Abstract
In the present study, the expression of β-catenin, leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5) and GATA6 was investigated during the transition from normal mucosa through to adenoma and adenocarcinoma in colorectal tissue sections obtained from 65 patients with a pathological diagnosis of colorectal adenocarcinoma and a history of adenoma. Immunohistochemical staining of β-catenin, LGR5 and GATA6 was performed and evaluated. The nuclear expression of β-catenin and the cytoplasmic expression of LGR5 and GATA6 were increased in samples as they progressed from normal mucosa to adenoma and adenocarcinoma. However, membrane-bound β-catenin and nuclear GATA6 expression decreased. Positive correlations were observed between the expression of LGR5 and cytoplasmic GATA6 in adenoma (P=0.0005; rs=0.48) and adenocarcinoma samples (P=0.007; rs=0.38): However, no significant association was observed in normal mucosa (P=0.399). The expression of nuclear β-catenin was significantly increased in the serosal layer compared with the invasive layers of the colorectal wall in samples of adenocarcinoma (P=0.042). The results of the present study suggest that the nuclear expression of β-catenin and LGR5 and the cytoplasmic expression of GATA6 function together during the development of colorectal carcinoma.
Keywords: colorectal cancer, tumorigenesis, β-catenin, leucine-rich repeat-containing G protein-coupled receptor 5, GATA6
Introduction
Worldwide, colorectal cancer (CRC) is a leading cause of cancer-associated mortality (1). Despite significant advances in medical and surgical treatment options, the mortality rate for CRC remains high (2), with a reported 812,000–855,000 mortalities resulting from colon and rectal cancer in 2015 (3). In order to address this issue and improve the prognosis of patients with CRC, a more thorough understanding of the underlying pathological processes of CRC carcinogenesis is required.
The adenoma-carcinoma sequence represents the process by which the majority of CRC arise (4,5). In 70–80% of colorectal tumors, this sequence is initiated by the mutation and inactivation of the adenomatous polyposis coli (APC) gene, which acts as the gatekeeper of colorectal tumorigenesis (6). The APC protein combines with glycogen synthase kinase 3β, axin and casein kinase 1α to form a ‘destruction complex’ that degrades β-catenin (7). As a consequence of APC protein inactivation, the Wnt/β-catenin signaling pathway is often aberrantly activated in CRC (6). The Wnt/β-catenin signaling pathway is important as it serves a key function in the regulation of intestinal stem cells (ISCs) (8). These are multipotent cells with the capacity to self-renew and thus sustain the proliferative capacity of the intestinal epithelium (9). Cancer stem cells (CSCs) act in a manner similar to normal ISCs to promote tumor expansion and progression in adenomas and adenocarcinomas (10,11).
In addition to Wnt/β-catenin, two other key signaling pathways regulate colorectal ISCs; the Notch signaling pathway, which is associated with generating cells with ISC-like properties (12,13), and the bone morphogenetic protein (BMP) signaling pathway, which counteracts the effects of the Wnt/β-catenin pathway (14,15). Cellular activation of the Wnt/β-catenin signaling pathway is characterized by the nuclear localization of β-catenin; these cells act as CSCs and are capable of tumorigenesis (6,12,16). Additionally, membrane-bound β-catenin is associated with the presence of epithelial cadherin, which maintains epithelial cell-cell adhesion; loss of membrane-bound β-catenin may contribute to the activation of the Wnt/β-catenin signaling pathway (17,18).
Leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5) acts downstream of the Wnt gene and has been identified as a marker of ISCs and CSCs in CRC (19–23). It has been reported that increased expression of LGR5 occurs in colon cancer cell lines as well as samples of colorectal adenoma and adenocarcinoma, compared with noncancerous controls (24). In addition, LGR5 silencing in colorectal cell lines has been reported to result in downregulated Notch signaling (22). Specific activation of the Wnt/β-catenin signaling pathway via the loss of APC in ISCs expressing LGR5 is sufficient to promote the formation of adenomas in the mouse intestine (21).
GATA6, a member of the zinc-finger DNA binding transcription factor family, has been identified as a regulator of the Wnt/β-catenin signaling pathway, the BMP pathway and LGR5 expression. GATA6 deficiency suppresses colonic tumorigenesis by antagonizing the β-catenin/transcription factor 4 complex by binding to the BMP4 regulatory region in APC null mice (25). Furthermore, GATA6 is able to bind to the LGR5 promoter region and, as such, knockdown of GATA6 decreases LGR5 mRNA levels to a similar extent as β-catenin knockdown (25). Decreasing the expression of LGR5 via suppressing GATA6 therefore inhibits the tumorigenic properties of CRC cells (26). Based on the available evidence, it was hypothesized in the present study that the nuclear translocation of β-catenin and increased expression of LGR5 and GATA6 may serve a cooperative role in human colorectal tumorigenesis. The aim of the present study was to investigate this cooperative role by observing the varied expression of β-catenin, LGR5 and GATA6 in a normal mucosa-adenoma-adenocarcinoma sequence in colorectal tissue samples.
Materials and methods
Subjects
A total of 65 patients with a pathological diagnosis of colorectal adenocarcinoma and a history of adenoma that underwent surgical resections at The First Affiliated Hospital of Jinzhou Medical University, Department of General Surgery (Liaoning, China) between June 2012 and September 2014 were retrospectively included in the present study. The Tumor-Node-Metastasis (TNM) stage of patients was determined according to the 7th edition of the American Joint Committee on Cancer staging manual (27,28). The baseline characteristics of the patients are presented in Table I.
Table I.
Characteristics of patients with colorectal adenoma and adenocarcinoma.
| Characteristic | n (%) |
|---|---|
| Age, years | |
| <65 | 31 (47.69) |
| ≥65 | 34 (52.31) |
| Sex | |
| Male | 47 (72.31) |
| Female | 18 (27.69) |
| Location of adenoma | |
| Colon | 25 (38.46) |
| Rectum | 40 (61.54) |
| Histological grade of adenomatous dysplasia | |
| Mild | 29 (44.62) |
| Moderate/severe | 31 (47.69) |
| Number of adenoma | |
| Single | 56 (86.15) |
| Multiple | 9 (13.85) |
| Location of adenocarcinoma | |
| Colon | 26 (40.00) |
| Rectum | 39 (60.00) |
| TNM staging of adenocarcinoma | |
| Stage I/II | 46 (70.77) |
| Stage III/IV | 19 (29.23) |
| pTNM T stage | |
| T1/T2 | 18 (27.69) |
| T3/T4 | 47 (72.31) |
| pTNM N stage | |
| N0 | 47 (72.31) |
| N1/N2 | 18 (27.69) |
pTNM, pathological Tumor-Node-Metastasis.
Adenoma, adenocarcinoma and adjacent normal tissue samples (3-µm thick) were fixed in 4% formalin overnight at 4°C and embedded in paraffin. The majority of adenomas (89.25%; n=58/65) were in the same location as adenocarcinomas diagnosed in the same patient. The present study was approved by The Ethics Committee of The First Affiliated Hospital of Jinzhou Medical University.
Immunohistochemistry (IHC)
The tissue sections were initially deparaffinized in an oven at 65°C for 2 h, immersed in xylene 3 times and subsequently heated to 100°C in 10 mM citrate buffer (Origene Technologies, Inc., Beijing, China), pH 6.0, for 30 min. Sections were blocked with 1% bovine serum albumin (A1933; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and 5% normal goat serum (S-1000; Vector Laboratories, Inc., Burlingame, CA, USA) in PBS plus 0.04% tween 20 at room temperature for 1 h. Sections were subsequently incubated with primary anti-LGR5 (1:100; ab75850; Abcam, Cambridge, UK), anti-GATA6 (1:150; sc-9055; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and anti-β-catenin antibodies (1:200; 51067-2-AP; ProteinTech Group, Inc., Chicago, IL, USA) at 4°C overnight. Samples were treated with a 2-step plus Poly-HRP anti-mouse/rabbit IgG detection system (cat. no. PV-9000; Origene Technologies, Inc.) and 3,3′-diaminobenzidine tetrahydrochloride (cat. no. ZLI-9032; Origene Technologies, Inc.), according to manufacturer's protocols. Finally, samples were counterstained with Mayer's hematoxylin (cat. no. MHS16; Sigma-Aldrich; Merck KGaA) at room temperature for 5 min and mounted with Histomount mounting medium (HS-103; National Diagnostics, Atlanta, GA, USA).
Evaluation of IHC staining
IHC results were evaluated as previously described (29). Images were evaluated in at least three fields of view at ×200 magnification using an Olympus BX53 light microscope (Olympus Corporation, Tokyo, Japan) which included either a whole section or whole layer of colorectal wall (mucosa-submucosa-muscularis-serosa) were evaluated and selected unanimously by two experienced pathologists. The pathologists also took into consideration the staining intensity and the proportion of positive staining. The intensity score refers to four grades of staining intensity: 0, negative staining; 1, weak/light yellow staining; 2, moderate/yellow staining; and 3, strong/dark yellow staining. The quantity score refers to the proportion of positively stained cells in the tissue sections: 0, 0–4% positively stained cells; 1, 5–24% positively stained cells; 2, 25–49% positively stained cells; 3, 50–74% positively stained cells; and 4, 75–100% positively stained cells. The final overall score ranged from 0 to 12 and was obtained by multiplying the intensity score by the quantity score. The mean of the overall scores of the selected fields was analyzed as the evaluative scores of staining. Tissue sections with unavailable staining were excluded from the final analysis.
Statistical analysis
SAS-JMP software (version 11; SAS Institute, Inc., Cary, NC, USA) was used for statistical analysis. For comparisons between groups, a Kruskal-Wallis test followed by Steel-Dwass method was performed for abnormally distributed data comprising more than two groups. A Wilcoxon rank-sum was used when there were two groups. Data are presented as the median [interquartile range, (IQR)]. Spearman rank correlation was used to analyze the correlation between β-catenin, LGR5 and GATA6 expression. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of β-catenin, LGR5 and GATA6 in a normal mucosa-adenoma-adenocarcinoma sequence of colorectal tissue
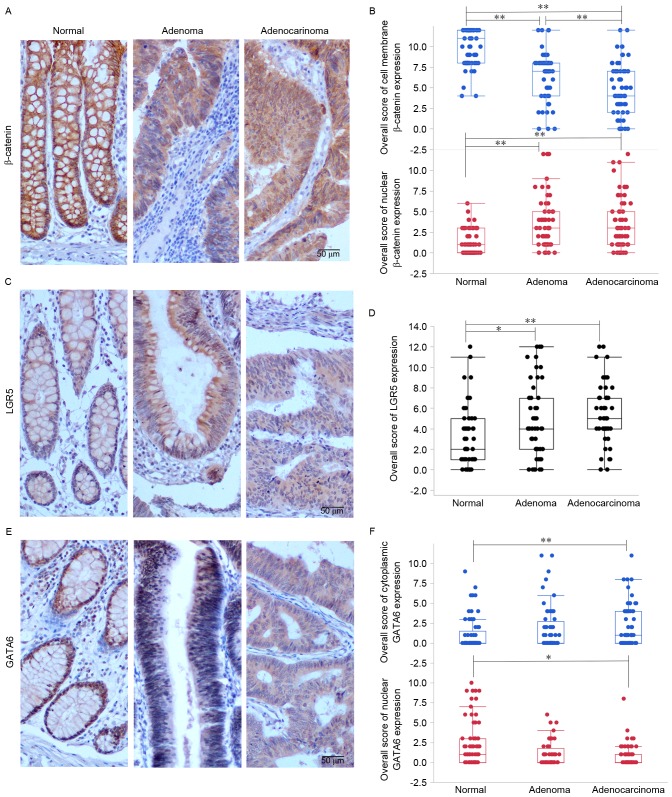
To simultaneously observe the effect of Wnt/β-catenin signaling pathway activation, LGR5 and GATA6 on colorectal tumorigenesis, the expression of β-catenin, LGR5 and GATA6 in adenocarcinoma, adjacent adenoma and normal tissue samples were evaluated. It was identified that β-catenin, LGR5 and GATA6 were expressed in distinct locations within intestinal epithelial cells: β-catenin was expressed on the cell membrane, in the cytoplasm and in the nucleus; LGR5 was predominantly expressed in the cytoplasm; and GATA6 was expressed in the cell nucleus and cytoplasm (Fig. 1).
Figure 1.
The expression and intracellular localization of β-catenin, LGR5 and GATA6 in a normal mucosa-adenoma-adenocarcinoma sequence. (A) Immunolabeling patterns and (B) quantitative analysis of β-catenin; (C) immunolabeling patterns and (D) quantitative analysis of LGR5; and (E) immunolabeling and (F) quantitative analysis of GATA6 in representative colorectal tissues, including normal mucosa, adenomas and adenocarcinomas obtained from the same patient. Scale bar=50 µm. *P<0.05, **P<0.01. LGR5, leucine-rich repeat-containing G protein-coupled receptor 5; GATA6, GATA-binding factor 6.
Nuclear expression of β-catenin increased in samples as they progressed from normal mucosa to adenoma to adenocarcinoma (P<0.0001), whereas membrane-bound β-catenin expression decreased (P<0.0001; Fig. 1A and B). A statistically significant difference was identified between all pairwise comparisons, with the exception of nuclear β-catenin expression in adenoma and adenocarcinoma samples. This suggests that increased nuclear β-catenin expression and decreased cell-membrane β-catenin expression may serve a function in the transformation from normal colorectal mucosa to adenoma.
To investigate the expression of LGR5 and its function in the normal mucosa-adenoma-adenocarcinoma transformation, IHC staining of LGR5 in tissue sections were evaluated. It was revealed that, similar to nuclear β-catenin expression, there was an increase in LGR5 expression as the tissue progressed from normal mucosa to adenocarcinoma (P=0.0003; Fig. 1C and D).
Similarly, cytoplasmic GATA6 was overexpressed in adenocarcinomas compared with normal mucosal samples (P=0.015; Fig. 1E and F). In contrast, nuclear GATA6 was downregulated in adenocarcinomas compared with normal mucosa (P=0.001; Fig. 1E and F).
These data demonstrate that nuclear β-catenin and cytoplasmic LGR5 expression is higher in adenoma and adenocarcinoma samples compared with normal mucosal samples, whereas cytoplasmic GATA6 expression is higher in adenocarcinoma samples compared with normal mucosal samples. These findings suggest that nuclear β-catenin and LGR5 may serve a function in the transition from normal mucosa to adenoma and increased expression of cytoplasmic GATA6 may promote the formation of adenocarcinoma.
Correlation between the expression of β-catenin, LGR5 and GATA6 in normal mucosa, adenoma and adenocarcinoma
Using normal mucosa, adenoma and adenocarcinoma tissue sections obtained from the same patient, the association between disease stage and β-catenin, LGR5 and GATA6 expression in distinct intracellular locations was investigated (Tables II–IV). In adenomas and adenocarcinomas, a significant positive correlation was observed between cytoplasmic GATA6 expression and LGR5 expression (P=0.001 rs=0.481, Table III; P=0.007, rs=0.377, Table IV). In contrast, there was a negative association between nuclear GATA6 expression and LGR5 expression in adenomas (P=0.022, rs=−0.329; Table V). No significant associations were observed in normal mucosa (Table II). These results suggest that there is an interaction between GATA6 and LGR5 expression in colorectal tumors but not in normal mucosa. No significant association between nuclear β-catenin and GATA6 expression was observed in any tissues (Tables II–IV).
Table II.
Correlation between β-catenin, LGR5 and GATA6 in colorectal normal mucosa.
| Protein | Cell membrane β-catenin | Nuclear β-catenin | LGR5 | Cytoplasmic GATA6 | Nuclear GATA6 |
|---|---|---|---|---|---|
| Cell membrane β-catenin | rs=1.000 | rs=0.085 | rs=0.146 | rs=−0.176 | rs=−0.048 |
| – | P=0.516 | P=0.308 | P=0.176 | P=0.715 | |
| Nuclear β-catenin | rs=0.085 | rs=1.000 | rs=0.134 | rs=−0.001 | rs=0.056 |
| P=0.516 | – | P=0.353 | P=0.993 | P=0.674 | |
| LGR5 | rs=0.146 | rs=0.134 | rs=1.000 | rs=0.122 | rs=0.012 |
| P=0.308 | P=0.353 | – | P=0.399 | P=0.934 | |
| Cytoplasmic GATA6 | rs=−0.176 | rs=−0.001 | rs=0.122 | rs=1.000 | rs=0.074 |
| P=0.176 | P=0.993 | P=0.399 | – | P=0.573 | |
| Nuclear GATA6 | rs=−0.048 | rs=0.056 | rs=0.012 | rs=0.074 | rs=1.000 |
| P=0.715 | P=0.674 | P=0.934 | P=0.573 | – |
Statistical analyses were performed using Spearman's rank correlation coefficient. LGR5, leucine-rich repeat-containing G protein-coupled receptor 5; GATA6, GATA-binding factor 6.
Table IV.
Correlation between β-catenin, LGR5 and GATA6 in colorectal adenocarcinoma.
| Cell membrane β-catenin | Nuclear β-catenin | LGR5 | Cytoplasmic GATA6 | Nuclear GATA6 | |
|---|---|---|---|---|---|
| Cell membrane β-catenin | rs=1.000 | rs=−0.141 | rs=−0.086 | rs=−0.056 | rs=−0.002 |
| – | P=0.270 | P=0.554 | P=0.665 | P=0.985 | |
| Nuclear β-catenin | rs=−0.141 | rs=1 | rs=−0.233 | rs=−0.212 | rs=0.251 |
| P=0.270 | – | P=0.103 | P=0.098 | P=0.049a | |
| LGR5 | rs=−0.086 | rs=−0.233 | rs=1 | rs=0.377 | rs=−0.270 |
| P=0.554 | P=0.103 | – | P=0.007b | P=0.058 | |
| Cytoplasmic GATA6 | rs=−0.056 | rs=−0.212 | rs=0.377 | rs=1 | rs=0.123 |
| P=0.665 | P=0.098 | P=0.007b | – | P=0.343 | |
| Nuclear GATA6 | rs=−0.002 | rs=0.251 | rs=−0.270 | rs=0.122 | rs=1.000 |
| P=0.985 | P=0.049a | P=0.058 | P=0.343 | – |
P<0.05
P<0.01. Statistical analyses were performed using Spearman's rank correlation coefficient. LGR5, leucine-rich repeat-containing G protein-coupled receptor 5; GATA6, GATA-binding factor 6.
Table III.
Correlation between β-catenin, LGR5 and GATA6 in colorectal adenoma.
| Protein | Cell membrane β-catenin | Nuclear β-catenin | LGR5 | Cytoplasmic GATA6 | Nuclear GATA6 |
|---|---|---|---|---|---|
| Cell membrane β-catenin | rs=1.000 | rs=0.022 | rs=−0.135 | rs=−0.168 | rs=0.305 |
| – | P=0.866 | P=0.367 | P=0.209 | P=0.020a | |
| Nuclear β-catenin | rs=0.022 | rs=1.000 | rs=−0.087 | rs=−0.170 | rs=0.148 |
| P=0.866 | – | P=0.560 | P=0.202 | P=0.269 | |
| LGR5 | rs=−0.135 | rs=−0.087 | rs=1.000 | rs=0.481 | rs=−0.329 |
| P=0.367 | P=0.560 | – | P=0.001b | P=0.022a | |
| Cytoplasmic GATA6 | rs=−0.168 | rs=−0.170 | rs=0.481 | rs=1.000 | rs=−0.235 |
| P=0.209 | P=0.202 | P=0.001b | – | P=0.071 | |
| Nuclear GATA6 | rs=0.305 | rs=0.148 | rs=−0.329 | rs=−0.235 | rs=1.000 |
| P=0.020a | P=0.269 | P=0.022a | P=0.071 | – |
P<0.05.
P<0.01. Statistical analyses were performed using Spearman's rank correlation coefficient. LGR5, leucine-rich repeat-containing G protein-coupled receptor 5; GATA6, GATA-binding factor 6.
Table V.
β-catenin, LGR5 and GATA6 expression in adenoma stratified by histological grade of dysplasia.
| Cell membrane β-catenin | Nuclear β-catenin | LGR5 | Cytoplasmic GATA6 | Nuclear GATA6 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dysplasia | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 |
| Mild | 27 | 8 (2) | 2.17 | 27 | 4 (3) | 4.33a | 24 | 4 (7) | 0.46 | 27 | 0 (4) | 0.02 | 27 | 0 (3) | 0.57 |
| Moderate/severe | 29 | 7 (6) | 29 | 2 (3) | 22 | 4 (4) | 29 | 1 (3) | 29 | 0 (1) | |||||
P<0.05. Statistical analyses were performed using a Wilcoxon signed-rank test. LGR5, leucine-rich repeat-containing G protein-coupled receptor 5; GATA6, GATA-binding factor 6; IQR, interquartile range.
Analysis of β-catenin, LGR5 and GATA6 expression in colorectal adenoma and adenocarcinoma stratified by pathological parameters
β-catenin, LGR5 and GATA6 expression was investigated in samples of adenomas and adenocarcinomas stratified by pathological parameters (Tables V and VI). Adenomas with mild-grade dysplasia exhibited increased levels of nuclear β-catenin expression compared with adenomas with moderate and severe-grade dysplasia (P=0.038; Table V). This suggests that upregulation of nuclear β-catenin may be an early event in the pathogenesis of colorectal tumors. When protein expression was evaluated in adenocarcinomas with distinct pathological TNM stages, nuclear GATA6 was observed to be significantly downregulated in stage T3 and T4 adenocarcinomas compared with stage T1 and T2 adenocarcinomas (P=0.024; Table VI).
Table VI.
β-catenin, LGR5 and GATA6 expression in adenocarcinoma stratified by pathological parameters.
| Cell membrane β-catenin | Nuclear β-catenin | LGR5 | Cytoplasmic GATA6 | Nuclear GATA6 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 |
| TNM staging | |||||||||||||||
| I/II | 44 | 5 (5) | 0.09 | 44 | 3 (5) | 0.04 | 38 | 5 (3) | 1.74 | 44 | 1 (4) | 0.97 | 44 | 1 (1) | 0.69 |
| III/IV | 19 | 4 (4) | 19 | 3 (4) | 12 | 6 (4) | 18 | 1 (4) | 18 | 0 (1) | |||||
| pTNM T stage | |||||||||||||||
| T1/T2 | 17 | 4 (5) | 0.04 | 17 | 4 (7) | 0.23 | 15 | 6 (3) | 0.10 | 17 | 1 (3) | 0.07 | 17 | 1 (3) | 5.10a |
| T3/T4 | 46 | 4 (5) | 46 | 3 (4) | 35 | 5 (3) | 45 | 1 (4) | 45 | 0 (1) | |||||
| pTNM N stage | |||||||||||||||
| N0 | 45 | 4 (5) | 0.64 | 45 | 2 (5) | 0.08 | 39 | 5 (3) | 0.09 | 45 | 1 (4) | 0.43 | 45 | 0 (2) | 0.36 |
| N1&N2 | 18 | 4 (5) | 18 | 3 (5) | 11 | 6 (3) | 17 | 1 (5) | 17 | 0 (1) | |||||
P<0.05. Statistical analyses were performed using a Wilcoxon signed-rank test. pTNM, pathological tumor-node-metastasis; LGR5, leucine-rich repeat-containing G protein-coupled receptor 5; GATA6, GATA-binding factor 6; IQR, interquartile range.
Nuclear β-catenin expression is positively correlated with the depth of invasion of colorectal adenocarcinomas, however LGR5 and GATA6 expression is not
To explore the association between β-catenin, LGR5 and GATA6 expression and the depth of tumor invasion, protein expression was evaluated at distinct invasive depths (mucosa, submucosa, muscularis and serosa) in the same sample of adenocarcinoma. Nuclear β-catenin expression was significantly increased in the serosal layer compared with all other layers (P=0.042; Table VII). No significant difference in the cell-membrane expression of β-catenin, or the cytoplasmic expression of LGR5 or GATA6, was observed among layers (Table VII). These results suggest that nuclear β-catenin expression may contribute to the invasive capacity of colorectal cancer. In addition, these results are consistent with those of previous reports, which demonstrated increased expression of nuclear β-catenin in the invasive front (30) and that the Wnt/β-catenin pathway regulates the epithelial to mesenchymal transition and increases the invasive capabilities of tumor cells (31,32).
Table VII.
β-catenin, LGR5 and GATA6 expression in different invasive depth of colorectal adenocarcinoma.
| Cell membrane β-catenin | Nuclear β-catenin | LGR5 | Cytoplasmic GATA6 | Nuclear GATA6 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 | n | Median (IQR) | χ2 |
| Mucosa | 28 | 4 (7) | 1.63 | 28 | 2 (7) | 8.23a | 14 | 7 (4) | 1.28 | 8 | 1 (4) | 3.79 | 8 | 2 (4) | 6.41 |
| Submucosa | 45 | 4 (4) | 45 | 2 (4) | 29 | 4 (3) | 29 | 1 (2) | 29 | 0 (1) | |||||
| Muscularis | 53 | 4 (6) | 53 | 3 (5) | 36 | 6 (4) | 42 | 2 (4) | 42 | 0 (1) | |||||
| Serosa | 10 | 2 (7) | 10 | 6 (9) | 7 | 4 (3) | 4 | 4 (6) | 4 | 0 (0) | |||||
P<0.05. Statistical analyses were performed using a Kruskal-Wallis test followed by the Steel-Dwass method. pTNM, pathological tumor-node-metastasis; LGR5, leucine-rich repeat-containing G protein-coupled receptor 5; GATA6, GATA-binding factor 6; IQR, interquartile range.
Discussion
Activation of the Wnt/β-catenin signaling pathway results in β-catenin translocation to the nucleus and transcription of Wnt target genes; this process has been implicated in the initiation of colorectal tumorigenesis (33,34). LGR5 is a target of the Wnt/β-catenin signaling pathway (19) and has been identified in colorectal CSCs with an expansive phenotype (35,36); furthermore, this protein may be regulated by GATA6 during CRC formation (26).
To evaluate the contribution and interactions of β-catenin, LGR5 and GATA6 in the adenoma-carcinoma sequence of human intestinal samples, matched samples of normal mucosa, adenoma and adenocarcinoma were used. The expression of these proteins in distinct intracellular locations and the associations between them were assessed. Compared with normal mucosal samples, adenoma and adenocarcinoma samples exhibited increased nuclear β-catenin and cytoplasmic LGR5 expression. These results are consistent with previous studies that have identified increased levels of nuclear β-catenin expression in colorectal tumors (16,30). Furthermore, suppression of the Wnt/β-catenin signaling pathway in CRC cells has been demonstrated to inhibit tumor formation (37), whereas silencing LGR5 in CRC cell lines decreases proliferation, migration and colony formation in vitro, as well as tumorigenic capacity in vivo (22). Furthermore, ISCs that are LGR5+ and in which the Wnt/β-catenin signaling pathway is activated tend to progress more efficiently towards intestinal adenoma (21). These results of this aforementioned study, together with the results of the present study, suggest that activation of the Wnt/β-catenin pathway and increased expression of LGR5 may occur in the early stages of colorectal tumorigenesis, and are sustained at high levels during the malignant transformation.
The present study also demonstrated that the expression of nuclear β-catenin in adenomas characterized by mild-grade dysplasia was significantly increased (P=0.038) compared with those characterized by moderate and severe-grade dysplasia, whereas there was no significant difference in the expression of LGR5 among adenomas of different stages. Taken together, these results suggest that the activated Wnt/β-catenin signaling pathway may serve an important function in the initiation of adenoma formation, whereas subsequent transcription of the LGR5 gene sustains self-renewing ISCs in order to propagate CRC development (20,22,38).
With respect to GATA6 expression, the results of the present study revealed that higher levels of cytoplasmic GATA6 expression were exhibited in adenocarcinomas compared with adenomas and normal mucosal samples. This suggests that there may be a cumulative effect of cytoplasmic GATA6 expression on colorectal tumorigenesis. A previous study also demonstrated strong cytoplasmic GATA6 staining in adenocarcinomas (39) and GATA6 was identified as a key regulator that sustains the tumorigenic capability of CRC cells (26). Taken together, these results suggest that overexpressed cytoplasmic GATA6 serves a role in the formation of colorectal adenocarcinomas.
In the present study, a positive correlation was identified between cytoplasmic GATA6 and LGR5 expression in colorectal adenomas and adenocarcinomas, but not in normal mucosa. This suggests that the expression of cytoplasmic GATA6 is associated with the expression of LGR5 in colorectal tumors. Indeed, it has previously been demonstrated that GATA6 is able to regulate LGR5 expression and is required for tumorigenesis in colon cancer cells with APC mutations, as characterized by aberrant activation of the Wnt/β-catenin signaling pathway (6,26). In summary, the results of these previous studies, and those of the present study, implicate LGR5 as an important regulator of colorectal tumorigenesis.
The expression of nuclear GATA6 was demonstrated to decrease in the normal mucosa-adenoma-adenocarcinoma sequence in the present study, which is inconsistent with the results of our previous study (39). Furthermore, the correlation between LGR5 and nuclear GATA6 expression was negative in adenomas. It has previously been reported that GATA6 may regulate the expression of LGR5 by binding to the lgr5 promoter (25). The reason for this difference may be due in part to the interplay between the expression of GATA6 in the cytoplasm and the nucleus during colorectal tumorigenesis, or due to distinct modes of regulation of LGR5. However, the mechanism of cytoplasmic GATA6 regulation of LGR5 during colorectal tumorigenesis is unclear. Future studies are warranted to investigate the expression of nuclear and cytoplasmic GATA6 in human CRC tissues.
In conclusion, the results of the present study suggest that aberrantly activated Wnt/β-catenin signaling and increased expression of LGR5 may act together to drive the transition from colorectal normal mucosa to adenoma and to sustain the expansive proliferation of tumor cells. Simultaneously, the cumulative increase in cytoplasmic GATA6 expression, which is correlated with LGR5 expression, may promote the progression to colorectal adenocarcinoma. It has been reported that the activated Wnt/β-catenin pathway, LGR5 and GATA6 exhibit pro-tumorigenic effects on colonic and rectal epithelial cells. The results of the present study provide additional evidence for the cooperative role of these proteins during colorectal tumorigenesis, and suggest that LGR5 may be a novel and useful target for CRC prevention and treatment. Nevertheless, additional studies are required to further investigate the interaction between these proteins during the transformation from normal colorectal mucosa to adenomas and adenocarcinomas.
Acknowledgements
The present study was supported by Aohongboze Graduate Sci-tech Innovation Foundation (the President Fund of Jinzhou Medical University; grant no. AH2015001).
Glossary
Abbreviations
- CRC
colorectal cancer
- ISCs
intestinal stem cells
- CSCs
cancer stem cells
- LGR5
leucine-rich repeat-containing G protein-coupled receptor 5
References
- 1.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et al. The Global Burden of Cancer, 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al. Global, regional and national cancer incidence, mortality, years of life lost, years lived with disability and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/S0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 5.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 6.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 7.Novellasdemunt L, Antas P, Li VS. Targeting Wnt signaling in colorectal cancer. A review in the theme: Cell signaling: Proteins, pathways and mechanisms. Am J Physiol Cell Physiol. 2015;309:C511–C521. doi: 10.1152/ajpcell.00117.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basu S, Haase G, Ben-Ze'ev A. Wnt signaling in cancer stem cells and colon cancer metastasis. F1000Res. 2016;5:F1000. doi: 10.12688/f1000research.7579.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright NA. Epithelial stem cell repertoire in the gut: Clues to the origin of cell lineages, proliferative units and cancer. Int J Exp Pathol. 2000;81:117–143. doi: 10.1046/j.1365-2613.2000.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Alea M Perez, Richel DJ, Stassi G, Medema JP. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity; Proc Natl Acad Sci USA; 2008; pp. 13427–13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeuner A, Todaro M, Stassi G, De Maria R. Colorectal cancer stem cells: From the crypt to the clinic. Cell stem cell. 2014;15:692–705. doi: 10.1016/j.stem.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Vermeulen L, De Sousa E, Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 13.Sikandar SS, Pate KT, Anderson S, Dizon D, Edwards RA, Waterman ML, Lipkin SM. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res. 2010;70:1469–1478. doi: 10.1158/0008-5472.CAN-09-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors; Proc Natl Acad Sci USA; 2007; pp. 15418–15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nature genetics. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 16.Serafino A, Moroni N, Zonfrillo M, Andreola F, Mercuri L, Nicotera G, Nunziata J, Ricci R, Antinori A, Rasi G, Pierimarchi P. WNT-pathway components as predictive markers useful for diagnosis, prevention and therapy in inflammatory bowel disease and sporadic colorectal cancer. Oncotarget. 2014;5:978–992. doi: 10.18632/oncotarget.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinck L, Näthke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: Novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2:a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, Clevers H. The intestinal Wnt/TCF signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 20.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 21.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch D, Barker N, McNeil N, Hu Y, Camps J, McKinnon K, Clevers H, Ried T, Gaiser T. LGR5 positivity defines stem-like cells in colorectal cancer. Carcinogenesis. 2014;35:849–858. doi: 10.1093/carcin/bgt377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemper K, Prasetyanti PR, De Lau W, Rodermond H, Clevers H, Medema JP. Monoclonal antibodies against Lgr5 identify human colorectal cancer stem cells. Stem cells. 2012;30:2378–2386. doi: 10.1002/stem.1233. [DOI] [PubMed] [Google Scholar]
- 24.Uchida H, Yamazaki K, Fukuma M, Yamada T, Hayashida T, Hasegawa H, Kitajima M, Kitagawa Y, Sakamoto M. Overexpression of leucine-rich repeat-containing G protein-coupled receptor 5 in colorectal cancer. Cancer Sci. 2010;101:1731–1737. doi: 10.1111/j.1349-7006.2010.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whissell G, Montagni E, Martinelli P, Hernando-Momblona X, Sevillano M, Jung P, Cortina C, Calon A, Abuli A, Castells A, et al. The transcription factor GATA6 enables self-renewal of colon adenoma stem cells by repressing BMP gene expression. Nat Cell Biol. 2014;16:695–707. doi: 10.1038/ncb2992. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji S, Kawasaki Y, Furukawa S, Taniue K, Hayashi T, Okuno M, Hiyoshi M, Kitayama J, Akiyama T. The miR-363-GATA6-Lgr5 pathway is critical for colorectal tumourigenesis. Nat Commun. 2014;5:3150. doi: 10.1038/ncomms5025. [DOI] [PubMed] [Google Scholar]
- 27.Edge SB, Byrd DR. Compton CC:AJCC cancer staging manual. 7th. New York, NY: Springer; 2010. [Google Scholar]
- 28.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 29.Chen Q, Cao HZ, Zheng PS. LGR5 promotes the proliferation and tumor formation of cervical cancer cells through the Wnt/beta-catenin signaling pathway. Oncotarget. 2014;5:9092–9105. doi: 10.18632/oncotarget.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki H, Masuda N, Shimura T, Araki K, Kobayashi T, Tsutsumi S, Asao T, Kuwano H. Nuclear beta-catenin expression at the invasive front and in the vessels predicts liver metastasis in colorectal carcinoma. Anticancer Res. 2008;28:1821–1830. [PubMed] [Google Scholar]
- 31.Hu TH, Yao Y, Yu S, Han LL, Wang WJ, Guo H, Tian T, Ruan ZP, Kang XM, Wang J, et al. SDF-1/CXCR4 promotes epithelial-mesenchymal transition and progression of colorectal cancer by activation of the Wnt/β-catenin signaling pathway. Cancer Lett. 2014;354:417–426. doi: 10.1016/j.canlet.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones S, Chen WD, Parmigiani G, Diehl F, Beerenwinkel N, Antal T, Traulsen A, Nowak MA, Siegel C, Velculescu VE, et al. Comparative lesion sequencing provides insights into tumor evolution; Proc Natl Acad Sci USA; 2008; pp. 4283–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/S0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 35.Vermeulen L, Snippert HJ. Stem cell dynamics in homeostasis and cancer of the intestine. Nat Rev Cancer. 2014;14:468–480. doi: 10.1038/nrc3744. [DOI] [PubMed] [Google Scholar]
- 36.Lin YU, Wu T, Yao Q, Zi S, Cui L, Yang M, Li J. LGR5 promotes the proliferation of colorectal cancer cells via the Wnt/β-catenin signaling pathway. Oncology Lett. 2015;9:2859–2863. doi: 10.3892/ol.2015.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanwar SS, Yu Y, Nautiyal J, Patel BB, Majumdar AP. The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres. Mol Cancer. 2010;9:212. doi: 10.1186/1476-4598-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 39.Belaguli NS, Aftab M, Rigi M, Zhang M, Albo D, Berger DH. GATA6 promotes colon cancer cell invasion by regulating urokinase plasminogen activator gene expression. Neoplasia. 2010;12:856–865. doi: 10.1593/neo.10224. [DOI] [PMC free article] [PubMed] [Google Scholar]