Significance
Hybridization between species can produce reproductively isolated lineages by combining parental genotypes in novel ways. Here, we used thousands of genetic markers to demonstrate that the recently rediscovered golden-crowned manakin represents an avian hybrid species from the Amazon basin. This hybrid species has a unique golden-colored crown patch used for display, which differs from the brilliant white coloration of the parental species. We used microscopy to demonstrate that, despite its unique coloration, the crown has intermediate color-producing morphological features at the nanoscale. We propose that these intermediate features disrupted the high reflectivity of the parental species, resulting in a dull hybrid population. Selection then sequestered carotenoids to the crown to compensate for its low reflectivity.
Keywords: hybrid speciation, structural color, ornamentation, Amazon, Lepidothrix vilasboasi
Abstract
Hybrid speciation is rare in vertebrates, and reproductive isolation arising from hybridization is infrequently demonstrated. Here, we present evidence supporting a hybrid-speciation event involving the genetic admixture of the snow-capped (Lepidothrix nattereri) and opal-crowned (Lepidothrix iris) manakins of the Amazon basin, leading to the formation of the hybrid species, the golden-crowned manakin (Lepidothrix vilasboasi). We used a genome-wide SNP dataset together with analysis of admixture, population structure, and coalescent modeling to demonstrate that the golden-crowned manakin is genetically an admixture of these species and does not represent a hybrid zone but instead formed through ancient genetic admixture. We used spectrophotometry to quantify the coloration of the species-specific male crown patches. Crown patches are highly reflective white (snow-capped manakin) or iridescent whitish-blue to pink (opal-crowned manakin) in parental species but are a much less reflective yellow in the hybrid species. The brilliant coloration of the parental species results from nanostructural organization of the keratin matrix feather barbs of the crown. However, using electron microscopy, we demonstrate that the structural organization of this matrix is different in the two parental species and that the hybrid species is intermediate. The intermediate nature of the crown barbs, resulting from past admixture appears to have rendered a duller structural coloration. To compensate for reduced brightness, selection apparently resulted in extensive thickening of the carotenoid-laden barb cortex, producing the yellow crown coloration. The evolution of this unique crown-color signal likely culminated in premating isolation of the hybrid species from both parental species.
Hybridization is a common phenomenon in nature and can either prevent or promote speciation. Introgressive gene flow erodes population distinctions and prevents speciation, but the introgression of select genes into new genetic backgrounds may also increase the genetic diversity on which evolution can act (1–4). Hybridization might also generate new species by combining parental genomes in novel ways that render hybrids reproductively isolated from parental species. Hybrid speciation occurs commonly in plants, and involves speciation by polyploidy and less often without polyploidy, the latter case known as “homoploid hybrid speciation” (5–7). Hybrid speciation in animals has generally been considered rare and of limited evolutionary significance (8–11). However, genome-wide assessments of nonmodel organisms have led to a number of suspected cases of homoploid hybrid speciation in animals (1, 10). For example, some evidence has been presented for insects (12–16), fishes (17–19), mammals (20–22), and birds (23–28). However, in most putative cases of hybrid speciation in animals it remains unknown whether hybridization itself produced reproductive isolation or whether hybrid populations became geographically isolated, with reproductive isolation evolving as a product of divergence in geographic isolation rather than from the original hybridization event. The former would generally be considered a strong case of hybrid speciation in which reproductive isolation evolved as a direct consequence of the initial admixture event (10). In the case of birds, three examples of potential hybrid species have been analyzed using genetic data—the Audubon’s warbler (23), the Italian sparrow (24–27, 29), and the Hawaiian duck (28)—and in all cases the putative hybrid species are morphologically intermediate and continue to hybridize with their parental species, thus demonstrating that hybridization has not yet led to full reproductive isolation.
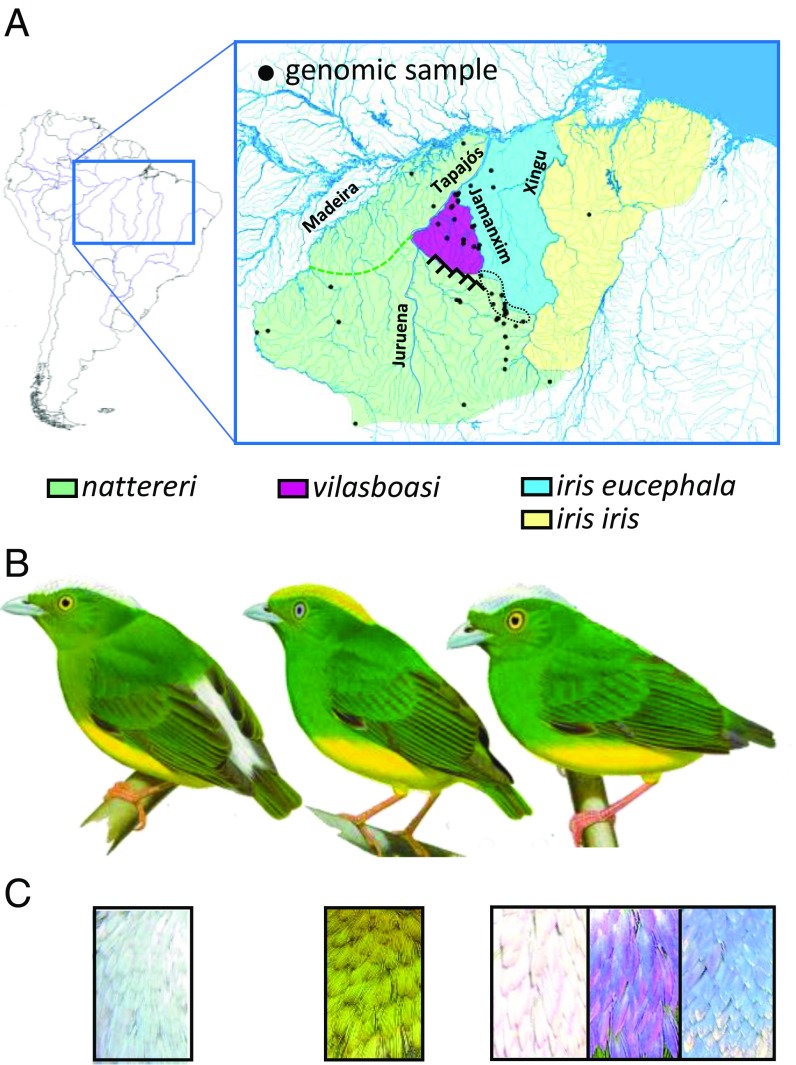
In 1957, the upland forest-dwelling passerine golden-crowned manakin (Lepidothrix vilasboasi) was discovered in a small geographic region in the headwaters of the Cururu-ri River in Pará state, Amazonian Brazil. The discovery was unusual, given that no other endemic species of birds are known from this region (30). The geographic range of L. vilasboasi lies between the ranges of two other congeners, the opal-crowned (Lepidothrix iris) and snow-capped (Lepidothrix nattereri) manakins (Fig. 1A), which form a superspecies (31) and were later identified as sister taxa (32). This led some ornithologists to suggest that L. vilasboasi might represent a rare hybrid phenotype between these two species (33). However, L. vilasboasi has a golden-colored crown, while L. nattereri and L. iris have shiny whitish and opalescent-colored crowns, respectively (Fig. 1 B and C). Given the difference in crown color and the importance of this trait for female mate choice in manakins, L. vilasboasi is generally considered a distinct biological species (30, 34). Determining the status of L. vilasboasi has been difficult because the species had not been observed since the 1950's until 2002, when it was rediscovered with a substantial but geographically restricted population (35). Preliminary phylogenetic analyses with mitochondrial DNA nest L. vilasboasi within adjacent populations of L. iris (36). The lack of distinctive mitochondrial DNA could be compatible either with very recent divergence of L. vilasboasi or with mitochondrial introgression between L. iris and L. vilasboasi. A third possibility is that L. vilasboasi represents a genetic admixture between L. nattereri and L. iris and therefore is of hybrid origin. Here we used a large genome-wide SNP dataset to assess whether L. vilasboasi represents its own independent evolutionary lineage, a hybrid zone between L. nattereri and L. iris, or a hybrid species. We show that L. vilasboasi represents a genetically admixed population from the two proposed parental species. We then used spectrometry and transmission electron microscopy to determine whether the structural elements that produce the distinctive crown coloration of L. vilasboasi have morphological components intermediate between the two parental species, which would suggest that reproductive isolation driven by crown color evolved as a consequence of the admixture event.
Fig. 1.
The three species of Lepidothrix manakin distributed east of the Madeira River in Amazonia. (A) Outside the headwater regions, rivers and mountains largely demarcate the boundaries of these species with the Tapajós River separating L. nattereri from the other species, the Jamanxim River possibly separating L. vilasboasi from L. iris (however, see text), and the Xingu River separating the two subspecies of L. iris. The Cachimbo range (inverted V’s) may provide a partial barrier separating L. nattereri and L. vilasboasi. All these taxa are known or presumed to come into geographic contact in headwater regions where rivers and mountains cease to demarcate taxa boundaries. A contact zone possessing both L. nattereri and L. iris eucephala individuals as well as hybrids between them is demarcated by the black dotted contour. (B and C) Males of these species differ in the presence (L. nattereri) or absence (other species) of a white rump patch (B) and in the color of the crown patch (C). The crown patch in L. iris is iridescent and varies from brilliant white (its usual look, which is very similar to L. nattereri) to blue or purple, depending on the angle of light. Males of the two subspecies of L. iris distributed on either side of the Xingu River are almost identical in plumage, with L. iris iris possessing a thin green strip between the upper mandible and the crown patch and with the crown patch extending all the way to the mandible in L. iris eucephala. Females (not shown) appear like males but lack the contrasting crown and rump patches and do not differ appreciably among species. The two subspecies of L. nattereri (L. nattereri nattereri north of the green dashed line and L. nattereri gracilis to the south) do not differ in male plumage. Illustrations of species used with permission from Handbook of Birds of World (30).
Results
Genetic Patterns.
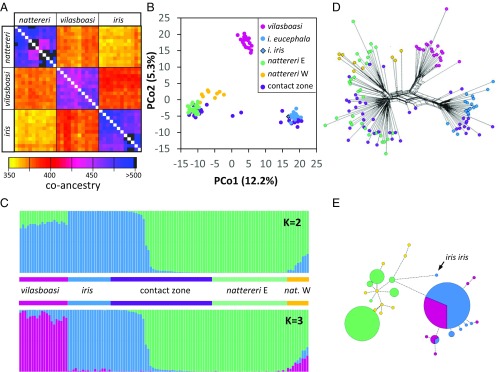
Genetic analyses from genome-wide SNP and mtDNA data suggest that L. vilasboasi is genetically admixed between L. nattereri and L. iris. First, for genome-wide SNPs, we find that L. vilasboasi individuals are genetically more similar to one or the other putative parental species than the parental species are to each other based on coancestry (P < 0.00001, two-tailed t test) (Fig. 2A and SI Appendix, Table S1) and fixation index (FST) (P = 0.000, based on 1,000 bootstrap datasets) (SI Appendix, Table S1), which is consistent either with a hybrid origin or with L. vilasboasi representing a distinct lineage that has had substantial gene flow from the other species. FST values were significantly greater than 0 but were low (0.14 to 0.2) compared with other Amazonian species pairs (37) suggesting recent divergence. Second, a principal coordinate analysis (PCoA) indicates an intermediate position of L. vilasboasi along the first principal coordinate (PCo1), as is consistent with genetic admixture (Fig. 2B). While the position of L. vilasboasi along PCo1 is similar to that of several L. iris x L. nattereri hybrids from a recently discovered contact zone (37), L. vilasboasi individuals are differentiated in PCo2 from both the parental species and their hybrids, as expected if L. vilasboasi has had sufficient time for sorting of some ancestral alleles or evolved distinct alleles following the initial admixture event. PCoA results were mirrored by Bayesian analyses of population structure and admixture (Fig. 2C). The best-fit models supported either two or three distinct populations (with only a modest increase in likelihood under the three-population model) (SI Appendix, section 3 and Fig. S2) differing in whether L. vilasboasi was treated as a distinct population. The three-population model recognized L. vilasboasi as distinct, albeit with substantial admixture with L. iris. In contrast, in the two-population model, L. vilasboasi was not a distinct population but instead derived 15–20% of its genome from L. nattereri and the remainder from L. iris. These results, for both the structure plots and PCoA, are consistent with a hybrid origin of L. vilasboasi resulting in a population that has become weakly differentiated genetically since its initial origin but could also be consistent with L. vilasboasi representing its own, recently differentiated independent lineage. Third, the phylogenetic network connecting the three species shows extensive reticulation, demonstrating that the three species likely do not share a bifurcating, tree-like history (Fig. 2D), although this analysis does not discriminate between reticulation derived from admixture versus ancestral polymorphism. Finally, our mtDNA haplotype network (Fig. 2E) indicates a lack of differentiation of L. vilasboasi haplotypes from adjacent L. iris haplotypes as previously reported (36). Our sole sequenced sample of L. iris iris found east of the Xingu River was highly differentiated in mtDNA from L. iris eucephala found west of the Xingu. In contrast, the two subspecies of L. iris were not differentiated in the genome-wide SNP data (Fig. 2B). This lack of nuclear differentiation might suggest that males freely cross the Xingu River, preventing differentiation in nuclear markers, while females are more dispersal limited, with the Xingu forming a barrier to gene flow and promoting mtDNA differentiation. If correct, then L. vilasboasi may have inherited the mtDNA of L. iris eucephala as a consequence of a past hybridization event. Alternatively, the mtDNA of L. iris eucephala might have originated in L. vilasboasi following its formation either as a hybrid species or as a nonhybrid lineage and then more recently introgressed across species boundaries into adjacent populations of L. iris west of the Xingu River. Both scenarios for the mtDNA data are consistent with hybridization playing a role in the formation of this species complex. We point out that results from each of these genetic analyses are consistent with an initial hybrid origin for L. vilasboasi, but some of these results could also be consistent with a nonhybrid origin. We next used coalescent modeling to test these alternatives.
Fig. 2.
Genetic analyses of Lepidothrix in southern Amazonia. (A) Coancestry matrix using the 12 individuals with the highest coverage and least amount of missing data for each species. (B) PCoA showing the first two coordinates and the percentage of variation explained by each. (C) Bayesian estimates of population structure and admixture obtained for two and three populations (K = 2 and K = 3). (D) Phylogenetic network showing genome reticulation. (E) Mitochondrial DNA haplotype network with the sole individual of L. iris iris indicated. In B–E, L. nattereri is divided into populations east (E) and west (W) of the Juruena River. Only nattereri E is included in A. Headwater populations of L. nattereri (nattereri E) come into geographic contact (contact zone) and form a narrow hybrid zone with an adjacent population of L. iris eucephala. Hybrids and parentals of both species were found in syntopy in this population. Contact zone individuals were not included in E.
Coalescent Test for Hybrid Origins.
Coalescent modeling of genome-wide, noncoding nuclear markers not closely linked to coding regions (and thus presumably neutral) clearly favored a hybrid speciation model leading to the formation of L. vilasboasi over models in which L. vilasboasi is sister to either L. nattereri or L. iris or is basal to these (Table 1). The maximum likelihood parameter estimates of the hybrid speciation model suggested that 62% (95% CI: 54–72%) of the genome of L. vilasboasi was derived from L. iris and 38% (28–46%) from L. nattereri (SI Appendix, Fig. S4), which is slightly different from the admixture proportions reported from our analyses of population structure (Fig. 2B). Assuming the neutral mutation rate calculated from pedigree analyses in Ficedula flycatchers (38) and a generation length of 2 y, L. nattereri and L. iris are estimated to have split 242 kya, with the hybrid-speciation event leading to L. vilasboasi occurring 158 kya. We also tested more complex models in which gene flow occurred among all three species. While a hybrid speciation model with gene flow received substantial support over a model without gene flow (SI Appendix, Table S3), the resulting parameter estimates relating to admixture proportions had broad CIs (SI Appendix, Fig. S4), suggesting that the signal in the data may not be sufficient to obtain precise estimates for this parameter from models of this complexity. Moreover, various issues related to genome reduction datasets are known to influence parameter estimates in coalescent models (39, 40), and so we treat all parameter estimates with caution. Nevertheless, the better fit of a hybrid speciation model with gene flow (SI Appendix, Table S3) suggests that gene flow among these species occurred following the hybrid-speciation event.
Table 1.
Support for coalescent models in which L. vilasboasi (V) arises with (A1) and without (T1, T2, T3) genetic admixture from L. nattereri (N) and L. iris (I)
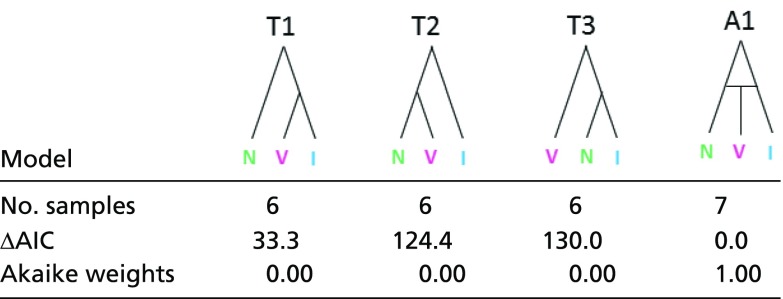 |
ΔAIC, change in Akaike information criterion.
Crown Feather Coloration.
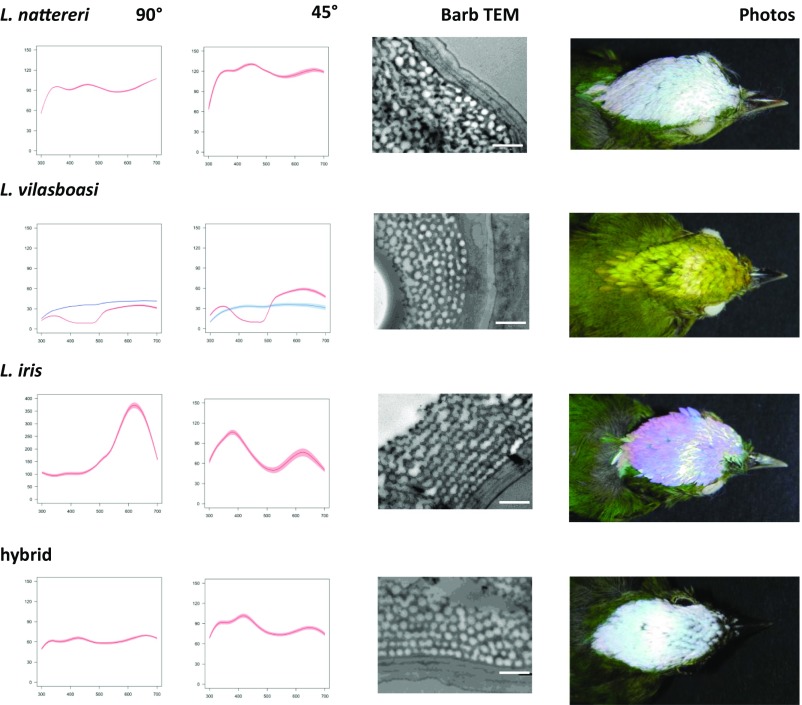
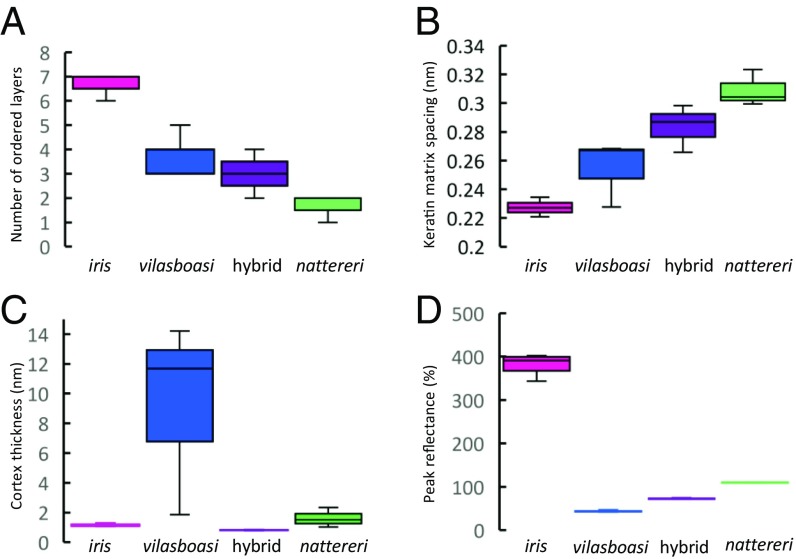
Feather coloration and the brilliance of the ornamental male crown patches differed in the three species (Figs. 3 and 4). The dramatic change in reflectance (with colors ranging from white to blue to pink) (Fig. 1C) with probe angle (UV/blue at 45° and red at 90°) in L. iris is indicative of strong iridescence, likely produced by the linearly arranged ordered layers of air and keratin in the spongy matrix (Fig. 3) (41–43). L. iris barbs also possessed a thin cortex (Fig. 4C) with some melanosomes present, while barbules were filled with melanosomes (SI Appendix, Fig. S5A). Melanosomes are thought to play little role in the crown coloration of L. iris (41). In contrast, L. nattereri and L. vilasboasi did not exhibit strong iridescence, with the former lacking or having few ordered layers in the spongy matrix and the latter having an intermediate number of layers (Figs. 3 and 4). L. nattereri possessed a brilliant white color with flatter reflectance spectra caused by incoherent scattering from a disordered matrix in the absence of melanosomes (Fig. 3 and SI Appendix, Fig. S5B). The melanin pigments in melanosomes can absorb incoherently scattered light at certain wavelengths (42–45). The absence of melanin allows a brilliant white coloration, as observed in albino individuals of normally blue- or black-colored avian species (44). Thus, both L. nattereri and L. iris possess brilliant crown coloration that is strongly influenced by the morphology of the spongy matrix, but the structural coloration of each species is obtained through different arrangements of the components of this matrix. In contrast, the sharp drop in reflectance below 500 nm in L. vilasboasi at both angles is characteristic of yellow pigmentation caused by carotenoids (46–48) that disappears after performing carotenoid extraction (Fig. 3). The unusually thick cortex of L. vilasboasi (Fig. 4C) contains structures that resemble carotenoid pigments reported for other bird species (46), and we did not observe these structures in L. iris or L. nattereri (SI Appendix, Fig. S5). Melanosomes were also present in the thinnest parts of the barb cortex and throughout the barbules (SI Appendix, Fig. S5C). The nanostructure of L. vilasboasi is mostly quasi-ordered, as in L. nattereri, and has an intermediate number of ordered layers of the keratin matrix (Fig. 4A). The observed yellow coloration is likely produced by a combination of light scattering by the keratin matrix and filtering by carotenoids as in yellow budgerigars (48). The average diameter of the air pockets in the keratin matrix of L. vilasboasi is also intermediate between the other two species (Fig. 4 A and B). L. vilasboasi exhibited much lower reflectance values (44% at peak) than L. nattereri (109%) or L. iris (380%), resulting in a much duller look both before and after carotenoid extraction. Crown feathers of the L. iris × L. nattereri F1-like hybrid have some iridescence, as in L. iris, but their reflectance is lower than in both parental species and is slightly higher than in L. vilasboasi (Fig. 4D). The nanostructure of the spongy matrix is also intermediate between that of the parental species (Fig. 3) with the spacing for the air pockets and the number of ordered layers very close to the values obtained for L. vilasboasi (Fig. 4 A and B). Unlike L. vilasboasi, the cortex of the hybrid is very thin (Fig. 4C) and contained few melanosomes (SI Appendix, Fig. S5D).
Fig. 3.
(Left) Percentage of reflectance (90° and 45° to feather). Percentage of reflectance is show before (red trace) and after (blue trace) carotenoid extraction in L. vilasboasi. Reflectance curves and their SEs are taken from five measures of two stacked feathers. (Center) Transmission electron micrographs (TEMs) of the barb cortex and keratin spongy layer of male crown patch. (Scale bars: 1 µm.) (Right) Crown patch photographs for three species of Lepidothrix and an F1-like L. nattereri × L. iris hybrid.
Fig. 4.
Properties of Lepidothrix male crown feathers shown as box-and-whisker plots. (A) The number of 2D layers. (B) Spacing of air pockets in the spongy keratin matrix of feather barbs. (C) Barb cortex thickness. (D) Peak reflectance measured from two stacked crown feathers on a dull black background at 90°.
Discussion
Hybrid speciation involves an admixture event between two species that produces a new lineage that, as a consequence of hybridization, is reproductively isolated from both parental species (1, 7, 10, 49, 50). Here, we present evidence that the geographically restricted and recently rediscovered L. vilasboasi represents a hybrid species of recent origin from the Amazon basin of Brazil. Our coalescent-based model-testing framework strongly favored a hybrid origin over nonhybrid scenarios (Table 1 and SI Appendix, Table S1), while a variety of genetic analyses add complementary support that this species is genetically admixed between L. iris and L. nattereri (Fig. 2). Haffer (51), one of the most influential Amazonian ornithologists of the 20th century, had earlier argued that L. vilasboasi might represent rare hybrid individuals along a contact zone between L. iris and L. nattereri. If L. vilasboasi represented a hybrid zone, then we would expect to find only F1 hybrids (all with hybrid indexes close to 0.5) if these hybrids were sterile or if later-generation hybrids were inviable or a range of hybrid genotypes with varying admixture proportions between the two parental species. Instead, our results show that the admixture proportions of L. vilasboasi depart from the 0.5 expectation for F1 hybrids and are tightly coupled around a value of 0.2 (ca. 80% composition of L. iris and 20% composition of L. nattereri) (Fig. 2B and SI Appendix, Fig. S3). Heterozygosity of L. vilasboasi is also lower than expected for early generation hybrids (SI Appendix, Fig. S3). Together, these results reject the hybrid zone hypothesis and instead demonstrate that L. vilasboasi is a uniquely evolving lineage of hybrid origin.
A hybrid origin of L. vilasboasi is surprising, given its unique yellow crown color, which differentiates it from all other members of its genus (30, 34). The higher reflectance observed in the crown patches of the parental species makes them highly visible in the dark forest interior where males form leks to attract females. The high reflectance of the parental species results from the organization of the keratin matrix of feather barbs, but this organization differed between these two species. The white crown color of L. nattereri is produced by incoherent scattering of light from the disordered array of air spaces in the keratin spongy layer. In contrast, in L. iris the air spaces are arranged in highly ordered layers—unique in the bird world—that produce the strong iridescent coloration in combination with a flattened barb shape, a restricted distribution of melanosomes, and the presence of vacuoles at the center of barb medullary cells (43). As expected for a hybrid lineage, L. vilasboasi is intermediate between the parental species in both the number of ordered layers and the distance of air spaces in the keratin spongy matrix (Fig. 4 A and B). In addition, it derives melanosomes in the barbules and barb cortex from L. iris but is unique in having a thickened barb cortex laden with carotenoids, which lend it its yellow appearance. The intermediate nature of the keratin matrix appears to have disrupted both the brilliant iridescence found in L. iris and the brilliant white found in L. nattereri, as suggested by the much lower reflectance values in the hybrid species. Extraction of the carotenoids of these feathers resulted in a dull grayish-white appearance in L. vilasboasi (Fig. 3 and SI Appendix, Fig. S6), which probably is close to the original appearance of the crown following the initial hybridization event, as also suggested by the pattern of crown reflectance recorded for L. iris × L. nattereri hybrids (see below). To compensate, we propose that sexual selection, probably mediated by female choice, resulted in extensive thickening of the carotenoid-laden barb cortex, with a yellow crown as the outcome (SI Appendix, Fig. S7). The evolution of this unique yellow crown color was thus not a direct consequence of hybridization but instead was selected to compensate for the significant loss of reflectance following hybridization. It is plausible that the yellow crown could have evolved in a relatively short period, given that sexual selection can accelerate the evolution of male plumage characters related to premating isolation (52). Preference for the novel yellow crown phenotype in hybrid females (not tested here) also could have evolved in parallel after the initial hybridization event. Experimental interspecific crosses in fishes (53) and insects (54–56) have demonstrated that early generation hybrid females often show mating preferences for novel hybrid phenotypes, and such mating preferences are believed to promote hybrid speciation (53, 54, 57). Furthermore, it is documented in other manakin species that female choice can promote the establishment of certain hybrid phenotypes in some populations (58), while in Darwin’s finches the preference for hybrid phenotypes has been documented to occur in as little as three generations following hybridization (59). As crown patches are displayed prominently during courtship of lekking males (30, 34), it is likely that this character plays a strong role in premating reproductive isolation between the Lepidothrix species. In contrast, song, which is used by the males to attract females to leks, is not known to differ among these species (60), and we have successfully used the songs of L. iris to attract L. nattereri females, showing that songs are not causing strong premating reproductive isolation.
One way to support the hypothesis for the dull origin of L. vilasboasi would be to observe hybrids between L. iris and L. nattereri. L. iris and L. nattereri currently form a narrow hybrid zone in the southern part of their distributions (37). The sole adult male F1-like hybrid that we collected had structural features of the barb keratin matrix in its crown that were intermediate between those in the parental species and closely matched L. vilasboasi (Fig. 3 and 4). Like L. vilasboasi, this hybrid individual had greatly reduced reflectance of the crown, although the color remained whitish and not yellow as in L. vilasboasi. The duller crown in the hybrid supports our hypothesis that L. vilasboasi began as a dull-crowned hybrid swarm before sequestering carotenoids in the crown feathers. The even duller look of L. vilasboasi compared with the early generation L. iris × L. nattereri hybrid might have derived from increased recombination of the parental genomes in later-generation hybrids that resulted in still further disruption of the structural elements fine-tuned to generate high reflectivity.
Despite species-specific ornamentation, reproductive isolation between L. nattereri, L. iris, and L. vilasboasi appears to be incomplete, as is often true of other morphologically distinctive manakins and for birds in general. First, coalescent model testing with and without gene flow favored a model with ongoing migration among each of the three species following the initial admixture event leading to L. vilasboasi. However, the maximum likelihood estimates of migration rates in SNPs far from coding regions (and which are presumably neutral) are slightly less than a single individual per generation (SI Appendix, Fig. S4). This level of gene flow is unlikely to result in homogenization of the gene pools of these species. Second, populations of L. nattereri west of the Tapajós and Juruena Rivers possess a considerable genome proportion of L. iris and/or L. vilasboasi origin, as shown by structure and PCoA plots (Fig. 2 A and B). The introgression appears to be one-sided, with L. nattereri genes not occurring in L. iris along the east bank of the Tapajós River. A likely explanation is that the Tapajós River, which forms a barrier between these and many other pairs of species (37, 51), may have originally flowed further west. A change in its course brought it east to its current position, transferring a small population of L. iris and possibly also L. vilasboasi to the west bank of the Tapajós, where they became genetically subsumed into adjacent populations of L. nattereri. The proportion of L. iris or L. vilasboasi genes in this L. nattereri population declines from north to south, consistent with this interpretation (in Fig. 2B these individuals are arranged from left to right corresponding to a south-to-north gradient). Changes in the course of the Tapajós River have also been proposed as an explanation for complex phylogeographical patterns of other bird species in the region (61–65).
A third indicator of incomplete reproductive isolation comes from a parapatric contact zone between L. nattereri and L. iris in the headwater region between the Teles Pires and Xingu Rivers (37). We found both species syntopically at a few localities in this contact zone (Fig. 1A). Genetic analyses identified only two early generation hybrids (F1, F2, or backcrosses of these with parentals) among our contact zone sample (SI Appendix, Fig. S3), although a small amount of introgression is seen in a substantial number of individuals of either species from the contact zone (Fig. 2 A and B). The small number of early generation hybrids suggests substantial reproductive isolation between L. iris and L. nattereri, which may include premating isolation (resulting in the formation of few early generation hybrids) and also might include postzygotic selection against early-generation hybrids (e.g., few early generation hybrids would be found). If the parental species possess genetic incompatibilities, then postzygotic isolation could rapidly be generated in the hybrid population, leading to L. vilasboasi through the mosaic inheritance of alleles at incompatibility loci (26, 27, 66), and tests are needed to assess levels of postzygotic isolation. The formation of these species, including the hybrid-speciation event leading to L. vilasboasi, are likely to represent cases in which moderate pre- and postmating isolating barriers are in place but fail to prevent small amounts of hybridization between species.
The combined effect of river barriers and wet forest retraction during past climatic oscillations may have provided periods of allopatry (33, 37, 67, 68) facilitating the origination of L. vilasboasi following hybridization of the parental species. L. vilasboasi is located in a very restricted geographical region west of the Jamanxim River and east of the Cachimbo range and Tapajós River. These geographic features may form partial barriers to gene flow in the north (although at one locality L. vilasboasi and L. iris occur syntopically along the east bank of the Jamanxim River in our samples, and contact between L. vilasboasi and L. nattereri around the north end of the Cachimbo range is likely, given continuous habitat and a lack of river barriers) (SI Appendix, Fig. S6), but there is currently no obvious barrier to the south where all three species are likely to come into contact (SI Appendix, Fig. S6). We suspect that during a past humid period L. nattereri and L. iris came into geographic contact and formed a hybrid zone in the region now occupied by L. vilasboasi. With the onset of the next glacial period, the southern edge of humid forest in Amazonia retracted to the north (37), isolating the hybrid zone populations in this region from the broader ranges of L. nattereri and L. iris and forcing them to collapse into a hybrid swarm. The disruption of the brilliant structural crown colors of the parental species in the hybrid swarm precipitated the sequestering of carotenoids in crown feathers, leading to the formation of the unique ornamentation of L. vilasboasi. During interglacials, humid forest expanded to the south, bringing L. vilasboasi back into contact with its parental species, as is likely at present (SI Appendix, Fig. S6). Although our estimates of the timing of these events vary depending on whether we allow postspeciation gene flow between species (i.e., migration essentially doubles the estimated dates), model estimates nevertheless place the separation of L. nattereri and L. iris within the past 600,000 y and the hybrid-speciation event within the past 260,000 y, coinciding with the Mid- to Late-Pleistocene severe climatic cycles that most likely caused oscillation of the southern extent of humid forest in Amazonia (69). It thus seems likely that, following its initial isolation, L. vilasboasi has come back into contact with the other parental species during one or two interglacial periods when humid forests expanded maximally to the south and has maintained its morphological distinction and species status despite this contact.
We have presented substantial evidence that L. vilasboasi is of hybrid origin. Whether L. vilasboasi also represents a hybrid species depends largely on how hybrid species are defined. A recent review (10) narrowly defined hybrid species as having had a hybrid origin, exhibiting reproductive isolation from parental species, and in which reproductive isolation originated as a direct consequence of hybridization. Here we demonstrate a hybrid origin and argue that the unique yellow crown likely results in substantial (but incomplete) premating reproductive isolation. However, we favor a scenario in which the yellow crown coloration originated as an indirect, rather than a direct, consequence of the hybrid event. We argue that the initial admixture event may have triggered a chain of events that ultimately and indirectly resulted in reproductive isolation, as proposed here, and that such examples should also be considered valid hybrid-speciation events. We do caution, however, that a key aspect of our scenario remains speculative. We have demonstrated that both L. vilasboasi and an early generation hybrid between the parental species have a duller crown color associated with an intermediate nanostructural organization of the keratin matrix of crown barbs. We argue that selection favored sequestration of carotenoids (which are present across the yellow body plumage of these birds) in crown feathers as a way to render dull-crowned males more attractive to females. However, an alternative is that the yellow crown coloration evolved for reasons unrelated to hybridization. Unfortunately, we are not aware of any way to test these alternatives, short of trying to replicate this chain of events through impractical laboratory-based formation of a hybrid swarm. Nevertheless, it seems highly unlikely that L. vilasboasi would have evolved a dull yellow crown from the brilliant white or opalescent crown of the parental species without the intermediate nanostructural arrangement of the barbs in the hybrids reducing both iridescence and the incoherently scattered white, as suggested by early generation hybrids between L. iris and L. nattereri.
In conclusion, we provide evidence of a hybrid origin for a morphologically distinctive Neotropical bird species. Our results highlight the importance of hybridization both as a source of genetic variation for the speciation process and as a source of evolutionary novelty. This study adds to the growing number of putative cases of homoploid hybrid speciation in terrestrial vertebrates, particularly birds. In contrast to other reported avian examples, such as the Italian sparrow (24–27, 29), the Audubon’s warbler (23) and the Hawaiian duck (28), the yellow-crown phenotype of L. vilasboasi is not intermediate between the parentals. This yellow crown coloration could represent a novel transgressive phenotype that arose purely through the recombination of parental alleles (1, 7, 49, 50, 70, 71). Instead, our analyses of crown feather coloration and nanostructure suggest that the intermediate and mosaic nature of the nanostructural elements of the hybrids resulted in a dull white-gray crown ornamentation with subsequent sexual selection resulting in the sequestration of carotenoids leading to the novel yellow coloration (SI Appendix, Fig. S7). Although our current understanding of the levels of both pre- and postzygotic isolation is incomplete, the tight coupling of admixture proportions and low levels of heterozygosity suggest our sample of L. vilasboasi does not contain any early generation hybrids with parental species despite the syntopic presence of L. vilasboasi and L. iris at one of our sampling localities and the likely presence of parapatric contact zones with the parental species in the western and southern portions of its range (SI Appendix, Fig. S6). These results are consistent with substantial reproductive isolation, as also suggested by the fairly low migration rates estimated between L. vilasboasi and L. iris/nattereri. Nevertheless, our modeling results support ongoing interspecies migration at neutral loci. The unique crown ornamentations of L. vilasboasi, L. iris, and L. nattereri appear to have evolved despite ongoing episodes of gene flow. Future work should (i) investigate the extent to which female L. vilasboasi prefer the yellow crown coloration over the crown color of the parental species; (ii) sample further putative parapatric contact zones to assess levels of hybridization; and (iii) evaluate whether genetic incompatibilities exist and are the result of mosaic inheritance of preexisting genetic variation found in the parental species, as suggested for other hybrid species.
Methods
Sampling Design.
We collected specimen-vouchered genetic samples (deposited at the Museu Paraense Emílio Goeldi) during four field trips to Pará and Mato Grosso states of Brazil during 2012 (see further details in ref. 37), 2014, and 2015 and obtained additional samples from museum collections for a total of 144 individuals with tissue samples [21 L. vilasboasi, 47 L. nattereri, 21 L. iris eucephala, 1 L. iris iris, and 54 individuals from the Xingu/Teles Pires headwaters contact zone between L. nattereri and L. iris eucephala (Fig. 1A and Dataset S1)]. Approval was obtained from the Brazilian Government (collecting permits 4253-1, 40173-1, and 6581-1).
DNA Sequencing.
A 966-bp fragment of the mtDNA gene cytochrome b was sequenced for 109 individuals using standard protocols (SI Appendix, section 2). Genome-reduction genotype-by-sequencing was used to obtain a genome-wide sample of SNPs. We generated three datasets that differed in the individuals included and in filtering strategy. Dataset 1 retained 16,281 SNPs for 12 individuals of each species. Dataset 2 retained 7,394 SNPs for 120 individuals. Dataset 3 down-sampled individuals at each of 10,298 SNPs to retain 42 gene copies per SNP (12 L. iris eucephala, 10 L. vilasboasi, 20 L. nattereri). For details, see SI Appendix, section 1.
Genetic Analyses.
Dataset 1 was used for coancestry estimates, and Dataset 2 was used to characterize genetic structure, population differentiation, and admixture patterns using PCoA, structure plots (72), phylogenetic networks, and pairwise FST. A haplotype network was generated for the mtDNA dataset. For details, see SI Appendix, section 2.
Coalescent Modeling.
We used coalescent modeling in a composite likelihood framework to compare the fit of six models for the origin of L. vilasboasi (see models in Table 1) using Dataset 3. These models differ in whether L. vilasboasi originated with or without genetic admixture between L. iris and L. nattereri and whether that admixture occurred as a point event in time, through ongoing gene flow, or a combination of the two. For details, see SI Appendix, section 3.
Spectrophotometry and Microscopy.
Reflectance of male crown feathers for each species was obtained with a spectrometer held at an angle of 90° and 45°. For L. vilasboasi, this was done both before and after carotenoid extraction. Transmission electron microscopy was used in one feather per species (and one from the early generation hybrid individual) to quantify the number of ordered layers perpendicular to the barb surface and the spacing of air cavities in the keratin matrix of the barbs, barb cortex thickness, and to corroborate the presence of melanosomes. For details, see SI Appendix, sections 4 and 5.
Supplementary Material
Acknowledgments
We thank the many landowners in Brazil who facilitated fieldwork and museums that donated samples for genetic analysis. Asher Cutter provided advice on estimating and interpreting linkage disequilibrium; and Irby Lovette, Bob Murphy, Belinda Chang, and Maya Faccio provided useful feedback on the manuscript. Maya Faccio and Paola Pulido-Santacruz assisted with field collection. Transmission electron microscopy was performed with the help of Bruno Chue (Centre for the Neurobiology of Stress, University of Toronto Scarborough). Computations were performed on the General Purpose and Sandy Bridge computing clusters at the SciNet High Performance Computing Consortium, which is funded by the Canadian Foundation for Innovation, the Government of Ontario, and the University of Toronto. Research funding was provided by a fellowship from the Mexican Council for Science and Technology (to A.O.B.-G.); Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant RGPIN-2016-06538 and NSERC Discovery Accelerator Grant 492890 (to J.T.W.); the University of Toronto Scarborough Office of the Vice-Principal Research Competitiveness Fund (J.T.W.); and Brazilian Research Council for Scientific and Technological Development Grants ‘‘INCT em Biodiversidade e Uso da Terra da Amazônia’’ 574008/2008-0, 471342/2011-4, 310888/2012-2, and 306843/2016-1 and Amazonia Foundation for Studies and Research Support Grant ICAAF 023/2011 (to A.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the National Center for Biotechnology Information GenBank database (accession nos. MG662263–MG662371) and the Sequence Read Archive (SRA) database (accession nos. SRR6370333–SRR6370542).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717319115/-/DCSupplemental.
References
- 1.Abbott R, et al. Hybridization and speciation. J Evol Biol. 2013;26:229–246. doi: 10.1111/j.1420-9101.2012.02599.x. [DOI] [PubMed] [Google Scholar]
- 2.Edwards SV, Potter S, Schmitt CJ, Bragg JG, Moritz C. Reticulation, divergence, and the phylogeography-phylogenetics continuum. Proc Natl Acad Sci USA. 2016;113:8025–8032. doi: 10.1073/pnas.1601066113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallet J, Besansky N, Hahn MW. How reticulated are species? BioEssays. 2016;38:140–149. doi: 10.1002/bies.201500149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedrick PW. Adaptive introgression in animals: Examples and comparison to new mutation and standing variation as sources of adaptive variation. Mol Ecol. 2013;22:4606–4618. doi: 10.1111/mec.12415. [DOI] [PubMed] [Google Scholar]
- 5.Rieseberg LH. Hybrid origins of plant species. Annu Rev Ecol Syst. 1997;28:359–389. [Google Scholar]
- 6.Gross BL, Rieseberg LH. The ecological genetics of homoploid hybrid speciation. J Hered. 2005;96:241–252. doi: 10.1093/jhered/esi026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott R, Hegarty M, Hiscock S, Brennan A. Homoploid hybrid speciation in action. Taxon. 2010;59:1375–1386. [Google Scholar]
- 8.Coyne JA, Orr HA. Speciation. Sinauer Associates; Sunderland, Massachusetts: 2004. [Google Scholar]
- 9.Servedio MR, Hermisson J, van Doorn GS. Hybridization may rarely promote speciation. J Evol Biol. 2013;26:282–285. doi: 10.1111/jeb.12038. [DOI] [PubMed] [Google Scholar]
- 10.Schumer M, Rosenthal GG, Andolfatto P. How common is homoploid hybrid speciation? Evolution. 2014;68:1553–1560. doi: 10.1111/evo.12399. [DOI] [PubMed] [Google Scholar]
- 11.Barton NH. The role of hybridization in evolution. Mol Ecol. 2001;10:551–568. doi: 10.1046/j.1365-294x.2001.01216.x. [DOI] [PubMed] [Google Scholar]
- 12.Gompert Z, et al. Admixture and the organization of genetic diversity in a butterfly species complex revealed through common and rare genetic variants. Mol Ecol. 2014;23:4555–4573. doi: 10.1111/mec.12811. [DOI] [PubMed] [Google Scholar]
- 13.Kunte K, et al. Sex chromosome mosaicism and hybrid speciation among tiger swallowtail butterflies. PLoS Genet. 2011;7:e1002274. doi: 10.1371/journal.pgen.1002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mavárez J, et al. Speciation by hybridization in Heliconius butterflies. Nature. 2006;441:868–871. doi: 10.1038/nature04738. [DOI] [PubMed] [Google Scholar]
- 15.Nice CC, et al. Hybrid speciation and independent evolution in lineages of alpine butterflies. Evolution. 2013;67:1055–1068. doi: 10.1111/evo.12019. [DOI] [PubMed] [Google Scholar]
- 16.Heliconius Genome Consortium Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature. 2012;487:94–98. doi: 10.1038/nature11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberici da Barbiano L, Gompert Z, Aspbury AS, Gabor CR, Nice CC. Population genomics reveals a possible history of backcrossing and recombination in the gynogenetic fish Poecilia formosa. Proc Natl Acad Sci USA. 2013;110:13797–13802. doi: 10.1073/pnas.1303730110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller I, et al. Population genomic signatures of divergent adaptation, gene flow and hybrid speciation in the rapid radiation of Lake Victoria cichlid fishes. Mol Ecol. 2013;22:2848–2863. doi: 10.1111/mec.12083. [DOI] [PubMed] [Google Scholar]
- 19.Stemshorn KC, Reed FA, Nolte AW, Tautz D. Rapid formation of distinct hybrid lineages after secondary contact of two fish species (Cottus sp.) Mol Ecol. 2011;20:1475–1491. doi: 10.1111/j.1365-294X.2010.04997.x. [DOI] [PubMed] [Google Scholar]
- 20.vonHoldt BM, et al. A genome-wide perspective on the evolutionary history of enigmatic wolf-like canids. Genome Res. 2011;21:1294–1305. doi: 10.1101/gr.116301.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen PA, Marchán-Rivadeneira MR, Baker RJ. Natural hybridization generates mammalian lineage with species characteristics. Proc Natl Acad Sci USA. 2010;107:11447–11452. doi: 10.1073/pnas.1000133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monzón J, Kays R, Dykhuizen DE. Assessment of coyote-wolf-dog admixture using ancestry-informative diagnostic SNPs. Mol Ecol. 2014;23:182–197. doi: 10.1111/mec.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brelsford A, Milá B, Irwin DE. Hybrid origin of Audubon’s warbler. Mol Ecol. 2011;20:2380–2389. doi: 10.1111/j.1365-294X.2011.05055.x. [DOI] [PubMed] [Google Scholar]
- 24.Elgvin TO, et al. Hybrid speciation in sparrows II: A role for sex chromosomes? Mol Ecol. 2011;20:3823–3837. doi: 10.1111/j.1365-294X.2011.05182.x. [DOI] [PubMed] [Google Scholar]
- 25.Hermansen JS, et al. Hybrid speciation in sparrows I: Phenotypic intermediacy, genetic admixture and barriers to gene flow. Mol Ecol. 2011;20:3812–3822. doi: 10.1111/j.1365-294X.2011.05183.x. [DOI] [PubMed] [Google Scholar]
- 26.Hermansen JS, et al. Hybrid speciation through sorting of parental incompatibilities in Italian sparrows. Mol Ecol. 2014;23:5831–5842. doi: 10.1111/mec.12910. [DOI] [PubMed] [Google Scholar]
- 27.Trier CN, Hermansen JS, Sætre G-P, Bailey RI. Evidence for mito-nuclear and sex-linked reproductive barriers between the hybrid Italian sparrow and its parent species. PLoS Genet. 2014;10:e1004075. doi: 10.1371/journal.pgen.1004075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavretsky P, Engilis A, Jr, Eadie JM, Peters JL. Genetic admixture supports an ancient hybrid origin of the endangered Hawaiian duck. J Evol Biol. 2015;28:1005–1015. doi: 10.1111/jeb.12637. [DOI] [PubMed] [Google Scholar]
- 29.Bailey RI, Tesaker MR, Trier CN, Saetre G-P. Strong selection on male plumage in a hybrid zone between a hybrid bird species and one of its parents. J Evol Biol. 2015;28:1257–1269. doi: 10.1111/jeb.12652. [DOI] [PubMed] [Google Scholar]
- 30.Snow D. Family Pipridae (manakins) In: del Hoyo J, Elliot A, Christie DA, editors. Handbook of the Birds of the World, Vol 9: Cotingas to Pipits and Wagtails. Lynx Edicions; Barcelona: 2004. pp. 110–169. [Google Scholar]
- 31.Haffer J. Avian Speciation in Tropical South America. Publications of the Nuttall Ornithological Club No. 14; Cambridge, MA: 1974. [Google Scholar]
- 32.Ohlson JI, Fjeldså J, Ericson PGP. Molecular phylogeny of the manakins (Aves: Passeriformes: Pipridae), with a new classification and the description of a new genus. Mol Phylogenet Evol. 2013;69:796–804. doi: 10.1016/j.ympev.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 33.Haffer J. Hypotheses to explain the origin of species in Amazonia. Braz J Biol. 2008;68(4) Suppl:917–947. doi: 10.1590/s1519-69842008000500003. [DOI] [PubMed] [Google Scholar]
- 34.Kirwan GM, Green G. Cotingas and Manakins. Princeton Univ Press; Princeton: 2011. [Google Scholar]
- 35.Olmos F, Pacheco J. Rediscovery of golden-crowned manakin Lepidotrix vilasboasi. Cotinga. 2003;20:48–50. [Google Scholar]
- 36.Bandeira RS, et al. 2008. Lepidothrix vilasboasi (Sick, 1959): Espécie válida ? Uso de marcadores moleculares no estudo de uma ave amazônica, endêmica e ameaçada. Resumos 54° Congr Bras Genética:148.
- 37.Weir JT, Faccio MS, Pulido-Santacruz P, Barrera-Guzmán AO, Aleixo A. Hybridization in headwater regions, and the role of rivers as drivers of speciation in Amazonian birds. Evolution. 2015;69:1823–1834. doi: 10.1111/evo.12696. [DOI] [PubMed] [Google Scholar]
- 38.Smeds L, Qvarnström A, Ellegren H. Direct estimate of the rate of germline mutation in a bird. Genome Res. 2016;26:1211–1218. doi: 10.1101/gr.204669.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnold B, Corbett-Detig RB, Hartl D, Bomblies K. RADseq underestimates diversity and introduces genealogical biases due to nonrandom haplotype sampling. Mol Ecol. 2013;22:3179–3190. doi: 10.1111/mec.12276. [DOI] [PubMed] [Google Scholar]
- 40.Shafer ABA, et al. Bioinformatic processing of RAD-seq data dramatically impacts downstream population genetic inference. Methods Ecol Evol. 2017;8:907–917. [Google Scholar]
- 41.Prum RO. Anatomy, physics and evolution of avian structural colors. In: Hill GE, McGraw KJ, editors. Bird Coloration, Volume 1, Mechanisms and Measurements. Harvard Univ Press; Cambridge, MA: 2006. pp. 295–353. [Google Scholar]
- 42.Yin H, et al. Iridescence in the neck feathers of domestic pigeons. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;74:051916. doi: 10.1103/PhysRevE.74.051916. [DOI] [PubMed] [Google Scholar]
- 43.Igic B, D’Alba L, Shawkey MD. Manakins can produce iridescent and bright feather colours without melanosomes. J Exp Biol. 2016;219:1851–1859. doi: 10.1242/jeb.137182. [DOI] [PubMed] [Google Scholar]
- 44.Shawkey MD, Hill GE. Significance of a basal melanin layer to production of non-iridescent structural plumage color: Evidence from an amelanotic Steller’s jay (Cyanocitta stelleri) J Exp Biol. 2006;209:1245–1250. doi: 10.1242/jeb.02115. [DOI] [PubMed] [Google Scholar]
- 45.Maia R, Caetano JVO, Báo SN, Macedo RH. Iridescent structural colour production in male blue-black grassquit feather barbules: The role of keratin and melanin. J R Soc Interface. 2009;6(Suppl 2):S203–S211. doi: 10.1098/rsif.2008.0460.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shawkey MD, Hill GE. Carotenoids need structural colours to shine. Biol Lett. 2005;1:121–124. doi: 10.1098/rsbl.2004.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shawkey MD, Hill GE, McGraw KJ, Hood WR, Huggins K. An experimental test of the contributions and condition dependence of microstructure and carotenoids in yellow plumage coloration. Proc Biol Sci. 2006;273:2985–2991. doi: 10.1098/rspb.2006.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’Alba L, Kieffer L, Shawkey MD. Relative contributions of pigments and biophotonic nanostructures to natural color production: A case study in budgerigar (Melopsittacus undulatus) feathers. J Exp Biol. 2012;215:1272–1277. doi: 10.1242/jeb.064907. [DOI] [PubMed] [Google Scholar]
- 49.Mallet J. Hybrid speciation. Nature. 2007;446:279–283. doi: 10.1038/nature05706. [DOI] [PubMed] [Google Scholar]
- 50.Nolte AW, Tautz D. Understanding the onset of hybrid speciation. Trends Genet. 2010;26:54–58. doi: 10.1016/j.tig.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Haffer J. Contact zones between birds of southern Amazonia. Ornithol Monogr. 1997;48:281–305. [Google Scholar]
- 52.Seddon N, et al. Sexual selection accelerates signal evolution during speciation in birds. Proc Biol Sci. 2013;280:20131065. doi: 10.1098/rspb.2013.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Selz OM, Thommen R, Maan ME, Seehausen O. Behavioural isolation may facilitate homoploid hybrid speciation in cichlid fish. J Evol Biol. 2014;27:275–289. doi: 10.1111/jeb.12287. [DOI] [PubMed] [Google Scholar]
- 54.Melo MC, Salazar C, Jiggins CD, Linares M. Assortative mating preferences among hybrids offers a route to hybrid speciation. Evolution. 2009;63:1660–1665. doi: 10.1111/j.1558-5646.2009.00633.x. [DOI] [PubMed] [Google Scholar]
- 55.Schwander T, Suni SS, Cahan SH, Keller L. Mechanisms of reproductive isolation between an ant species of hybrid origin and one of its parents. Evolution. 2008;62:1635–1643. doi: 10.1111/j.1558-5646.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- 56.Segura DF, et al. Assortative mating among Anastrepha fraterculus (Diptera: Tephritidae) hybrids as a possible route to radiation of the fraterculus cryptic species complex. Biol J Linn Soc Lond. 2011;102:346–354. [Google Scholar]
- 57.Sánchez AP, et al. An introgressed wing pattern acts as a mating cue. Evolution. 2015;69:1619–1629. doi: 10.1111/evo.12679. [DOI] [PubMed] [Google Scholar]
- 58.Stein AC, Uy JAC. Unidirectional introgression of a sexually selected trait across an avian hybrid zone: A role for female choice? Evolution. 2006;60:1476–1485. [PubMed] [Google Scholar]
- 59.Lamichhaney S, et al. Rapid hybrid speciation in Darwin’s finches. Science. November 23, 2017 doi: 10.1126/science.aao4593. [DOI] [PubMed] [Google Scholar]
- 60.Marantz C, Zimmer K. 2006. Bird voices of Alta Floresta and southeastern Amazonian Brazil [audio CD] (Cornell Laboratory of Ornithology, Ithaca, NY)
- 61.Fernandes AM. Fine-scale endemism of Amazonian birds in a threatened landscape. Biodivers Conserv. 2013;22:2683–2694. [Google Scholar]
- 62.Fernandes AM, Wink M, Aleixo A. Phylogeography of the chestnut-tailed antbird (Myrmeciza hemimelaena) clarifies the role of rivers in Amazonian biogeography. J Biogeogr. 2012;39:1524–1535. [Google Scholar]
- 63.Fernandes AM, Gonzalez J, Wink M, Aleixo A. Multilocus phylogeography of the wedge-billed woodcreeper Glyphorynchus spirurus (Aves, Furnariidae) in lowland Amazonia: Widespread cryptic diversity and paraphyly reveal a complex diversification pattern. Mol Phylogenet Evol. 2013;66:270–282. doi: 10.1016/j.ympev.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 64.Fernandes AM, Cohn-Haft M, Hrbek T, Farias IP. Rivers acting as barriers for bird dispersal in the Amazon. Rev Bras Ornitol. 2014;22:361–371. [Google Scholar]
- 65.Fernandes AM, Wink M, Sardelli CH, Aleixo A. Multiple speciation across the Andes and throughout Amazonia: The case of the spot-backed antbird species complex (Hylophylax naevius/Hylophylax naevioides) J Biogeogr. 2014;41:1094–1104. [Google Scholar]
- 66.Schumer M, Cui R, Rosenthal GG, Andolfatto P. Reproductive isolation of hybrid populations driven by genetic incompatibilities. PLoS Genet. 2015;11:e1005041. doi: 10.1371/journal.pgen.1005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Capparella AP. Neotropical avian diversity and riverine barriers. Acta Congr Int Ornithol. 1991;20:307–316. [Google Scholar]
- 68.Ayres JM, Clutton-Brock TH. River boundaries and species range size in Amazonian primates. Am Nat. 1992;140:531–537. doi: 10.1086/285427. [DOI] [PubMed] [Google Scholar]
- 69.Mayle FE, Burn MJ, Power M, Urrego DH. 2009. Vegetation and fire at the last glacial maximum in tropical South America. Past Climate Variability in South America and Surrounding Regions, Developments in Paleoenvironmental Research (Springer, Dordrecht, The Netherlands), pp 89–112.
- 70.Buerkle CA, Morris RJ, Asmussen MA, Rieseberg LH. The likelihood of homoploid hybrid speciation. Heredity (Edinb) 2000;84:441–451. doi: 10.1046/j.1365-2540.2000.00680.x. [DOI] [PubMed] [Google Scholar]
- 71.Dittrich-Reed DR, Fitzpatrick BM. Transgressive hybrids as hopeful monsters. Evol Biol. 2013;40:310–315. doi: 10.1007/s11692-012-9209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.