Twenty years ago, Ray Blanchard and Anthony Bogaert demonstrated that the probability of a boy growing up to be gay increases for each older brother born to the same mother, the so-called fraternal birth order (FBO) effect. Their first investigation indicated that each older brother increased the probability of being gay by about 33% (1). This startling phenomenon was confirmed in multiple studies based on independent populations totaling over 10,000 subjects, and a meta-analysis indicated that between 15% and 29% of gay men owe their sexual orientation to this effect (2). Despite this compelling evidence, a mechanism to account for the effect remained elusive. In PNAS, Bogaert et al. (3) present direct biochemical evidence indicating that the increased incidence of homosexuality in males with older brothers results from a progressive immunization of the mother against a male-specific cell-adhesion protein that plays a key role in cell–cell interactions, specifically in the process of synapse formation, during development called neuroligin 4 Y-linked, or NLGN4Y. This study provides the first data-based explanation for the FBO effect and adds a significant chapter to growing evidence indicating that sexual orientation is heavily influenced by prenatal biological mechanisms rather than by unidentified factors in socialization.
The nature–nurture debate still rages in the minds of many scientists and scholars, despite the consensus that these are complementary rather than mutually exclusive explanations. However, no field of research subject to this debate generates more heated controversies than those probing the proximate causes of sexual orientation, particularly its less-frequent and thus, perhaps, more-perplexing form: homosexuality. Why the obverse questions probing the causes of heterosexuality attracts no attention remains enigmatic. Theories relying mainly on psychological and social mechanisms contend that the newborn is essentially neutral and that sexual orientation develops during infancy and childhood through a variety of socializing influences. In contrast, many scientists are now convinced that biological processes during embryonic and early postnatal life play a major role in the control of sexual orientation.
Three types of biological mechanisms have been identified in this context (4–7). First, work in a variety of animal models has shown that sexual partner preference can be experimentally modified by perinatal treatments with sex steroids: masculinization [i.e., development of gynephilia (attraction to females)] following exposure to testosterone or its estrogenic metabolites, and feminization [development of androphilia (attraction to males)] in the (relative) absence of these steroids during a critical period of development. Correlative studies suggest that these mechanisms are also at play in humans. Endocrine pathologies that modify the embryonic hormonal environment are associated with increased incidence of homosexuality. Additionally, numerous epidemiological studies have shown that gay men and lesbians display a partial sex-reversal of morphological, physiological, and behavioral/cognitive traits that are sexually differentiated, and in many cases known to develop under the early influence of sex steroids (6). Second, there is strong evidence, from studies of family trees and of monozygotic and dizygotic twins, of a genetic component to the control of sexual orientation, even if attempts to identify the specific genes involved have met so far with little success (5, 8, 9). Recent publications also suggest that epigenetic mechanisms could be involved (10) but the specifics have so far remained elusive (11). Third, and directly relevant to Bogaert et al. (3), homosexuality in males has been consistently and repeatedly linked to the presence of older brothers born to the same mother, the FBO effect (1, 2, 12). While FBO represents the best-documented biological influence on sexual orientation, the underlying mechanisms had remained completely speculative so far.
Several explanations were initially suggested, including older age of the mother or the father, and the social interactions between multiple boys raised in the same family. However, the large number of studies confirming this phenomenon, based on huge numbers of subjects, allowed partial regression techniques to reject these interpretations. For example, older biological brothers raised apart still affect the odds of the proband being gay, while older step-brothers living in the same home appear to have no effect. The FBO effect on sexual orientation is also associated with a decreased body and brain weight at birth, further suggesting that the developmental process triggered by older brothers starts prenatally (2).
After considering the possibilities over the years, Bogaert and Skorska (12) concluded that the most plausible explanation was based on a progressive immunization of the mother bearing male embryos against a male antigen; antibodies would accumulate over successive pregnancies and increasingly interfere with the development of the embryonic brain of subsequent sons. This maternal immune hypothesis (MIH) (12) would be similar to what happens in the hemolytic disease of the newborn where a mother with a rhesus negative (Rh−) blood type mounts an immune response against the Rh factor upon giving birth to a Rh+ offspring; the resulting antibodies attack the red blood cells and cause anemia in subsequent Rh+ offspring.
Bogaert and Skorska (12) further elaborated on this hypothesis and reasoned that for the MIH to be viable, a number of conditions should be fulfilled, including: (i) embryonic material should enter the mother’s circulation; (ii) this material should contain male-specific proteins causing immune responses in females; (iii) these proteins should play a role in the sexual differentiation of the brain; (iv) the maternal immune response to a male protein should affect fetal development, including sexual differentiation of the brain; and (v) the maternal immune reaction should display an incremental response to previous male fetuses and the immune response to the male antigen should persist for years in the mother’s blood. Based on the existing biomedical literature, Bogaert and Skorska identified four male-specific proteins fulfilling these criteria.
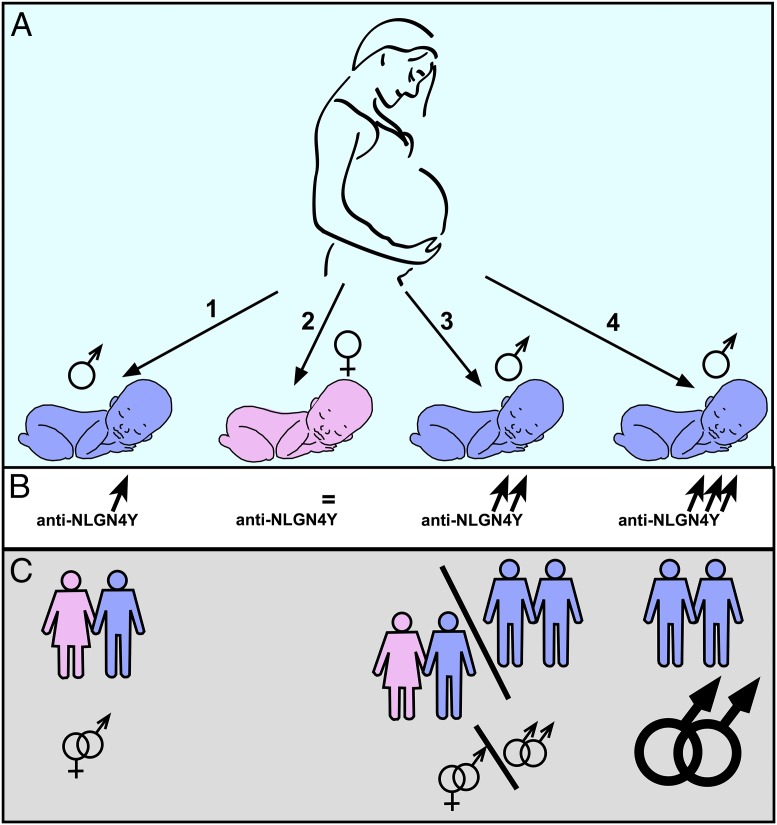
Now the research group (3) has tested the MIH by quantifying the concentration of antibodies directed against two of the male-linked proteins initially selected by deductive reasoning: PCDH11Y and NLGN4Y. The authors demonstrate that, overall, women have a higher blood concentration of anti-NLGN4Y antibodies than men and, more importantly, that after statistically controlling for the number of pregnancies, mothers of gay sons—especially those with older brothers—have significantly higher anti-NLGN4Y levels than control women, including mothers of heterosexual sons (3) (Fig. 1).
Fig. 1.
Schematic illustration of how pregnant women might mount a progressive immune response to the male-linked protein NLGN4Y during the gestation of male, but not female, embryos (A). The accumulation of anti-NLGN4Y antibodies (B) would then increase the relative incidence of a gay orientation among subsequent sons (C). Note, however, that this mechanism potentially explains only a fraction of gay males, so other mechanisms must also be at work.
In fact, there is a progressive increase of anti-NLGN4Y antibodies across groups of mothers: women with no sons < mothers of heterosexual sons < mothers of gay sons with no older brothers < mothers of gay sons with older brothers. Bogaert et al. (3) thus bring, for the first time, direct experimental support for the maternal immune theory to explain the FBO effect on the incidence of gay males.
The Bogaert et al. (3) report also provides additional support for biological theories of sexual orientation. Note that the FBO effect accounts for only a maximum of 29% of gay males, or possibly a bit more if one assumes that a fraction of the primiparous mothers who
In PNAS, Bogaert et al. present direct biochemical evidence indicating that the increased incidence of homosexuality in males with older brothers results from a progressive immunization of the mother against a malespecific cell-adhesion protein
had a gay son had unknowingly miscarried male embryos previously (12). Similarly, the other biological mechanisms implicated in the control of sexual orientation, the effects of early steroid hormones, and genetic background, also explain only a fraction of the cases of male homosexuality (6). Whether a unifying theory can be derived from the available experimental evidence thus remains unclear and will require additional investigations. Either the diverse biological mechanisms (hormonal, genetic, and immune) each explain a fraction of the cases and homosexuality is a multifactorial phenotype that can have multiple independent origins, or these different mechanisms interact and complement each other to control a phenotype that is otherwise essentially homogeneous. The endocrine and genetic mechanisms can easily be seen as interacting; for example, if a genetic mutation or variant affects the secretion or action of sex steroids in the brain. A gay orientation in males has been linked to the terminal Xq28 region of the X chromosome (8, 9), and this region contains a gene coding for protein MAGE-11 (melanoma-associated antigen), which is a coactivator of the androgen receptor (13). A mutation of this gene in males could thus modify testosterone action during brain development, although such an interaction still raises a number of questions (14). An interaction between the immune mechanisms discussed here and the endocrine or genetic mechanisms is less obvious, but not inconceivable.
Alternatively, perhaps the prenatal/perinatal biological factors do not by themselves determine sexual orientation but interact with specific aspects of the postnatal environment to reveal their full effect. There is, for example, recent evidence that the prenatal androgenization of girls with congenital adrenal hyperplasia modifies the way in which these girls respond to information about gender-appropriate behavior (15). This finding brings us back to the nature–nurture debate, demonstrating that these two approaches to the control of sexual orientation are not mutually exclusive but clearly cooperate to determine the adult phenotype. Adult differences in behavioral or cognitive abilities always result from cooperation between genetic or perinatal biological influences and postnatal experiences. Furthermore, as discussed in volume 5 of the novel Millenium (16), these two types of influences are not independent: the exact same postnatal environment of an individual is not shared with anybody, even brothers and sisters. Each individual creates his or her own environment, actively seeking out what interests or pleases him or her and developing related skills or cognitive abilities. Nature and nurture thus cooperate to forge the adult phenotype of each individual and this is probably also true for sexually differentiated traits, such as sexual orientation. The current Bogaert et al. (3) study adds an important piece to this puzzle by identifying a specific biological mechanism associated with the FBO effect.
Footnotes
The author declares no conflict of interest.
See companion article on page 302.
References
- 1.Blanchard R, Bogaert AF. Homosexuality in men and number of older brothers. Am J Psychiatry. 1996;153:27–31. doi: 10.1176/ajp.153.1.27. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard R. Quantitative and theoretical analyses of the relation between older brothers and homosexuality in men. J Theor Biol. 2004;230:173–187. doi: 10.1016/j.jtbi.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Bogaert AF, et al. Male homosexuality and maternal immune responsivity to the Y-linked protein NLGN4Y. Proc Natl Acad Sci USA. 2017;115:302–306. doi: 10.1073/pnas.1705895114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeVay S. Gay, Straight, and the Reason Why. The Science of Sexual Orientation. Oxford Univ Press; New York: 2010. [Google Scholar]
- 5.Ngun TC, Ghahramani N, Sánchez FJ, Bocklandt S, Vilain E. The genetics of sex differences in brain and behavior. Front Neuroendocrinol. 2011;32:227–246. doi: 10.1016/j.yfrne.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balthazart J, Young LJ. Mate selection, sexual orientation and pair bonding. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill’s Physiology of Reproduction. Vol 2. Elsevier; Amsterdam: 2015. pp. 2157–2210. [Google Scholar]
- 7.Balthazart J. Sex differences in partner preferences in humans and animals. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150118. doi: 10.1098/rstb.2015.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamer DH, Hu S, Magnuson VL, Hu N, Pattatucci AML. A linkage between DNA markers on the X chromosome and male sexual orientation. Science. 1993;261:321–327. doi: 10.1126/science.8332896. [DOI] [PubMed] [Google Scholar]
- 9.Sanders AR, et al. Genome-wide scan demonstrates significant linkage for male sexual orientation. Psychol Med. 2015;45:1379–1388. doi: 10.1017/S0033291714002451. [DOI] [PubMed] [Google Scholar]
- 10.Rice WR, Friberg U, Gavrilets S. Homosexuality as a consequence of epigenetically canalized sexual development. Q Rev Biol. 2012;87:343–368. doi: 10.1086/668167. [DOI] [PubMed] [Google Scholar]
- 11.Ngun TC, Vilain E. The biological basis of human sexual orientation: Is there a role for epigenetics? Adv Genet. 2014;86:167–184. doi: 10.1016/B978-0-12-800222-3.00008-5. [DOI] [PubMed] [Google Scholar]
- 12.Bogaert AF, Skorska M. Sexual orientation, fraternal birth order, and the maternal immune hypothesis: A review. Front Neuroendocrinol. 2011;32:247–254. doi: 10.1016/j.yfrne.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Karpf AR, Bai S, James SR, Mohler JL, Wilson EM. Increased expression of androgen receptor coregulator MAGE-11 in prostate cancer by DNA hypomethylation and cyclic AMP. Mol Cancer Res. 2009;7:523–535. doi: 10.1158/1541-7786.MCR-08-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balthazart J, Court L. Human sexual orientation: The importance of evidentiary convergence. Arch Sex Behav. 2017;46:1595–1600. doi: 10.1007/s10508-017-0997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hines M, et al. Prenatal androgen exposure alters girls’ responses to information indicating gender-appropriate behaviour. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150125. doi: 10.1098/rstb.2015.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagercrantz D. 2017. The Girl Who Takes an Eye for an Eye: Continuing Stieg Larsson’s Millennium Series, Vol 5 (Hachette UK, London)