Significance
Confocal microscopy provides high-resolution images of highly curved interfaces with the surprising result that the morphology of phase-separated lung surfactant monolayers changes dramatically as the interfacial curvature approaches alveolar dimensions (radius, ∼100 µm). This is due to the anisotropic bending properties of the semicrystalline liquid-condensed (LC) domains that lead to a transition between discrete, discontinuous domains on a flat interface to an interconnected meshwork on alveolar-size bubbles. This may have important implications toward understanding the dilatational properties of the monolayer-covered interfaces which can dictate lung stability during breathing.
Keywords: Survanta, phosphatidylcholine, palmitic acid, anisotropy, capillary pressure
Abstract
The morphology of surfactant monolayers is typically studied on the planar surface of a Langmuir trough, even though most physiological interfaces are curved at the micrometer scale. Here, we show that, as the radius of a clinical lung surfactant monolayer-covered bubble decreases to ∼100 µm, the monolayer morphology changes from dispersed circular liquid-condensed (LC) domains in a continuous liquid-expanded (LE) matrix to a continuous LC linear mesh separating discontinuous LE domains. The curvature-associated morphological transition cannot be readily explained by current liquid crystal theories based on isotropic domains. It is likely due to the anisotropic bending energy of the LC phase of the saturated phospholipids that are common to all natural and clinical lung surfactants. This continuous LC linear mesh morphology is also present on bilayer vesicles in solution. Surfactant adsorption and the dilatational modulus are also strongly influenced by the changes in morphology induced by interfacial curvature. The changes in morphology and dynamics may have physiological consequences for lung stability and function as the morphological transition occurs at alveolar dimensions.
Surfactant monolayers have been studied on planar air–water interfaces since the pioneering work of Agnes Pockels, who published the first observations of the relationships between molecular area and surface tension, γ, in 1891 (1, 2). Langmuir and Blodgett expanded on Pockels’ work by investigating the morphology and dynamics of interfacial monolayers on planar air–aqueous interfaces by introducing the Langmuir–Blodgett trough (3), which remains the method of choice today (4–12). However, highly curved surfactant-covered interfaces are ubiquitous in physiology and technology in emulsions (13), foams (14), aerosols (15), and at the air–liquid interface of lung alveoli (16). Curved pendant drops (17, 18) and sessile bubbles (19) have been used to determine the surface tension of various pure liquids and surfactant-coated interfaces, but imaging highly curved interfaces has been challenging (20). As a result, monolayer properties on curved interfaces of bubbles and drops have often been explained using findings from the planar interface, assuming minimal effects of curvature, even though a curved monolayer-covered interface has additional thermodynamic degrees of freedom (21) compared with the planar interface (2, 4–6, 22).
We find that the morphology and dynamics of monolayers of the clinical lung surfactant Survanta change dramatically as the bubble radius R (curvature, ∼1/R) decreases to <100 µm. Survanta monolayers separate into coexisting semicrystalline “liquid-condensed” (LC) and disordered “liquid-expanded” (LE) phases over a wide range of surface tension and temperature (16, 23, 24). The saturated dipalmitoylphosphatidylcholine (DPPC) and palmitic acid (PA) cocrystallize in the LC domains in a tilted hexagonal lattice leading to anisotropic viscoelastic properties (25, 26). On planar interfaces, the LC domains have a long-range electrostatic dipole–dipole repulsion (7–11) and do not coalesce and remain discrete, even at high area fractions, for hours to days, even though there is a measurable “line tension” that acts to minimize the domain perimeter and favors coalescence (10, 11, 27–31). This is typical for any monolayer with LC–LE coexistence, regardless of composition (7–11). However, as the radius of a monolayer-covered bubble decreases, the domain connectivity is reversed from LE continuous on the planar surface to LC continuous as the bubble radius shrinks to alveolar dimensions at a fixed surface tension. The LC domains change shape from circles to stripes, even though the electrostatic repulsion between domains persists. Unlike similar circle-to-stripe transitions in monolayers that are a signature of liquid–liquid miscibility critical points (32), an ordered, semicrystalline LC phase and a disordered LE phase cannot have a critical transition. This change in morphology is likely a consequence of coupling the interfacial curvature to the anisotropic properties of the LC domains. This dramatic change in morphology and connectivity of the LC phase also alters the dynamic properties of the monolayer, in particular the dilatational modulus, , which relates the dynamic change in surface tension with change in bubble surface area. The LC continuous monolayers on the alveolar sized bubbles (R ∼ 100 µm) have a significantly larger dilatational modulus than the LE continuous monolayers on larger bubbles or flat interfaces. While there are examples of coupling molecular shape to the local curvature at the nanometer scale, such as spontaneous vesicles (33, 34) or modulated phases in monolayers and bilayers (35, 36), we are not aware of other examples of how curvature on the 100-μm scale modifies monolayer morphology and dynamics.
Results
To image the static and dynamic effects of interfacial curvature, we designed a bubble-based curvature modulation (BBCM) instrument (37) to examine the properties of monolayer-covered bubbles with the laser-scanning confocal fluorescence microscope (Fig. 1). Hemispherical air–water interfaces were created by applying a controlled capillary pressure (38), P, to generate air bubbles at the end of a hydrophobic microneedle (inner diameter range from 10 to 500 µm) in a polytetrafluoroethylene (PTFE) chamber filled with an aqueous buffered surfactant solution. This measured capillary pressure determines the stress across the bubble interface (39, 40). The bubble radius, R, which is measured by a second microscope, is coupled to P and the surface tension, by the Laplace equation, , in the limit that the Bond number The bubble is centered under a water-immersion objective lens; the laser-scanning confocal microscope allows for optical sectioning from the end of the needle at which the bubble is pinned, to the apex of the bubble, at submicron resolution. On formation of a fresh bubble, surfactant in the aqueous solution adsorbs to the interface at a fixed capillary pressure, causing the bubble radius to decrease along with the surface tension, before reaching a steady radius and surface tension (Fig. 2). Confocal imaging is done on immobile bubbles firmly pinned to the microneedle that have a stable radius and surface tension set by the controlled capillary pressure; only Brownian motion of the interfacial domains is observed, consistent with a uniform surface tension across the interface (Fig. S1 and Movies S1–S4).
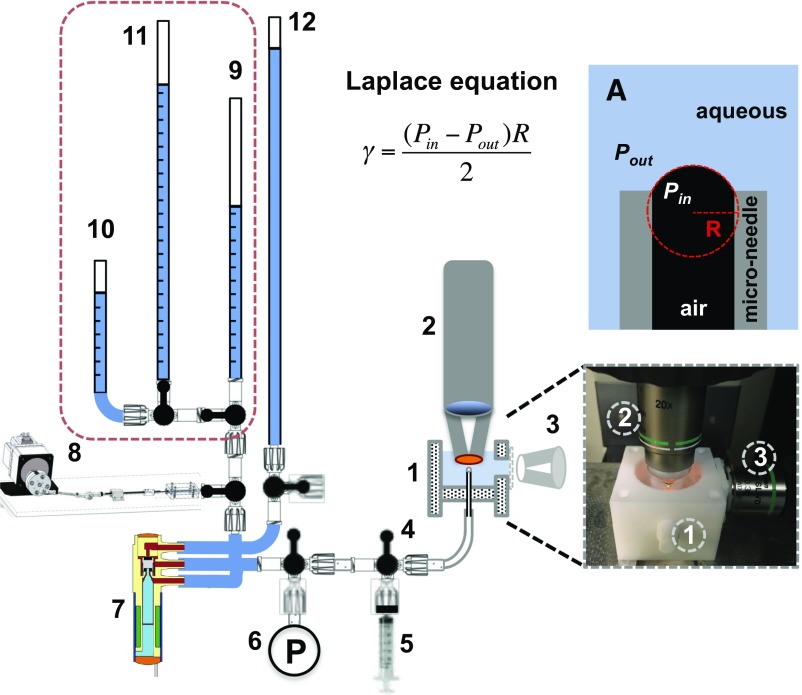
Fig. 1.
Schematic diagram of BBCM apparatus. (A) The difference between the internal, Pin, and external pressure, Pout, determines the capillary pressure, P = Pin − Pout. The capillary pressure, surface tension, and bubble radius are related by the Laplace equation. (Inset) Photograph of PTFE sample chamber (#1), water-immersion lens for confocal microscopy of curved interface of bubble (#2), and SLWD objective lens for bright-field imaging of bubble (for radius determination) (#3). PTFE tubing and Luer locks (#4) connect the bubble in the cell to a 0- to 6,900-Pa pressure transducer (P) (#6) and a three-way solenoid valve (SV) (#7). (#8) Mechanism for oscillating the capillary pressure in the bubble. The pressure transducer measures and records the pressure (Pin) applied to the bubble through the various hydrostatic pressure heads (#9–12). Syringe (#5) is used to rapidly purge the lines between experiments.
Fig. 2.
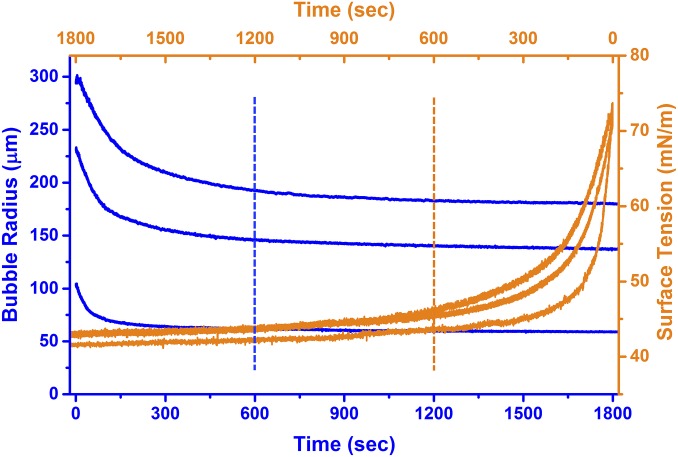
Representative adsorption kinetics of Survanta surfactant on selected sizes of bubbles. The bubble radius decreases from an initial radius, Ri, when freshly pinned and clean (time t = 0), to a final radius, Re, as Survanta adsorbs to the bubble. The measured surface tension reaches the same value of 45 ± 1 mN/m in about 10 min (indicated by dotted lines) for all bubble sizes. After 60 min, the equilibrium surface tension was 39 ± 1 mN/m for all bubbles. The bubble radius and surface tension are related by Laplace’s equation: P = 2γ/R.
Survanta, a clinical replacement human lung surfactant, was used as a model system to form monolayers of coexisting LE and LC phases by spontaneous adsorption from a 100 µg/mL surfactant in saline buffer solution. Survanta is used for treating premature infants suffering from neonatal respiratory distress syndrome (41) and contains a significant fraction of saturated phospholipids, primarily DPPC and PA, along with smaller fractions of unsaturated phospholipids (41). Survanta also contains a small fraction (<1 wt%) of the surfactant-specific proteins SP-B and SP-C that facilitate adsorption to the air–aqueous interface. Survanta at 100 µg/mL concentration reduces the normal air–water surface tension of γW = 72 ± 1 mN/m on a fresh bubble to 45 ± 1 mN/m after 10 min and to an equilibrium surface tension, γE = 39 ± 1 mN/m, regardless of the final bubble radius (Fig. 2). Survanta was chosen as a model surfactant because of its facile adsorption to the interface; pure saturated phospholipids or phospholipid mixtures adsorb slowly, if at all (42), and other clinical lung surfactants with smaller fractions of saturated lipids do not have such a wide range of LE–LC coexistence on adsorption (Supporting Information and Fig. S3) (42, 43).
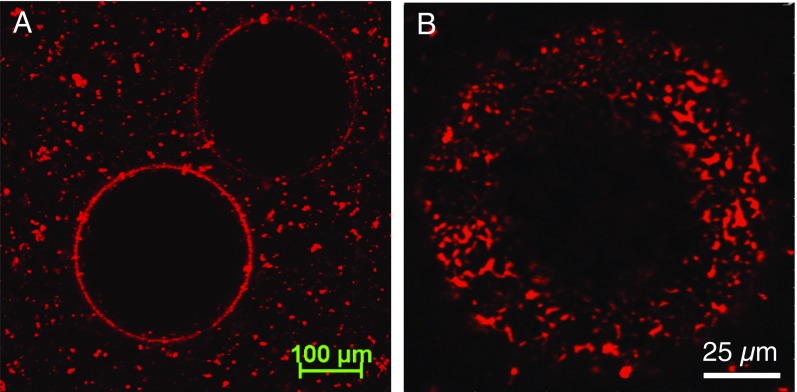
On a planar interface (Fig. 3A), Survanta separates into circular semicrystalline LC domains (black) in a continuous disordered LE phase (red). Contrast in the confocal fluorescence images is due to the exclusion of the Texas Red 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt (TR-DHPE) lipid dye (concentration, 0.4 wt%) from the semicrystalline LC phase domains, which appear black; the fluorescent lipid dye is concentrated in the disordered LE phase, which appears red (7). The LC domains consist primarily of saturated DPPC and PA as shown by grazing incidence X-ray diffraction and pack into a tilted hexagonal lattice with short ranged correlations, very similar to the lattice structure of binary DPPC–PA mixtures (26, 44, 45). The LE phase contains the unsaturated lipids [mainly mono- and di-unsaturated phosphatidylcholines and phosphatidylglycerols (41)] and the SP-B and C proteins. This LE continuous morphology with isolated LC domains is typical for LC–LE phase separation in a number of lipid-only mixtures on the Langmuir trough (25, 26). The relative fraction of LE and LC phases is set by the surface tension and temperature as the molecules in the various domains are in exchange equilibrium (7); this guarantees a uniform surface tension across any static or quasistatic interface. The LC domains undergo a constant Brownian motion, but the long-range electrostatic repulsion between domains keeps them separated (Movie S2).
Fig. 3.
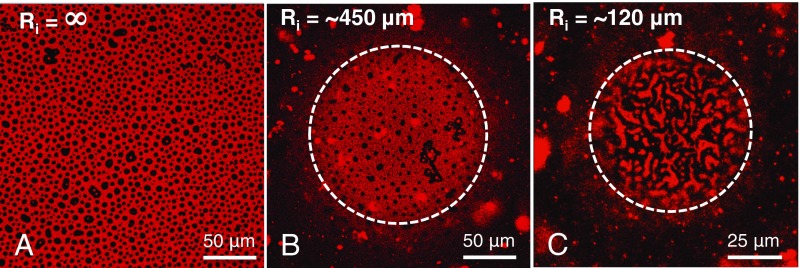
Evolution of morphology after adsorption of Survanta at planar and curved air–aqueous interfaces. (A) Confocal fluorescence microscopy image of a planar interface, Ri = ∞, shows black, circular LC phase domains dispersed in a continuous red LE phase. (B) On the curved interface of a bubble of Ri ∼ 450 µm, the morphology is similar to the planar surface (area within the dotted white line). (C) On smaller bubbles, Ri ∼ 120 µm, the domain morphology changes to a meshwork of linear LC phase domains separating the red LE phase domains. The surface tension is 45 ± 1 mN/m for all three interfaces.
To study the morphology of Survanta films on curved interfaces, we made bubbles of different radii, from ∼450 to ∼25 µm at the tip of hydrophobic capillaries of different inner radii by applying fixed capillary pressures. These small bubbles are sections of hemispheres as the shape is dominated by the surface tension, with negligible distortions due to gravity as the Bond number (39). Fig. 3B shows the phase morphology on a typical bubble of initial radius Ri ∼ 450 µm after 10 min of adsorption (radius, ∼ 280 µm), which is similar to the planar interface, with circular black LC domains in a continuous red LE matrix. In sharp contrast, when Survanta is adsorbed onto a Ri ∼ 120-µm bubble (Re ∼ 75 µm; Fig. 3C) to the same surface tension, the black LC phase organizes into interconnected, furcated stripes, with a small number of small LC domains trapped within the red LE phase that form discontinuous patches, inverting the planar morphology. Movie S1 shows a different Ri ∼ 120-µm bubble in which the LC phase forms an immobile interconnected network; the disconnected LC domains undergo Brownian motion, although they are confined to “corrals” by the LC network.
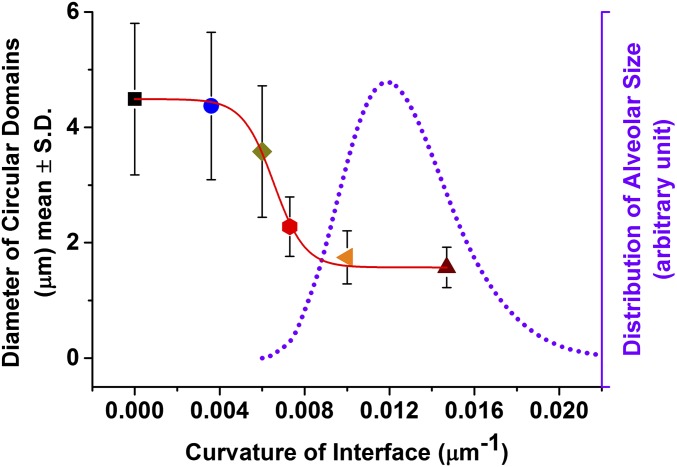
Quantitative analysis of the size of circular domains formed on hundreds of bubbles of different radius showed the impact of curvature on the LC phase organization. Fig. 4 shows that as the equilibrium bubble radius, Re, decreased from 300 to 100 µm (the curvature, 1/Re, increased from 0.003 to 0.01 µm−1), the characteristic domain size and polydispersity abruptly decreased. Also plotted on Fig. 4 is the measured alveolar size distribution of rabbit pups (46). The mean radius of the rabbit alveolus is similar to the human alveolus, R ∼ 100 µm, or a curvature of 0.01 µm−1 (47). Surprisingly, the morphological transition we observe occurs when the bubbles approach alveolar dimensions.
Fig. 4.
Impact of interfacial curvature on the size of circular domains. Quantitative analysis of circular domains observed on interfaces of different curvature (equilibrated to same surface tension, 45 ± 1 mN/m) shows that the diameter and polydispersity of LC domains sharply decrease with increasing curvature. The redrawn alveolar size distribution of rabbit pups from ref. 42 on the graph reveals overlap between the range of bubble curvatures where morphological transition occurs and the alveolar dimensions. The mean curvature of human alveoli is ∼0.01 µm−1 (43).
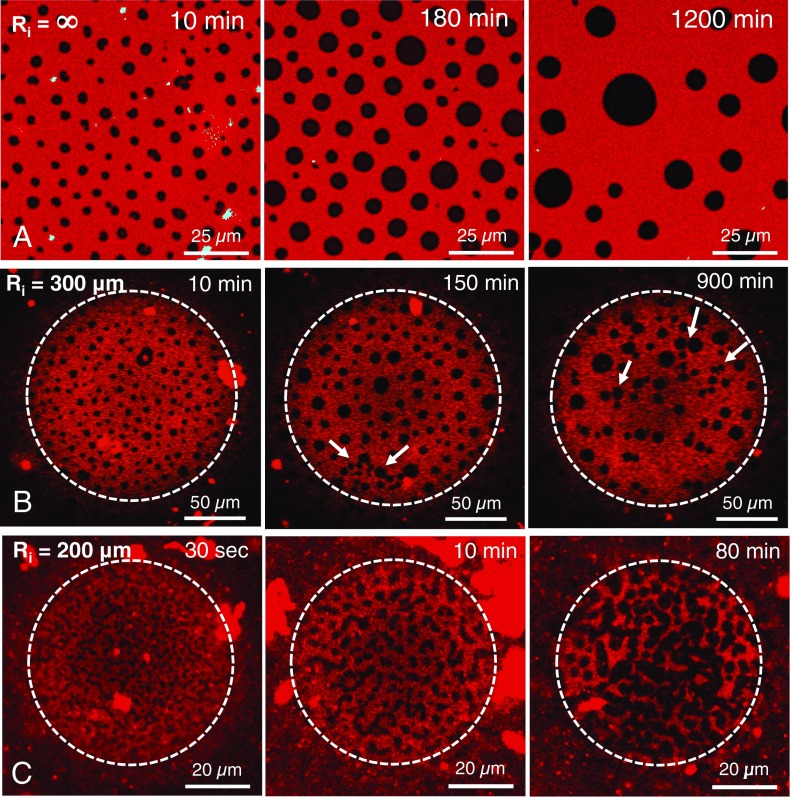
On a planar interface, the black circular LC domains grow by apparent Ostwald ripening over the course of hours, but remain circular and dispersed in a continuous red LE phase (Fig. 5A). The domains do not coalesce, as there is a repulsive electrostatic interaction between the LC domains that keeps them dispersed (Movie S2) (7, 48). Similarly, LC domains on bubbles of Ri > 400 µm remain circular and dispersed over long period of time (analyzed up to 24 h). Bubbles of Ri ∼ 300 µm (Re ∼ 160 µm) initially show formation of circular domains (10-min image in Fig. 5B), as on the flat surface (Fig. 5A). However, 150 min after fresh interface formation, some domains appear coalescing into linear patterns (white arrows in 150-min image in Fig. 5B). Image at 900 min shows that the clustering has proceeded further and longer linear features appear (white arrows in 900-min image in Fig. 5B). Movie S5, a time series of the cross-sections of the equilibrated bubble surface, reveals a coalescence phenomenon happening across the bubble surface. Fig. 5C shows that, for a bubble with Ri ∼ 200 µm, the black domains are not circular even 30 s after adsorption. The domain shapes evolve from disconnected elliptical, dog bone, and star-like shapes into thicker, elongated stripes (10-min image), and begin to link into an interconnected network (80-min image), similar to Fig. 3C. This suggests that the equilibrium domain morphology is different on highly curved interfaces than on planar interfaces.
Fig. 5.
Evolution of monolayer morphology with time on a flat and the curved interfaces. (A) On a planar interface, the domains age by Ostwald ripening, but remain circular and dispersed even after 20 h. (B) On a Ri ∼ 300-µm bubble, there are indications of domain coalescence at 150 min that continues at 900 min (white arrows). See Movie S5 and Fig. S1 for phenomenon across the bubble surface. (C) On a Ri ∼ 200-µm bubble, the domains are not circular even immediately after adsorption and form extended linear structures as they age, suggesting that the equilibrium organization changes with curvature.
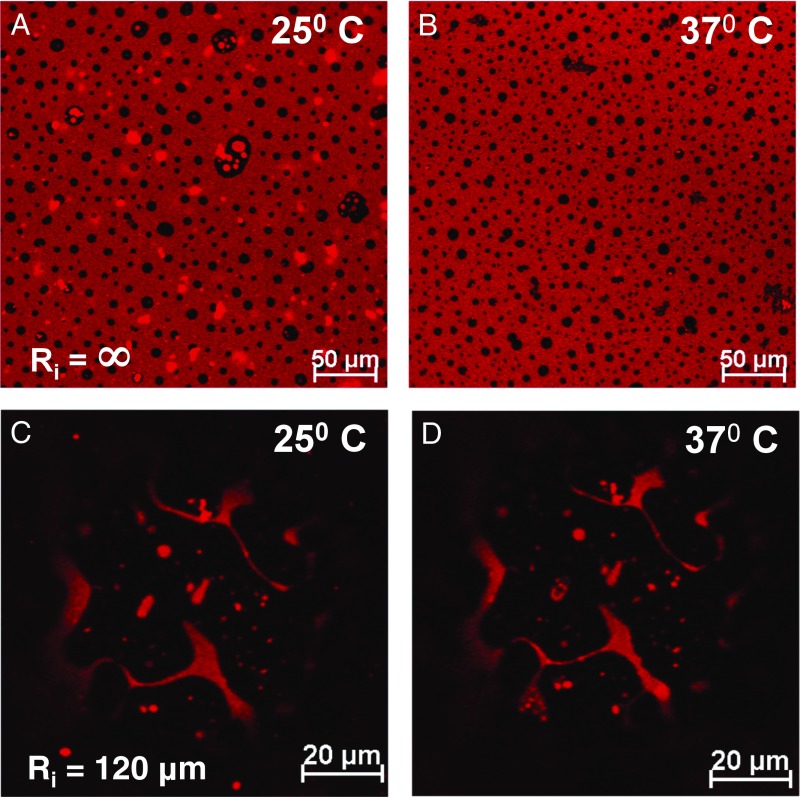
Fig. 6 shows that increasing the temperature from 25 °C to the more physiologically relevant 37 °C has minimum effect on the phase behavior and morphology of Survanta either on flat interfaces (Fig. 6 A and B) or highly curved bubbles (Fig. 6 C and D). Previous work has shown that the LC phase of DPPC and PA is stable to >40 °C, and there is minimal change in the surface pressure–area isotherms or shear viscosity of Survanta monolayers from 25 to 37 °C (44, 45, 49).
Fig. 6.
Impact of temperature on the phase morphology patterns. Changing the temperature from 25 to 37 °C has minimal effect on the morphology on flat, Ri = ∞, interface (A and B), or a highly curved bubble interface (C and D) (Ri = 120 µm). This is consistent with the LC phase being primarily a cocrystal of DPPC and PA that has a phase transition temperature of >40 °C.
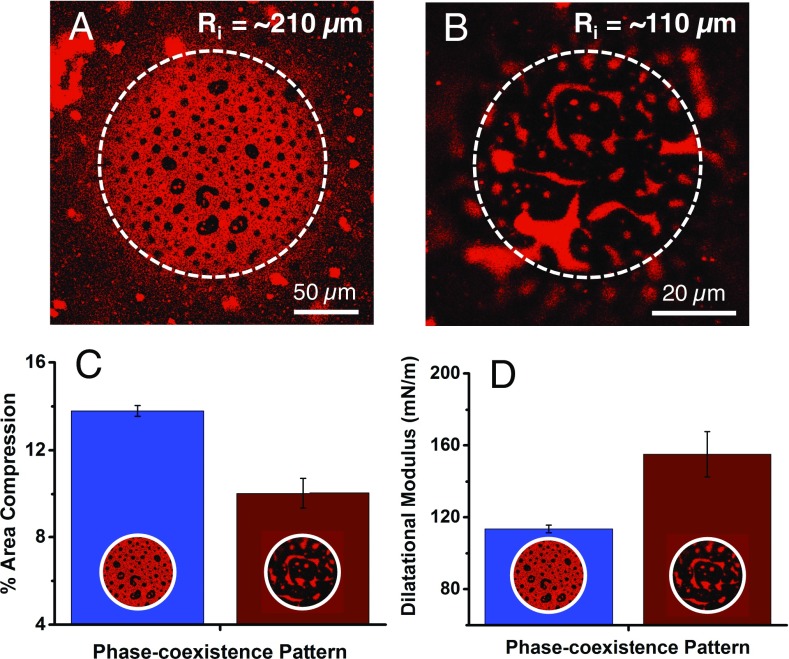
The dynamic responses of monolayers on bubbles of different radius, and hence morphology, were also significantly different (Fig. 7). Bubbles with larger radius (Fig. 7A), which had a continuous LE phase (red), had a much larger relative change in interfacial area than the smaller radius bubbles with a continuous LC phase (Fig. 7B) for the same normalized capillary pressure variation (ΔP/P) (Fig. 7C). The continuous network of LC phase on smaller bubbles (Movie S1) is more resistant to deformation than the continuous LE phase on larger bubbles or a planar interface (Movie S2). The dilatational modulus, , which describes the dynamic change in surface tension with oscillatory, small changes in interfacial area, also changes with bubble size. For small-amplitude bubble oscillations, , in which A0 is the equilibrium surface area at the equilibrium surface tension, . is the change in surface tension for an area change of . Fig. 7D shows that the complex dilatational modulus, , is smaller for the larger bubbles, which have a continuous LE phase.
Fig. 7.
Modulation of interfacial dynamics with curvature and phase morphology. (A) For Ri ∼ 210-µm bubbles, the LC phase domains (black) are discrete and dispersed in a continuous LE phase (red). (B) For Ri ∼120-µm bubbles, the LC phase is continuous and the LE phase is dispersed. (C) For a given change in capillary pressure, P, larger bubbles with a LE continuous morphology (Inset image A) compress significantly more than smaller bubbles with a LC continuous morphology (Inset image B). (D) The complex dilatational modulus is found smaller for the larger bubbles than for the smaller bubbles; the LC phase mesh likely resists compression and expansion (Movies S1 and S2).
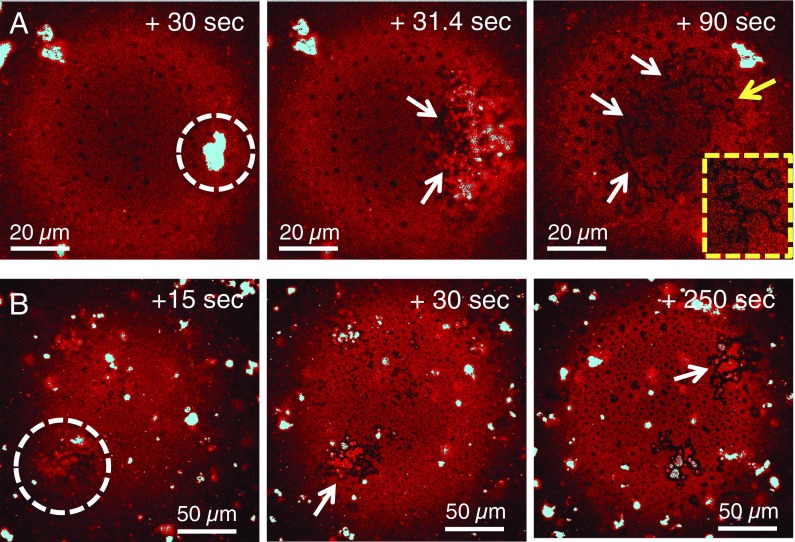
Fig. 8 (and Movies S3 and S4) shows that the adsorption and spreading of Survanta from multilamellar aggregates (cyan-color patches in Fig. 8) in suspension also depend on interfacial curvature. At about 30 s, Fig. 8A shows that a cyan multilamellar Survanta aggregate contacts the Ri ∼ 120-µm bubble (the bubble has already adsorbed some Survanta as can be observed from its red color). A second later, the multilamellar Survanta aggregate has begun to spread across the bubble surface in the form of an advancing front of multilayers (indicated by the varying fluorescence intensity) that by 90 s has converted into a monolayer segregating into linear black LC phase structures (white arrows; Inset provides an enlarged view of the morphology at the yellow arrow). For Ri ∼ 450 µm, Fig. 8B shows that, when a similar Survanta aggregate contacts the larger bubble, spreading from multilayers to monolayers is slower. The new patch of multilayered interface does not spread over the surface even after 250 s. The smaller bubbles immediately show furcated stripe patterns of ordered phase on the interface, while on larger bubbles such stripes are missing and the adsorbed materials are trapped in fluctuating domain shapes that eventually break up and evolve into circular LC phase domains.
Fig. 8.
The rate and pattern of evolution of LC domains at high and low curvature interfaces as the surfactants adsorb. (A) Multilamellar Survanta aggregate (the cyan color of the aggregates indicates saturated pixels on detector due to higher levels of dye in the aggregates) contacts a 120-µm bubble and within seconds has converted into a multilayered patch on the bubble surface (arrows). By 90 s, the aggregate has spread into a monolayer, with linear LC phase domains (Inset). (B) On a 450-µm bubble, an aggregate (dotted circle) has just adsorbed onto the bubble but does not spread into a monolayer even after 250 s (arrow). The irregular LC domains fluctuate and eventually break up and evolve into circular domains.
While most Survanta aggregates in solution showed little detail regarding lateral phase separation in the bilayers (Fig. 8), we occasionally observed giant multilamellar vesicles (MLVs) in the Survanta suspensions that did show phase separation (Fig. 9A). Fig. 9 shows that the bilayer morphology of the Survanta MLV of ∼100-µm radii also has a continuous LC phase (black) separating the red LE phase into discrete islands, which is consistent with the monolayer morphology found on the high curvature interface. Movie S6 shows time series of a section of the MLV surface. This LC continuous texture is similar to those observed by Dimova and coworkers (50, 51) who have observed similar striped patterns in giant unilamellar vesicles of ternary mixtures of dioleyolphosphatidylglycerol, egg sphingomyelin, and cholesterol that form coexisting LC–LE phases.
Fig. 9.
Phase morphology of multilamellar vesicles (MLVs) (curved bilayer model) of Survanta. (A) Circular cross-section of two spontaneously formed MLVs in Survanta suspension. Small red spots are typical multilamellar aggregates as in Fig. 8. (B) High-resolution confocal section of a MLV (∼100-µm radius) showing the continuous LC with discontinuous LE (red regions) phase morphology pattern, as seen for monolayers on bubbles of this size.
Discussion and Conclusions
The morphology of coexisting isotropic domains on a planar interface is governed by a balance between the line tension between domains, , which acts to minimize the total domain perimeter, and the dipole density difference between phases, , which acts to make individual domains smaller (7, 36, 48). These opposing forces lead to an optimal domain diameter, D0:
| [1] |
is the dielectric constant of water, is the permittivity of free space, is the distance between molecular dipoles, and is the exponential (7, 48). Movie S1 shows that, while the LC phase meshwork is immobile on the interface, disconnected LC phase domains in the fluid phase regions move by Brownian motion and are repelled from each other and the LC phase meshwork, and fluctuate around the center of the fluid-phase regions. Movie S2 shows a similar Brownian domain motion on the planar interface that induces a collective motion of the repulsive domains and prevents them from coalescing (52). Any variations in the local surface tension induce a directional flow of the domains. For isotropic domains, noncircular shapes have higher energies and are metastable (7, 48). However, when the temperature or surface tension is rapidly altered, the dimensionless ratio can change, thereby changing D0 in Eq. 1. During Survanta adsorption, the bubble radius and surface tension change over the course of several minutes before equilibrium is achieved (Fig. 2). If changes during the adsorption period, D0 may decrease as the bubble equilibrates. If an existing domain has D > D0, the circular domain shape is unstable and the domain will undergo fluctuations into elliptical, stripe, or more complex shapes (48). However, these shapes are higher in energy than circles, and on a planar interface, the monolayer adjusts over time to form the minimum energy domains with D0 defined by Eq. 1. However, Figs. 3C and 5C show that monolayers on small bubbles not only retain their linear, mesh-like morphology, but the linear features grow in time as the bubble age, unlike on the planar interface. If the noncircular features were metastable, a reversion to circular domains would occur on equilibration (7), which we do not observe. Rather, Fig. 5C shows the linear features lengthen and thicken, consistent with a different equilibrium morphology on curved interfaces.
As the bubbles grow smaller, the monolayer curves more; the associated Helfrich curvature energy, Ec, of an isotropic monolayer is as follows (53):
| [2] |
and are the bending and Gaussian curvature moduli, respectively, and R0 is the spontaneous radius of curvature; these material parameters depend on monolayer composition, phase behavior, temperature, and surface tension (34, 53). If the monolayer conforms to the spherical bubble, both radii of curvature equal the bubble radius, R1 = R2 = R. Hence, in the absence of a spontaneous curvature , the curvature energy of a domain is independent of the bubble radius: , in which is the solid angle that subtends the domain, which is independent of the shape of domains of similar area. For a nonzero spontaneous curvature, ; the bending energy is minimized when R0 = R, but remains independent of the domain shape.
Hence, the curvature energy does not distinguish between stripes or circles if the monolayer is conformal; but can a sufficiently rigid monolayer locally deform a bubble? The ratio of bending modulus and the surface tension gives a curvature–capillary crossover length, . For fluid DPPC bilayers, ∼ 10−19 J (53), which for = 40 mN/m, gives a lower bound for Lc ∼ 2 nm. As an upper bound, we estimate the bending modulus of a lamellae of single-crystal polyethylene of thickness, t: , in which E is the Young’s modulus and is Poisson’s ratio (0.4 for polyethylene). For oriented crystalline chains of polyethylene, E ∼ 300 GPa (54); for a monolayer thickness of ∼1 nm, ∼ 3 × 10−17 J, which gives 0.03 µm. Even a crystalline monolayer has a crossover length orders of magnitude smaller than the micrometer-size LC phase domains we observe (see Supporting Information and Fig. S1 for more detailed analysis). At the needle tip, where the bubble is pinned, the bubble deformation cannot be purely dilatational, as the radial strain is confined by the needle, while the axial strain is not. However, a similar analysis suggests that this strain imbalance also only propagates a distance of order Lc, and the dominant fraction of the bubble undergoes pure dilatation (42).
Experimentally, we fit the top portion of the bubble surface (well away from the pinning site) to a circle with a resolution of 0.12 µm using a second microscope (Fig. 1) while simultaneously imaging the distribution and shape of domains across the entire interface with the confocal microscope (Fig. S1); we see no variations in the spherical shape across the surface, even up to the circular pinning site on the needle. The surface tension across the interface also rapidly equilibrates via Marangoni-type flows across the interface; our measurements are made after any such flow ceases. The interface maintains its hemispherical shape and the relationship between capillary pressure, surface tension, and radius can be described by the Young–Laplace equation (39). However, micrometer-thick, cross-linked, purely elastic films grown on pendant liquid drops can have Lc on the same order as the drop dimensions, resulting in more complex, and even wrinkled shapes that are not governed by the Young–Laplace equation (17, 18). On the opposite extreme, bilayer vesicles in solution can have an effective surface tension of zero (equivalent to a zero osmotic pressure difference across the bilayer). The competition between line tension (λ ∼ 1 pN) and bending energy ( ∼ 10−19 J) can lead to budding and nonspherical vesicle shapes, but only for fluid monolayers with low bending energies (28) for domains larger than ∼ 100 nm.
Our analysis shows that isotropic LC phase domains should not undergo the morphological transition we observe with changing interfacial curvature (Fig. 3). However, X-ray diffraction of the LC phase domains reveals that the alkane chains are tilted with respect to the monolayer normal with an anisotropic short-range positional order of ∼10 lattice repeats in the tilted direction and ∼100 in the untilted direction (25, 44). Hence, the molecular packing in the tilted direction is less ordered (more liquid-like) than in the untilted direction (more crystalline), which suggests that bending modulus in the tilted direction is likely smaller than the bending modulus in the untilted direction. The spontaneous curvature, which also depends on the local molecular packing (34), might also be different in the tilted and untilted direction. The resultant anisotropic bending energy may alter the balance between the line tension and dipole density difference to cause the domains to elongate into stripes to minimize the overall energy as the interface curves. Anisotropy in the line tension or the dipole density difference (7, 26) can lead to noncircular domains on planar interfaces (Fig. S2), but Survanta domains do not show anisotropy until the bubbles have sufficient curvature.
The meshwork structure seen on the small bubbles may provide a mechanism of resistance to alveolar collapse at the end of exhalation and store elastic energy that may help reexpand the alveoli on inhalation. The mesh would minimize monolayer flow out of the alveoli, which may provide the necessary resistance to possible Marangoni flows due to the surface tension gradients between the deep lung and the bronchi.
The morphology and connectivity of the LC phase also causes the dilatational modulus, , to change with bubble radius. In the lungs, if were constant, smaller alveoli of radius RS would deflate due to their increased Laplace pressure, , relative to slightly larger alveoli of radius RL, which would overinflate. This Laplace instability would cause alveolar collapse, which is a symptom of acute respiratory distress syndrome, which affects 150,000 per year in the United States, with a mortality rate of ∼40% (55). However, the Laplace instability is eliminated if For > 0, the smaller alveoli no longer have a higher internal pressure than the larger alveoli, which stabilizes the lung. However, during inspiration, lung surfactant must respread to cover the expanding interface, which is enhanced by a low dilatational modulus (38), suggesting that an optimal, but as still unknown, range of is required for lung function.
Materials and Methods
Water with a resistivity of 18.2 MΩ·cm at 25 °C was purified with a Millipore Direct Q 3UV-R (Millipore) system. Sodium chloride (NaCl), calcium chloride (CaCl2), and 3-(N-morpholino) propanesulfonic acid sodium salt (MOPS) were purchased in powder form from Sigma-Aldrich and used to prepare MOPS saline buffer (150 mM NaCl, 2 mM CaCl2, 1 mM MOPS, and pH 7.0). TR-DHPE fluorescent dye was purchased from Life Technologies and used as received.
Survanta (AbbVie) was purchased from Boynton Health Pharmacy. Survanta (beractant) is sold as aqueous suspension in 0.9% sodium chloride solution. The resultant formulation contains ∼65–75% (by weight) saturated phospholipids, primarily DPPC, ∼5–10% fatty acids, mainly PA, and <1 wt% (<1.0 mg/mL) protein. Its protein content consists of two hydrophobic, surfactant-associated proteins commonly known as SP-B and SP-C. There is negligible cholesterol in Survanta (41). The 100 µg lipids per milliliter working solutions are prepared by diluting the purchased stock of Survanta (∼25 mg phospholipids per milliliter, suspended in 0.9% sodium chloride) in the appropriate amount of MOPS buffer; Survanta solutions are stored at 4 °C and allowed to come to room temperature before use. Survanta is organized as multilamellar aggregates in suspension. Previous work has shown that the LC–LE coexistence of Survanta does not change significantly with temperature from 20 to 40 °C (44).
Stainless-steel needles with inner diameters of 30, 50, 110, 160, 185, 210, 310, and 410 µm were purchased from Hamilton Company. Polymer microtubes of PTFE and natural polyether-ether ketone (PEEK) materials with inner diameters of 20, 50, 75, 100, 125, and 150 µm were purchased from Cole-Parmer and VICI Metronics. Fittings for PTFE and PEEK microtubes were bought from Swagelok Minnesota and Valco Instruments, respectively.
Design and Fabrication of BBCM Assembly.
Fig. 1 shows the key features of the BBCM apparatus (37), which is designed to fit under a customized laser-scanning confocal fluorescence microscope head. A dedicated cell (#1 in the schematic and photo in Fig. 1) of outer dimensions 5 × 5 × 4 cm is machined from a single block of hydrophobic and chemically inert PTFE. The PTFE cell has an inner sample chamber of 3-cm diameter and 1.5-cm depth, which contains a 1-cm-wide window on one wall. The top opening in the sample chamber is sized to allow for a water-immersion lens (#2) to be oriented vertically over the bubble at the end of the needle. A stainless-steel microneedle or PEEK or PTFE microtube of a desired diameter is inserted through a port on the bottom of the chamber, which is capable of holding adapters for leak-proof fitting of microneedles of different outer diameters. A borosilicate or Pyrex glass disk (Chemglass Life Sciences) is clamped on the outer wall of 1-cm-wide window using PTFE screws to create a transparent viewing window for a lens and low-resolution camera (#3) to visualize the entire bubble to measure the bubble radius and validate the spherical shape and pinning conditions at the needle tip.
PTFE tubing and Luer locks (#4) connect the bubble in the cell to a 0- to 6,900-Pa pressure transducer (P) (#6) and a three-way solenoid valve (SV) (#7). The pressure transducer measures and records the pressure (Pin) applied to the bubble through the various hydrostatic pressure heads (#9–11). The pressure external to the bubble (Pout) is kept constant by keeping the level of aqueous subphase in the sample chamber at constant height (4- to 5-mm water head or 40–50 Pa). The second port of the SV (normally open) is connected to the primary hydrostatic pressure column (#9). The water column height Ho (± 0.5-mm water head or ±5 Pa) is used to determine Pin and set the initial bubble radius (Ri) and curvature (C = 1/Ri) (Inset sketch A). The third port of the SV is connected to a high pressure (i.e., taller) water column (#12), which is used to quickly blow out the old bubble before forming a new bubble. The primary hydrostatic pressure column (#9) is connected to two additional hydrostatic pressure columns by valves: one is set so Hs < Ho (#10) and the second is set so HL > Ho (#11). This allows for a rapid, fixed change in Pin and a rapid change in bubble radius. All connections are made using PTFE tubing. Fittings, needles, and tubes connected to the inner chamber of PTFE cell are thoroughly cleaned multiple times in an ultrasonic bath using hydrocarbon and polar solvents and subsequently rinsing in Milli-Q water. Gauge pressure transducers of 25-cm water (2,000-Pa) range and 0- to 1-psi range (7,000 Pa) were purchased from Omega Engineering; the three-way SV was purchased from The Lee Company; and the peristaltic pump was purchased from Cole-Parmer.
A sinusoidal pressure oscillation mechanism is built using a brushless DC motor, capable of 4–200 rpm (Oriental Motor), an external motor shaft hub, and microsyringe (37). The shaft hub fits on the gear head of the motor and moves the piston of the microsyringe periodically to generate oscillatory pressure waves by changing the Pin. Different torque lengths on the shaft hub and/or microsyringes of different volumes are used for achieving the desired amplitude and frequency of the pressure oscillations. Oscillation frequencies range from 4 to 200 cycles per min.
Bright-Field Imaging of Bubble.
A high-speed optical imaging system (#3 in Fig. 1) (nominal image pixel size of 0.12 µm) is used for real-time tracking of the bubble radius, R, which, when combined with the pressure measurement from the transducer, Pin, allows for calculation of the dynamic surface tension (γ) at the bubble interface, as well as measurement of the dilatational moduli, ε. An in-line optical assembly InfiniTube (Edmund Optics) is connected to a Nikon T Plan SLWD 20× objective lens at one end and a Chameleon3 Color USB3 Vision camera (55 fps at a resolution of 2,048 × 1,536 pixels; 3.2 megapixels total) on the other. The lens and camera system is oriented next to the 1-cm-wide window of PTFE chamber on a three-axis translation stage with standard micrometers mounted on a rigid stand with platform (Thorlabs).
A computer interface is written in LabView 13.0 [National Instruments (NI)] for fitting the edge of the bubble to a circle to evaluate the bubble radius, R, from real-time bubble images and for calculating the surface tension (γ) in combination with the pressure measurements from the transducer. Bubble images are acquired at ∼60 fps at 512 × 512 resolution through the Chameleon3 Color USB3 Vision camera, and the radius from each bubble image/frame is instantaneously calculated using a NI-Vision development module. Deviation from the spherical shape of the bubble is monitored by means of “goodness-of-fit” values. All bubbles used in experiments were fit as a spherical cap with no systematic deviations in curvature over the entire bubble. A NI-DAQ device was used to port the pressure transducer data to the LabView program. The pressure transducer records the hydrostatic pressure, , applied to the bubble. The surface tension across the curved interface is calculated from the Laplace equation in real time from the measured values of the capillary pressure and the bubble radius, in which .
The dilatational modulus is calculated off-line from the surface tension, pressure, and radius data. The surface area of the spherical interface of bubble, A, is calculated using the following equation for a spherical cap:
| [3] |
in which r is the inner radius of the microneedle and h is the height of the apex of the bubble above the needle. As the bubble radius decreases, the exposed bubble surface area increases. The bubble surface area is greatest when the bubble radius equals the needle radius, R = r, with A = 2πr2, and is a minimum when the interface is planar (r/R << 1), with A = πr2.
The apparent dilatational modulus, ε, is calculated from the change in surface tension (Δγ) for a periodic change in interfacial area, ΔA:
| [4] |
Ao is the bubble surface area before oscillation. For the small bubbles we use, the effects of gravity, g, are eliminated as the Bond number and the bubble takes on a spherical shape. We measure the capillary pressure difference across the bubble, which gives a direct measure of the interfacial stress for any type of interface, including viscoelastic interfaces. Before, during, and after the area oscillations, we image the entire 3D shape of the bubble, so as to identify any nonspherical deformations; maintaining the spherical bubble shape ensures a purely dilatational deformation. At the needle tip, where the bubble is pinned, the bubble deformation cannot be purely dilatational, as the radial strain is confined by the needle, while the axial strain is not. However, this strain imbalance only propagates a distance of order of the curvature–capillary crossover length, , and the fraction of the bubble not undergoing pure dilatation is ∼Lc/R << 1 (42). As such, any anisotropic strains imposed by the pinning site dissipate over tens of nanometers, which makes them <1% of the film surface area; this is why our bubbles appear spherical down to the pinning site on the needle (Fig. S1). The amplitude of the periodic pressure modulation is adjusted to achieve the desired ΔA (56). For the complex dilatational modulus data presented in Fig. 7, the film is oscillated at a frequency of 0.234 Hz and ΔA ∼ 0.05–0.1 Ao. These parameters were kept constant when comparing different types of interfacial films. To compare the relative area compression, a large-amplitude pressure modulation, ΔP/P ∼ 0.35, was applied, while other experimental parameters were kept the same. All measurements are performed at room temperature (23.5 ± 0.5 °C).
Preparing a Clean “Planar” Interface.
To prepare a clean planar interface, the interface is aspirated using suction through a clean glass pipette. The surface tension on a planar air–aqueous interface is measured using a Wilhelmy filter paper tensiometer (Riegler and Kirstein). A clean air–water interface yields a surface tension γ = 72.5 ± 0.5 mN/m at room temperature. Adsorption of Survanta to the air–aqueous interface begins as soon as the surfactant solution is added to the sample chamber.
Preparing Clean “Spherical” Interfaces.
A new bubble is generated by connecting the high-pressure hydrostatic line (#12 in Fig. 1) via the solenoid valve for >20 ms. This process expels the old bubble from the microneedle and pins a fresh bubble of a desired curvature with a clean air–aqueous interface when the valve is closed. Surface tension calculation of a clean air–water interface of a freshly pinned bubble in Milli-Q water or MOPS buffer (control experiments) yields 72.5 ± 0.5 mN/m at room temperature, which is in good agreement with known values. We found that pinning a fresh bubble with clean interface becomes difficult if a high concentration of Survanta surfactant is present in the chamber. Therefore, after multiple trials, 100 µg lipids per milliliter was chosen as an optimum Survanta concentration for this study.
Survanta adsorption to the bubble causes a continuous decrease in the bubble radius (i.e., increase in curvature) as the surface tension is reduced (Fig. 2). We refer to the radius of the clean bubble at formation as the initial radius, Ri (time = 0 in Fig. 2). The radius and surface tension decreases quickly for the first 10 min of adsorption, reaching an equilibrium radius, Re, after about 60 min. From the measured radius and capillary pressure, the surface tension is calculated from Laplace’s equation, , and it reaches 45 ± 1 mN/m for all bubbles after about 10 min and 39 ± 1 after 60 min. If the bubble attains a hemispherical shape (R = r) before reaching the minimum surface tension, the bubble pops out of the needle.
Laser-Scanning Confocal Fluorescence Microscopy.
A C1 confocal scan head fitted on a Nikon Eclipse 80i upright microscope (Nikon Instruments) was used for imaging. The microscope was controlled with Nikon EZ-C1 software. On planar interfaces, a Nikon Plan Apochromatic 20× air-immersion objective, corrected for spherical aberration with a thin glass disk, was used for confocal imaging. For imaging the bubble interface, water-immersion Nikon Fluor 20× differential interference contrast (DIC) and Nikon Fluor 60× DIC objective lenses were used. An absolute magnification calibration for each objective lens in their working medium and conditions was determined before imaging.
Image contrast was due to the preferential segregation of 0.4 wt% TR-DHPE between the ordered and disordered phases of Survanta monolayers. TR-DHPE lipid dye was dissolved in ethanol and added to the diluted Survanta dispersion (100 µg lipids per milliliter). The lipid dye quickly partitioned into the Survanta bilayers. Disordered phase monolayers appear red, while ordered domains exclude the dye and appear black. The black LC phase domains are primarily DPPC and PA, while the red liquid phase contains the unsaturated lipids and surfactant proteins. Movies are from compiled images taken at a frame rate of ∼1.15 s, with each frame 512 × 512 pixels. Movies are either played at original frame rate or 2× or 4× or 8× faster than real time.
For high-speed confocal fluorescence microscopy, Nikon FN1 A1RMP confocal microscope equipped with a hybrid A1R laser-scanning confocal head that incorporates both an ultrahigh-speed resonant scanner and a high-resolution galvano scanner was used. The system is equipped with 405-, 488-, 457-, 514-, 561-, and 640-nm laser lines. A motorized prior stage and piezo Z drive control sample positioning and focus. For imaging at planar interface, Nikon Plan Apochromatic 20× air-immersion objective corrected for spherical aberration with a thin glass disk, and for imaging at curved interfaces, water-immersion Nikon Fluor 20× DIC or Nikon Fluor 60× DIC objective lenses were used. The resonant scanner in-band mode was used with 2–16× averaging to obtain suitable frame rate. All images were acquired at resolution of 512 × 512 pixels. Pixel size was adjusted by zooming in with the laser. For recording multiple consecutive Z stacks, step size of 1 µm was chosen. The microscope was controlled with NIS Elements 4.6 software.
Imaging of Survanta MLVs (Bilayer Model).
A closed but transparent chamber filled with Survanta surfactant was prepared to image the phase morphology of Survanta MLVs, the curved bilayers. The top opening of PTFE chamber, part of BBCM assembly, was covered using ∼170-µm-thick clean glass coverslip, and then Survanta surfactant was injected in the closed chamber using microneedle of lower gauge size (>20 gauge) through the bottom port. Z stacks of the adhered Survanta MLVs on the top glass coverslip were imaged using Nikon Plan Apochromatic 20× air-immersion objective. If MLVs were not found adhered on the glass coverslip, the filled chamber was mildly shaken to transfer the MLVs toward top region.
Effect of Temperature on Phase Morphology.
The effect of temperature was studied on the equilibrated Survanta monolayer formed at room temperature on planar and curved interfaces. The subphase was exchanged with preheated buffer to desired temperatures. After exchange of buffer, monolayer was observed until minimum of 2 h at the elevated temperature.
Curvature Modulation Experiment.
A rapid change in the interfacial curvature of an already pinned bubble is done by exploiting the valves present between the three hydrostatic pressure columns. Swapping between columns #9 and #11 (Fig. 1) changes the applied hydrostatic pressure on the pinned bubble, which induces a rapid increase or decrease in the capillary pressure and bubble radius.
Supplementary Material
Acknowledgments
We thank Benjamin Stottrup, Todd Squires, David Morse, Ka Yee Lee, and Sarah Keller for their insights into monolayer morphology and dynamics, and Lynn Walker, Shelley Anna, and their students for their help in building the experimental apparatus and an introduction to dilatational rheology. High-speed confocal imaging was done at the University of Minnesota Imaging Center. We acknowledge support from National Institutes of Health Grants HL 51177 and HL 135065 and National Science Foundation Grant CBET 170378.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1715830115/-/DCSupplemental.
References
- 1.Pockels A. On the relative contamination of the water-surface by equal quantities of different substances. Nature. 1892;46:418–419. [Google Scholar]
- 2.Pockels A, Rayleigh L. Surface tension. Nature. 1891;43:437–439. [Google Scholar]
- 3.Blodgett KB. Films built by depositing successive monomolecular layers on a solid surface. J Am Chem Soc. 1935;57:1007–1022. [Google Scholar]
- 4.Takamoto DY, et al. Stable ordering in Langmuir-Blodgett films. Science. 2001;293:1292–1295. doi: 10.1126/science.1060018. [DOI] [PubMed] [Google Scholar]
- 5.Zasadzinski JA, Viswanathan R, Madsen L, Garnaes J, Schwartz DK. Langmuir-Blodgett films. Science. 1994;263:1726–1733. doi: 10.1126/science.8134836. [DOI] [PubMed] [Google Scholar]
- 6.Kim K, Choi SQ, Zell ZA, Squires TM, Zasadzinski JA. Effect of cholesterol nanodomains on monolayer morphology and dynamics. Proc Natl Acad Sci USA. 2013;110:E3054–E3060. doi: 10.1073/pnas.1303304110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McConnell HM. Structures and transitions in lipid monolayers at the air-water interface. Annu Rev Phys Chem. 1991;42:171–195. [Google Scholar]
- 8.McConnell HM, Tamm LK, Weis RM. Periodic structures in lipid monolayer phase transitions. Proc Natl Acad Sci USA. 1984;81:3249–3253. doi: 10.1073/pnas.81.10.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min Y, et al. Critical and off-critical miscibility transitions in model extracellular and cytoplasmic myelin lipid monolayers. Biophys J. 2011;100:1490–1498. doi: 10.1016/j.bpj.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DW, et al. Relating domain size distribution to line tension and molecular dipole density in model cytoplasmic myelin lipid monolayers. Proc Natl Acad Sci USA. 2011;108:9425–9430. doi: 10.1073/pnas.1106368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhar P, et al. Lipid-protein interactions alter line tensions and domain size distributions in lung surfactant monolayers. Biophys J. 2012;102:56–65. doi: 10.1016/j.bpj.2011.11.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachan AK, Galla HJ. Bidirectional surface analysis of monomolecular membrane harboring nanoscale reversible collapse structures. Nano Lett. 2013;13:961–966. doi: 10.1021/nl303928m. [DOI] [PubMed] [Google Scholar]
- 13.Utada AS, et al. Monodisperse double emulsions generated from a microcapillary device. Science. 2005;308:537–541. doi: 10.1126/science.1109164. [DOI] [PubMed] [Google Scholar]
- 14.Saye RI, Sethian JA. Multiscale modeling of membrane rearrangement, drainage, and rupture in evolving foams. Science. 2013;340:720–724. doi: 10.1126/science.1230623. [DOI] [PubMed] [Google Scholar]
- 15.Rossignol S, et al. Atmospheric photochemistry at a fatty acid-coated air-water interface. Science. 2016;353:699–702. doi: 10.1126/science.aaf3617. [DOI] [PubMed] [Google Scholar]
- 16.Lipp MM, Lee KYC, Zasadzinski JA, Waring AJ. Phase and morphology changes in lipid monolayers induced by SP-B protein and its amino-terminal peptide. Science. 1996;273:1196–1199. doi: 10.1126/science.273.5279.1196. [DOI] [PubMed] [Google Scholar]
- 17.Knoche S, et al. Elastometry of deflated capsules: Elastic moduli from shape and wrinkle analysis. Langmuir. 2013;29:12463–12471. doi: 10.1021/la402322g. [DOI] [PubMed] [Google Scholar]
- 18.Nagel M, Tervoort TA, Vermant J. From drop-shape analysis to stress-fitting elastometry. Adv Colloid Interface Sci. 2017;247:33–51. doi: 10.1016/j.cis.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Schürch S, Green FHY, Bachofen H. Formation and structure of surface films: Captive bubble surfactometry. Biochim Biophys Acta. 1998;1408:180–202. doi: 10.1016/s0925-4439(98)00067-2. [DOI] [PubMed] [Google Scholar]
- 20.Sugden S. XCVII.—The determination of surface tension from the maximum pressure in bubbles. J Chem Soc Trans. 1922;121:858–866. [Google Scholar]
- 21.Li D, Neumann AW. Phase rule for capillary systems. Adv Colloid Interface Sci. 1994;49:147–195. [Google Scholar]
- 22.Blodgett KB. Monomolecular films of fatty acids on glass. J Am Chem Soc. 1934;56:495–495. [Google Scholar]
- 23.Longo ML, Bisagno AM, Zasadzinski JA, Bruni R, Waring AJ. A function of lung surfactant protein SP-B. Science. 1993;261:453–456. doi: 10.1126/science.8332910. [DOI] [PubMed] [Google Scholar]
- 24.Lipp MM, Lee KYC, Takamoto DY, Zasadzinski JA, Waring AJ. Coexistence of buckled and flat monolayers. Phys Rev Lett. 1998;81:1650–1653. [Google Scholar]
- 25.Choi SQ, et al. Influence of molecular coherence on surface viscosity. Langmuir. 2014;30:8829–8838. doi: 10.1021/la501615g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sachan AK, et al. Interfacial rheology of coexisting solid and fluid monolayers. Soft Matter. 2017;13:1481–1492. doi: 10.1039/c6sm02797k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian A, Johnson C, Wang W, Baumgart T. Line tension at fluid membrane domain boundaries measured by micropipette aspiration. Phys Rev Lett. 2007;98:208102. doi: 10.1103/PhysRevLett.98.208102. [DOI] [PubMed] [Google Scholar]
- 28.Baumgart T, Hess ST, Webb WW. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425:821–824. doi: 10.1038/nature02013. [DOI] [PubMed] [Google Scholar]
- 29.Heinrich MC, Levental I, Gelman H, Janmey PA, Baumgart T. Critical exponents for line tension and dipole density difference from lipid monolayer domain boundary fluctuations. J Phys Chem B. 2008;112:8063–8068. doi: 10.1021/jp7116246. [DOI] [PubMed] [Google Scholar]
- 30.Ursell TS, Klug WS, Phillips R. Morphology and interaction between lipid domains. Proc Natl Acad Sci USA. 2009;106:13301–13306. doi: 10.1073/pnas.0903825106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veatch SL, Keller SL. Organization in lipid membranes containing cholesterol. Phys Rev Lett. 2002;89:268101. doi: 10.1103/PhysRevLett.89.268101. [DOI] [PubMed] [Google Scholar]
- 32.Honerkamp-Smith AR, Veatch SL, Keller SL. An introduction to critical points for biophysicists; observations of compositional heterogeneity in lipid membranes. Biochim Biophys Acta. 2009;1788:53–63. doi: 10.1016/j.bbamem.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaler EW, Murthy AK, Rodriguez BE, Zasadzinski JAN. Spontaneous vesicle formation in aqueous mixtures of single-tailed surfactants. Science. 1989;245:1371–1374. doi: 10.1126/science.2781283. [DOI] [PubMed] [Google Scholar]
- 34.Safran SA, Pincus P, Andelman D. Theory of spontaneous vesicle formation in surfactant mixtures. Science. 1990;248:354–356. doi: 10.1126/science.248.4953.354. [DOI] [PubMed] [Google Scholar]
- 35.Zasadzinski JA, Schneir J, Gurley J, Elings V, Hansma PK. Scanning tunneling microscopy of freeze-fracture replicas of biomembranes. Science. 1988;239:1013–1015. doi: 10.1126/science.3344420. [DOI] [PubMed] [Google Scholar]
- 36.Seul M, Andelman D. Domain shapes and patterns: The phenomenology of modulated phases. Science. 1995;267:476–483. doi: 10.1126/science.267.5197.476. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez NJ, Walker LM, Anna SL. A microtensiometer to probe the effect of radius of curvature on surfactant transport to a spherical interface. Langmuir. 2010;26:13310–13319. doi: 10.1021/la101870m. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez NJ, Anna SL, Saigal T, Tilton RD, Walker LM. Interfacial dynamics and rheology of polymer-grafted nanoparticles at air-water and xylene-water interfaces. Langmuir. 2012;28:8052–8063. doi: 10.1021/la300737p. [DOI] [PubMed] [Google Scholar]
- 39.Kotula AP, Anna SL. Regular perturbation analysis of small amplitude oscillatory dilatation of an interface in a capillary pressure tensiometer. J Rheol (NYNY) 2015;59:85–117. [Google Scholar]
- 40.Lin GL, et al. Interfacial dilatational deformation accelerates particle formation in monoclonal antibody solutions. Soft Matter. 2016;12:3293–3302. doi: 10.1039/c5sm02830b. [DOI] [PubMed] [Google Scholar]
- 41.Bernhard W, et al. Commercial versus native surfactants. Surface activity, molecular components, and the effect of calcium. Am J Respir Crit Care Med. 2000;162:1524–1533. doi: 10.1164/ajrccm.162.4.9908104. [DOI] [PubMed] [Google Scholar]
- 42.Kotula AP, Anna SL. Insoluble layer deposition and dilatational rheology at a microscale spherical cap interface. Soft Matter. 2016;12:7038–7055. doi: 10.1039/c5sm03133h. [DOI] [PubMed] [Google Scholar]
- 43.Walther FJ, Waring AJ, Sherman MA, Zasadzinski JA, Gordon LM. Hydrophobic surfactant proteins and their analogues. Neonatology. 2007;91:303–310. doi: 10.1159/000101346. [DOI] [PubMed] [Google Scholar]
- 44.Stenger PC, et al. X-ray diffraction and reflectivity validation of the depletion attraction in the competitive adsorption of lung surfactant and albumin. Biophys J. 2009;97:777–786. doi: 10.1016/j.bpj.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee KYC, et al. Influence of palmitic acid and hexadecanol on the phase transition temperature and molecular packing of dipalmitoylphosphatidyl-choline monolayers at the air-water interface. J Chem Phys. 2002;116:774–783. [Google Scholar]
- 46.Carnibella RP, Kitchen MJ, Fouras A. 2014. Single-shot X-ray measurement of alveolar size distributions. Proceedings of Medical Imaging 2014: Biomedical Applications in Molecular, Structural, and Functional Imaging, SPIE, eds Molthen RC, Weaver JB (SPIE, International Society for Optical Engineering, Bellingham, WA), Vol 9038, No. 90380V.
- 47.Ochs M, et al. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169:120–124. doi: 10.1164/rccm.200308-1107OC. [DOI] [PubMed] [Google Scholar]
- 48.McConnell HM, Moy VT. Shapes of finite two-dimensional lipid domains. J Phys Chem. 1988;92:4520–4525. [Google Scholar]
- 49.Alonso C, Waring A, Zasadzinski JA. Keeping lung surfactant where it belongs: Protein regulation of two-dimensional viscosity. Biophys J. 2005;89:266–273. doi: 10.1529/biophysj.104.052092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pataraia S, Liu Y, Lipowsky R, Dimova R. Effect of cytochrome c on the phase behavior of charged multicomponent lipid membranes. Biochim Biophys Acta. 2014;1838:2036–2045. doi: 10.1016/j.bbamem.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 51.Vequi-Suplicy CC, Riske KA, Knorr RL, Dimova R. Vesicles with charged domains. Biochim Biophys Acta. 2010;1798:1338–1347. doi: 10.1016/j.bbamem.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 52.McConnell HM, Rice PA, Benvegnu DJ. Brownian motion of lipid domains in electrostatic traps in monolayers. J Phys Chem. 1990;94:8965–8968. [Google Scholar]
- 53.Helfrich W. Elastic properties of lipid bilayers: Theory and possible experiments. Z Naturforsch C. 1973;28:693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- 54.Li P, Hu L, McGaughey AJH, Shen S. Crystalline polyethylene nanofibers with the theoretical limit of Young’s modulus. Adv Mater. 2014;26:1065–1070. doi: 10.1002/adma.201304116. [DOI] [PubMed] [Google Scholar]
- 55.Bellani G, et al. LUNG SAFE Investigators ESICM Trials Group Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800, and erratum (2016) 316:350. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 56.Reichert MD, et al. The importance of experimental design on measurement of dynamic interfacial tension and interfacial rheology in diffusion-limited surfactant systems. Colloids Surf A. 2015;467:135–142. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.