Abstract
Pancreatic cancer is one of the leading causes of cancer-associated mortality. The understanding of the expression pattern of key protein factors and their function in pancreatic cancer cells is therefore vital for the diagnosis and treatment of this malignancy. The results of the present study reveal that the levels of neurogenic locus notch homolog protein 2 (Notch2) and phosphorylated (p)-SMAD family member 2 decreased, whereas the expression of Notch3 and phosphoinositide-3 kinase catalytic subunit-γ protein increased in human pancreatic cancer tissues compared with tumor-adjacent tissues. Using the human pancreatic cancer MIA PaCa-2 cell line, it was observed that retinoblastoma-associated protein (RB) and p-RB expression were inhibited and p-AKT was upregulated when Notch signaling was activated in MIA PaCa-2 cells. Furthermore, inhibition of phosphoinositide-3 kinase catalytic subunit-γ (PIK3CG) activity by AS-605240 was able to block the growth and migration of MIA PaCa-2 cells. In conclusion, the results of the present study demonstrate that the Notch signal pathway may be involved in pancreatic carcinogenesis by modulating RB and p-AKT. PIK3CG may therefore be a potential target gene for the treatment of pancreatic cancer.
Keywords: Notch signaling, retinoblastoma protein, phosphoinositide-3 kinase catalytic subunit-γ, SMAD family member 2, pancreatic cancer
Introduction
Pancreatic cancer is the fourth-leading cause of cancer-associated mortality, with an overall 5-year survival rate less than 5% (1). There are ~40,000 Americans annually that are diagnosed with pancreatic cancer, and there is around an equivalent number of patients that succumb to the disease (2). Pancreatic cancer prognosis is so poor because the majority of patients have locally advanced or distant metastatic disease at the time of presentation (3). Although patient outcomes have been improved by curative resection, chemotherapy and biological therapies, the majority of patients succumb to disease within 5 years owing to the early recurrence and metastasis of the disease (4). Therefore, a further understanding the molecular mechanisms involved in pancreatic cancer is required for improved diagnosis and treatment of the disease.
Previous research demonstrated that multiple signaling pathways are implicated in the pathogenesis of pancreatic cancer, including the neurogenic locus notch homolog protein (Notch), hedgehog, Wnt/β-catenin and transforming growth factor-β (TGF-β) (5–7). The Notch receptor family includes Notch1-4, which control pancreatic differentiation during development and also participates in the initiation and progression of pancreatic cancer (8). A previous study indicates that Notch1 functions as a tumor suppressor in a model of GTPase KRas (K-RAS)-induced pancreatic ductal adenocarcinoma (PDAC) (9). Notch2 is required for the progression of pancreatic intraepithelial neoplasia and development of PDAC (10). Notch3 may be a prognostic biomarker in PDAC. Moreover, several Notch receptors modify proteins; for example, lunatic fringe (LFNG), which is a potential tumor suppressor that regulates the activity of different Notch receptors and can also serve important roles in pancreatic adenocarcinoma (3). However, the function and expression pattern of these Notch receptors in pancreatic cancer remain poorly understood.
Dysregulation of the phosphoinositide-3 kinase (PI3K)/AKT pathway is implicated in a number of human diseases, including cancer, diabetes, cardiovascular disease and neurological diseases (11). Activation is often mediated by mutations occurring in the PIK3CA gene, which encodes the p110α catalytic subunit of PI3Kα, a heterodimeric class IA PI3K that is one of the most frequently mutated genes (16%) in colorectal cancer (12). Certain reports demonstrate that phosphoinositide-3 kinase catalytic subunit-γ (PIK3CG) can also have important roles in sarcomagenesis (13–15). A previous study suggested that PIK3CG can be regulated by Notch signal transduction and is tightly associated with stemness and migration of claudin-low breast cancer cells (16). According to these results, the level of PIK3CG expression seems to be tightly associated with the metastasis and malignancy of different tumors. Therefore, it is critical to investigate whether aberrant expression of PIK3CG is associated with the initiation of pancreatic cancer and its metastasis, advanced-stage diagnosis and resistance to the majority of therapies (3).
The retinoblastoma gene (RB), a tumor suppressor gene, is dysfunctional in several types of cancer (17). One function of RB is to prevent cell proliferation by inhibiting cell cycle progression and recent studies have revealed a substantial role for RB and its downstream effectors, particularly the E2F family of transcription factors, in regulating various aspects of tumor progression, angiogenesis and metastasis (18). In pancreatic cancer, dysfunction of RB1 enables TGF-β to promote cancer cell proliferation, and pancreatic carcinogenesis can be accelerated by the deletion of RB (19,20). A previous study reported that Notch1 exhibits oncogene-like characteristics by inducing dephosphorylation of Rb in esophageal squamous carcinoma cells (21). However, the mechanism of the crosstalk between Notch, TGF-β and RB requires further clarification.
In the present study, clinical pancreatic cancer sections and the human pancreatic cancer MIA PaCa-2 cell line were examined to assess the expression pattern of the Notch receptors, P-Smad2, RB and PIK3CG and the crosstalk between these proteins in pancreatic cancer cells to understand further the mechanisms involved in pancreatic carcinogenesis.
Materials and methods
Tissue samples
A total of 20 patients (male, 16; female, 4) with pancreatic cancer were included in this study. The median patient age was 56 years (range, 23–79 years). All tumor samples were obtained in December 2015 from the Department of Pathology, The Second Xiangya Hospital of Central South University (Changsha, China). Tumor samples were obtained during surgery and in routine diagnostic biopsies. The samples were fixed in 10% formalin for 24 h at room temperature and paraffin-embedded. All research biopsies were evaluated by pathologists specialized in pancreatic cancer diagnostics and ensured adequate quantity of tumor tissues were used for analysis. Adjacent tissue samples were located within 1 cm of the tumor margin and were confirmed to be noncancerous by pathological examination. Ethical approval for the present study was provided by the Ethics Committee at The Second Xiangya Hospital of Central South University (Hunan, China).
Cell culture
The pancreatic cancer MIA PaCa-2 cell line was purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). The siRNA-Lfng and Notch3 intracellular domain (N3IC)-overexpressing MIA PaCa-2 cell models were established as previously described (3). The cells were cultured according to ATCC protocols in Dulbecco's modified Eagle's medium (DMEM) (Corning Incorporated, Corning, NY, USA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Inc., Flowery Branch, GA, USA) at 37°C with 100 U/ml penicillin and 100 µg/ml streptomycin maintained in a humidified environment containing 5% CO2. For cell counting, MIA PaCa-2 cells were respectively treated with 100, 50, 25, 12, 6, 3, 1.5, 0.8, 0.4, 0.2 and 0.1 µM PI3K inhibitor AS-605240 (catalog no. S1410; Selleck Chemicals, Houston, TX, USA) at 37°C for 72 h. For wound healing assays, MIA PaCa-2 cells were treated with AS-605240 at a final concentration of 5 µM for 48 h.
Cell counting
For cell counting, MIA PaCa-2 cells were incubated in 10% cell counting kit-8 solution (catalog no. 40203ES60; Shanghai Yeasen Biotechnology Co., Ltd, Shanghai, China) diluted in DMEM at 37°C for 3 h. The absorbance of each well was measured with a microplate reader, set at an absorbance wavelength of 450 nm. All experiments were performed in triplicate. The cell growth curve was performing using GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Data are represented as the mean ± standard error of three independent experiments.
Immunohistochemistry (IHC)
Formalin-fixed paraffin-embedded pancreatic tissues were processed for histology and IHC as described previously (22,23). For each sample, two adjacent tissue sections (5-µm thickness) were mounted on one glass slide. Then, the tissue sections were deparaffinized and rehydrated using xylene and a graded ethanol series. Sections were then heated at 100°C in a 10 mM citrate buffer solution (pH 6.0) for 30 min for antigen retrieval. Endogenous peroxidase activity was quenched by immersing the sections in 3% H2O2 for 10 min. Next, the staining was performed using the ImmunoCruz™ ABC Staining system (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) according to the manufacturer's protocol. The specimens for 1 h in 5% normal blocking serum in PBS at room temperature. Following removal of blocking reagent from the slides, the samples were incubated with primary antibodies for overnight at 4°C. Following washing three times with PBS for 5 mins each, incubation for 30 min at room temperature with biotin-conjugated secondary antibody at approximately 1 µg/ml was performed. Then the samples were washed three times with PBS for 5 min each. Incubation for 30 mins at room temperature with the ABC reagent was performed, followed by washing three times with PBS for 5 min each. Subsequently, incubation in peroxidase substrate and chromogen mixture until the desired stain intensity developed was performed. Then sections were washed in deionized H2O. When appropriate, dehydration with alcohols and xylenes was performed as follows: Soak in 90% ethanol twice for 3 mins each, 100% ethanol twice for 3 min each, then xylene three times for 10 sec each and excess xylene was wiped off. Immediately 1–2 drops of permanent mounting medium was added, covered with a glass coverslip and observed by light microscopy. A brown color was indicative of a positive result. Primary antibodies used for immunostaining were as follows: Notch1 (catalog no. bTAN 20; 1:200;, Developmental Studies Hybridoma Bank (DSHB), University of Iowa, IA, USA), Notch2 (catalog no. C651.6DbHN; DSHB; 1:200), Notch3 (catalog no. 55114-1-AP; ProteinTech Group, Inc., Chicago, IL, USA; 1:100), PI3K p110γ (catalog no. 5405; Cell Signaling Technology, Inc., Danvers, MA, USA; 1:100), p-Smad2 (catalog no. 3101; Cell Signaling Technology, Inc; 1:100). Cytoplasmic and nuclear staining was considered positive if >10% cells were positively stained. Surrounding fibrous and inflammatory tissue was not scored. The slides were graded independently by two observers blinded to the clinical data.
Western blotting
For western blotting, the MIA PaCa-2 cells were lysed in radioimmunoprecipitation buffer (Boston BioProducts Inc., Ashland, MA, USA) with protease inhibitor cocktail (Roche Applied Science, Penzberg, Germany). Protein concentrations were determined using the BCA protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Samples of cell lysate supernatant (50 µg protein) were resolved by 8% SDS-PAGE and electrotransferred to nitrocellulose membranes. The membranes were blocked in TBST buffer with 5% w/v non-fat dry milk at room temperature for 1 h and then probed at 4°C overnight with indicated antibodies with the following dilutions: Akt (catalog no. 4691; Cell Signaling), phospho-Akt (Ser473) (catalog no. 4060; Cell Signaling), phospho-RB (catalog no. 8516; Cell Signaling), RB (catalog no. 9309; Cell Signaling), PI3K p110γ (catalog no. 5405; Cell Signaling) and β-actin (catalog no. sc-81178; Santa Cruz Biotechnology, Inc.), all at 1:1,000 dilution. The secondary goat anti-rabbit IgG-HRP (catalog no. sc-2004) and goat anti-mouse IgG-HRP (catalog no. sc-2005) (both Santa Cruz Biotechnology, Inc.) were used at a 1:4,000 dilution at room temperature for 2 h. Detection was performed using ECL Western Blotting Detection reagents (catalog no. PI32106; Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). All antibodies were used in accordance with the manufacturer's instructions.
Wound-healing experiment
Collective cell migration was measured using wound-healing assay. The cells were seeded in a 6-well plate at a density of 5×105 cells per well. After incubation at 37°C for 12 h, a portion of the monolayer was scratched with a 1,000 µl pipette tip, and examined for resealing of the ‘wounded’ monolayer after 48 h using a light microscope.
Statistical analysis
Data from all experiments are presented as the mean ± standard deviation of at least three independent experiments performed in duplicate for each construct. Statistical analysis was performed using the two-tailed Student's t-test. The software used for statistical analysis was GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.001 was considered to indicate a statistically significant difference.
Results
Changes in Notch receptor expression in in pancreatic cancer tissues compared with tumor-adjacent tissues
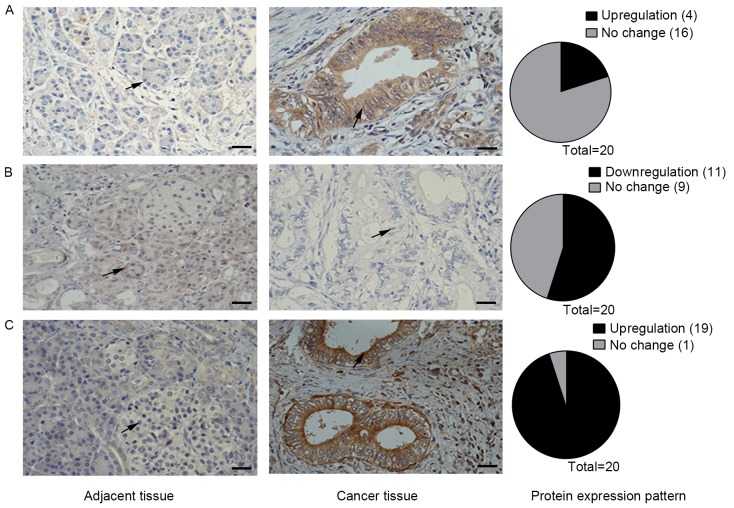
The expression levels of all Notch receptors has been previously found to be upregulated in the KrasLSL-G12D/+;Pdx1-Cre pancreatic mouse model (3). However, the present study observed that the expression pattern of Notch receptors in human pancreatic tissue samples is different from the expression pattern in mouse tumor tissues (Fig. 1). Using IHC analysis of samples from 20 patients, Notch1 staining was found to be positive in 4/20 (20%) pancreatic cancer tissues, whereas the adjacent tissues were all negative for staining (Fig. 1A). Staining for Notch3 was positive in 19/20 pancreatic cancer tissues (95%) and negative in all adjacent tissues (Fig. 1C). However, Notch2 staining results were negative in 11/20 (55%) pancreatic cancer tissues, but positive in all adjacent tissues (Fig. 1B). Therefore, the Notch2 receptor may serve a different role to Notch1 and Notch3 in human pancreatic cancer.
Figure 1.
Notch receptor expression patterns in human pancreatic cancer and tumor-adjacent tissues. (A) IHC staining for Notch1 in human pancreatic cancer and tumor-adjacent tissues. Arrows point to cells showing staining result of Notch1. (B) IHC staining expression for Notch2 expression in human pancreatic cancer tissues and tumor-adjacent tissues. Arrows point to cells showing staining result of Notch2. (C) IHC staining showing expression of Notch3 in human pancreatic cancer tissue and tumor-adjacent tissues. Arrows point to cells showing staining result of Notch3. Scale bars, 50 µm. Notch, neurogenic locus notch homolog protein. IHC, immunohistochemistry.
PIK3CG and TGF-β signal play important roles in pancreatic carcinogenesis
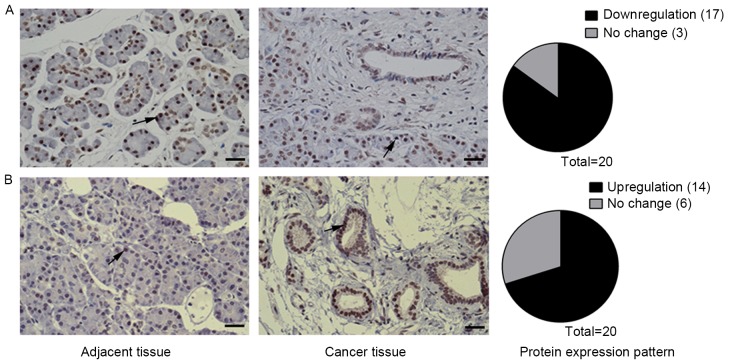
Notch signaling antagonizes TGF-β signaling in the mouse pancreas and in PDAC cells through downregulation of Tgfb and Tgfbr gene expression (3). The IHC results of the present study revealed that nuclear staining of p-smad2 was weaker or the p-smad2 expression decreased in pancreatic cancer tissues compared with the adjacent tissues in 17/20 (85%) patients (Fig. 2A), which was consistent with results in mice found in a previous study (3). Staining for PIK3CG was found to be stronger in pancreatic cancer tissue compared with adjacent tissues in 14/20 (70%) patients. To the best of our knowledge, the results of the present study revealed that PIK3CG expression is upregulated in human pancreatic cancer for the first time (Fig. 2B) and that PIK3CG may serve an important role in human pancreatic cancer.
Figure 2.
PIK3CG and p-Smad2 expression patterns in human pancreatic cancer and tumor-adjacent tissues. (A) IHC staining for p-Smad2 expression in human pancreatic cancer and tumor-adjacent tissues. Arrows point to cells showing staining result of p-Smad2. (B) IHC staining for PIK3CG expression in human pancreatic cancer tissues and tumor-adjacent tissues. Arrows point to cells showing staining result of PIK3CG. Scale bars, 50 µm. PIK3CG, phosphoinositide-3 kinase catalytic subunit-γ; Smad2, SMAD family member 2. IHC, immunohistochemistry.
Notch signaling pathway modulates RB and p-AKT expression in pancreatic cancer cells
In pancreatic cancer, RB1 dysfunction enabled TGF-β signaling, which was modulated by Notch signaling to promote cancer cell proliferation (19). The present authors previously found that PIK3CG gene expression was modulated by Notch signaling in claudin-low breast cancer (16). Using the siRNA-Lfng MIA PaCa-2 cell model, where Notch signaling is activated (3), it was observed by western blotting that the protein expression level of p-RB and pan-RB was downregulated compared with the control, the PIK3CG expression level remained the same and p-AKT expression was upregulated (Fig. 3A and B). These results indicated that RB1 and p-AKT, but not PIK3CG, were the downstream targets of the Notch signaling pathway in the siRNA-Lfng MIA PaCa-2 cell model, suggesting that P-AKT may be regulated by Notch signaling through pathways other than PIK3CG/AKT. Western blot analysis was also performed to assess the level of RB expression, using another MIA PaCa-2 cell model in which Notch3IC was over-expressed. The results confirmed that RB expression was inhibited when Notch signaling was activated in MIA PaCa-2 cells (Fig. 3C).
Figure 3.
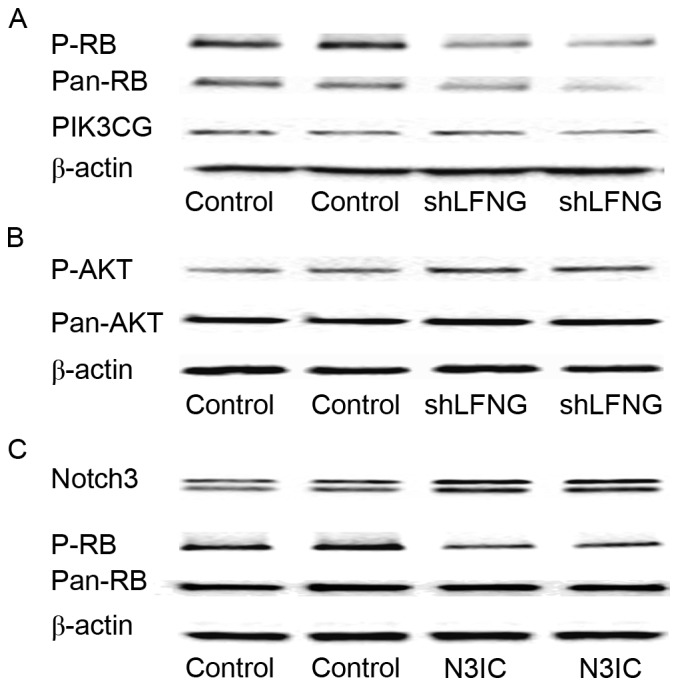
RB and p-AKT but not PIK3CG expression can be regulated by Notch signaling in pancreatic cancer. (A) Western blot analysis of pan-RB, p-RB and PIK3CG in shLFNG-MIA PaCa-2 and control cells. β-actin was included as loading control. (B) Western blot analysis of p-AKT and pan-AKT in shLFNG-MIA PaCa-2 and control cells. β-actin was included as loading control. (C) Western blot analysis of Notch3, RB and p-RB in MIA PaCa-2 cells overexpressing Notch3IC. β-actin was included as loading control. RB, retinoblastoma-associated protein; p-AKT, phosphorylated RAC-α serine/threonine-protein kinase; PIK3CG, phosphoinositide-3 kinase catalytic subunit-γ; Notch, neurogenic locus notch homolog protein; shLFNG, short hairpin RNA targeted at lunatic fringe; P-, phosphorylated.
Inhibition of PIK3CG blocks the growth and migration of MIA PaCa-2 cells
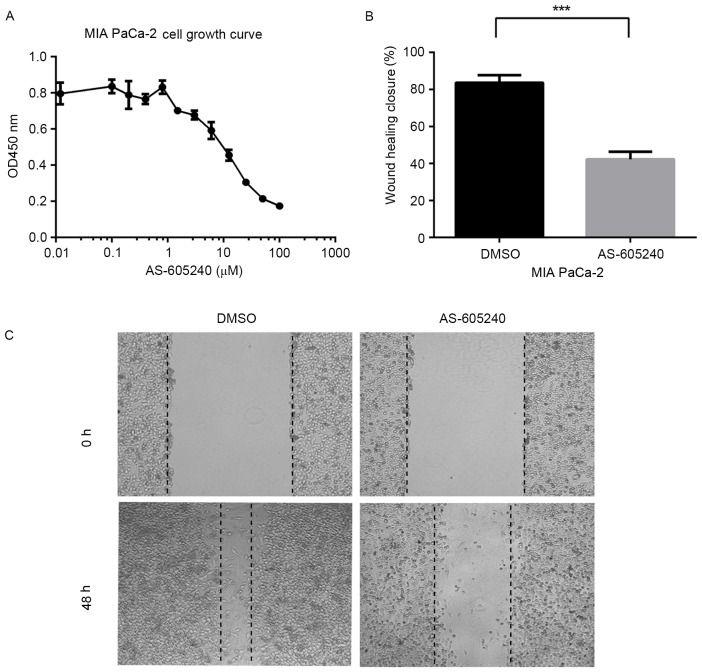
PIK3CG has been tightly associated with claudin-low breast cancer migration and the potential targets of multiple types of cancer, including sarcoma and gastric cancer (24). The results of the present study demonstrated that PIK3CG was a key gene involved in the growth and migration of pancreatic cancer cells. The growth of MIA PaCa-2 cells was markedly reduced upon treatment with the PIK3CG-specific inhibitor AS-605240 (Fig. 4A). The migration of MIA PaCa-2 cells was significantly blocked when the cells were incubated with 5 µM AS-605240 for 48 h, as assessed using wound healing experiments (Fig. 4B and C).
Figure 4.
MIA PaCa-2 cell growth and migration were blocked by treatment with phosphoinositide-3 kinase catalytic subunit-γ inhibitor AS-605240. (A) MIA PaCa-2 cells were treated with different concentration of AS-605240 for three days. Data are shown as the mean ± standard deviation derived from triplicate experiments using Cell Counting Kit-8 assay. (B) Wound healing assays using DMSO vehicle or 5 µM AS-605240. Resealing of the ‘wounded’ monolayer was examined after 48 h. (C) Representative images (magnification, ×100) of wound-healing assays in MIA PaCa-2 cells treated with vehicle (DMSO) or 5 µM AS-605240 for two days. ***P<0.001. OD, optical density; DMSO, dimethyl sulfoxide.
Discussion
Recently, increasing evidence has demonstrated that the Notch signaling pathway has important roles in the initiation, progression and metastasis of a variety of human malignancies, including pancreatic cancer (25,26). However, the mechanism by which this happens remains poorly understood. The present study focused on the current understanding of the molecular pathogenesis of pancreatic cancer, particularly on the Notch signaling pathway and its novel molecular targets, including RB and PIK3CG. The current study may aid the prevention and treatment of pancreatic cancer.
Pancreatic cancer is a malignant cancer that frequently metastasizes prior to diagnosis, meaning there is a pressing requirement to understand the mechanism by which it initiates and progresses. Data from a previous study demonstrated that deletion of Lfng in the KrasLSL-G12D/+;Pdx1-Cre mouse model caused increased activation of Notch3 throughout the initiation and progression of PDAC (3). In the present study, the expression pattern of Notch1-3 receptors was first assessed using human clinical samples to confirm the function of the Notch signaling pathway in pancreatic cancer. The results of the present study demonstrated that Notch3 expression was upregulated in the majority of tumor tissues assessed, and Notch1 staining was observed to be positive in a number of pancreatic cancer tissues by IHC staining. The degree of Notch1 and Notch3 staining was consistent with previous data from mouse models and clinical specimens (27). The current study found that Notch2 expression was downregulated in tumor tissues, meaning that Notch receptors have different functions in the process of pancreatic carcinogenesis and that use of a γ-secretase inhibitor to block the Notch signaling pathway for the treatment of pancreatic cancer should be used cautiously.
A previous study by the present authors demonstrated that TGF-β, which is regulated by Notch signaling, is tightly associated with the metastasis of pancreatic cancer (3). The present study revealed that the gene expression level of p-Smad2 and PIK3CG was altered in pancreatic cancer tissues compared with the adjacent tissues. By over-activating the Notch signaling pathway in pancreatic cancer MIA PaCa-2 cells, the expression of RB was downregulated and P-AKT was upregulated, whereas PIK3CG expression did not change. These results demonstrated that Notch signaling and PIK3CG may have important roles in the initiation and progression of pancreatic cancer. Notch signaling can be an upstream regulator of RB but not PIK3CG in the pancreas, although PIK3CG could be a target for Notch signaling in triple-negative breast cancer. In pancreatic cancer, PIK3CG may function independent of the Notch signaling pathway and the mechanism involved in the process is worthy of being further clarified. It is very promising that both cell growth and migration rate decreased when MIA PaCa-2 cells were treated with the PIK3CG inhibitor AS-605240. These data reveal that PIK3CG may be a potential target for the treatment of malignant pancreatic cancer. For future studies, the present authors will investigate the function of AS-605240 using more pancreatic cell lines and KrasLSL−G12D/+; Pdx1-Cre mouse models.
In conclusion, the present study revealed the expression pattern of the Notch receptors, RB and PIK3CG in pancreatic cancer and adjacent tissue samples, although more pancreatic cancer tissue samples are required to confirm the experimental data of the study further. The Notch signaling pathway and PIK3CG are involved in pancreatic carcinogenesis. Cell model results revealed that Notch signal pathway is able to modulate RB expression but not PIK3CG, which is tightly associated with cell proliferation and migration in pancreatic cancer cells. The use of more pancreatic cell lines to confirm the present results is required, as is investigating the different transcription factor-binding patterns of the PIK3CG promoter between pancreatic cancer and claudin-low breast cancer cells. Finally, the finding of PIK3CG function in pancreas cancer provides new insights into the functions of this gene, which may be a novel target gene for the treatment of pancreatic cancer.
Acknowledgements
The present study was supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, the State Education Ministry and the Fund of the National Forestry Bureau, China.
References
- 1.Chen Huang, Jiawei Du, Keping Xie. FOXM1 and its oncogenic signaling in pancreatic cancer pathogenesis. Biochim Biophys Acta. 2014;1845:104–116. doi: 10.1016/j.bbcan.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Chung WC, Xu K. Lunatic Fringe is a potent tumor suppressor in Kras-initiated pancreatic cancer. Oncogene. 2016;35:2485–2495. doi: 10.1038/onc.2015.306. [DOI] [PubMed] [Google Scholar]
- 4.Boyle J, Czito B, Willett C, Palta M. Adjuvant radiation therapy for pancreatic cancer: a review of the old and the new. J Gastrointest Oncol. 2015;6:436–444. doi: 10.3978/j.issn.2078-6891.2015.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunnimalaiyaan S, Trevino J, Tsai S, Gamblin TC, Kunnimalaiyaan M. Xanthohumol-mediated suppression of Notch1 signaling is associated with antitumor activity in human pancreatic cancer cells. Mol Cancer Ther. 2015;14:1395–1403. doi: 10.1158/1535-7163.MCT-14-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marechal R, Bachet JB, Calomme A, Demetter P, Delpero JR, Svrcek M, Cros J, Bardier-Dupas A, Puleo F, Monges G, et al. Sonic hedgehog and Gli1 expression predict outcome in resected pancreatic adenocarcinoma. Clin Cancer Res. 2015;21:1215–1224. doi: 10.1158/1078-0432.CCR-14-0667. [DOI] [PubMed] [Google Scholar]
- 7.Jiang S, Zhu L, Tang H, Zhang M, Chen Z, Fei J, Han B, Zou GM. Ape1 regulates WNT/β-catenin signaling through its redox functional domain in pancreatic cancer cells. Int J Oncol. 2015;47:610–620. doi: 10.3892/ijo.2015.3048. [DOI] [PubMed] [Google Scholar]
- 8.Court H, Amoyel M, Hackman M, Lee KE, Xu R, Miller G, Bar-Sagi D, Bach EA, Bergo MO, Philips MR. Isoprenylcysteine carboxylmethyltransferase deficiency exacerbates KRAS-driven pancreatic neoplasia via Notch suppression. J Clin Invest. 2013;123:4681–4694. doi: 10.1172/JCI65764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanlon L, Avila JL, Demarest RM, Troutman S, Allen M, Ratti F, Rustgi AK, Stanger BZ, Radtke F, Adsay V, et al. Notch1 functions as a tumor suppressor in a model of K-ras-induced pancreatic ductal adenocarcinoma. Cancer Res. 2010;70:4280–4286. doi: 10.1158/0008-5472.CAN-09-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazur PK, Einwachter H, Lee M, Sipos B, Nakhai H, Rad R, Zimber-Strobl U, Strobl LJ, Radtke F, Klöppel G, et al. Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma; Proc Natl Acad Sci USA; 2010; pp. 13438–13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao HF, Wang J, Tony To SS. The phosphatidylinositol 3-kinase/Akt and c-Jun N-terminal kinase signaling in cancer: Alliance or contradiction? (Review) Int J Oncol. 2015;47:429–436. doi: 10.3892/ijo.2015.3052. [DOI] [PubMed] [Google Scholar]
- 12.Manceau G, Marisa L, Boige V, Duval A, Gaub MP, Milano G, Selves J, Olschwang S, Jooste V, le Legrain M, et al. PIK3CA mutations predict recurrence in localized microsatellite stable colon cancer. Cancer Med. 2015;4:371–382. doi: 10.1002/cam4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin D, Galisteo R, Molinolo AA, Wetzker R, Hirsch E, Gutkind JS. PI3Kγ mediates kaposi's sarcoma-associated herpesvirus vGPCR-induced sarcomagenesis. Cancer Cell. 2011;19:805–813. doi: 10.1016/j.ccr.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Wang K, Jiang YZ, Chang XW, Dai CF, Zheng J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) inhibits human ovarian cancer cell proliferation. Cell Oncol (Dordr) 2014;37:429–437. doi: 10.1007/s13402-014-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, et al. COSMIC: Mining complete cancer genomes in the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2011;39((Database Issue):D945–D950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Chung WC, Wu G, Egan SE, Miele L, Xu K. Manic fringe promotes a claudin-low breast cancer phenotype through notch-mediated PIK3CG induction. Cancer Res. 2015;75:1936–1943. doi: 10.1158/0008-5472.CAN-14-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphree AL, Benedict WF. Retinoblastoma: Clues to human oncogenesis. Science. 1984;223:1028–1033. doi: 10.1126/science.6320372. [DOI] [PubMed] [Google Scholar]
- 18.Schaal C, Pillai S, Chellappan SP. The Rb-E2F transcriptional regulatory pathway in tumor angiogenesis and metastasis. Adv Cancer Res. 2014;121:147–182. doi: 10.1016/B978-0-12-800249-0.00004-4. [DOI] [PubMed] [Google Scholar]
- 19.Gore AJ, Deitz SL, Palam LR, Craven KE, Korc M. Pancreatic cancer-associated retinoblastoma 1 dysfunction enables TGF-γ to promote proliferation. J Clin Invest. 2014;124:338–352. doi: 10.1172/JCI71526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carriere C, Gore AJ, Norris AM, Gunn JR, Young AL, Longnecker DS, Korc M. Deletion of Rb accelerates pancreatic carcinogenesis by oncogenic Kras and impairs senescence in premalignant lesions. Gastroenterology. 2011;141:1091–1101. doi: 10.1053/j.gastro.2011.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kagawa S, Natsuizaka M, Whelan KA, Facompre N, Naganuma S, Ohashi S, Kinugasa H, Egloff AM, Basu D, Gimotty PA, et al. Cellular senescence checkpoint function determines differential Notch1-dependent oncogenic and tumor-suppressor activities. Oncogene. 2015;34:2347–2359. doi: 10.1038/onc.2014.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Zhao YJ, Zou QY, Zhang K, Wu YM, Zhou C, Wang K, Zheng J. Preeclampsia does not alter vascular growth and expression of CD31 and vascular endothelial cadherin in human placentas. J Histochem Cytochem. 2015;63:22–31. doi: 10.1369/0022155414558063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao YJ, Zou QY, Li Y, Li HH, Wu YM, Li XF, Wang K, Zheng J. Expression of G-protein subunit γ-14 is increased in human placentas from preeclamptic pregnancies. J Histochem Cytochem. 2014;62:347–354. doi: 10.1369/0022155414521213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Z, Song Y, Piao D, Liu T, Zhao L. Identification of genes and long non-coding RNAs associated with the pathogenesis of gastric cancer. Oncol Rep. 2015;34:1301–1310. doi: 10.3892/or.2015.4129. [DOI] [PubMed] [Google Scholar]
- 25.Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146:245–1256. doi: 10.1053/j.gastro.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/S1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 27.Mann CD, Bastianpillai C, Neal CP, Masood MM, Jones DJ, Teichert F, Singh R, Karpova E, Berry DP, Manson MM. Notch3 and HEY-1 as prognostic biomarkers in pancreatic adenocarcinoma. PLoS One. 2012;7:e51119. doi: 10.1371/journal.pone.0051119. [DOI] [PMC free article] [PubMed] [Google Scholar]