Significance
The maintenance of taste sensory organs (taste buds) in the tongue has been known for 140 years to depend on sensory innervation from distant neurons by an unknown mechanism. We find that maintenance and regeneration of taste receptor cells (TRCs) within taste buds requires neuronal delivery of the Sonic hedgehog (Shh) protein signal, thus explaining loss of taste sensation associated with Hedgehog pathway antagonism in patients and illustrating the principle that spatial patterning of TRC regeneration is specified by the projection pattern of Shh-expressing sensory neurons. We also find that pharmacologic Hedgehog pathway activation accelerates TRC recovery, suggesting a means to ameliorate the loss of taste sensation and appetite and the associated delay in recovery in cancer patients undergoing chemotherapy.
Keywords: Hedgehog signaling, regeneration, genetics, neurobiology, taste
Abstract
How organs maintain and restore functional integrity during ordinary tissue turnover or following injury represents a central biological problem. The maintenance of taste sensory organs in the tongue was shown 140 years ago to depend on innervation from distant ganglion neurons, but the underlying mechanism has remained unknown. Here, we show that Sonic hedgehog (Shh), which encodes a secreted protein signal, is expressed in these sensory neurons, and that experimental ablation of neuronal Shh expression causes loss of taste receptor cells (TRCs). TRCs are also lost upon pharmacologic blockade of Hedgehog pathway response, accounting for the loss of taste sensation experienced by cancer patients undergoing Hedgehog inhibitor treatment. We find that TRC regeneration following such pharmacologic ablation requires neuronal expression of Shh and can be substantially enhanced by pharmacologic activation of Hedgehog response. Such pharmacologic enhancement of Hedgehog response, however, results in additional TRC formation at many ectopic sites, unlike the site-restricted regeneration specified by the projection pattern of Shh-expressing neurons. Stable regeneration of TRCs thus requires neuronal Shh, illustrating the principle that neuronal delivery of cues such as the Shh signal can pattern distant cellular responses to assure functional integrity during tissue maintenance and regeneration.
Taste sensation permits the discrimination of substances whose ingestion sustains us from others that are detrimental to our well-being. Such discrimination is critical for survival but requires continual exposure to substances or conditions that may cause taste receptor cell (TRC) loss, necessitating robust mechanisms for replacement of TRCs within taste sensory organs by cells from surrounding epithelium (1). Disturbances of taste sensation likely due to TRC loss accompany cancer chemotherapy in up to 85% of patients, and recovery from this loss also requires TRC replacement (2, 3). Input from sensory neurons has long been known as a critical factor in TRC maintenance, as crush injury or transection of the innervating processes causes TRC degeneration within a period of several weeks (4, 5). Replacement of such lost TRCs following nerve injury can occur but depends on reestablishment of the innervation (6, 7). Although the molecular mechanisms by which neurons induce TRC maintenance and regeneration remain unknown, a potential involvement of the Hedgehog protein signal is suggested by loss or alteration of taste sensation in cancer patients systemically treated with any of several drugs that block response to the Hedgehog signal (8–11).
The potential role of neurons in supplying Hedgehog signal for TRC maintenance and regeneration is further suggested by neuronal expression of Hedgehog in several developmental contexts. In Drosophila, for example, Hedgehog protein expressed peripherally in developing photoreceptors influences neurogenesis in the central brain targets of these photoreceptor projections (12). In addition, projections from the sensory ganglia in mice have been reported to elicit a response in hair follicle (13), incisor (14), and touch dome of the skin (15, 16).
Here, we describe the expression of Sonic hedgehog (Shh), a member of the mammalian Hedgehog gene family, in sensory ganglion neurons that innervate the taste sensory organs, and we examine the role of this signal in TRC maintenance and in regeneration. Our study confirms recent reports on the importance of Hh response in TRC maintenance (17, 18). Beyond maintenance, our study examines de novo TRC regeneration following TRC ablation with the use of a Hh pathway antagonist that is particularly effective in mice. We found that TRCs regenerate naturally following such ablation and were thus able to demonstrate the role of neuronally delivered Shh not only in stimulating regeneration but also in spatially restricting TRC regeneration to the taste buds. We also found that pharmacologic Hh pathway activation accelerates TRC regeneration, suggesting a possible approach to the loss of taste sensation in patients with TRC loss.
Results
Nerve-Dependent Maintenance of Organized Hedgehog Signaling in Adult Taste Buds.
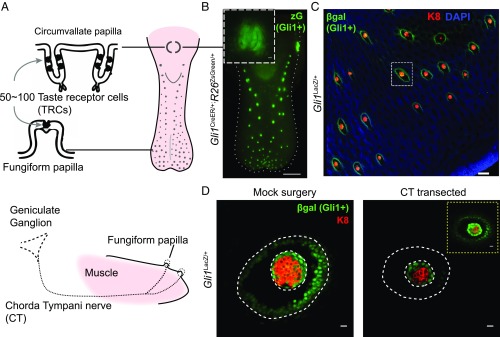
We hypothesized that neurons might provide a signal that elicits responses in lingual epithelium to promote TRC replacement. To investigate the role of Hedgehog signaling in mediating neuronal contributions to TRC replacement, we focused on the mouse fungiform papillae, which are dispersed in a grid-like pattern in the anterior two-thirds of the tongue (Fig. 1A and Fig. S1 A and B). Each papilla houses a single taste bud containing 50–100 TRCs, with ipsilateral innervation from the chorda tympani branch of the facial nerve, which projects from the geniculate ganglion (Fig. 1A). These regularly spaced fungiform papillae constitute an anatomically simpler system for analysis compared with the taste buds of the circumvallate papilla, with their bilateral innervation and compact localization at the back of the tongue (Fig. 1A). We therefore analyzed the relationship between chorda tympani innervation and Hedgehog response in lingual epithelium of the fungiform papillae. Hedgehog signal response, indicated by β-galactosidase staining in Gli1LacZ/+ animals, is localized in epithelial cells surrounding the TRCs within taste buds of all fungiform papillae (Fig. 1 B and C and Fig. S1C). We found that this β-galactosidase activity is significantly reduced upon transection of the chorda tympani nerve (Fig. 1D and Fig. S2). Transection of the distinct sensory projections that innervate sensory organs of the fungiform papillae thus results in the loss of Hedgehog response within the corresponding taste buds.
Fig. 1.
Nerve-dependent maintenance of organized Hedgehog signaling in adult taste buds. (A) Taste receptor cells (TRCs) reside within taste buds of the circumvallate papilla and fungiform papillae. Fungiform papilla receives innervation from geniculate ganglion through the chorda tympani nerve. (B) Genetic marking of Hedgehog-responsive cells. Epifluorescence imaging of a tongue from Gli1CreER/+;R26ZsGreen/+ mouse, 1 wk after tamoxifen injection. (Scale bar: 1 mm.) Inset shows circumvallate papilla. (Scale bar: 100 μm.) (C) Immunofluorescence staining of β-galactosidase (green), K8 (red), and DAPI (blue) of peeled epithelium from Gli1LacZ/+ mice (see Fig. S1 for more details). (D) Reduced βgal and K8 staining 5 d after chorda tympani nerve transection. Inset shows an overexposed βgal staining. See Fig. S2 for quantifications. [Scale bars: 100 μm (C); 10 μm (D).]
Neuronal and Epithelial Shh Are the Main Sources of Hedgehog Signal in Taste Bud Homeostasis.
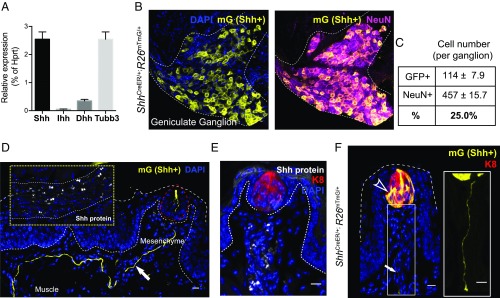
These observations raise the question of how taste organ innervation results in the stimulation of Hedgehog response. We considered the possibility that lingual response may be elicited by Hedgehog signals delivered directly by innervating sensory projections. This hypothesis is consistent with association of the Hedgehog protein with the extracellular surface of the plasma membrane due to lipid modification during secretion and biogenesis (19); the mature, processed Hedgehog signal thus may gain access to distant taste sensory organs of the tongue by traveling along the membranes of sensory axonal projections. We indeed found by RT-PCR analysis that Shh, one of three mammalian Hedgehog family members, is expressed in neurons of the geniculate ganglion (Fig. 2A). We marked these Shh-expressing cells by administering tamoxifen to ShhCreER/+;R26mTmG/+ mice, leading to Cre-mediated ablation of the membrane-tethered tdTomato fluorescent protein (mT) and expression of membrane-tethered GFP fluorescent protein (mG) in Shh-expressing cells. By costaining for expression of the neuronal marker, NeuN (20), we found that at least 25% of neurons in the geniculate ganglion express Shh (Fig. 2 B and C). The membrane association of mG further permits visualization of processes projecting continuously all of the way from Shh-expressing cell bodies in the geniculate ganglion through lingual muscle and mesenchyme to TRCs within the fungiform papillae of the lingual epithelium (Fig. 2D and Fig. S3B). Remarkably, these projections from Shh-expressing neurons selectively enter into and terminate within the lingual epithelium at the locations of the TRCs, and not in other lingual epithelial appendages (Fig. 2D and Fig. S3 B and C). We also verified the presence of the Shh protein along the membranes of these sensory axonal projections by immunohistochemistry (Inset in Fig. 2 D and E). By the same marking strategy, we also observed that Shh is produced by a small subset of cells within the taste bud (Fig. 2F and Fig. S3A), as also noted by others (21–24).
Fig. 2.
Neuronal and epithelial Shh are the main sources of Hedgehog signal in taste bud homeostasis. (A) qPCR analysis of Shh, Indian Hedgehog (Ihh), Desert Hedgehog (Dhh), and a ubiquitous neuronal marker betaIII-tubulin (Tubb3) expression in the geniculate ganglion plotted as relative percentages to Hprt1. n = 5∼6 dissected ganglia from 3 mice were pooled into one sample; total number of mice: n = 9. (B) Genetic marking of Shh-expressing cells. Immunofluorescence labeling of GFP (mG, yellow) and neuronal marker (NeuN, magenta) in the geniculate ganglion from ShhCreER/+;R26mTmG mice. (C) Quantification of GFP-positive and NeuN-positive cell numbers in geniculate ganglion (n = 8∼12 ganglia from 6 adult ShhCreER/+;R26mTmG mice). (D) A tongue section containing a fungiform papilla receiving a bundle of mGFP-labeled nerve fiber (white arrow). Inset shows immunostaining of SHH protein (white) on a nerve bundle in tongue muscle. TRCs are denoted with red circles. (E) Immunostaining of SHH protein (white) within the fungiform papilla. (F) Marking of Shh-producing basal cells (arrowhead) and sensory projections from Shh-expressing neurons (arrow; also magnified in single-channel view) in ShhCreER/+;R26mTmG mice. (Scale bars, 10 μm.) Note that the mG marking of Shh-expressing cells within the taste bud is driven by the CAG promoter inserted at the R26 locus. The intensity of mG antibody staining thus is stronger than that of SHH protein in the basal cells (Fig. S3A).
Shh Expressed from Sensory Neurons Mediates Long-Term Maintenance of TRCs.
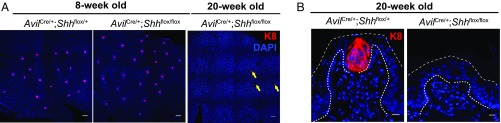
To test the functional role of neuronal Shh in TRC maintenance, we used a Cre recombinase allele under control of the Advillin promoter (AvilCre), specifically expressed in sensory neurons (25), to ablate a conditional Shh allele (Shhflox) (26). We found a nearly complete decline of TRC-containing taste buds in AvilCre/+;Shhflox/flox mutants from 8 to 20 wk of age, in sharp contrast to the persistence of TRCs in heterozygous control mice (AvilCre/+;Shhflox/+ mice; Fig. 3), or in adult mice subjected to conditional ablation of Shh in the epithelium (K5CreER/+;Shhflox/flox mutants; Fig. S4A). Interestingly, although the AvilCre driver is expressed beginning in embryogenesis, AvilCre;Shhflox/flox mice do not exhibit extensive TRC loss until later in adulthood, indicating that neuronal Shh expression functionally contributes to maintenance of TRCs after their initial establishment within the lingual epithelium. We also used a conditional neuronal driver, Thy1CreER, to ablate Shh (Thy1CreER; Shhflox) and found a significant, albeit less complete, TRC loss 12 wk after mice were given tamoxifen. The less extensive TRC loss observed here may be due to incomplete Cre-mediated recombination and consequent partial knockdown of Shh transcript in the geniculate ganglia (Fig. S4 B and C). Nevertheless, our results with two independent genetic ablation methods together indicate that neuronal Shh gene function is important for long-term maintenance of TRCs.
Fig. 3.
Shh expressed from sensory neurons mediates long-term maintenance of TRCs. (A) Peeled epithelium from neuronal Shh knockout mice at either 8 or 12 wk of age, immunostained with K8 (red) and DAPI (blue). Note that TRCs in AvilCre/+;Shhflox/flox mice are present at 8 wk but are eliminated in ∼85% of fungiform papillae at 20 wk. Fungiform papillae with partial TRC loss are denoted by yellow arrows. (Scale bars, 100 μm.) (B) Representative cryosections of fungiform papillae stained with K8 (red) and DAPI (blue). (Scale bars, 10 μm.)
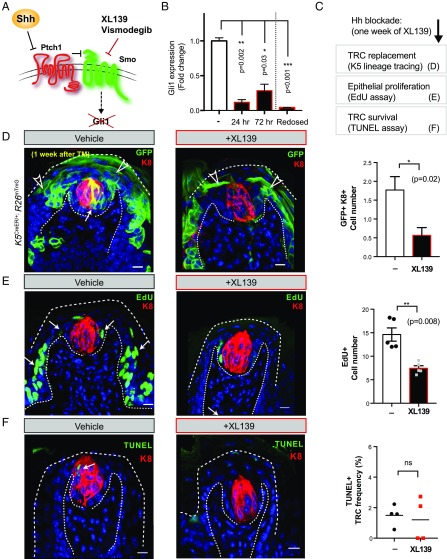
Hh Blockade Impedes TRC Renewal by Reducing Epithelial Cell Proliferation and Differentiation.
Having demonstrated the importance of neuronally derived Hedgehog ligand in TRC maintenance, we investigated how loss of Hedgehog response disrupts TRC maintenance by imposing pharmacologic blockade on pathway activity. For this purpose, we used the cyclopamine mimic XL139 (27), an antagonist of the essential pathway component Smoothened (Fig. 4A) that we have found to be highly effective in mice with dosing every third day (relative to vismodegib, compared in Fig. S5 A and B). Confirming its effectiveness in the lingual epithelium, we found that expression of Gli1 was reduced 8.5-fold 24 h after the first dosing (0.12 ± 0.04 relative to vehicle treatment, P = 0.002), and remained largely suppressed at 72 h (0.29 ± 0.09 relative to vehicle, P = 0.001) with full suppression restored by redosing at 72 h (−24 fold; 0.04 ± 0.004 relative to vehicle, P = 0.004; Fig. 4B). Postmitotic TRCs are replaced every 8–24 d from surrounding epithelium (28), and this process can be tracked by marking of basal epithelial cells, which express K5 (Fig. S5C). To investigate whether Hedgehog pathway suppression affects TRC replacement, we labeled K5-expressing cells by treating K5CreER/+;R26mTmG mice with tamoxifen, followed by 1 wk of XL139 blockade. The frequency of TRCs derived from the K5+ epithelial lineage in XL139-treated mice was one-third of that in untreated mice (from 1.8 ± 0.4 to 0.6 ± 0.2 cells per fungiform papilla, P = 0.02; Fig. 4 C and D). As proliferation of progenitor cells is a key requirement for renewal of TRCs (29), we also measured the frequency of 5-ethynyl-2′-deoxyuridine (EdU) incorporation in epithelial cells of fungiform papillae of mice during XL139 blockade. We found that EdU+ cells decreased by one-half (from 14.2 ± 1.4 to 7.4 ± 0.5, P = 0.008; Fig. 4E), indicating that proliferation is dependent on Hedgehog response. Increased loss of TRCs by induction of apoptosis does not appear to play a role, as we did not observe increased TUNEL labeling of the TRCs under Hh blockade (Fig. 4F), even at 1 wk of XL139 treatment, when TRC loss is ongoing (Fig. S5D). Taken together, these data show that Hedgehog pathway blockade affects taste sensory organ maintenance by impeding replacement of TRCs rather than by facilitating their decay (Fig. S5E).
Fig. 4.
Hh blockade impedes TRC renewal by reducing epithelial cell proliferation and differentiation. (A) Diagram of Hedgehog signal transduction mechanism and usage of Smoothened antagonists Vismodegib (GDC-0449) and XL139 (BMS-833923) for Hh blockade, resulting in loss of downstream target Gli1 expression. (B) qRT-PCR analysis of Gli1 expression levels measured from isolated epithelial cells at indicated time points and normalized to the vehicle-treated group. The fourth bar (Redosed) denotes a group of mice from which Gli1 expression was measured 4 h after a second dose of XL139. (C) Experimental scheme. (D) Representative fungiform papillae from K5CreER/+;R26mTmG mice 1 wk after tamoxifen injection treated with vehicle or XL139. Note that K5-expressing lineage (GFP+) gives rise to TRCs (with GFP+K8+ double label; white arrow) and keratinocytes (with GFP+, single-label; open arrowhead). Panel, number of traced K5-lineage cells differentiating into GFP+K8+ double-positive cells per fungiform papilla (n = 26 vehicle- and 23 XL139-treated fungiform papillae; *P = 0.02). (E) Representative fungiform papilla 2 d after last dose of EdU (green, arrows) from mice treated with vehicle or XL139. Panel, number of EdU+ cells per fungiform papilla (n = 149 vehicle- and 118 XL139-treated; 5 mice in each group; **P = 0.008). (F) Immunofluorescence of TUNEL labeling (green) to detect apoptotic TRCs in fungiform papillae. Mice were treated with vehicle or XL139 for 7 d. Panel, frequency of TUNEL+ TRCs among total number of TRCs within each fungiform papilla (n = 56 vehicle- and 81 XL139-treated fungiform papillae from 3 or 4 mice in each group; ns, not significant). (Scale bars, 10 μm.)
Hh Pathway Activity Is Critical for Taste Cell Regeneration.
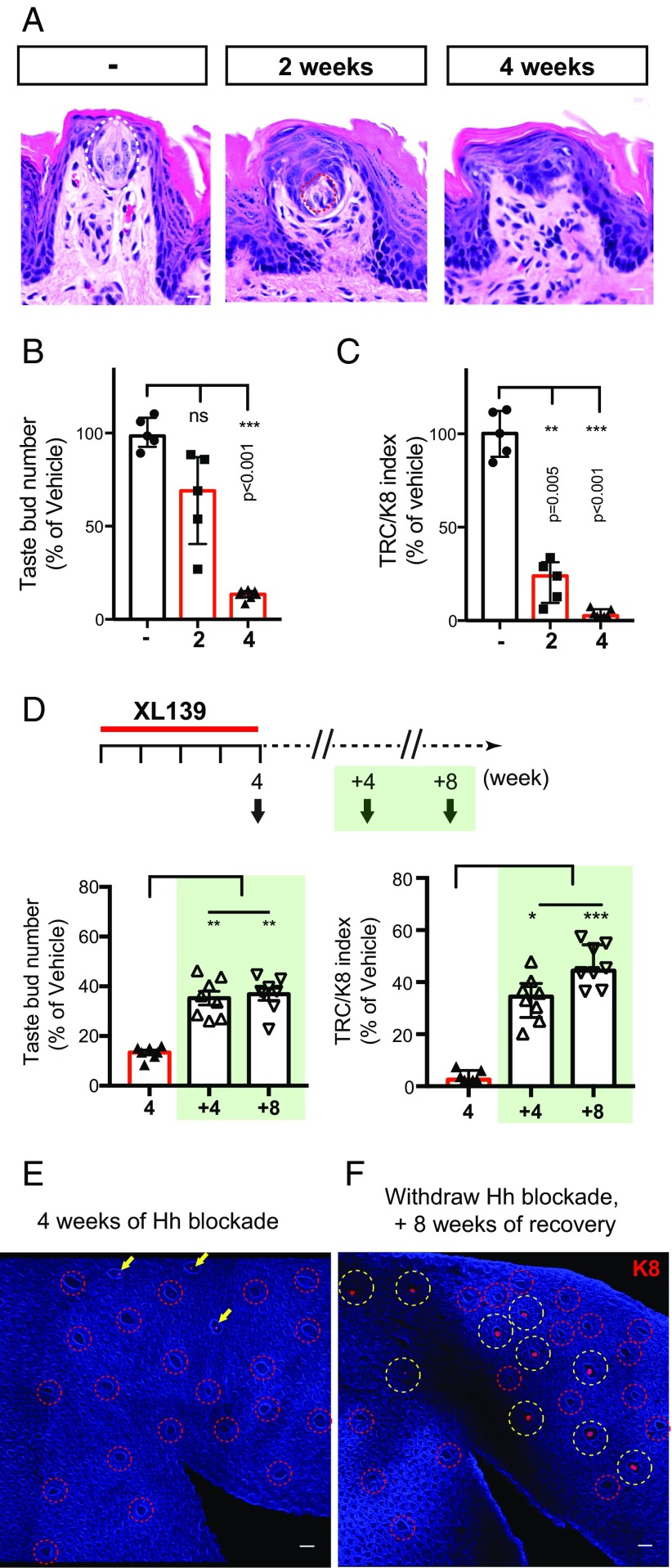
The great majority of cancer patients receiving Hedgehog pathway antagonists for more than a month report loss or severe disturbance of taste sensation, and this loss is reversed upon cessation of treatment (8–11). To determine whether the impaired TRC replacement we noted above might account for such a loss, we exposed mice to extended XL139 blockade. We indeed found that mice undergoing XL139 treatment showed a progressive reduction of TRCs; thus, by 4 wk of treatment, only 13% (±0.9%) of fungiform papillae contained TRCs, with only 3.8% (±0.8%) of overall TRCs remaining (Fig. 5 A–C). Extended blockade of Hedgehog pathway activity also affects taste organs in the circumvallate papilla, although not as rapidly (Fig. S6 A–D vs. E–I). To determine whether TRC loss can be reversed upon lifting of the Hedgehog pathway blockade, as suggested by the recovery of taste sensation in patients that undergo drug withdrawal, we monitored TRCs in mice treated with XL139 for 4 wk followed by 4- and 8-wk recovery periods (Fig. 5 D–F). We found that the relative percentage of TRC-containing taste bud number increased to 35.3% (±2.7%) and 36.8% (±2.4%) after 4 and 8 wk of recovery, respectively (Fig. 5D, Left); the overall TRCs in these regenerated papillae respectively increased to 33.7% (±3.1%) and 46.3% (±2.8%) (Fig. 5D, Right).
Fig. 5.
Hh pathway activity is critical for TRC maintenance. (A) Representative H&E-stained examples of fungiform papilla from untreated and 2- and 4-wk XL139-treated animals. (B and C) Number of TRC-containing fungiform papillae, and TRC/K8+ index estimating relative TRC numbers (Materials and Methods) using immunofluorescence images of whole-mount peeled epithelium from mice treated with vehicle or XL139 for 2 or 4 wk. n = 5, 5, and 7 mice in each group, respectively. (D) Mice treated with XL139 for 4 wk, and allowed to recover for 4 or 8 wk after XL139 withdrawal. n = 7, 8, and 8 mice in each group. (E and F) Immunofluorescence image of whole-mount tongue epithelium stained with K8 (red) and DAPI (blue). (E) Analysis immediately following 4-wk XL139 treatment (no recovery period). Note fungiform papillae are devoid of TRCs (red circles) or show reduced K8+ intensity (arrows). (F) A 4-wk XL139 treatment followed by an 8-wk recovery period. K8 staining returned to ∼40% of the fungiform papillae as quantified in D. Fungiform papillae with regenerated TRCs are denoted with yellow circles. [Scale bars: 10 μm (A); 100 μm (E and F).]
Pharmacologic Activation of Hedgehog Pathway Facilitates TRC Regeneration.
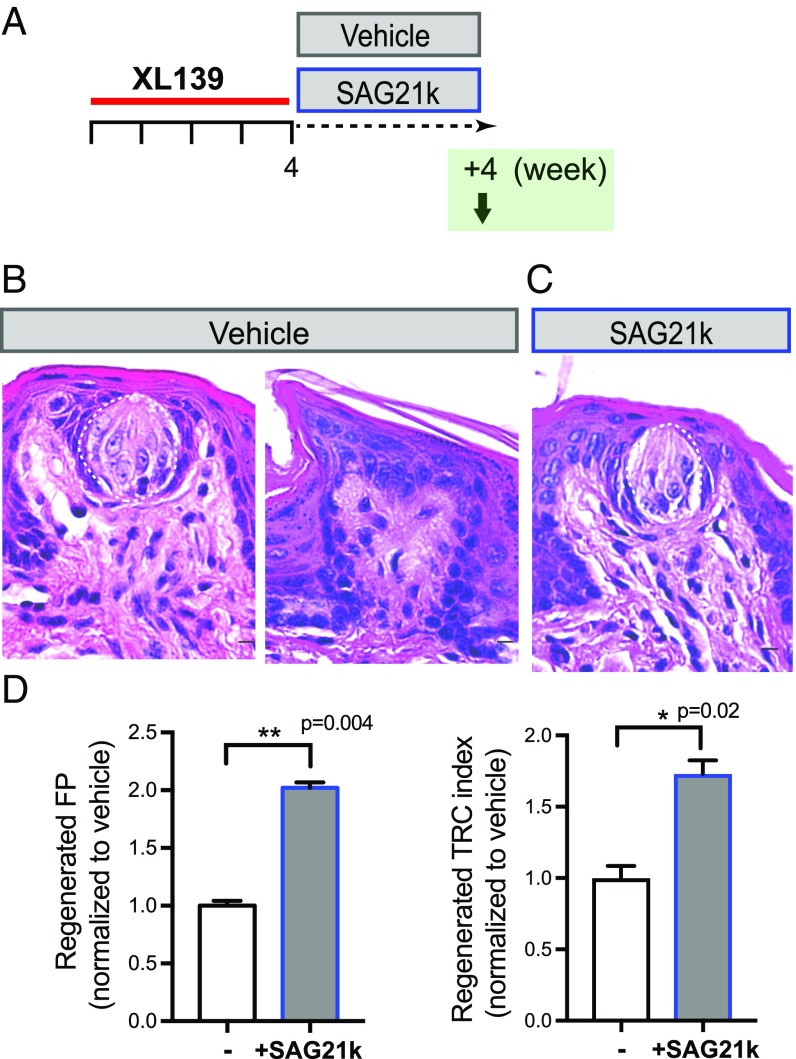
To determine whether increased Hedgehog pathway activity can enhance TRC regeneration within fungiform papillae, we administered the Smoothened agonist SAG21k (30, 31) during the 4-wk recovery period (Fig. 6 A and B). We noted a twofold increase in the number of taste buds containing TRCs (from 29 ± 1.2% to 58.6 ± 1.4% with SAG21k treatment, P = 0.004), and a 1.7-fold increase in the overall TRCs (from 41.3 ± 3.5% to 71.5 ± 3.9% with SAG21k treatment, P = 0.02). We thus find that, in addition to a requirement for Hedgehog pathway in TRC maintenance, increased pathway activity can substantially augment TRC regeneration after near-complete degeneration, an observation that may be clinically useful in the setting of chemotherapy-induced loss of taste sensation.
Fig. 6.
Pharmacologic activation of Hedgehog pathway facilitates TRC regeneration. (A) Experimental design to examine the effect of Hh agonist on TRC regeneration. (B and C) Representative H&E-stained fungiform papillae from mice treated with vehicle (B) or SAG21k (C) after a 4-wk recovery period following 4 wk of XL139 treatment. (D) Quantification of TRC regeneration as frequency of fungiform papillae (FP) containing TRCs (Left), and TRC index (Right). Shown as relative frequency normalized to vehicle-treated group. n = 5 and 6 mice in each group. [Scale bars: 10 μm (B and C).]
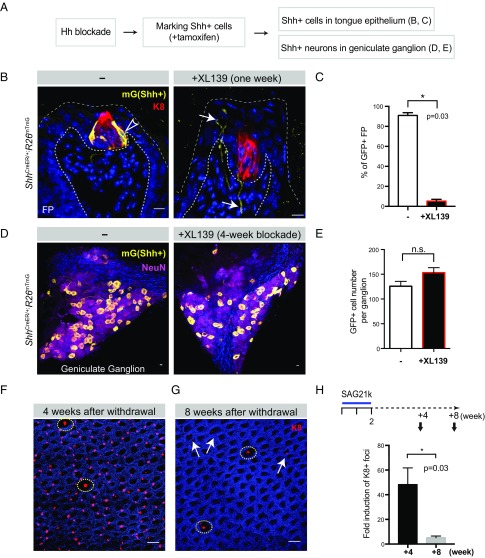
Hh Antagonism Causes Loss of Local Shh-Producing Cells in the Taste Buds.
To examine the effect of Hh antagonism on Shh production, we subjected ShhCreER/+; R26mTmG mice to XL139 blockade before giving tamoxifen to label Shh-producing cells in the taste bud as well as in sensory neurons (Fig. 7A), as we have found that genetic marking is more definitive in identification of Shh-expressing cells than antibody-based detection. We found that local expression of Shh within basal cells is lost in 94% of the taste buds after a 2-wk treatment with XL139 (Fig. 7 B and C), probably due to cell turnover within the taste bud and consistent with our observation of impaired TRC replacement upon XL139 treatment (Fig. 4 D–F). Correspondingly, Shh expression assayed by qRT-PCR in isolated lingual epithelium was also dramatically reduced (Fig. S7). In contrast, Shh expression persists within neurons of the geniculate ganglion, as the number of Shh-producing neurons by genetic marking was unaffected by XL139 treatment (Fig. 7 D and E).
Fig. 7.
Hh antagonism causes loss of local Shh-producing cells in the taste buds. (A) Experimental design to determine the effect of prior Hh pathway blockade. (B) GFP staining of fungiform papillae from ShhCreER/+;R26mTmG mice. GFP+ (Shh-expressing) cells in the Shh-expressing basal cells marked in vehicle-treated group are denoted by open arrowheads. In XL139-treated group, GFP markings of Shh-producing neurons are denoted by arrows. (C) Quantified percentage of GFP-marked Shh-producing cells in fungiform papillae; tamoxifen was given after 2 wk of Hh blockade: 90.9 ± 2.81% vs. 5.3 ± 1.81%; P = 0.03; n = 4 mice in each group. (D) GFP+ (Shh-expressing) cells in the geniculate ganglion (mGFP in yellow; NeuN in magenta). (E) Quantification of GFP+ cell numbers per ganglion: 125.8 ± 9.8 vs. 153.3 ± 10.1; ns, not significant. Sample size, n = 12 and 7 ganglia from 6 and 4 mice in vehicle- and XL139-treated (4 wk), respectively. (F) Immunofluorescence image of tongue epithelium stained with K8 (red) and DAPI (blue). Normal TRC-containing fungiform papillae are denoted by yellow circles. (G) Residual ectopic K8+ foci after 4 wk are denoted by arrows. (H) Total number of K8+ foci normalized to the number of K8+ fungiform papillae quantified as 4 and 8 wk after removal of SAG21k treatment, normalized to untreated group (as 1, not plotted). n = 4 mice in each group (42 ± 11.7-fold; 4.5 ± 1.2-fold, P = 0.03). [Scale bars: 10 μm (B and D); 100 μm (F).]
Shh from Sensory Neurons Specifies the Spatial Patterning of TRC Regeneration.
We also tested the effect of systemic SAG21k administration on normal lingual epithelium and noted the appearance of K8+ TRCs at many ectopic sites (Fig. 7F), in a manner reminiscent of that noted upon ectopic expression of Shh within the lingual epithelium in the absence of innervation (32). Because we activated the Hedgehog pathway pharmacologically, we were able to terminate ectopic pathway activation by withdrawing SAG21k, thus producing the interesting effect that nearly all ectopic TRCs degenerated within 8 wk after SAG21k withdrawal, leaving predominantly TRCs at their normal locations within the fungiform papillae (Fig. 7 G and H). The ability of SAG21k treatment to induce ectopic TRCs suggests that Hedgehog response is sufficient to specify TRC fate in cells of the lingual epithelium. The decay of ectopic TRCs upon withdrawal of SAG21k reinforces the finding that Hedgehog pathway activity is required for TRC maintenance (Fig. 5). The restriction of TRC regeneration to fungiform papillae in the absence of SAG21k treatment (Fig. 5F) suggests that Hedgehog signal, which activates the pathway, is normally restricted to the environment of the fungiform papillae and must be maintained there following Hh pathway blockade to specify TRC regeneration. We indeed found that Shh expression persists within the neurons of the geniculate ganglion and their processes that project to the fungiform papillae, even with a 4-wk XL139 treatment (Fig. S8).
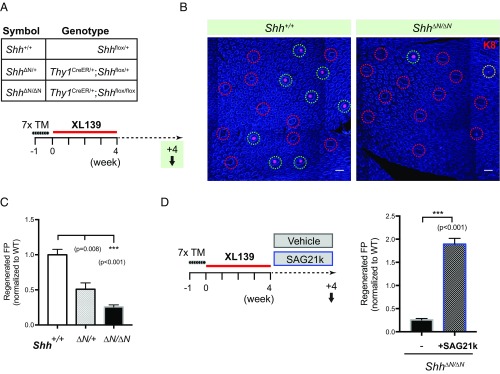
Shh from Sensory Neurons Is Required for TRC Regeneration.
As noted above, the Shh signal that contributes to maintenance of TRCs is neuronal in origin (Figs. 2 and 3). To determine whether the Shh signal required for regeneration following inhibitor-mediated ablation of TRCs is also from this neuronal source, we used Thy1CreER, a neuronal-specific, tamoxifen-dependent Cre recombinase to ablate Shh in sensory neurons in mice of the genotype Thy1CreER/+;Shhflox/flox, hereafter referred to as Shh∆N/∆N. We indeed found a marked dose dependence of taste bud regeneration on Shh function, with a reduction of TRC-containing fungiform papillae to 50.9% (±9.1%, P = 0.008) of WT in heterozygous Shh∆N/+ mice, and to 25.7% (±2.9%, P < 0.001) of WT in Shh∆N/∆N mice (Fig. 8 A–C). Normal regeneration of TRCs following ablation by Hedgehog pathway antagonist treatment thus requires Shh expression in distant sensory neurons. Similar to the effect of Hh agonism in WT mice during the 4-wk regeneration period above (Fig. 6), SAG21k treatment of Shh∆N/∆N mice also resulted in a 1.9-fold increase (±0.12-fold, P < 0.001; Fig. 8D) of TRC-containing fungiform papillae, demonstrating pharmacologic rescue of a TRC regeneration deficit caused by absence of neuronal Shh expression. This result indicates that pharmacologically stimulated Hh response suffices to rescue TRC regeneration in the absence of neuronal Shh signal.
Fig. 8.
Shh from sensory neurons specifies the spatial patterning of TRC regeneration. (A) Genotype of mice used and experimental scheme to introduce neuronal Shh ablation before TRC regeneration. (B) Immunofluorescence images of tongue epithelia showing regenerated taste buds (green circles) in Shh+/+ and Shh∆N/∆N animals (partially regenerated taste buds denoted by yellow circles). (Scale bars, 100 μm.) Shown as composites of tile-scanned images stitched together by Zeiss ZEN software, as described in Materials and Methods. (C) Quantification of regenerated fungiform papillae as a function of Shh allele dosage, normalized to Shh+/+; n = 7, 5, and 15 mice in each group. (D) Experimental scheme to activate Hh pathway pharmacologically during TRC regeneration. Quantification of regenerated fungiform papillae in Shh∆N/∆N mice, analyzed after a 4-wk recovery period with either vehicle (DMSO) or SAG21k treatment. Shown as ratio normalized to Shh+/+ group in Fig. 6C. Number of mice, n = 15 and 11.
Discussion
A role for Hh signaling in the taste system emerged from the recent development of Hh pathway antagonists for use in treatment of advanced basal cell carcinoma and other cancers. Patient trials with this class of drugs, including the Food and Drug Administration-approved antagonist vismodegib, produced the surprising finding that a disturbance or loss of taste occurs in most patients within 2 mo after initiation of treatment (8–10). These clinical observations are consistent with loss of TRCs associated with systemic blockade of Hedgehog response in the mouse (18, 33, 34) (Figs. 4 and 5). The basis for this failure of TRC maintenance is not an accelerated demise of TRCs, as no increase in apoptosis was observed (Fig. 4F). Instead, we noted a deficiency in replacement of TRCs, associated with reduced specification of TRC fates from lingual epithelial progenitors and reduced proliferation of these progenitors (Fig. 4 D and E). Reduced epithelial cell proliferation upon effective pharmacologic blockade, as noted here, is consistent with a previous study demonstrating loss of proliferation upon transcriptional suppression of Gli targets (17). Given the kinetics of TRC turnover in the mouse [8–24 d (28)], the observed 2- to 4-wk time for loss of TRCs (Fig. 5 A–C) is consistent with impaired TRC replacement as a mechanism for Hedgehog antagonist treatment.
A role for neuronal innervation in taste system maintenance was described 140 years ago, in experiments showing that taste buds in rabbits degenerate upon transection of innervating sensory projections (4). Our results align with and extend these and subsequent nerve transection experiments (5–7) by showing that neuronal expression of Shh, occurring in geniculate ganglion neurons that project into the taste buds (Fig. 2), contributes to the maintenance of TRCs (Fig. 3 and Fig. S4C). Our results confirm and extend those of Castillo-Azofeifa et al. (18), who observed no TRC defects within 35 d (7 wk) following neuronal Shh ablation. We also observed few if any defects in TRC maintenance in 8-wk-old AvilCre;Shhflox/flox mice, but did see extensive loss of TRCs after a longer 20-wk period (Fig. 3 A and B), or a less extensive loss after a 12-wk period with the less efficiently ablating Thy1CreER;Shhflox/flox mice. We speculate that the long-term requirement for neuronally provided Hh may relate to the greater stability of Shh expression in ganglion neurons compared with local expression of Shh within cells of the taste bud (Fig. 7 B–E). This local taste bud expression is lost upon Hh inhibition and may also be lost during TRC injury and normal turnover (Fig. S4 B and C).
With near-complete pharmacologic ablation of TRCs, mediated by XL139 (Fig. 5 A–C), we were able to analyze regeneration of TRCs in an epithelial field largely devoid of TRCs (Fig. 5 D–F) or Shh-expressing cells (Fig. 7 B and C) within the taste buds. We found that regeneration depends critically upon neuronal Shh expression, as Shh ablation in neurons impaired TRC recovery in a manner depending on the remaining WT Shh gene dosage (Fig. 8 A–C). The critical role of Hedgehog pathway activity in TRC regeneration is also evident from the observation that pharmacologic activation of Hedgehog pathway activity accelerates regeneration of TRCs within taste buds (Fig. 6), and also suffices to induce TRC fates ectopically throughout the lingual epithelium (Fig. 7 F and H). Interestingly, TRCs in ectopic locations are lost upon agonist withdrawal, but are maintained in the fungiform papillae (Fig. 7 G and H). Correspondingly, regeneration in the absence of pharmacologic manipulation is also restricted to the fungiform papillae (Fig. 4 D–F).
Our results indicate that the unique attribute of fungiform papillae that supports regenerative activity is the presence of Hedgehog delivered by sensory processes from distant neurons, as demonstrated by disruption of TRC regeneration upon neuronal ablation of Shh. Neuronal provision of Hedgehog signal thus provides the basis for understanding not only loss of taste in cancer patients treated with Hedgehog pathway antagonists but also the spatially correct specification of regenerative activity upon antagonist withdrawal. In a clinical context, it may be possible to make use of Hedgehog pathway agonist to accelerate the return of lost taste sensation, whether caused by chemotherapy, radiation, or Hedgehog pathway inhibition.
The neuronal provision of Hedgehog signal at precise locations to specify the architecture of a regenerating organ represents a principle of tissue regeneration that may have broader application. Other examples may be nerve-dependent limb regeneration in amphibians (35) and digit regeneration in mice (36), although in these cases the identity of the neuronally supplied signal(s) is not known. Dual lipid modification of the mature Hedgehog protein signal and consequent association with the membrane make it particularly well suited for delivery by neuronal processes; other such signals could include the Wnt proteins, which are also lipid modified (37, 38), and Notch or Notch ligands (39), which contain transmembrane domains. Such signals might be delivered to distant sites by their association with and movement along the membranes of neuronal processes, thus providing a robust and durable template for the maintenance and regeneration of tissue targets.
Materials and Methods
See Supporting Information for additional materials and methods.
Mice.
The following mouse strains were obtained from The Jackson Laboratory: WT C57BL/6J (000664), Gli1LacZ (008211) (40), Gli1CreERT2 (007913) (41), RosaZsGreen (007906) (42), ShhCreERT2 (005623) (43), RosamTmG (007576) (44), K5CreER (018394) (45), Thy1CreER (12708) (46), Shhflox (004293) (26), and TaumGFP (021162) (47). WT FVB/NCrl (207) mice were obtained from Charles River. AvilCre (25) was kindly provided by the Yin Liu/Krasnow Laboratory at Stanford University (Stanford, CA). All procedures were performed under Institutional Animal Care and Use Committee-approved protocol at Stanford University.
Image Acquisition.
Epifluorescence imaging was performed on a fluorescence stereomicroscope (Leica; M205 FA). H&E-stained sections were imaged on an upright microscope (Leica; DM5500 B). Immunofluorescence imaging was performed on laser-scanning confocal microscopes (Zeiss; LSM 700 or LSM 800). For whole-mount stained lingual epithelia, images were acquired by tile scan with z stacks using 10× objective lens.
Supplementary Material
Acknowledgments
We thank Liqun Luo, Yin Liu, Greg Nachtrab, and the P.A.B. laboratory members for critical comments on the manuscript. This work was supported by postdoctoral fellowships to W.-J.L. from Damon Runyon Cancer Research Foundation, California Institute for Regenerative Medicine, the Siebel Foundation, and NIH [Grant R21 NS093556 (to P.A.B.)]. P.A.B. was an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719109115/-/DCSupplemental.
References
- 1.Okubo T, Clark C, Hogan BLM. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells. 2009;27:442–450. doi: 10.1634/stemcells.2008-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukherjee N, Delay ER. Cyclophosphamide-induced disruption of umami taste functions and taste epithelium. Neuroscience. 2011;192:732–745. doi: 10.1016/j.neuroscience.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JB, Smutzer G, Doty RL. Understanding the impact of taste changes in oncology care. Support Care Cancer. 2016;24:1917–1931. doi: 10.1007/s00520-016-3083-8. [DOI] [PubMed] [Google Scholar]
- 4.Vintschgau MV, Hönigschmied J. Nervus glossopharyngeus und Schmeckbecher. Archiv Gesamte Physiol Menschen Tiere. 1877;14:443–448. [Google Scholar]
- 5.Olmsted JMD. Effects of cutting the lingual nerve of the dog. J Comp Neurol. 1921;33:149–154. [Google Scholar]
- 6.Guth L. Taste buds on the cat’s circumvallate papilla after reinnervation by glossopharyngeal, vagus, and hypoglossal nerves. Anat Rec. 1958;130:25–37. doi: 10.1002/ar.1091300104. [DOI] [PubMed] [Google Scholar]
- 7.Cheal M, Oakley B. Regeneration of fungiform taste buds: Temporal and spatial characteristics. J Comp Neurol. 1977;172:609–626. doi: 10.1002/cne.901720405. [DOI] [PubMed] [Google Scholar]
- 8.Von Hoff DD, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 9.Sekulic A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang JY, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodon J, et al. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin Cancer Res. 2014;20:1900–1909. doi: 10.1158/1078-0432.CCR-13-1710. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z, Kunes S. Hedgehog, transmitted along retinal axons, triggers neurogenesis in the developing visual centers of the Drosophila brain. Cell. 1996;86:411–422. doi: 10.1016/s0092-8674(00)80114-2. [DOI] [PubMed] [Google Scholar]
- 13.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao H, et al. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 2014;14:160–173. doi: 10.1016/j.stem.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson SC, et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16:400–412. doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao Y, et al. Neural hedgehog signaling maintains stem cell renewal in the sensory touch dome epithelium. Proc Natl Acad Sci USA. 2015;112:7195–7200. doi: 10.1073/pnas.1504177112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ermilov AN, et al. Maintenance of taste organs is strictly dependent on epithelial hedgehog/GLI signaling. PLoS Genet. 2016;12:e1006442. doi: 10.1371/journal.pgen.1006442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillo-Azofeifa D, et al. Sonic hedgehog from both nerves and epithelium is a key trophic factor for taste bud maintenance. Development. 2017;144:3054–3065. doi: 10.1242/dev.150342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mann RK, Beachy PA. Novel lipid modifications of secreted protein signals. Annu Rev Biochem. 2004;73:891–923. doi: 10.1146/annurev.biochem.73.011303.073933. [DOI] [PubMed] [Google Scholar]
- 20.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 21.Miura H, et al. Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech Dev. 2001;106:143–145. doi: 10.1016/s0925-4773(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 22.Thirumangalathu S, Harlow DE, Driskell AL, Krimm RF, Barlow LA. Fate mapping of mammalian embryonic taste bud progenitors. Development. 2009;136:1519–1528. doi: 10.1242/dev.029090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H-X, et al. Multiple Shh signaling centers participate in fungiform papilla and taste bud formation and maintenance. Dev Biol. 2013;382:82–97. doi: 10.1016/j.ydbio.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miura H, Scott JK, Harada S, Barlow LA. Sonic hedgehog-expressing basal cells are general post-mitotic precursors of functional taste receptor cells. Dev Dyn. 2014;243:1286–1297. doi: 10.1002/dvdy.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.da Silva S, et al. Proper formation of whisker barrelettes requires periphery-derived Smad4-dependent TGF-beta signaling. Proc Natl Acad Sci USA. 2011;108:3395–3400. doi: 10.1073/pnas.1014411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis PM, et al. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105:599–612. doi: 10.1016/s0092-8674(01)00369-5. [DOI] [PubMed] [Google Scholar]
- 27.Gendreau SB, et al. Abstract B192: Preclinical characterization of BMS-833923 (XL139), a hedgehog (HH) pathway inhibitor in early clinical development. Mol Cancer Ther. 2009;8(12 Suppl):B192. [Google Scholar]
- 28.Perea-Martinez I, Nagai T, Chaudhari N. Functional cell types in taste buds have distinct longevities. PLoS One. 2013;8:e53399. doi: 10.1371/journal.pone.0053399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beidler LM, Smallman RL. Renewal of cells within taste buds. J Cell Biol. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunton SA, et al. Potent agonists of the hedgehog signaling pathway. Bioorg Med Chem Lett. 2009;19:4308–4311. doi: 10.1016/j.bmcl.2009.05.096. [DOI] [PubMed] [Google Scholar]
- 31.Lee JJ, et al. Stromal response to hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci USA. 2014;111:E3091–E3100. doi: 10.1073/pnas.1411679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castillo D, et al. Induction of ectopic taste buds by SHH reveals the competency and plasticity of adult lingual epithelium. Development. 2014;141:2993–3002. doi: 10.1242/dev.107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumari A, et al. Hedgehog pathway blockade with the cancer drug LDE225 disrupts taste organs and taste sensation. J Neurophysiol. 2014;113:1034–1040. doi: 10.1152/jn.00822.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H, et al. Vismodegib, an antagonist of hedgehog signaling, directly alters taste molecular signaling in taste buds. Cancer Med. 2014;4:245–252. doi: 10.1002/cam4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer M. The influence of the nerve in regeneration of the amphibian extremity. Q Rev Biol. 1952;27:169–200. doi: 10.1086/398873. [DOI] [PubMed] [Google Scholar]
- 36.Rinkevich Y, et al. Clonal analysis reveals nerve-dependent and independent roles on mammalian hind limb tissue maintenance and regeneration. Proc Natl Acad Sci USA. 2014;111:9846–9851. doi: 10.1073/pnas.1410097111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: Its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bray SJ. Notch signalling in context. Nat Rev Mol Cell Biol. 2016;17:722–735. doi: 10.1038/nrm.2016.94. [DOI] [PubMed] [Google Scholar]
- 40.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 41.Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 42.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harfe BD, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 45.Indra AK, et al. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: Comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young P, et al. Single-neuron labeling with inducible Cre-mediated knockout in transgenic mice. Nat Neurosci. 2008;11:721–728. doi: 10.1038/nn.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hippenmeyer S, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.