Significance
The ability to remember a new place is crucial for survival. The locus coeruleus (LC) in the brain stem is known to respond to novel sensory stimuli and can facilitate hippocampus-dependent memory, although the circuit and the role that LC plays in novelty-associated memory is unknown. We performed circuit-specific optogenetic inhibition and found that the hippocampal CA3 subregion is the crucial target of LC projections during the encoding of a novel context. Furthermore, we show with activity-dependent labeling and in vivo calcium imaging that LC inputs are necessary to provide stable neuronal representations of the context. This study provides evidence that LC neuromodulation, especially to the CA3 subregion, plays a crucial role in memory formation of a new context.
Keywords: locus coeruleus, novelty, memory formation, hippocampus, neuromodulation
Abstract
The memory for a new episode is formed immediately upon experience and can last up to a lifetime. It has been shown that the hippocampal network plays a fundamental role in the rapid acquisition of a memory of a one-time experience, in which the novelty component of the experience promotes the prompt formation of the memory. However, it remains unclear which neural circuits convey the novelty signal to the hippocampus for the single-trial learning. Here, we show that during encoding neuromodulatory input from locus coeruleus (LC) to CA3, but not CA1 or to the dentate gyrus, is necessary to facilitate novel contextual learning. Silencing LC activity during exposure to a novel context reduced subsequent reactivation of the engram cell ensembles in CA3 neurons and in downstream CA1 upon reexposure to the same context. Calcium imaging of the cells reactivated in both novel and familiar contexts revealed that suppression of LC inputs at the time of encoding resulted in more variable place fields in CA3 neurons. These results suggest that neuromodulatory input from LC to CA3 is crucial for the formation of a persistent memory in the hippocampus.
The ability to acquire memories for a one-time experience in a new environment is crucial for adapting to an ever-changing world. Novel information must be learned quickly with a single experience and must persist to be useful. Theoretical studies have proposed that modifiable synaptic connections among hippocampal pyramidal neurons could support the rapid formation of a memory for a novel episode with a single experience (1, 2). In particular, it has been suggested that the recurrent collaterals in the CA3 subregion of the hippocampus facilitate this function through their mutually excitatory inputs, which exhibit rapid induction of long-term potentiation (3, 4). Multiple behavioral experiments strongly support the idea that recurrent collateral patterns permit elements of incoming sensory input to be stored associatively in CA3 after only a single experience, without the need for repetition (5–7).
More specifically, CA3-specific NMDA receptor-knockout mice demonstrated impaired spatial learning only when these mice were trained in a novel environment but displayed normal learning when the training environment was familiar (5). These data indicate that in response to novelty CA3 receives inputs from other brain regions that promote changes in synaptic plasticity within the CA3 microcircuit. This raises the question of which inputs convey the novelty signal to CA3 enabling the single-trial learning.
The hippocampus receives neuromodulatory input from multiple brain areas that can regulate synaptic plasticity. Among those, one neuromodulatory center known to respond to novelty and to regulate hippocampal plasticity is the locus coeruleus (LC). It was recently shown that optogenetic stimulation of the LC can enhance the retention of everyday memories in a familiar environment (8). Interestingly, it is known that activity in the LC switches from tonic firing at low frequency to phasic burst firing when novel stimuli occur, and the intensity of the burst firing wears off when the novelty of the experience wanes (8–11). Another source of neuromodulation in the hippocampus is the ventral tegmental area (VTA), which also responds to novelty. However, studies have shown that VTA projections to dorsal hippocampus are scarce, whereas LC projections are abundant (8, 12, 13). In addition, dopamine, which is the most prominently implicated neuromodulator in novelty signaling (14), was demonstrated to be released from the LC, along with norepinephrine in the cortex (15) and in the hippocampus (8, 12, 16). Therefore, we hypothesized that the LC input to the CA3 network plays a pivotal role in single-trial learning of novel experience.
In the present study, we tested the neuromodulatory influence of LC activity during encoding of a one-time experience and found that LC neuromodulation is crucial for stable CA3 ensemble activity in support of rapid memory acquisition of a novel environment. A circuit-specific genetic manipulation experiment revealed the causal role of LC input targeted to the hippocampal CA3 subregion in single-trial learning of a novel context.
Results
Anatomical Connectivity Between LC and Hippocampal Subregions.
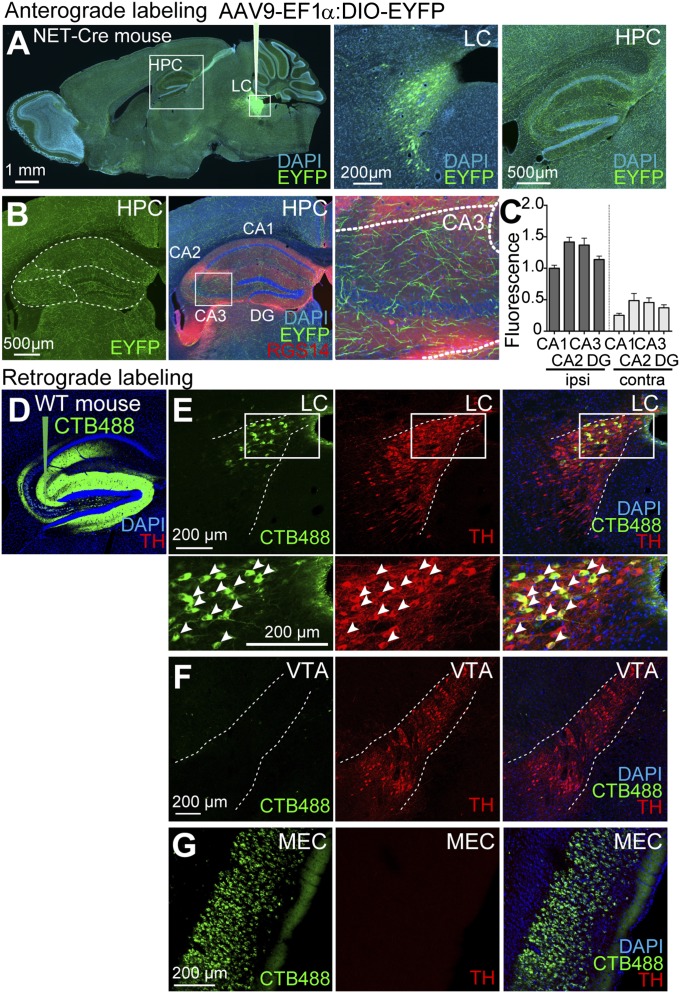
We generated an LC-specific Cre transgenic mouse line in which Cre expression is driven by the norepinephrine transporter (NET; slc6a2) promoter (NET-Cre) (Materials and Methods). The expression of Cre was primarily found in the LC of the NET-Cre mouse (Fig. S1). We characterized the projections of LC neurons in the hippocampal subregions of NET-Cre mice using the anterograde tracer AAV9-EF1α:DIO-EYFP injected unilaterally into the LC (Fig. 1 A and B). CA boundaries are defined by the expression of RGS14, a hippocampal CA2 marker (Fig. 1B) (17). While previous studies have shown LC projections to the hippocampus (12, 18), comparative analysis of LC fibers between hippocampal subregions have not yet been reported. Strong expression of enhanced yellow fluorescent protein (EYFP) in LC-specific Cre mice with double-floxed inverted orientation (DIO) virus enabled us to observe small fibers and to quantify relative fluorescence in each hippocampal subregion (Fig. 1B). Using this anterograde viral approach, we found that CA3 and CA2 have higher fiber density than CA1 and the dentate gyrus (DG) (Fig. 1C). To confirm the axonal projections of LC cells to hippocampal CA3, we injected the retrograde tracer cholera toxin B (CTB) conjugated to a fluorophore Alexa-488 (CTB488) targeting dorsal CA3 unilaterally (Fig. 1D). We observed CTB488 mostly in CA3 and found that it spreads toward the DG and the proximal part of CA1. We also found that CA3/DG was strongly innervated by dorsal LC neurons in the ipsilateral hemisphere (Fig. 1E). On the other hand, there was little detectable innervation from the VTA (Fig. 1F). In addition, we observed that CA3 receives major inputs from medial entorhinal cortex (MEC) (Fig. 1G).
Fig. 1.
Anatomical connectivity between the LC and hippocampal subregions. (A–C) A NET-Cre transgenic mouse injected with AAV9-EF1α:DIO-EYFP in the LC. (A) Sagittal sections showing EYFP expressions in the LC cell bodies and fibers in the hippocampus (HPC). (B) Coronal sections showing EYFP+ fibers (green) in hippocampal subregions. Immunostaining with anti-RGS14 (red) indicates CA2. (C) Quantification of EYFP+ fiber density in the hippocampal subregions (n = 5 per group); contra, contralateral; ipsi, ipsilateral. Data are presented as mean ± SEM. (D) Injection site of the retrograde tracer CTB488 (green) in the CA3 of a wild-type mouse. (E–G) Sagittal sections of the LC (E), VTA (F), and MEC (G) showing retrogradely labeled cell bodies with CTB488 (green) and immunostaining with anti-TH (red).
Optogenetic Inhibition of LC Axonal Terminals in CA3 Impairs Contextual Memory Formation at the Encoding Stage.
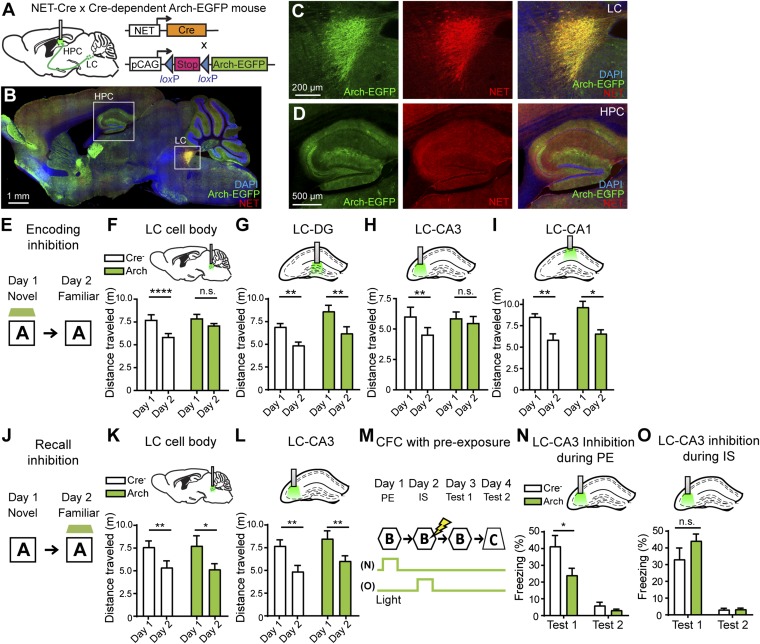
To assess the role of the LC in novel context learning, we investigated the circuit-specific effect of silencing LC input in the hippocampus. We generated a double-transgenic mice by crossing NET-Cre mice and Cre-dependent Arch mice: pCAG-loxP-STOP-loxP-Arch-EGFP (19) (Fig. 2A). The expression of Arch-EGFP in NET-Cre mice was well restricted to the NET+ LC cells (98.6 ± 1.87%, n = 6) (Fig. 2 B and C), and no EGFP expression was observed in other catecholaminergic cells, such as those in the VTA or substantia nigra. We observed Arch-EGFP+ fibers in the hippocampal subregions (Fig. 2D) consistent with the anatomical experiments performed with anterograde and retrograde tracers (Fig. 1). We investigated the effects of LC cell body inhibition during the encoding of novel contextual memory (Fig. 2 E and F). To assess the degree of learning of a new context, we employed a novel-context recognition test using an open field (Materials and Methods) (20). When repeatedly exposed to an initially novel context (chamber A), control mice explored the now familiar chamber A significantly less on day 2, than on day 1, when it was novel (Fig. S2A). In contrast, mice with inhibition of LC cell bodies on day 1 displayed the same level of exploration in the chamber on day 2 as on day 1, indicating a deficit in novel contextual memory (Fig. 2F). Next, we examined the effects of LC terminal inhibition in DG, CA3, and CA1 during the encoding of contextual memory (Fig. 2 G–I). We found that inhibition of LC input to CA3 resulted in equal exploration on day 1 and day 2, suggesting deficits in memory encoding of a novel environment (Fig. 2H), whereas inhibition of LC input to either the DG or CA1 did not affect the expected behavior (Fig. 2 G and I). Moreover, optogenetic inhibition of either LC cell bodies or of the LC input to CA3 during the day 2 recall test session did not increase the exploration level of the animals on day 2 (Fig. 2 J–L). These results are consistent with previous studies showing that CA3 is crucial for memory encoding of a one-time experience but not for memory recall or repeated-trial learning (5, 21). LC inhibition did not affect locomotor activity in animals, as control and Arch groups showed the same levels of exploration when light was delivered during novel context exposure on day 1 (Fig. 2F) and during familiar context exposure on day 2 (Fig. 2K). These results therefore suggest that the LC-to-CA3 pathway is critical during memory encoding for single-trial learning of a novel context.
Fig. 2.
Optogenetic inhibition of LC axonal terminals in CA3 impairs contextual memory formation at the encoding stage. (A) Double-transgenic mice were generated by crossing NET-Cre mice with Cre-dependent Arch-EGFP mice. (B–D) Images of sagittal sections show LC-specific expression of Arch-EGFP fusion protein (green) and immunoreactivity with anti-NET antibody (red). (E) Behavioral schedule used for the novel-context recognition test to test memory encoding. The green trapezoid represents the timing of light delivery. The photoinhibition was given for the entire sampling sessions. (F–I, Upper) The placement of optic fibers targeting the LC cell body (day × genotype, F1,22 = 4.749, P < 0.05; n = 13 for control and n = 11 for the Arch group) (F), DG (day × genotype, F1,10 = 0.5853, P = 0.4619; day, F1,10 = 78.27, P < 0.0001; genotype, F1,10 = 4.112, P = 0.0701; n = 6 per group) (G), CA3 (day × genotype, F1,12 = 6.543, P < 0.05; n = 7 per group) (H), and CA1 (day × genotype, F1,10 = 0.1798, P = 0.6805; day, F1,10 = 33.25, P < 0.0005; genotype, F1,10 = 2.144, P = 0.1739; n = 6 per group) (I). (Lower) Travel distances were tracked and averaged over 4-min sessions. (J) Behavioral schedule to test memory recall. (K and L, Upper) The placement of optic fibers targeting the LC cell body (day × genotype, F1,10 = 0.1032, P = 0.7547; day, F1,10 = 21.14, P < 0.001; genotype, F1,10 = 0.0007817, P = 0.9782; n = 6 per group) (K) and CA3 (day × genotype, F1,12 = 0.2397, P = 0.6332; day, F1,12 = 45.59, P < 0.0001; genotype, F1,12 = 1.115, P = 0.3118; n = 6 per group) (L). (Lower) Travel distances were tracked and averaged over 4-min sessions. (M) Behavioral schedule for preexposure-dependent CFC. Green bars represent the timing of light delivery. (N and O) Freezing levels were averaged over 5-min test sessions in the conditioned context B (test 1) and unconditioned context C (test 2). Photoinhibition was delivered at context preexposure (PE) sessions (n =7 per group) (N) or at IS sessions (n = 8 per group) (O). Statistical comparisons were performed using two-way repeated-measures ANOVA and post hoc Sidak’s multiple comparisons test (F–I, K, and L) and unpaired t tests (N and O); ****P < 0.001 **P < 0.01; *P < 0.05; n.s., not significant. Data are presented as mean ± SEM.
To examine the identity of the neurotransmitter released from LC terminals into CA3 that enables single-trial learning, we implanted cannulae bilaterally in CA3 and infused dopaminergic or noradrenergic antagonists 20 min before encoding sessions on day 1 (Fig. S3A). In a novel context recognition test, contextual learning was blocked by intrahippocampal infusion of a selective dopamine D1-like receptor antagonist, SCH23390, but not by the β-adrenergic antagonist propranolol or by saline (Fig. S3B). These results demonstrate the release of dopamine elicited by the activation of LC neurons under physiological conditions, as supported by recent gain-of-function stimulation studies (8, 12), and reveal the importance of LC projections to hippocampal CA3 in single-trial learning.
Next, we investigated the role of the LC-to-CA3 pathway in the association between a conditioned stimulus (CS) and an unconditioned stimulus (US) by using contextual fear conditioning (CFC). To address the effect of novelty, we employed a preexposure-mediated CFC paradigm (Fig. 2M) (7, 22, 23) in which the contextual representation is formed on day 1 while the context-shock association occurs on day 2 (Materials and Methods). In an immediate-shock (IS) protocol with 8-s exposure to context before shock delivery, mice are unable to acquire a full contextual representation rapidly in the fear-conditioning chamber. However, a 10-min preexposure session enables an association between the CS represented by the context and the US represented by the shock during the IS session. We applied this protocol of CFC with preexposure as it allows clear separation of the encoding of a novel context from contextual recall and CS–US association and precise temporal regulation of optogenetic inhibition. In this way, it is also possible to avoid rebound effects that were observed in LC neurons expressing light-sensitive opsins (8). Freezing deficits in the conditioned context were present in the Arch group with inhibition of LC–CA3 input during the entire context-preexposure session (Fig. 2N) but not during association between context and shock (Fig. 2O). These data suggest that LC activity plays a crucial role in the formation of a novel contextual memory; however, once the initial contextual representation is formed, LC activity does not seem to be necessary for recall of the previously experienced context.
Formation of Stable Neuronal Ensembles Is Impaired Under Inhibition of LC Input.
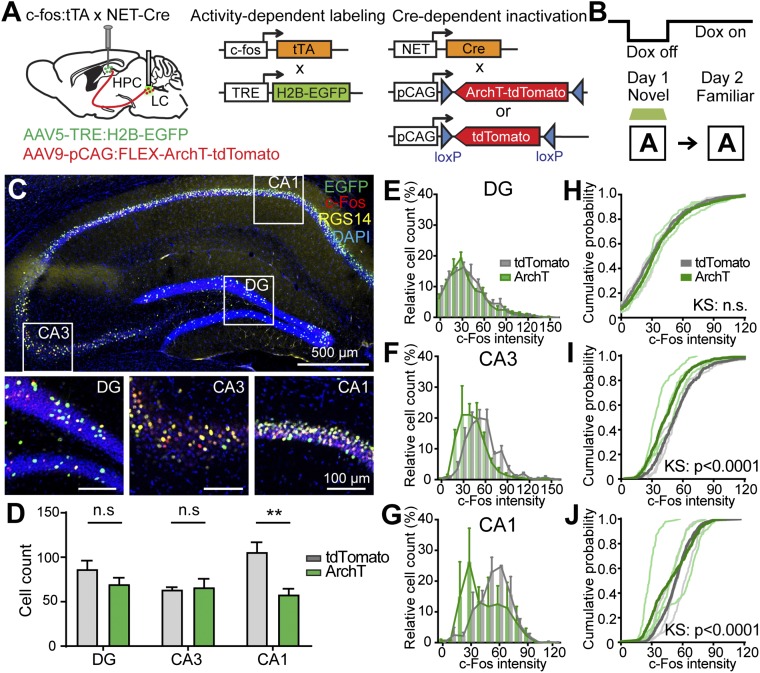
Previous studies using a c-Fos–driven labeling technique have shown that neuronal ensembles corresponding to a specific memory trace, or engram, emerge as a result of experience and that these engram cells are reactivated by recall (24, 25). To investigate the cellular activity ensembles of a given context, we employed activity-dependent labeling methods to track neuronal ensembles activated during novel contextual learning and subsequent recall. We generated a double-transgenic mouse by crossing c-fos:tTA mice with NET-Cre mice to label c-Fos+ cells with H2B-EGFP during a restricted time period while NET+ LC cells were optogenetically inhibited (Fig. 3A). We bilaterally injected AAV5-TRE:H2B-EGFP into the DG, CA3, and CA1 and injected AAV9-pCAG:FLEX-ArchT-tdTomato into the LC (Fig. 3A). Mice were then exposed to a novel context A during doxycycline withdrawal (Dox off) to label active cell populations for memory of context A at the same time that LC activity was inhibited or not inhibited by green light delivered bilaterally (Fig. 3B). After context exposure, these mice were immediately placed back in their home cage and kept on Dox to prevent further labeling. The next day, the mice were exposed to the same context A and were killed 90 min later to examine c-Fos–dependent EGFP expression as a readout of neural activation (Fig. 3B). We subsequently identified cells activated by the first exposure to context A by the expression of Tet response element (TRE)-dependent H2B-EGFP (green) and cells reactivated by the second exposure to context A by immunostaining of endogenous c-Fos (red) (Fig. 3C). First, optogenetic suppression of the LC did not affect the total numbers of H2B-EGFP+ cells in the DG and CA3 but did reduce the total number of H2B-EGFP+ cells in CA1 (Fig. 3D), consistent with a deficit in the encoding phase in CA3. Indeed, CA1 neurons show increased physiological activity and c-Fos expression when animals are exposed to novel environments (26, 27). These data suggest that inhibition of LC input suppresses the effect of novelty on CA1 cells. Next, the degree of reactivation was determined by the intensity of endogenous c-Fos expression among the cells that were active during first exposure to context A (Fig. 3 E–G). Silencing the LC input during the novel context exposure impaired reactivation of the neuronal ensemble in CA3 (Fig. 3 F and I) and CA1 (Fig. 3 G and J) compared with a light-off control group, while reactivation of DG cells was not affected (Fig. 3 E and H). These results demonstrate that optogenetic inhibition of LC activity disrupts the formation of an engram ensemble at the encoding phase on day 1, resulting in the altered ensemble in CA3 and CA1 at recall phase on day 2. Overall, these results indicate that the neuromodulation provided by LC is necessary to encode a contextual memory in a new pattern of engram cells.
Fig. 3.
The formation of stable neuronal ensembles is impaired with the inhibition of LC input. (A) Double-transgenic mice were generated by crossing c-fos:tTA mice with NET-Cre mice. The mice were injected with AAV5-TRE:H2B-EGFP and AAV9-pCAG:FLEX-ArchT-tdTomato into the hippocampus and the LC, respectively. (B) On day 1, mice were taken off Dox and exposed to novel context A to label the activated cells with EGFP while LC activity was inhibited by green light illumination. Then Dox was resumed, and mice were exposed to the same context A on day 2. (C, Upper) Confocal images showing TRE-driven labeling of c-Fos+ cells on day 1 (green) and c-Fos immunopositive cells representing endogenous c-Fos expression on day 2 (red). Immunostaining with anti-RGS14 (yellow) indicates CA2. (Lower) Magnified images of the boxed areas in the upper panel. (D) The number of EGFP+ cells in the DG, CA3, and CA1 (unpaired t test; n.s., not significant; n = 3 subjects per group; n = 6 sections for the tdTomato group and n = 8 sections for the ArchT group). (E–G) Among EGFP+ cells, the distributions of c-Fos immunoreactivity intensity were plotted (n = 3 subjects each; n = 6 sections for the tdTomato group and n = 8 sections for the ArchT group). (H–J) Cumulative probability histograms of E–G, respectively. KS, Kolmogorov–Smirnov test; n.s., P value not significant. Data are presented as mean ± SEM.
Silencing LC Activity During Encoding Disrupts the Formation of a Stable Representation of Space.
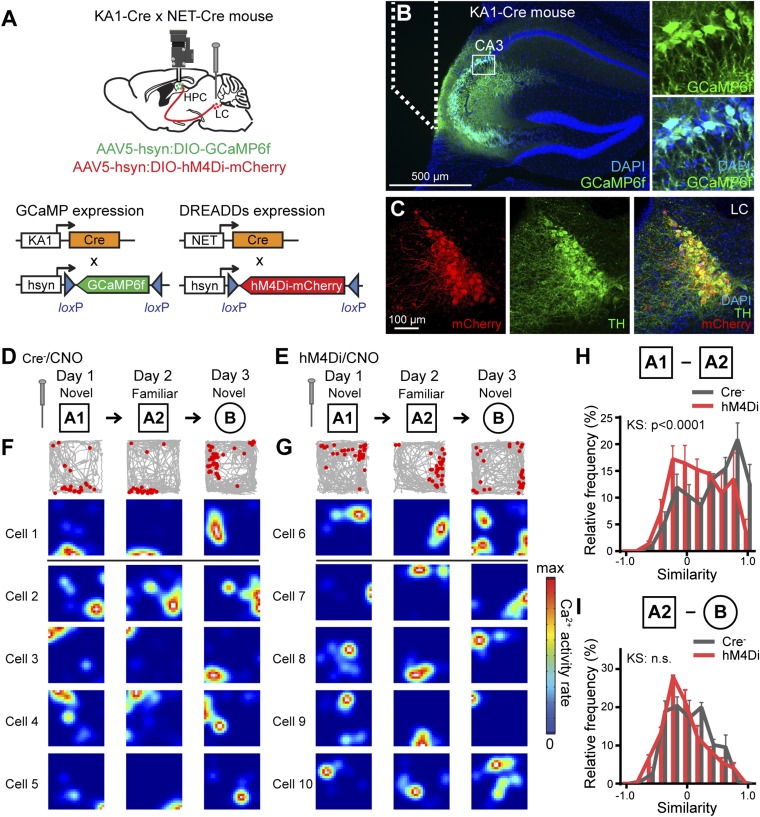
The encoding of novel contextual information requires the formation of new place cells, which are reactivated upon reexposure to the same context (28). Spatial coding of place cells is subject to remapping not only by a change of location but also by a change of contextual cues (29). To understand the role of the LC in the encoding of a spatial map, we investigated place fields of CA3 pyramidal cells in mice with silencing of LC activity using the DREADDs (designer receptors exclusively activated by designer drugs) system during exposure to a novel context. We generated double-Cre transgenic mice by crossing NET-Cre mice with CA3-specific KA1-Cre mice (21) and injected AAV5-hsyn:DIO-GCaMP6f and AAV5-hsyn:DIO-hM4Di-mCherry into CA3 and the LC, respectively (Fig. 4A). An inhibitory DREADDs variant hM4Di, which is an engineered version of the muscarinic acetylcholine receptor, binds clozapine-N-oxide (CNO) and results in membrane hyperpolarization. We then implanted a microprism lens probe targeting the pyramidal cell layer of CA3 (Fig. 4B). We found that 93.1 ± 2.47% (n = 3) of TH+ cells are labeled with hM4Di-mCherry in NET-Cre mice injected with AAV5-DIO-hM4Di-mCherry (Fig. 4C). We confirmed that there is no Cre-dependent expression in nontarget regions by injections of AAV5-hsyn:DIO-EYFP in CA3 of NET-Cre mice and in the LC of KA1-Cre mice (Fig. S5). We also showed that novel contextual memory is impaired under LC inhibition using the DREADDs system (Fig. S6). Together with previous studies demonstrating electrophysiological and behavioral characterization using the DREADDs system to manipulate LC neurons (30, 31), our results validate the effectiveness of DREADDs in silencing LC activity. We monitored Ca2+ activity in CA3 cells over 3 d in an experimental design in which mice explored a novel context A for 30 min for two consecutive days and then explored a second novel context B on the following day, in the order A1/A2/B (Fig. 4 D and E). We examined spatially tuned Ca2+ events in CA3 cells under silenced LC conditions using the DREADDs system (Materials and Methods) and Cre− control mice. We tracked the positions of the mice, which were sorted into 5 cm × 5 cm spatial bins, and the Ca2+ event rate of each neuron determined for each bin produced a rate map (Materials and Methods and Fig. 4 F and G) (32). We calculated a similarity score between two run sessions, A1/A2 and A2/B, using a Pearson correlation of the bin-by-bin comparison of the rate maps across the two sessions for each individual neuron. The distribution of similarity scores of the A1/A2 sessions demonstrated that in hM4Di mice a greater proportion of cells represented different place fields when the mice were reexposed to the familiar context (Fig. 4H). The similarity score of the A2/B sessions is distributed around zero in both the hM4Di and control groups, indicating that the majority of cells represented distinct place fields in both groups (Fig. 4I). These data demonstrate that LC activity has a crucial role in the encoding of a persistent spatial representation in CA3 pyramidal neurons.
Fig. 4.
Silencing LC activity during encoding disrupts the formation of a stable representation of space in CA3. (A) The double-transgenic mice were generated by crossing KA1-Cre mice with NET-Cre mice. The mice were injected with AAV5-hsyn:DIO-GCaMP6f in CA3 and AAV5-hsyn:DIO-hM4Di-mCherry in the LC. (B) GCaMP6f expression in the CA3 cells of a double-Cre mouse. Dotted lines indicate the placement of microprism lens probes. (Insets) Magnified images of GCaMP-expressing cells in the boxed area in the main panel. (C) Expression of hM4Di-mCherry in LC neurons. (D and E) CNO was injected i.p. 30 min before the first exposure to novel context A to suppress the activity of LC neurons. On the following day, these mice were exposed to the same context A and then to the distinct novel context B. (F and G) Examples of the activities of individual CA3 neurons in the open-field chambers. (Top Row) Gray lines indicate traces of mouse movement, and red dots indicate each calcium event captured by the microendoscope over 3 d. (Other Rows). Place maps for individual CA3 neurons corresponding to the calcium events. (H and I) Similarity of place fields between A1 and A2 (n = 4 mice per group; n = 362 cells for the Cre− group and n = 460 cells for the hM4Di group) (H) and between A2 and B (n = 4 mice per group; n = 344 cells for the Cre− group and n = 219 cells for the hM4Di group) (I). KS, Kolmogorov–Smirnov test; n.s., P value not significant. Data are presented as mean ± SEM.
Discussion
We discovered that LC input to dorsal CA3 during exposure to a novel environment has a crucial role in supporting the rapid acquisition of a memory of a one-time experience, whereas LC inputs to dorsal CA1 and DG are not required. Using activity-dependent labeling methods to track neuronal ensembles activated during contextual learning, we found that LC activity is crucial for the formation of a new engram cell ensemble, which must be reactivated for memory retrieval. Furthermore, we discovered that LC activity plays a key role in the formation of an enduring spatial map, suggesting that LC inputs are fundamental in driving plastic changes in the CA3 microcircuit.
Here, we demonstrated the pivotal role of LC neuromodulation in the hippocampus for single-trial learning in a physiologically relevant behavioral task (Fig. 2). Our findings revealed that CA3 is the indispensable target of LC input, which provides the neuromodulatory signals to relevant stimuli associated with novelty. Furthermore, we observed that in the novel-context recognition test the effect of LC neuromodulation in CA3 is dopaminergic-dependent but not noradrenergic-dependent (Fig. S3). It is widely accepted that the LC is the major source of norepinephrine, and although we cannot rule out the existence of an indirect source of dopamine, recent studies support the hypothesis that the LC could release dopamine, alongside norepinephrine, in the hippocampus (8, 12, 16, 33). Our data are consistent with previous studies showing that the dopaminergic neuromodulatory system enhances the encoding of novel information by modulating hippocampal synaptic strength (14, 34, 35). If LC neurons can corelease dopamine and norepinephrine, under what conditions does the LC release dopamine in addition to norepinephrine? In a quiet awake state, LC neurons fire tonically at relatively low frequency (1–3 Hz) (36). However, LC neurons burst fire at ∼10–15 Hz when animals are exposed to novelty (9). In the pathway for synthesis of norepinephrine, dopamine is transported to vesicles, where dopamine-β-hydroxylase converts it to norepinephrine. It is conceivable that burst firing could elicit immature processing in vesicles in the terminal of LC neurons, leading to the release of dopamine along with norepinephrine as a result. Our data support this possibility and are consistent with previous studies that mimicked burst firing with ChR2 in LC neurons and elicited dopamine release (8, 12).
In this study, we demonstrated that neuromodulatory input from the LC to CA3 plays a pivotal role in the single-trial learning of a novel context (Fig. 2). Our terminal inhibition experiments in the novelty recognition test demonstrated that LC inputs to CA3 are indispensable for contextual learning, whereas LC inputs to the DG and to CA1 do not seem to have an obligatory role in the mnemonic process (Fig. 2 G–I). Furthermore, CFC with preexposure experiments demonstrated that LC inputs to CA3 are crucial during the encoding of a novel context but not during recall or for association of the contextual information with negative stimuli (Fig. 2 N and O). Interestingly, inhibition of LC cell bodies resulted in reduced reactivation of newly formed neuronal ensembles in both CA3 and downstream CA1 (Fig. 3 G–J). CA1 receives major inputs from CA3 pyramidal neurons via the Schaffer collaterals, and these projections are thought to govern processing for spatial information during spatial exploration (37). CA3 input is crucial for refined spatial-tuning properties of CA1 place cells (5, 7), suggesting a role for plasticity at CA3 synapses in the establishment of place fields in CA1. With optogenetic inactivation of the LC, CA3 cells may fail to provide the highly tuned drive to CA1 and thus result in poorly tuned place-cell activities. We also demonstrated a great reduction in the number of activated CA1 engram cells caused by LC suppression (Fig. 3D), which is consistent with previous studies reporting that environmental novelty increases physiological activity and c-Fos induction in CA1 cells (26, 27). These data suggest that novelty-induced changes in CA1 activity are induced by LC inputs. The present results combined with those previously reported (5, 7, 37) indicate that neuromodulatory input from LC to CA3 may indirectly contribute to spatial tuning in CA1.
Our calcium imaging study demonstrates that the activity of the LC is crucial to form a stable place field in CA3 place cells. During encoding, a CA3 neural representation is formed by sensory inputs, and LC inputs activated by exposure to novelty seem to be necessary to allow plastic changes to occur. These plastic changes allow the reactivation of a stable cellular ensemble in the CA3 subregion. The CA3 circuit is characterized by three major inputs: the recurrent collateral operating within the CA3 subregion, the mossy fiber input from the DG subregion, and direct input from the stellate cells in entorhinal cortex layer II (ECII). While NMDA receptors in CA3 are required for contextual learning in a novel environment (5), synaptic plasticity in the mossy fibers from the DG to CA3 does not require NMDA receptors (38), ruling out a primary role of mossy fiber plasticity in the novelty-associated learning. Further study is needed to identify the synaptic target of LC-mediated plastic changes, which could be at CA3 recurrent collaterals, inputs from ECII, or both. However, based on the projection pattern of LC terminals in CA3 (Fig. 1) (18), it is likely that plastic changes occurring at both CA3–CA3 and ECII–CA3 synapses are impaired by the suppression of LC input.
A recent study (39) demonstrated that c-Fos–tagged neurons in CA1 that were reactivated upon memory recall (i.e., engram cells) hold place fields. Moreover, silencing the engram-bearing place cells active in a specific environment unmasked a subset of quiet neurons, which in turn enabled the emergence of an alternative map in those neurons. These data suggest two things: first, that a subset of CA1 engram cells are place cells, and second, that a subset of CA1 nonengram cells can turn into place cells when the original ensembles are unavailable (39). Our results indicate that LC neuromodulation supports these dynamic changes in a newly generated ensemble activity (Fig. 3) and in firing patterns of individual place cells (Fig. 4). Neuromodulatory systems of the LC, with its diverse projections in the brain, have been implicated in other cognitive functions such as attention and emotion, as well as novelty (40, 41). Our study provides a circuit mechanism by which behaviorally relevant stimuli are specifically encoded into long-term memory traces, ensuring that important stimuli are stored preferentially over incidental ones.
Materials and Methods
Methods of mouse generation, immunohistochemistry, optogenetics, behavior, activity-dependent cell labeling, and in vivo calcium imaging are described in SI Materials and Methods. Animal experiments were performed in accordance with US NIH guidelines and the MIT Department of Comparative Medicine and Committee on Animal Care. Materials requests may be submitted to tonegawa@mit.edu; all reasonable requests will be granted through a materials transfer agreement.
Supplementary Material
Acknowledgments
We thank C. Twiss, A. Hamalian, C. Ragion, and N. Nayyar for technical assistance; J. Young, M. Pignatelli, T. Kitamura, and D. Roy for discussion and comments; L. Brenner for proofreading; and all members of the S.T. laboratory for their support. This work was supported by the RIKEN Brain Science Institute, the Howard Hughes Medical Institute, and the JPB Foundation (S.T.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714082115/-/DCSupplemental.
References
- 1.Marr D. Simple memory: A theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 2.McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 3.Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- 4.Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 5.Nakazawa K, et al. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 6.Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- 7.Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi T, et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature. 2016;537:357–362. doi: 10.1038/nature19325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sara SJ, Vankov A, Hervé A. Locus coeruleus-evoked responses in behaving rats: A clue to the role of noradrenaline in memory. Brain Res Bull. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 11.Vankov A, Hervé-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci. 1995;7:1180–1187. doi: 10.1111/j.1460-9568.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- 12.Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci USA. 2016;113:14835–14840. doi: 10.1073/pnas.1616515114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNamara CG, Tejero-Cantero Á, Trouche S, Campo-Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci. 2014;17:1658–1660. doi: 10.1038/nn.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lisman JE, Grace AA. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Devoto P, Flore G, Pani L, Gessa GL. Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol Psychiatry. 2001;6:657–664. doi: 10.1038/sj.mp.4000904. [DOI] [PubMed] [Google Scholar]
- 16.Smith CC, Greene RW. CNS dopamine transmission mediated by noradrenergic innervation. J Neurosci. 2012;32:6072–6080. doi: 10.1523/JNEUROSCI.6486-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohara K, et al. Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat Neurosci. 2014;17:269–279. doi: 10.1038/nn.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oleskevich S, Descarries L, Lacaille JC. Quantified distribution of the noradrenaline innervation in the hippocampus of adult rat. J Neurosci. 1989;9:3803–3815. doi: 10.1523/JNEUROSCI.09-11-03803.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madisen L, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamura T, et al. Hippocampal function is not required for the precision of remote place memory. Mol Brain. 2012;5:5. doi: 10.1186/1756-6606-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakazawa K, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fanselow MS, Sigmundi RA. Species-specific danger signals, endogenous opioid analgesia, and defensive behavior. J Exp Psychol Anim Behav Process. 1986;12:301–309. [PubMed] [Google Scholar]
- 23.Fanselow MS. Factors governing one-trial contextual conditioning. Anim Learn Behav. 1990;18:264–270. [Google Scholar]
- 24.Liu X, et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonegawa S, Liu X, Ramirez S, Redondo R. Memory engram cells have come of age. Neuron. 2015;87:918–931. doi: 10.1016/j.neuron.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson MP, Frank LM. Network dynamics underlying the formation of sparse, informative representations in the hippocampus. J Neurosci. 2008;28:14271–14281. doi: 10.1523/JNEUROSCI.4261-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VanElzakker M, Fevurly RD, Breindel T, Spencer RL. Environmental novelty is associated with a selective increase in Fos expression in the output elements of the hippocampal formation and the perirhinal cortex. Learn Mem. 2008;15:899–908. doi: 10.1101/lm.1196508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford Univ Press; Oxford: 1978. [Google Scholar]
- 29.Leutgeb JK, et al. Progressive transformation of hippocampal neuronal representations in “morphed” environments. Neuron. 2005;48:345–358. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Vazey EM, Aston-Jones G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci USA. 2014;111:3859–3864. doi: 10.1073/pnas.1310025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCall JG, et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87:605–620. doi: 10.1016/j.neuron.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun C, et al. Distinct speed dependence of entorhinal island and ocean cells, including respective grid cells. Proc Natl Acad Sci USA. 2015;112:9466–9471. doi: 10.1073/pnas.1511668112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devoto P, Flore G. On the origin of cortical dopamine: Is it a co-transmitter in noradrenergic neurons? Curr Neuropharmacol. 2006;4:115–125. doi: 10.2174/157015906776359559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- 35.Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors contribute to de novo hippocampal LTD mediated by novel spatial exploration or locus coeruleus activity. Cereb Cortex. 2012;22:2131–2138. doi: 10.1093/cercor/bhr297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed OJ, Mehta MR. The hippocampal rate code: Anatomy, physiology and theory. Trends Neurosci. 2009;32:329–338. doi: 10.1016/j.tins.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris EW, Cotman CW. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci Lett. 1986;70:132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- 39.Trouche S, et al. Recoding a cocaine-place memory engram to a neutral engram in the hippocampus. Nat Neurosci. 2016;19:564–567. doi: 10.1038/nn.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 41.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 42.McLaren IPL, Mackintosh NJ. An elemental model of associative learning: I. Latent inhibition and perceptual learning. Anim Learn Behav. 2000;28:211–246. [Google Scholar]
- 43.Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakashiba T, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.