Significance
Efficient reduction of carbon dioxide to useful fuels and chemicals is an important research goal in artificial photosynthesis. Significant progress has been made for the C1 products, CO and HCOO−. We report here a procedure based on the use of ultrasmall, monodispersed Cu and Ag bimetallic nanoparticles on thin, electrochemically polymerized poly-Fe(vbpy)3(PF6)2 films. They reduce CO2 to acetate at pH 7 in aqueous HCO3− solutions at relatively high efficiencies with significant rate enhancements with added benzotriazole. In the sequence of clusters, the most efficient results for acetate production were obtained in films of (Cu)2,(Ag)3 with a faradaic efficiency of 21.2% for acetate from CO2 at −1.33 V vs. reversible hydrogen electrode in 0.5 M KHCO3 with 8 ppm of added benzotriazole at 0 °C.
Keywords: CO2 reduction, electrocatalysis, electrodeposition, bimetallic nanoparticles, artificial photosynthesis
Abstract
Monodispersed mixtures of 6-nm Cu and Ag nanoparticles were prepared by electrochemical reduction on electrochemically polymerized poly-Fe(vbpy)3(PF6)2 film electrodes on glassy carbon. Conversion of the complex to poly-Fe(vbpy)2(CN)2 followed by surface binding of salts of the cations and electrochemical reduction gave a mixture of chemically distinct clusters on the surface, (Cu)m,(Ag)n|polymer|glassy carbon electrode (GCE), as shown by X-ray photoelectron spectroscopy (XPS) measurements. A (Cu)2,(Ag)3|(80-monolayer-poly-Fe(vbpy)32+|GCE electrode at −1.33 V vs. reversible hydrogen electrode (RHE) in 0.5 M KHCO3, with 8 ppm added benzotriazole (BTA) at 0 °C, gave acetate with a faradaic efficiency of 21.2%.
Efficient reduction of carbon dioxide to useful fuels and chemicals is an important research goal of artificial photosynthesis (1–7). Solar or electrochemical reduction of CO2 is complicated by the large overpotentials required for one-electron transfer reduction to CO2− [with E(CO2/CO2−) = −1.9 V vs. normal hydrogen electrode (NHE) at pH 7] and a high reorganizational energy (8). Subsequent steps to give formate or CO are energetically more favorable. A growing number of catalytic systems for CO2 reduction have been identified that give C1 products (9–13) but obtaining multicarbon (C2+) products has proven more difficult due to the high barrier for C–C bond formation on electrode surfaces (14).
Current research has led to robust catalysts for CO2 reduction, including molecular (15, 16), metallic (10, 17, 18), and nonmetallic catalysts (9) with an extensive literature on metal catalysts (19, 20). Cu has proven to be the most effective metallic CO2 reduction catalyst with an ability to form a variety of high-energy-density products including high-value-added, multiple carbon, C2+, products (20). At Cu electrodes, Jaramillo and coworkers (21) have identified 16 CO2 reduction products, including 12 multicarbon products. In follow-up experiments, the lack of selectivity by Cu has led to the synthesis of Cu-based bimetallic catalysts (3, 22, 23) or to decreases in the size of the Cu catalysts (24). In these experiments, difficulties arise from complicated and time-consuming synthetic procedures by contamination by added surfactants and catalysts (25).
Electrochemical deposition provides a fast and convenient approach for depositing nanoparticles on electrode surfaces (26). The procedure can be less time-consuming than wet chemical synthesis, but there are difficulties in obtaining small, confined nanoparticles and an extended range in size from hundreds of nanometers to micrometers in diameter (27). Here we utilize a procedure described earlier for surface binding and cluster formation (24) to prepare ∼6-nm clusters of Cu and Ag on the surfaces of electropolymerized films. We also report that surface-dependent, electrochemical reduction of CO2 occurs on these surfaces with C–C coupling to give acetate as a significant product.
Results and Discussion
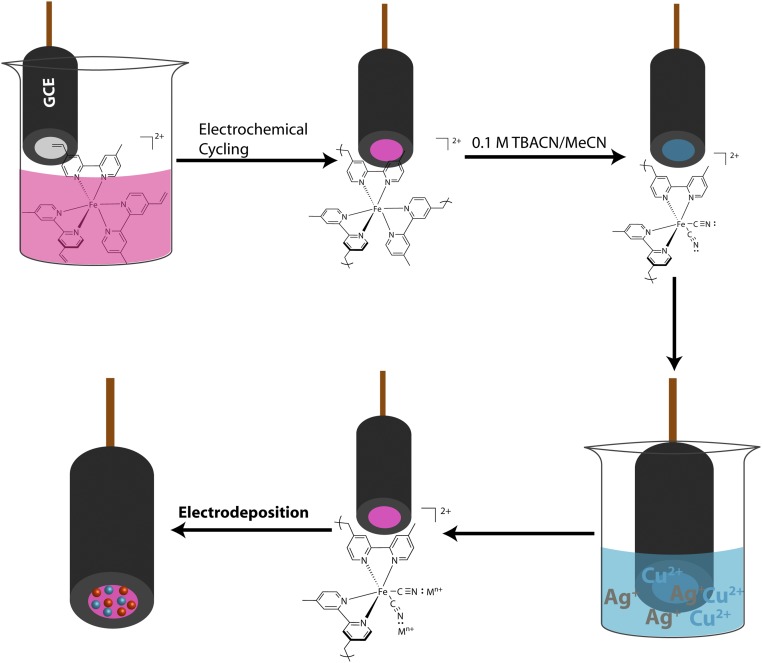
A general procedure for preparation of the electrodes has been described previously (2, 28) and is shown in an adapted form in Scheme 1. In the procedure, the vinyl-derivatized salt [Fe(vbpy)3][PF6]2 (vbpy is 4-methyl-4′-vinyl-2,2′-bipyridine) is electropolymerized on the surface of a glassy carbon electrode (GCE). Subsequent addition of CN− to an external solution causes displacement of a –vbpy ligand by CN− by immersing the polymerized electrode in 0.1 M [TBA][CN] for 20 min (28). The CN− ligands act as a bridge to added cations in solution and exposure of the films gives the ligand-bridged film adducts FeII-CN-Mm+ (2, 28). Further reduction of the films at potentials sufficient to reduce the coordinated metal ions results in reduction and transfer of the reduced metals to the surface of the films, Eq. 1, where nanoparticles form. For films loaded with multiple metal ions, composite nanoclusters form on the electrode surface providing a basis for multiple surface interactions and cluster formation. On surfaces on which multiple nanoparticles are added, the ratio of available metals can be tuned by the metal ion composition in the precursor solution.
| [1] |
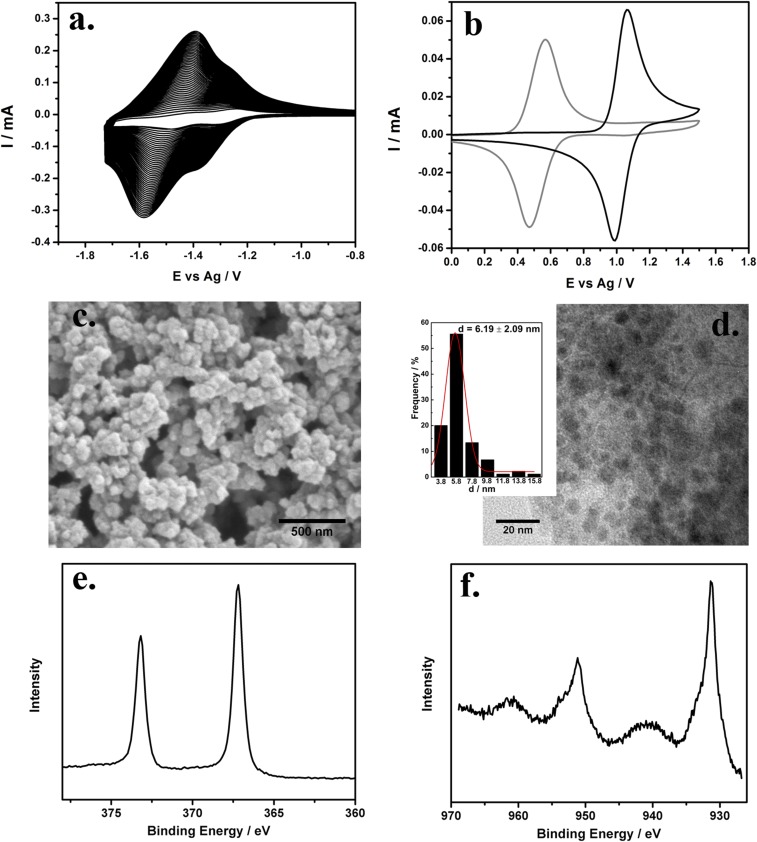
Polymerization of [Fe(vbpy)3]2+ onto a 0.071 cm2 GCE in 1 mM solution, in 0.1 M TBAPF6 MeCN, was monitored by cyclic voltammogram (CV) scanning at 250 mV s−1 (Fig. 1A). Well-defined redox waves for the couples poly-[Fe(vbpy)3]2+/1+ and poly-[Fe(vbpy)3]1+/0 at E1/2 = −1.28 V and −1.45 V vs. Ag/Ag+ were observed. As shown, the peak currents increase with the number of cycles, consistent with the growth of poly-Fe(vbpy)32+ on the electrode surface. The latter occurs by reduction of the vbpy ligands to vinyl radicals, initiating C–C bond formation (2, 28, 29). Given the presence of multiple polymerizable vinyl substituents, polymerization is accompanied by extensive cross-linking as the polymer deposits on the electrode surface to form the porous open structure shown in Fig. 1E. The surface coverage of poly-Fe(vbpy)32+ was estimated to be 6.44 ± 0.48 nmol/cm2 from peak current integrations of the poly-[Fe(vbpy)3]3+/2+ wave at E1/2 = 1.03 V vs. Ag/Ag+ (Fig. 1B). Based on this analysis, the coverage used in this study was ∼80 molecular layers with a monolayer surface coverage of 8 × 10−11 mol/cm2 (2, 28, 29). The thickness of the polymer film on the electrode was ∼250 nm (Fig. S1).
Scheme 1.
Fabrication of Cu,Ag-polymer-GCE electrodes.
Fig. 1.
(A) Room-temperature CVs showing the electropolymerization of 1 mM [Fe(vbpy)3](PF6)2 on a GCE in 0.1 M TBAPF6/MeCN at 250 mV s−1. (B) CV of the poly-[Fe(vbpy)3]3+/2+ couple before replacement of -vbpy (black solid line) and after replacement (gray solid line) at 100 mV s−1 in 0.1 M TBAPF6/MeCN, room temperature. (C) SEM image for a (Cu)m,(Ag)n/80-layer-poly[Fe(vbpy)3](PF6)2/GCE surface. (D) TEM image and size distribution (Inset) for (Cu)2,(Ag)3 nanoparticles; and XPS spectra for Ag (E) and Cu (F) in (Cu)2,(Ag)3-polymer-GCE.
Following the −CN displacement step, the poly-[Fe(vbpy)3]3+/2+ couple shifts cathodically by 0.51–0.52 V vs. Ag/Ag+, coincident with −bpy replacement by –CN− (28). The change in film structure is also shown in UV-vis spectra (Fig. S2). There is a red shift in the visible absorption bands for the dπ(FeII) → π*(bpy) metal-to-ligand charge-transfer transitions following –CN replacement. The metal ions were added by immersing poly[Fe(vbpy)3](CN)2|GCE in solutions containing different ratios of Cu2+ and Ag+ in CH3CN which leads to the rapid incorporation of Cu(II) and Ag(I). Addition of the metal ions was evident by shifts in UV-vis spectra with a blue shift observed upon addition of the ions. After binding to the metal ions, the cyano group becomes a better π-acceptor, stabilizing the dπ levels, decreasing the dπ → π*energy gap (28). Following addition of the ions, a single-pulse electrodeposition at −1.7 V vs. Ag+ for 30 s was carried out which led to rapid reduction of the cyano-bound Cu(II)/Ag(I) ions to Cu(0)/Ag(0).
Fig. 1C characterizes the surface morphology of the polymerized electrode. The polymer film has an open porous structure which presumably enhances mass transport during electrochemical reactions (30). The nanoparticulate structure is not obvious in the cross-sectional image in Fig. S1 because the particle sizes were too small to resolve by SEM. The average (Cu)2,(Ag)3 particle diameter was 6 ± 2 nm as characterized by the transmission electron microscope (TEM) images in Fig. 1D. This small particle size is documented in uniform distributions of particles prepared by direct electrodeposition (Table 1). The X-ray photoelectron spectroscopy (XPS) spectrum in Fig. 1E shows the characteristic metallic Ag 3d 2/5 band at 366.8 eV and a mix of metallic Cu and its oxide form by XPS in Fig. 1F (31, 32). The appearance of oxide in the Cu sample is not surprising because the Cu nanoparticles are easily oxidized, especially when small (31, 33). Based on the binding energies for Cu and Ag from XPS measurements, compared with their monometallic forms (Fig. S3), in the Cu–Ag particle mixtures, there are separate Cu and Ag nanoparticle clusters rather than mixed-metal alloys. This in contrast to earlier results described for clusters of CuxPdy where electrochemical formation of clusters of the alloy was observed (2). The difference in behavior may be due to differences in the electrochemical deposition methods. Single-pulse electrodeposition provides a large driving force over a short period of time, initially with selective deposition of the more reducible metal. The surface atomic ratio of Cu and Ag was determined by XPS with at least three parallel measurements for each sample.
Table 1.
Properties of electrodeposited of Cu and Ag nanoparticles
| Material | d/nm | Condition | Substrate | Technique | Ref. |
| Cu | 25.1 | 0.029 g Cu(BF4)2·xH2O and 0.058 g of dodecylbenzene sulfonic acid sodium salt | Au | Electrochemical cycling | (41) |
| 51 | 1 mM CuSO4 in 0.1 M H2SO4 | HOPG | Double-pulse deposition | (42) | |
| 20–80 | 2.0 mM CuSO4 in 0.1 M Na2SO4 | GCE/CNTs | Electrochemical cycling | (43) | |
| 50–80 | 10 g/L CuSO4·5H2O, 12 g/L NaCl and 20 g/L H3BO3, 50 °C | TiO2-NT | Direct electrodeposition | (44) | |
| Ag | 50–150 | 1.0 mM AgNO3 in 0.1 M KNO3 | GCE/ZnO | Double-pulse deposition | (45) |
| 50–80 | 0.43 mg/mL AgNO3 in 0.1 M NaNO3 | Au | Electrochemical cycling | (46) | |
| 16.6 | 5 mM AgNO3 in 0.1 M KNO3 with poly (N-vinylpyrrolidone) as the additive | Pt | Electrodeposition | (47) |
During the electrodeposition process, nucleation and growth from within the particle occur sufficiently rapidly to separate the two effects completely (34). Under normal conditions, with high concentrations of metal ions in the external solution at high applied potentials, large particle sizes with a broad size distribution would be expected (34). In the strategy adopted here, CN− coordination plays a critical role in dictating particle size and distribution. Electrodeposition, under the same condition but without the CN− ligand, resulted in the appearance of a film for Cu and a large flake for Ag as shown in Fig. S4. Presumably, the diluted concentration of metal ions in the electrodes, by coordination to the CN− ligand, decreases local interactions resulting in a decrease in particle size (34).
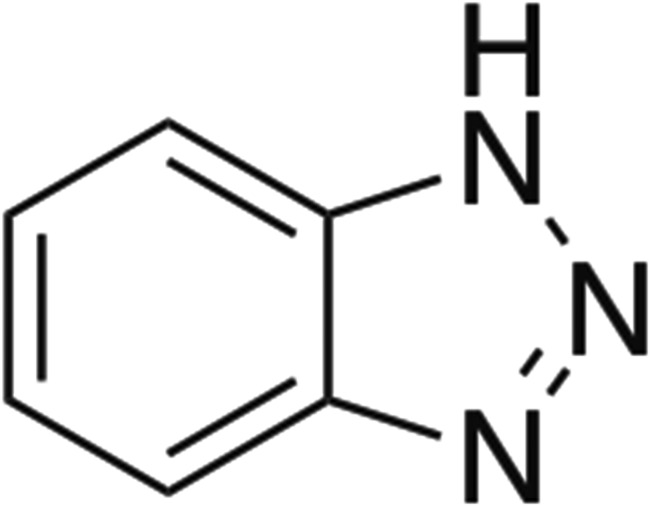
The electrocatalytic reactivities of the nanoparticles of the polymer-stabilized, (Cu)m,(Ag)n/GCE electrodes for CO2 reduction were evaluated in 0.5 M KHCO3 with 8 ppm of benzotriazole (BTA, Scheme 2) added. To increase the solubility of CO2, all experiments were performed at 0 °C (35). The products of CO2 reduction were identified and quantified by 1H NMR in the liquid phase, and by GC for the headspace gaseous products. This (Cu)m,(Ag)n/polymer/GCE assembly showed a stable catalytic current over extended electrolysis periods as shown in Fig. S5.
Scheme 2.
Structure of BTA.
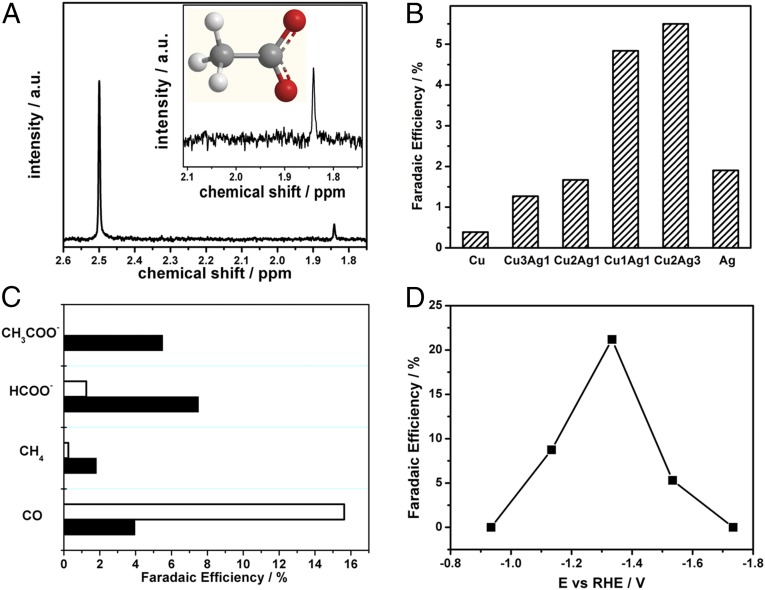
The 8e− product (Table S1), CH3COO− (Fig. 2A), is a highly reduced product. Its appearance is unusual because of the difficulties in overcoming high-energy barriers for C–C formation on surfaces at low temperatures. Its appearance as the major liquid product at low temperatures is one of the few examples in CO2 reduction chemistry (21). Other products that appear include formate in the liquid phase and CO and methane in the gas phase. A control experiment was conducted using the identical parameters except N2 was used in place of CO2. Under this condition, no product peaks were observed in the 1H NMR (Fig. S6). The major product in the background is hydrogen from reduction of the solvent.
Fig. 2.
(A) NMR spectrum of acetate (δ = 1.83 ppm) with DMSO (δ = 2.5 ppm) as the internal standard. (B) Efficiency of acetate formation as a function of the atom ratios of Cu and Ag in (Cu)m,(Ag)n/polymer/GCE in CO2-saturated 0.5 M KHCO3, −1.53 V vs. RHE after 3,600 s, 0 °C. (C) Product distribution with (solid) without (empty) 8 ppm BTA on (Cu)2, (Ag)3|80-layer-poly[Fe(vbpy)3](PF6)2|GCE electrode, condition as above. (D) As above, influence of the applied potential on acetate production for a (Cu)2,(Ag)3/polymer/GCE electrode.
The appearance of acetate by the Cum,Agn clusters led to a more detailed investigation. The results of a study in which the surface metal atom composition was varied are shown in Fig. 2B. Data in the figure appear for acetate generation from the clusters Cu, Cu3–Ag, Cu2–Ag, Cu2–Ag3, and Ag in CO2 saturated 0.5 M KHCO3 at 0 °C at E = −1.53 vs. RHE over periods of 1 h. Based on the data in the figure, there was an increase in the appearance of acetate as Ag was added to the nanoparticles with a maximum reached at 5.5% for Cu2–Ag3 clearly showing that surface metal composition plays an important role in acetate formation.
The influence of BTA on CO2 reduction was also investigated with the reactive Cu2–Ag3 cluster mixture. As shown in Fig. 2C, CO2 reduction by the Cu2–Ag3/polymer/GCE electrode, at 0 C at −1.53 V vs. RHE for 1 h, gave acetate and methane at 5.5% and 1.8% with 8 ppm added BTA. Without BTA the values were 0% acetate and 0.3% methane. Similar results were obtained for a Cu/polymer/GCE (Fig. S7) film. Without added BTA, acetate 0.23% and methane appeared in 23% yields and with acetate the yields were 0.7% with methane at 3.10%. The role of BTA was selective. For example, there was no impact of added BTA on Ag/polymer/GCE nanoparticle reductions. Its role may arise from its adsorption on Cu with chemical changes at the electrode interface. BTA has been shown to adsorb on metallic Cu surfaces by physical and chemical adsorption (36).
The potential dependence of acetate generation by the (Cu)2,(Ag)3 cluster was also investigated. Faradaic efficiencies were evaluated as a function of applied potential with results shown in Fig. S8. At −1.33 V vs. RHE, acetate was generated in 21.2% yield. Decrease of the surface potential to −0.93 V resulted in no acetate. The appearance of acetate increases with applied potential with the highest efficiency of 21.2% found at −1.33 V vs. RHE. Further decreases in potential resulted in decreased formation of acetate with no acetate formed by −1.73 V. A related behavior has been reported by Jaramillo and coworkers at Cu electrodes with CH3COO− maximized as a product at −1.1 V vs. RHE and H2 the dominant product at more negative potentials.
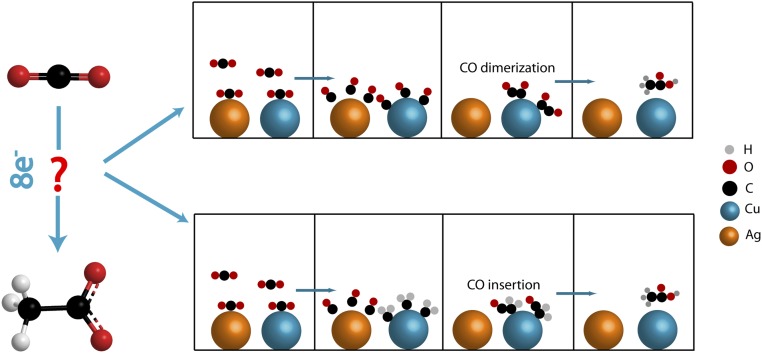
The mechanism of C–C formation is still debatable. From the data in Fig. 2B, addition of Ag clusters to the surface plays an important role with acetate maximized as a product for the cluster pair Cu2–Ag3. Ag is a known catalyst for CO2 reduction to CO with insight on mechanism from calculations by Peterson and Nørskov (37). Once formed by loss of CO2 from the surface, CO(gas) is favored thermodynamically as shown in Scheme 3. Cu, on the other hand, has a moderate ability to adsorb and desorb CO (37). A possible contributor to acetate production may be Ag-catalyzed formation of CO followed by its capture on neighboring Cu sites for C–C bond formation, Scheme 3. A similar observation was made by Yeo and coworkers (3) for phase-segregated CuZn nanoparticles.
Scheme 3.
Possible reaction pathways for CO2 reduction to acetate on (Cum),(Ag)n/polymer/GCE electrodes.
In the mechanism in the scheme, acetate could be produced after a series of hydroxylation and electron transfer steps (21). In an alternate route, CO insertion (3, 38, 39) as observed by Kenis and coworkers (38) on pure Cu surfaces could also occur in ethanol generation. Following CO2 reduction to –CH2 on the surface of Cu, dimerization of the latter with free CO from Ag would occur to give the C–C bond on the surface. Once formed on the surface, further reduction of acetaldehyde would give acetate as the final product (40).
Conclusion
We have introduced here the details of a well-defined electrochemical procedure for preparing a family of (Cu)m,(Ag)n clusters on an underlayer of poly[Fe(vbpy)3](PF6)2|GCE. Monodispersed, ultrasmall, ∼6-nm particles were obtained by electrodeposition within the films to give nanoparticles that are among the smallest particle sizes available by this technique. In their reduction chemistry, a key factor is reduction of CO2 with an enhancement by added BTA. The ability of clusters to undergo CO2 reduction to acetate is highly dependent on the mole fraction ratio in the (Cu)m,(Ag)n/polymer/GCE mixtures. In the sequence of clusters, the most efficient results for acetate were obtained for (Cu)2,(Ag)3/polymer/GCE films with a faradaic efficiency of 21.2% for acetate at −1.3 V vs. RHE in 0.5 M KHCO3 with 8 ppm of BTA at 0 °C.
Methods
Electrochemical Measurements.
All of the electrochemical measurements were performed on a CHI601 D potentiostat station (CH Instruments, Inc.) with a three-electrode setup by using a Pt mash as the counterelectrode, a Ag (for nonaqueous system) and a saturated calomel electrode (for aqueous system) as the reference electrode, and a GCE as the working electrode. The GCE was first polished with a decreasing size of 1.0- and 0.05-μm alumina lapping compounds for 5 min each and followed by sonication in an ultrasound bath before use. The potential used for CO2 reduction reaction (CO2RR) is corrected to RHE to make comparison with literature.
Electrode Fabrication.
The polymerization process was carried out in a nitrogen-saturated solution containing 1 mM [Fe(vbpy)3](PF6)2 in 0.1 M TBAPF6/CH3CN. The GCE was then immersed into this well degassed solution to perform consecutive scans to get poly-[Fe(vbpy)3]/GCE. This electrode was then transferred into 0.1 M TBACN/MeCN solution and left for 20 min to allow full replacement of –CN with –bpy. Before being put into solution with Cu/Ag, the formed dicyano film/GCE was washed with MeCN and dried. After 20 min of incorporation in the metal ion precursor solution, the electrode was cleaned with MeCN and transferred into a nitrogen-saturated 0.1 M TBAPF6/MeCN to run electrodeposition under −1.7 V vs. Ag for 30 s. Aggregation of particles were observed with longer electrodeposition time.
Detailed product analysis and CVs are presented in Supporting Information.
Supplementary Material
Acknowledgments
We thank Dr. Carrie Donley and Dr. Amar Kumbhar for assistance with XPS and SEM measurements. This research was supported solely by the UNC EFRC: Center for Solar Fuels, an Energy Frontier Research Center funded by the US Department of Energy Office of Science, Office of Basic Energy Science under Award DE-SC0001011. This work made use of instrumentation at the Chapel Hill Analytical and Nanofabrication Laboratory, a member of the North Carolina Research Triangle Nanotechnology Network, which is supported by the National Science Foundation (Grant ECCS-1542015) as part of the National Nanotechnology Coordinated Infrastructure.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1713962115/-/DCSupplemental.
References
- 1.Liu Y, Chen S, Quan X, Yu H. Efficient electrochemical reduction of carbon dioxide to acetate on nitrogen-doped nanodiamond. J Am Chem Soc. 2015;137:11631–11636. doi: 10.1021/jacs.5b02975. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, et al. Polymer-supported CuPd nanoalloy as a synergistic catalyst for electrocatalytic reduction of carbon dioxide to methane. Proc Natl Acad Sci USA. 2015;112:15809–15814. doi: 10.1073/pnas.1522496112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren D, Ang BS-H, Yeo BS. Tuning the selectivity of carbon dioxide electroreduction toward ethanol on oxide-derived CuxZn catalysts. ACS Catal. 2016;6:8239–8247. [Google Scholar]
- 4.Sahara G, et al. Photoelectrochemical reduction of CO2 coupled to water oxidation using a photocathode with a Ru(II)-Re(I) complex photocatalyst and a CoOx/TaON photoanode. J Am Chem Soc. 2016;138:14152–14158. doi: 10.1021/jacs.6b09212. [DOI] [PubMed] [Google Scholar]
- 5.Morimoto T, et al. CO2 capture by a rhenium(I) complex with the aid of triethanolamine. J Am Chem Soc. 2013;135:16825–16828. doi: 10.1021/ja409271s. [DOI] [PubMed] [Google Scholar]
- 6.Sekizawa K, Maeda K, Domen K, Koike K, Ishitani O. Artificial Z-scheme constructed with a supramolecular metal complex and semiconductor for the photocatalytic reduction of CO2. J Am Chem Soc. 2013;135:4596–4599. doi: 10.1021/ja311541a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar B, et al. Photochemical and photoelectrochemical reduction of CO2. Annu Rev Phys Chem. 2012;63:541–569. doi: 10.1146/annurev-physchem-032511-143759. [DOI] [PubMed] [Google Scholar]
- 8.Kang P, Chen Z, Brookhart M, Meyer TJ. Electrocatalytic reduction of carbon dioxide: Let the molecules do the work. Top Catal. 2015;58:30–45. [Google Scholar]
- 9.Zhang S, et al. Polyethylenimine-enhanced electrocatalytic reduction of CO2 to formate at nitrogen-doped carbon nanomaterials. J Am Chem Soc. 2014;136:7845–7848. doi: 10.1021/ja5031529. [DOI] [PubMed] [Google Scholar]
- 10.Zhu W, et al. Active and selective conversion of CO2 to CO on ultrathin Au nanowires. J Am Chem Soc. 2014;136:16132–16135. doi: 10.1021/ja5095099. [DOI] [PubMed] [Google Scholar]
- 11.Maurin A, Robert M. Noncovalent immobilization of a molecular iron-based electrocatalyst on carbon electrodes for selective, efficient CO2-to-CO conversion in water. J Am Chem Soc. 2016;138:2492–2495. doi: 10.1021/jacs.5b12652. [DOI] [PubMed] [Google Scholar]
- 12.Medina-Ramos J, Pupillo RC, Keane TP, DiMeglio JL, Rosenthal J. Efficient conversion of CO2 to CO using tin and other inexpensive and easily prepared post-transition metal catalysts. J Am Chem Soc. 2015;137:5021–5027. doi: 10.1021/ja5121088. [DOI] [PubMed] [Google Scholar]
- 13.Zhu W, et al. Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO. J Am Chem Soc. 2013;135:16833–16836. doi: 10.1021/ja409445p. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Fugate EA, Mueanngern Y, Baker LR. Photoelectrochemical CO2 reduction to acetate on iron–copper oxide catalysts. ACS Catal. 2017;7:177–180. [Google Scholar]
- 15.Kang P, Chen Z, Nayak A, Zhang S, Meyer TJ. Single catalyst electrocatalytic reduction of CO2 in water to H2+CO syngas mixtures with water oxidation to O2. Energy Environ Sci. 2014;7:4007–4012. [Google Scholar]
- 16.Sampson MD, Kubiak CP. Manganese electrocatalysts with bulky bipyridine ligands: Utilizing Lewis acids to promote carbon dioxide reduction at low overpotentials. J Am Chem Soc. 2016;138:1386–1393. doi: 10.1021/jacs.5b12215. [DOI] [PubMed] [Google Scholar]
- 17.Verdaguer-Casadevall A, et al. Probing the active surface sites for CO reduction on oxide-derived copper electrocatalysts. J Am Chem Soc. 2015;137:9808–9811. doi: 10.1021/jacs.5b06227. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Kang P, Meyer TJ. Nanostructured tin catalysts for selective electrochemical reduction of carbon dioxide to formate. J Am Chem Soc. 2014;136:1734–1737. doi: 10.1021/ja4113885. [DOI] [PubMed] [Google Scholar]
- 19.Azuma M, Hashimoto K, Hiramoto M, Watanabe M, Sakata T. Electrochemical reduction of carbon dioxide on various metal electrodes in low-temperature aqueous KHCO3 media. J Electrochem Soc. 1990;137:1772–1778. [Google Scholar]
- 20.Hori Y. Electrochemical CO2 reduction on metal electrodes. In: Vayenas C, editor. Modern Aspects of Electrochemistry. Vol 42. Springer; New York: 2008. pp. 89–187. [Google Scholar]
- 21.Kuhl KP, Cave ER, Abram DN, Jaramillo TF. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ Sci. 2012;5:7050–7059. [Google Scholar]
- 22.Watanabe M, Shibata M, Kato A, Azuma M, Sakata T. Design of alloy electrocatalysts for CO2 reduction different Cu alloys. J Electrochem Soc. 1991;138:3382–3389. [Google Scholar]
- 23.Neaţu Ş, Maciá-Agulló JA, Concepción P, Garcia H. Gold-copper nanoalloys supported on TiO2 as photocatalysts for CO2 reduction by water. J Am Chem Soc. 2014;136:15969–15976. doi: 10.1021/ja506433k. [DOI] [PubMed] [Google Scholar]
- 24.Reske R, Mistry H, Behafarid F, Roldan Cuenya B, Strasser P. Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles. J Am Chem Soc. 2014;136:6978–6986. doi: 10.1021/ja500328k. [DOI] [PubMed] [Google Scholar]
- 25.Peng Z, Kisielowski C, Bell AT. Surfactant-free preparation of supported cubic platinum nanoparticles. Chem Commun (Camb) 2012;48:1854–1856. doi: 10.1039/c2cc16962b. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Laborda E, Ward KR, Tschulik K, Compton RG. A kinetic study of oxygen reduction reaction and characterization on electrodeposited gold nanoparticles of diameter between 17 nm and 40 nm in 0.5 M sulfuric acid. Nanoscale. 2013;5:9699–9708. doi: 10.1039/c3nr02340k. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Kang P, Zhang M-T, Stoner BR, Meyer TJ. Cu(II)/Cu(0) electrocatalyzed CO2 and H2O splitting. Energy Environ Sci. 2013;6:813–817. [Google Scholar]
- 28.Bakir M, MacKay SG, Linton RW, Sullivan BP, Meyer TJ. Binding and reduction of silver ions in thin polymeric films of poly-[Fe(vbpy)2(CN)2],poly-vbpy. Inorg Chem. 1994;33:3945–3951. [Google Scholar]
- 29.Calvert JM, Sullivan BP, Facci JS, Meyer TJ, Murray RW. Synthetic and mechanistic investigations of the reductive electrochemical polymerization of vinyl-containing complexes of iron(II), ruthenium(II), and osmium(II) Inorg Chem. 1983;22:2151–2162. [Google Scholar]
- 30.Compton RG, Banks CE. Understanding Voltammetry. World Scientific; Singapore: 2011. pp. 226–231. [Google Scholar]
- 31.Jeong S, et al. Controlling the thickness of the surface oxide layer on Cu nanoparticles for the fabrication of conductive structures by ink-jet printing. Adv Funct Mater. 2008;18:679–686. [Google Scholar]
- 32.Ghodselahi T, Vesaghi MA, Shafiekhani A, Baghizadeh A, Lameii M. XPS study of the Cu@Cu2O core-shell nanoparticles. Appl Surf Sci. 2008;255:2730–2734. [Google Scholar]
- 33.Grouchko M, Kamyshny A, Magdassi S. Formation of air-stable copper–silver core–shell nanoparticles for inkjet printing. J Mater Chem. 2009;19:3057–3062. [Google Scholar]
- 34.Penner RM. Mesoscopic metal particles and wires by electrodeposition. J Phys Chem B. 2002;106:3339–3353. [Google Scholar]
- 35.Hori Y. Modern Aspects of Electrochemistry. Springer; New York: 2008. Electrochemical CO2 reduction on metal electrodes; pp. 89–189. [Google Scholar]
- 36.Kokalj A. Ab initio modeling of the bonding of benzotriazole corrosion inhibitor to reduced and oxidized copper surfaces. Faraday Discuss. 2015;180:415–438. doi: 10.1039/c4fd00257a. [DOI] [PubMed] [Google Scholar]
- 37.Peterson AA, Nørskov JK. Activity descriptors for CO2 electroreduction to methane on transition-metal catalysts. J Phys Chem Lett. 2012;3:251–258. [Google Scholar]
- 38.Ma S, et al. One-step electrosynthesis of ethylene and ethanol from CO2 in an alkaline electrolyzer. J Power Sources. 2016;301:219–228. [Google Scholar]
- 39.Lessner DJ, et al. An unconventional pathway for reduction of CO2 to methane in CO-grown Methanosarcina acetivorans revealed by proteomics. Proc Natl Acad Sci USA. 2006;103:17921–17926. doi: 10.1073/pnas.0608833103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiebel F, et al. Self-assembly of acetate adsorbates drives atomic rearrangement on the Au(110) surface. Nat Commun. 2016;7:13139. doi: 10.1038/ncomms13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ko W-Y, Chen W-H, Cheng C-Y, Lin K-J. Highly electrocatalytic reduction of nitrite ions on a copper nanoparticles thin film. Sens Actuators B Chem. 2009;137:437–441. [Google Scholar]
- 42.Huang L, Lee E-S, Kim K-B. Electrodeposition of monodisperse copper nanoparticles on highly oriented pyrolytic graphite electrode with modulation potential method. Colloids Surf A Physicochem Eng Asp. 2005;262:125–131. [Google Scholar]
- 43.Kang X, Mai Z, Zou X, Cai P, Mo J. A sensitive nonenzymatic glucose sensor in alkaline media with a copper nanocluster/multiwall carbon nanotube-modified glassy carbon electrode. Anal Biochem. 2007;363:143–150. doi: 10.1016/j.ab.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Li X, et al. Nickel/copper nanoparticles modified TiO2 nanotubes for non-enzymatic glucose biosensors. Sens Actuators B Chem. 2013;181:501–508. [Google Scholar]
- 45.Wang Q, Zheng J. Electrodeposition of silver nanoparticles on a zinc oxide film: Improvement of amperometric sensing sensitivity and stability for hydrogen peroxide determination. Mikrochim Acta. 2010;169:361–365. [Google Scholar]
- 46.Du D, et al. Recognition of dimethoate carried by bi-layer electrodeposition of silver nanoparticles and imprinted poly-o-phenylenediamine. Electrochim Acta. 2008;53:6589–6595. [Google Scholar]
- 47.Yin B, Ma H, Wang S, Chen S. Electrochemical synthesis of silver nanoparticles under protection of poly(N-vinylpyrrolidone) J Phys Chem B. 2003;107:8898–8904. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.