Significance
The symptoms of neurological diseases such as autism and schizophrenia are often attributed to a loss of excitatory/inhibitory balance of neural network function. By showing that ATR and ATM impact inhibitory and excitatory vesicle trafficking differently, our work expands the known repertoire of cytoplasmic functions for the two kinases and provides a new perspective on the origins of the symptoms of ataxia-telangiectasia (A-T) and Seckel syndrome (ATM and ATR deficiency, respectively). While these findings have their most immediate implications for the neurologic and cognitive symptoms of A-T and Seckel syndrome, they have potential relevance to a much broader range of neurologic conditions.
Keywords: vesicle trafficking, endocytosis, clathrin, E/I balance, neurodegeneration
Abstract
ATM (ataxia-telangiectasia mutated) and ATR (ATM and Rad3-related) are large PI3 kinases whose human mutations result in complex syndromes that include a compromised DNA damage response (DDR) and prominent nervous system phenotypes. Both proteins are nuclear-localized in keeping with their DDR functions, yet both are also found in cytoplasm, including on neuronal synaptic vesicles. In ATM- or ATR-deficient neurons, spontaneous vesicle release is reduced, but a drop in ATM or ATR level also slows FM4-64 dye uptake. In keeping with this, both proteins bind to AP-2 complex components as well as to clathrin, suggesting roles in endocytosis and vesicle recycling. The two proteins play complementary roles in the DDR; ATM is engaged in the repair of double-strand breaks, while ATR deals mainly with single-strand damage. Unexpectedly, this complementarity extends to these proteins’ synaptic function as well. Superresolution microscopy and coimmunoprecipitation reveal that ATM associates exclusively with excitatory (VGLUT1+) vesicles, while ATR associates only with inhibitory (VGAT+) vesicles. The levels of ATM and ATR respond to each other; when ATM is deficient, ATR levels rise, and vice versa. Finally, blocking NMDA, but not GABA, receptors causes ATM levels to rise while ATR levels respond to GABA, but not NMDA, receptor blockade. Taken together, our data suggest that ATM and ATR are part of the cellular “infrastructure” that maintains the excitatory/inhibitory balance of the nervous system. This idea has important implications for the human diseases resulting from their genetic deficiency.
Ataxia-telangiectasia mutated (ATM) and ataxia-telangiectasia and Rad3 related (ATR) are serine/threonine kinases belonging to the phosphatidylinositol 3-kinase–related kinase (PIKK) family. ATM and ATR are more closely related to each other than to the other members of PIKK family, sharing a common architecture (1–4) and similar molecular weights (300–370 kDa). Mutations in ATM result in ataxia-telangiectasia (A-T), a multisystem disease that includes immune deficiency, predisposition to cancer, hypersensitivity to ionizing radiation (IR), and a prominent neurodegenerative phenotype most visibly affecting the cerebellum (5–9). Null mutations of ATR are lethal during embryonic development. Hypomorphic ATR mutations lead to a distinct CNS developmental disorder known as Seckel syndrome (10–14). Befitting their roles in the DNA damage response, both ATM and ATR proteins are found in the neuronal nucleus, where, after DNA damage, they phosphorylate downstream targets involved in cell cycle arrest and DNA repair (3, 15, 16). However, it is difficult to ascribe the complex clinical phenotypes of A-T and Seckel syndrome solely to deficiencies in DNA repair, and, perhaps not surprisingly, both proteins are also found in the neuronal cytoplasm. ATM has been localized to vesicular structures (17, 18), and most recently to synaptic vesicles (19, 20). ATR is also cytoplasmic and biochemically associated with ATM and the synaptic vesicle proteins VAMP2 (synaptobrevin) and synapsin-1 (19). The specific function of these two proteins in the neuronal synapse remains unclear, however.
Most neural networks have two basic types of synapses, excitatory and inhibitory, which use glutamate and γ-amino butyric acid (GABA), respectively, as neurotransmitters. Acting through ligand-gated cation channels, glutamate depolarizes the postsynaptic membrane, thereby favoring the initiation of an action potential. GABA opens ligand-gated anionic (Cl−) channels, hyperpolarizing the postsynaptic cell and making it less likely to fire. Clearly, the balance of excitatory and inhibitory neurotransmitter release must be precisely controlled. Indeed, excitatory/inhibitory (E/I) imbalance has been suggested as a contributing factor to several neurodevelopmental disorders, including autism spectrum disorders, amyotrophic lateral sclerosis, and schizophrenia (21–23).
In this paper, we report that ATM and ATR contribute to the E/I balance of the CNS by regulating the dynamics of different populations of synaptic vesicles. Both in vivo and in vitro, this effect is mediated by the segregation of ATM and ATR to different classes of vesicles—ATM with excitatory vesicles, ATR with inhibitory vesicles. Our study thus reveals unexpected roles for ATM and ATR in neuronal function and provides insight into the pleiotropic neurologic symptoms of A-T and Seckel syndrome.
Results
ATM or ATR Loss Causes Synaptic Deficiency.
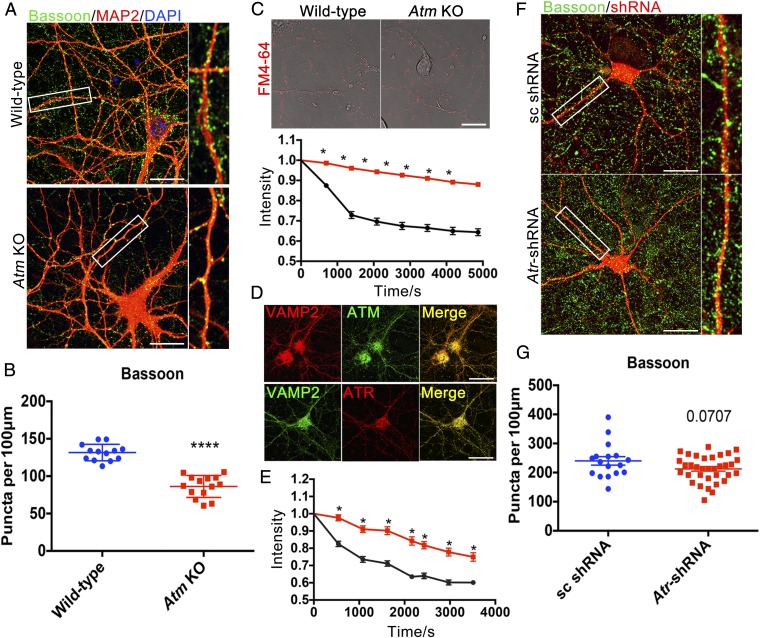
We measured synaptic density by analyzing the number of immunostained puncta of the presynaptic protein Bassoon. We found that the density of synapses in Atm−/− neuronal cultures was 30–40% less than that found in wild type (WT) (Fig. 1 A and B). We next examined the rate of spontaneous FM4-64 dye release from individual puncta along the fine processes of the neuronal dendrites (Fig. 1 C and D). Dye release was significantly slower from puncta on Atm−/− cultured neurons (Fig. 1C), confirming and extending previous observations that examined only release from the cell body (19, 20). As we normalized our dye release data to the initial levels of dye loading (Fig. 1C), the slower release was likely not caused solely by the reduced synaptic density. Thus, ATM is necessary for the maintenance of normal synaptic density and function.
Fig. 1.
ATM or ATR loss causes synaptic deficiency. (A) Cultured cortical neurons from WT or Atm KO mice were immunolabeled with anti-Bassoon (green) and anti-MAP2 (red) antibodies. (Scale bar: 20 μm.) (B) Quantification of Bassoon puncta in WT and Atm KO cortical neurons. n = 13–14 neurons from three batches of neuronal cultures. Error bars represent SEM. ****P < 0.0001, unpaired t test. (C) Representative images and quantification results of FM4-64 dye spontaneous release from neurites of WT (black) and Atm KO (red) cultures. (Scale bar: 20 μm.) n = 102–200 individual measurements from four independent neuron cultures. Error bars represent SEM. Significance of the difference between the curves, *P < 0.05 (multiple t tests). (D) Confocal images of WT cortical neurons stained with anti-VAMP2 (red), anti-ATM [2C1(1A1)] (green), or anti-ATR (red) antibodies. (Scale bar: 20 μm.) (E) Atr knockdown (red) with shRNA. n = 40–60 individual measurements from four separate neuronal cultures. Error bars represent SEM. Significance of the difference between the curves, *P < 0.05 (multiple t tests). (F) Scrambled (sc) or Atr-shRNA (red) transfected cortical neurons immunolabeled with anti-Bassoon (green) antibody. (Scale bar: 20 μm.) (G) Quantification of Bassoon (presynaptic puncta) density in control and Atr knockdown neurons. n = 17∼34 neurons from three different neuronal cultures. Error bars represent SEM. P = 0.0707, unpaired t test.
We next showed that the loss of ATR had similar effects. ATR also colocalized with VAMP2 (Fig. 1D), and after Atr knockdown with Atr-shRNA, spontaneous release of FM4-64 dye was significantly reduced (Fig. 1E). We found only a nonsignificant decrease of synaptic density in Atr knockdown neurons, however, perhaps because shRNA did not totally eliminate ATR protein (Fig. 1 F and G).
ATM and ATR Protein Levels Show a Reciprocal Relationship.
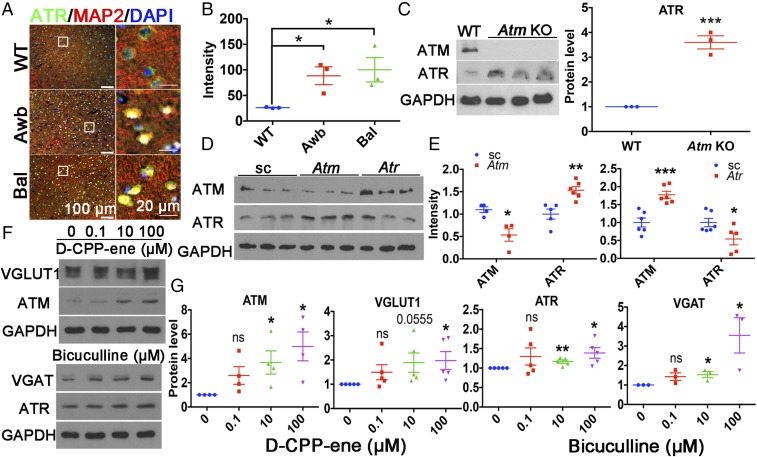
Since ATR and ATM are closely related kinases, we asked whether each could compensate for a deficiency of the other. We estimated ATR protein levels by immunohistochemistry (Fig. 2A) and Western blot analysis (Fig. 2C) in the cortex of two different Atm mutants (Awb or Bal homozygotes). In both genotypes, we found a nearly threefold increase in ATR protein levels (Fig. 2 B and C). The reverse experiment is difficult, since Atr KO mice are embryonic-lethal; thus, we used HEK293T cells transfected with Atm-shRNA or Atr-shRNA as our assay system. At 48 h after transfection, ATR levels increased following Atm knockdown (Fig. 2 D and E) and ATM protein increased after Atr knockdown, thus identifying a reciprocal feedback relationship (Fig. 2 D and E).
Fig. 2.
ATM and ATR protein levels show a reciprocal relationship. (A) Brain sections from 2-mo-old WT, Atmtm1Awb/Atmtm1Awb (Awb), and Atmtm1Bal/Atmtm1Bal (Bal) mice were labeled with ATR (green) or MAP2 (red) antibodies and counterstained with DAPI (blue). (Scale bars: as marked.) (B) ATR immunostaining intensity. n = 3 animals for each group. Error bars represent SEM. *P = 0.0232; *P = 0.036, unpaired t test. (C) Western blots of Atm KO (Awb) and WT cortical lysates. Actin served as a loading control. n = 3 animals for each group. Error bars represent SEM. ***P = 0.0006, unpaired t test. (D) Western blots of HEK293T cells lysates obtained at 48 h after transfection with Atm-shRNA or Atr-shRNA. GAPDH served as a loading control. (E) Quantification of the blots shown in D. n = 4–6 independent cultures. Error bars represent SEM. ***P = 0.0007; *P = 0.01; *P = 0.0445; **P = 0.0032, unpaired t test. (F) Western blots of ATM, ATR, VGLUT1, and VGAT of lysates from 21 DIV cortical neurons treated with D-CPP-ene or bicuculline for 24 h. (G) Quantification of the blots shown in F. n = 3∼4 independent neuronal cultures. Error bars represent SEM. For ATM, *P = 0.0317, *P = 0.0152; for VGLUT1, *P = 0.03; for ATR, **P = 0.0046, *P = 0.0213; for VGAT, *P = 0.0293, *P = 0.0479, unpaired t test.
We next asked whether the activity of the neuronal network itself might dynamically regulate ATM or ATR levels. We treated WT neuronal cultures with l-glutamate and found a significant decrease of ATM protein after 4 h, along with a corresponding increase in ATR (Fig. S1). Similarly, neurons treated with the NMDA receptor antagonist D-CPP-ene showed higher ATM and VGLUT1 protein levels (Fig. 2 F and G), while treatment with the GABA receptor antagonist bicuculline led to higher neuronal ATR and VGAT protein levels (Fig. 2 F and G). Thus, ATM responds primarily to changes in the activity of excitatory neurons, while ATR responds mostly to changes in the activity of inhibitory neurons.
ATM or ATR Deficiency Leads to E/I Imbalance.
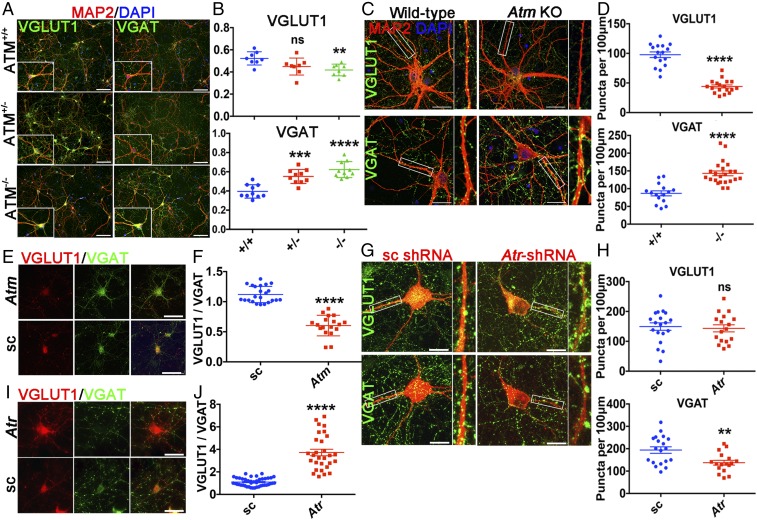
We immunostained cortical neuronal cultures established from WT (Atm+/+) and Bal mice (both Atm+/− and Atm−/−) using VGLUT1 or VGAT antibodies. The fraction of VGLUT1+ neuronal cell bodies was significantly smaller in the homozygous mutant cultures and trended lower in the heterozygote cultures (Fig. 3 A and B). To extend these observations in the cell soma, we quantified VGLUT1 and VGAT puncta in the dendrites. We found 50% fewer VGLUT1+ puncta in the neuropil of Atm−/− cultures (Fig. 3 C and D). Indeed, in Atm−/− cultures, while VGLUT1 puncta were seen along the main dendritic branches, the finer dendritic branches were nearly blank (Fig. 3 C and D). Although VGLUT1+ was decreased in Atm−/− cultures, VGAT+ cell bodies and synaptic puncta were both increased in heterozygous cultures and even more so in homozygous cultures (Fig. 3 A and C). Nearly identical results were achieved with the independent GABAergic neuron marker GAD65 (Fig. S2).
Fig. 3.
ATM or ATR deficiency leads to excitatory/inhibitory (E/I) imbalance. (A) 21 DIV cortical neurons isolated from Bal mice—heterozygous (Atm+/−), homozygous (Atm−/−), and WT (Atm+/+)— were stained with the indicated antibodies. (Scale bar: 100 μm.) (B) Percentages of VGLUT1+ and VGAT+ neurons in these cultures. n = 8–10 coverslips of neurons from four independent cultures. Error bars represent SEM. ns, P = 0.0512; **P = 0.0024; ***P = 0.0001; ****P < 0.0001, unpaired t test. (C) Representative confocal images of WT and Atm KO cortical neurons, stained with MAP2 (red), VGLUT1 (green), or VGAT (green) antibodies. (Scale bar: 20 μm.) High-magnification images show VGLUT1 or VGAT puncta on MAP2+ neurites. (D) Quantification of VGLUT1 and VGAT puncta in C. n = 15–23 neurons from four independent cultures. Error bars represent SEM. ****P < 0.0001, unpaired t test. (E) Atm or scrambled shRNA-transfected neurons immunolabeled by anti-VGLUT1 (red) and anti-VGAT (green) antibodies. (Scale bar: 50 μm.) (F) Ratio of VGLUT1 to VGAT protein intensity in E. n = 18–23 neurons from four independent cultures. Error bars represent SEM. ****P < 0.0001, unpaired t test. (G) Confocal images of scrambled or Atr shRNA (red)-transfected neurons, stained with anti-VGLUT1 (green) or anti-VGAT (green). (Scale bar: 20 μm.) High-magnification images show VGLUT1 or VGAT puncta on neurites. (H) Quantification of VGLUT1 and VGAT puncta in G. n = 17–19 neurons from three independent cultures. Error bars represent SEM. ns, P = 0.7341; **P = 0.0041, unpaired t test. (I) Atr or scrambled shRNA-transfected neurons immunolabeled with VGLUT1 (red) and VGAT (green) antibodies. (Scale bar: 50 μm.) (J) Ratio of VGLUT1 to VGAT protein intensity in I. n = 29–43 neurons from four independent cultures. Error bar represents SEM. ****P < 0.0001, unpaired t test.
Using these percentages, we estimated E/I ratios of approximately 1.5 in WT, 1.0 in heterozygote, and 0.67 in Atm−/− cultures. We next asked whether the reduced E/I balance was due to an intrinsic shift in the identity of the ATM-deficient neurons, or whether E/I regulation by ATM was more dynamic. We found that Atm shRNA-transfected neurons had higher VGAT (green) and lower VGLUT1 (red) expression levels compared with scrambled shRNA controls (Fig. 3E and Fig. S3B). Quantification revealed an almost 1:1 ratio of VGLUT1 to VGAT staining in WT neuronal soma. This ratio decreased to 0.5:1 by just 48 h after Atm knockdown (Fig. 3F). Thus, ATM helps maintain the E/I balance of a culture, and does so in a dynamic fashion. With ATR, the findings were reversed, with an increase in the E/I ratio after ATR knockdown. WT neurons transfected with Atr shRNA showed decreased VGAT signals in both cell bodies (Fig. 3I and Fig. S3C) and at synapses (Fig. 3G), but increased VGLUT1, compared with control shRNA. Quantification of the excitatory (VGLUT1) or inhibitory (VGAT) signals from both cell bodies (Fig. 3J) and synapses (Fig. 3H) clearly showed that loss of ATM protein decreases, while loss of ATR protein increases, the E/I ratio in cultured cortical neurons.
E/I Balance Is Independent of ATM and ATR Kinase Activity.
We treated cultured cortical neurons with the ATM inhibitor KU-60019 (1 μM) or the ATR inhibitor VE-822 (0.5 μM) for 48 h, then fixed and immunostained the cultures (Fig. S4A). Despite the inhibition of kinase activity, we found no significant difference in the VGLUT1/VGAT ratio (Fig. S4B). Moreover, kinase inhibition did not alter synaptic density or cause individual synapses to assume dual functionality (24–26). Confocal microscopy revealed the same nonoverlapping pattern of VGLUT1 and VGAT immunostaining in treated and untreated cultures (Fig. S4C). The densities of VGLUT1+ and VGAT+ synapses (puncta) were also comparable (Fig. S4D). Thus, it appears that the E/I balance of a cell is independent of ATM and ATR kinase activity.
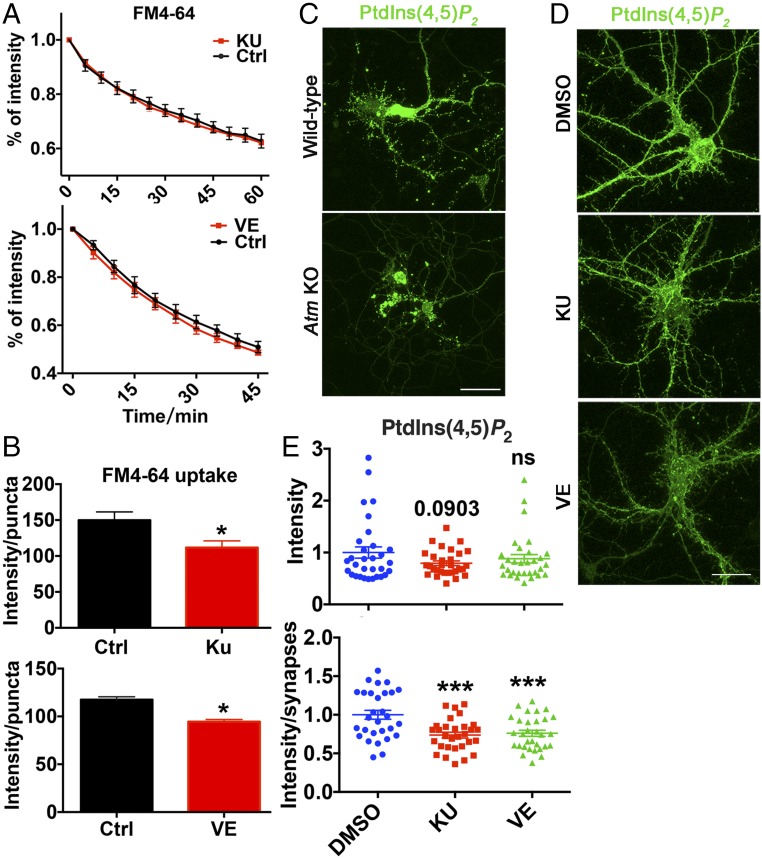
Synaptic Vesicle Endocytosis, but Not Release, Depends on ATM/ATR Kinase Activity.
As the vesicle content of the synapses did not seem to be affected by ATM or ATR kinase activity, we asked whether synaptic function was altered in the presence of their kinase inhibitors. We found that neither KU-60019 nor VE-822 had any effect on spontaneous dye release kinetics (Fig. 4A). This was in contrast to our earlier findings with Atm−/− cells and with Atr shRNA-mediated protein knockdown (Fig. 1 C and E), and suggests that the altered vesicle release properties might be a secondary consequence of a defect in vesicle recycling by endocytosis and an altered releasable pool of vesicles. Thus, we measured the uptake of FM4-64 dye into synaptic regions of cultures treated with KU-60019 or VE-822. In the presence of either inhibitor, we observed reduced dye uptake capacity (Fig. 4B and Fig. S4E), consistent with a deficiency in the endocytic process. As recycling occurs in PI-rich membrane regions, we also measured phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] levels with the GFP-C1-PLCdelta-PH fluorescent biosensor. Atm−/− neurons showed reduced PtdIns(4,5)P2 levels, especially in synaptic regions (Fig. 4C), which would be expected to impair vesicle endocytosis. Neither KU-60019 nor VE-822 decreased the overall intensity of signal from the GFP-C1-PLCdelta-PH reporter (Fig. 4 D and E), but the intensity of PtdIns(4,5)P2 in synaptic regions was significantly decreased. The picture that emerges from these data is that ATM/ATR kinase activities regulate synaptic endocytosis by regulating PtdIns(4,5)P2.
Fig. 4.
Synaptic vesicle endocytosis, but not release, is kinase-dependent. (A) FM4-64 spontaneous release from 21 DIV WT neurons was unaffected by 48 h of KU-60019 or VE-822 treatment. n = 2∼3 independent neuronal cultures. Error bars represent SEM. (Upper) P = 0.3874; (Lower) P = 0.0554, two-way ANOVA. (B) FM4-64 dye uptake intensity per puncta in indicated neuron cultures. n = 2∼3 batches of neuronal cultures. Error bars represent SEM. *P = 0.0317; *P = 0.0245, unpaired t test. (C) WT and Atm KO cortical neurons transfected with GFP-C1-PLCdelta-PH reporter. (Scale bar: 20 μm.) (D) Representative images of WT cortical neurons transfected with GFP-C1-PLCdelta-PH, followed by DMSO, KU-60019, or VE-822 treatment for 48 h. (Scale bar: 20 μm.) (E) PtdIns(4,5)P2 intensity in neurons treated with DMSO, KU-60019, or VE-822 measured using Fiji software. n = 29–31 neurons each group from four batches of different neuronal cultures. Error bars represent SEM. ns, P > 0.05, unpaired t test; ***P = 0.0001, one-way ANOVA.
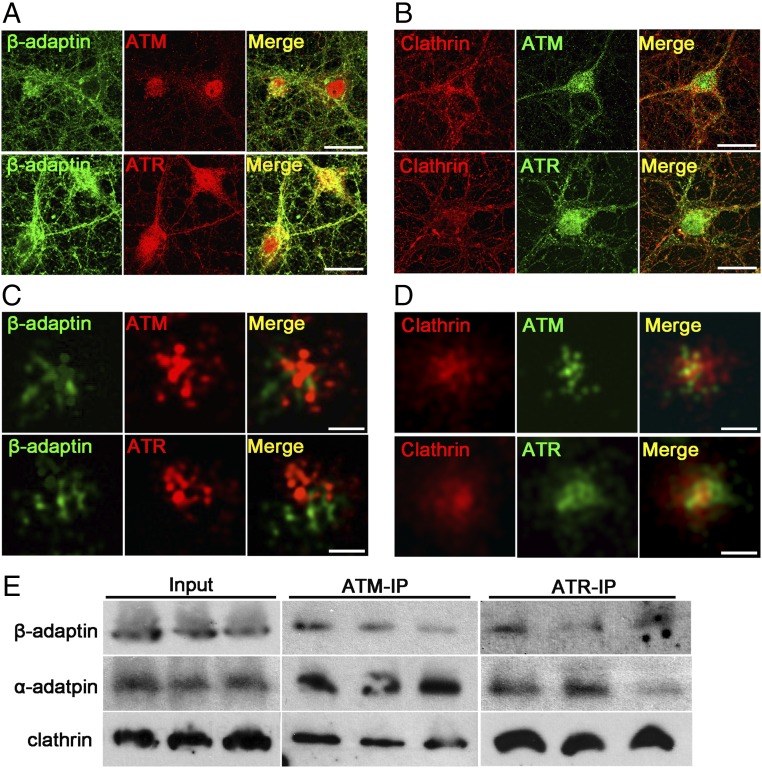
One of the first ATM-interacting proteins identified was β-adaptin (18, 27). Along with α-adaptin and other subunits of the AP-2 complex, β-adaptin plays a critical role in synaptic vesicle endocytosis. Using confocal microscopy, we confirmed that both ATM and ATR colocalized with β-adaptin on vesicular structures (Fig. 5A). We next examined the role of clathrin, a major vesicular coat protein involved in endocytosis, and found that it also colocalized with both ATM and ATR (Fig. 5B). Using superresolution microscopy to increase our resolving power to 20 nm, it became clear that both β-adaptin and clathrin colocalized with both ATM and ATR on vesicular structures with diameters of ∼60 nm (Fig. 5 C and D). As a final confirmation, we performed immunoprecipitation from adult mouse brain cortical lysates using anti-ATM and anti-ATR antibodies. Western blots of these immunoprecipitates probed with antibodies against β-adaptin confirmed the direct physical interaction between both ATM and ATR in the mouse brain (Fig. 5E). We probed replicate blots with α-adaptin as well as clathrin, and found that both of these endocytosis-related proteins were also strongly associated with ATR and ATM (Fig. 5E).
Fig. 5.
ATM and ATR show physical associations with clathrin and β-adaptin. (A and B) Representative confocal images of 21 DIV cortical neurons labeled with indicated antibodies. (Scale bar: 20 μm.) (C and D) Representative SRLM images from neurites of 21 DIV cortical neurons labeled with indicated antibodies. (Scale bar: 60 nm.) (E) Immunoprecipitation with ATM [2C1(1A1)] or ATR antibodies of cortical lysates from 3-mo-old WT mice analyzed by Western blot analysis probed with β-adaptin, α-adaptin, and clathrin antibodies as indicated.
To date, ATM protein associations have been studied mostly in peripheral tissues (28–30). To explore the situation in the nervous system, we analyzed ATM immunoprecipitates from whole mouse brain by mass spectroscopy. We found several known ATM-interacting synaptic vesicle proteins, such as VAMP2, as well as several new candidates, including syntaxin, synaptojanin, and synaptotagmin. In addition to the α and β subunits of the AP-2 complex, the μ subunit was also identified as an ATM-interacting protein. As a control for antibody specificity, none of the foregoing proteins were found in the control pulldowns from Atm−/− brain lysates. Thus, ATM and ATR are physically associated with many of the proteins involved in clathrin-mediated synaptic vesicle endocytosis.
ATR Associates with a Wider Range of Vesicles than ATM.
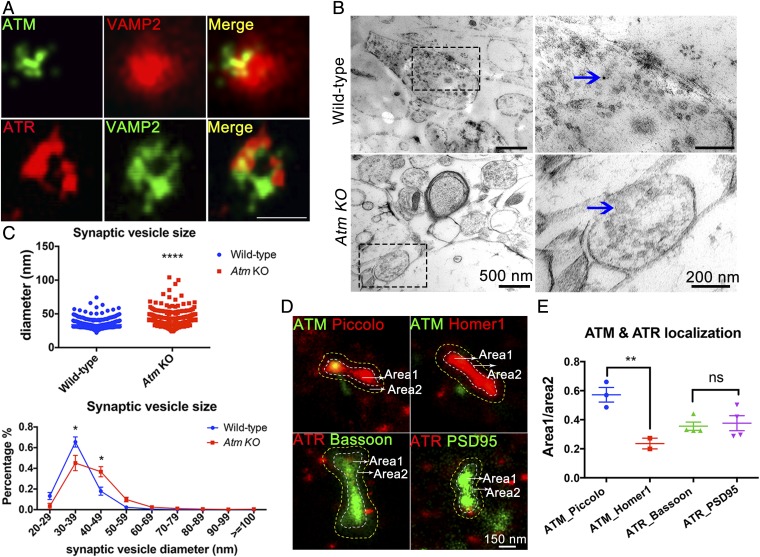
As shown previously (19, 20), ATM colocalizes with VAMP2+ vesicles in both conventional (Fig. 1D) and superresolution microscopy (Fig. 6A). A second anti-ATM antibody (Y170) showed a similar colocalization pattern with VAMP2 (Fig. S5A). We next used immunoelectron microscopy to validate these findings and found clear labeling of synaptic vesicles in WT mice (Fig. 6B, Upper), but not in Atm−/− mice (Fig. 6B, Lower). In our EM study, we noticed that the synaptic vesicles in the EM images of Atm KO synapses appeared larger than those seen in the WT. Quantification of vesicular size in the two genotypes confirmed this difference (Fig. 6C). The implications of this finding are discussed below.
Fig. 6.
ATM and ATR associate with synaptic vesicles and show different subsynaptic localizations. (A) SRLM images of WT cortical neurons stained with anti-VAMP2 (red) and anti-ATM [2C1(1A1)] (green) or anti-VAMP2 (green) and anti-ATR (red) antibodies. (Scale bar: 60 nm.) (B) TEM images from neocortices of WT and Atm KO mice stained by anti-ATM [2C1(1A1)] primary antibody revealed with 6-nm gold nanoparticles. (Scale bars: as marked.) (C) Average of synaptic vesicle diameters and percentages of different-sized vesicles (418–654 vesicles/sample). n = 3 animals. Error bars represent SEM. ****P < 0.0001, unpaired t test; *P < 0.0001, multiple t tests. (D) SRLM preparation double-stained with ATM (green)-Piccolo (red), ATM (green)-Homer1 (red), ATR (red)-Bassoon (green), or ATR (red)-PSD95 (green). (E) Quantification of ATM molecules localized in close proximity to Piccolo or Homer1 areas, and ATR molecules localized at Bassoon or PSD95 areas. Images are from two to four independent cultures. Error bars represent SEM. **P = 0.0077; ns, P = 0.7396, one-way ANOVA.
As with ATM, we found that ATR also closely associated with VAMP2+ 60-nm vesicular structures in superresolution localization microscopy (SRLM) images (Fig. 6A). Unlike ATM (Fig. S5B), however, ATR immunostaining was also found associated with larger vesicular structures surrounding chromogranin A (CgA), a cargo protein typically found in large dense-core vesicles (Fig. S5C). Perhaps because of this dual localization, we found that, unlike ATM, ATR was located in close proximity to presynaptic proteins, such as VAMP2 (Fig. 6A) and Bassoon (Fig. S5D), as well as to postsynaptic proteins, such as PSD95 (Fig. S5E). We quantified these observations using two-color SRLM (Fig. 6D). We found no substantial presynaptic/postsynaptic difference in ATR localization (Fig. 6E), in contrast to ATM, which was located significantly closer to Piccolo (presynaptic)- than to Homer1 (postsynaptic)-labeled vesicular structures (Fig. 6 D and E). These data indicate that while ATM is predominately presynaptic, the distribution of ATR is broader. We note that CgA is highly expressed in GABAergic neurons (31), and that dense-core CgA-containing vesicles are usually located away from the synaptic active zone. This predicts that some fraction of the vesicle-bound ATR protein would be located at a distance from the synaptic active zone, and perhaps explains the lack of ATR-Bassoon association (Fig. 6D).
ATM and ATR Associate with Different Synaptic Vesicle Populations.
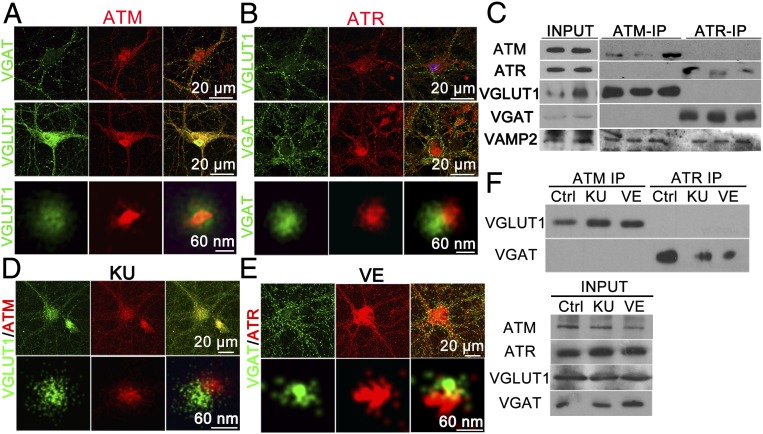
ATM is expressed in neuron cultures in which both excitatory and inhibitory neurons can be found (Fig. S3A). In glutamate-containing excitatory synapses, synaptic vesicles load their neurotransmitter with the transporter protein, VGLUT1. In GABA-containing inhibitory synapses, synaptic vesicles are loaded with GABA using VGAT. As expected, SRLM imaging of the synaptic regions of neuronal culture revealed that both VGLUT1 and VGAT stained vesicular structures (Fig. S6A) and were never found colocalized on a single vesicle. As expected, ATM and VGLUT1 regularly double-immunostained vesicles (Fig. 7A, Lower), but surprisingly, ATM never colocalized with VGAT+ vesicles (Fig. 7A, Upper). Even more surprisingly, SRLM imaging revealed that ATR stained in an exactly complementary pattern; it was localized with VGAT+, but not VGLUT1+, vesicles (Fig. 7B, Lower). Moreover, SRLM images of ATM, VAMP2, and ATR triple-staining showed that ATM-VAMP2 double-positive vesicles were not colocalized with vesicular ATR-VAMP2 structures (Fig. S6B).
Fig. 7.
ATM and ATR associate with different synaptic vesicle populations. (A) 21 DIV cultured cortical neurons immunostained with ATM [2CA(1A1)] (red), VGLUT1 (green), or VGAT (green) antibodies imaged by confocal microscopy (Upper and Middle) and SRLM (Lower). (Scale bars: as marked.) (B) Confocal (Upper and Middle) and SRLM images (Lower) of cultured cortical neurons immunostained with ATR (red), VGAT (green), or VGLUT1 (green) antibodies. (Scale bars: as marked.) (C) Western blots of ATM [2C1(1A1)], ATR, VGLUT1, VGAT, and VAMP2 after immunoprecipitation by ATM [2C1(1A1)] or ATR antibodies in cortical extracts from WT mice. (D and E) Confocal (Upper) and SRLM (Lower) images of KU-60019– or VE-822–treated cortical neurons labeled with anti-ATM and anti-VGLUT1 or with anti-ATR and anti-VGAT antibodies. (F) Western blots of VGLUT1 and VGAT after immunoprecipitation by anti-ATM [2C1(1A1)] or anti-ATR antibodies from lysates of 21 DIV cortical neurons treated with either KU-60019 or VE-822. (Note: There is an empty lane between the ATM and ATR samples in the VAMP2 blot.)
Finally, we immunoprecipitated ATM or ATR from WT mouse cortical lysates and analyzed the precipitates by Western blot analysis. ATM antibody was able to pull down VGLUT1, but not VGAT, from the lysates (Fig. 7C), In contrast, the ATR immunoprecipitates labeled robustly with VGAT, but not with VGLUT1 (Fig. 7C). Blotting with anti-VAMP2 antibody showed positive signals from both ATM and ATR immunoprecipitates (Fig. 7C), consistent with previously reported data (19). Taken together, these data strongly suggest that ATM is located in association with excitatory vesicles, while ATR is associated with inhibitory vesicles, consistent with their differential effects on the E/I balance in our cultures.
To explore whether these associations required kinase activity, 19 days in vitro (DIV) cortical neurons were treated with KU-60019 or VE-822 for 48 h, then fixed and double-immunolabeled with either ATM-VGLUT1 or ATR-VGAT. We observed no significant change in either the colocalization of ATM with VGLUT1 (Fig. 7D) or the colocalization of ATR with VGAT in the presence of either inhibitor (Fig. 7E). Kinase inhibitors did alter the quantitative aspects of these physical associations, however. Both KU-60019 and VE-822 led to a decrease in the total levels of VGLUT1 protein and an increase in the levels of VGAT (Fig. 7F, input panel). Despite this, both KU-60019 and VE-822 enhanced the interaction between ATM with VGLUT1 but decreased the interaction between ATR and VGAT (Fig. 7F). Thus, while the specificity of the interaction appears unaltered, the strength of the VGLUT1 and VGAT associations appears to be negatively regulated by the inhibition of ATM and ATR kinase activity, respectively.
Discussion
The results reported here deepen the appreciation of the role of ATM and ATR in CNS function. Both kinases play a role in synaptic vesicle recycling and have complementary roles in excitatory and inhibitory synapses. We found that ATR is specifically localized to inhibitory, but not excitatory, neuronal vesicles, while ATM has the reverse pattern, present on excitatory vesicles but absent on inhibitory vesicles. The complementarity of ATM and ATR in the synapse is reminiscent of their relationship in the DNA damage response, where ATM responds primarily to double-strand breaks while ATR is mostly responsive to single-strand breaks. Thus, in both nucleus and cytoplasm, ATM and ATR support each other in the service of an important cellular function.
In the synapse, the ATM-ATR complementarity is made even more meaningful by our finding that the levels of the two proteins appear to be in a dynamic balance with each other. Deficiency of either ATM or ATR leads to a compensatory increase in the level of the other protein. Other laboratories have examined the interplay of ATR and ATM, but almost always from the standpoint of activity (e.g., ref. 32), not protein levels as we have done here. The compensation in the brain that we describe occurs rapidly and thus can quickly influence the E/I balance of neuronal networks. The E/I balance then feeds back into the ATM-ATR relationship, as demonstrated by our finding that altering neuronal activity can also affect the levels of both ATM and ATR proteins. This suggestion of a dynamic interplay between the ATM/ATR ratio and neuronal activity is in full agreement with a recent report showing that reduced levels of ATM in mouse hippocampus, both in vivo and in vitro, shift the E/I balance toward a more inhibitory tone (33), which we believe was due to a complementary increase in ATR levels. Thus, through their differential involvement with excitatory and inhibitory synaptic vesicles, ATM and ATR may form a part of their identity and guide their differential trafficking dynamics.
Evidence suggests that E/I imbalance is highly related to neural network dysfunctions, such as epilepsy, anxiety, disorders and memory impairment (21–23, 34). In addition to cerebellar degeneration, some patients with A-T also show degeneration in brain regions, such as the motor cortex (35), and suffer from both cerebellar and noncerebellar cognitive deficits (36, 37). Our findings thus provide a valuable new perspective on the multivalent neurologic phenotype of A-T. Our work also predicts that ATR deficiency should cause an opposite response in the brain, namely an increased E/I ratio (Fig. 3). The data are rudimentary in this regard, as complete ATR deficiency leads to embryonic lethality. However, patients with Seckel syndrome (10, 13, 14) have reduced ATR levels, and in addition to developmental problems such as microcephaly, some individuals reportedly experience epileptic seizures. An increased E/I ratio has been reported in epilepsy (34, 38), which is consistent with the hypothesis that the epilepsy observed in patients with Seckel syndrome may be due in part to an increased E/I balance.
The association of ATM and ATR with subunits of the AP-2 complex as well as with clathrin indicates their possible roles in clathrin-mediated synaptic vesicle endocytosis (Fig. 5). Previous studies (18, 27) had hinted at this type of relationship. β-adaptin is a member of the AP-2 complex that plays a major role in clathrin-mediated endocytosis. While Atm−/− neurons show decreased FM4-64 dye release rates in vitro, we found that inhibition of ATM kinase activity in WT neurons had no effect, even while kinase inhibition significantly compromised dye uptake (Fig. 4). These findings imply that impaired dye release may reflect a more primary defect in vesicle endocytosis and a smaller ready-release pool of vesicles. The finding of reduced numbers of presynaptic puncta in Atm−/− neurons supports this suggestion.
This situation of reduced vesicle recycling causing problems in vesicle release is reminiscent of the deficiency of Tweek (39), a large Drosophila neuromuscular junction protein that was identified in a screen for mutants that could not maintain synaptic function during intense stimulation. Tweek is a large, evolutionarily conserved protein with little obvious domain structure. Intriguingly, given that ATM and ATR are members of the PIKK family, Tweek was found to regulate PtdIns(4,5)P2 availability and levels at synapses, thereby regulating synaptic vesicle endocytosis (39). Atm KO neurons also showed reduced PtdIns(4,5)P2 levels at the plasma membrane, especially at synapses (Fig. 4C). Deepening the analogy, synaptic vesicles in the nerve terminals of tweek flies are larger than those in WT flies, the same phenotype that we found in the neocortex of Atm KO mice (Fig. 6 B and C). Although there are many differences in the three proteins, these analogies open the possibility that ATM and ATR, perhaps through phosphoinositide, regulate synaptic vesicle recycling as their primary synaptic function.
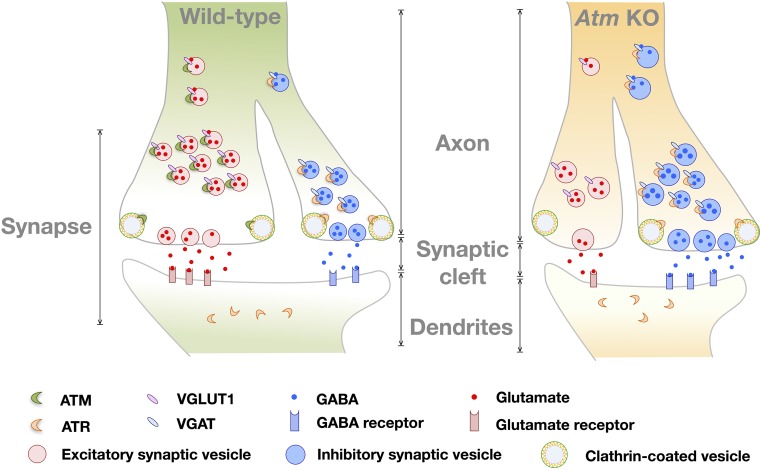
In sum, our work reveals roles of ATM and ATR beyond their function as DNA damage repair-related kinases. In our model (Fig. 8), both proteins have important functions in synaptic vesicle trafficking, most likely in vesicle endocytosis. Importantly, their complementary roles in excitatory and inhibitory vesicles may provide important insights into the symptoms of A-T and perhaps other neurodegenerative diseases (40).
Fig. 8.
Diagram of the vesicle trafficking model of WT and Atm KO synapses. Our model proposes that in synapses, ATM protein localizes predominately in presynaptic terminals, while ATR localizes on both side of synapse. ATM binds exclusively to VGLUT1+ vesicles, while ATR localizes only to VGAT+ vesicles. Compared with WT, Atm KO neurons show decreased E/I balance, impaired clathrin-mediated synaptic vesicle endocytosis, increased ATR protein levels, and larger vesicle size.
Experimental Procedures
Animals.
C57BL/6J and two ATM mutant lines, Awb (129S6/SvEvTac-Atmtm1Awb/J) and Bal (B6;129S4-Atmtm1Bal/J), were obtained from Jackson Laboratory. All mice used in this study were maintained and bred in the Animal and Plant Care Facility of The Hong Kong University of Science and Technology (HKUST). Awb primers and genotyping method were described previously (20). To genotype mice from the Bal line, the following PCR primers were used: PGK35: 5′-GGA AAA GCG CCT CCC CTA CCC -3′; Bal AT9: 5′-CCT CCT CAT ATT TGT AAC ACG CTG-3′; Bal AT12: 5′-TGT AAT GTG CCT TAA AGA ACC TGG -3′. PCR analysis was performed using the PCR ReadyMix Kit (E3004; Sigma-Aldrich) (Fig. S7).
Colonies were maintained by intercrossing heterozygous mice. All animal experiment protocols were approved by the Animal Ethics Committee at HKUST, and animal care was provided in accordance with both institutional and Hong Kong guidelines that include government legislation, Hong Kong's Code of Practice for Care and Use of Animals for Experimental Purposes, as well as International Guides and Codes of Practice on the Care and Use of Animals in Research.
Primary Neuronal Culture.
Cultures of mouse cortical neurons were prepared from embryonic day 16 (E16) C57BL/6J WT mouse embryos as described previously (41–43). Cortexes were collected in ice-cold PBS/glucose solution, then incubated in 1× trypsin solution for 10 min (37 °C). DMEM/10% FBS was used to inactivate the trypsin. The cells were then placed into Neurobasal medium (Thermo Fisher Scientific), supplemented with 2% B27 (Thermo Fisher Scientific), 1% Glutamax (Thermo Fisher Scientific), and 1% penicillin-streptomycin (10,000 U/mL; Thermo Fisher Scientific) at a density of 8,500 cells/cm2 at 37 °C in a humidified incubator with 5% CO2/95% air. Cultures were grown either on poly-l-lysine–coated glass coverslips for immunocytochemistry studies or in six-well plates (MW6; Thermo Fisher Scientific) for protein analysis.
Histochemistry.
Mice were deeply anesthetized by i.p. administration of 1.25% avertin (30 mL/kg) and then perfused transcardially with chilled PBS for 3 min. After perfusion, the whole brain was dissected free of the skull and bisected down the midline. One half was placed in 4% paraformaldehyde fixative (P6148-500G; Sigma-Aldrich) for immunohistochemistry, while the other half was snap-frozen in dry ice and stored at −80 °C until homogenization for protein chemistry, DNA/RNA extraction, or immunoprecipitation.
Chemicals and Molecular Biologicals.
KU-60019 (ATM kinase inhibitor; S1570) and VE-822 (ATR kinase inhibitor; S7102) were obtained from Selleckchem. FM4-64 dye (T13320) and Dynabeads Protein G (30 mg/mL; 10004D) were obtained from Life Technologies. D-CPP-ene (NMDA receptor antagonist; 1265) was purchased from Tocris, and bicuculline (GABA receptor antagonist) was purchased from Sigma-Aldrich. For siRNA and ORF plasmids, Atr-siRNA (sc-29764) was obtained from Santa Cruz Biotechnology, GFP-Atm-shRNA (TL320267) and GFP-Atr-shRNA (TL519184) were supplied by Origene, mCherry-Atm-shRNA (MSH026597) was obtained from Genecopoeia, GFP-C1-PLCdelta-PH (21179) was provided by Addgene.
Immunocytochemistry and Immunohistochemistry.
Coverslips were fixed in 4% paraformaldehyde for 15 min, washed three times with PBS, and incubated in 10% donkey serum with 0.1% Triton X-100 for 2 h at room temperature to block nonspecific binding. After blocking, primary antibodies were applied in blocking buffer, and the coverslips were incubated at 4 °C overnight. Coverslips were then washed three times with PBS and incubated with secondary antibodies (Alexa Fluor 488, Alexa Fluor 555, Alexa Fluor 647, or Alexa Fluor 750; Life Technologies) at room temperature for 1–2 h. Coverslips were washed five times with PBS, then fixed with 3% paraformaldehyde/0.05% glutaraldehyde (G7776-10 mL; Sigma-Aldrich) for 20 min. Stained coverslips were mounted with Hydromount (HS-106; National Diagnostics) for confocal imaging or placed in imaging buffer with freshly added 25 mM Tris(2-carboxyethyl)phosphine (TCEP) (646547-10 × 1 mL; Sigma-Aldrich) before acquiring SRLM images.
After overnight fixation in 4% paraformaldehyde followed by 30% sucrose in PBS for cryoprotection, each brain was embedded in Shandon Cryomatrix Frozen Embedding Medium (6769006; Thermo Fisher Scientific) and then quickly frozen on dry ice. Then 10-μm cryostat sections (CryoStar NX70; Thermo Fisher Scientific) were individually mounted on glass slides and stored at −80 °C. Before immunostaining, brain slices were washed in PBS, placed in citrate buffer (10 mM citric acid/0.05% Tween20, pH 6.0) at 95 °C for 10 min, then cooled to room temperature. Sections were then incubated in 10% donkey serum with 0.1% Triton X-100 for 2 h at room temperature, followed by antibody staining and mounting procedures as above.
SRLM Imaging.
Superresolution images were acquired with an SRLM system specially designed for the three-channel imaging of Alexa Fluor 561-, Alexa Fluor 647-, and Alexa Fluor 750-labeled samples (44–46). Each superresolution image was reconstructed from a file containing 30,000 frames recorded at 33 Hz, during which time the dye molecules alternated between dark and bright states in TCEP-containing imaging buffer. The resulting “winking” was used to calculate a 2D Gaussian distribution assumed to be centered on the location of a single dye molecule (sampled an average of 4,000 times). Moderate excitation laser intensities (4 kW/cm2 at 656 nm for Alexa Fluor 647 and 4.5 kW/cm2 at 750 nm for Alexa Fluor 750) were applied to minimize photo bleaching. The final resolution was determined to be <20 nm in both channels based on average fitting error. Active sample locking (44) was used to stabilize the sample with 1 nm-accuracy during imaging.
Transmission Electron Microscopy Imaging.
The procedures for sample preparation, thin sectioning, immunogold labeling and transmission electron microscopy (TEM) imaging were performed as described previously (47). For high-pressure freezing, small pieces of dissected mouse brain were rapidly frozen in a high-pressure freezing machine (EM HPM100; Leica). Ultrathin sections (∼70 nm) of the samples were cut on a UC7 Ultramicrotome (Leica). Immunolabeling was performed with primary antibodies against ATM (100 mg/mL). The corresponding gold-coupled secondary antibodies were used at 1:50 dilution. After immunogold labeling, the samples were poststained with aqueous uranyl acetate/lead citrate, and TEM images were captured on a Hitachi H7650 transmission electron microscope.
FM4-64 Spontaneous Dye Release and Uptake Test.
Release assay.
After removing culture medium, 15–21 DIV primary neurons were incubated with 10 µM FM4-64 dye in 45 mM KCl Tyrode’s solution for 90 s, then perfused with dye-free 4 mM KCl Tyrode’s solution for 15 min. After rinsing, neurons were subjected to live cell imaging by confocal microscopy (Leica) at a rate of 5 s per frame and analyzed with LAS X software. Circular 3-µm regions of interest (ROIs) were defined on neuronal dendrites or over nearby cell-free regions as background controls. The total dye intensity of each ROI was measured by the LAS X software.
Uptake assay.
After removing culture medium, 15–21 DIV primary neurons were incubated with 10 µM FM4-64 dye in sterile 45 mM KCl Tyrode’s solution for 90 s, and then washed three times with dye-free sterile 4 mM KCl Tyrode’s solution. The stained cells were then immediately imaged by confocal microscopy (Leica) in 10 randomly selected fields. FM4-64 intensity and puncta numbers were analyzed by Fiji software.
Cell Culture and Transfection.
HEK293T cells (Thermo Fisher Scientific) were cultured in DMEM with 10% FBS plus penicillin/streptomycin (Gibco). Primary neurons were isolated as above. DNA constructs were transfected with Lipofectamine 2000 or Lipofectamine LTX with Plus Reagent (Thermo Fisher Scientific). Primary neurons were transfected at 10 DIV. At 4∼6 h after transfection, fresh culture medium was added, and the cells were incubated for another 48 h (HEK293T) or longer (primary neurons; 5–10 d) to allow recovery and construct expression. For siRNA treatments, Atr-siRNA (Santa Cruz Biotechnology) was added, and the cells were incubated for an additional 48 h, then harvested in RIPA buffer.
Immunoprecipitation, SDS/PAGE, and Western Blot Analysis.
Cultured cells or isolated brain tissues were homogenized in ice-cold RIPA buffer (EMD Millipore) with 1× PhosSTOP phosphatase inhibitor mixture (Roche Applied Science) and 1× complete protease inhibitor mixture (Roche Applied Science) and then centrifuged for 20 min at 21,000 × g. The protein concentration of the supernatant was determined by the Bradford assay (Bio-Rad). For immunoprecipitation, 1 mg of total protein was incubated for 30 min with control IgG (Santa Cruz Biotechnology), precleared with 50 μL of Dynabeads Protein G (Invitrogen), then incubated overnight at 4 °C with primary antibody. Beads were collected with DynaMag-2 magnets (Life Technologies) and washed three times, followed by elution in 50 μL of 4× bromophenol blue dye with β mercaptoethanol as well as 150 μL of RIPA buffer containing 1× cOmplete protease inhibitor mixture (Roche Applied Science) and 1× PhosSTOP phosphatase inhibitor mixture (Roche Applied Science). The eluted proteins were subjected to 5–12% SDS/PAGE. For Western blot analysis, 40 μg of protein was run on SDS/PAGE and transferred to Immuno-Blot PVDF membranes (Bio-Rad). After blocking, membranes were probed with primary antibodies and visualized with SuperSignal West Pico, Dura, or Femto chemiluminescent substrate (Thermo Fisher Scientific).
Mass Spectrometry.
After immunoprecipitation, eluted proteins were resolved by 7.5% SDS/PAGE. Lanes containing immunoprecipitated proteins were cut into four segments. Each segment was digested with trypsin and analyzed with an LTQ Velos mass spectrometer (Thermo Fisher Scientific), coupled to an Accela liquid chromatography system (Thermo Fisher Scientific) with a nanoelectrospray ionization source. Peptides and proteins were identified and quantified using MaxQuant version 13.0.5 with the Andromeda search engine, along with proteome discovery with Mascot and SEQUEST search engines (Thermo Fisher Scientific). MS/MS spectra were searched against the Swiss-Prot/National Center for Biotechnology Information database, and the taxonomy was set as mouse. The search criteria were set as follows: trypsin digestion with two allowed missed cleavages, carbamidomethylation on cysteines as a fixed modification, and oxidation on methionine as a variable modification.
Statistical Analysis.
All data were obtained from at least three independent preparations. Quantifications were performed in a blinded manner. Differences between groups were analyzed using the unpaired t test, one-way ANOVA, or multiple t tests. Two-way ANOVA was used to determine the difference of two predicted variables. All statistical analyses were performed with GraphPad Prism 6. A P value ≤0.05 was considered significant. Results are reported as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Ron Hart and Mark R. Plummer (Rutgers, the State University of New Jersey) for helpful comments on the manuscript, and Dr. Daiying Xu (NanoBioImaging Ltd., Hong Kong) and Lujia Yu for help with the three-color SRLM imaging. We also thank NanoBioImaging Ltd. for the SRLM image analysis software. This work was supported by the Research Grants Council, Hong Kong Special Administrative Region (Grants HKUST12/CRF/13G, GRF660813, GRF16101315, GRF16124916, AoE/M-05/12, and C6030-14E); the Offices of Provost, Vice-President for Research and Graduate Studies, and Dean of Science, The Hong Kong University of Science and Technology (HKUST; Grant VPRGO12SC02); the National Key Basic Research Program of China (Grant 2013CB530900), the US National Institutes of Health (Grant NS70193), an RGC/HKUST Initiation Grant (IGN16SC02), and a HKUST Institute for Advanced Study Junior Fellowship (to H.-M.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716892115/-/DCSupplemental.
References
- 1.Bosotti R, Isacchi A, Sonnhammer EL. FAT: A novel domain in PIK-related kinases. Trends Biochem Sci. 2000;25:225–227. doi: 10.1016/s0968-0004(00)01563-2. [DOI] [PubMed] [Google Scholar]
- 2.Keith CTSS, Schreiber SL. PIK-related kinases: DNA repair, recombination, and cell cycle checkpoints. Science. 1995;270:50–51. doi: 10.1126/science.270.5233.50. [DOI] [PubMed] [Google Scholar]
- 3.Rivera-Calzada A, et al. Structure and assembly of the PI3K-like protein kinases (PIKKs) revealed by electron microscopy. AIMS Biophys. 2015;2:36–57. [Google Scholar]
- 4.Brewerton SC, Doré AS, Drake AC, Leuther KK, Blundell TL. Structural analysis of DNA-PKcs: Modelling of the repeat units and insights into the detailed molecular architecture. J Struct Biol. 2004;145:295–306. doi: 10.1016/j.jsb.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y, Chong MJ, McKinnon PJ. Ataxia telangiectasia mutated-dependent apoptosis after genotoxic stress in the developing nervous system is determined by cellular differentiation status. J Neurosci. 2001;21:6687–6693. doi: 10.1523/JNEUROSCI.21-17-06687.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavin MF, Shiloh Y. The genetic defect in ataxia-telangiectasia. Annu Rev Immunol. 1997;15:177–202. doi: 10.1146/annurev.immunol.15.1.177. [DOI] [PubMed] [Google Scholar]
- 7.Chun HH, Gatti RA. Ataxia-telangiectasia, an evolving phenotype. DNA Repair (Amst) 2004;3:1187–1196. doi: 10.1016/j.dnarep.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 8.McKinnon PJ. ATM and ataxia telangiectasia. EMBO Rep. 2004;5:772–776. doi: 10.1038/sj.embor.7400210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor AM, Byrd PJ. Molecular pathology of ataxia telangiectasia. J Clin Pathol. 2005;58:1009–1015. doi: 10.1136/jcp.2005.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alderton GK, et al. Seckel syndrome exhibits cellular features demonstrating defects in the ATR-signalling pathway. Hum Mol Genet. 2004;13:3127–3138. doi: 10.1093/hmg/ddh335. [DOI] [PubMed] [Google Scholar]
- 11.Murga M, et al. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat Genet. 2009;41:891–898. doi: 10.1038/ng.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka A, et al. Germline mutation in ATR in autosomal-dominant oropharyngeal cancer syndrome. Am J Hum Genet. 2012;90:511–517. doi: 10.1016/j.ajhg.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogi T, et al. Identification of the first ATRIP-deficient patient and novel mutations in ATR define a clinical spectrum for ATR-ATRIP Seckel syndrome. PLoS Genet. 2012;8:e1002945. doi: 10.1371/journal.pgen.1002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valdés-Sánchez L, et al. ATR localizes to the photoreceptor connecting cilium and deficiency leads to severe photoreceptor degeneration in mice. Hum Mol Genet. 2013;22:1507–1515. doi: 10.1093/hmg/dds563. [DOI] [PubMed] [Google Scholar]
- 15.Hiom K. DNA repair: How to PIKK a partner. Curr Biol. 2005;15:R473–R475. doi: 10.1016/j.cub.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Weber AM, Ryan AJ. ATM and ATR as therapeutic targets in cancer. Pharmacol Ther. 2015;149:124–138. doi: 10.1016/j.pharmthera.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Watters D, et al. Localization of a portion of extranuclear ATM to peroxisomes. J Biol Chem. 1999;274:34277–34282. doi: 10.1074/jbc.274.48.34277. [DOI] [PubMed] [Google Scholar]
- 18.Lim DS, et al. ATM binds to β-adaptin in cytoplasmic vesicles. Proc Natl Acad Sci USA. 1998;95:10146–10151. doi: 10.1073/pnas.95.17.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Han YR, Plummer MR, Herrup K. Cytoplasmic ATM in neurons modulates synaptic function. Curr Biol. 2009;19:2091–2096. doi: 10.1016/j.cub.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vail G, et al. ATM protein is located on presynaptic vesicles, and its deficit leads to failures in synaptic plasticity. J Neurophysiol. 2016;116:201–209. doi: 10.1152/jn.00006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bateup HS, et al. Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron. 2013;78:510–522. doi: 10.1016/j.neuron.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foerster BR, et al. An imbalance between excitatory and inhibitory neurotransmitters in amyotrophic lateral sclerosis revealed by use of 3-T proton magnetic resonance spectroscopy. JAMA Neurol. 2013;70:1009–1016. doi: 10.1001/jamaneurol.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudenko A, et al. Loss of cyclin-dependent kinase 5 from parvalbumin interneurons leads to hyperinhibition, decreased anxiety, and memory impairment. J Neurosci. 2015;35:2372–2383. doi: 10.1523/JNEUROSCI.0969-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landis SC. Rat sympathetic neurons and cardiac myocytes developing in microcultures: Correlation of the fine structure of endings with neurotransmitter function in single neurons. Proc Natl Acad Sci USA. 1976;73:4220–4224. doi: 10.1073/pnas.73.11.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furshpan EJ, MacLeish PR, O’Lague PH, Potter DD. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: Evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci USA. 1976;73:4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spitzer NC. Neurotransmitter switching? No surprise. Neuron. 2015;86:1131–1144. doi: 10.1016/j.neuron.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman LS, McKeever MO, Okano HJ, Darnell RB. Beta-NAP, a cerebellar degeneration antigen, is a neuron-specific vesicle coat protein. Cell. 1995;82:773–783. doi: 10.1016/0092-8674(95)90474-3. [DOI] [PubMed] [Google Scholar]
- 28.Smith RJ, Savoian MS, Weber LE, Park JH. Ataxia telangiectasia mutated (ATM) interacts with p400 ATPase for an efficient DNA damage response. BMC Mol Biol. 2016;17:22. doi: 10.1186/s12867-016-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandes N, et al. DNA damage-induced association of ATM with its target proteins requires a protein interaction domain in the N terminus of ATM. J Biol Chem. 2005;280:15158–15164. doi: 10.1074/jbc.M412065200. [DOI] [PubMed] [Google Scholar]
- 30.Lavin MF, et al. Functional consequences of sequence alterations in the ATM gene. DNA Repair (Amst) 2004;3:1197–1205. doi: 10.1016/j.dnarep.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Schafer MK, Mahata SK, Stroth N, Eiden LE, Weihe E. Cellular distribution of chromogranin A in excitatory, inhibitory, aminergic and peptidergic neurons of the rodent central nervous system. Regul Pept. 2010;165:36–44. doi: 10.1016/j.regpep.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jazayeri A, et al. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- 33.Pizzamiglio L, et al. New role of ATM in controlling GABAergic tone during development. Cereb Cortex. 2016;26:3879–3888. doi: 10.1093/cercor/bhw125. [DOI] [PubMed] [Google Scholar]
- 34.Eichler SA, Meier JC. E-I balance and human diseases: From molecules to networking. Front Mol Neurosci. 2008;1:2. doi: 10.3389/neuro.02.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahama I, et al. Motor pathway degeneration in young ataxia telangiectasia patients: A diffusion tractography study. Neuroimage Clin. 2015;9:206–215. doi: 10.1016/j.nicl.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoche F, et al. Neurodegeneration in ataxia telangiectasia: What is new? What is evident? Neuropediatrics. 2012;43:119–129. doi: 10.1055/s-0032-1313915. [DOI] [PubMed] [Google Scholar]
- 37.Hoche F, et al. Cognitive phenotype in ataxia-telangiectasia. Pediatr Neurol. 2014;51:297–310. doi: 10.1016/j.pediatrneurol.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 38.Fritschy JM. Epilepsy, E/I balance and GABA(A) receptor plasticity. Front Mol Neurosci. 2008;1:5. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verstreken P, et al. Tweek, an evolutionarily conserved protein, is required for synaptic vesicle recycling. Neuron. 2009;63:203–215. doi: 10.1016/j.neuron.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen X, Chen J, Li J, Kofler J, Herrup K. Neurons in vulnerable regions of the Alzheimer’s disease brain display reduced ATM signaling. eNeuro. 2016;3:ENEURO.0124-15.2016. doi: 10.1523/ENEURO.0124-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cicero S, Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J Neurosci. 2005;25:9658–9668. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almeida CG, et al. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Caldeira MV, et al. Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem. 2007;282:12619–12628. doi: 10.1074/jbc.M700607200. [DOI] [PubMed] [Google Scholar]
- 44.Zhao T, et al. A user-friendly two-color super-resolution localization microscope. Opt Express. 2015;23:1879–1887. doi: 10.1364/OE.23.001879. [DOI] [PubMed] [Google Scholar]
- 45.Betzig EP, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 46.Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat Methods. 2006;3:793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui Y, et al. Activation of the Rab7 GTPase by the MON1-CCZ1 complex is essential for PVC-to-vacuole trafficking and plant growth in Arabidopsis. Plant Cell. 2014;26:2080–2097. doi: 10.1105/tpc.114.123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.