Abstract
Data on prognostic factors and treatment outcomes for chest wall soft tissue sarcomas (STS) are sparse. Wide resections with negative margins are the mainstay of therapy, but the prognostic impact of surgical margins remains controversial. The purpose of the present study was to determine the significance of microscopic margins through a long-term follow-up. The associations between local recurrence-free survival (LRFS), overall survival (OS) and potential prognostic factors were retrospectively assessed in a consecutive series of 110 patients who were suitable for surgical treatment with curative intent. Potential prognostic factors were assessed using univariate and multivariate analyses. The median follow-up time following primary diagnosis was 9.6 years [95% confidence interval (CI), 7.2–10.5]. In the entire cohort, the 5-year estimates of the OS and LRFS rates were 66.0% (95% CI, 55.9–74.3) and 60.6% (95% CI, 50.3–69.4), respectively. A total of 27 patients (24.5%) developed distant metastases with a median survival time of 0.9 years following the diagnosis of metastasis. Surgical margins attained at the initial resection and eventual re-excisions significantly influenced OS in univariate analysis (5-year OS, R0 69.9% vs. R1/R2 38.5%; P=0.046), but this failed to reach statistical significance in the multivariate analysis. In the multivariate analysis, significant adverse prognostic features of LRFS included angiosarcoma subtype, G2 and G3 histology. For OS, the only independent significant predictors were age >50 years, tumor size >5 cm, angiosarcoma subtype and G3 histology. The results of the present study suggest that tumor biology, as reflected by the histological grade, influences the final outcome in patients with chest wall STS. Surgical margins failed to reach statistical significance in multivariate analysis as they demonstrated a dependency towards the independent predictors of OS. Subsequently, a positive margin status may be a result rather than a cause of biological aggressiveness, and it may not influence the outcome directly.
Keywords: sarcoma, chest wall, survival, margin
Introduction
Soft tissue sarcomas (STSs) are a heterogeneous group of rare tumors of mesenchymal origin, accounting for ~1% of all adult malignancies (1). Approximately 20% of all STS arise in the chest wall (2).
At present, there have been several analyses of the prognostic factors influencing survival in patients with soft tissue and bone sarcomas of the chest wall (2–6). Among these factors, histological grade, age, tumor size, depth and histological subtype are considered the most significant. The majority of these analyses involved heterogeneous patient cohorts due to varying inclusion criteria. Certain studies evaluated the outcome of chest wall STS together with STS of the abdominal wall, pelvis and extremities (6–8). Numerous other studies only included patients who underwent full-thickness chest wall resections; however, no rationale for the exclusion of patients with superficial chest wall STS was provided (9–11). Thus, patients with STS that infiltrated the bony chest wall structures were assessed in these previous studies, resulting in a specific patient population with advanced local disease. Furthermore, these studies mixed patients with soft tissue and bone sarcomas in the analyses, although chondrosarcomas and other bone sarcomas exhibit different clinical behaviors compared with STS (12). Furthermore, only certain studies have specifically focused on the outcomes of patients with chest wall STS (13).
Although negative margins are commonly sought in the surgical treatment of chest wall STS, the prognostic significance of surgical margins remains controversial. None of the large studies on chest wall STS assessed the prognostic significance of microscopic surgical margins; instead these studies compared the outcomes of patients with wide and marginal excisions as defined by the surgeons rather than the pathologists (5,13). Thus, the effect of microscopic surgical margins on the outcome of chest wall STS warrants evaluation, and the question remains whether wide resections with clear margins at any cost or more conservative resections should be performed.
To further understand the clinical behavior of chest wall STS, the present study reviewed the demographic, tumor and treatment characteristics of 110 patients who underwent surgical treatment with curative intent at the BG-University Hospital Bergmannsheil (Bochum, Germany). The potential prognostic indicators of survival and with focus on the effect of surgical margins on disease outcome were assessed.
Patients and methods
Patients
A total of 122 patients with chest wall STS were treated consecutively at the BG-University Hospital Bergmannsheil between June 1999 and May 2016. Only patients presenting with primary chest wall STS and no simultaneous distant metastases were included in the present study. All patients underwent surgical treatment with curative intent. Chest wall tumors extending into the region bordered superiorly by the clavicles and inferiorly by the rib margins were included. From this cohort, 3 patients were excluded due to essential data regarding the initial surgical procedure, including tumor size or margin status, not being available. Furthermore, 9 patients, including those from other countries, were lost to follow-up. Thus, the analyses were restricted to 110 participants with full information available on the outcome, histology and surgical margins of the initial procedures. Patient follow-up results were obtained from the BG-University Hospital Bergmannsheil database, medical records and patient correspondence. The study was approved by the BG-University Hospital Bergmannsheil Ethics Committee with the registration no. 4782-13.
Treatment
Preoperatively, computed tomography scans and/or contrast-enhanced magnetic resonance imaging (MRI) of the chest wall and the tumor site were routinely performed. The goal of surgical treatment for all patients was resection of the primary tumor with negative margins. A lateral clear margin of 2 cm of healthy tissue was ensured wherever possible. In epifascial lesions, a deep clear margin of one fascial layer was intended. Full-thickness chest wall resections were performed on lesions infiltrating the ribs or intercostal space. Plastic reconstructive surgery involving skin grafts and local flaps was performed for coverage of resulting soft tissue defects following partial- and full-thickness chest wall resections.
Several patients received adjuvant radiation and/or chemotherapy. The indication for adjuvant radiation or chemotherapy was given at the discretion of the interdisciplinary tumor board of the BG-University Hospital Bergmannsheil or referral institutions.
Following surgical treatment, the follow-up management for all patients included clinical examinations, chest X-rays and contrast-enhanced MRIs every 3 months for the first 2 years, and then every six months for the next 3 years. The decision of whether follow-up MRIs and chest X-rays would be continued following five years for every six or twelve months was based on previous tumor behavior and the decision of the informed patient.
Histopathological classification
All STS were diagnosed and classified according to the French Federation of Cancer Centers and the most recent World Health Organization guidelines (14,15). Surgical margins were assessed following fixation of the pathological specimen (sample thickness 5 µm) with formalin (10%) and staining the surface with ink (TMD™ Tissue Marking Dye, Blue; General Data Company, Inc., Cincinnati, OH, USA). All pathological slides and the according surgical margin widths were analyzed or reviewed for consensus diagnosis by an experienced soft tissue pathologist at the BG-University Hospital Bergmannsheil.
Statistical analysis
All patients were retrospectively analyzed regarding possible prognostic factors influencing survival (Tables I and II). Overall survival (OS) was defined as the time period between the date of surgery for primary disease and the date of mortality from any cause or the date of the last follow-up assessment in living patients. The local recurrence-free survival (LRFS) was calculated as the time period between the date of surgery for the primary disease and the date of first recurrence or the date of the last follow-up assessment in recurrence-free patients. Survival rates were estimated according to the Kaplan-Meier method with 95% confidence intervals (CIs), and were compared using the log-rank test. Multivariate analyses and regression analysis of surgical margin widths were performed using Cox's proportional hazards model and the Wald test. Variables that were associated with P<0.05 in the univariate analysis were included in the multivariate regression analysis to assess independent prognostic factors for LRFS and OS. Data analyses were performed using Stata software (version 11.2; StataCorp LP, College Station, TX, USA). Mean data are presented. P<0.05 was considered to indicate a statistically significant difference.
Table I.
Results of the univariate analyses to determine factors predictive of LRFS.
| Clinicopathological characteristic | N | No. of local recurrences | 1-year LRFS (95% CI) | 2-year LRFS (95% CI) | 5-year LRFS (95% CI) | P-value (log-rank) |
|---|---|---|---|---|---|---|
| All patients | 110 | 55 | 77.3 (68.1–84.2) | 70.4 (60.6–78.2) | 60.6 (50.3–69.4) | |
| Age, years | 0.116 | |||||
| ≤50 | 39 | 18 | 81.6 (65.3–90.8) | 79.0 (62.4–88.9) | 70.4 (52.9–82.5) | |
| >50 | 71 | 37 | 74.9 (62.7–83.6) | 65.3 (52.4–75.4) | 54.7 (41.5–66.1) | |
| Gender | 0.007 | |||||
| Female | 57 | 35 | 74.1 (60.1–83.8) | 64.3 (49.9–75.6) | 49.2 (34.7–62.2) | |
| Male | 53 | 20 | 80.7 (67.1–89.1) | 76.6 (62.4–86.0) | 72.3 (57.7–82.6) | |
| Tumor size, cm | 0.104 | |||||
| ≤5 | 37 | 17 | 89.1 (73.5–95.8) | 83.4 (66.6–92.2) | 73.9 (55.6–85.6) | |
| >5 | 73 | 38 | 71.0 (58.7–80.2) | 63.4 (50.7–73.6) | 53.4 (40.6–64.7) | |
| Tumor depth | 0.109 | |||||
| Epifascial | 52 | 26 | 87.8 (74.9–94.3) | 81.7 (67.8–90.0) | 71.1 (56.1–81.8) | |
| Subfascial | 58 | 29 | 68.1 (54.3–78.6) | 60.3 (46.1–71.8) | 51.1 (36.6–63.8) | |
| Grading | <0.001a | |||||
| G1 | 32 | 8 | 100 (−) | 96.8 (79.2–99.5) | 89.5 (70.8–96.5) | |
| G2 | 32 | 18 | 77.4 (58.4–88.5) | 67.3 (47.7–80.9) | 53.5 (34.3–69.3) | |
| G3 | 46 | 29 | 60.7 (44.5–73.5) | 52.8 (36.7–66.6) | 44.1 (28.3–58.8) | |
| Subtype | ||||||
| NOS | 31 | 18 | 64.5 (45.2–78.5) | 57.9 (38.7–73.0) | 57.9 (38.7–73.0) | 0.166 |
| Angiosarcoma | 21 | 17 | 73.7 (47.9–88.1) | 57.9 (33.2–76.3) | 26.3 (9.6–46.8) | <0.001 |
| Liposarcoma | 19 | 4 | 88.1 (60.2–96.9) | 88.1 (60.2–96.9) | 88.1 (60.2–96.9) | 0.007 |
| Leiomyosarcoma | 10 | 4 | 80.0 (40.9–94.6) | 80.0 (40.9–94.6) | 66.7 (27.2–88.1) | 0.361 |
| Margin status | 0.275 | |||||
| (Primary tumor) | ||||||
| R0 | 96 | 47 | 76.7 (66.8–84.0) | 71.2 (60.8–79.2) | 61.5 (50.6–70.7) | |
| R1/R2 | 14 | 8 | 82.5 (46.1–95.3) | 61.9 (27.0–83.9) | 49.5 (16.9–75.7) | |
| Full-thickness resection received | 0.523 | |||||
| No | 89 | 45 | 80.3 (70.3–87.3) | 71.9 (61.0–80.2) | 62.7 (51.2–72.2) | |
| Yes | 21 | 10 | 64.6 (39.6–81.4) | 64.6 (39.6–81.4) | 52.2 (27.7–72.0) | |
| Wound closure | 0.917 | |||||
| Primary closure | 90 | 46 | 80.5 (70.5–87.4) | 72.0 (61.2–80.3) | 63.1 (51.7–72.5) | |
| Plastic surgical tissue transfer | 20 | 9 | 63.3 (38.1–80.6) | 63.3 (38.1–80.6) | 47.5 (22.1–69.3) | |
| Adjuvant radiotherapy received (Primary tumor) | 0.799 | |||||
| No | 73 | 38 | 77.0 (65.3–85.3) | 72.6 (60.5–81.6) | 61.2 (48.4–71.8) | |
| Yes | 37 | 17 | 78.2 (61.1–88.5) | 65.9 (47.7–79.1) | 59.5 (41.1–73.8) | |
| Adjuvant chemotherapy received (Primary tumor) | 0.519 | |||||
| No | 96 | 47 | 78.2 (68.3–85.4) | 71.4 (60.8–79.5) | 63.8 (52.7–72.9) | |
| Yes | 14 | 8 | 71.4 (40.6–88.2) | 63.5 (33.1–83.0) | 39.7 (14.8–64.0) |
Global log-rank test for trend of survivor functions. LRFS, local recurrence-free survival; CI, confidence interval; NOS, sarcoma not otherwise specified.
Table II.
Results of the univariate analyses to determine factors predictive of OS.
| Clinicopathological characteristic | N | No. of mortalities | 1-year OS (95% CI) | 2-year OS (95% CI) | 5-year OS (95% CI) | P-value (log-rank) |
|---|---|---|---|---|---|---|
| All patients | 110 | 44 | 90.7 (83.3–94.9) | 80.0 (71.0–86.5) | 66.0 (55.9–74.3) | |
| Age, years | 0.003 | |||||
| ≤50 | 39 | 8 | 94.8 (80.8–98.7) | 89.4 (74.1–95.9) | 80.9 (64.0–90.4) | |
| >50 | 71 | 36 | 88.3 (77.9–94.0) | 74.6 (62.3–83.4) | 57.6 (44.7–68.5) | |
| Gender | 0.267 | |||||
| Female | 57 | 25 | 87.1 (74.8–93.6) | 75.6 (61.7–85.1) | 59.5 (44.8–71.5) | |
| Male | 53 | 19 | 94.3 (83.5–98.1) | 84.5 (71.4–91.9) | 72.5 (58.0–82.7) | |
| Tumor size, cm | 0.022 | |||||
| ≤5 | 37 | 10 | 94.6 (80.1–98.6) | 89.2 (73.7–95.8) | 80.5 (63.3–90.2) | |
| >5 | 73 | 34 | 88.6 (78.5–94.1) | 75.0 (62.8–83.7) | 58.0 (45.2–68.8) | |
| Tumor depth | 0.098 | |||||
| Epifascial | 52 | 18 | 96.0 (85.1–99.0) | 86.0 (72.9–93.1) | 75.6 (60.9–85.3) | |
| Subfascial | 58 | 26 | 85.9 (73.8–92.7) | 74.6 (60.8–84.1) | 57.3 (43.0–69.3) | |
| Grading | 0.003a | |||||
| G1 | 32 | 7 | 100 (−) | 100 (−) | 88.9 (69.3–96.3) | |
| G2 | 32 | 13 | 87.5 (70.0–95.1) | 84.3 (66.2–93.1) | 64.0 (44.4–78.3) | |
| G3 | 46 | 24 | 86.3 (72.0–93.6) | 62.3 (46.0–75.0) | 50.3 (34.5–64.2) | |
| Subtype | ||||||
| NOS | 31 | 18 | 64.5 (45.2–78.5) | 57.9 (38.7–73.0) | 57.9 (38.7–73.0) | 0.166 |
| Angiosarcoma | 21 | 17 | 73.7 (47.9–88.1) | 57.9 (33.2–76.3) | 26.3 (9.6–46.8) | <0.001 |
| Liposarcoma | 19 | 4 | 88.1 (60.2–96.9) | 88.1 (60.2–96.9) | 88.1 (60.2–96.9) | 0.007 |
| Leiomyosarcoma | 10 | 4 | 80.0 (40.9–94.6) | 80.0 (40.9–94.6) | 66.7 (27.2–88.1) | 0.361 |
| Margin status (Primary tumor) | 0.046 | |||||
| R0 | 96 | 36 | 92.5 (85.0–96.4) | 82.6 (73.1–88.9) | 69.9 (59.2–78.3) | |
| R1/R2 | 14 | 8 | 76.9 (44.2–91.9) | 61.5 (30.8–81.8) | 38.5 (14.1–62.8) | |
| Full-thickness resection | 0.025 | |||||
| No | 89 | 32 | 96.5 (89.6–98.9) | 84.5 (74.7–90.7) | 70.7 (59.5–79.3) | |
| Yes | 21 | 12 | 66.7 (42.5–82.5) | 61.9 (38.1–78.8) | 46.4 (24.4–65.9) | |
| Wound closure | 0.352 | |||||
| Primary closure | 90 | 35 | 94.3 (86.9–97.6) | 82.5 (72.7–89.1) | 68.0 (56.9–76.9) | |
| Plastic surgical tissue transfer | 20 | 9 | 73.7 (47.9–88.1) | 68.4 (42.8–84.4) | 55.8 (30.2–75.2) | |
| Adjuvant radiotherapy received (Primary tumor) | 0.383 | |||||
| No | 73 | 27 | 90.0 (80.2–95.1) | 79.8 (68.3–87.5) | 70.5 (58.0–79.9) | |
| Yes | 37 | 17 | 91.9 (76.9–97.3) | 80.4 (63.2–90.2) | 57.4 (39.6–71.8) | |
| Adjuvant chemotherapy received (Primary tumor) | 0.479 | |||||
| No | 96 | 37 | 89.3 (81.0–94.1) | 80.4 (70.7–87.2) | 66.6 (55.7–75.3) | |
| Yes | 14 | 7 | 100 (−) | 76.9 (44.2–91.9) | 61.5 (30.8–81.8) |
Global log-rank test for trend of survivor functions. OS, overall survival; CI, confidence interval; NOS, sarcoma not otherwise specified.
Results
Patient and tumor characteristics
The median age of patients at the time of primary diagnosis was 59.8 years (range, 17.3–91.7) for the entire cohort. There were 57 female (51.8%) and 53 male (48.2%) individuals. Only patients with primary STS of the chest wall were included in the present study. A total of 61 patients (55.5%) presented with untreated primary tumors at the BG-University Hospital Bergmannsheil. However, several patients were referred to the BG-University Hospital Bergmannsheil following ‘whoops’ procedures. A total of 49 patients (44.5%) underwent previous inadequate resections of their primary tumors. Of these, 38 (77.6%) underwent previous R1 resections and 4 patients (8.1%) underwent resections with R2 margins. In the remaining 7 patients (14.3%) the margin status was unclear. All of these 49 patients underwent subsequent re-excisions at the BG-University Hospital Bergmannsheil.
A total of 55 patients (50.0%) developed ≥1 local recurrence, whereas 30 patients (27.3%) had ≥2 local recurrences (range, 2–16) during the course of disease. Over time, 27 patients (24.5%) developed distant metastases. Of these patients, 15 presented with pulmonary metastases. The median survival time following diagnosis of the initial metastasis was 0.9 years (95% CI, 0.3–1.7).
The distribution of the histological grading was as follows: G1 in 32 cases (29.1%); G2 in 32 cases (29.1%); G3 in 46 cases (41.8%). Primary tumors were located epifascially in 52 patients (47.3%), while 58 patients (52.7%) presented with subfascial tumors. In 73 patients (66.4%), the primary tumors were >5 cm. Among the entire cohort, the most frequent histotypes were as follows: 31 sarcoma not otherwise specified (sarcoma NOS; 28.2%); 21 angiosarcoma (19.1%); 19 liposarcoma (17.3%).
Treatment characteristics
Surgical resection of the primary tumor in one or two steps resulted in microscopically negative margins (R0) in 96 patients (87.3%), whereas 10 patients (9.1%) exhibited microscopically positive margins (R1) and 4 patients (3.6%) exhibited macroscopically positive margins (2). In the patients with positive margins, tumors infiltrated critical anatomical structures or were too advanced and widespread for complete resection, which may have resulted in increased morbidity or more extensive surgery. Thus, positive margins were tolerated consensually in these patients. Continuous follow-ups were performed in these patients to monitor tumor progression.
Full-thickness chest wall resections were performed in 21 patients (19.1%) presenting with primary tumors. Following the resection of the primary tumor, soft tissue defects had to be covered in 16 patients (14.5%) with local flaps, while 4 patients (3.6%) underwent transplantation with split-thickness skin grafts due to skin defects.
A total of 37 patients (33.7%) received adjuvant radiotherapy following resection of their primary tumor, with a median overall dose of 57.6 Gy (range, 44.8–70.0). Of these 37 patients, 18 (48.6%) had G3 lesions while 17 (45.9%) had G2 tumors. A total of 2 patients (5.4%) with R1-resected G1 tumors were also radiated postoperatively. No patients were treated preoperatively with radiation.
A total of 14 patients received adjuvant anthracycline-based chemotherapy following resection of the primary tumor. A total of 10 of these patients (71.4%) had G3 tumors and 4 patients (28.6%) had G2 tumors. All patients treated with adjuvant chemotherapy had subfascially localized tumors >5 cm.
Follow-up
As of June 2016 (cut-off date), the median follow-up was 5.4 years and the reverse Kaplan-Meier estimate of the median follow-up following primary diagnosis was 9.6 years (95% CI, 7.2–10.5) (16,17). At the cut-off date, 61 patients (55.5%) had no evidence of disease whereas 5 patients (4.5%) were alive with residual localized disease. During follow-up, 44 patients (40.0%) had succumbed to the disease.
Univariate analysis of LRFS
The 5-year rate of LRFS was 60.6% (95% CI, 50.3–69.4) for the entire cohort. Female gender, histological grade, and the angiosarcoma and liposarcoma subtypes were the only factors with a statistically significant effect on LRFS following univariate analysis (Table I). Female patients exhibited a significantly decreased LRFS compared with male patients [5-year LRFS, 49.2% (34.7–62.2) vs. 72.3% (57.7–82.6); P=0.007). Regarding the histological grade, G1 tumors had a significantly more favorable local outcome compared with G2 and G3 lesions [5-year LRFS, G1 89.5% (70.8–96.5) vs. G2 53.5% (34.3–69.3) vs. G3 44.1% (28.3–58.8); P<0.001].
Angiosarcomas were associated with a significantly diminished LRFS [5-year LRFS, 26.3% (9.6–46.8) vs. 69.2% (58.1–77.9); P<0.001]. By contrast, liposarcomas displayed the lowest rates of local recurrence [5-year LRFS, 88.1% (60.2–96.9) vs. 55.6% (44.4–65.5); P=0.007].
When analyzing the treatment characteristics, the surgically attained margin status had no statistically significant effect on LRFS [5-year LRFS, R0 61.5% (50.6–70.7) vs. R1/R2 49.5% (16.9–75.7); P=0.275]. Adjuvant radiation (P=0.799) and chemotherapy (P=0.519) also had no significant influence on LRFS.
Univariate analysis of OS
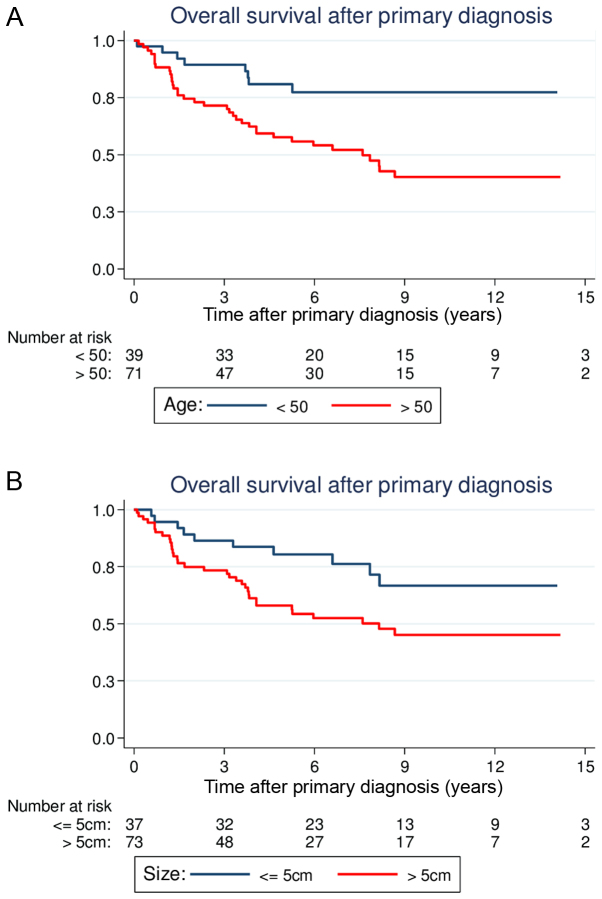
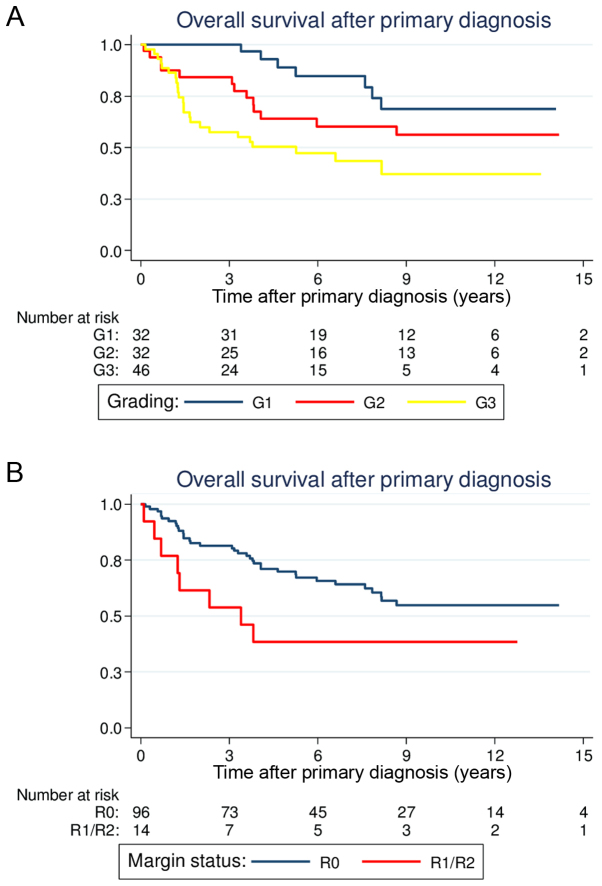
Overall, the 5-year estimate of the OS rate was 66.0% (95% CI, 55.9–74.3). Age, tumor size, histological grade and full-thickness resections were demonstrated to be statistically significant predictors of OS in the univariate analysis (Table II). Patients >50 years old at the time of primary diagnosis had significantly reduced OS compared with younger patients [5-year OS, 57.6% (44.7–68.5) vs. 80.9% (64.0–90.4); P=0.003; Fig. 1A]. Large tumor size (>5 cm) was also associated with a significantly reduced OS compared with smaller tumor sizes [5-year OS, 58.0% (45.2–68.8) vs. 80.5% (63.3–90.2); P=0.022; Fig. 1B]. Similar to the results for LRFS, patients with G1 lesions had more favorable prognoses compared with patients with G2 or G3 lesions [5-year OS, G1 88.9% (69.3–96.3) vs. G2 64.0% (44.4–78.3) vs. G3 50.3% (34.5–64.2); P=0.003; Fig. 2A]. Regarding the different histological subsets, patients with liposarcomas tended to have a more favorable OS (P=0.007), whereas patients with angiosarcomas had reduced survival (P<0.001). The other histological subtypes were not associated with a significantly altered outcome.
Figure 1.
Estimated overall survival curves following primary diagnosis according to (A) age and (B) tumor size.
Figure 2.
Estimated overall survival curves following primary diagnosis according to (A) histological grade and (B) margin status.
The surgical margin status following treatment of the primary tumor reached prognostic significance in the univariate analysis. Patients with R0 margins had a significantly better OS compared with patients with positive margins [5-year OS, 69.9% (59.2–78.3) vs. 38.5% (14.1–62.8); P=0.046; Fig. 2B]. Notably, patients who underwent full-thickness chest wall resections for the primary tumors had a significantly diminished OS [5-year OS, 46.4% (24.4–65.9) vs. 70.7% (59.5–79.3); P=0.025]. Similar to the results for LRFS, adjuvant radiation (P=0.383) and chemotherapy (P=0.479) did not significantly alter OS.
Multivariate analysis of LRFS
In the Cox's hazard regression model, the significant and independent prognostic factors for the local outcome were identified as histological grade and angiosarcoma subtype (Table III). The hazard ratio (HR) for local recurrence was 2.65 (95% CI, 1.15–6.11; P=0.022) for G2 and 3.99 (95% CI, 1.86–8.54; P<0.001) for G3 lesions. Angiosarcomas presented an HR of 1.96 (95% CI, 1.02–3.78; P=0.043).
Table III.
Results of multivariate analysis on LRFS according to Cox's proportional hazards model.
| Category: (reference) | Hazard ratio for recurrence | 95% confidence interval | P-value |
|---|---|---|---|
| Gender: Female (vs. male) | 1.16 | 0.58–2.30 | 0.679 |
| Histological grade: G2 (vs. G1) | 2.65 | 1.15–6.11 | 0.022 |
| Histological grade: G3 (vs. G1) | 3.99 | 1.86–8.54 | <0.001 |
| Histological subtype: Angiosarcoma (vs. other) | 1.96 | 1.02–3.78 | 0.043 |
| Histological subtype: Liposarcoma (vs. other) | 0.36 | 0.10–1.27 | 0.113 |
Multivariate analysis of OS
Multivariate analysis revealed age, histological grade, tumor size and angiosarcoma subtype as independent prognostic factors of OS (Table IV). The HR for mortality was 2.84 (95% CI, 1.27–6.39; P=0.011) in patients >50 years. Regarding the prognostic significance of tumor size and histological grade, the HR for mortality was 2.43 (95% CI, 1.13–5.23; P=0.023) for tumors >5 cm and 3.02 (95% CI, 1.35–6.76; P=0.0007) for G3 tumors when compared with G1 lesions. Angiosarcomas presented a HR of 2.32 (95% CI, 1.20–4.44; P=0.012).
Table IV.
Results of multivariate analysis on OS according to Cox's proportional hazards model.
| Clinicopathological characteristic: (reference) | Hazard ratio for mortality | 95% confidence interval | P-value |
|---|---|---|---|
| Age: >50 years (vs. ≤50 years) | 2.84 | 1.27–6.39 | 0.011 |
| Margin status: R1/R2 (vs. R0) | 1.50 | 0.62–3.64 | 0.370 |
| Histological grade: G2 (vs. G1) | 1.40 | 0.61–3.20 | 0.423 |
| Histological grade: G3 (vs. G1) | 3.02 | 1.35–6.76 | 0.007 |
| Tumor size: >5 cm (vs. ≤5 cm) | 2.43 | 1.13–5.23 | 0.023 |
| Histological subtype: Angiosarcoma (vs. other) | 2.31 | 1.20–4.44 | 0.012 |
| Full-thickness resection: Yes (vs. no) | 1.59 | 0.66–3.84 | 0.303 |
Although margin status and full-thickness resection were found to be statistically significant predictors of OS in univariate analysis, they failed to reach statistical significance in multivariate analysis because they were dependent on histological grade and tumor size. In other words, positive margins and full-thickness resections were more frequent in those cases where tumors were large and high-grade. Therefore, none of the assessed treatment characteristics was an independent predictor of OS in multivariate analysis.
Regression analysis of non-categorized surgical margin width
In the subgroup of patients with R0 margins, the impact of negative margin widths was assessed. The closest negative margin width (median, 0.6 cm) was assessed histologically at the BG-University Hospital Bergmannsheil for 59/96 patients with R0-resected tumors. Cox's regression analysis was performed to evaluate the prognostic significance of non-categorized clear margin widths in the R0 subgroup, which identified that the closest surgical margin width did not significantly influence OS. The HR for mortality following the Wald test was 0.52 (95% CI, 0.23–1.21) for wide margins, which did not reach statistical significance (P=0.129). LRFS was also unaffected by the surgical margin width. The HR for tumor recurrence was 0.45 (95% CI, 0.18–1.08) for wide margins (P=0.074). Thus, close and wide negative margins resulted in similar OS and LRFS.
Discussion
In the present study, the outcome of 110 patients who underwent surgical resection of primary chest wall STS with curative intent was analyzed. NOS (28.2%), angiosarcoma (19.1%) and liposarcoma (17.3%) were the most frequent histological subtypes in our series. The majority of the tumors were high-grade (G3, 41.8%) and large (>5 cm, 66.4%). Surgical treatment of the primary tumor resulted in microscopic negative margins in 87.3% of all patients. Despite surgical resection, 24.5% developed distant metastases during the disease course. The median survival time following the diagnosis of metastasis was 0.9 years. In the multivariate analysis, age, tumor size, histological grade and angiosarcoma subtype were identified as independent predictors of OS. Several previous studies also confirmed the prognostic significance of histological grade with respect to OS, although these studies involved smaller patient cohorts (2–5). Regarding the local outcome, angiosarcoma subtype and histological grade were identified as independent prognostic factors. In the univariate analysis, female gender was demonstrated to be a statistically significant predictor of LRFS, but did not reach significance in the multivariate analysis because it demonstrated a dependency towards the angiosarcoma subtype. In the present study, all 21 patients with angiosarcoma were female.
One of the main aims of the present study was to determine the prognostic significance of surgical margins. To the best of our knowledge, this is the first study to analyze the prognostic effects of surgical margins on survival in >100 patients with chest wall STS. Notably, margins were revealed to be significant predictors of OS in univariate analysis whereby the 5-year OS rate was 69.9% for patients with microscopic negative margins and 38.5% for those with positive margins. However, surgical margins did not to reach statistical significance in multivariate analysis as they demonstrated a dependency towards being independent predictors of OS. The data of the present study suggest that it may have been the factor ‘R0 resectability’ and not the R0 resection itself that resulted in the improved outcome. Conversely, tumors that could not be completely resected were larger and exhibited more aggressive biological features compared with completely resectable tumors. More specifically, it was the inherent aggressiveness of the tumor itself that influenced the surgically attainable margin status and the final outcome. Subsequently, a positive margin status may be a result rather than a cause of biological aggressiveness and may not influence the outcome directly.
The question remains whether an aggressive surgical approach would result in a survival benefit and should generally be required in the treatment of chest wall STS. Presently, to the best of our knowledge, no prospective studies have assessed this question regarding chest wall STS or extremity STS. In the current study, the results from the multivariate analysis suggest that tumor biology dictates survival and that the quality of surgery has a minor effect on the final outcome. Hence, radical surgery with the aim of clear margins at any price cannot be justified by the presented findings in order to improve OS. However, the translation of retrospective data into clinical decisions is only possible to a limited extent and possesses several problems. On the one hand, whether the achievement of negative margins at any cost would have improved OS in those patients with positive margins cannot be assessed. On the other hand, the outcome of those patients with negative margins if they had been treated with inadequate margins cannot be estimated. Nevertheless, given the diminished outcome of patients left with positive margins, it appears reasonable that surgical efforts should aim for complete resections with negative margins wherever feasible.
Reviewing the literature on chest wall STS, several retrospective studies were identified, but they involved varied patient cohorts and were difficult to compare (Table V). In the largest specific study on chest wall STS, Gross et al (3) analyzed the outcomes of 55 surgically treated patients in the Hospital do Cancer in Sao Paulo. In this study, histological grade and tumor size were identified to be significant prognostic factors for OS in the univariate analysis; however, only histological grade emerged as an independent prognostic factor. Notably, a 5-year OS rate of 87% was reported, which is higher compared with the rate of 66% identified in the current study. The two studies reported comparable median follow-up durations (52 vs. 65 months) and had similar rates of high-grade sarcomas. However, the study by Gross et al (3) did not include any patients with angiosarcoma, whereas these patients accounted for 19.1% of the patient population in the present study. The study by Gross et al (3), which reviewed patients between 1964 and 1996, did not reflect the patient distribution reported in the present study. During the last few years, the incidence of secondary angiosarcomas has risen due to the increased use of adjuvant radiation in the treatment of breast cancer (18,19). In the present study, 14.5% of the entire cohort and 76.2% of all patients with angiosarcoma had secondary angiosarcomas, a previous history of breast cancer and adjuvant radiation treatment. Furthermore, the median age was 47.5 years in the study of Gross et al (3), while that in the present study was 59.8 years. Although the two studies focused on chest wall STS without bone sarcomas, they are not comparable.
Table V.
Overview of retrospective analyses on primary chest wall sarcomas.
| Authors | No. of patients | Median follow-up (months) | Sarcoma type | M (%) | 5-OS (%) | Microscopic margins available | Prognostic effect of microscopic margins on OS | (Refs.) |
|---|---|---|---|---|---|---|---|---|
| Present study | 110 | 65 | STS | 24 | 66 | + | + | |
| Kachroo et al | 51 | NR | STS/bone/AF | 23 | 66 | + | − | (2) |
| Oksuz et al | 26 | 82 | STS | 42 | 69 | + | − | (4) |
| Tsukushi et al | 44 | 57 | STS | NR | 89 | − | NA | (5) |
| Gross et al | 55 | 52 | STS | 18 | 87 | − | NA | (3) |
| Van Geel et al | 60 | 20 | STS/bone | 48 | 46 | + | − | (9) |
| Wouters et al | 83 | 73 | STS/bone | 25 | 63 | + | NR | (10) |
| Gordon et al | 149 | NA | STS/AF | 35 | 66 | − | NA | (13) |
| McMillan et al | 192 | 51 | STS/AF | 15 | 73 | + | NR | (20) |
M, metastasis; 5-OS, 5-year rate of overall survival; AF, aggressive fibromatosis; NR, not reported; NA, data not available; OS, overall survival.
The remaining studies on chest wall STS by Oksuz et al (4) and Tsukushi et al (5) revealed age and histological grade to be significant prognostic factors of survival based on univariate analyses. Tsukushi et al (5) performed a study on 44 patients with chest wall STS involving a high proportion of dermatofibrosarcoma protuberans (27.2 vs. 7.5% in the current study), which rarely metastasizes and, therefore, may have resulted in the high 5-year OS rate of 89%. The distribution of histological subtypes in the series of Oksuz et al (4) was comparable with that in the patient population of the present study, resulting in similar 5-year OS rates (69 vs. 66%). The study by Oksuz et al (4) is the only analysis on chest wall STS to date that determined the prognostic effects of surgical margins. In the analysis of 26 patients, microscopic negative margins failed to reach statistical significance in a univariate analysis.
To date, there have been two studies with larger patient cohorts than those in the present study. These two studies were performed at the Memorial Sloan-Kettering Cancer Center (MSKCC) in New York. The MSKCC study in 1991 reviewed the outcomes of 189 patients; however, it did not delineate the prognostic role of surgical margins (13). It is notable that 32.2% of all patients included in the survival analysis had aggressive fibromatosis, which is a semi-malignant mesenchymal tumor that does not metastasize. The more recent MSKCC study (2013) involving 192 patients also included a high proportion of aggressive fibromatosis patients (17.2%) and only assessed the local recurrence patterns (20). In this study, an association between surgical margins and local outcomes was not able to be established.
Finally, a large retrospective study was performed by Wouters et al (10), in which the outcomes of 83 patients with primary chest wall STS and bone sarcomas who were treated at two institutions were analyzed. Although data on surgical margins were available for the majority of patients, Wouters et al (10) did not investigate the prognostic impact of margins. Furthermore, patients with chondrosarcoma or osteosarcoma constituted 43% of the patient population. As stated previously, STS and bone sarcomas possess different clinical behaviors. Regarding chondrosarcomas, there have been two well-characterized retrospective studies that outlined the prognostic significance of negative margins (21,22).
Finally, similar to many other retrospective analyses of STS, the present study also had several limitations. Although it was one of the largest analyses on chest wall STS to date, the assessed subgroups in this study remained relatively small. Despite the exclusion of bone sarcomas and aggressive fibromatosis, the distribution of the histological subtypes remained heterogeneous. Furthermore, the present analysis only included patients with STS who were suitable for further surgical treatment with curative intent. Patients with extensive tumors that could not be approached surgically due to rapid disease progression and, therefore, less favorable outcomes, were not assessed in this study. Thus, the results of the current study are only applicable to the group of patients in whom further surgical treatment was possible and not to all patients with chest wall STS. This implies a study selection bias that must be acknowledged.
In conclusion, the present study provides long-term follow-up data that may provide clinicians with a more detailed insight into the clinical behavior and prognosis of patients with chest wall STS. Adverse prognostic features identified include age >50 years, tumor size >5 cm, high histological grade and an angiosarcoma subtype. The data from this study was not able to underscore the long-term benefit of negative margins achieved following resection of the primary tumor. When the aim of achieving negative margins requires extensive surgery with a high risk of morbidity, the postoperative consequences should be clearly discussed with the patient, as these can be highly subjective. The final decision should be made in each case based on the histological grade and progression of the tumor, the health status of the patient and the decision of the informed patient.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Kachroo P, Pak PS, Sandha HS, Lee C, Elashoff D, Nelson SD, Chmielowski B, Selch MT, Cameron RB, Holmes EC, et al. Single-institution, multidisciplinary experience with surgical resection of primary chest wall sarcomas. J Thorac Oncol. 2012;7:552–558. doi: 10.1097/JTO.0b013e31824176df. [DOI] [PubMed] [Google Scholar]
- 3.Gross JL, Younes RN, Haddad FJ, Deheinzelin D, Pinto CA, Costa ML. Soft-tissue sarcomas of the chest wall: Prognostic factors. Chest. 2005;127:902–908. doi: 10.1378/chest.127.3.902. [DOI] [PubMed] [Google Scholar]
- 4.Oksuz DC, Ozdemir S, Kaydihan N, Dervisoglu S, Hiz M, Tuzun H, Mandel NM, Koca S, Dincbas FO. Long-term treatment results in soft tissue sarcomas of the thoracic wall treated with pre-or-postoperative radiotherapy-a single institution experience. Asian Pac J Cancer Prev. 2014;15:9949–9953. doi: 10.7314/APJCP.2014.15.22.9949. [DOI] [PubMed] [Google Scholar]
- 5.Tsukushi S, Nishida Y, Sugiura H, Nakashima H, Ishiguro N. Soft tissue sarcomas of the chest wall. J Thorac Oncol. 2009;4:834–837. doi: 10.1097/JTO.0b013e3181a97da3. [DOI] [PubMed] [Google Scholar]
- 6.Salas S, Bui B, Stoeckle E, Terrier P, Ranchere-Vince D, Collin F, Leroux A, Guillou L, Michels JJ, Trassard M, et al. Soft tissue sarcomas of the trunk wall (STS-TW): A study of 343 patients from the French Sarcoma Group (FSG) database. Ann Oncol. 2009;20:1127–1135. doi: 10.1093/annonc/mdn757. [DOI] [PubMed] [Google Scholar]
- 7.Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A, Jørgensen PH, Hansen BH, Baerentzen S, Pedersen AB, Keller J. Prognostic factors for local recurrence and mortality in adult soft tissue sarcoma of the extremities and trunk wall: A cohort study of 922 consecutive patients. Acta Orthop. 2014;85:323–332. doi: 10.3109/17453674.2014.908341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stojadinovic A, Leung DH, Hoos A, Jaques DP, Lewis JJ, Brennan MF. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235:424–434. doi: 10.1097/00000658-200203000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Geel AN, Wouters MW, Lans TE, Schmitz PI, Verhoef C. Chest wall resection for adult soft tissue sarcomas and chondrosarcomas: Analysis of prognostic factors. World J Surg. 2011;35:63–69. doi: 10.1007/s00268-010-0804-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wouters MW, van Geel AN, Nieuwenhuis L, van Tinteren H, Verhoef C, van Coevorden F, Klomp HM. Outcome after surgical resections of recurrent chest wall sarcomas. J Clin Oncol. 2008;26:5113–5118. doi: 10.1200/JCO.2008.17.4631. [DOI] [PubMed] [Google Scholar]
- 11.Walsh GL, Davis BM, Swisher SG, Vaporciyan AA, Smythe WR, Willis-Merriman K, Roth JA, Putnam JB., Jr A single-institutional, multidisciplinary approach to primary sarcomas involving the chest wall requiring full-thickness resections. J Thorac Cardiovasc Surg. 2001;121:48–60. doi: 10.1067/mtc.2001.111381. [DOI] [PubMed] [Google Scholar]
- 12.Smith GM, Johnson GD, Grimer RJ, Wilson S. Trends in presentation of bone and soft tissue sarcomas over 25 years: Little evidence of earlier diagnosis. Ann R Coll Surg Engl. 2011;93:542–547. doi: 10.1308/147870811X13137608455055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon MS, Hajdu SI, Bains MS, Burt ME. Soft tissue sarcomas of the chest wall. Results of surgical resection. J Thorac Cardiovasc Surg. 1991;101:843–854. [PubMed] [Google Scholar]
- 14.Coindre JM. Grading of soft tissue sarcomas: Review and update. Arch Pathol Lab Med. 2006;130:1448–1453. doi: 10.5858/2006-130-1448-GOSTSR. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher CD. The evolving classification of soft tissue tumours-an update based on the new 2013 WHO classification. Histopathology. 2014;64:2–11. doi: 10.1111/his.12267. [DOI] [PubMed] [Google Scholar]
- 16.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-X. [DOI] [PubMed] [Google Scholar]
- 17.Clark TG, Bradburn MJ, Love SB, Altman DG. Survival analysis part I: Basic concepts and first analyses. Br J Cancer. 2003;89:232–238. doi: 10.1038/sj.bjc.6601117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coindre JM, Terrier P, Guillou L, Le Doussal V, Collin F, Ranchère D, Sastre X, Vilain MO, Bonichon F, Bui N'Guyen B. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: A study of 1240 patients from the French Federation of cancer centers sarcoma group. Cancer. 2001;91:1914–1926. doi: 10.1002/1097-0142(20010515)91:10<1914::AID-CNCR1214>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Mery CM, George S, Bertagnolli MM, Raut CP. Secondary sarcomas after radiotherapy for breast cancer: Sustained risk and poor survival. Cancer. 2009;115:4055–4063. doi: 10.1002/cncr.24462. [DOI] [PubMed] [Google Scholar]
- 20.McMillan RR, Sima CS, Moraco NH, Rusch VW, Huang J. Recurrence patterns after resection of soft tissue sarcomas of the chest wall. Ann Thorac Surg. 2013;96:1223–1228. doi: 10.1016/j.athoracsur.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Marulli G, Duranti L, Cardillo G, Luzzi L, Carbone L, Gotti G, Perissinotto E, Rea F, Pastorino U. Primary chest wall chondrosarcomas: Results of surgical resection and analysis of prognostic factors. Eur J Cardiothorac Surg. 2014;45:e194–e201. doi: 10.1093/ejcts/ezu095. [DOI] [PubMed] [Google Scholar]
- 22.Widhe B, Bauer HC. Scandinavian Sarcoma Group: Surgical treatment is decisive for outcome in chondrosarcoma of the chest wall: A population-based Scandinavian Sarcoma Group study of 106 patients. J Thorac Cardiovasc Surg. 2009;137:610–614. doi: 10.1016/j.jtcvs.2008.07.024. [DOI] [PubMed] [Google Scholar]