc.275.8.5379. [DOI] [PubMed] [Google Scholar]
- 28.Frasor J, El-Shennawy L, Stender JD, Kastrati I. NFκB affects estrogen receptor expression and activity in breast cancer through multiple mechanisms. Mol Cell Endocrinol. 2015;418:235–239. doi: 10.1016/j.mce.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babu RL, et al. Effect of estrogen and tamoxifen on the expression pattern of AP-1 factors in MCF-7 cells: Role of c-Jun, c-Fos, and Fra-1 in cell cycle regulation. Mol Cell Biochem. 2013;380:143–151. doi: 10.1007/s11010-013-1667-x. [DOI] [PubMed] [Google Scholar]
- 30.White RE, Gerrity R, Barman SA, Han G. Estrogen and oxidative stress: A novel mechanism that may increase the risk for cardiovascular disease in women. Steroids. 2010;75:788–793. doi: 10.1016/j.steroids.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang A, Sun D, Kaley G, Koller A. Estrogen preserves regulation of shear stress by nitric oxide in arterioles of female hypertensive rats. Hypertension. 1998;31:309–314. doi: 10.1161/01.hyp.31.1.309. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Tollefsbol TO. DNA methylation detection: Bisulfite genomic sequencing analysis. Methods Mol Biol. 2011;791:11–21. doi: 10.1007/978-1-61779-316-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.