Abstract
The aim of the present study was to evaluate angiogenesis by determining the micro vascular density (MVD) and the expression of vascular endothelial growth factor (VEGF-A) in advanced non-small cell lung cancer (NSCLC) tumor samples, and to analyze their associations with clinical parameters and survival. Tumor tissue specimens of fifty patients (41 males and 9 females), who underwent radical surgical treatment for NSCLC in stage IIIA (T1-3N2) were collected for immunohistochemical analysis. MVD evaluation was performed using an anti-CD31 monoclonal antibody and VEGF-A expression using a polyclonal anti-VEGF-A antibody. The results were associated with two-year survival. Statistical analysis revealed significant associations in the level of angiogenesis (high MVD) and shorter survival of patients with NSCLC (P=0.0007). VEGF-A expression showed no association with micro vascular density (P=0.51) or survival (P=0.68). There was no significant association between MVD and VEGF-A. The measurable, clinical MVD parameters could be used as a reliable prognostic factor for the survival of patients with advanced NSCLC.
Keywords: angiogenesis, blood vessel density, vascular endothelial growth factor-A, lung cancer, survival
Introduction
Lung cancer is the most common malignant tumor and the leading cause of death from malignancies. Five-year survival of the lung cancer is around 15% (1). In patients with no distant metastases (M0), the status of mediastinal lymph nodes is crucial for the prognosis of the disease. Two-year and five-year survival of a patient with metastasis spreading to the mediastinal lymph nodes (stage IIIA-N2) treated with surgery and adjuvant chemotherapy is 50 and 22%, respectively (2). TNM classification of malignant tumors, including lung cancer, is still the most accurate way of estimating the survival of patients with malignant tumors.
Angiogenesis is defined as the formation of new blood vessels from preexisting vessels. It is determined by complex interaction of multiple pro-angiogenic and anti-angiogenic factors. Angiogenesis is a prerequisite for tumor growth beyond 1–2 mm in diameter, when tumor cells can no longer be supplied with oxygen and nutrients by simple diffusion (3,4). In order to increase in size, tumors undergo an angiogenic switch, where the action of pro-angiogenic factors leads to angiogenesis and tumor growth (5). Tumor blood vessels are irregular in shape and branching, disordered and have fragmented basement membrane, which makes them permeable to tumor cells and thus enable metastasis (6). Angiogenesis is estimated by determining the average density of tumor blood vessels (MVD).
VEGF-A is the most potent and specific pro-angiogenic factor. It plays a critical role in tumor growth and metastasis of epithelial tumors including colorectal cancer, breast cancer, renal cell cancer, cervical cancer as well as head and neck tumors and lung cancer (7–11). The binding of VEGF-A to its receptors triggers multiple signaling cascades resulting in endothelial cell proliferation, migration and differentiation. VEGF-A mediates increased vascular permeability promoting tumor dissemination via circulation. By induction of anti-apoptotic signals, such as bcl-2 and surviving, VEGF-A protects new vasculature from destruction (12,13).
Although numerous studies point out that MVD is associated with the outcome of malignant tumors, the significance of MVD as a prognostic factor in patients with advanced NSCLC, who were initially surgically treated, was never investigated before (14). Furthermore, results of studies evaluating the prognostic significance of VEGF-A in NSCLC are controversial. High expression of VEGF-A, as a powerful pro-angiogenic factor, was correlated with high MVD in most studies (15–17).
In this retrospective study, we evaluated the prognostic significance of MVD and VEGF-A and their correlation with clinical parameters in advanced NSCLC (stage IIIA). MVD was assessed using CD31 and platelet endothelial cell adhesion molecule (PECAM-1), a protein found on the surface of endothelial cells. Immunohistochemical analysis was performed to evaluate the expression of CD 31 using a monoclonal anti-CD31 antibody and VEGF-A expression using polyclonal anti-VEGF-A antibody.
Materials and methods
Tissues
From the archives of the Department of Pathology in Zadar General Hospital, 50 formalin fixed, paraffin embedded cancer specimens were obtained from patients with histologically confirmed metastases to mediastinal lymph nodes, without distant metastases. The surgery on these patients was performed at the Department of Thoracic Surgery, Zadar General Hospital, Croatia between March1, 2007 and March 1, 2009. Samples from patients who died within 2 months of surgery were excluded from the study to eliminate perioperative complications as the cause of death. No patient underwent neoadjuvant chemotherapy or radiation therapy.
Histo-pathological properties of tumors were classified according to the World Health Organization. All patients received the same chemotherapy protocols postoperatively. The organization and stage of tumors were determined according to the guidelines of the International Association for the Study of Lung Cancer (IASLC) (18). Immunohistochemical analysis of tumor tissue samples was performed at the Department of Pathology, University of Rijeka School of Medicine. Patient survival was followed for a period of two years. Data on patients' survival were obtained from the Croatian National Cancer Registry. This study was approved by the Ethics Committee of Zadar General Hospital.
Immunohistochemical analysis
In each paraffin block, new sections were obtained and stained with hematoxylin eosin (HE) to determine a representative tumor area. The selected area of tumor tissue was transferred to the donor paraffin block and samples were taken from three different areas, to create tissue microarrays (TMA). With MTA Booster OI (Alphelys, Plaisir, France), 3 tissue cores, each measuring 1 mm in diameter, were taken from the marked spot on donor block and transferred into a new recipient paraffin block (recipient block microarrays) at given coordinates. The recipient paraffin block was left overnight at 45°C for tissue cores from the donor block to interconnect with the recipient block. Four µm thick sections were cut from the TMA block for immunohistochemical analysis. After drying, samples were deparaffinized in xylene and rehydrated in ethanol.
Sections were incubated with anti-CD31 monoclonal antibodies (1/50 dilution, JC70; NeoMarkers, Freemont, California, USA) at room temperature for 30 min in order to visualize the blood vessels. Positive controls were endothelial cells in normal blood vessels of the lungs.
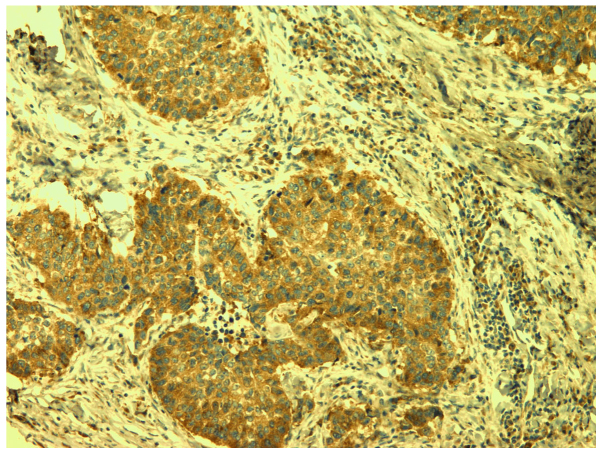
Sections were incubated with the anti-VEGF-A polyclonal antibodies (A-20, 1/200 dilution; Santa Cruz Biotechnology, Santa Cruz, California, USA) at room temperature for two h to determine VEGF-A (Fig. 1).
Figure 1.
Vascular endothelial growth factor-A positive staining (magnification, ×100).
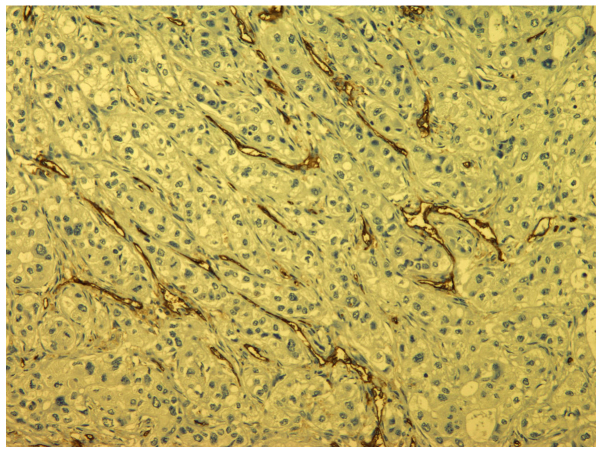
Microvessel counting was used to evaluate angiogenesis. Tumor sections stained with CD 31 were examined at low magnification (×40) to determine areas with the greatest vessel density (hot spots). Counting of blood vessels to determine MVD was performed at high magnification (×400) in selected areas (Fig. 2).
Figure 2.
CD31 immunostaining (magnification, ×100).
Individual endothelial cells or clusters of endothelial cells with barely visible lumen or no lumen were considered individual vessels and therefore included in the analysis.
VEGF-A expression was assessed semi quantitatively by estimating the percentage of tumor cells stained on tumors slides. Four groups were formed as follows: 1) Positive staining in less than 25% of tumor cells; 2) positive staining in 25 to 50% of tumor cells; 3) positive staining in 50 to 75% of e tumor cells and 4) positive staining in more than 75% of tumor cells. The intensity of smooth muscle cells' staining in the blood vessels walls was positive internal control.
‘Statistica 10’ software program (StatSoft, Inc, Tulsa, Oklahoma, USA) was used for statistical analysis. Chi-square test with Yeates's correction was used to assess the correlations between MVD and VEGF-A with clinical parameters and with survival. Values of P<0.05 were considered statistically significant. Survival curves were determined by Kaplan-Meier's method.
Results
The average age of patients was 63 years (ranging between 41 and 80), and there were 9 women and 41 men. There were 28 adenocarcinomas (56%) and 22 squamous cell carcinomas (44%) in our tissue sample. Macroscopic features of the primary tumors were estimated as follows: T1 tumors were found in 8 (16%) patients, T2 in 19 patients (38%) and T3 tumors in 23 patients (46%). Noninvasive and invasive ‘staging’ was conducted with all patients whereby tumor dissemination in mediastinal lymphatic nodes was not proved and neither in distant metastases.
The median MVD was 16.5 (range 8–30) and the mean of MVD values was 16.7, so 17 is taken as cut-off value. Patients were classified into two groups: With low MVD (<17) and high MVD (≥17). Low MVD was present in 26 (52%) patients and high MVD in 24 (48%) patients.
The overall two-year survival of patients in the study group was 52%. The two-year survival of patients with high MVD was 21% and of patients with low MVD was 77%. The results indicate a significantly increased survival of patients with low MVD (χ2=11.48; P=0.0007) (Table I).
Table I.
Associations between micro vascular density, and clinicopathological parameters and survival.
| MVD | |||
|---|---|---|---|
| Clinicopathological parameters | ≥17, n (%) | <17, n (%) | P-value |
| Age, years | |||
| >63 | 9 (18) | 14 (28) | 0.2400 |
| <63 | 15 (30) | 12 (24) | |
| Gender | |||
| Male | 17 (34) | 24 (48) | 0.5900 |
| Female | 5 (10) | 4 (8) | |
| pT | |||
| 1,2 | 12 (24) | 15 (30) | 0.3900 |
| 3 | 13 (26) | 10 (20) | |
| Histological type of NSCLC | |||
| Adenocarcinoma | 12 (24) | 13 (26) | 1.0000 |
| Squamous cell carcinoma | 12 (24) | 13 (26) | |
| Differentiation | |||
| Good (G1, 2) | 11 (22) | 16 (32) | 0.2600 |
| Poor (G3, 4) | 13 (26) | 10 (20) | |
| Survival | |||
| <24 months | 18 (36) | 6 (12) | 0.0007 |
| >24 months | 6 (12) | 20 (40) | |
| Expression of VEGF-A | |||
| 1 (<25%) | 1 (2) | 1 (2) | 0.5900 |
| 2 (25–50%) | 4 (8) | 3 (6) | |
| 3 (50–75%) | 11 (22) | 13 (26) | |
| 4 (>75%) | 11 (22) | 6 (12) | |
MVD, micro vascular density; NSCLC, non-small cell lung cancer; VEGF-A, vascular endothelial growth factor-A.
None of the standard clinically-relevant parameters (age, gender, histological type, pT and tumor differentiation) was significantly associated with MVD (Table I).
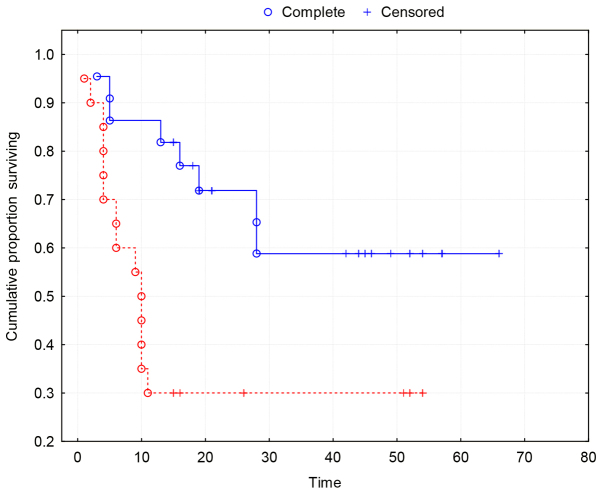
Kaplan-Meier survival curves show a strong association between MVD and two-year survival (P=0.0035) (Fig. 3).
Figure 3.
Kaplan-Meier's survival curves demonstrating a strong association between MVD and the two-year survival (P=0.0035). Group 0 (red line), tumors with MVD≥17. Group 1 (blue line), tumors with MVD<17. MVD, micro vascular density.
VEGF-A was highly expressed (positive staining in more than 50% of tumor cells) in the majority of tumors (68%), but no association was found between VEGF-A expression and high MVD (P=0.59) (Table I).
VEGF-A expression was not associated with standard clinical parameters nor with survival (P>0.05) (Table II).
Table II.
Associations between vascular endothelial growth factor-A expression, and clinicopathological parameters and survival.
| Expression of VEGF-A (%) | |||||
|---|---|---|---|---|---|
| Clinicopathological parameters | Group 1 (<25%) | Group 2 (25–50%) | Group 3 (50–75%) | Group 4 (>75%) | P-value |
| Age (years) | |||||
| >63 | 1 | 7 | 12 | 6 | 0.77 |
| <63 | 1 | 4 | 10 | 9 | |
| Gender | |||||
| Male | 1 | 8 | 19 | 13 | 0.95 |
| Female | 1 | 3 | 3 | 2 | |
| pT | |||||
| 1,2 | 0 | 6 | 11 | 7 | 0.92 |
| 3 | 2 | 5 | 11 | 8 | |
| Histological type of NSCLC | |||||
| Adenocarcinoma | 1 | 5 | 14 | 6 | 0.82 |
| Squamous cell carcinoma | 1 | 6 | 8 | 9 | |
| Differentiation | |||||
| Good (G1, 2) | 1 | 7 | 17 | 9 | 0.87 |
| Poor (G3, 4) | 1 | 4 | 5 | 6 | |
| Survival (months) | |||||
| <24 | 1 | 3 | 12 | 8 | 0.62 |
| >24 | 1 | 8 | 10 | 7 | |
VEGF-A, vascular endothelial growth factor-A; NSCLC, non-small cell lung cancer.
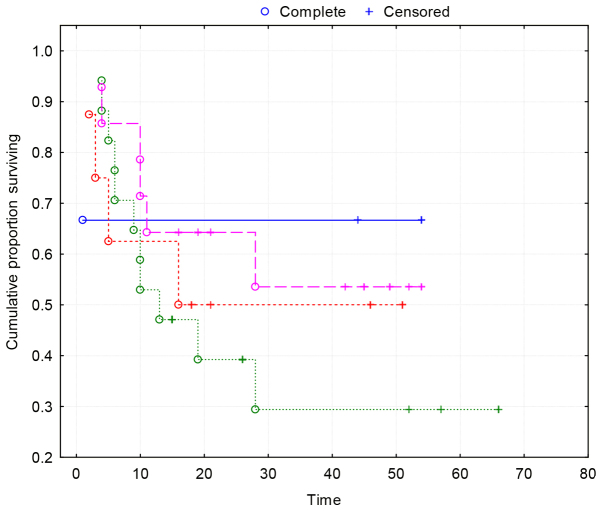
Kaplan-Maier survival curves show no correlation between VEGF-A and survival (Fig. 4).
Figure 4.
Kaplan-Meier curves demonstrating no association between VEGF-A and survival (P=0.73). Group 1 (blue line), VEGF-A expression <25%; Group 2 (red line), VEGF-A expression 25–50%; Group 3 (green line), VEGF-A expression 50–75%; Group 4 (purple line), VEGF-A expression >75%. VEGF-A, vascular endothelial growth factor-A.
Discussion
Lung cancer is the most common malignant tumor and the leading cause of death from malignancies. Five-year survival of lung cancer is around 15% (1). Despite the overall decreasing trend, Croatia is still among the European countries with the highest male lung cancer incidence and mortality (19). TNM stage is the most important predictor of outcome in patients with NSCLC. However, patients within the same stage or tumor usually have a very variable prognosis, which depends not only on the stage, but also on the biological characteristics of the tumor. Angiogenesis was validated as an independent prognostic factor in a variety of solid malignancies. Several studies demonstrated that angiogenesis, assessed by MVD, is a significant prognostic factor for patients with NSCLC (3,7,20–23). However, most studies have been conducted on patients with NSCLC in early stages and studies dealing specifically with the prognostic impact of angiogenesis in NSCLC with metastasis in mediastinal lymph nodes are lacking. The only study, to our knowledge, which examines the impact of angiogenesis on prognosis of patients in stage IIIA (T1-3N2), who were initially surgically treated, was performed by Angeletti and al. in 1996 and in this study MVD was identified as a significant prognostic factor (23).
Several studies have not found MVD to be a predictor of survival (24–26).
Since those papers did not prove the correlation MVD and survival it is obvious that the role of tumor angiogenesis prognosis in the disease's survival is still controversial, we decided to examine the correlation MVD and survival with our patients by using CD 31 monoclonal antibodies to visualize the blood vessels instead of anti-factor VII polyclonal antibodies which were used in the mentioned researches.
The results of studies which examined the correlation of VEGF-A and survival of patients with NSCLC are controversial. Although many data support the statement that the VEGF-A is correlated with MVD and survival (16,25–27), others did not confirm VEGF-A as an independent factor of survival (28–30). The prognostic significance of VEGF-A in patients with advanced NSCLC, who were primary surgically treated, has never been investigated. In our study, we observed the high expression of VEGF-A in most patients, but there was no correlation with MVD and survival. This lack of correlation indicates that other angiogenic factors may have an important role in angiogenesis in advanced NSCLC.
Our study is retrograded and includes patients with metastases to mediastinal lymph nodes that were discovered during thoracotomy and subsequent histopathological examination, and for these reasons were not subjected to neoadjuvant chemotherapy.
All patients included in the study received adjuvant chemotherapy according to the appropriate protocol and in full dose. Not one of the patients was subjected to anti-VEGF antibodies (bevacizumab) therapy. Our results show that patients who were classified by TNM classification in the same prognostic stage have a highly variable prognosis. We found that high MVD has a strong negative prognostic value for survival of patients with lung cancer.
The introduction of anti-angiogenic therapy in cancer treatment makes methods of angiogenesis evaluation even more important. Patients with highly vascularized tumors, due to their worse prognosis, may be candidates for more aggressive therapy especially neoadjuvant multimodal chemotherapy. Assessment of angiogenesis can become an integral part of the tumor staging and ultimately helping to choose the most efficient therapeutic approach.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu Y, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Pisters KM, Darling G. The IASLC lung cancer staging project: ‘The Nodal Zone’. J Thorac Oncol. 2007;2:583–584. doi: 10.1097/JTO.0b013e31807a2fce. [DOI] [PubMed] [Google Scholar]
- 3.Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9–19. [PMC free article] [PubMed] [Google Scholar]
- 4.Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- 5.Cox G, Jones JL, Walker RA, Steward WP, O'Byrne KJ. Angiogenesis and non-small cell lung cancer. Lung Cancer. 2000;27:81–100. doi: 10.1016/S0169-5002(99)00096-3. [DOI] [PubMed] [Google Scholar]
- 6.Fox SB, Gatter KC, Harris AL. Tumour angiogenesis. J Pathol. 1996;179:232–237. doi: 10.1002/(SICI)1096-9896(199607)179:3<232::AID-PATH505>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 7.Mineo TC, Ambrogi V, Baldi A, Rabitti C, Bollero P, Vincenzi B, Tonin G. Prognostic impact of VEGF, CD31, CD34, and CD105 expression and tumour vessel invasion after radical surgery for IB-IIA non-small cell lung cancer. J Clin Pathol. 2004;57:591–597. doi: 10.1136/jcp.2003.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Jawdeh GM, Faix JD, Niloff J, Tognazzi K, Manseau E, Dvorak HF, Brown LF. Strong expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in ovarian borderline and malignant neoplasms. Lab Invest. 1996;74:1105–1115. [PubMed] [Google Scholar]
- 9.Claffey KP, Brown LF, del Aguila LF, Tognazzi K, Yeo KT, Manseau EJ, Dvorak HF. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res. 1996;56:172–181. [PubMed] [Google Scholar]
- 10.Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Sowa M. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996;77:858–863. doi: 10.1002/(SICI)1097-0142(19960301)77:5<858::AID-CNCR8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- 12.Maharaj AS, Saint-Geniez M, Maldonado AE, D'Amore PA. Vascular endothelial growth factor localization in the adult. Am J Pathol. 2006;168:639–648. doi: 10.2353/ajpath.2006.050834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrera N. Vascular endothelial growth factor. Trends Cardiovasc Med. 1993;3:244–250. doi: 10.1016/1050-1738(93)90046-9. [DOI] [PubMed] [Google Scholar]
- 14.Meert AP, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout JM, Lafitte JJ, Mascaux C, Sculier JP. The role of microvessel density on the survival of patients with lung cancer: A systematic review of the literature with meta-analysis. Br J Cancer. 2002;87:694–701. doi: 10.1038/sj.bjc.6600551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadota K, Huang CL, Liu D, Ueno M, Kushida Y, Haba R, Yokomise H. The clinical significance of lymphangiogenesis and angiogenesis in non-small cell lung cancer patients. Eur J Cancer. 2008;44:1057–1067. doi: 10.1016/j.ejca.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Fontanini G, Vignati S, Boldrini L, Chinè S, Silvestri V, Lucchi M, Mussi A, Angeletti CA, Bevilacqua G. Vascular endothelial growth factor is associated with neovascularization and influences progression of non-small cell lung carcinoma. Clin Cancer Res. 1997;3:861–865. [PubMed] [Google Scholar]
- 17.Han H, Silverman JF, Santucci TS, Macherey RS, d'Amato TA, Tung MY, Weyant RJ, Landreneau RJ. Vascular endothelial growth factor expression in stage I non-small cell lung cancer correlates with neoangiogenesis and a poor prognosis. Ann Surg Oncol. 2001;8:72–79. doi: 10.1007/s10434-001-0072-y. [DOI] [PubMed] [Google Scholar]
- 18.IASLC Staging Manual in Thoracic Oncology. Orange Park: Editorial Rx Press; 2009. International Association for the Study of Lung Cancer and Peter Goldstraw. [Google Scholar]
- 19.Janković M, Samarzija M, Jakopović M, Kulis T, Znaor A. Trends in lung cancer incidence and mortality in Croatia, 1988–2008. Croat Med J. 2012;53:93–99. doi: 10.3325/cmj.2012.53.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuyama K, Chiba Y, Sasaki M, Tanaka H, Muraoka R, Tanigawa N. Tumor angiogenesis as a prognostic marker in operable non-small cell lung cancer. Ann Thorac Surg. 1998;65:1405–1409. doi: 10.1016/S0003-4975(97)01416-1. [DOI] [PubMed] [Google Scholar]
- 21.Macchiarini P, Fontanini G, Hardin MJ, Squartini F, Angeletti CA. Relation of neovascularization to metastasis of non-small cell lung cancer. Lancet. 1992;340:145–146. doi: 10.1016/0140-6736(92)93217-B. [DOI] [PubMed] [Google Scholar]
- 22.Giatromanolaki A, Koukourakis MI, Theodossiou D, Barbatis K, O'Byrne K, Harris AL, Gatter KC. Comparative evaluation of angiogenesis assessment with anti-factor-VIII and anti-CD31 immunostaining in non-small cell lung cancer. Clin Cancer Res. 1997;3:2485–9224. [PubMed] [Google Scholar]
- 23.Angeletti CA, Lucchi M, Fontanini G, Mussi A, Chella A, Ribechini A, Vignati S, Bevilacqua G. Prognostic significance of tumoral angiogenesis in completely resected late stage lung carcinoma (stage IIIA-N2). Impact of adjuvant therapies in a subset of patients at high risk of recurrence. Cancer. 1996;78:409–415. doi: 10.1002/(SICI)1097-0142(19960801)78:3<409::AID-CNCR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 24.Chandrachud LM, Pendleton N, Chisholm DM, Horan MA, Schor AM. Relationship between vascularity, age and survival in non-small-cell lung cancer. Br J Cancer. 1997;76:1367–1375. doi: 10.1038/bjc.1997.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pastorino U, Andreola S, Tagliabue E, Pezzella F, Incarbone M, Sozzi G, Buyse M, Menard S, Pierotti M, Rilke F. Immunocytochemical markers in stage I lung cancer: Relevance to prognosis. J Clin Oncol. 1997;15:2858–2865. doi: 10.1200/JCO.1997.15.8.2858. [DOI] [PubMed] [Google Scholar]
- 26.Imoto H, Osaki T, Taga S, Ohgami A, Ichiyoshi Y, Yasumoto K. Vascular endothelial growth factor expression in non-small-cell lung cancer: Prognostic significance in squamous cell carcinoma. J Thorac Cardiovasc Surg. 1998;115:1007–1014. doi: 10.1016/S0022-5223(98)70398-8. [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki A, Kuwahara M, Yoshinaga Y, Shirakusa T. Basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) levels, as prognostic indicators in NSCLC. Eu J Cardio-Thoracic Surg. 2004;25:443–448. doi: 10.1016/j.ejcts.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 28.Bonnesen B, Pappot H, Holmstav J, Skov BG. Vascular endothelial growth factor A and vascular endothelial growth factor receptor 2 expression in non-small cell lung cancer patients: Relation to prognosis. Lung Cancer. 2009;66:314–318. doi: 10.1016/j.lungcan.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Yano T, Tanikawa S, Fujie T, Masutani M, Horie T. Vascular endothelial growth factor expression and neovascularisation in non-small cell lung cancer. Eur J Cancer. 2000;36:601–609. doi: 10.1016/S0959-8049(99)00327-5. [DOI] [PubMed] [Google Scholar]
- 30.Decaussin M, Sartelet H, Robert C, Moro D, Claraz C, Brambilla C, Brambilla E. Expression of vascular endothelial growth factor (VEGF) and its two receptors (VEGF-R1-Flt1 and VEGF-R2-Flk1/KDR) in non-small cell lung carcinomas (NSCLCs): Correlation with angiogenesis and survival. J Pathol. 1999;188:369–377. doi: 10.1002/(SICI)1096-9896(199908)188:4<369::AID-PATH381>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]