Significance
In laboratory experiments, Wolbachia (wMel strain)-infected Aedes aegypti are refractory to disseminated arboviral infections. Yet previous characterizations of wMel-mediated blocking have not considered several biologically and ecologically important factors likely to influence the virus–mosquito interaction. After direct feeding on 141 viremic dengue patients, we demonstrate wMel lowers dengue virus (DENV) transmission potential and lengthens the extrinsic incubation period. Subsequently, using established field populations of wild-type and wMel-infected Ae. aegypti, we compared field- versus laboratory-rearing conditions on mosquito susceptibility to disseminated DENV infection. The magnitude of wMel-mediated virus blocking was even greater when mosquitoes developed under field conditions. These clinically and ecologically relevant findings support Wolbachia introgression into Ae. aegypti populations as a biocontrol method to reduce the transmission of DENV and other arboviruses.
Keywords: wMel Wolbachia, dengue virus, Aedes aegypti mosquito, extrinsic incubation period, virus transmission
Abstract
The wMel strain of Wolbachia can reduce the permissiveness of Aedes aegypti mosquitoes to disseminated arboviral infections. Here, we report that wMel-infected Ae. aegypti (Ho Chi Minh City background), when directly blood-fed on 141 viremic dengue patients, have lower dengue virus (DENV) transmission potential and have a longer extrinsic incubation period than their wild-type counterparts. The wMel-infected mosquitoes that are field-reared have even greater relative resistance to DENV infection when fed on patient-derived viremic blood meals. This is explained by an increased susceptibility of field-reared wild-type mosquitoes to infection than laboratory-reared counterparts. Collectively, these field- and clinically relevant findings support the continued careful field-testing of wMel introgression for the biocontrol of Ae. aegypti-born arboviruses.
Dengue is a self-limiting arboviral disease caused by any of the four dengue virus (DENV) serotypes. There is no specific treatment for the disease. The only licensed dengue vaccine has a complex efficacy profile against symptomatic and asymptomatic infections (1–3) and even if used programmatically is not projected to result in elimination of DENV transmission (4). Disease control via suppression of the primary mosquito vector, Aedes aegypti, is the cornerstone of the public health response but has manifestly failed to control the incidence of dengue in most endemic countries. New approaches to control transmission are needed—both improved vaccines and novel vector control approaches.
In general, Ae. aegypti mosquito vector competence studies have been performed using laboratory-based approaches, which fail to consider several biologically and ecologically important factors that are likely to influence the virus–mosquito interaction. For example, laboratory studies typically use mosquitoes reared under optimized laboratory conditions producing large adults, uncompromised by pathogens, and suboptimal nutritional or environmental conditions during their development (5–8). Such stresses experienced during immature stages can have “carryover” effects into adulthood, altering immune system regulation and adult susceptibility to virus infection (7, 8), among other life history traits (9, 10). Further, laboratory studies typically use virus passaged in cell culture, which is spiked into animal or human blood; neither virus nor blood reflect the composition of viremic blood from a symptomatic dengue case.
In laboratory studies, Ae. aegypti mosquitoes infected with the wMel strain of Wolbachia, an obligate intracellular bacterium, are refractory to disseminated DENV, chikungunya (CHIKV), and Zika virus (ZIKV) infections (11–18). The mechanisms through which wMel confers resistance to these viruses is likely multifactorial and includes priming of the immune system (19), competition for cellular resources (e.g., cholesterol) (20), and regulating the production of reactive oxygen species (21). Mathematical modeling suggests that if wMel-infected Ae. aegypti become stably established in a given setting, then local dengue transmission should cease under most circumstances (16). The World Mosquito Program (WMP, https://www.worldmosquitoprogram.org) is developing scalable methods to introgress wMel into mosquitoes in disease-endemic settings as a public health intervention that is complementary to other approaches to disease control.
The purpose of this study was to employ clinical and field entomology approaches to deliver the most relevant description of the vector competence phenotype of wMel-infected Ae. aegypti. To this end, we first conducted clinical research to compare the vector competence of laboratory-reared wild-type (WT) and wMel-infected Ae. aegypti (Ho Chi Minh City background) via direct human–mosquito feeding experiments on Vietnamese dengue patients. Second, we drew upon a field-established wMel-infected Ae. aegypti population in central Vietnam to determine if field-reared WT and wMel-infected mosquitoes differ in their DENV transmission potential compared with their laboratory-reared counterparts.
Results
Phenotype of WT and wMel-Infected Ae. aegypti After Direct Human–Mosquito Feeding on Viremic Dengue Cases.
We performed direct feeding of cohorts of mosquitoes on 141 Vietnamese dengue patients to test the hypothesis that wMel-infected Ae. aegypti (HCMC background) would have lower DENV transmission potential under such “natural history”-like conditions. The demographic and virological profiles of the patient population are described in Fig. S1 and Table S1. DENV-1 and DENV-4 were the most prevalent DENV serotypes. Two hundred and eleven independent mosquito feeding events on 141 patients (70 patients with two serial exposures, 71 patients with a single exposure) were performed between March 2015 and January 2016 for a total of 11,178 blood-fed females (Fig. S2). Surviving females from each feeding event were harvested at preassigned, randomly allocated intervals (see Methods for details of randomization) of either 10 and 14 d, or 12 and 16 d postexposure (Table S2).
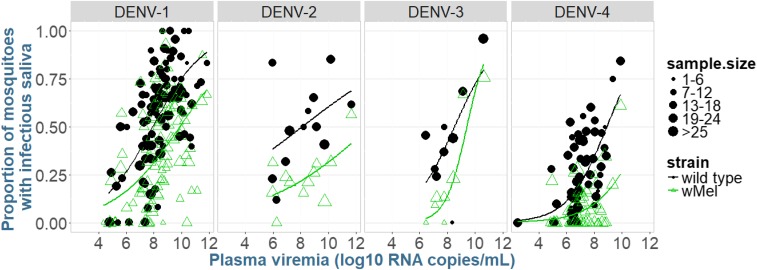
wMel-infected mosquitoes had overall reduced odds of developing a DENV infection in the abdomen [odds ratio (OR) = 0.53, 95% CI = 0.44–0.63, P < 0.0001], with mean percentages of mosquitoes with DENV-infected abdomens being 71.5% for WT and 58.7% for wMel. The same held true for wMel mosquitoes having an infectious virus in saliva (OR = 0.45, 95% CI = 0.38–0.53, P < 0.0001; Fig. 1); the percentage of mosquitoes with virus in their saliva was 38.5% and 22.8% for WT and wMel, respectively. Results from adjusted marginal logistic regression demonstrated that a higher plasma viremia (measured as RNA copies per milliliter) increased the odds of mosquitoes developing an abdomen infection and infectious saliva. DENV-4 produced fewer mosquitoes with virus in saliva than did DENV-1. Each additional day increase in the time since blood feeding increased the odds of detecting infectious virus in the mosquitoes’ saliva by more than 10% (see Table S3 for full details).
Fig. 1.
Proportion of mosquitoes with infectious virus in their saliva, as a function of log10 plasma viremia (RNA copies per milliliter). Within each panel, the scatterplots and corresponding smooth curves based on logistic regression are shown, with panels separating virus serotypes. Data points represent the proportion of mosquitoes in a given unique cohort of WT or wMel mosquitoes that had infectious saliva on the day of harvesting. Data are pooled from day 10, 12, 14, and 16 harvesting time points. The size of the dot indicates the number of mosquitoes in that cohort.
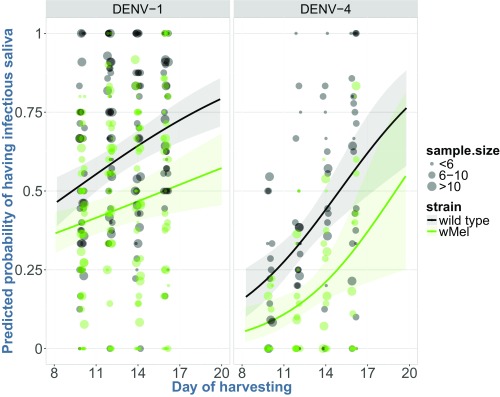
We estimated the extrinsic incubation period (EIP50), the time at which 50% of mosquitoes have infectious virus in their saliva, for each mosquito strain with a marginal logistic regression model, using saliva data acquired on days 10, 12, 14, and 16 (Fig. 2). The predicted EIP50 of WT and wMel mosquitoes exposed to DENV-1 was 9.3 d and 15.8 d, respectively (difference = 6.5 d, 95% CI = 2.02–11.09, P = 0.004). For DENV-4, the corresponding values were 14.9 and 19 d for WT and wMel mosquitoes (difference = 4.1 d, 95% CI = −0.35 to 8.43, P = 0.072). Unfortunately, there was insufficient data available to predict serotype-specific changes in the EIP50 of mosquitoes exposed to DENV-2 and DENV-3. Nevertheless, wMel infection in the HCMC mosquito background appears to extend the EIP50 for DENV-1 and -4 infection by 4–7 d, depending on serotype.
Fig. 2.
Predicted probability of mosquitoes with DENV-positive saliva for WT and wMel Ae. aegypti mosquitoes, based on a marginal logistic regression model. Each dot point shows the proportion of all mosquitoes that had DENV in their saliva among all mosquitoes that took a blood meal. The corresponding smoothing curves and shading (representing 95% CIs) illustrate the predicted probability of having virus in saliva between days 8 and 20 postexposure to DENV-1 and DENV-4 serotypes (DENV-2 and DENV-3 were excluded due to the small numbers of patients infected with these serotypes).
Field-Rearing Conditions Exacerbate the Difference Between WT and wMel Mosquitoes in Their Susceptibility to Disseminated and Transmissible DENV Infection.
We then tested the hypothesis that rearing conditions modify the transmission potential of WT and wMel Ae. aegypti for DENV. To this end, we performed weekly collections of eggs (via ovitraps placed in the field) and late-instar larvae/pupae (via manual sampling from field containers) in Nha Trang City (a Wolbachia-free area) and Tri Nguyen village (where wMel has been established since 2014), in Khanh Hoa Province, central Vietnam. Ovitrapped WT and wMel mosquito eggs were hatched and reared under laboratory conditions. Field-collected WT and wMel late-instar larvae/pupae were held in the same water in which they were collected (with no added nutrition) until emergence. Only those females that emerged as adults within 3 d of removal from the field were retained. Age-matched, 2–3-d-old adult females, achieved through synchronous emergence of laboratory- and field-reared cohorts, were used for blood feeding. As expected, the wing lengths of field-reared WT and wMel mosquitoes were significantly smaller (by 14% and 13%, respectively) than their laboratory-reared counterparts; there was no difference between wing lengths of WT- and wMel-infected mosquitoes. The prevalence of field-reared mosquitoes (WT and wMel) with DENV in their saliva was compared with laboratory-reared equivalents, 10 and 14 d after synchronous indirect human viremic blood feeding. A total of 3,214 mosquitoes were harvested after feeding on viremic blood from 55 dengue cases infected with each of the four serotypes (Fig. S3). Details of the patient and mosquito cohorts are detailed in Fig. S4.
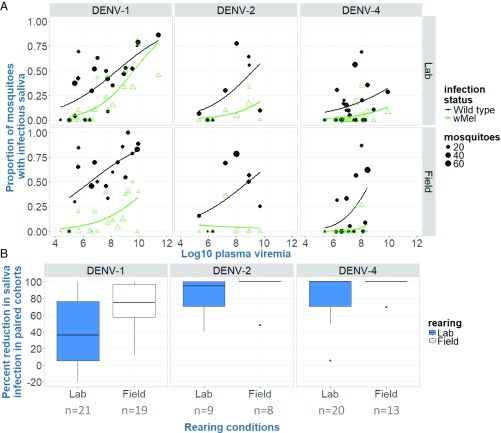
The proportion of mosquitoes with infectious virus in their saliva after human viremic blood feeding is described in Fig. 3A (with full details of all tissue types in Table S4). We observed that field-reared WT females had increased odds of having infectious virus in their saliva, compared with their laboratory-reared counterparts (point estimates = 49.7% vs. 30.8%, OR = 1.68, 95% CI = 1.33–2.12, P < 0.001) (Table S5). This result was supported by a marginal logistic regression model, with field- and laboratory-reared WT and wMel-infected mosquito type as the main covariate, adjusting for plasma viremia levels, days of harvesting, and serotype (Table S6).
Fig. 3.
Outcomes of field- and laboratory-rearing vector competence experiments for WT and wMel-infected Aedes aegypti. (A) Proportion of field- and laboratory-reared WT and wMel-infected mosquitoes with infectious saliva after indirect feeding on viremic human blood, as a function of log10 plasma viremia (RNA copies per milliliter). Data are stratified by rearing conditions and the serotype to which the mosquitoes were exposed. Data points represent cohorts of mosquitoes exposed to a single patient blood meal; the size of the dot indicates the mosquito cohort size. (B) wMel-mediated percentage reduction in mosquitoes with infectious saliva, under field- and laboratory-rearing conditions. The boxplot depicts the percentage reductions (medians and interquartile range) in DENV infection rates in saliva, between paired WT/wMel-infected mosquito cohorts (the number of pairs within a group, indicated along the axis). Positive estimates indicate that Wolbachia reduced the relative infection rate; negative estimates indicate a paired cohort had higher infection rates in Wolbachia-infected mosquitoes compared with WT females. Note: DENV-3 is excluded due to small sample sizes.
We then calculated the difference (increase or decrease) in the proportion of wMel mosquitoes with infectious virus in their saliva versus WT counterparts, stratified by rearing conditions (Fig. 3B). Compared with their WT counterparts, the relative reduction in the proportion of wMel mosquitoes with infectious virus in their saliva was significantly greater in field-reared (mean reduction = 85.9% ± 6.3 SE) versus laboratory-reared mosquitoes (67.9% ± 5.2 SE, P = 0.033). These data demonstrate that laboratory-rearing conditions underestimate the wMel-mediated anti-DENV effect in mosquitoes.
Discussion
The transmission potential of Ae. aegypti for DENV is postulated to be influenced by the specific combination of mosquito and virus genotypes (22–24). Here we demonstrate that in a cognate “ecosystem” of naturally infected Vietnamese dengue patients directly fed upon by WT or wMel-infected Ae. aegypti mosquitoes (HCMC background), wMel conferred resistance to DENV transmission and extended the virus EIP. Additionally, we provide evidence that field-rearing conditions increase the difference in the susceptibility of wMel-infected and WT Ae. aegypti to DENV transmission potential. These clinically and field-relevant data reinforce expectations that wMel introgression could deliver major reductions in disease incidence (16). Further, they highlight that the field environment is a variable in arbovirus:mosquito:Wolbachia biology that is often poorly represented in laboratory experiments.
Several wholly laboratory-based studies have reported that wMel-infected mosquitoes are almost entirely refractory to disseminated arbovirus infection (e.g., refs. 13, 15, 18, and 25). In contrast, we have shown previously (16), and again in this study, that feeding on the viremic blood of patients results in infection of wMel-infected mosquitoes and sometimes this includes detection of infectious virus in the saliva. The different outcomes for wMel-infected mosquitoes in laboratory studies versus direct feeding might be attributable to the intrinsic infectiousness of the virus population in the blood of dengue patients compared with laboratory-cultured virus that is spiked into human blood.
Laboratory-reared wMel-infected Ae. aegypti from Cairns (Australia) that had fed upon Vietnamese dengue patient blood samples had significantly reduced susceptibility to DENV infection in saliva (16). Here we expand on these results and provide greater relevance by having mosquitoes (laboratory-reared WT or wMel) from HCMC feed directly on viremic Vietnamese dengue patients with a study design that permitted estimates of the EIP. As expected, wMel was associated with a lower prevalence of mosquitoes with infectious saliva. Importantly, modeling-based estimates suggested that wMel extended the EIP by 6.5 and 4 d for DENV-1 and DENV-4, respectively. We hypothesize that the basis for the EIP delay is likely due to the general wMel-mediated “antiviral” state in mosquito tissues (19–21) that delays the time that virus titers reach a critical threshold in salivary glands such that infectious virus can be detected in the saliva. Ye et al. (26) also observed that wMel extended the EIP of Ae. aegypti challenged with DENV-1 by 1–2 d, however our study suggested an even greater delay when using patient-derived viremic blood meals, across more serotypes. We suggest that the EIP extensions observed in this study will be epidemiologically significant given the importance of this parameter in shaping overall vectorial capacity (27). Further modeling within already established frameworks will be needed to address this (16).
Tri Nguyen island in Khanh Hoa Province is home to a community of ∼800 households that has been entomologically characterized (28–30). wMel was introgressed into Ae. aegypti mosquitoes on Tri Nguyen island, Khanh Hoa province, in 2014 and has remained established since that time (WMP, https://www.worldmosquitoprogram.org). Using field- and laboratory-reared wMel and WT mosquitoes and indirect feeding on viremic blood from patients, we found that wMel confers relatively greater protection against DENV when mosquitoes are reared naturally, compared with in the laboratory. The major impact of field-rearing is a significant increase in the susceptibility of WT mosquitoes, thus leading to a greater difference between WT and wMel mosquitoes. Despite DENV-1 being the most challenging serotype for wMel to inhibit under laboratory conditions [Ferguson et al., 2015 (16) and the present study], wMel-infected Ae. aegypti reared in the field still reduced the median saliva infection rate by 40%. Field-reared mosquitoes used in this study were collected from containers with variable nutritional reserves and larval densities from week to week (and container to container). The containers were neither monitored nor controlled, producing females authentically field-reared. That field conditions should influence a mosquito’s transmission potential for DENV might be attributable to larval environmental conditions such as temperature, rearing density, and available nutrition; these variables interact during development to influence an adult mosquito’s size, microbiota, and immune system (6, 8, 25, 31–33). We suggest the reason why field-reared WT mosquitoes are more susceptible to DENV infection than laboratory-reared WT counterparts is multifactorial and stems from immune and nutritional effects (6, 34, 35). Thus, despite being unable to identify the precise factor(s) contributing to this difference in susceptibility, these field-based results deliver realistic estimates of wMel-mediated antiviral effects and underscore the importance of field observations in assessing new biological interventions. Further research into the effect of field conditions on the mosquito immune system and nutritional resources are needed to promote our understanding of the variables that govern the dissemination and transmissibility of DENV in both WT and wMel mosquitoes.
A bias of this study is that we enrolled hospitalized dengue patients for mosquito feeding; these patients have higher viremias than ambulatory cases (36). Increasing virus concentration in the plasma is a parameter that we (36) and others (37, 38) have found to be positively associated with increased risk of human-to-mosquito infection. Intriguingly though, Duong et al. (38) recently concluded that for a given viremia, asymptomatically infected humans were more successful at infecting mosquitoes than symptomatic cases. This observation needs replication and an explanatory mechanism, but potentially it suggests other scenarios of human-to-mosquito infection need to be considered in research studies of Wolbachia.
In summary, this study demonstrates wMel-mediated inhibition of DENV infection in Ae. aegypti, under conditions of direct mosquito–human feeding, and reliable extension of the EIP across serotypes. Using indirect patient-derived blood meals, we demonstrate that the magnitude of wMel-mediated virus blocking is greater when mosquitoes are reared in field containers in a community where wMel has been established since 2014. Collectively, these endemic country-based observations provide highly relevant and supportive underpinning science favoring the careful trialing of the wMel introgression approach as a means of eliminating dengue transmission.
Methods
Ethics Statement and Patient Cohorts.
All work was conducted at the Hospital for Tropical Diseases (HTD), Ho Chi Minh City, Vietnam. The work involved patients from a total of three studies; each was approved by the Ethics Committee of the HTD (HTD EC), the Oxford Tropical Research Ethics Committee (OxTREC), and the University of Melbourne Human Research Ethics Committee (UoM HREC) as appropriate (SI Methods). Written informed consent was obtained from all patients participating in both studies and was taken by qualified staff from the HTD for all studies.
Inclusion criteria for the direct blood feeding study were (i) ≥15 y of age, (ii) an inpatient at HTD with <96 h fever at the time of screening, (iii) clinical signs and symptoms consistent with dengue, (iv) virological confirmation of DENV infection (NS1 rapid test positive or RT-PCR positive), and (v) written informed consent. Exclusion criteria were (i) patients in intensive care or with intellectual disabilities who in the attending clinicians’ judgment cannot provide fully informed consent, (ii) patients who report a history of hypersensitive reactions to mosquito bites or with dermatological conditions, and (iii) pregnant women. Of the cohort of 141 patients, all participants received at least one exposure to mosquitoes. If patients were enrolled with <72 h of illness, they were eligible for a second exposure; only two of 72 eligible patients declined a second exposure. Demographic and clinical information were recorded, with disease severity classified based on the WHO dengue classification guidelines from 2009.
Patient-derived blood meals for the field- and laboratory-rearing comparison came from participants who (i) were ≥15 y of age, (ii) were inpatients or outpatients at HTD with <96 h fever, (iii) showed clinical signs and symptoms consistent with dengue, (iv) had virological confirmation of DENV infection (NS1 rapid test positive), and (v) provided written informed consent. Patients were not unconscious or severely ill and were not pregnant.
Direct Human Feeding Studies.
Derivation of mosquitoes.
Mosquitoes used in these direct feeding experiments were all of HCMC genetic background. WT mosquitoes were colonized in the laboratory for three generations (as described in ref. 37). The wMel-infected colony was produced with five generations of backcrossing from the original Cairns wMel background (15) onto the HCMC background. After completion of the backcrossing, outcrossing of both colonies was performed with 10% WT males every second generation. Wolbachia infection status was confirmed in both WT and wMel-infected females. All mosquitoes were maintained at 28 °C, 70–80% rH, and a 12:12 h light:dark cycle, with access to sucrose solution ad libitum. Mosquitoes used in these experiments ranged from G2–G6.
Human–mosquito exposures.
For blood-feeding experiments, ∼25 unfed WT females and ∼25 unfed wMel females (1–4 d old) were prepared in opposing sides of a purpose-designed glass mosquito chamber. Secured by a mesh cover, mosquitoes were transported to the hospital wards where they were held against the patient’s forearm for the 5-min exposure. Mosquitoes were then returned to the insectary (<250 m from the ward) and cold anesthetized. Fully engorged females were maintained for later harvesting.
Clinical adverse events.
Patient responses to mosquito feeding were monitored for adverse events. Severe adverse events (SAEs) in this study were defined as clinically significant if a patient required a clinical intervention, prolonged stay in hospital, or admission to the intensive care unit, when either possibly, probably, or definitely related to the exposure of Ae. aegypti mosquitoes during the course of this study.
Mosquito harvesting and detection of DENV in abdomen and saliva.
All laboratory assays of insects were performed by technicians blinded to the clinical and virological details of the patients. Mosquitoes were killed by cold exposure. The abdomen and saliva of individual females were collected for testing. Saliva was inoculated into naïve, WT Ae. aegypti for the purpose of amplification of any infectious virus particles that were expectorated; inoculated mosquitoes were maintained for 7 d in the environmental conditions described above before harvesting. All abdomens were scored for DENV infection using a quantitative, internally controlled RT-PCR assay (17), with the results expressed as copies per tissue. Recipient mosquitoes from a single index mosquito were pooled before testing for DENV by RT-PCR. A positive result indicated that the index mosquito successfully transmitted infectious virus to one or more recipient mosquitoes in their saliva.
Comparison of Field- and Laboratory-Rearing Conditions on Virus Susceptibility.
Origin of mosquitoes.
All mosquitoes used in experiments were field-derived (F0) females. Both larvae and egg collections were conducted in two field sites: Nha Trang city, which was Wolbachia-free, and Tri Nguyen, a village on Hon Mieu Island (off the coast of Nha Trang). Hon Mieu is the site of wMel releases for the WMP in Vietnam.
Mosquito deliveries and rearing.
Both eggs and larvae collected in the field (F0 mosquitoes) were transported to HCMC for subsequent use in DENV challenge experiments. Late instar larvae and pupae were collected on a weekly basis in Nha Trang and Tri Nguyen and transported to HCMC by courier, taking around 18 h. The delivery was received every Friday morning. To minimize the time that immature mosquitoes were exposed to laboratory conditions, only those mosquitoes that had emerged as adults by the Sunday morning were used in blood feedings in the first 2–3 d of that week. No additional food was given to the larvae after entering the laboratory; the water in which they arrived was the water in which they emerged. For the laboratory-reared mosquitoes, ovitraps located close to the larval breeding sites collected eggs laid by field females. These eggs were collected on a weekly basis and transported alongside the larvae to HCMC. They were then reared under controlled environmental conditions with ample food supply, as per our standard rearing conditions for Ae. aegypti colonies. Eggs collected in the previous week were hatched to synchronize their emergence with the larvae/pupae that arrived the following week. Once emerged, all females were maintained under the same conditions as described above. All mosquitoes were confirmed as Ae. aegypti (both visually and by RT-PCR) and for Wolbachia infection status (details in Dengue Diagnostics).
Indirect blood feeding.
Mosquitoes of each of the four groups (laboratory- and field-reared mosquitoes from both Nha Trang and Tri Nguyen) were each maintained in separate cups and offered the blood of NS1-positive dengue patients in parallel, via artificial membrane feeders. Venous blood, drawn into an EDTA tube, was collected from patients in the hospital, and the blood was transported directly to the insectary, where an aliquot of the blood was taken for quantification of virus and serotyping; the rest was offered to mosquitoes within 1 h of the blood draw.
Mosquito harvesting and detection of DENV in abdomen, head/thorax, and saliva.
Only fully engorged females were maintained for incubation. Individual mosquitoes were collected at days 10 and 14 after exposure to the virus. Mosquitoes were processed in the same way as described above (abdomen + saliva collections), with the addition of the head/thorax tissue being collected as well. The wings of 1,333 field- and laboratory-reared DENV-exposed females were measured (39) in duplicate, by independent technicians. Quantitative and binary measures of DENV infection in the abdomen and head/thorax samples were made, with estimates of virus transmission based on a binary positive/negative of inoculated mosquito pools. All mosquitoes harvested were tested for DENV and Wolbachia infection. For analysis purposes, mosquitoes were grouped within field- and laboratory-reared groups according to their PCR-confirmed infection status for wMel, as opposed to their origin of collection, leading to four strains in the analysis: laboratory-reared WT, laboratory-reared wMel, field-reared WT, and field-reared wMel.
Dengue Diagnostics.
For the direct human blood-feeding experiments, classification of primary and secondary dengue in patients was based on serology results (IgM and IgG antibody capture ELISAs; Panbio). For both sets of experiments, DENV plasma viremia levels were measured by a validated, quantitative serotype-specific RT-PCR assay that has been described previously (40). The ratio between genome copies per milliliter and plaque-forming units per milliliter was 214:1 for DENV-1, 73:1 for DENV-2, 436:1 for DENV-3, and 101:1 for DENV-4. Mosquitoes in both studies were tested for DENV infection in a triplex-PCR, designed to amplify all four DENV serotypes, a Wolbachia-specific target, as well as an internal Ae. aegypti control gene (RPS17) (17).
Statistical Analysis.
All statistical analyses were performed in R, V3.2.4 Revised (R Foundation for Statistical Computing) and SPSS V23.0.0.2 (IBM).
Direct human blood-feeding experiment.
The probability of successful human–mosquito transmission in abdomen and similarly in saliva was first modeled based on a marginal logistic regression model with mosquito type (wMel vs. WT) as the main covariate, and then the model was subsequently adjusted for the additional effects of virus serotype, and plasma viremia and day of harvesting on the DENV infection status. Graphical descriptive analyses were also performed. Further details of these analyses can be found in SI Methods.
Field- and laboratory-reared mosquito comparisons.
Analysis of the field-laboratory comparison data was approached in the same way as the direct human blood feeding. We first compared the effect of field and laboratory rearing within WT and wMel mosquitoes, before performing an adjusted marginal logistic regression with additional parameters. Full details are provided in SI Methods.
Supplementary Material
Acknowledgments
We thank the patients and their families for their participation in this study; the staff of the HTD; and Huynh Anh Huy, Huynh Le Phuong Thuy, Nguyen Thi Van Thuy, Huynh Thi Xuan Trang, Huynh Thi Thuy Van, and Vu Ty Hang for providing outstanding technical assistance. The field staff based in Nha Trang, particularly Luong Ngo Dong Vien and Tran Nguyen Bao Duy, provided weekly collections of both eggs and larvae; this work could not have been done without them. This work was supported by the Wellcome Trust (UK) and the National Health and Medical Research Council (Australia).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1715788115/-/DCSupplemental.
References
- 1.Sabchareon A, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 2.Hadinegoro SR, et al. CYD-TDV Dengue Vaccine Working Group Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 3.Olivera-Botello G, et al. CYD-TDV Vaccine Trial Group Tetravalent dengue vaccine reduces symptomatic and asymptomatic dengue virus infections in healthy children and adolescents aged 2-16 years in Asia and Latin America. J Infect Dis. 2016;214:994–1000. doi: 10.1093/infdis/jiw297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson NM, et al. Benefits and risks of the Sanofi-Pasteur dengue vaccine: Modeling optimal deployment. Science. 2016;353:1033–1036. doi: 10.1126/science.aaf9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell-Foster K, et al. The influence of larval density, food stress, and parasitism on the bionomics of the dengue vector Aedes aegypti (Diptera: Culicidae): Implications for integrated vector management. J Vector Ecol. 2012;37:221–229. doi: 10.1111/j.1948-7134.2012.00220.x. [DOI] [PubMed] [Google Scholar]
- 6.Alto BW, Reiskind MH, Lounibos LP. Size alters susceptibility of vectors to dengue virus infection and dissemination. Am J Trop Med Hyg. 2008;79:688–695. [PMC free article] [PubMed] [Google Scholar]
- 7.Muturi EJ, Blackshear M, Jr, Montgomery A. Temperature and density-dependent effects of larval environment on Aedes aegypti competence for an alphavirus. J Vector Ecol. 2012;37:154–161. doi: 10.1111/j.1948-7134.2012.00212.x. [DOI] [PubMed] [Google Scholar]
- 8.Price DP, Schilkey FD, Ulanov A, Hansen IA. Small mosquitoes, large implications: Crowding and starvation affects gene expression and nutrient accumulation in Aedes aegypti. Parasit Vectors. 2015;8:252. doi: 10.1186/s13071-015-0863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beserra EB, Fernandes CRM, Ribeiro PS. [Larval density as related to life cycle, size and fecundity of Aedes (Stegomyia) aegypti (L.) (Diptera: Culicidae) in laboratory] Neotrop Entomol. 2009;38:847–852. doi: 10.1590/s1519-566x2009000600020. [DOI] [PubMed] [Google Scholar]
- 10.Koella JC, Offenberg J. Food availability and parasite infection influence the correlated responses of life history traits to selection for age at pupation in the mosquito Aedes aegypti. J Evol Biol. 1999;12:760–769. [Google Scholar]
- 11.Aliota MT, Peinado SA, Velez ID, Osorio JE. The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci Rep. 2016;6:28792. doi: 10.1038/srep28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutra HLC, et al. Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe. 2016;19:771–774. doi: 10.1016/j.chom.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreira LA, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Aliota MT, et al. The wMel strain of Wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLoS Negl Trop Dis. 2016;10:e0004677. doi: 10.1371/journal.pntd.0004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker T, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson NM, et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med. 2015;7:279ra37. doi: 10.1126/scitranslmed.3010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joubert DA, et al. Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathog. 2016;12:e1005434. doi: 10.1371/journal.ppat.1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frentiu FD, et al. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis. 2014;8:e2688. doi: 10.1371/journal.pntd.0002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rancès E, Ye YH, Woolfit M, McGraw EA, O’Neill SL. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 2012;8:e1002548. doi: 10.1371/journal.ppat.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caragata EP, et al. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 2013;9:e1003459. doi: 10.1371/journal.ppat.1003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan X, et al. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2012;109:E23–E31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambrechts L. Quantitative genetics of Aedes aegypti vector competence for dengue viruses: Towards a new paradigm? Trends Parasitol. 2011;27:111–114. doi: 10.1016/j.pt.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Dickson LB, Sanchez-Vargas I, Sylla M, Fleming K, Black WC., 4th Vector competence in West African Aedes aegypti is Flavivirus species and genotype dependent. PLoS Negl Trop Dis. 2014;8:e3153. doi: 10.1371/journal.pntd.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fansiri T, et al. Genetic mapping of specific interactions between Aedes aegypti mosquitoes and dengue viruses. PLoS Genet. 2013;9:e1003621. doi: 10.1371/journal.pgen.1003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kho EA, Hugo LE, Lu G, Smith DD, Kay BH. Effects of larval nutrition on Wolbachia-based dengue virus interference in Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2016;53:894–901. doi: 10.1093/jme/tjw029. [DOI] [PubMed] [Google Scholar]
- 26.Ye YH, et al. Wolbachia reduces the transmission potential of dengue-infected Aedes aegypti. PLoS Negl Trop Dis. 2015;9:e0003894. doi: 10.1371/journal.pntd.0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brady OJ, et al. Vectorial capacity and vector control: Reconsidering sensitivity to parameters for malaria elimination. Trans R Soc Trop Med Hyg. 2016;110:107–117. doi: 10.1093/trstmh/trv113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffery JAL, et al. Characterizing the Aedes aegypti population in a Vietnamese village in preparation for a Wolbachia-based mosquito control strategy to eliminate dengue. PLoS Negl Trop Dis. 2009;3:e552. doi: 10.1371/journal.pntd.0000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hugo LE, et al. Adult survivorship of the dengue mosquito Aedes aegypti varies seasonally in central Vietnam. PLoS Negl Trop Dis. 2014;8:e2669. doi: 10.1371/journal.pntd.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen TH, et al. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasit Vectors. 2015;8:563. doi: 10.1186/s13071-015-1174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muturi EJ, Kim CH, Alto BW, Berenbaum MR, Schuler MA. Larval environmental stress alters Aedes aegypti competence for Sindbis virus. Trop Med Int Health. 2011;16:955–964. doi: 10.1111/j.1365-3156.2011.02796.x. [DOI] [PubMed] [Google Scholar]
- 32.Alto BW, Bettinardi D. Temperature and dengue virus infection in mosquitoes: Independent effects on the immature and adult stages. Am J Trop Med Hyg. 2013;88:497–505. doi: 10.4269/ajtmh.12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linenberg I, Christophides GK, Gendrin M. Larval diet affects mosquito development and permissiveness to Plasmodium infection. Sci Rep. 2016;6:38230. doi: 10.1038/srep38230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sumanochitrapon W, Strickman D, Sithiprasasna R, Kittayapong P, Innis BL. Effect of size and geographic origin of Aedes aegypti on oral infection with dengue-2 virus. Am J Trop Med Hyg. 1998;58:283–286. doi: 10.4269/ajtmh.1998.58.283. [DOI] [PubMed] [Google Scholar]
- 35.Buckner EA, Alto BW, Lounibos LP. Larval temperature-food effects on adult mosquito infection and vertical transmission of dengue-1 virus. J Med Entomol. 2016;53:91–98. doi: 10.1093/jme/tjv145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyet MN, et al. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc Natl Acad Sci USA. 2013;110:9072–9077. doi: 10.1073/pnas.1303395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett KE, et al. Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am J Trop Med Hyg. 2002;67:85–92. doi: 10.4269/ajtmh.2002.67.85. [DOI] [PubMed] [Google Scholar]
- 38.Duong V, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci USA. 2015;112:14688–14693. doi: 10.1073/pnas.1508114112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nasci RS. Relationship between adult mosquito (Diptera, Culicidae) body size and parity in field populations. Environ Entomol. 1986;15:874–876. [Google Scholar]
- 40.Hue KDT, et al. Validation of an internally controlled one-step real-time multiplex RT-PCR assay for the detection and quantitation of dengue virus RNA in plasma. J Virol Methods. 2011;177:168–173. doi: 10.1016/j.jviromet.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.