Significance
The subsurface seabed is a great anaerobic bioreactor where organic matter deposited from the ocean’s water column is slowly being degraded by microorganisms. It is uncertain how rates of organic matter degradation progress through different geochemical zones, and the deep production of methane and CO2 in the global seabed therefore remains poorly constrained. With highly depth-resolved analyses of geochemistry and microbial activities, we demonstrate that rates of organic matter degradation decrease continuously with sediment age, irrespective of the prevailing redox zonation and associated changes in the degradation pathway. Moreover, our results show that the microbial food chain leading to methane does not proceed directly through the general key intermediate acetate, but rather through an additional, as of yet unidentified, syntrophic step.
Keywords: marine sediment, organic matter mineralization, sulfate reduction, methanogenesis, syntrophic acetate oxidation
Abstract
The degradation of organic matter in the anoxic seabed proceeds through a complex microbial network in which the terminal steps are dominated by oxidation with sulfate or conversion into methane and CO2. The controls on pathway and rate of the degradation process in different geochemical zones remain elusive. Radiotracer techniques were used to perform measurements of sulfate reduction, methanogenesis, and acetate oxidation with unprecedented sensitivity throughout Holocene sediment columns from the Baltic Sea. We found that degradation rates transition continuously from the sulfate to the methane zone, thereby demonstrating that terminal steps do not exert feedback control on upstream hydrolytic and fermentative processes, as previously suspected. Acetate was a key intermediate for carbon mineralization in both zones. However, acetate was not directly converted into methane. Instead, an additional subterminal step converted acetate to CO2 and reducing equivalents, such as H2, which then fed autotrophic reduction of CO2 to methane.
Microbial processes in most of the global seabed are directly or indirectly coupled to the degradation of organic matter (1). With increasing depth and age in the sediment, the remaining organic matter becomes increasingly recalcitrant to further degradation by microorganisms (2). Sulfate is quantitatively the most important terminal electron acceptor for the anaerobic oxidation of organic carbon in ocean margin sediments (3). When sulfate is depleted at depth, the terminal process of degradation shifts to methanogenesis with the production of methane and CO2. Microbial sulfate reduction rates (SRR) and methanogenesis rates (MGR) can be directly determined from experiments using radioactively labeled tracers. Such measurements show that SRR drop with increasing sediment depth and age according to a reactive continuum that may be simulated by a simple power law function (3–5). This relationship reflects diagenetic alteration and increasing recalcitrance during aging of the organic matter, which limits the hydrolytic attack by extracellular enzymes and thereby the release of substrates that feed the microbial food chain (2, 6). In contrast to SRR, MGR have been difficult to determine experimentally in sediments due to extremely low rates and due to outgassing of supersaturated methane. The deep production of methane in the global seabed and the associated organic matter degradation are therefore poorly constrained.
It has been questioned whether extracellular hydrolysis of the organic matter is always limiting the rate of degradation (7, 8) or if downstream fermentative or terminal respiratory steps may also affect the overall rates (9). If the terminal electron acceptor does not affect the kinetics of organic matter degradation, then the trend of rate versus age determined in the sulfate zone should continue down into the methane zone. Thus, by extrapolation of this trend, the distribution of organic matter degradation and methane production deep within the methane zone could be estimated. To test this, we performed radiotracer-based experiments to measure (i) SRR using [35S]-labeled sulfate, (ii) MGR using [14C]-labeled dissolved inorganic carbon (MGRDIC), (iii) MGR using [2-14C]-labeled acetate (MGRAC), and (iv) acetate oxidation rates using [2-14C]-labeled acetate (AOR).
Results and Discussion
The sampling sites were located at a water depth of 88–96 m in the Bornholm Basin, SE Baltic Sea, which is characterized by seasonally hypoxic bottom water (stations BB01 to BB04; Table S1) (10). Guided by preceding seismoacoustic surveys and exploratory coring campaigns, we have carefully chosen each station according to organic matter burial history. The shallow depth of sulfate depletion (from 0.4 mbsf at BB03 to 4 mbsf at BB04) and the high rates of methane production within the top few meters (up to 140 nmol CH4·cm−2·d−1 at BB03) depended primarily on the thickness of the organic-rich Holocene mud layer. The Holocene mud layer was formed by stable sedimentation during the past 7,000 y and reached 4–9 m thickness, depending on the topography of the underlying organic-poor, postglacial clay from >8,500 y B.P. (Fig. S1) (10). Thus, the composition of the deposited mud was uniform across the flat basin floor, while only the sedimentation rate, 0.5–1 mm·y−1, varied between stations.
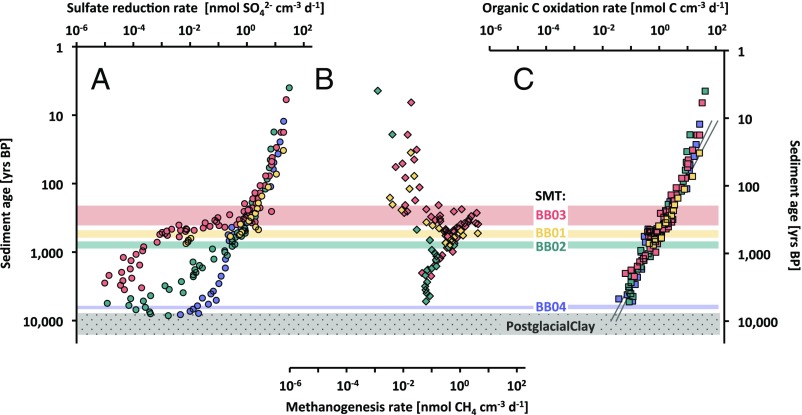
SRR decreased in a log–log linear relationship with sediment age at all stations in accordance with an expected control by organic matter age on the overall metabolic rate (Fig. 1A) (2). This trend continued down toward the sulfate–methane transition (SMT), where sulfate dropped below 1 mM with a concomitant rise of methane. Below the SMT, SRR dropped sharply away from the log–log linear depth trend. In the sulfate zone, above the SMT, MGRDIC were detectable but very low, <10−1 nmol CH4·cm−3·d−1, or two to three orders of magnitude lower than SRR (Fig. 1B). The MGRDIC rose sharply and peaked in the SMT with rates similar to the SRR in that zone (up to ∼4 nmol CH4·cm−3·d−1 at station BB03). Thus, the highest rates of MGRDIC occurred in the SMT because the organic matter here had the youngest age and the highest reactivity. In the methane zone below, MGRDIC continued to drop along the same log–log linear depth trend of organic carbon mineralization as was found for SRR in the sulfate zone. By contrast, MGRAC were extremely low throughout the sediment at all investigated stations (<10−3 nmol CH4·cm−3·d−1) (Figs. S7–S9).
Fig. 1.
Rates of anaerobic carbon mineralization in Bornholm Basin sediments. Double logarithmic plots of rates versus age in the sediment for four stations (BB01–BB04): (A) SRR, (B) total MGR (MGRDIC + MGRAc), (C) total anaerobic organic carbon oxidation rate (COR). Shaded zones indicate the SMT at each individual station using the same color coding for station number as the data symbols. The two gray lines in C depict the upper and lower 95% CI for the best fitted power law function to the data (Eq. 1). Detailed biogeochemical and prokaryotic activity profiles of individual stations are shown in Figs. S3–S10.
We calculated total anaerobic carbon oxidation rates (COR) from the sum of SRR and MGR based on their stoichiometry during organic carbon mineralization (Fig. S2) (11). The resulting vertical depth trend of COR (Fig. 1C) revealed that rates of organic carbon mineralization decreased smoothly and continuously with organic matter age throughout the sediment, irrespective of the prevailing redox zonation and the associated change in the terminal pathway of the microbial food chain. This trend has previously been assumed but not experimentally demonstrated. The data thereby provide direct support for the validity of commonly applied organic matter decay models, such as the time-continuous and reactive-continuum approaches (2, 6), without the necessity to correct organic matter degradation rate with pathway-specific inhibition factors (1). The depth trend, based on activity measurements from all four stations in the Bornholm Basin, corresponded to the following power law dependence on sediment age (in years B.P.):
| [1] |
In a uniform and continuously deposited sediment, such a relationship can be extrapolated to estimate organic carbon mineralization also in deeper sediment layers where process rates are difficult to quantify by radiotracer methods due to exceedingly low microbial activity and due to supersaturation of methane. Total depth-integrated MGR contributed to the total depth-integrated COR in the Holocene mud layer from 8% at BB02 to 40% at BB03—that is, a relatively low contribution, although the methanogenic zone covered 74–94% of the Holocene mud layer (Figs. S1 and S3–S6). This low contribution of methanogenesis was a consequence of the steep decrease in organoclastic activity with sediment depth and age. There was an abrupt shift from no methane to a well-developed methane zone between the neighboring stations, BB04 and BB02, triggered by a moderate increase in sedimentation rate from 0.051 cm·y−1 at BB04 to 0.055 cm·y−1 at BB02. Such an abrupt shift is due to a positive feedback whereby a slight enhancement of the upwards diffusion of methane lifts up the SMT and causes a strong enhancement of methanogenesis in shallower and much more active layers (5).
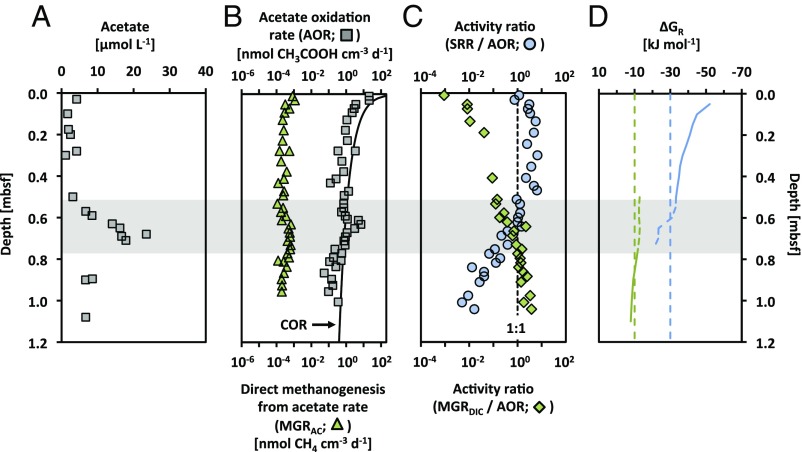
Degradation of sedimentary organic matter should lead to fermentative production of acetate, as the main single end product, plus some H2 and CO2 (12, 13). Accordingly, it could be expected that most of the methane production originates from acetoclastic methanogenesis (13). Indeed, we found that the rate of acetate consumption corresponded to about one-third of the rate of sulfate reduction above the SMT and about half of the rate of methanogenesis below the SMT (Fig. 2B). Intriguingly, however, our incubations with [14C]-DIC showed that CO2 reduction accounted for more than 99% of total methanogenesis, while incubations with [2-14C]-acetate showed no transfer of labeled methyl-carbon from acetate to methane. Instead, [2-14C]-acetate was converted to [14C]-CO2 in the methane zone at rates that closely followed CO2 reduction to methane (Fig. 2C). We attribute this to the only known alternative degradation pathway for acetate under methanogenic conditions—a syntrophic coupling where acetate is first oxidized to CO2 plus reducing equivalents (e.g., H2), followed by hydrogenotrophic methanogenesis (Fig. 3) (14, 15).
Fig. 2.
Acetate turnover in station BB03 sediment. (A) Down-core profile of acetate concentrations in the pore water. (B) AOR accounted for about one-third and half of the COR above and below the SMT (shaded zone), respectively, and they were about 1,000-fold higher than the rates of direct methanogenesis from acetate. (C) Activity ratios indicate the electron/mass balance between SRR and AOR or MGRDIC and AOR, with the dashed line indicating a 1:1 ratio. (D) Gibbs energy yield (ΔGR) for acetate metabolism via sulfate reduction (blue line) or via methane production (green line) is shown relative to suggested minimum energy levels of sulfate-reducing prokaryotes (dashed blue line) (29) and methanogenic archaea (dashed green line) (26). Data for other stations are shown in Figs. S7–S9.
Fig. 3.
Pathways of acetate turnover. The experiments with radiotracers (red) show how the key intermediate, acetate, may be degraded in the sulfate zone and the methane zone: (A) When sulfate was above a threshold concentration, acetate was oxidized to CO2, coupled with sulfate reduction to sulfide. (B) Depletion of sulfate did not lead to direct methane production from acetate. (C) Instead, acetate was completely oxidized to CO2. (D) Reducing equivalents, symbolized by electrons [e−], from acetate were used for methane production from CO2.
This two-step process was first proposed by Barker (1936) (14) and was rediscovered half a century later by Zinder and Koch (1984) (15) in a thermophilic coculture isolated from an anaerobic digestion reactor. Since then it has been primarily regarded as a niche process that only occurs at high temperature or under conditions inhibitory for acetoclastic methanogens, such as excessively high NH4+ concentrations (16, 17) that are about 100 times higher than those measured in Bornholm Basin sediments (<5 mmol·L−1; Figs. S3–S6). However, the detection of syntrophic acetate oxidation coupled to methanogenesis in batch incubations of lake sediment (18, 19) and oil reservoir fluids (20) has indicated broader environmental relevance. The process corresponds to a reversal of acetogenesis (from H2/CO2), and the enzymatic requirements—that is, variations of the Wood–Ljungdahl pathway—might therefore be widespread among anaerobic prokaryotes, including sulfate reducers and methanogens (17, 21, 22).
Consistent with our findings, incubation studies and investigations of stable carbon isotopic compositions of CH4 in marine sediments suggested a predominance of methanogenesis via CO2 reduction in marine sediments (4, 13, 23, 24), thus indicating a global relevance of syntrophic methanogenesis from acetate. However, minor contributions of acetoclastic methanogenesis (<20% of total methane production) have also been detected in marine sediments (4, 24, 25), thereby leading to the question of what controls the relative contribution of the two acetate degradation pathways in methanogenic environments. There is no obvious thermodynamic rationale for a syntrophic coupling between acetate oxidation and hydrogenotrophic methanogenesis, as there is no difference in the overall energy yield of the sum of these processes compared with that of direct acetoclastic methanogenesis. In Bornholm Basin sediments, methanogenesis from acetate was exergonic beneath the SMT (Fig. 2D) but was close to the proposed energetic limit of methanogenic archaea (26), −10 kJ·mol−1. If H2 is the exchanged reducing equivalent, then the syntrophic relationship would require a narrow concentration range of 1–4 nM to allow both reactions to be exergonic so that the low overall energy yield can be shared between the partner organisms. Such low H2 concentrations have, indeed, been reported below the SMT (e.g., <10 nM in the Cape Lookout Bight) (26). Consistent with our findings, that site was also characterized by a conspicuously high contribution of methanogenesis via CO2 reduction (70–80% of total methane production) (25) and by similar energy yields for methane production from acetate (27).
Our results highlight the importance of a syntrophic relationship between acetate oxidizers and methanogens to enable acetate degradation under sulfate-limiting conditions. Intriguingly, at stations BB04 and BB02, where the methane zone is still in early development, very high acetate concentrations (up to >1 mmol·L−1; Figs. S5 and S6) indicate a non-steady state situation where syntrophic degradation is not yet able to balance, at the energetic limit, the continuous acetate production by fermenters.
The predominance of acetate oxidizing prokaryotes over acetoclastic methanogens might be due to substrate kinetics and/or other competitive factors, such as the ability to engage in alternative forms of energy metabolism like acetogenesis and fermentation (17). Methanogens have a very narrow substrate spectrum (28), and the direct conversion of acetate to methane has so far only been ascribed to two microbial groups, Methanosarcina and Methanosaeta, of which only the latter is reported to grow at in situ acetate concentrations (<0.2 mM) (29, 30). Thus, the conversion of high levels of [2-14C]-acetate to [14C]-CO2 in batch incubations of manure and wastewater sludge was only detected in the absence of Methanosaeta (19).
Our results have important implications for the interpretation of organic matter degradation in the methane zone of marine sediments: (i) The terminal process of anaerobic organic matter mineralization does not control the overall rate of degradation, (ii) fermentation produces acetate as the main potential substrate for the terminal degradation step in both the sulfate and the methane zones, and (iii) methanogenesis from acetate proceeds through a syntrophic pathway via CO2 rather than by a simple conversion of the methyl-carbon in acetate to CH4. The reasons for this indirect pathway and the key parameters controlling it remain to be identified. In fact, it also remains to be shown whether the oxidation of acetate, as the single most important substrate feeding sulfate reduction, may also partly take place in syntrophy. Similar to methanogenesis, a syntrophic acetate oxidation coupled to sulfate reduction would be exergonic for both partners under the low H2 concentrations available.
Materials and Methods
Site Description and Sediment Coring.
Cruises were undertaken with the R/V Aurora to the Bornholm Basin (Stations BB01, BB02, BB03, and BB04; Table S1). After initial exploratory sampling campaigns in 2014 and 2015, stations were revisited in 2016 with a keen focus on the transition in mineralization processes across the SMT. If not indicated otherwise, each site was sampled with a gravity corer of up to 9 m in length and complementary Rumohr cores (31). All cores at each station were sampled with the vessel positioned within 5 m diameter. The Rumohr corer allows nearly undisturbed sampling of the top meter of sediment below the seafloor. The extent of the unavoidable sediment loss from the top of the gravity cores (ca. 10–20 cm) was determined by aligning the sulfate concentration profiles of parallel gravity and Rumohr cores. All cores were processed on board the R/V Aurora, with high priority given to samples for methane and microbial activity analysis to ensure that samples could be taken within minutes after core retrieval. Gravity cores were cut into 1-m or 2-m subsections, capped, and processed immediately depending on the type of analysis, as described below. In Rumohr cores, sediment material was sampled by stepwise extrusion from the core liner with a piston, while sediment material in gravity-core subsections was sampled, starting from the bottom, by drilling holes (for methane samples) or by cutting windows in the core liner (for microbial activity samples). The outermost part of the sediment that had been in contact with the core liner was avoided during sampling.
Methane Analysis.
A 2.5-mL sediment sample was taken with a cutoff plastic syringe, immediately transferred to 20-mL preweighed glass vials containing 4 mL of saturated NaCl solution, and crimp-capped with butyl rubber stoppers. Samples were then shaken thoroughly and stored upside down at −20 °C until analysis. Before analysis, the samples were weighed, thawed, and shaken again to equilibrate methane between slurry and headspace of the glass vial. We injected 100–500 µL of the headspace with a glass syringe into a gas chromatograph equipped with a 0.9 m packed silica gel column (3.1 mm inner diameter) and a flame ionization detector (GC-FID; SRI 310C; SRI Instruments).
Pore-Water Analyses.
Rhizon samplers (32) for pore-water analysis were flushed with at least 50 mL Milli-Q water, dried, and stored in gas-tight foil bags until use. In Rumohr and gravity cores, small holes were drilled into the core liner of undisturbed sections to collect pore water with the Rhizon samplers by creating a vacuum with a 20-mL syringe. The first 1 mL of the extracted pore water was discarded, and then up to ∼10 mL was collected before the samples were aliquoted for analysis of individual pore-water parameters. For sulfate analysis, the extracted pore water was acidified and stripped of hydrogen sulfide (H2S) by bubbling with a stream of moist CO2. Sulfate samples were stored at 4 °C until analysis as described before (33). DIC samples were stored in 2-mL glass vials without headspace at 4 °C. Samples were transferred to sealed Exetainers, acidified with 85% (v:v) phosphoric acid, and after 24 h of equilibration time, the produced CO2 was measured from the headspace of the Exetainer by a DeltaV isotope ratio mass spectrometer. Samples for acetate analysis were stored in combusted glass vials at −80 °C until analysis by 2D ion chromatography–mass spectrometry (29, 34). Pore water for ammonium (NH4+) analysis was stored in 2 mL Eppendorf vials at −20 °C, and NH4+ was measured photometrically according to the Danish Standard DS 224 protocol adapted for seawater samples (35). The detailed pore-water geochemistry and the measurements of microbial activity were derived from different cores. To ensure comparable geochemistry between replicate cores, additional pore-water samples for sulfate and acetate analysis were extracted from selected sediment samples before radiotracer incubation. For this, sediment aliquots (up to 1 g) were centrifuged at 14,000 × g with an Eppendorf Minispin benchtop centrifuge, and the supernatant was stored and processed as described above.
Diffusive Fluxes and Stoichiometric Ratios During Organic Matter Mineralization.
Diffusive fluxes of methane were calculated from the concentration gradients using Fick’s First Law:
where J is the diffusive flux, C is the concentration, z is depth (cm), Ds is the whole-sediment diffusion coefficient, and φ is porosity. Ds was calculated from porosity, φ, and molecular diffusion coefficients of individual species (36), Di, at 8 °C in situ temperature and a salinity of 15 (DCH4 = 1.08 cm2·d−1, DSO42− = 0.566 cm2·d−1, DHCO3− = 0.609 cm2·d−1), as described before [Ds = Di/(1 + 3(1 − φ))] (37).
The concentration profiles of pore-water DIC and SO42− (Fig. S2) were used to calculate the stoichiometry of organic carbon oxidation to sulfate reduction (COR:SRR, or rC:SO42−) in the sediment (11):
where dDIC/dSO42− is the slope of the linear regression. Because rC:SO42− is determined by the oxidation state of the organic matter (ox), we can use rC:SO42− to calculate the stoichiometry of organic carbon oxidation to methanogenesis (COR:MGR, or rC:CH4) (11):
Process Rate Measurements Using Radiotracers.
As processing under strictly temperature-controlled conditions was not possible on board, the temperature of the cores was monitored continuously. Over the course of the entire sampling procedure (1–2 h), the temperature in the cores increased by only 1–3 °C.
SRR were determined in subcores collected in butyl rubber-stoppered 5-mL sterile cutoff polycarbonate syringes, as described before (33). Each syringe subcore was injected with 5 µL carrier-free [35S]-sulfate solution containing up to 140 kBq of radioactivity, depending on the expected microbial activity.
MGRDIC or MGRAc, as well as AOR, were determined in subcores collected with butyl rubber-stoppered glass tubes equipped with acrylic plungers similar to the cut syringes. Each subcore was injected with 5 µL [14C]-DIC solution, containing up to 170 kBq of 14C radioactivity, or 5 µL [2-14C]-acetate solution, containing up to 10 kBq of 14C radioactivity. The addition of [2-14C]-acetate led to an increase of the acetate concentration by only ∼20%. All samples for measurement of microbial activity were incubated at the in situ temperature of 8 °C (281 K) within gas-tight plastic bags containing an Oxoid AnaeroGenTM pad (Oxoid A/S). This oxygen scrubber effectively removes O2 but does not generate H2. The incubation times were chosen according to the expected microbial activity and differed from 12 h to 30 h. For example, in samples from the shallow Rumohr cores, we expected a fast turnover of the typically small acetate pool, and therefore, incubations were kept relatively short (∼12 h).
SRR incubations in cut syringes were terminated by freezing the intact samples at −20 °C, without opening the bags. After thawing, the total reduced inorganic sulfur (TRIS = H2S, S0, FeS, and FeS2) was separated from the 35SO42− by a single-step cold chromium distillation (33, 38). The radioactivities of [35S]-sulfate and 35S-TRIS were counted in 15 mL scintillation mixture (Gold Star, Meridian Biotechnologies) on a TriCarb 2900TR liquid scintillation analyzer (Packard Instrument Company).
To terminate [14C]-DIC and [2-14C]-acetate incubations, the entire glass syringe was transferred into glass vials, containing 5 mL NaOH (2.5%), without extruding the mud, and immediately crimp-capped with butyl rubber stoppers. The glass vials were then shaken thoroughly to wash the sample out of the glass syringe and stored upside down at −20 °C until analysis. 14CH4 in these vials was determined by flushing the headspace with CO2-free air at 25 mL·min−1 for 15 min and oxidation of the 14CH4 in the evolving gas stream to 14CO2 in a quartz glass tube containing CuO pellets, heated to 900 °C. The efficiency of methane oxidation was tested by adding known amounts of CH4 to a reaction vessel and following its conversion to CO2 in the exhaust gas by GC-FID (see Methane Analysis). Conversion efficiencies were always >99%. 14CO2 from the oven exhaust gas was trapped in 5 mL Carbosorb (Perkin-Elmer). In contrast to our earlier application of 14C for determination of methanogenesis, the gas stream was subjected to a wash step in 1 M NaOH before combustion to prevent trace amounts of labeled DIC to penetrate into the oven. In addition, the entire gas line was made of glass, which does not absorb CO2. These precautions lowered the background by at least two orders of magnitude and thus enhanced the sensitivity of the method by a similar factor. This improvement of the method was a prerequisite for the precise measurement of extremely low rates of methanogenesis in the deep part of the sediment.
To determine the [14C]-DIC remaining in the glass vial, after extraction of CH4, a 0.5-mL subsample of sediment slurry was transferred into a new glass vial, crimp-capped with butyl rubber stoppers, and acidified with 2 mL of HCl (6 M). All 14CO2 produced was flushed out of the vial headspace with N2 at 25 mL·min−1 for 35 min and trapped in 5 mL Carbosorb. The radioactivity of 14CO2 was counted in a 5 mL scintillation mixture (Permafluor, PerkinElmer) on a TriCarb 2900TR liquid scintillation analyzer (PerkinElmer). To determine the residual 14C-acetate in [2-14C]-acetate incubations, an additional 1-mL subsample of the acidified and flushed supernatant was counted in 10 mL Gold Star scintillation mixture on a TriCarb 2900TR liquid scintillation analyzer. In control experiments to determine abiotic radiotracer conversion and/or carryover during sample processing, radiotracer was added to sediments, immediately frozen, and processed in the same way as the incubated samples.
The SRR were calculated as described before (33, 39):
where [SO42−] is the sulfate concentration, ATRIS is the radioactivity of TRIS at the end of the incubation, ASO42− is the radioactivity of SO42− at the end of the incubation, and t is the length of the incubation. Similarly, the MGRDIC were calculated as described before (25, 40):
where [DIC] is the DIC concentration, ACH4 is the radioactivity of CH4 at the end of the incubation, and ADIC is the radioactivity of DIC at the end of the incubation. In contrast to incubations with [14C]-DIC, a significant fraction of the [2-14C]-acetate tracer was consumed during the incubation of samples close to the sediment surface, where activities were high and acetate concentrations were low. To account for this consumption, we assumed a first-order decrease in the specific radioactivity of the acetate pool (25, 40). The acetate turnover rate (ATR) was therefore calculated as
where [Ac] is the acetate concentration and AAc is the radioactivity of acetate at the end of the incubation. MGRAc and AOR were then calculated as
and
COR were calculated from SRR and MGR based on the estimated stoichiometry of organic matter mineralization during sulfate reduction and methanogenesis (rC:SO42− = rC:CH4 = 1.37 ± 0.16; see Diffusive Fluxes and Stoichiometric Ratios During Organic Matter Mineralization and Fig. S2) (11):
The power law correlation lines describing the age or depth trend of COR were generally calculated under omission of elevated rates within the SMT, fed by upwards diffusing methane, and those from 0 to 10 cmbsf (0–100 y sediment age) where sulfate might not be the main electron acceptor. The linear regression after log–log transformation was corrected for logarithmic skewing as described before (5).
Calculation of Gibbs Free Energy.
The standard Gibbs energy (∆G0insitu) of sulfate reduction from acetate (SO42− + CH3COO− → HS− + 2HCO3−) and methanogenesis from acetate (direct: CH3COO− + H2O → CH4 + HCO3−; or syntrophic: CH3COO− + 4H2O → 4H2 + 2HCO3−+ H+, coupled to 4H2 + HCO3−+ H+ → CH4 + 3H2O) was calculated using the SUPCRT92 software package (41) and reported thermodynamic data (42–44) for a mean pressure of 1.1 MPa and 8 °C in situ temperature. The energy of reactions at nonstandard conditions (∆GR) was calculated according to
where R is the ideal gas constant, T is the in situ temperature, and Q denotes the activity quotient of the reactants and reaction products. Activities in the saline pore fluids were estimated by multiplying the measured concentration of the species with activity coefficients (0.245 for SO42−, 0.725 for HCO3−, 0.699 for HS−, 0.725 for CH3COO−, 1.08 for CH4, and 1 for H2O) calculated from an extended version of the Debye–Hückel equation (45) for an ionic strength of I = 0.28 and at 8 °C using the Geochemists Workbench Software (www.gwb.com).
Supplementary Material
Acknowledgments
The authors thank captain Torben Vang and the crew of the R/V Aurora for sampling; Susanne Nielsen, Jeanette Pedersen, Karina Bomholt Oest, and Julie Rotschi (Aarhus University) for analytical work and technical support; André Pellerin, Alexander Brice Olson Michaud, Karen-Marie Hilligsøe (Aarhus University), and Gilad Antler (Cambridge University) for help during sampling; and Julia Beulig for constructive comments on the text. The research was funded by the Danish National Research Foundation Grant DNRF104 and FP7 European Research Council (ERC) Advanced Grant 294200 (to B.B.J.) and Danish Center for Marine Research Grant “Cryptic Biogeochemistry in the Bornholm Basin” (to H.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1715789115/-/DCSupplemental.
References
- 1.Arndt S, et al. Quantifying the degradation of organic matter in marine sediments: A review and synthesis. Earth Sci Rev. 2013;123:53–86. [Google Scholar]
- 2.Middelburg JJ. A simple rate model for organic matter decomposition in marine sediments. Geochim Cosmochim Acta. 1989;53:1577–1581. [Google Scholar]
- 3.Jørgensen BB. Mineralization of organic matter in the sea bed—The role of sulphate reduction. Nature. 1982;296:643–645. [Google Scholar]
- 4.Jørgensen BB, Parkes RJ. Role of sulfate reduction and methane production by organic carbon degradation in eutrophic fjord sediments (Limfjorden, Denmark) Limnol Oceanogr. 2010;55:1338–1352. [Google Scholar]
- 5.Flury S, et al. Controls on subsurface methane fluxes and shallow gas formation in Baltic Sea sediment (Aarhus Bay, Denmark) Geochim Cosmochim Acta. 2016;188:297–309. [Google Scholar]
- 6.Boudreau BP, Ruddick BR. On a reactive continuum representation of organic matter diagenesis. Am J Sci. 1991;291:507–538. [Google Scholar]
- 7.Arnosti C. Speed bumps and barricades in the carbon cycle: Substrate structural effects on carbon cycling. Mar Chem. 2004;92:263–273. [Google Scholar]
- 8.Abell J, Laverman AM, Cappellen PV. Bioavailability of organic matter in a freshwater estuarine sediment: Long-term degradation experiments with and without nitrate supply. Biogeochemistry. 2009;94:13–28. [Google Scholar]
- 9.Burdige DJ, Gardner KG. Molecular weight distribution of dissolved organic carbon in marine sediment pore waters. Mar Chem. 1998;62:45–64. [Google Scholar]
- 10.Andrén E, Andrén T, Sohlenius G. The Holocene history of the southwestern Baltic Sea as reflected in a sediment core from the Bornholm Basin. Boreas. 2000;29:233–250. [Google Scholar]
- 11.Burdige DJ. Geochemistry of Marine Sediments. Princeton Univ Press; Princeton: 2006. [Google Scholar]
- 12.Sørensen J, Christensen D, Jørgensen BB. Volatile fatty acids and hydrogen as substrates for sulfate-reducing bacteria in anaerobic marine sediment. Appl Environ Microbiol. 1981;42:5–11. doi: 10.1128/aem.42.1.5-11.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conrad R. Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiol Ecol. 1999;28:193–202. [Google Scholar]
- 14.Barker HA. On the biochemistry of the methane fermentation. Arch Mikrobiol. 1936;7:404–419. [Google Scholar]
- 15.Zinder SH, Koch M. Non-aceticlastic methanogenesis from acetate: Acetate oxidation by a thermophilic syntrophic coculture. Arch Microbiol. 1984;138:263–272. [Google Scholar]
- 16.Schnürer A, Zellner G, Svensson BH. Mesophilic syntrophic acetate oxidation during methane formation in biogas reactors. FEMS Microbiol Ecol. 1999;29:249–261. [Google Scholar]
- 17.Hattori S. Syntrophic acetate-oxidizing microbes in methanogenic environments. Microbes Environ. 2008;23:118–127. doi: 10.1264/jsme2.23.118. [DOI] [PubMed] [Google Scholar]
- 18.Nüsslein B, Chin K-J, Eckert W, Conrad R. Evidence for anaerobic syntrophic acetate oxidation during methane production in the profundal sediment of subtropical Lake Kinneret (Israel) Environ Microbiol. 2001;3:460–470. doi: 10.1046/j.1462-2920.2001.00215.x. [DOI] [PubMed] [Google Scholar]
- 19.Karakashev D, Batstone DJ, Trably E, Angelidaki I. Acetate oxidation is the dominant methanogenic pathway from acetate in the absence of Methanosaetaceae. Appl Environ Microbiol. 2006;72:5138–5141. doi: 10.1128/AEM.00489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayumi D, et al. Carbon dioxide concentration dictates alternative methanogenic pathways in oil reservoirs. Nat Commun. 2013;4:1998. doi: 10.1038/ncomms2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozuolmez D, et al. Methanogenic archaea and sulfate reducing bacteria co-cultured on acetate: Teamwork or coexistence? Front Microbiol. 2015;6:492. doi: 10.3389/fmicb.2015.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lever MA. Acetogenesis in the energy-starved deep biosphere–A paradox? Front Microbiol. 2012;2:284. doi: 10.3389/fmicb.2011.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiticar MJ, Faber E, Schoell M. Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation—Isotope evidence. Geochim Cosmochim Acta. 1986;50:693–709. [Google Scholar]
- 24.Parkes RJ, et al. Biogeochemistry and biodiversity of methane cycling in subsurface marine sediments (Skagerrak, Denmark) Environ Microbiol. 2007;9:1146–1161. doi: 10.1111/j.1462-2920.2006.01237.x. [DOI] [PubMed] [Google Scholar]
- 25.Crill PM, Martens CS. Methane production from bicarbonate and acetate in an anoxic marine sediment. Geochim Cosmochim Acta. 1986;50:2089–2097. [Google Scholar]
- 26.Hoehler TM, Alperin MJ, Albert DB, Martens CS. Thermodynamic control on hydrogen concentrations in anoxic sediments. Geochim Cosmochim Acta. 1998;62:1745–1756. [Google Scholar]
- 27.Hoehler TM, Alperin MJ, Albert DB, Martens CS. Field and laboratory studies of methane oxidation in an anoxic marine sediment: Evidence for a methanogen-sulfate reducer consortium. Global Biogeochem Cycles. 1994;8:451–463. [Google Scholar]
- 28.Ferry JG, Lessner DJ. Methanogenesis in marine sediments. Ann N Y Acad Sci. 2008;1125:147–157. doi: 10.1196/annals.1419.007. [DOI] [PubMed] [Google Scholar]
- 29.Glombitza C, et al. Formate, acetate, and propionate as substrates for sulfate reduction in sub-arctic sediments of Southwest Greenland. Front Microbiol. 2015;6:846. doi: 10.3389/fmicb.2015.00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jetten MSM, Stams AJM, Zehnder AJB. Methanogenesis from acetate: A comparison of the acetate metabolism in Methanothrix soehngenii and Methanosarcina spp. FEMS Microbiol Rev. 1992;8:181–197. [Google Scholar]
- 31.Meischner D, Rumohr J. A light-weight, high-momentum gravity corer for subaqueous sediments. Senckenb Marit. 1974;6:105–117. [Google Scholar]
- 32.Seeberg-Elverfeldt J, Schlüter M, Feseker T, Kölling M. Rhizon sampling of porewaters near the sediment-water interface of aquatic systems. Limnol Oceanogr Methods. 2005;3:361–371. [Google Scholar]
- 33.Røy H, Weber HS, Tarpgaard IH, Ferdelman TG, Jørgensen BB. Determination of dissimilatory sulfate reduction rates in marine sediment via radioactive 35S tracer. Limnol Oceanogr Methods. 2014;12:196–211. [Google Scholar]
- 34.Glombitza C, Pedersen J, Røy H, Jørgensen BB. Direct analysis of volatile fatty acids in marine sediment porewater by two-dimensional ion chromatography-mass spectrometry. Limnol Oceanogr Methods. 2014;12:455–468. [Google Scholar]
- 35.Bower CE, Holm-Hansen T. A salicylate–Hypochlorite method for determining ammonia in seawater. Can J Fish Aquat Sci. 1980;37:794–798. [Google Scholar]
- 36.Boudreau BP. Diagenetic Models and Their Implementation: Modelling Transport and Reactions in Aquatic Sediments. Springer; Berlin: 1997. [Google Scholar]
- 37.Iversen N, Jørgensen BB. Diffusion coefficients of sulfate and methane in marine sediments: Influence of porosity. Geochim Cosmochim Acta. 1993;57:571–578. [Google Scholar]
- 38.Kallmeyer J, Ferdelman TG, Weber A, Fossing H, Jørgensen BB. A cold chromium distillation procedure for radiolabeled sulfide applied to sulfate reduction measurements. Limnol Oceanogr Methods. 2004;2:171–180. [Google Scholar]
- 39.Jørgensen BB. A comparison of methods for the quantification of bacterial sulfate reduction in coastal marine sediments. Geomicrobiol J. 1978;1:11–27. [Google Scholar]
- 40.Hansen LK, Jakobsen R, Postma D. Methanogenesis in a shallow sandy aquifer, Rømø, Denmark. Geochim Cosmochim Acta. 2001;65:2925–2935. [Google Scholar]
- 41.Johnson JW, Oelkers EH, Helgeson HC. SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000°C. Comput Geosci. 1992;18:899–947. [Google Scholar]
- 42.Shock EL, Helgeson HC. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Standard partial molal properties of organic species. Geochim Cosmochim Acta. 1990;54:915–945. [Google Scholar]
- 43.Shock EL, Helgeson HC. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Correlation algorithms for ionic species and equation of state predictions to 5 kb and 1000°C. Geochim Cosmochim Acta. 1988;52:2009–2036. [Google Scholar]
- 44.Shock EL. Organic acids in hydrothermal solutions: Standard molal thermodynamic properties of carboxylic acids and estimates of dissociation constants at high temperatures and pressures. Am J Sci. 1995;295:496–580. doi: 10.2475/ajs.295.5.496. [DOI] [PubMed] [Google Scholar]
- 45.Helgeson HC. Thermodynamics of hydrothermal systems at elevated temperatures and pressures. Am J Sci. 1969;267:729–804. [Google Scholar]
- 46.Jensen JB, Moros M, Endler R. IODP Expedition 347 Members The Bornholm Basin, southern Scandinavia: A complex history from late Cretaceous structural developments to recent sedimentation. Boreas. 2016;46:3–17. [Google Scholar]
- 47.Andrén T, Barker Jorgensen B, Cotterill C, Green S, Slomp C. IODP expedition 347: Baltic Sea basin paleoenvironment and biosphere. Sci Drill. 2015;20:1–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.