Abstract
To date, little is known regarding the etiology and disease mechanisms of Alzheimer’s disease (AD). There is a general urgency for novel approaches to advance AD research. In this study, we analyzed blood RNA from female patients with advanced AD and matched healthy controls using genome-wide gene expression microarrays. Our data showed significant alterations in 3,944 genes (≥2-fold, FDR≤1%) in AD whole blood, including 2,932 genes that are involved in broad biological functions. Importantly, we observed abnormal transcripts of numerous tissue-specific genes in AD blood involving virtually all tissues, especially the brain. Of altered genes, 157 are known to be essential in neurological functions, such as neuronal plasticity, synaptic transmission and neurogenesis. More importantly, 205 dysregulated genes in AD blood have been linked to neurological disease, including AD/dementia and Parkinson’s disease, and 43 are known to be the causative genes of 42 inherited mental retardation and neurodegenerative diseases. The detected transcriptional abnormalities also support robust inflammation, profound ECM impairments, broad metabolic dysfunction, aberrant oxidative stress, DNA damage and cell death. While the mechanisms are currently unclear, this study demonstrates strong blood-brain correlations in AD. The blood transcriptional profiles reflect the complex neuropathological status in AD, including neuropathological changes and broad somatic impairments. The majority of genes altered in AD blood have not previously been linked to AD. We believe that blood genome-wide transcriptional profiling may provide a powerful and minimally invasive tool for the identification of novel targets beyond Aβ and tauopathy for AD research.
Keywords: Alzheimer’s disease, blood-brain transcriptional association, genome-wide expression arrays, neuropathology
Introduction
Alzheimer’s disease (AD) is the most common age-related progressive neurodegenerative disorder, the primary cause of dementia in the elderly and a major global healthcare burden [1, 2]. The majority of AD patients are diagnosed when they are over 65 years of age (late onset), and only 1–5% of AD cases are familial and/or early onset. The characteristic clinical presentation of AD is a progressive loss of memory and specific cognitive functioning, ultimately leading to the loss of independence and death. As yet, therapies for AD have been palliative in nature, with limited symptomatic benefits.
The hallmark neuropathological changes in AD are neuritic plaques [amyloid-β (Aβ) deposition], neurofibrillary tangles (tauopathy) and neuronal loss most prominent in specific temporal, parietal and frontal regions of the brain. To date, the majority of AD research has focused on changes associated with Aβ deposition and tauopathy, mostly using postmortem brain tissues. While significant progress has been made, the etiologies and detailed mechanisms of AD remain unclear. Genetic studies have identified autosomal dominant mutations in familial AD, including mutations in Amyloid precursor protein (APP), presenilin 1 (PSEN1) and PSEN2. The most prominent genetic component that correlates with late onset AD is apolipoprotein E (APOE) (specifically the ε4 allele [3]), a protein involved in the clearance of Aβ [4, 5]. Multiple environmental factors have been considered as potential risks for AD, including cardiovascular diseases, hypertension, high cholesterol, diabetes, obesity, smoking and traumatic brain injuries [6–10]. The diversity of possible risk factors shows that AD is a complex chronic disorder and may involve multiple factors, rather than a single cause. Numerous studies support the hypothesis that AD pathology is more complex than Aβ and tau accumulation, indicating the involvement of inflammation,[11, 12] prionopathy,[13] oxidative stress,[14] and metabolic abnormalities [15, 16] in the brain. The hallmark AD neuropathological lesions, Aβ and tau deposition, also occur in many other neurodegenerative diseases, such as Lewy Body Dementia and neuropathic lysosomal storage diseases [17–21], suggesting that Aβ and tau deposition are secondary pathologies in neurodegenerative diseases, which may be triggered by different causes. Novel research strategies are consequently needed to investigate AD etiology and mechanisms, targeting changes beyond and prior to Aβ and tau deposition.
Impediments to AD etiology and mechanistic research largely stem from the lack of animal models that effectively recapitulate the disease and limited access to human brain tissues. There is a general urgency for approaches that can detect all of the changes during AD progression, in order to separate causative factors from downstream pathologies and to better understand the detailed disease mechanisms[22, 23]. Currently available genome-wide gene expression microarray techniques offer an excellent tool allowing the detection of even small changes in the expression of all currently identified and unidentified genes. Previous studies have shown the biomarker potential of blood transcriptional profiles using the genome-wide array approach in autism spectrum disorders (ASD) [24], schizophrenia [25, 26], and early-onset major depressive disorder (MDD)[27]. The AD transcriptional profile studies by microarray have mostly tested postmortem brain tissues [28, 29]. Blood transcriptional profiling of AD has been performed using diverse probe sets, mostly in mixed AD patient populations [30–35]. Studies by Maes et al using microarrays targeting selected genes suggest that peripheral blood monocytes (PBMC) may reflect molecular events germane to the neuropathophysiology of AD [30, 31]. This is supported by more recent studies reporting that blood genome-wide splice arrays (GWSA) can generate AD-associated signatures [33] and identify drug-treatment-specific differences [32].
In the present study, we assayed blood RNA samples from patients with advanced AD, using up-to-date genome-wide gene expression microarrays, to assess the association between blood transcriptional abnormalities and AD pathology. Our results demonstrate that genome-wide transcriptional profiles in the blood reflect the complex biopathophysiological status of AD, suggesting the potential for investigating causative factors, disease mechanisms, biomarkers, and novel therapeutic targets for AD.
Materials and Methods
Study subjects
Study subjects were all female, including patients with advanced AD (n=9, age 79.3±12.3 years) and age–matched female healthy controls (n=10, age 72.1±13.1 years). Table 1 shows the demographic distribution of the study subjects. The AD diagnoses were made by the Neurobehavior and Memory Disorders Clinic at the Ohio State University Wexner Medical Center (NMDC-OSUWMC), following the revised NIH Diagnostic Guidelines for Alzheimer’s disease and Related Disorders. All recruited AD subjects were nursing home residents at Forest Hills Center, an all dementia facility, and all were completely dependent or bed-ridden, with severe clinical dementia rating 2–3 at the time of recruitment. The enrolled AD patient subjects had no other neurological conditions and had been monitored for years by MDC-OSUWMC after their diagnosis. Healthy controls were recruited among female spouses and primary caregivers of afflicted male dementia patients seen at MDC-OSUWMC, and were excluded of dementia, acute or chronic infection, inflammation, and diabetes. An IRB approval of this study was obtained from the OSU Biomedical Sciences Human Subject Institutional Review Board. All study subjects were recruited strictly following the approved IRB protocol. Informed consents were provided by all healthy control subjects. Legally authorized representatives signed the informed consents for AD patients, because these subjects did not have the capacity to give informed consent, due to their significant cognitive impairment.
Table 1.
Demographic distribution of study subjects
| Subjects* | n | Ethnic background | Age (years±SE) |
|
|---|---|---|---|---|
|
| ||||
| Caucasian | African American |
|||
| AD | 9 | 7 | 2 | 79.3±12.3 |
| Controls | 10 | 10 | 0 | 72.1±13.1 |
AD: patients with advanced AD; Controls: non-AD healthy controls;
: All subjects were females.
Blood RNA extraction
Peripheral blood samples (5ml) were collected using Paxgene blood tubes (Qiagen) from each study subject by venipuncture via median cubital vein. Total RNA was isolated from blood cells using the Paxgene Blood RNA kit (Qiagen), following the protocols provided by the manufacturer. To ensure the RNA quality, each extracted total RNA sample was processed to deplete α and β-globin mRNA using GLOBINclear™-Human Kit (Ambion, NY), and then further purified using Qiagen RNeasy columns. RNA concentration was determined using the NanoDrop spectrophotometer (NanoDrop products, DE). Each sample was also carefully analyzed for integrity using an Agilent 2100 bioanalyzer (Agilent Technologies, CA) prior to array analysis. The blood RNA sample from one AD patient did not pass quality control, and was excluded from all further analyses (analyzed AD n=9).
Genome wide gene expression microarray
Qualified RNA samples from study subjects were assayed for gene expression with greater than 60,000 probes using the Sureprint G3 Human Gene Expression 8×60k v2 microarrays (Agilent Technologies, CA), which provide full coverage of currently identified and unidentified human genes and transcripts, including large intergenic non-coding RNAs. Array hybridizations were performed in-house by the Biomedical Genomics Core in the Research Institute at Nationwide Children’s Hospital. In brief, the RNA samples were labeled with Cy3, purified using Qiagen columns and checked for labeling efficiency using the NanoDrop spectrophotometer (NanoDrop products). The labeled test and control samples were fragmented and hybridized to the array overnight. Microarray slides were then washed and scanned with the Agilent G2505C Microarray Scanner, at 2 µM resolution. Images were analyzed with Feature Extraction 10.10 (Agilent Technologies). Median foreground intensities were then obtained for each spot and imported into the mathematical software package “R”, which was used for all data input, diagnostic plots, normalization and quality checking steps of the analysis process using scripts developed in-house for this analysis. The data was normalized by the loess method using the LIMMA (Linear models for microarray data) package in “R” as described [36]. The median value for the replicate probes was used as the expression measurement for that given mRNA. Complete statistical analysis of microarray data was performed in “R” using both the LIMMA and Siggenes Bioconductor packages (Significance Analysis of Microarrays, http://www-stat.stanford.edu/~tibs/SAM/), to determine the False Discovery Rate (FDR) and q-value for each transcript and combined with a fold change (FC) cutoff (a difference ≥ 2 fold) to identify the final list of differentially expressed transcripts.
Functional analysis of gene expression
Transcriptional pathway changes were analyzed using the Ingenuity Pathway Analysis (IPA) software (Ingenuity Systems, www.ingenuity.com). The functional analysis identifies the biological functions and/or diseases that were most significant to the data set (FC≥2, FDR≤1%). Molecules from the dataset underwent unsupervised analysis for biological function and/or disease associations via the Ingenuity Knowledge Base. Right-tailed Fisher’s exact test was used to calculate a p-value determining the probability that each disease assigned to that data set was not due to chance alone. A more directed analysis was also performed in parallel by applying the microarray data to functional pathways and pathways specifically known to be involved in Alzheimer’s disease pathology. Using this same cutoff, transcripts were identified as characterized genes and assigned tissue expression categories by the Database for Annotation, Visualization and Integrated Discovery (DAVID) according to enrichment analysis using DAVID on UniProt (UP_TISSUE, EASE score P=7.14E-08, FDR 1.01E-04)[37]. Heat maps were generated by MultiExperiment Viewer software (MeV, MA), using the average linkage clustering algorithm and the Pearson correlation as a measure of similarity.
Real-time Quantitative RT-PCR (qRT-PCR)
Total blood RNA from each study subject was assayed by qRT-PCR for gene expression using sequence-specific primers and probes, to confirm the quality and accuracy of microarray data. Reverse transcriptase reactions were performed using the Superscript III First Strand Synthesis Kit (Life Technologies, CA). Quantitative real-time PCR reactions were performed using Absolute Blue QPCR Mix (Thermo Scientific, MA) and Applied Biosystems 7000 Real-Time PCR System (Life technologies, CA) following the procedures recommended by the manufacturers. Primers and probes used are listed in Supplemental Table S1. All reactions were run in triplicate as a duplex reaction, with probes conjugated to either Fam or Hex (Sigma, MO). Primers for human GAPDH were used as an internal control because no change was observed in GAPDH transcripts in AD blood using arrays. The levels of target mRNAs relative to GAPDH mRNA were determined, as based on the on-plate standard curve, and presented as patients vs. controls.
Statistical analyses
The False Discovery Rate (FDR≤1%) and q-value for each transcript, combined with a fold change cutoff (a difference ≥ 2 fold) were used to identify the final list of differentially altered transcripts for the microarrays in AD. For qRT-PCR results, significance (p>0.05) was assessed by two-tailed T-tests (GraphPad Software, CA) comparing data from AD patients to controls.
Results
In order to focus more specifically on disease-related gene expression changes, and eliminate potential “noise” stemming from sex-related variations, all subjects in this study were females, comprising patients with late-stage AD and age-matched non-AD healthy controls (Table 1). The total peripheral blood RNA samples from each individual study subject were analyzed using genome-wide gene expression microarrays of >60,000 probes, to assess the potential association of blood molecular abnormalities with AD pathology. Functional analysis of array data was performed using IPA software and DAVID Bioinformatics analysis.
Genome-wide microarrays provide decisive AD blood transcriptional profiles
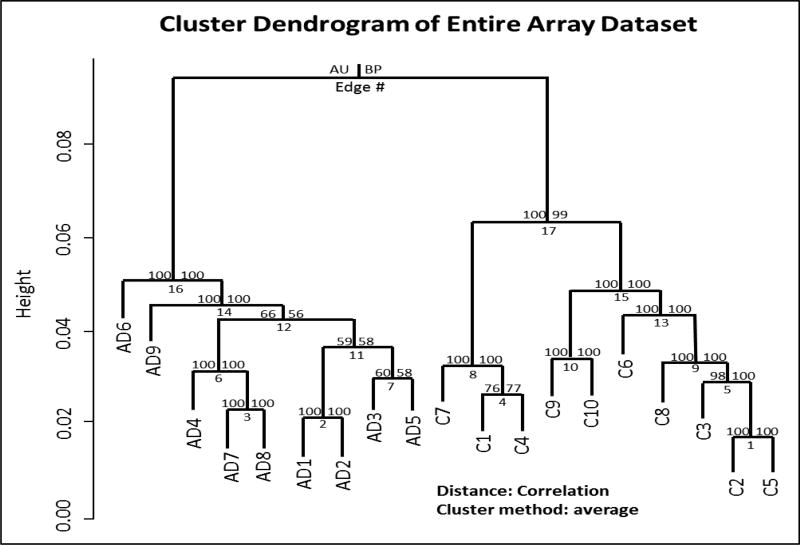
Unsupervised hierarchical cluster analysis of the overall gene expression array data generated a cluster dendrogram that showed clear separation of blood transcriptional profiles in AD patients from non-AD healthy controls (Fig. 1). Further, we confirmed our array data by qRT-PCR analyses of selected genes (Table 2).
Fig. 1. Clear group separation of blood transcript profiles.
Blood total RNAs from female patients with late-stage AD (n=9) and matched healthy control (n=10) were assayed by genome-wide gene expression microarrays using 60,000 probes. Array data were analyzed by unsupervised hierarchical cluster analysis. Values at branches are approximately unbiased (AU) p-values (left), bootstrap probability (BP) estimates (right) and cluster distance labels (bottom). Clusters with AU >95 were considered significant. AD1–9: AD patients; C1–10: healthy controls.
Table 2.
Microarray data confirmation
| Genes | Transcription (fold of change) | |
|---|---|---|
|
| ||
| Array | qRT-PCR | |
| APBB2 | −2.2 | −1.3 |
| ATRX | 3.8 | 3.9* |
| CLU | −5.2 | −3.0* |
| LRRK2 | 2.1 | 1.2* |
| PINK1 | −3.1 | −1.4* |
| PLAU | 3.5 | 1.1 |
| PRNP | 3.1 | 1.3* |
| SERPINA3 | −2.7 | −4.4* |
| SLC6A4 | −2.1 | −1.6* |
| SNCA | −4.9 | −12.0* |
qRT-PCR was performed to confirm microarray results. Fold change for qRT-PCR was calculated using the ΔCT method.
: p<0.05
Differential transcript alterations in blood present complex biopathophysiological status of AD
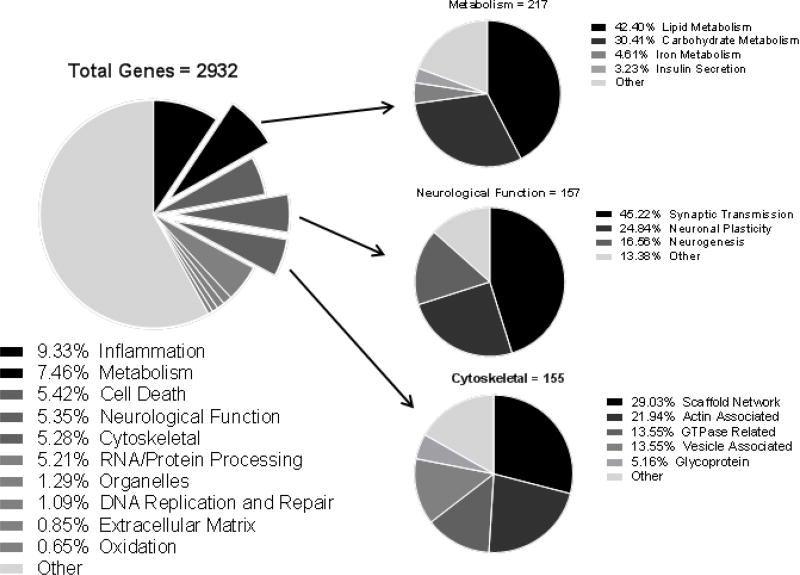
Using genome-wide human gene expression arrays, we detected highly significant alterations in numerous transcripts from AD patient blood. Our data showed that 3,944 genes were altered significantly (≥2-fold, FDR≤1%), of which 2,932 were well characterized known genes and 1,012 were genes whose functions have yet to be identified (Fig. 2, Supplementary Table S3). Further functional analyses on these 2,932 dysregulated known genes reflect the complex pathophysiological status of AD across a wide spectrum of functional and disease pathways. Importantly, numerous blood transcript abnormalities detected in AD patients have been previously linked to AD neuropathology in the brain (Fig. 2&3, Supplementary Table S3).
Fig. 2. Blood transcriptional abnormalities in AD patients affect broad biological functions.
Blood RNAs from AD patients (n=9) and healthy controls (n=10) were assayed by genome-wide gene expression microarrays. Based on array data, 2,932 significantly altered known genes (≥2-fold, FDR<1%) were analyzed by IPA for functional associations and used to generate pie charts.
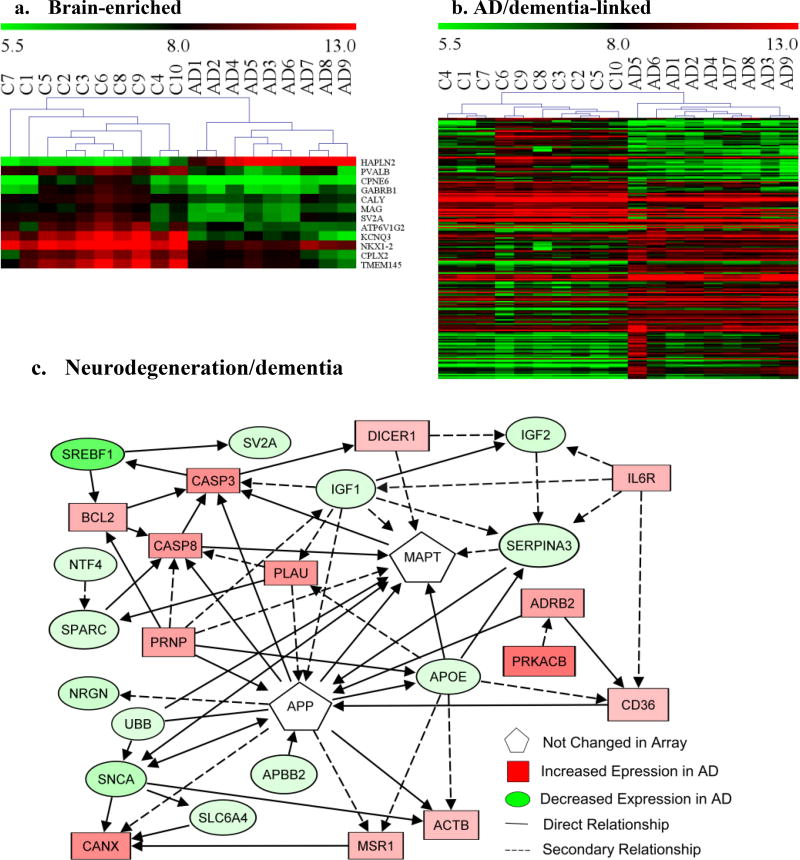
Fig. 3. Potential links of blood transcriptional profiles to the brain in AD patients.
Total RNA from peripheral blood of AD patients (n=9) and healthy controls (n=10) were assayed by genome-wide gene expression microarrays. Significantly altered genes (≥2-fold, FDR≤1%) detected by microarrays in AD blood were analyzed for functional association using IPA. Heat maps were generated using MeV analysis software: a. Brain-enriched genes (12); b. Genes (108) linked to neuropathology in AD/dementia. AD1–9: AD patients; C1–10: healthy controls; Red: up-regulated genes in AD; Green: down regulated genes in AD; color intensity: degrees of changes. c. Functional interaction network analyses using IPA on key altered blood genes that are linked to neurodegeneration/dementia: solid line: direct relationship; dash line: secondary relationship. *Gene symbols and the direction of gene alteration are shown Supplementary Table S2.
An additional 2,631 genes were shown to be significantly altered (1.5–1.99 fold, FDR≤1%), with functional association similar to that of the genes changed ≥2-fold. Given the large volume of information in our data, the following detailed results are focused mainly on the analyses of 2,932 known genes with ≥2-fold alterations (FDR≤1%). Table 3 lists the major functional categories of detected major gene alterations in AD blood. Supplementary Table S3 presents the direction of changes in all genes in each major functional categories of interest.
Table 3.
Examples of functional involvement of blood transcriptional abnormalities in AD
| Gene functions | Genes altered (≥2fold, FDR≤1%) |
|---|---|
| Neurological functions | CNR1, NRGN, SYNGR1, CPNE6, GABRB1, GRIN3A, GABRP, SLC6A4, NES, SLC6A9, SLC6A5, GAN, NAV3, DBI, CRBN, CNTNAP1, PCP4, OPRL1, NEGR1, NCAM2, CRLF1, DOCK7, INA, MOG, CNP, MAG, GMFB |
| Brain-specific/enriched | HAPLN2, PVALB, CPNE6, GABRB1, CALY, MAG, SV2A, ATP6V1G2, KCNQ3, NKX1-2, CPLX2, TMEM145, INA, *SNCB |
| Somatic-specific/enriched | AHSG, SERPINA6, UMOD, MYL4, VIL1, CASQ1, TNNI1, CLPS, CELA2B, CPB1, *HR, DIO2, LALBA |
| Diabetes-linked | ICA1, GAL, IGF1, CASP3, ADRB2, CLU, PTPN22, ADIPOR1 |
| Inflammation | CD4, CD28, CD3G, IL17F, CTLA4, ITK, LTA, GZMA, GNLY, IL15, LST1, *CD70, CD69, CXCR4, TIA1, CCR2, CCR3, CCR4, CXCR4, CD80, *CD24, IL7, CSF1, MSR1, #HLA-DQA2, CXCL16, IFNG, IL37, CD226, GZMA, GNLY, KLRF1, KIR2DL4, C9, C2, C4B, CFH, CR1, C5AR2, CD46, IRAK1, MAP3K8, SIKE, UNC93B1, CHUK, CD36, CTSE, LTA, TNFAIP8, TNFSF8, C1QTNF5, TNFRSF14, FAS, TAB2, TAB3, MAPK3, MAPK6, MAPK8, MAPK9, MAP2K3, MAP3K2, MAP3K7, MAP3K8, MAP3K11, MAP4K5, TGFB1I1, NKIRAS2, NKIRAS2, NKAPL, NKRF, IKBKB, CHUK, SIKE, ICA1, SSB, TINAGL1, PNMA5, BST2, FCN3, SP100, SLC25A16, COL4A3BP |
| Cytoskeletal | MAG, MOG, TAGLN, CLDN10, MACF1, PRNP |
| Extracellular matrix components | HSPG2, HYAL3, HAPLN2, AHSG, CSPG4, GPC4, CHI3L1, LRG1, GP1BB, TNXB, SV2A, SV2C, LAMA3, LAMB1, LAMB2, LAMC3, ELN, EMILIN3, COL4A4, COL7A1, COL11A2, COL19A1, COL27A1, AGRN, MMP9, MMP19, ADAM11, ADAM21, *ADAM22, ADAM33, PLAU, CLU |
| Ion Channels | SLC5A6, SLC5A2, SLC17A4, SLC5A3, SLC4A7, SLC8A1, SLC24A3, SCNN1D,, SLC9A7, CAMK2D, CACNG8, CACNG6, SLC24A3, CASK, KCNA3, *KCNAB2, KCNQ3, KCNC3, KCND3, KCTD12, KCNH2, KCNH8, KCNK15, KCNK16, KCNK7, KCNQ2 |
| Oxidative stress/redox | CMC1, CA5B, SLC25A24, SLC25A16, UCP3, TOMM7, MTCH1, DIABLO, MCAT, ATP5I, FIS1, GPD2, ABCD4, ABCB10, SMOX, CYP2F1, CYP2R1, CYP1B1, CYP2B6, CYP20A1, CYP4F2, CYP4F30P, CYP21A2, CYP4V2, GPX1, GPX4, GPX5, GPX2, OXR1, PON2, PRDX6, PRDX2, CYGB, PSIP1, TXNRD1, BLVRB, NAPRT1, COX6C, COX6B2, COX11, COX7A2L, COX7C, COX7B, COX16, COX17, ALDH3B1, NDUFS4, *MDH1, ADH5 |
| DNA damage/repair | ATM, ATR, BRCC3, CHEK2, MDC1, MRE11A, XRCC4, TONSL, RRM2B, ERCC2, XPA, DCLRE1A, SFR1 |
| Apoptosis/cell death | ATK1, CASP3, CASP8, MAPK3, MAPK9, FAS, KIR2DL4, PDCD7, CCNL2, SIAH2 SIAH1 |
| Cell survival/repair | SMNDC1, CCAR1, DDX3X, PRKAA1, MST4 |
| Oncogenes | KRAS, NRAS, WNT4, MYCNOS, BCL2, BCL11A |
| Tumor suppressor genes | RB1, OSM, TP53INP1, TP53RK, APC, TNK1, RERG, RRAS2, BNIP3L, BAG6 |
Total RNA from peripheral blood of AD patients (n=9) and healthy controls (n=10) were assayed by genome-wide gene expression microarrays. Functional analyses of array data were performed using IPA and GeneCards database Listed are examples of major functional involvement of dysregulated genes in the blood of AD patients. Underlined: known autoantigens. Unless indicated, all AD blood genes were altered ≥2 fold, FDR≤1%.
: Genes altered ≥2 fold, FDR≤2%.
: Genes altered ≥2 fold, FDR≤8%.
Blood transcriptional abnormalities reflect AD neuropathology
Of the 2,932 dysregulated known genes in AD blood, 1,151 were identified as genes expressed in the brain, and 157 genes were known to be involved in neurological functions, such as neuronal plasticity, synaptic transmission, neurogenesis and myelination (Table 3, Fig. 2, Supplementary Table S2&3). Of altered genes in AD blood, 14 are predominantly expressed in the brain (Fig. 3a, Table 3), and 205 were linked to neurological diseases, of which 108 have strong association with AD (Fig. 3b&c, Supplementary Table S2&3), including APBB2, SNCA, CASP3, CLU, PLAU, and APOE. While the altered blood transcripts do not include the primary gene for Aβ or Tau, they do form a cohesive pathway surrounding both (Fig. 3c). Alterations were also observed in 50 genes linked to Parkinson’s disease (PD) (Supplementary Table S2). Interestingly, we also revealed numerous dysregulated genes that are implicated as causative genes for a broad spectrum of inherited mental retardation and neurodegenerative disorders (Supplementary Table S2, Table 4). Taken together, these data support a strong blood-brain transcriptional correlation in AD.
Table 4.
Potential association of AD blood transcriptional alterations with inherited mental retardation and neurodegenerative genes
| Diseases | Genes* | Gene alteration (Fold) |
|---|---|---|
| X-linked | ||
| Fragile X mental retardation 1 | FMR1 | 3.1 |
| Rett syndrome | MECP2 | −2.0 |
| Alpha thalassemia/mental retardation syndrome X-linked (ATRX) | ATRX | 3.8 |
| X-linked adrenoleukodystrophy (X-ADL), Zellweger syndrome | ABCD2 | 2.6 |
| Coffin-Lowry syndrome (CLS). | RPS6KA3 | 3.2 |
| Mental retardation, X-linked 59 (MRX59) | AP1S2 | 2.9 |
| FG syndrome 4 | CASK | 2.2 |
| Nonsyndromic MRX (X-linked mental retardation) | IQSEC2 | −6.7 |
| Mental retardation X-linked type 95 (MRX95). | MAGT1 | 3.5 |
| Mental Retardation, X-Linked 63 [MRX63 (Alport syndrome)] | ACSL4 | 2.8 |
| Mental Retardation, X-Linked 63 [MRX63 (Alport syndrome)] | GATA1 | −6.9 |
| Menkes disease, X-linked cutis laxa, occipital horn syndrome | ATP7A | 3.2 |
| Mental retardation X-linked type 45(MRX45). | ZNF81 | 3.3 |
| Mental retardation syndromic X-linked Siderius type (MRXSSD). | PHF8 | 3.3 |
| X-linked syndromic mental retardation, Turner type (MRX-Turner) | HUWE1 | 2.1 |
| X-linked mental retardation with growth hormone deficiency (MRX-GHD) | SOX3 | 2.5 |
| Others | ||
| Amyotrophic lateral sclerosis 2 (ALS2) | ALS2CR8 | 2.8 |
| Amyotrophic lateral sclerosis 15 (ALS15) | UBQLN2 | 3.3 |
| Amyotrophic lateral sclerosis with frontal mental retardation (ALS-FTD) | C9orf72 | 4.6 |
| Leukodystrophy | EIF2B3 | 2.4 |
| Mental retardation autosomal dominant type 4 (MRD4) | KIRREL3 | −9.4 |
| Autosomal recessive nonsyndromic mental retardation (ARNSMR) | CRBN | 2.6 |
| Peroxisomal biogenesis disorders (Zellweger syndrome) | PEX13 | 2.5 |
| Machado-Joseph disease | ATXN3 | 3.1 |
| Spinocerebellar ataxia-8 (SCA8) | ATXN8 | −2.9 |
| Spinocerebellar ataxia-10 (SCA10) | ATXN10 | −2.5 |
| Spinocerebellar ataxias (SCAs) | ATXN2L | −2.0 |
| Wolf-Hirschhorn syndrome | ZNF141 | 9.2 |
| Autosomal dominant spastic paraplegia 6 | NIPA1 | 3.3 |
| Trichorhinophalangeal syndrome I (TRPS) | TRPS1 | 2.5 |
| Williams-Beuren syndrome | WBSCR17 | −2.2 |
| Imprinted in Prader-Willi syndrome (non-protein coding) | IPW | 2.1 |
| Giant axonal neuropathy | GAN | 2.6 |
| Adrenoleukodystrophy | ABCD2 | 2.6 |
| Primary microcephaly (MCPH) | CASC5 | 3.5 |
| Spastic paraplegia autosomal dominant type 31 | REEP1 | −11.7 |
| Segawa syndrome | TH | −2.6 |
| Polymicrogyria, optic nerve hypoplasia | TUBA8 | −3.4 |
| Charcot-Marie-Tooth Disease, neuropathy | GDAP1 | 2.1 |
| Familial neonatal convulsions type 2 (BFNC2) | KCNQ3 | −25.8 |
| Down syndrome | DYRK1A | 3.7 |
Total RNAs from peripheral blood of AD patients (n=9) and healthy controls (n=10) were assayed by genome-wide gene expression microarrays. Functional and disease association were analyzed using IPA and GeneCards database.
: Genes altered ≥2 fold (FDR≤1%) in AD patients.
Somatic tissue correlation with blood gene alterations in AD
In addition to the brain-enriched genes, our data indicated significant changes in somatic tissue-enriched genes in AD blood (Table 3), involving diverse tissues, including liver, kidney, heart, intestine, skeletal muscle, pancreas, skin, adrenal gland, thyroid and breast (Table 3). These data further indicate the complexity of AD and blood-tissue links in our system.
Robust inflammation in AD
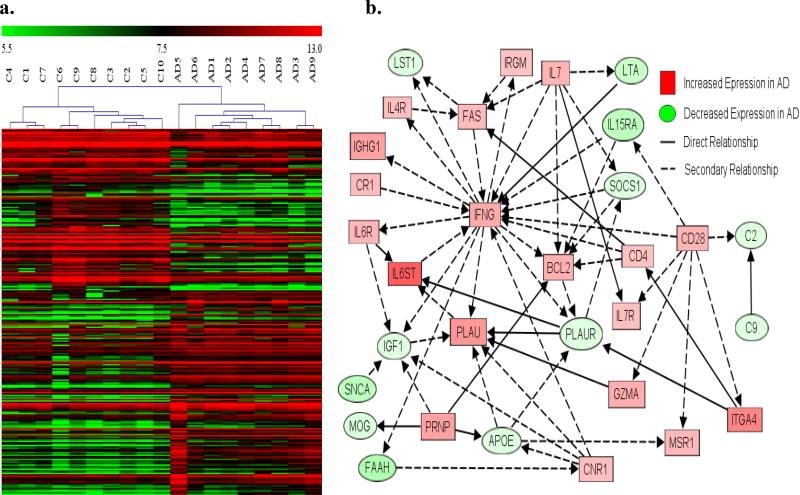
In AD patients, more than 10% of dysregulated blood genes (255) were immune genes, involving virtually all components of the immune system (Table 3, Fig. 2&4, Supplemental table S2&3). The results identify numerous cell makers and activation signals for T-cells, B-cells, macrophages, dendritic cells, NK cells, Toll-like receptors, complement system, and MHC Class II. Our data also showed abnormal transcripts in major cytokine signaling pathways, such as interferon, the tumor necrosis factor (TNF) superfamily, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸB) signaling. In addition, we observed significant increases in transcripts of many known autoantigens that are thought to be linked to autoimmune disorders (Table 3).
Fig. 4. Profound inflammation in AD.
RNA from peripheral blood of AD patients (n=9) and healthy controls (n=10) were assayed by genome-wide gene expression microarrays. Significantly altered genes (≥2-fold FDR≤1%) detected by microarrays in AD blood were analyzed for functional association, using IPA. a. Heat maps on immune gene alterations (273 gene) were generated using MeV analysis software: AD1–9: AD patients; C1–10: healthy controls; Red: up-regulated genes in AD; Green: down regulated genes in AD; Color intensity: degrees of changes. b. Functional interactions of significantly altered immune genes associated with AD neuropathology were identified using IPA: solid line: direct relationship; dash line: secondary relationship. *Gene symbols and the direction of gene alteration are shown Supplementary Table S2.
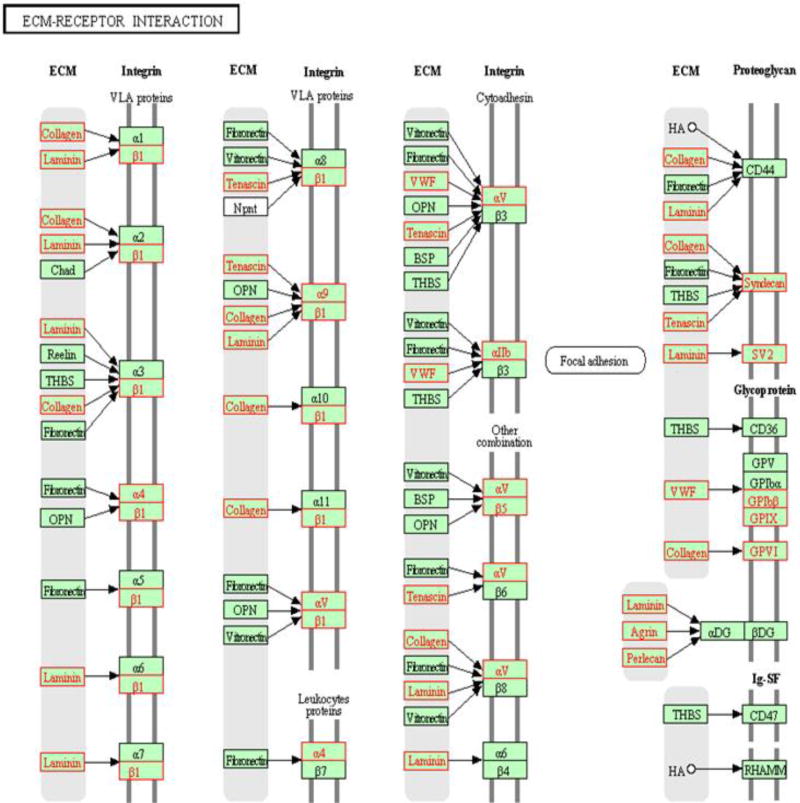
Broad cytoskeleton and extracellular matrix (ECM) dysfunction
We observed in AD blood the significant dysregulation of numerous (>155) genes associated with cytoskeletal maintenance and ECM (Table 3, Fig. 2&5, Supplementary Table S2). Importantly, many detected abnormal cytoskeleton-associated transcripts are brain-enriched, such as myelin associated protein (MAG) and myelin oligodendrocyte protein (MOG). We also detected blood transcript abnormalities involving broad ECM components, including glycoproteins, laminin, elastin, collagen, agrin, matrix metalloproteinases (MMP), plasminogen activator urokinase (PLAU), and clusterin (CLU) (Table 4, Fig. 5).
Fig. 5. Broad extracellular matrix genes dysregulation in the blood in AD patients.
RNA from peripheral blood of AD patients (n=9) and healthy controls (n=10) were assayed by genome-wide gene expression microarrays. Significant alterations in the ECM-receptor pathways (≥2-fold, FDR≤1%) were identified by IPA software, and presented by the KEGG pathway viewer. Red: significant alterations detected by arrays in AD blood; Black: unchanged.
Profound metabolic impairments
Functional analyses identified dysregulation of 219 genes in AD blood that are associated with metabolic functions, involving pathways of lipids (92), carbohydrates (66), iron (10) and others (Fig. 2, Supplementary Table S2, Fig. S1). The observed gene dysregulations affected the metabolism of fatty acids, lipids, and cholesterol (Supplemental Table S2, Fig. S1a). The carbohydrate metabolic changes in AD blood involved wide-ranging molecular interaction networks, from glycosylation to the metabolism of various sugars (glucose, fructose, lactose and glycosaminoglycans)(supplemental Table S3, Fig. S1b). Importantly, 123 transcript changes have been linked to diabetes, such as ICA1, ADIPOR1 and IGF1 (supplemental Table S3, Fig. S1c). We also observed significant transcriptional alterations in numerous genes of ion channels, involving Na+, Ca++ and K+ channels (Table 3).
Aberrant changes in oxidative stress
Our data showed numerous blood gene alterations in AD patients that are associated with mitochondrial structure and function, cytochrome P450 superfamily, and oxidation-reduction (redox) reactions (Table 3). These alterations suggest an extensive disruption in oxidative stress responses and widespread cellular damage in AD patients.
Widespread alterations in DNA damage and repair, apoptosis, cell death and survival
We also observed the dysregulation of >32 genes in the blood that are known to be involved in DNA damage and repair (Table 3, Fig. 2, Supplementary Table S2). The dysregulated genes included those involved in double-strand break, single-strand break, homologous recombination repair, nucleotide excision, cross-link, and P53/TP53-dependent DNA repair, encompassing virtually all phases of the cell cycle (Table 3). These data indicate widespread ongoing DNA damage in AD. It is therefore not surprising to see alterations in numerous genes that have been linked to various cancers, including both oncogenes and tumor suppressor genes (Table 3).
In addition, we detected changes in 165 genes associated with apoptosis and cell death (Table 3, Fig. 2, Supplementary Table S2). In parallel, significant transcriptional alterations were also seen in genes linked to cell proliferation, survival and cellular repair.
Discussion
We demonstrate here that using genome-wide gene expression microarrays allows for the decisive identification of AD-specific transcriptional abnormalities in the blood from patients with late-stage AD. The dysregulated genes observed in AD patients include numerous well characterized genes (74%) and currently unidentified genes (26%), supporting the notion that AD is much more complex than is currently understood. Importantly, blood transcript alterations reflect the complex pathophysiological status of AD, linking to known AD neuropathologies as well as previously unidentified changes. Given our limited understanding of the disease, and the extreme complexity of AD, it is therefore important to determine the role of the detected transcript abnormalities, including novel genes. Our results suggest that blood transcriptional profiling offer an invaluable tool for AD etiology and disease mechanism research. As demonstrated in this study, quality blood microarray data can be readily generated with quality RNA samples from carefully recruited comparable cohorts of patients with well-defined disease status. Minimizing individual variations by recruiting only female subjects and patients with well-defined disease severity increases the power of the study cohorts and allows the use of small sample sizes to generate significant gene profiles associated with AD.
The most important finding in this study is the demonstration of a strong blood-brain correlation in AD. Many of the significantly altered genes in AD blood are known to be critical for neurological functions, such as neuronal plasticity (SYNGR1, CPNE6), synaptic transmission (CNR1, GABRB1, SLC6A4, GRIN3A), neurogenesis (NTF4, DOCK7, NAV3, NEGR1, NES), and myelination (MOG, MAG). Under normal conditions, many genes altered in AD blood are highly or predominantly expressed in the brain, such as HAPLN2, SV2A, and CPLX2. A hyaluronan-binding protein, HAPLN2, was previously shown to play a pivotal role in the formation of the hyaluronan-associated matrix in the CNS that facilitates neuronal conduction and general structural stabilization [38–40]. SV2A is a unique modulator found in neurons and endocrine cells that mediates calcium-stimulated neurotransmitter release by regulating the expression and trafficking of the calcium sensor protein synaptotagmin [41, 42]. CPLX2 is a cytosolic protein that regulates synaptic vesicle exocytosis [43]. It is worth noting that many of the altered brain-enriched genes revealed by arrays in AD blood, including HAPLN2 (115-fold), SV2A (−2.9-fold), and CPLX2 (−4.4-fold), have not been previously linked to AD. None of these genes have a known role in blood cells, suggesting that changes in these transcript levels are a response to conditions in the CNS, either by altered transcription activity in blood or by as yet unknown mechanisms. We hypothesize that these blood transcriptional profiles reflect pathophysiological status in AD and may offer the potential of finding new targets in AD neuropathology. Further, ~7% of the total transcriptional changes in AD blood are associated with neurological diseases, and ~4% are known to be involved in interaction networks linked to neuropathology of AD and dementia, such as APBB2 (−2.2-fold), SNCA (−4.9-fold), APOE (−2.5), CASP3 (3.8-fold), CLU (−5.2-fold), and PLAU (3.5-fold)[44–46]. This finding is supported by a previously published study using a human array probing 6,424 unique genes in peripheral blood monocytes (PBMC)[30], suggesting that blood expression profiles may reflect molecular events germane to AD neurophysiology. Furthermore, our data show that SNCA was up-regulated while SNCB was down-regulated in AD blood. While SNCA aggregates represent a major component of Lewy body inclusions in PD and the major non-Aβ component of Aβ plaques in AD [47], SNCB may act as a regulator of the SNCA aggregation process, and has not been found in either Lewy bodies in PD or in Aβ plaque in AD. Given the functional differences and abundance of both SNCA and SNCB in the brain, their opposite transcriptional alterations in AD blood could very well be associated with neuropathology in the brain, though it is currently unclear how blood and brain transcripts correlate.
Strikingly, 43 significantly dysregulated genes in AD blood in this study are causative genes for a broad spectrum of inherited mental retardation (MR) and neurodegenerative disorders. In general, patients with these diseases have a mutation that causes defects in a specific gene, though the majority of these genes were up-regulated in AD blood in this study. It is unknown whether these genes were up- or down-regulated in the brain of our AD patient subjects. Our recently published data in a mouse model of a neuropathic lysosomal storage disease showed an inverse blood-brain correlation in multiple genes that are key components of neurodegeneration.[48] One thing in common between late-stage AD and inherited neurological disorders is severe cognitive defects with neurodegeneration. It is possible that the cognitive defects and neurodegeneration in these diseases are a consequence of impairments in similar pathways triggered by different causes. Therefore, investigating the functional interaction networks among these genes may have the potential for the identification of critical pathways up-stream of Aβ aggregation and tauopathy. Taken together, these data support the hypothesis that blood transcriptional profiles reflect the complex neuropathophysiological status in AD.
We demonstrate here that blood transcripts reflect biopathophysiological status throughout the system, not only in the CNS but also in virtually all tissues, as supported by the dysregulation of somatic enriched genes in AD blood. These gene dysregulations involved liver (ASHG, SERPINA6), kidney (UMOD), heart (MYL4), intestine (VIL1), skeletal muscle (CASQ, TNN1), pancreas (CLPS, CELA2B, CPB1), skin (HR), thyroid (DIO2) and breast (LALBA). Multiple risk factors have been linked to AD, including cardiovascular diseases, hypertension, high cholesterol, diabetes, obesity, smoking and traumatic brain injuries.[6–10] However, the detailed associations between the neurological and somatic disorders in AD remain unknown. We believe that blood transcriptional profiling may provide a powerful tool for better understanding the involvement of somatic abnormalities in disease progression of AD.
This study also demonstrated a profound inflammatory status in AD patients, involving virtually all components of the immune system, including T-cells, B-cells, macrophages, dendritic cells, complement, toll-like receptors, and cytokines. More interestingly, we detected the dysregulation of multiple known autoantigens, which are linked to autoimmune diseases, such as ICA1 to insulin-dependent diabetes mellitus[49], SSB to Sjogren syndrome [50], TINAGL1 to tubulointerstitial Nephritis [51], SP100 to primary biliary cirrhosis[52], and SLC25A16 to Graves disease [53]. Neuroinflammation has been reported to play a role in AD neuropathology[54, 55]. Our data here demonstrates broad involvement of immune system in AD, though it is unclear how these blood immune transcript alterations correlate to the inflammatory state of the CNS and somatic tissues. However, the blood RNA in this study was from nucleated blood cells, predominantly white blood cells (WBCs). Functionally, WBCs are mixed circulating immune cells. We therefore hypothesize that the broad blood transcriptional abnormalities observed in AD patients in this study are a consequence of immune surveillance under complex disease conditions. Thus, the immune system may respond not only to classical insults (infections, injuries and cell/protein/DNA damage), but may also sense and report molecular abnormalities in tissues. More research is needed to test this hypothesis and identify the specific immune cells that are responsible.
Our finding of profound dysfunction of cytoskeletal and ECM interaction networks, support severe damages to cell morphology, homeostasis and function in AD. These involve virtually all major ECM components, including both structural and soluble ECM proteins, indicating broad ECM dysfunction. It is unclear whether and how the blood ECM gene alterations relate to AD neuropathology. However, ECM abnormalities have previously been reported in AD postmortem brains, including the presence of all heparan sulfate proteoglycans (HSPGs) in the hallmark extracellular Aβ deposits, and the overexpression of MMPs (MMP1, 2, 3, 9, 10 and 12)[40]. The ECM is the composite material around and between cells, constituting the microenvironment for all cells outside of circulation. Blood cells are circulating cells, and are therefore considered to lack ECM. Therefore, the AD blood ECM transcript abnormalities reported herein may signal pathological changes in tissues, including the brain. This is supported by our novel results of the dysregulation in HAPLN2, SV2A, PLAU and CLU in AD blood. As discussed above, HAPLN2 was the second most up-regulated transcript in our data set, and while neither HAPLN2 nor SV2A has been linked to AD, PLAU is involved in degradation of ECM and has been linked to late-onset AD.[56, 57] CLU is a secreted versatile ECM chaperone that is highly expressed in the brain and was shown to co-localize with fibrillar Aβ plaque in AD.[58] It is therefore likely that the blood ECM transcript alterations in this study do indeed reflect profound ECM impairment in the CNS and also possibly in somatic tissues in AD.
Blood transcriptional profiles in this study indicate extensively impaired metabolic functions in AD patients, involving virtually all aspects of metabolism, including carbohydrates, lipids, cholesterol, proteins and iron metabolism. Importantly, such broad metabolic dysfunctions have previously been linked to AD neuropathology [14–16, 59–61]. It is particularly noteworthy that our results showed alteration of 123 genes that are associated with diabetes, given that type 2 diabetes has been considered a key risk factor of AD [7, 62, 63]. The AD-diabetes link is further supported by our finding of abnormal transcription in ICA1, an autoantigen in insulin-dependent diabetes mellitus [64]. While it is unclear what triggered these metabolic abnormalities, our results suggest that metabolic impairments in AD are much more profound than previously reported in a study probing 3,240 genes in blood lymphocytes[65] and another targeting 6,424 genes in PBMC.[30]
Furthermore, we demonstrate widespread transcript abnormalities affecting virtually all ion channels, including Ca++, Na+, K+ channels and their ligands. Ion channels are critical in many biological processes and are prominent components of nervous system underlying synaptic signal transmission and conduction. It is therefore possible that ion channel dysregulation in the blood may correlate with the loss of neurological function and complex tissue damage in AD. Previous studies have indicated that the impairment of Na+ and Ca++ signaling may contribute to neuronal dysfunction and cognitive deficits in AD [66, 67]. Further investigation is needed to determine the association between ion channel dysregulation and AD neuropathology.
Given the robust inflammation, profound metabolic dysfunction and broad ECM impairments found in AD blood in this study, it is not surprising to see such abundant molecular evidence of oxidative stress, widespread DNA damage/repair and cell death. Correlative changes associated with these impairments have been linked to AD neuropathology [68], and a few of them have been previously identified in PBMC from AD patients using arrays probing selected genes [30]. These data further demonstrate that the molecular and cellular damage in AD are much more broad and severe than previously thought, and likely affect many different tissues during the later disease stages.
It is worth noting that in this study 26% of the altered transcripts in AD blood were that whose functions are currently unidentified. Indeed, the functions of the most up-regulated (195-fold) and the most down-regulated (−143-fold) trancripts are unknown. Because we know very little about the etiology and disease mechanisms of AD, it is important to further investigate the biological functions of these understudied transcripts and reveal their potential role in AD.
In summary, using genome-wide transcriptional profiling of the blood, we demonstrate strong blood-brain associations and an extremely complex pathophysiological status in AD, involving not only the CNS but also the somatic tissues. Importantly, the majority of the detected gene dysregulations have not been linked to AD. While the mechanisms of the observed blood-brain and blood-tissue links are unclear, we believe this genome-wide blood transcriptional profiling approach may significantly advance our understanding of AD by enabling the detection of numerous ongoing previously unknown abnormalities beyond Aβ aggregation and tauopathy.
Supplementary Material
Acknowledgments
We would like to thank all patients, healthy control individuals and their families for their contribution to this study, and the Biomedical Genomics Core at NCHRI for providing microarray services. Funding: BN, FJD, DAM, AM and HF were supported by a translational research grant from NIN/NINDS (U01NS069626). DM was supported by grants from NIH (R01CA172713, R21NS081173).
Footnotes
Author contributions: HF and DMM were responsible for study design, data analyses, sample acquisition and manuscript preparation. DWS was responsible for clinical aspect of this study and recruitment of study subjects. NS performed informed consents and sample acquisition. BN conducted sample process, qRT-PCR, data analyses and participated manuscript preparation. FJD participated in sample processing, data analyses and manuscript preparation. PW and late DN performed array assays and data analyses. DAM and AM contributed to sample analyses. All authors contributed to manuscript preparation.
Competing interest: The authors declare no conflict of interest.
Note: The entire array data set will be submitted to NCBI Gene Expression Omnibus (GEO) database upon the publication of this manuscript.
References
- 1.Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 3.Waring SC, Rosenberg RN. Genome-wide association studies in Alzheimer disease. Arch Neurol. 2008;65:329–334. doi: 10.1001/archneur.65.3.329. [DOI] [PubMed] [Google Scholar]
- 4.Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martins IJ, Hone E, Foster JK, Sunram-Lea SI, Gnjec A, Fuller SJ, Nolan D, Gandy SE, Martins RN. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer's disease and cardiovascular disease. Mol Psychiatry. 2006;11:721–736. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Lara JM, Aguilar-Navarro S, Gutierrez-Robledo LM, Avila-Funes JA. The metabolic syndrome, diabetes, and Alzheimer's disease. Rev Invest Clin. 2010;62:343–349. [PubMed] [Google Scholar]
- 8.Anstey KJ, von Sanden C, Salim A, O'Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166:367–378. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- 9.Lye TC, Shores EA. Traumatic brain injury as a risk factor for Alzheimer's disease: a review. Neuropsychol Rev. 2000;10:115–129. doi: 10.1023/a:1009068804787. [DOI] [PubMed] [Google Scholar]
- 10.Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66:343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg PB. Clinical aspects of inflammation in Alzheimer's disease. Int Rev Psychiatry. 2005;17:503–514. doi: 10.1080/02646830500382037. [DOI] [PubMed] [Google Scholar]
- 12.Medeiros R, Laferla FM. Astrocytes: Conductors of the Alzheimer disease neuroinflammatory symphony. Exp Neurol. 2013;239:133–138. doi: 10.1016/j.expneurol.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Biasini E, Turnbaugh JA, Unterberger U, Harris DA. Prion protein at the crossroads of physiology and disease. Trends Neurosci. 2012;35:92–103. doi: 10.1016/j.tins.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dasuri K, Zhang L, Keller JN. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic Biol Med. 2012 doi: 10.1016/j.freeradbiomed.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Orth M, Bellosta S. Cholesterol: its regulation and role in central nervous system disorders. Cholesterol. 2012;2012:292598. doi: 10.1155/2012/292598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah K, Desilva S, Abbruscato T. The Role of Glucose Transporters in Brain Disease: Diabetes and Alzheimer’s Disease. Int J Mol Sci. 2012;13:12629–12655. doi: 10.3390/ijms131012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotzbauer PT, Cairns NJ, Campbell MC, Willis AW, Racette BA, Tabbal SD, Perlmutter JS. Pathologic Accumulation of alpha-Synuclein and Abeta in Parkinson Disease Patients With Dementia. Arch Neurol. 2012:1–6. doi: 10.1001/archneurol.2012.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Van Deerlin V, Lee VM, Leverenz JB, Montine TJ, Duda JE, Hurtig HI, Trojanowski JQ. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012;72:587–598. doi: 10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomperts SN, Locascio JJ, Marquie M, Santarlasci AL, Rentz DM, Maye J, Johnson KA, Growdon JH. Brain amyloid and cognition in Lewy body diseases. Mov Disord. 2012;27:965–973. doi: 10.1002/mds.25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohmi K, Zhao HZ, Neufeld EF. Defects in the medial entorhinal cortex and dentate gyrus in the mouse model of Sanfilippo syndrome type B. PLoS One. 2011;6:e27461. doi: 10.1371/journal.pone.0027461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamano K, Hayashi M, Shioda K, Fukatsu R, Mizutani S. Mechanisms of neurodegeneration in mucopolysaccharidoses II and IIIB: analysis of human brain tissue. Acta Neuropathol. 2008;115:547–559. doi: 10.1007/s00401-007-0325-3. [DOI] [PubMed] [Google Scholar]
- 22.Corbett A, Pickett J, Burns A, Corcoran J, Dunnett SB, Edison P, Hagan JJ, Holmes C, Jones E, Katona C, Kearns I, Kehoe P, Mudher A, Passmore A, Shepherd N, Walsh F, Ballard C. Drug repositioning for Alzheimer's disease. Nat Rev Drug Discov. 2012;11:833–846. doi: 10.1038/nrd3869. [DOI] [PubMed] [Google Scholar]
- 23.Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- 24.Kong SW, Collins CD, Shimizu-Motohashi Y, Holm IA, Campbell MG, Lee IH, Brewster SJ, Hanson E, Harris HK, Lowe KR, Saada A, Mora A, Madison K, Hundley R, Egan J, McCarthy J, Eran A, Galdzicki M, Rappaport L, Kunkel LM, Kohane IS. Characteristics and predictive value of blood transcriptome signature in males with autism spectrum disorders. PLoS One. 2012;7:e49475. doi: 10.1371/journal.pone.0049475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glatt SJ, Stone WS, Nossova N, Liew CC, Seidman LJ, Tsuang MT. Similarities and differences in peripheral blood gene-expression signatures of individuals with schizophrenia and their first-degree biological relatives. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:869–887. doi: 10.1002/ajmg.b.31239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumarasinghe N, Tooney PA, Schall U. Finding the needle in the haystack: a review of microarray gene expression research into schizophrenia. Aust N Z J Psychiatry. 2012;46:598–610. doi: 10.1177/0004867412442405. [DOI] [PubMed] [Google Scholar]
- 27.Pajer K, Andrus BM, Gardner W, Lourie A, Strange B, Campo J, Bridge J, Blizinsky K, Dennis K, Vedell P, Churchill GA, Redei EE. Discovery of blood transcriptomic markers for depression in animal models and pilot validation in subjects with early-onset major depression. Transl Psychiatry. 2012;2:e101. doi: 10.1038/tp.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheubert L, Lustrek M, Schmidt R, Repsilber D, Fuellen G. Tissue-based Alzheimer gene expression markers - comparison of multiple machine learning approaches and investigation of redundancy in small biomarker sets. BMC Bioinformatics. 2012;13:266. doi: 10.1186/1471-2105-13-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva AR, Grinberg LT, Farfel JM, Diniz BS, Lima LA, Silva PJ, Ferretti RE, Rocha RM, Filho WJ, Carraro DM, Brentani H. Transcriptional alterations related to neuropathology and clinical manifestation of Alzheimer's disease. PLoS One. 2012;7:e48751. doi: 10.1371/journal.pone.0048751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maes OC, Xu S, Yu B, Chertkow HM, Wang E, Schipper HM. Transcriptional profiling of Alzheimer blood mononuclear cells by microarray. Neurobiol Aging. 2007;28:1795–1809. doi: 10.1016/j.neurobiolaging.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Maes OC, Schipper HM, Chertkow HM, Wang E. Methodology for discovery of Alzheimer's disease blood-based biomarkers. J Gerontol A Biol Sci Med Sci. 2009;64:636–645. doi: 10.1093/gerona/glp045. [DOI] [PubMed] [Google Scholar]
- 32.Calciano MA, Zhou W, Snyder PJ, Einstein R. Drug treatment of Alzheimer's disease patients leads to expression changes in peripheral blood cells. Alzheimers Dement. 2010;6:386–393. doi: 10.1016/j.jalz.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Fehlbaum-Beurdeley P, Jarrige-Le Prado AC, Pallares D, Carriere J, Guihal C, Soucaille C, Rouet F, Drouin D, Sol O, Jordan H, Wu D, Lei L, Einstein R, Schweighoffer F, Bracco L. Toward an Alzheimer's disease diagnosis via high-resolution blood gene expression. Alzheimers Dement. 2010;6:25–38. doi: 10.1016/j.jalz.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Chen KD, Chang PT, Ping YH, Lee HC, Yeh CW, Wang PN. Gene expression profiling of peripheral blood leukocytes identifies and validates ABCB1 as a novel biomarker for Alzheimer's disease. Neurobiol Dis. 2011;43:698–705. doi: 10.1016/j.nbd.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 35.Rye PD, Booij BB, Grave G, Lindahl T, Kristiansen L, Andersen HM, Horndalsveen PO, Nygaard HA, Naik M, Hoprekstad D, Wetterberg P, Nilsson C, Aarsland D, Sharma P, Lonneborg A. A novel blood test for the early detection of Alzheimer's disease. J Alzheimers Dis. 2011;23:121–129. doi: 10.3233/JAD-2010-101521. [DOI] [PubMed] [Google Scholar]
- 36.Smyth GK, Speed T. Normalization of cDNA microarray data. Methods. 2003;31:265–273. doi: 10.1016/s1046-2023(03)00155-5. [DOI] [PubMed] [Google Scholar]
- 37.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 38.Oohashi T, Hirakawa S, Bekku Y, Rauch U, Zimmermann DR, Su WD, Ohtsuka A, Murakami T, Ninomiya Y. Bral1, a brain-specific link protein, colocalizing with the versican V2 isoform at the nodes of Ranvier in developing and adult mouse central nervous systems. Mol Cell Neurosci. 2002;19:43–57. doi: 10.1006/mcne.2001.1061. [DOI] [PubMed] [Google Scholar]
- 39.Bekku Y, Vargova L, Goto Y, Vorisek I, Dmytrenko L, Narasaki M, Ohtsuka A, Fassler R, Ninomiya Y, Sykova E, Oohashi T. Bral1: its role in diffusion barrier formation and conduction velocity in the CNS. J Neurosci. 2010;30:3113–3123. doi: 10.1523/JNEUROSCI.5598-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soleman S, Filippov MA, Dityatev A, Fawcett JW. Targeting the neural extracellular matrix in neurological disorders. Neuroscience. 2013;253:194–213. doi: 10.1016/j.neuroscience.2013.08.050. [DOI] [PubMed] [Google Scholar]
- 41.Nowack A, Yao J, Custer KL, Bajjalieh SM. SV2 regulates neurotransmitter release via multiple mechanisms. Am J Physiol Cell Physiol. 2010;299:C960–967. doi: 10.1152/ajpcell.00259.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, Scheller RH, Chavkin C, Bajjalieh SM. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A) Proc Natl Acad Sci U S A. 1999;96:15268–15273. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMahon HT, Missler M, Li C, Sudhof TC. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 44.Berchtold NC, Coleman PD, Cribbs DH, Rogers J, Gillen DL, Cotman CW. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer's disease. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao PJ, Zhu M, Pyun EI, Brooks AI, Therianos S, Meyers VE, Coleman PD. Defects in expression of genes related to synaptic vesicle trafficking in frontal cortex of Alzheimer's disease. Neurobiol Dis. 2003;12:97–109. doi: 10.1016/s0969-9961(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 46.Barr TL, Alexander S, Conley Y. Gene expression profiling for discovery of novel targets in human traumatic brain injury. Biol Res Nurs. 2011;13:140–153. doi: 10.1177/1099800410385671. [DOI] [PubMed] [Google Scholar]
- 47.Kim S, Seo JH, Suh YH. Alpha-synuclein, Parkinson's disease, and Alzheimer's disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S9–13. doi: 10.1016/j.parkreldis.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Naughton BJ, Duncan FJ, Murrey D, Ware T, Meadows A, McCarty DM, Fu H. Amyloidosis, Synucleinopathy, and Prion Encephalopathy in a Neuropathic Lysosomal Storage Disease: The CNS-Biomarker Potential of Peripheral Blood. PLoS One. 2013;8:e80142. doi: 10.1371/journal.pone.0080142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaedigk R, Karges W, Hui MF, Scherer SW, Dosch HM. Genomic organization and transcript analysis of ICAp69, a target antigen in diabetic autoimmunity. Genomics. 1996;38:382–391. doi: 10.1006/geno.1996.0641. [DOI] [PubMed] [Google Scholar]
- 50.Barcellos KS, Nonogaki S, Enokihara MM, Teixeira MS, Andrade LE. Differential expression of Ro/SSA 60 kDa and La/SSB, but not Ro/SSA 52 kDa, mRNA and protein in minor salivary glands from patients with primary Sjogren's syndrome. J Rheumatol. 2007;34:1283–1292. [PubMed] [Google Scholar]
- 51.Nelson TR, Charonis AS, McIvor RS, Butkowski RJ. Identification of a cDNA encoding tubulointerstitial nephritis antigen. J Biol Chem. 1995;270:16265–16270. doi: 10.1074/jbc.270.27.16265. [DOI] [PubMed] [Google Scholar]
- 52.Worman HJ, Courvalin JC. Antinuclear antibodies specific for primary biliary cirrhosis. Autoimmun Rev. 2003;2:211–217. doi: 10.1016/s1568-9972(03)00013-2. [DOI] [PubMed] [Google Scholar]
- 53.Rossi E, Zarrilli R, Zuffardi O. Regional assignment of the gene coding for a human Graves' disease autoantigen to 10q21.3–q22.1. Hum Genet. 1993;90:653–654. doi: 10.1007/BF00202485. [DOI] [PubMed] [Google Scholar]
- 54.Griffin WS. Neuroinflammatory cytokine signaling and Alzheimer's disease. N Engl J Med. 2013;368:770–771. doi: 10.1056/NEJMcibr1214546. [DOI] [PubMed] [Google Scholar]
- 55.Griffin WS. Alzheimer's - Looking beyond plaques. F1000 Med Rep. 2011;3:24. doi: 10.3410/M3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riemenschneider M, Konta L, Friedrich P, Schwarz S, Taddei K, Neff F, Padovani A, Kolsch H, Laws SM, Klopp N, Bickeboller H, Wagenpfeil S, Mueller JC, Rosenberger A, Diehl-Schmid J, Archetti S, Lautenschlager N, Borroni B, Muller U, Illig T, Heun R, Egensperger R, Schlegel J, Forstl H, Martins RN, Kurz A. A functional polymorphism within plasminogen activator urokinase (PLAU) is associated with Alzheimer's disease. Hum Mol Genet. 2006;15:2446–2456. doi: 10.1093/hmg/ddl167. [DOI] [PubMed] [Google Scholar]
- 57.Pesaresi M, Batelli S, Prato F, Polito L, Lovati C, Scarpini E, Quadri P, Mariani C, Albani D, Forloni G. The urokinase-type plasminogen activator polymorphism PLAU_1 is a risk factor for APOE-epsilon4 non-carriers in the Italian Alzheimer's disease population and does not affect the plasma Abeta(1–42) level. Neurobiol Dis. 2007;25:609–613. doi: 10.1016/j.nbd.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 58.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, Heun R, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O'Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell Biochem. 2008;49:241–268. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hare D, Ayton S, Bush A, Lei P. A delicate balance: Iron metabolism and diseases of the brain. Front Aging Neurosci. 2013;5:34. doi: 10.3389/fnagi.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Correia SC, Santos RX, Carvalho C, Cardoso S, Candeias E, Santos MS, Oliveira CR, Moreira PI. Insulin signaling, glucose metabolism and mitochondria: major players in Alzheimer's disease and diabetes interrelation. Brain Res. 2012;1441:64–78. doi: 10.1016/j.brainres.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 62.Vagelatos NT, Eslick GD. Type 2 Diabetes as a Risk Factor for Alzheimer's Disease: The Confounders, Interactions, and Neuropathology Associated With This Relationship. Epidemiol Rev. 2013 doi: 10.1093/epirev/mxs012. [DOI] [PubMed] [Google Scholar]
- 63.Ristow M. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med (Berl) 2004;82:510–529. doi: 10.1007/s00109-004-0552-1. [DOI] [PubMed] [Google Scholar]
- 64.Karges W, Pietropaolo M, Ackerley CA, Dosch HM. Gene expression of islet cell antigen p69 in human, mouse, and rat. Diabetes. 1996;45:513–521. doi: 10.2337/diab.45.4.513. [DOI] [PubMed] [Google Scholar]
- 65.Kalman J, Kitajka K, Pakaski M, Zvara A, Juhasz A, Vincze G, Janka Z, Puskas LG. Gene expression profile analysis of lymphocytes from Alzheimer's patients. Psychiatr Genet. 2005;15:1–6. doi: 10.1097/00041444-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Corbett BF, Leiser SC, Ling HP, Nagy R, Breysse N, Zhang X, Hazra A, Brown JT, Randall AD, Wood A, Pangalos MN, Reinhart PH, Chin J. Sodium channel cleavage is associated with aberrant neuronal activity and cognitive deficits in a mouse model of Alzheimer's disease. J Neurosci. 2013;33:7020–7026. doi: 10.1523/JNEUROSCI.2325-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woods NKPJ. In: Advances in Experimental Medicine and Biology. Islam M, editor. Springer Sci.; 2012. pp. 1193–1217. [DOI] [PubMed] [Google Scholar]
- 68.Dasuri K, Zhang L, Keller JN. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic Biol Med. 2013;62:170–185. doi: 10.1016/j.freeradbiomed.2012.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.