Significance
Flowering time is a critical determinant of crop adaptation to local environments. As a result of natural and artificial selection, maize has evolved a reduced photoperiod sensitivity to adapt to regions over 90° of latitude in the Americas. Here we show that a distant Harbinger-like transposon acts as a cis-regulatory element to repress ZmCCT9 expression to promote flowering under the long days of higher latitudes. The transposon at ZmCCT9 and another functional transposon at a second flowering-time gene, ZmCCT10, arose sequentially following domestication and were targeted by selection as maize spread from the tropics to higher latitudes. Our results demonstrate that new functional variation created by transposon insertions helped maize to spread over a broad range of latitudes rapidly.
Keywords: maize adaptation, flowering time, transposable element, domestication, ZmCCT9
Abstract
From its tropical origin in southwestern Mexico, maize spread over a wide latitudinal cline in the Americas. This feat defies the rule that crops are inhibited from spreading easily across latitudes. How the widespread latitudinal adaptation of maize was accomplished is largely unknown. Through positional cloning and association mapping, we resolved a flowering-time quantitative trait locus to a Harbinger-like transposable element positioned 57 kb upstream of a CCT transcription factor (ZmCCT9). The Harbinger-like element acts in cis to repress ZmCCT9 expression to promote flowering under long days. Knockout of ZmCCT9 by CRISPR/Cas9 causes early flowering under long days. ZmCCT9 is diurnally regulated and negatively regulates the expression of the florigen ZCN8, thereby resulting in late flowering under long days. Population genetics analyses revealed that the Harbinger-like transposon insertion at ZmCCT9 and the CACTA-like transposon insertion at another CCT paralog, ZmCCT10, arose sequentially following domestication and were targeted by selection for maize adaptation to higher latitudes. Our findings help explain how the dynamic maize genome with abundant transposon activity enabled maize to adapt over 90° of latitude during the pre-Columbian era.
Flowering time is a major determinant of the local adaptation of crops (1–10). Maize (Zea mays ssp. mays) was domesticated in southwestern Mexico ∼9,000 y ago from its wild progenitor, teosinte (Zea mays ssp. parviglumis), a tropical species that exhibits substantial photoperiod sensitivity and requires short-day (SD) conditions to flower (6, 11). As a result of natural and artificial selection, maize has evolved a reduced photoperiod sensitivity to adapt to long-day (LD) environments from latitude 58° north in Canada to 40° south in Chile (12–14). This widespread expansion over 90° of latitude across the North–South axis of the Americas was a remarkable achievement in the pre-Columbian era compared with the spread of other New World crops (i.e., potato and cotton), which remained in narrower latitudinal ranges before the era of modern breeding (15, 16). Understanding how this feat was achieved specifically with maize is an important question in the history of crop domestication.
Transposable elements were first discovered in maize by Barbara McClintock (17) and have since been shown to play important roles in shaping genome evolution and gene regulatory networks of many species (18). Compared with other crops, maize is exceptionally abundant in transposon activity, and nearly 85% of the maize genome consists of transposable elements (19, 20). This abundant transposon activity helps generate substantial genetic diversity upon which selection can act during evolution, as inferred by Barbara McClintock decades ago (21). Although many loci affecting natural variation in flowering time have been detected in maize using various types of mapping populations (6, 13, 22), only two of them, Vgt1 (23–25) and ZmCCT (6, 7, 26), have been well characterized and found to contribute to flowering-time adaptation. Interestingly, both loci are associated with the regulatory roles of transposable elements. Despite this progress, whether transposable elements play a prevalent role in driving maize flowering-time adaptation remains to be further tested and elucidated.
In this study, we report the cloning and characterization of a flowering-time QTL (qDTA9) that was previously mapped in a maize-teosinte experimental population (27). We show that qDTA9 is underlain by a Harbinger-like transposable element located 57 kb upstream of a CCT domain-containing gene, ZmCCT9. The Harbinger-like transposable element functions as a repressor of ZmCCT9 to promote flowering under LD conditions. ZmCCT9 confers LD-dependent flowering repression by negatively regulating the expression of florigen ZCN8. We finally show that the Harbinger-like transposable element arose more recently compared with the CACTA-like transposon insertion at another CCT paralog, ZmCCT10 (6, 7, 26). Both transposon insertions were targeted by selection and have played crucial roles as maize spread geographically from its tropical origin to higher latitudes.
Results and Discussion
Positional Cloning of qDTA9.
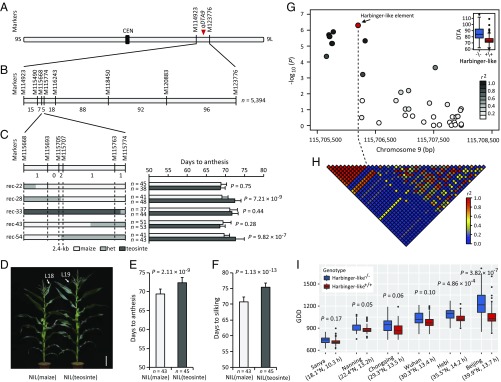
Using a large population of 866 maize-teosinte BC2S3 recombinant inbred lines (RILs), we previously performed quantitative trait locus (QTL) mapping for days to anthesis (DTA) under LD conditions (27) and detected a QTL (qDTA9) between markers M114923 and M123776 on chromosome 9 (Fig. 1A). To fine-map qDTA9, we selected a heterogeneous inbred family (HIF) that was heterozygous only at qDTA9 (SI Appendix, Fig. S1) and used it to generate a large near-isogenic line (NIL) population (n = 5,394). Following a recombinant-derived progeny testing strategy (6, 27, 28), qDTA9 was delimited to a 2.4-kb noncoding region between markers M115705 and M115707 (Fig. 1 B and C).
Fig. 1.
Fine mapping and association analysis of qDTA9. (A) Chromosomal location of qDTA9 on chromosome 9. CEN, centromere. (B) Fine mapping of qDTA9 using a NIL population (n = 5,394) derived from a HIF that was heterozygous only at qDTA9. The number of recombinants between the adjacent markers is indicated below the linkage map. (C) Progeny testing of recombinants delimited the qDTA9 locus to a 2.4-kb noncoding region between markers M115705 and M115707. (Left) The graphical genotypes of the five critical recombinants. (Right) The homozygous progenies (homozygous recombinants and homozygous nonrecombinants) identified within each recombinant-derived F3 family. Light-gray, gray, and dark-gray bars represent regions homozygous for maize, heterozygous regions, and regions homozygous for teosinte, respectively. Homozygous progenies of each recombinant were phenotyped and compared to determine the genotype of qDTA9 of the parental recombinant. (D) Gross morphologies of NIL(maize) and NIL(teosinte). The uppermost leaf (L) is indicated by a white arrow. (Scale bar, 20 cm.) (E and F) Phenotypic comparison between NIL(maize) and NIL(teosinte). (E) Days to anthesis. (F) Days to silking. (G) Association analysis of the 2.4-kb causative region for qDTA9 in a panel of 513 diverse maize inbred lines. The Harbinger-like element showing the most significant association is indicated by a red dot. The intensity of gray shading indicates the level of linkage disequilibrium (r2) between the Harbinger-like element and other variants identified in the 2.4-kb region. (H) Pairwise linkage disequilibrium analysis of variants identified in the 2.4-kb region. The intensity of red shading indicates the level of linkage disequilibrium (r2) between variants. (I) Association testing of the Harbinger-like element with flowering time scored in five other locations at different latitudes. Days to anthesis were converted to growing degree days (GDD) to account for the effect of temperature differences among environments. The latitude and the mean day length of each location are indicated in parentheses. The data in C, E, and F are shown as means ± SD; P values were determined by Student’s t test. Harbinger-like+/+ and Harbinger-like−/− in G and I denote inbred lines containing the Harbinger-like element and inbred lines lacking the Harbinger-like element, respectively.
At the same time, a pair of NILs that is homozygous for the maize and teosinte alleles across the qDTA9 region, designated NIL(maize) and NIL(teosinte), was developed from the HIF for subsequent analysis. Field examination under natural LD conditions showed that NIL(maize) flowered approximately 4 d earlier than NIL(teosinte) (Fig. 1 D–F), along with reduced plant height and leaf number (SI Appendix, Fig. S2). However, no significant differences in flowering time were observed when NIL(maize) and NIL(teosinte) were grown under natural SD conditions (SI Appendix, Fig. S3). These results suggest that qDTA9 is regulated by photoperiod.
Association Mapping for qDTA9.
To further identify potential causative variants responsible for qDTA9, we performed an association analysis by resequencing the 2.4-kb causative region in an association panel containing 513 diverse maize inbred lines that was scored for DTA under LD conditions in Beijing (39.9°N, 116.4°E) (7). In total, 41 variants (SNPs and insertions and deletions) with minor allele frequencies ≥0.05 were identified and tested for association with DTA using a mixed linear model (29). Overall, 13 variants exhibited significant association with DTA after Bonferroni multiple test correction (P ≤ 2.44 × 10−4) (Fig. 1G). Interestingly, a 360-bp Harbinger-like transposable element that is present in 41.3% of maize inbred lines showed the strongest association with DTA (P = 5.26 × 10−7), with the Harbinger-like element promoting flowering (Fig. 1G). As expected, the maize parent of the BC2S3 population carried the Harbinger-like element, whereas the teosinte parent lacked the Harbinger-like element (SI Appendix, Fig. S4 A and B). The Harbinger-like element is in strong linkage disequilibrium (r2 > 0.5) with all other significant variants, creating a linkage disequilibrium block flanking the Harbinger-like element (Fig. 1H). We also tested the association of the Harbinger-like element with flowering time scored in five other locations at diverse latitudes (7). To account for the effect of temperature differences among environments on the observed flowering times, DTA were converted to growing degree days (30). Interestingly, the Harbinger-like element exhibited significant associations with flowering time only at higher latitudes (Fig. 1I), potentially indicating a role for the Harbinger-like element in latitudinal adaptation.
According to the B73 reference genome (AGPv2), the Harbinger-like transposable element is located 57 kb upstream of GRMZM2G004483 (SI Appendix, Fig. S4A), which encodes a CCT domain protein homologous to the rice photoperiod response regulator Ghd7 (SI Appendix, Fig. S5) (3). GRMZM2G004483 shows 36% protein sequence identity to ZmCCT (SI Appendix, Fig. S6), a CCT paralog residing on chromosome 10 that was recently shown to play an important role in the regulation of maize photoperiod flowering (6, 7, 26). To distinguish these two CCT genes, we hereafter refer to them as ZmCCT9 and ZmCCT10 based on the chromosomes on which they reside. We speculate that ZmCCT9 is most likely the target gene regulated by qDTA9 and that the Harbinger-like element in the 2.4-kb causative region for qDTA9 is a distant cis-regulatory variant of ZmCCT9 that alters ZmCCT9 expression to control flowering time.
Knockout of ZmCCT9 Causes Early Flowering Under Long Days.
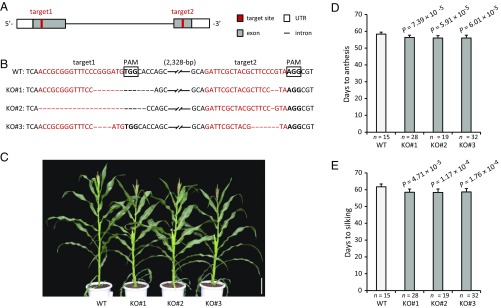
To validate the function of ZmCCT9, we knocked out the endogenous ZmCCT9 gene in maize inbred line ZC01 using CRISPR/Cas9 technology (31, 32). Two 20-bp sequences in the first and second exons of ZmCCT9 were selected as target sites for Cas9 cleavage (Fig. 2A). PCR and sequencing analysis of 20 independent first-generation (T0) transgenic lines identified three homozygous mutations with deletions in the target sites that truncated the ZmCCT9 ORF; these are referred to as KO#1, KO#2, and KO#3 (Fig. 2B and SI Appendix, Fig. S7). These three null mutants were self-pollinated, and the resulting T1 progenies were planted in natural LD conditions to investigate their flowering times. Notably, all three null mutants (KO#1, KO#2, and KO#3) flowered significantly earlier than wild-type plants under LD conditions (Fig. 2 C–E), strongly indicating that ZmCCT9 is involved in the regulation of flowering time in maize.
Fig. 2.
Knockout of ZmCCT9 by CRISPR/Cas9 causes early flowering under natural LD conditions. (A) Gene structure of ZmCCT9 and the two target sites (red boxes) in the first and second exons of ZmCCT9 for CRISPR/Cas9 editing. (B) Sequences of three homozygous knockout lines with deletions in target sites that truncated the ZmCCT9 ORF (KO#1, KO#2, and KO#3). The wild-type sequence is shown at the Top. Target sites and protospacer-adjacent motif (PAM) sequences are highlighted in red and boldface fonts, respectively, and deletions are indicated by dashes. The sequence gap length is shown in parentheses. (C) Gross morphologies of wild-type and knockout lines. (Scale bar, 20 cm.) (D and E) Phenotypic comparison of wild-type and three knockout lines under natural LD conditions. (D) Days to anthesis. (E) Days to silking. The data in D and E are shown as means ± SD; P values were determined by Student’s t test.
ZmCCT9 Is Diurnally Regulated and Exhibits Differential Expression Between the Maize Allele and the Teosinte Allele.
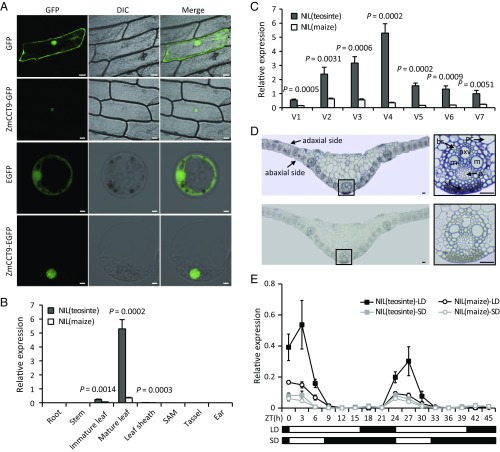
To elucidate the cellular localization of ZmCCT9, we introduced the ZmCCT9-GFP and ZmCCT9-EGFP fusion genes under the control of the CaMV35S promoter into onion epidermal cells and maize protoplasts, respectively. The results showed that the GFP signal is localized to the nucleus (Fig. 3A), supporting a role for ZmCCT9 as a nuclear transcription factor. To determine whether the upstream Harbinger-like element altered ZmCCT9 expression, we investigated the spatiotemporal expression patterns of ZmCCT9 in NIL(maize) and NIL(teosinte) under LD conditions. ZmCCT9 was expressed mainly in mature leaves (Fig. 3B) and showed higher expression in NIL(teosinte) than in NIL(maize) at all examined growth stages, with a maximum difference in expression at the V4 stage (Fig. 3C). RNA in situ hybridization analysis showed that ZmCCT9 primarily accumulates in leaf vascular bundles and sclerenchyma fiber cells (Fig. 3D).
Fig. 3.
ZmCCT9 expression features. (A) ZmCCT9 protein is localized in the nucleus in onion epidermal cells (Top two rows) (Scale bars, 50 μm.) and maize protoplasts (Bottom two rows). (Scale bars, 5 μm.) (B) Spatial expression pattern of ZmCCT9 in NIL(maize) and NIL(teosinte) in various tissues under natural LD conditions. (C) Temporal expression pattern of ZmCCT9 in mature leaves of NIL(maize) and NIL(teosinte) at various growth stages under natural LD conditions. (D) RNA in situ hybridization analysis of ZmCCT9. (Top) The hybridization signal obtained using a ZmCCT9 DIG-labeled antisense probe in transverse sections of leaf blade tips from NIL(teosinte) at the V4 stage under natural LD conditions. (Bottom) The negative control for hybridization using a sense probe. An individual vascular bundle from near the midrib (indicated by the outlined square) is shown on the Right. (Scale bars, 50 μm.) bs, bundle sheath; m, metaxylem vessels; p, phloem; pc, parenchyma cells; pxv, protoxylem vessels; sf, sclerenchyma fibers. (E) Diurnal expression patterns of ZmCCT9 in mature leaves of NIL(maize) and NIL(teosinte) under artificial LD and SD conditions. The black bars and white bars indicate the dark period and the light period, respectively. ZT, zeitgeber time. The data in B, C, and E are relative to the control gene ZmTubulin1 and represent means ± SEM of three biological replicates; P values were determined by Student’s t test.
Genes involved in photoperiod flowering are diurnally regulated (2–4, 7, 33–37). We therefore examined the diurnal expression patterns of ZmCCT9 in mature leaves of NIL(maize) and NIL(teosinte) under artificial LD and SD conditions. Under LD conditions, ZmCCT9 transcript levels in NIL(teosinte) showed strong diurnal oscillation, with the highest expression 3 h after dawn; in contrast, the amplitude of ZmCCT9 expression in NIL(maize) was significantly attenuated (Fig. 3E). Under SD conditions, ZmCCT9 in NIL(maize) and NIL(teosinte) showed similar weak diurnal expression patterns (Fig. 3E). These results suggest that ZmCCT9 is diurnally regulated and that the maize allele exhibits a much reduced photoperiod sensitivity relative to the teosinte allele, likely due to the repressive effect of the Harbinger-like element in the 2.4-kb causative region for qDTA9.
The Harbinger-Like Element Acts in Cis to Repress ZmCCT9 Expression to Promote Flowering Under Long Days.
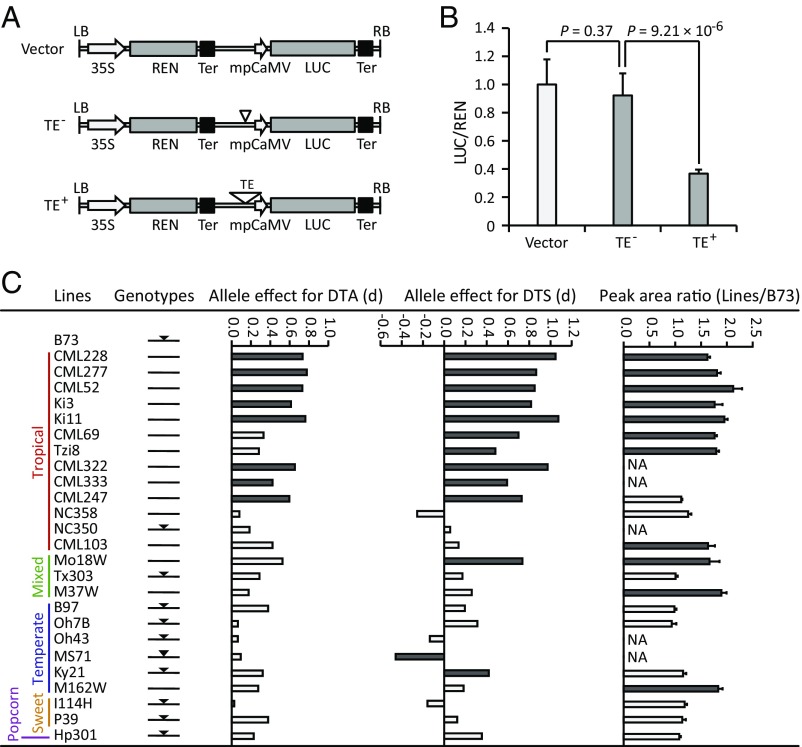
To test the effect of the Harbinger-like element on gene expression, we cloned the maize and teosinte sequences that differed only at the Harbinger-like element (SI Appendix, Fig. S8) into reporter constructs upstream of the minimal promoter of the cauliflower mosaic virus (mpCaMV), the firefly luciferase (LUC) ORF, and the nopaline synthase terminator (Fig. 4A) and compared their effect on gene expression in maize leaf protoplasts (Fig. 4B). The results showed that the maize construct containing the Harbinger-like element significantly repressed luciferase activity relative to the teosinte construct without the Harbinger-like element and the empty construct (Fig. 4B). However, no significant differences between the teosinte construct and the empty construct were detected (Fig. 4B). These results suggest that the Harbinger-like element represses LUC expression and, by inference, ZmCCT9 expression in vivo.
Fig. 4.
The Harbinger-like element acts in cis to regulate ZmCCT9 expression. (A) Constructs used to test the effect of the Harbinger-like element on gene expression in transient expression assays in maize leaf protoplasts. TE+ represents the construct with the maize sequence containing the Harbinger-like element cloned upstream of the mpCaMV; TE− represents the construct with the teosinte sequence lacking the Harbinger-like element cloned upstream of the mpCaMV. The maize and teosinte sequences used in the transient expression assay are 484 bp and 124 bp long, respectively, and they differ only at the Harbinger-like element. (B) The maize construct containing the Harbinger-like element significantly repressed luciferase activity relative to the teosinte construct without the Harbinger-like element and the empty construct. The data are normalized with respect to the average values of the empty construct and are shown as means ± SEM; P values were determined by Student’s t test. (C) Congruence between the Harbinger-like genotypes and the patterns of QTL segregation and relative ZmCCT9 expression across NAM founders. The black inverted triangle indicates that the corresponding NAM founder contains the Harbinger-like element. The allele effects for DTA and days to silking (DTS) at qDTA9 across the 25 NAM founders were obtained from the previous NAM flowering-time mapping study (13). The last column indicates allele-specific ZmCCT9 transcript abundance in mature leaves of F1 crosses between the 25 NAM founders and the reference B73. The dark-gray bars indicate significant differences (P < 0.05), and the light-gray bars indicate no significant differences.
The importance of qDTA9 has been observed consistently in previous flowering-time mapping studies (13, 22, 27, 30, 38). qDTA9 was one of the largest-effect flowering-time QTLs detected under LD conditions in a maize nested association mapping (NAM) population consisting of 5,000 RILs derived from crossing 25 diverse inbred lines to a common parent, B73 (13). We found that the Harbinger-like genotypes across the 26 NAM founders were highly consistent with the QTL segregation patterns across the NAM population (Fig. 4C). The effect of qDTA9 was detected mainly in tropical NAM founders that lack the Harbinger-like element, and these lines consistently exhibited delayed flowering relative to the reference temperate line B73 harboring the Harbinger-like element (Fig. 4C). Conversely, NAM founders carrying the Harbinger-like element tended to have no effect at qDTA9 (Fig. 4C).
Cis-acting variants typically affect the transcription of their target genes in an allele-specific manner (6, 23, 39, 40). We measured allele-specific ZmCCT9 transcript abundance in mature leaves of F1 crosses between the 25 NAM founders and the reference B73. The results showed that the NAM founders lacking the Harbinger-like element, which are mostly tropical lines, consistently exhibited higher expression of ZmCCT9 than the B73 allele under LD conditions (Fig. 4C), which highly corresponds with the QTL segregation patterns across the NAM founders. Taken together, these data further demonstrate that the Harbinger-like element acts in cis to repress ZmCCT9 expression to promote flowering under LD conditions.
ZmCCT9 Functions Upstream of ZCN8 to Regulate Flowering Time.
To determine the regulatory relationship of ZmCCT9 in the maize photoperiod pathway, we examined the expression patterns of several known photoperiod genes (Gigz1a, ZCN8, ZmCCT10, and CONZ1) (6, 7, 26, 37, 41, 42) in mature leaves of NIL(maize) and NIL(teosinte) under LD conditions. Interestingly, of these genes, only ZCN8, which encodes the maize florigen and functions as a floral activator (37, 42), exhibited significant difference in expression in the two NILs; NIL(maize) showed higher expression of ZCN8 than NIL(teosinte), indicating that ZCN8 is negatively regulated by ZmCCT9 under LD conditions (SI Appendix, Fig. S9). Consistent with this result, ZCN8 expression was significantly elevated in CRISPR/Cas9-engineered null mutants compared with wild-type plants under LD conditions (SI Appendix, Fig. S10). Analysis of diurnal expression of these photoperiod-related genes further showed that only ZCN8 exhibited differential diurnal expression in NIL(maize) and NIL(teosinte) under LD conditions (SI Appendix, Fig. S11A). However, ZCN8 showed similar diurnal expression patterns in NIL(maize) and NIL(teosinte) under SD conditions (SI Appendix, Fig. S11B). Taken together, these results suggest that ZmCCT9 functions as a critical LD suppressor connecting ZCN8 expression and the circadian network.
The Harbinger-Like Element Arose After Initial Domestication and Was Targeted by Selection.
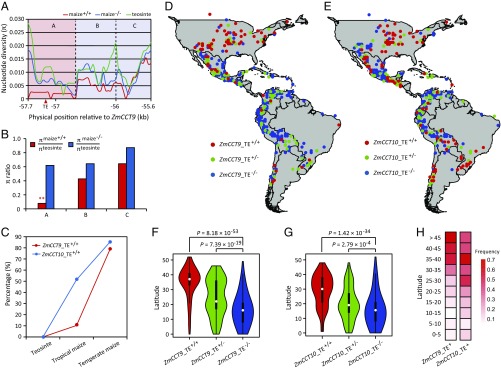
To examine the evolutionary origin of the Harbinger-like element, we genotyped the Harbinger-like locus in 73 diverse teosinte accessions (Z. mays ssp. parviglumis) (SI Appendix, Table S1). Interestingly, none of the investigated teosintes carried the Harbinger-like element, indicating that the Harbinger-like transposon insertion is most likely a de novo mutation that occurred after initial domestication. To determine whether selection has acted on the Harbinger-like element, we analyzed the nucleotide diversity across the 2.4-kb region in a panel of 27 diverse maize inbred lines and 19 teosinte (Z. mays ssp. parviglumis) accessions (Fig. 5A and SI Appendix, Table S2). Interestingly, in the A region flanking the Harbinger-like element, maize lines carrying the Harbinger-like element retained only 7.8% of the nucleotide diversity of teosinte (Fig. 5B). A coalescence simulation incorporating the maize domestication bottleneck (43–45) showed that this severe loss of genetic diversity cannot be explained by the maize domestication bottleneck alone, indicating a strong past selection near the Harbinger-like element. No significant selection signals were detected at regions B and C (Fig. 5B). Phylogenetic analysis of the A region clearly separated lines carrying the Harbinger-like element from lines lacking it, such that all teosintes cluster together with maize lines lacking the Harbinger-like element (SI Appendix, Fig. S12).
Fig. 5.
Evolutionary features of the Harbinger-like transposon insertion at ZmCCT9. (A) Nucleotide diversity across the 2.4-kb causative region for qDTA9. The red, blue, and green lines indicate nucleotide diversity (π) of maize lines carrying the Harbinger-like element (maize+/+), maize lines lacking the Harbinger-like element (maize−/−), and teosinte accessions, respectively. Base-pair positions are relative to the start codon of ZmCCT9. The red triangle indicates the position of the Harbinger-like element. (B) The Harbinger-like element shows evidence of selection. In the A region flanking the Harbinger-like element, maize lines carrying the Harbinger-like element retained only 7.8% of the nucleotide diversity in teosinte, a finding that cannot be explained by the neutral domestication bottleneck alone. The double asterisks represent significance difference determined by the coalescent simulations at P < 0.01. (C) Allele-frequency distribution of the Harbinger-like transposon insertion at ZmCCT9 and the CACTA-like transposon insertion at ZmCCT10 in teosinte, temperate maize inbred lines, and tropical maize inbred lines. (D and E) Geographic distribution of the Harbinger-like transposon insertion at ZmCCT9 (D) and the CACTA-like transposon insertion at ZmCCT10 (E) in 1,008 maize landraces native to the Americas. (F and G) The Harbinger-like transposon insertion at ZmCCT9 (F) and the CACTA-like transposon insertion at ZmCCT10 (G) predominantly accumulate at higher latitudes. In the violin plots, a violin shape indicates the kernel-density curve, a white node in the center indicates the median, and a black box inside the violin indicates a box-and-whisker plot. P values were determined by Student’s t test. (H) Frequency distributions of the Harbinger-like transposon insertion at ZmCCT9 and the CACTA-like transposon insertion at ZmCCT10 along latitudinal gradients.
The Two Transposon Insertions at ZmCCT9 and ZmCCT10 Arose Sequentially Following Domestication and Exhibited Strong Latitudinal Adaptation.
We previously cloned ZmCCT10, which controls the largest-effect flowering-time QTL, qDTA10, in the same maize-teosinte BC2S3 population and the maize NAM population (6). A CACTA-like transposon insertion at the promoter of ZmCCT10 was found to be the causative variant (7). Interestingly, both the Harbinger-like transposon insertion at ZmCCT9 and the CACTA-like transposon insertion at ZmCCT10 are de novo mutations that occurred after initial domestication and show evidence of selection. To reveal how these two transposable elements contributed to maize flowering-time adaptation, we analyzed their allele distribution in the 513 modern maize inbred lines that were used in the association mapping. We found that 51.4% of tropical inbred lines carried the CACTA-like transposon at ZmCCT10 and that this percentage further increased to 85.4% in temperate inbred lines (Fig. 5C). However, the Harbinger-like transposon insertion at ZmCCT9 remained at a low frequency (10.3%) in tropical inbred lines but increased to 79% in temperate inbred lines (Fig. 5C). These contrasting frequency distributions suggest that these two transposons might be associated with distinct patterns of geographic dispersal. To test this hypothesis, we investigated the distribution of these two transposons among 1,008 maize landrace accessions (SI Appendix, Table S3) representing the entire pre-Columbian range of maize races native to the Americas (46, 47). As expected, both transposons showed strong associations with latitude, with both predominantly accumulating at higher latitudes (Fig. 5 D–H). However, closer inspection showed that the two transposons exhibit different frequency distributions along latitudes (Fig. 5H). Compared with the Harbinger-like transposon insertion at ZmCCT9, which occurs at low frequency at low latitudes, the CACTA-like transposon insertion at ZmCCT10 occurs at a relatively higher frequency at low latitudes (Fig. 5H), suggesting that the CACTA-like transposon insertion at ZmCCT10 arose earlier than the Harbinger-like transposon insertion at ZmCCT9. To verify this, we performed molecular dating using previously described methods (48). The results showed that the CACTA-like transposon insertion and the Harbinger-like transposon insertion arose ∼7,269 and ∼4,645 y B.P., respectively. These estimates suggest that both transposon insertions arose postdomestication and that the Harbinger-like transposon insertion at ZmCCT9 arose ∼2,624 y later than the CACTA-like transposon insertion at ZmCCT10, thereby leading to the differences in the geographic range.
Conclusions
In summary, this work uncovered a distant Harbinger-like transposon acting in cis to alter ZmCCT9 expression to regulate maize flowering time. ZmCCT9 confers LD-dependent flowering repression by negatively regulating the expression of the florigen ZCN8. The two transposon insertions at ZmCCT10 and ZmCCT9 arose sequentially after the initial domestication of maize and were targeted by selection as maize spread from its tropical origin to higher latitudes. Additionally, a transposable element insertion at Vgt1, a third locus involved in maize adaption to higher latitudes, has also been identified as a candidate causal variant (23–25). These findings further demonstrate the important roles of transposable elements in creating functional variation and driving species evolution (7, 23, 48–53), as inferred by Barbara McClintock decades ago (21). Moreover, our results help explain how the dynamic maize genome with abundant transposon activity enabled maize to “break the rule” that latitude constrains crop diffusion and was spread by its Native American inventors over 90° of latitude from Chile to Canada during the pre-Columbian era. The selection of natural regulatory variation at paralogs with similar biological functions might represent an efficient and prevalent strategy for local adaptation of crops.
Materials and Methods
Plant materials are described in SI Appendix, SI Materials and Methods. Details of the methods and experimental procedures are provided in SI Appendix, SI Materials and Methods, including fine mapping, association mapping, transgenic analysis, RNA extraction, quantitative real-time PCR analysis, RACE, subcellular localization, RNA in situ hybridization, protoplast transient expression assay, allele-specific expression assay, nucleotide diversity analysis, selection test, molecular dating analysis, and phylogenetic analysis.
Supplementary Material
Acknowledgments
We thank Jigang Li (State Key Laboratory of Plant Physiology and Biochemistry, China Agricultural University) and Lubin Tan (Department of Plant Genetics and Breeding, China Agricultural University) for helpful discussions and advice on molecular experiments. This work was supported by grants from the National Key Research and Development Program of China (2016YFD0100404 and 2016YFD0100303); the National Natural Science Foundation of China (31771806 and 91535108); and the Recruitment Program of Global Experts and the Fundamental Research Funds for the Central Universities.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. MG233400–MG233921).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718058115/-/DCSupplemental.
References
- 1.Yan L, et al. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science. 2004;303:1640–1644. doi: 10.1126/science.1094305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner A, Beales J, Faure S, Dunford RP, Laurie DA. The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science. 2005;310:1031–1034. doi: 10.1126/science.1117619. [DOI] [PubMed] [Google Scholar]
- 3.Xue W, et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet. 2008;40:761–767. doi: 10.1038/ng.143. [DOI] [PubMed] [Google Scholar]
- 4.Murphy RL, et al. Coincident light and clock regulation of pseudoresponse regulator protein 37 (PRR37) controls photoperiodic flowering in sorghum. Proc Natl Acad Sci USA. 2011;108:16469–16474. doi: 10.1073/pnas.1106212108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comadran J, et al. Natural variation in a homolog of Antirrhinum CENTRORADIALIS contributed to spring growth habit and environmental adaptation in cultivated barley. Nat Genet. 2012;44:1388–1392. doi: 10.1038/ng.2447. [DOI] [PubMed] [Google Scholar]
- 6.Hung HY, et al. ZmCCT and the genetic basis of day-length adaptation underlying the postdomestication spread of maize. Proc Natl Acad Sci USA. 2012;109:E1913–E1921. doi: 10.1073/pnas.1203189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Q, et al. CACTA-like transposable element in ZmCCT attenuated photoperiod sensitivity and accelerated the postdomestication spread of maize. Proc Natl Acad Sci USA. 2013;110:16969–16974. doi: 10.1073/pnas.1310949110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SJ, et al. Optimization of crop productivity in tomato using induced mutations in the florigen pathway. Nat Genet. 2014;46:1337–1342. doi: 10.1038/ng.3131. [DOI] [PubMed] [Google Scholar]
- 9.Soyk S, et al. Variation in the flowering gene SELF PRUNING 5G promotes day-neutrality and early yield in tomato. Nat Genet. 2017;49:162–168. doi: 10.1038/ng.3733. [DOI] [PubMed] [Google Scholar]
- 10.Lu S, et al. Natural variation at the soybean J locus improves adaptation to the tropics and enhances yield. Nat Genet. 2017;49:773–779. doi: 10.1038/ng.3819. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka Y, et al. A single domestication for maize shown by multilocus microsatellite genotyping. Proc Natl Acad Sci USA. 2002;99:6080–6084. doi: 10.1073/pnas.052125199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuleshov NN. World’s diversity of phenotypes of maize. Agron J. 1933;25:688–700. [Google Scholar]
- 13.Buckler ES, et al. The genetic architecture of maize flowering time. Science. 2009;325:714–718. doi: 10.1126/science.1174276. [DOI] [PubMed] [Google Scholar]
- 14.Swarts K, et al. Genomic estimation of complex traits reveals ancient maize adaptation to temperate North America. Science. 2017;357:512–515. doi: 10.1126/science.aam9425. [DOI] [PubMed] [Google Scholar]
- 15.Diamond J. Guns, Germs, and Steel: The Fates of Human Societies. Norton; New York: 1997. [Google Scholar]
- 16.Diamond J. Evolution, consequences and future of plant and animal domestication. Nature. 2002;418:700–707. doi: 10.1038/nature01019. [DOI] [PubMed] [Google Scholar]
- 17.McClintock B. The origin and behavior of mutable loci in maize. Proc Natl Acad Sci USA. 1950;36:344–355. doi: 10.1073/pnas.36.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slotkin RK, Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet. 2007;8:272–285. doi: 10.1038/nrg2072. [DOI] [PubMed] [Google Scholar]
- 19.Schnable PS, et al. The B73 maize genome: Complexity, diversity, and dynamics. Science. 2009;326:1112–1115. doi: 10.1126/science.1178534. [DOI] [PubMed] [Google Scholar]
- 20.Jiao Y, et al. Improved maize reference genome with single-molecule technologies. Nature. 2017;546:524–527. doi: 10.1038/nature22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClintock B. The significance of responses of the genome to challenge. Science. 1984;226:792–801. doi: 10.1126/science.15739260. [DOI] [PubMed] [Google Scholar]
- 22.Chardon F, et al. Genetic architecture of flowering time in maize as inferred from quantitative trait loci meta-analysis and synteny conservation with the rice genome. Genetics. 2004;168:2169–2185. doi: 10.1534/genetics.104.032375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salvi S, et al. Conserved noncoding genomic sequences associated with a flowering-time quantitative trait locus in maize. Proc Natl Acad Sci USA. 2007;104:11376–11381. doi: 10.1073/pnas.0704145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ducrocq S, et al. Key impact of Vgt1 on flowering time adaptation in maize: Evidence from association mapping and ecogeographical information. Genetics. 2008;178:2433–2437. doi: 10.1534/genetics.107.084830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castelletti S, Tuberosa R, Pindo M, Salvi S. A MITE transposon insertion is associated with differential methylation at the maize flowering time QTL Vgt1. G3 (Bethesda) 2014;4:805–812. doi: 10.1534/g3.114.010686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ducrocq S, et al. Fine mapping and haplotype structure analysis of a major flowering time quantitative trait locus on maize chromosome 10. Genetics. 2009;183:1555–1563. doi: 10.1534/genetics.109.106922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D, et al. The genetic architecture of leaf number and its genetic relationship to flowering time in maize. New Phytol. 2016;210:256–268. doi: 10.1111/nph.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu G, et al. Complex genetic architecture underlies maize tassel domestication. New Phytol. 2017;214:852–864. doi: 10.1111/nph.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu J, et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat Genet. 2006;38:203–208. doi: 10.1038/ng1702. [DOI] [PubMed] [Google Scholar]
- 30.Coles ND, McMullen MD, Balint-Kurti PJ, Pratt RC, Holland JB. Genetic control of photoperiod sensitivity in maize revealed by joint multiple population analysis. Genetics. 2010;184:799–812. doi: 10.1534/genetics.109.110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 32.Belhaj K, Chaparro-Garcia A, Kamoun S, Patron NJ, Nekrasov V. Editing plant genomes with CRISPR/Cas9. Curr Opin Biotechnol. 2015;32:76–84. doi: 10.1016/j.copbio.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Suárez-López P, et al. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- 34.Kojima S, et al. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 2002;43:1096–1105. doi: 10.1093/pcp/pcf156. [DOI] [PubMed] [Google Scholar]
- 35.Valverde F, et al. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi Y, Weigel D. Move on up, it’s time for change: Mobile signals controlling photoperiod-dependent flowering. Genes Dev. 2007;21:2371–2384. doi: 10.1101/gad.1589007. [DOI] [PubMed] [Google Scholar]
- 37.Meng X, Muszynski MG, Danilevskaya ON. The FT-like ZCN8 gene functions as a floral activator and is involved in photoperiod sensitivity in maize. Plant Cell. 2011;23:942–960. doi: 10.1105/tpc.110.081406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li YX, et al. Identification of genetic variants associated with maize flowering time using an extremely large multi-genetic background population. Plant J. 2016;86:391–402. doi: 10.1111/tpj.13174. [DOI] [PubMed] [Google Scholar]
- 39.Clark RM, Wagler TN, Quijada P, Doebley J. A distant upstream enhancer at the maize domestication gene tb1 has pleiotropic effects on plant and inflorescent architecture. Nat Genet. 2006;38:594–597. doi: 10.1038/ng1784. [DOI] [PubMed] [Google Scholar]
- 40.Springer NM, Stupar RM. Allele-specific expression patterns reveal biases and embryo-specific parent-of-origin effects in hybrid maize. Plant Cell. 2007;19:2391–2402. doi: 10.1105/tpc.107.052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller TA, Muslin EH, Dorweiler JE. A maize CONSTANS-like gene, conz1, exhibits distinct diurnal expression patterns in varied photoperiods. Planta. 2008;227:1377–1388. doi: 10.1007/s00425-008-0709-1. [DOI] [PubMed] [Google Scholar]
- 42.Lazakis CM, Coneva V, Colasanti J. ZCN8 encodes a potential orthologue of Arabidopsis FT florigen that integrates both endogenous and photoperiod flowering signals in maize. J Exp Bot. 2011;62:4833–4842. doi: 10.1093/jxb/err129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eyre-Walker A, Gaut RL, Hilton H, Feldman DL, Gaut BS. Investigation of the bottleneck leading to the domestication of maize. Proc Natl Acad Sci USA. 1998;95:4441–4446. doi: 10.1073/pnas.95.8.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tenaillon MI, U’Ren J, Tenaillon O, Gaut BS. Selection versus demography: A multilocus investigation of the domestication process in maize. Mol Biol Evol. 2004;21:1214–1225. doi: 10.1093/molbev/msh102. [DOI] [PubMed] [Google Scholar]
- 45.Wright SI, et al. The effects of artificial selection on the maize genome. Science. 2005;308:1310–1314. doi: 10.1126/science.1107891. [DOI] [PubMed] [Google Scholar]
- 46.Vigouroux Y, et al. Population structure and genetic diversity of New World maize races assessed by DNA microsatellites. Am J Bot. 2008;95:1240–1253. doi: 10.3732/ajb.0800097. [DOI] [PubMed] [Google Scholar]
- 47.van Heerwaarden J, et al. Genetic signals of origin, spread, and introgression in a large sample of maize landraces. Proc Natl Acad Sci USA. 2011;108:1088–1092. doi: 10.1073/pnas.1013011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Studer A, Zhao Q, Ross-Ibarra J, Doebley J. Identification of a functional transposon insertion in the maize domestication gene tb1. Nat Genet. 2011;43:1160–1163. doi: 10.1038/ng.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bejerano G, et al. A distal enhancer and an ultraconserved exon are derived from a novel retroposon. Nature. 2006;441:87–90. doi: 10.1038/nature04696. [DOI] [PubMed] [Google Scholar]
- 50.Xiao H, Jiang N, Schaffner E, Stockinger EJ, van der Knaap E. A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science. 2008;319:1527–1530. doi: 10.1126/science.1153040. [DOI] [PubMed] [Google Scholar]
- 51.Naito K, et al. Unexpected consequences of a sudden and massive transposon amplification on rice gene expression. Nature. 2009;461:1130–1134. doi: 10.1038/nature08479. [DOI] [PubMed] [Google Scholar]
- 52.González J, Karasov TL, Messer PW, Petrov DA. Genome-wide patterns of adaptation to temperate environments associated with transposable elements in Drosophila. PLoS Genet. 2010;6:e1000905. doi: 10.1371/journal.pgen.1000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray MM, Sutter NB, Ostrander EA, Wayne RK. The IGF1 small dog haplotype is derived from Middle Eastern grey wolves. BMC Biol. 2010;8:16. doi: 10.1186/1741-7007-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.