Abstract
Damage to DNA from the metabolites of drugs and pollutants constitutes a major human toxicity pathway known as genotoxicity. Metabolites can react with metal ions and NADPH to oxidize DNA or participate in SN2 reactions to form covalently linked adducts with DNA bases. Guanines are the main DNA oxidation sites, and 8-oxo-7,8-dihydro-2-deoxyguanosine (8-oxodG) is the initial product. Here we describe a novel electrochemiluminescent (ECL) microwell array that produces metabolites from test compounds and measures relative rates of DNA oxidation and DNA adduct damage. In this new array, films of DNA, metabolic enzymes, and an ECL metallopolymer or complex assembled in microwells on a pyrolytic graphite wafer are housed in dual microfluidic chambers. As reactant solution passes over the wells, metabolites form and can react with DNA in the films to form DNA adducts. These adducts are detected by ECL from a RuPVP polymer that uses DNA as a coreactant. Aryl amines also combine with Cu2+ and NADPH to form reactive oxygen species (ROS) that oxidize DNA. The resulting 8-oxodG was detected selectively by ECL-generating bis(2,2′-bipyridine)-(4-(1,10-phenanthrolin-6-yl)-benzoic acid)Os(II). DNA/enzyme films on magnetic beads were oxidized similarly, and 8-oxodG determined by LC/MS/MS enabled array standardization. The array limit of detection for oxidation was 720 8-oxodG per 106 nucleobases. For a series of aryl amines, metabolite-generated DNA oxidation and adduct formation turnover rates from the array correlated very well with rodent 1/TD50 and Comet assay results.
Graphical Abstract
Human metabolic conversion of environmental pollutants and drugs by cytochrome P450s (cyt P450) and other enzymes can lead to chemically reactive metabolites in a process called bioactivation. Metabolites may react with DNA to form covalent nucleobase adducts that can also lead to abasic sites and strand breaks.1,2 Some redox active metabolites in conjunction with metal ions and NADPH promote formation of reactive oxygen species (ROS) that oxidize DNA, forming primary product 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) on the DNA strands.3,4 These processes constitute major toxicity pathways known as genotoxicity.
Predicting the toxicity of drug and environmental chemical candidates early in the discovery process is vital for bringing new products to market at reasonable cost.5–7 Covalent DNA adducts and 8-oxodG are biomarkers for DNA damage, and detecting them can provide important input for the safety evaluation of new chemicals and drugs.8–10 In vitro screening assays that uncover possible genotoxic chemistry pathways are valuable tools to complement toxicity bioassays to predict drug and pollutant toxicity.11,12
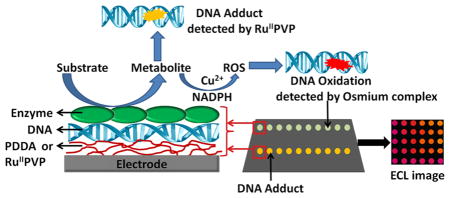
Previously, we reported a microfluidic voltammetric genotoxicity screening array that detects DNA adducts and oxidation by square wave voltammetry (SWV) in an eight sensor format.13 Electrochemiluminescence (ECL) using microwell-printed pyrolytic graphite (PG) arrays provides a simpler technology than voltammetry that allows larger arrays and simple camera detection.14 ECL does not require individually addressable sensors and detection electronics as in voltammetry. We have shown that oxidized DNA can be detected using ECL-producing osmium complexes with electrocatalytic pathways that employ 8-oxodG in DNA as a coreactant.15,16 Osmium complexes selectively oxidize 8-oxoG in the presence of guanine because of its low oxidation potential as shown in Scheme S1A. Ruthenium complexes can similarly be used to detect DNA adducts by ECL using intact guanine as a coreactant.11,17 Here on applying a potential of 1.25 V RuIIPVP is oxidized to RuIIIPVP, which then oxidizes intact guanines on the DNA, yielding an electronically excited RuIIPVP* that emits ECL (Scheme S1B). Mass spectrometry on similar reactions revealed that slopes of ECL increase vs reaction times are proportional to relative formation rates of 8-oxodG or specific nucleobase adducts, showing that ECL slopes monitor relative rates of the DNA reactions.11
In this paper, we describe a new ECL array with 30 microwells that detects both oxidized and metabolite-adducted DNA in a two-channel microfluidic format. These new arrays are evaluated with four aryl amines whose metabolites induce both DNA oxidation and nucleobase adduction. Detection of both forms of DNA damage and evaluation of the influence of bioactivation by different human cyt P450s were demonstrated for these aryl amines.
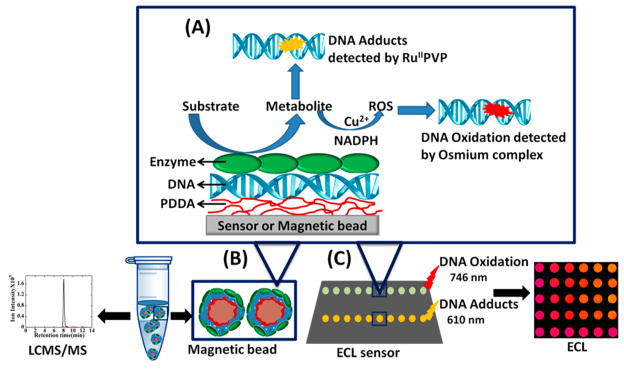
Microwells on the array chip contain films of DNA and metabolic enzymes grown by alternate layer-by-layer (LbL) electrostatic assembly.11 Ruthenium(II) poly(vinylpyridine), {[Ru(bpy)2(PVP)10]2+, RuPVP} was incorporated into the LbL films to detect DNA adducts and strand breaks in microwells of the first channel.11,14,18 Bis(2,2′-bipyridine)-(4-(1,10-phenanthrolin-6-yl)-benzoic acid)Os(II) {[Os-(bpy)2(phen-benz-COOH)]2+}16 was used to detect DNA oxidation in microwells in the second channel (Scheme 1). We used enzyme/DNA films on magnetic biocolloid reactor beads11 to oxidize DNA and determine 8-oxodG content by LC/MS/MS for array calibration.
Scheme 1. Array Strategy for Screening Genotoxic Pathways Using (A) DNA/Enzyme Films, (B) Biocolloid Reactors, and (C) ECL Arrays#.
# Metabolites are generated in DNA/enzyme films from reactant solutions by applying voltage to an array or by an NADPH regenerating system with the beads to activate cyt P450s. Reactive metabolites that were formed reacted with DNA to give DNA adducts, and they were detected by ECL from RuPVP. Oxidative DNA damage from a Cu2+-metabolite mediated redox pathway was detected by ECL using [Os(bpy)2(phen-benz-COOH)]2+. Calibration for oxidation product 8-oxodG was established by using LC/MS/MS generated standards.
4-Aminobiphenyl (4ABP), 2-aminofluorene (AF), o-anisidine (anisidine), and 2-naphthylamine (NA) were examined as test compounds. Metabolites of aromatic amines19,20 are key players in DNA adduct formation and oxidation. For oxidations, metabolites mediate generation of ROS such as superoxide and hydroxyl radicals via a Cu2+ mediated redox pathway involving the reductant NADPH.21–24 A genotoxicity pathway profile identifying enzymes important for bioactivation was developed and compared using liver microsomes, cyt P450 1A2 and cyt P450 1A1 supersomes, and N-acetyl transferase1 (NAT), which
EXPERIMENTAL SECTION
Chemicals and Materials
Ruthenium metallopolymer [Ru(bpy)2(PVP)10]2+ (RuIIPVP (bpy = 2,2-bipyridyl); PVP = 2+ poly(4-vinylpyridine)) and [Os(bpy)2(phen-benz-COOH)] (bis(2,2′-bipyridine)-(4-(1,10-phenanthrolin-6-yl)-benzoic acid)Os(II) were synthesized and characterized as described previously.16,17 Pyrolytic graphite (PG, 4.5 × 2.5 × 0.3 cm) was from the Graphite store (http://www.graphitestore.com). Sources of other chemicals are in the Supporting Information (SI) file.
Microfluidic Reactor
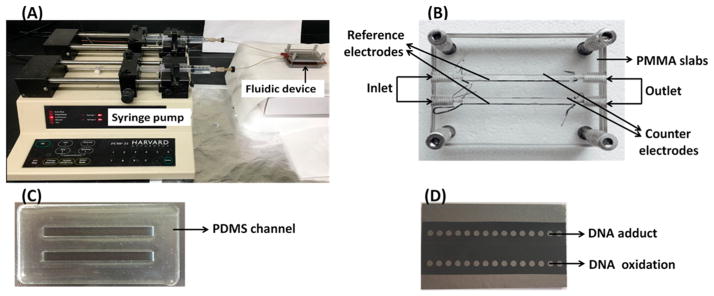
The fluidic system features two molded polydimethylsiloxane (PDMS) channels positioned directly above a PG chip featuring two rows of 15 printed microwells. Microwells were printed from toner ink using a laser printer onto glossy paper as neat transferred to the PG, providing 2 mm diameter wells 10–15 nm deep.26 The PDMS channel and PG chip (Schemes 2C, D) were positioned between two machined poly(methyl methacrylate) (PMMA) plates and screwed together to provide two sealed microfluidic channels (Scheme 2). Each microwell can hold a 1.0 ± 0.1 μL aqueous droplet, and the toner serves as a hydrophobic barrier to prevent cross-contamination during film deposition. Fluidic channels (Scheme 2C) are 4 mm wide, 4 cm long, and can hold 202 μL of volume. The top PMMA plate is connected to 0.2 mm i.d. polyether ether ketone (PEEK) tubing for inlets and outlets. The Ag/AgCl reference and Pt counter electrode wires run along lengths of both the channels on the top PMMA plate so that they both surround all microwells symmetrically to minimize crosstalk to negligible levels (Scheme 2B). A copper plate placed beneath the PG chip provided an electrical connection. The voltage for cyt P450 activation and ECL measurement was applied using a CHI 660 electrochemical analyzer.
Scheme 2. Photographs of Fluidic Array Featuring Two PDMS Channels in a PMMA Housing∇.
∇ Ag/AgCl reference and Pt counter wire electrodes are symmetrically placed along the lengths of the channels. (A) Fluidic reactor shown with dual syringe pump to deliver buffer and reactants solutions to the array. (B) Fluidic reactor showing channels and auxiliary electrodes. (C) PDMS slab showing two fluidic channels. (D) Pyrolytic graphite (PG) chip showing array of two rows of microwells.
Film Construction and Characterization
LbL films with individual layers deposited in the order (PDDA/DNA)3/cyt P450 source/PDDA/DNA were formed one layer at a time by depositing 1 μL droplets of PDDA, DNA, or enzyme solutions in microwells sequentially with washing between each deposition. Composition of solutions and deposition times were optimized previously to achieve steady state adsorption for each layer.17,18,27 Enzymes sources were rat liver microsomes (RLM), human liver microsomes (HLM), and supersomes cyt P450 1A2 (1A2), cyt P450 1A1 (1A1), and cyt P450 2E1 (2E1). The films are abbreviated as (PDDA/DNA)3/cyt P450 source/PDDA/DNA, with enzyme sources as RLM, HLM or 1A2, 1A1, 2E1. Specific films were grown in selected microwells on one row of the sensor chip. It was not possible to include ECL generating Os(bpy)2(phen-benz-COOH)]2+ in the detection layer to detect DNA oxidation and simultaneously maintain full enzyme and ECL activity, so this ECL dye was added in the solution phase just before the detection step to measure 8-oxodG.
The second row of microwells on the sensor chip was used to detect DNA-metabolites adducts. LbL assembly of the (RuPVP/DNA)3/cyt P450 source/RuPVP/conjugative enzyme source/DNA was done in selected microwells. N-acetyl transferase1 (NAT) was included as a conjugation enzyme due to its involvement in arylamine metabolism. Films for DNA adduct detection are denoted as (RuPVP/DNA)3/cyt P450 source/RuPVP/NAT/DNA.
Optimized compositions of solutions and time used to make films were: PDDA, 2 mg mL−1 in 0.05 M NaCl was incubated for 20 min.; RuIIPVP, 2.5 mg mL−1 50% V/V ethanol/water was incubated for 15 min.; salmon testes DNA, 2 mg mL−1 in 10 mM TRIS + 0.5 M NaCl, pH 7.4; HLM, 20 mg mL−1 in 250 mM sucrose; RLM, 20 mg mL−1 in 250 mM sucrose; cyt P450 supersomes, 4.5 mg mL−1 in 100 mM potassium phosphate buffer of pH 7.4. DNA and enzymes were incubated for 30 min each. A quartz crystal microbalance (QCM, USI Japan) was used to measure the mass densities and nominal thickness of the films.15,28 See SI file for full details.
Enzyme Bioactivation and ECL Measurement
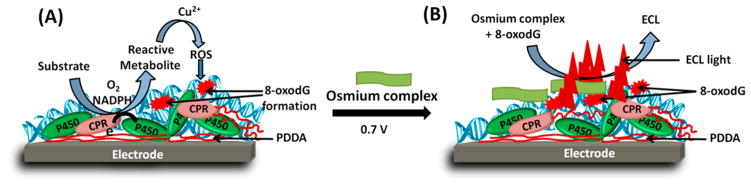
The four aryl amines used were 4-aminobiphenyl (4ABP), aminofluorene (AF), o-anisidine (anisidine), and 2-naphthylamine (NA). For DNA oxidation, ROS were generated inside the fluidic device by flowing solutions of 3 mM 4ABP, 3 mM AF, 3 mM anisidine, or 5 mM NA in 50 mM phosphate buffer pH 7.4 including an NADPH regenerating system (1 U/mL G6PDH enzyme, 2.5 mM G6P, 0.5 mM NADP+, 1 mM Mg2+) and 1 mM CuCl2 at 250 μL min−1 into the array with microwells containing films of (PDDA/DNA)3/P450 enzyme source/PDDA/DNA (Scheme 3A). After washing the channel for 2 min with 50 mM phosphate buffer and 0.1 M NaCl in pH 7.4 buffer, 2 mM [Os(bpy)2(phen-benz-COOH)]2+ was introduced into the channel and incubated for 2 min while flow was stopped to react completely with the oxidized DNA. Amperometry was done at 0.7 V vs Ag/AgCl (0.14 M KCl) and ECL light was captured using a charged coupled device (CCD) camera (G:BOX, Syngene), as depicted in Scheme 3B. For DNA adduct detection, metabolites of the aryl amines were generated by flowing solutions of 4ABP (0.2 mM), AF (0.2 mM), anisidine (0.2 mM), or NA (0.3 mM) containing 0.1 mM acetyl coenzyme A (AcCoA) and its regenerating system (4.6 mM acetyl-D,L-carnitine hydrochloride and 0.06 units of carnitine acetyltransferase)29 into the second channel with microwells filled with (RuPVP/DNA)3/cyt P450 source/RuPVP/NAT/DNA films at 250 μL min−1 while applying a constant potential of −0.65 V vs Ag/AgCl (0.14 M KCl) at room temperature to activate cyt P450s.11,30 The channel was then washed with 50 mM phosphate buffer and 0.1 M NaCl pH 7.4 for 2 min at 250 μL min−1. Then 1.25 V vs Ag/AgCl was applied to the array for 120 s to generate an ECL signal, which was detected by a CCD camera.14,18 Since the time scales of responses of the two types of DNA damage were different and detecting them requires different applied potentials, DNA oxidation measurements were done first and DNA adducts, detected at more positive potential, were done second.
Scheme 3. Schematic Representation of One Microwell Containing PDDA/Cyt P450 Source/PDDA/DNA Film⠭.
⠭ (A) Represents enzyme bioactivation process in the presence of oxygenated substrate, NADPH and Cu2+ leading to 8-oxodG formation; (B) Reaction of 8-oxodG with [Os(bpy)2(phen-benz-COOH)]2+ and ECL generation upon applying a potential of 0.7 V vs Ag/AgCl. liver.18,25
UPLC/MS/MS
LbL films of DNA, PDDA, and enzymes analogous to those above, but with no ECL dyes, were coated onto 1 μm carboxylate functionalized magnetic beads (Invitrogen Dynabeads) by the LbL method, with final film architecture PDDA/cyt P450 source/PDDA/DNA. Magnetic bead dispersions in 10 mM Tris buffer (200 μL, pH 7.0) were incubated with 4ABP, AF, or o-anisidine (3 mM) or 5 mM NA with the NADPH regeneration system and 1 mM CuCl2 at 37 °C to generate metabolites and ROS that oxidize DNA. After incubation, the supernatant was discarded and beads were washed twice with 10 mM Tris buffer.13,14,16 DNA was enzymatically hydrolyzed off the beads by incubating with deoxyribonuclease I (400 unit mg−1 of DNA), phosphodiesterase I from snake venom (0.2 unit mg−1 of DNA), phosphodiesterase II (0.01 unit mg−1 of DNA), 10 μL of 10 mM MgCl2, and phosphatase alkaline (1.2 unit mg−1 of DNA) at 37 °C for 12 h. The released oxidized products were vacuum filtered using a 3 kDa MW cutoff, 96 well filtration plate. The oxidized bases were analyzed by UPLC/MS/MS (See SI file for details).13,16
RESULTS AND DISCUSSION
Film Characterization
Films used in the arrays were grown on 9 MHz gold-coated quartz resonators and characterized by quartz crystal microbalance (QCM). A linear decrease in frequency after each layer was deposited and dried suggested stable and reproducible film growth (Figures S1 and S2). QCM frequency shifts were used to calculate the mass densities and nominal film thicknesses.14,18,27 Films were nominally 22–48 nm thick depending on layer mass densities (Table S2 and S3).
Oxidation and Adduct Damage of DNA by Aryl Amines
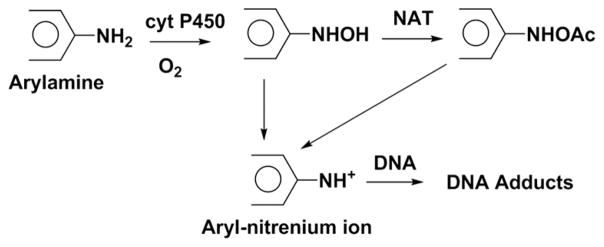
These compounds were chosen for study because their metabolites facilitate both DNA adduct formation and oxidation. The multienzyme metabolic pathway for arylamine bioactivation involves oxidation catalyzed by cyt P450s to N-hydroxylamines which are then converted by N-acetyl trans-ferase1 (NAT) to highly reactive O-substituted N-hydroxyl-amines (Scheme 4). The latter species undergoes spontaneous heterolysis of the N–O bond to aryl nitrenium ions that react with DNA predominantly at C8 positions of guanines to form covalent DNA adducts.27,31,32 The major DNA adduct found for 4ABP is N-(2′-deoxyguanosin-8-yl)-4-aminobiphenyl,33 for AF is N-(2′-deoxyguanosin-8-yl)-2-aminofluorene,34 for anisidine is N-(2′-deoxyguanosin-8-yl)-2-methoxyaniline,35 and for NA is N-(2′-deoxyguanosin-8-yl)-2-naphthylamine.36 However, in the presence of Cu2+ and NADPH, N-hydroxy metabolic intermediates induce DNA oxidation by ROS formed in a redox cycling pathway (Scheme 5).23–25,37,38
Scheme 4.
Major Metabolic Pathway of Aryl Amines Causing DNA Adduct Formation18
Scheme 5.
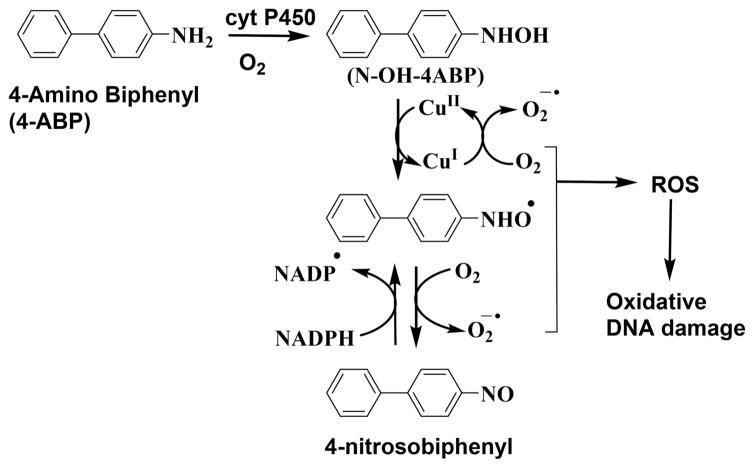
Proposed Pathway Involving Cu2+ and NADPH for ROS Formation Mediated by 4-ABP Leading to DNA Oxidation21
We previously monitored formation of covalent DNA adducts from arylamine metabolites using nonfluidic ECL arrays employing RuPVP.18 In this paper, we focused on including simultaneous measurements of DNA oxidation and adduct formation into a combined microfluidic system. For both types of DNA damage experiments, enzymes used for the metabolite generation were (a) HLM, (b) RLM, (c) cyt P450 1A2, and (d) cyt P450 1A1, except cyt P450 1A1 was replaced for anisidine by cyt P450 2E1, which is more active for its bioactivation.25 For DNA adduct damage experiments, the bioconjugation enzyme NAT was used for complete bio-activation of the aryl amines.
4ABP is classified as a group I carcinogen by the International Agency for Research on Cancer (IARC) and is found in synthetic dyes, rubber, and tobacco smoke. Evidence from in vitro and in vivo studies supports a role for oxidative stress (presumably involving ROS) in ABP-induced mouse liver carcinogenesis.4,25 In vitro, N–OH-ABP (N-hydroxyl-ABP) produces hydrogen peroxide and oxidizes DNA in the presence of Cu2+ ions and NADPH (Scheme 5).23,39
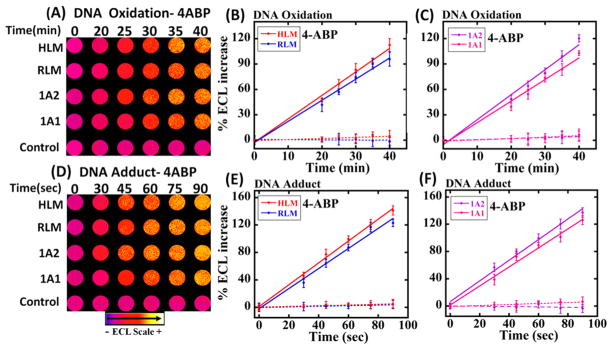
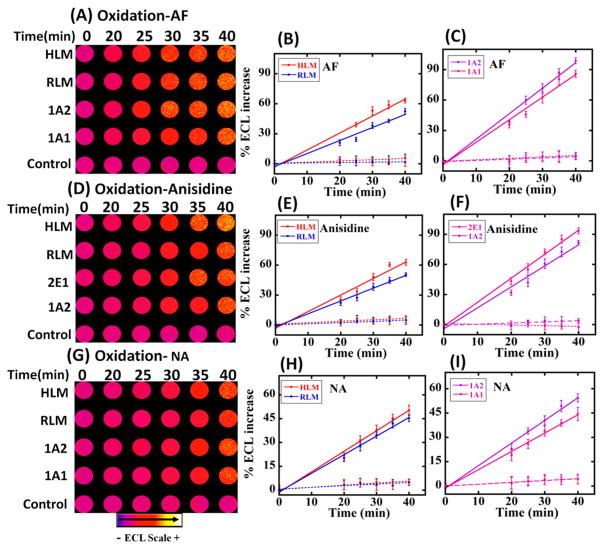
Essentially, the microfluidic system features microwells with thin films of metabolic enzymes, DNA and ECL reagents, and flowing solutions that facilitate either DNA adduct formation with the metabolite or DNA oxidation facilitated by the metabolite; Cu2+ and NADPH as shown in Schemes 1 and 2. One lane is designed to detect DNA oxidation, and the second lane is designed to detect adduct formation of DNA bases. Figure 1 shows reconstructed, recolorized ECL array images and intensities using 4ABP for DNA oxidation and adduct damage using four different metabolic enzyme sources. An increase in ECL intensity with reaction time was found for both lanes. Figure 1A–C reflects the results for the oxidation of dG in DNA to 8-oxodG measured selectively by ECL from Os(bpy)2(phen-benz-COOH)]2+. Figure 1D–F show an increased ECL intensity due to the formation of metabolite–DNA adducts in RuPVP/DNA/enzymes films. The slopes of the % ECL increase vs enzyme reaction reflects the relative rate of DNA oxidation (Figure 1B,C) and the relative rate of DNA adduct formation (Figure 1E,F). Note that the time scales are very different, and under our conditions, DNA oxidation is much slower than adduct formation. Control wells exposed to only buffer or only NADPH + Cu2+ with no 4ABP gave negligible changes in ECL (Figure 1B,C, dotted lines). No increase in ECL was seen for the adduct formation controls using only buffer or only 4ABP with no electrolysis (Figure 1E, F dotted lines).
Figure 1.
Recolorized, reconstructed images of ECL for DNA oxidation (1A) and DNA adduct formation (1D) using 4ABP. For DNA oxidation microwells, DNA/enzyme films were reacted with 3 mM 4ABP in pH 7.4 PBS + NADPH + Cu2+ ions. Then, 2 mM Os(bpy)2(phen-benz-COOH)]2+ is delivered to the microwell reactor and incubated, and 0.7 V vs Ag/AgCl is applied to generate ECL. For DNA adduct formation, wells containing RuPVP/Enzyme/DNA films were reacted with 4ABP in pH 7.4 PBS with bioelectronic activation of supersomal and microsomal enzymes at −0.65 V vs Ag/AgCl.14 1.25 V vs Ag/AgCl was applied to generate ECL in a dark box. (B), (C), (E), and (F) show influence of enzyme reaction time on %ECL increase for these reactions. Control experiments lacked 4ABP or enzymes.
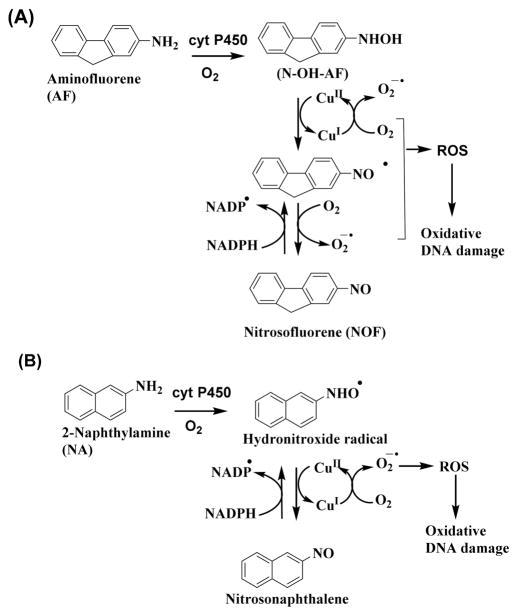
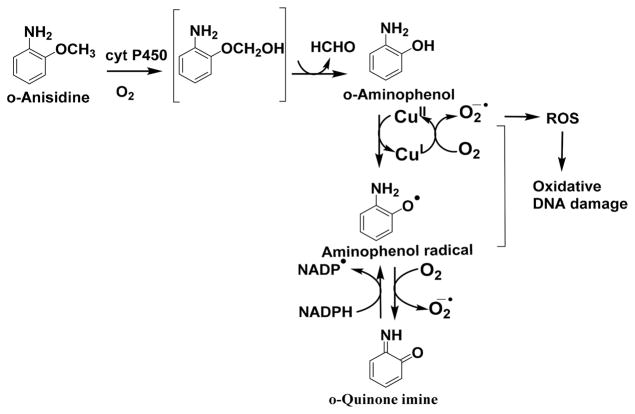
AF was originally developed as a pesticide but was never used, as it was discovered to be an animal carcinogen.40 NA was previously used commercially as an intermediate in the manufacture of dyes and as an antioxidant in the rubber industry; however, because of its carcinogenicity, its manufacture and use have been prohibited. AF and NA are also metabolized to N-hydroxyl derivatives like 4ABP and undergo redox cyclic pathways with Cu2+ and NADPH to generate ROS and to oxidize DNA (Scheme 6).21,22,24 o-Anisidine is a group 2B carcinogen used as an intermediate in the manufacturing of azo and naphthol pigments and dyes. It is also a constituent of cigarette smoke.41 Phenols are prominent products of o-anisidine metabolism involving direct oxidation of the aromatic ring or from the radical cation that is the precursor to the N-hydroxylamine (Scheme 7). The aminophenol can undergo spontaneous or metal catalyzed oxidation in the presence of NADPH to a quinone imine. This aminophenol/quinone imine redox couple generates ROS, which causes oxidative DNA damage.4,23,42,43 Thus, anisidine forms ROS in a aminophenol/quinone imine redox pathway with Cu2+ and NADPH (Scheme 7).4,23
Scheme 6.
Proposed Mechanism of DNA Oxidation by Aminofluorene (AF) and 2- Naphthylamines (NA) in Redox Cycles with Cu2+ and NADPH22,24
Scheme 7.
Proposed Pathway for DNA Oxidation Caused by o-Anisidine, Cu2+, and NADPH4,23
ECL arrays featuring different cyt P450 sources showed increases in ECL with reaction time for AF, anisidine, and NA (Figure 2A,D,G) in the presence of Cu2+ and the NADPH regenerating system. Slopes of ECL intensity vs enzyme reaction time are proportional to the amount of DNA oxidation forming 8-oxodG in the presence of AF, anisidine, and NA as shown in Figure 2B,C,E,F,H,I.
Figure 2.
Reconstructed, recolorized ECL images for DNA oxidation caused by (A) AF, (D) o-anisidine, and (G) NA. DNA/enzyme films were reacted with 3 mM AF or anisidine or 5 mM NA in pH 7.4 PBS + NADPH regenerating system + Cu2+. Os(bpy)2(phen-benz-COOH)]2+was delivered to the reactor and incubated, and 0.7 V was applied to obtain ECL. % ECL vs enzyme reaction time shown in (B), (E), (H) and (C), (F), (I) with different enzyme sources. Controls lack test compounds or enzymes.
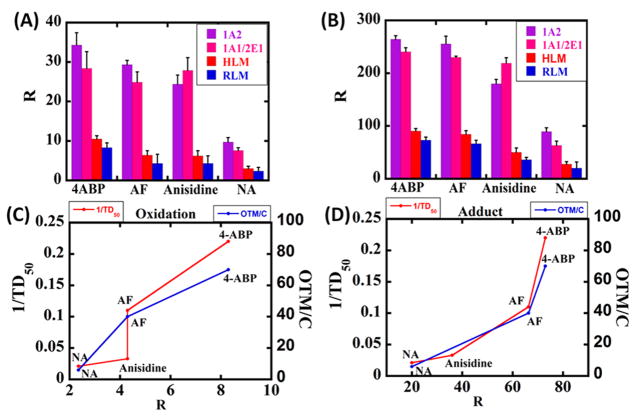
DNA adduct detection done on the same array for the above aryl amines also showed increases in % ECL with enzyme reaction time (Figure S3). Relative DNA damage and oxidation rates are proportional to the slopes of % ECL increase vs time11 and were converted to relative turnover rates (R) by dividing the initial slopes (t−1) by the total amount of protein in each film (μg) and the amount of substrate used (mM).18
Higher relative DNA oxidation and adduction rates (Figure 3A,B) were observed for supersomes compared to those for microsomal enzymes due to the higher concentrations of specific cyt P450 isoforms in supersomal fractions responsible for the metabolism of the carcinogens.27 HLM induced faster DNA oxidation and adduction rates than RLM for all the aryl amines, in agreement with previous work using a non-microfluidic manual array.18 Supersome 1A2 had the largest relative DNA oxidation and adduction rates for 4-ABP, AF, and NA, while for anisidine, the 2E1 isoform caused slightly more bioactivation for DNA oxidation and adduction than 1A2, consistent with other in vitro studies.25,44
Figure 3.
Bar graphs showing (A) relative DNA oxidation rate and (B) relative DNA adduction rate as R ({μg of protein}−1 min−1 mM−1) from exposure of 4-ABP, AF, anisidine, and NA to different cyt P450 sources in the array. Line graphs showing correlations of relative DNA damage rates (R) from human liver microsomes found by using the ECL array with the reciprocal of rodent liver TD50 values and comet assay values (OTM/C in AU*mM−1) from human urinary bladder cell lines for 4-ABP and NA45 and human peripheral lymphocytes for AF.18,46 (C) and (D) shows correlation with relative DNA oxidation rate and relative DNA adduction rates for 4-ABP, AF, anisidine, and NA.
The relative rate of DNA damage estimated as turnover rate (R) correlated very well with the in vitro Comet assay results and the reciprocal of the rodent in vivo liver tumor TD50 values (Figure 3C,D). Comet assays measure relative DNA damage in test cells from toxic chemicals. In this assay, broken DNA fragments move away from the nucleus during electrophoresis compared to intact DNA, creating a “tail”. The product of tail length and the fractional amount of DNA in the tail is defined as the olive tail moment (OTM) and is used to measure DNA damage.47 The carcinogenic potency value TD50 used here is the “dose-rate (mg kg−1 body weight per day), which, if administered chronically for the standard lifespan of the species, will have the probability of 50% of test animals remaining tumorless throughout that period”.48,49 Low TD50 values are indicative of high toxicity, so inverse TD50 values (1/TD50), which increase with toxicity level, were used in the correlation plot. The ECL array for DNA oxidation and adduction showed good correlation with the both the Comet assay and 1/TD50 values (Figures 3C,D and S10).
Elucidation of Metabolite Formation and DNA Oxidation by UPLC/MS/MS
Formation of the reactive metabolites of aryl amines, which undergo redox cycling with Cu2+ and NADPH (Schemes 5, 6 and 7) to form ROS, were confirmed by mass spectrometry after reaction with cyt P450s (Figures S6–9). In positive-ion electrospray mass spectra, 4-ABP showed the protonated molecule at m/z 170.0 (Figure S6), while its metabolites N–OH 4-ABP ((M+H)+ = 186.0), hydronitroxide radical of 4-ABP ((M+H)+ = 185.0), and 4-nitrosobiphenyl ((M+H)+ = 184.0) were found after incubation of 4-ABP with cyt P450s, NADPH, and Cu2+. This confirms the redox cyclic pathway that forms ROS (Scheme 5) to oxidize DNA on the arrays. Incubation of AF ((M+H)+ = 182.0) with cyt P450, NADPH, and Cu2+ gave metabolites N–OH AF ((M +H)+ = 198.0) and nitrosofluorene ((M+H)+ = 196.0 (Figure S7, Scheme 6). Metabolism of o-anisidine ((M+H)+ = 124.0) was demonstrated by detecting o-aminophenol ((M+H)+ = 110.0) as the main metabolite (Figure S8, Scheme 7). NA ((M +H)+ = 144.0) was metabolized to the hydronitroxide radical ((M+H)+ = 159.0) and nitroso naphthalene ((M+H)+ = 158.0) (Figure S9, Scheme 6).
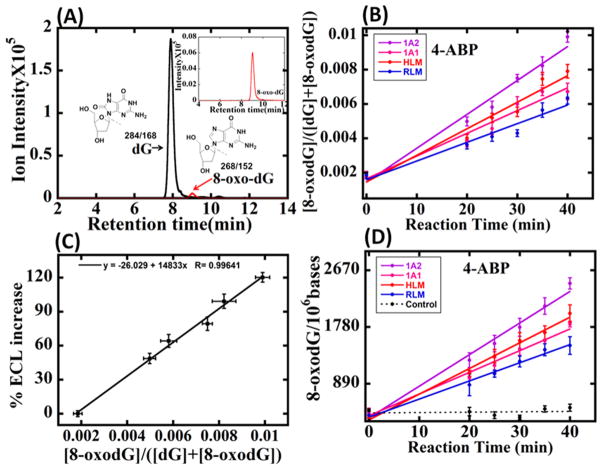
Relative amounts of 8-oxodG formed after bioactivation of test compounds with various enzymes sources, NADPH, and Cu2+ were also measured by UPLC/MS/MS. Magnetic beads (1 μm) coated with PDDA/DNA/enzyme (HLM or RLM or 1A2 or 1A1 or 2E1) films as used in ECL arrays served as biocolloid reactors to generate 8-oxodG, followed by hydrolysis of the DNA and LC/MS/MS quantitation of 8-oxodG.13 Multiple reaction monitoring (MRM) provided the amounts of dG and 8-oxodG. Figures 4A and S4 show the MRM chromatograms for the transition from m/z 268 to 152 at 7.8 min corresponding to peaks of dG and the transition from m/z 284 to 168 at 8.5 min for 8-oxodG. Figures 4B and S5A, B, C show ratios of 8-oxodG to total dG vs reaction time obtained from an UPLC/MS/MS assay for each test compound. These UPLC/MS/MS data were used to validate the sensor arrays and to develop a calibration plot for the sensor array, that is, % ECL increase vs ratio of 8-oxodG to total dG (Figure 4C). This calibration curve was used for the quantitation of array results by converting % ECL data to the relative amount of 8-oxodG formed. Relative amounts of 8-oxodG (number of 8-oxodG per 106 nucleobases) for each test compound metabolized by cyt P450 1A2, 1A1, HLM, and RLM enzymes for different oxidation times were obtained from these results. Figures 4D and S5D–F show the amount of 8-oxodG formed per 106 nucleobases when 4-ABP, anisidine, AF, and NA were exposed to DNA/enzyme films for different time intervals, respectively. The LC/MS/MS limit of detection (LOD) for 8-oxodG was 0.032 pmol of 8-oxodG per 15.5 pmol of DNA (one 8-oxodG per 106 nucleobases). The ECL array LOD was 720 8-oxodG per 106 nucleobases.
Figure 4.
UPLC/MS/MS results for DNA oxidation. (A) MRM chromatogram with mass transition m/z 268–152 indicating dG and m/z 284–168 indicating 8-oxodG for DNA/enzyme magnetic biocolloid reactors incubated with 4-ABP, Cu2+, and NADPH for 40 min followed by hydrolysis of the DNA. (B) Ratio of 8-oxodG to total dG from magnetic biocolloid reactors reacted with 4-ABP in the presence of Cu2+ and NADPH. (C) Calibration curve for sensor % ECL increase vs relative amount of 8-oxodG. (D) Influence of reaction time on 8-oxodG per 106 bases formation when DNA/enzyme films were incubated with 4-ABP in the presence of Cu2+ and NADPH from array data. Control experiments contained Cu2+ and NADPH but no 4-ABP.
The results described above demonstrate new microfluidic ECL microwell arrays based on DNA/enzymes/ECL dye films that can sequentially elucidate relative rates of metabolite-driven DNA oxidation and nucleobase adduct reactions (Figures 1–3). The two-channel microfluidic platform facilitated detection of DNA oxidation and DNA adduction in the same array (Figures 1 and 2). ECL sensors equipped with HLM and RLM microsomes, cyt P450 supersomes, and NAT reproduced important metabolic bioactivation events for the test aryl amines. The supersomal cyt P450s, consisting of one cyt P450 important in arylamine metabolism plus its accompanying P450 reductase, gave the highest relative turnover rates (R, Figure 3A,B), while HLM and RLM supported smaller turnover rates. While R is normalized for the amount of enzyme, it is likely that the single supersomal enzymes, chosen for their known participation in arylamine metabolism,20,50,51 have higher activity for arylamine oxidation than the mixture of eight or more cyt P450s in HLM and RLM. Also, relative DNA adduction was found to be 7–10 times faster than the relative DNA oxidation rate. While experimental design can be a factor, this result may suggest that DNA adduct formation is a more important mechanism for DNA damage4 in living systems than metabolite-driven oxidation. This issue will require additional study.
An excellent correlation of relative turnover rates R with rodent tumorgenicity metric 1/TD50 and the Comet assay, which measures DNA damage in cells, was found (Figure 3C–F). Compounds such as 4-ABP and AF, which are highly toxic by conventional bioassays, give high R values, and chemicals with relative lower levels of toxicity (e.g., NA and anisidine) in bioassays give smaller R values. These correlations suggest that array R values are consistent with existing genotoxicity metrics and could be valuable for toxicity screening in combination with bioassays. The relative DNA adduction rate for aryl amines were in the order of 4-ABP > AF > anisidine > NA, which is consistent with previous findings,18 while relative DNA oxidation rates were in the order of 4-ABP > AF ≈ anisidine > NA, which has not been reported previously.
LC/MS/MS results confirmed the specific metabolic activity of the enzymes used in the array and was used to measure the relative amount of 8-oxodG formation (Figures 4B and S5A–C). Detection of known metabolites of 4-ABP, AF, anisidine, and NA by mass spectrometry (Figures S6–S9) is consistent with the known metabolic pathways and with the concomitant generation of ROS to oxidize dG to 8-oxodG.20–23 A relative amount of 8-oxodG formation was measured by LC/MS/MS in the presence of the same enzyme system as used in the arrays. For 4-ABP, AF, and NA, cyt 1A2 was responsible for more DNA oxidation than cyt P450 1A1, while for o-anisidine cyt 2E1 was responsible for more DNA oxidation than cyt P450 1A2, in good agreement with the ECL results (Figure 3). The amount of 8-oxodG found per 106 nucleotides also correlates well with the % ECL increase data vs reaction time in the ECL arrays and validates our ECL sensor (Figures 4D and S5D–F). The limit of detection (LOD) of the array for DNA oxidation was 720 8-oxodG per 106 bases.
CONCLUSIONS
In summary, a new microfluidic genotoxicity screening ECL sensor array was developed that sequentially detects DNA oxidation and DNA adduct formation by ECL on unhydrolyzed DNA for the first time. Detection was achieved with 1 μg of DNA directly with no sample workup. Multiple enzyme reactions can be run simultaneously on the array to provide insight into metabolic enzymes that are involved in the molecular genotoxicity pathways for test compounds. Species differences can be assessed, demonstrated here by using RLM and HLM. These ECL genotoxicity arrays will be valuable in conjunction with established bioassays11 in early screening of unknown chemicals and drugs.
Supplementary Material
Acknowledgments
Funding
The authors thank the National Institute of Environmental Health Sciences (NIEHS), NIH, USA, Grant No. ES03154 for financial support.
Footnotes
Notes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.anal-chem.7b03528.
Quartz crystal microbalance results; ECL results for DNA adduct formation caused by AF, o-anisidine, and NA; UPLC/MS/MS results for DNA oxidation; mass spectra of carcinogens and their metabolites (PDF)
References
- 1.Park BK, Boobis A, Clarke S, Goldring CEP, Jones D, Kenna JG, Lambert C, Laverty HG, Naisbitt DJ, Nelson S, Nicoll-Griffith DA, Obach RS, Routledge P, Smith DA, Tweedie DJ, Vermeulen N, Williams DP, Wilson ID, Baillie TA. Nat Rev Drug Discovery. 2011;10:292–307. doi: 10.1038/nrd3408. [DOI] [PubMed] [Google Scholar]
- 2.Farmer PB, Brown K, Tompkins E, Emms VL, Jones DJ, Singh R, Phillips DH. Toxicol Appl Pharmacol. 2005;207:293–301. doi: 10.1016/j.taap.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Spencer WA, Vadhanam MV, Jeyabalan J, Gupta RC. Chem Res Toxicol. 2012;25:305–314. doi: 10.1021/tx200356v. [DOI] [PubMed] [Google Scholar]
- 4.Murata M, Kawanishi S Front. Biosci, Landmark Ed. 2011;16:1132–1143. doi: 10.2741/3739. [DOI] [PubMed] [Google Scholar]
- 5.Bowes J, Brown AJ, Hamon J, Jarolimek W, Sridhar A, Waldron G, Whitebread S. Nat Rev Drug Discovery. 2012;11:909–922. doi: 10.1038/nrd3845. [DOI] [PubMed] [Google Scholar]
- 6.Nassar AEF, Kamel AM, Clarimont C. Drug Discovery Today. 2004;9:1055–1064. doi: 10.1016/S1359-6446(04)03297-0. [DOI] [PubMed] [Google Scholar]
- 7.Rusling JF, Hvastkovs EG, Schenkman JB. Curr Opin Drug Discovery Dev. 2007;10:67–73. [PubMed] [Google Scholar]
- 8.Stevens JL. Chem Res Toxicol. 2006;19:1393–1401. doi: 10.1021/tx060213n. [DOI] [PubMed] [Google Scholar]
- 9.Kramer JA, Sagartz JE, Morris DL. Nat Rev Drug Discovery. 2007;6:636–649. doi: 10.1038/nrd2378. [DOI] [PubMed] [Google Scholar]
- 10.Farmer PB, Singh R. Mutat Res, Rev Mutat Res. 2008;659:68–76. doi: 10.1016/j.mrrev.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Hvastkovs EG, Schenkman JB, Rusling JF. Annu Rev Anal Chem. 2012;5:79–105. doi: 10.1146/annurev.anchem.111808.073659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hvastkovs EG, Rusling JF. Anal Chem. 2016;88:4584–4599. doi: 10.1021/acs.analchem.5b04772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song B, Shen M, Jiang D, Malla S, Mosa MI, Choudhary D, Rusling JF. Analyst. 2016;141:5722–5729. doi: 10.1039/c6an01237j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasalathanthri DP, Malla S, Bist I, Tang CK, Faria RC, Rusling JF. Lab Chip. 2013;13:4554–4562. doi: 10.1039/c3lc50698c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dennany L, Forster RJ, White B, Smyth, Rusling JF. J Am Chem Soc. 2004;126:8835–8841. doi: 10.1021/ja048615+. [DOI] [PubMed] [Google Scholar]
- 16.Bist I, Song B, Mosa IM, Keyes TE, Martin A, Forster RJ, Rusling JF. ACS Sens. 2016;1:272–278. doi: 10.1021/acssensors.5b00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dennany L, Forster RJ, Rusling JF. J Am Chem Soc. 2003;125:5213–5218. doi: 10.1021/ja0296529. [DOI] [PubMed] [Google Scholar]
- 18.Pan S, Zhao L, Schenkman JB, Rusling JF. Anal Chem. 2011;83:2754–2760. doi: 10.1021/ac200050n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D, Guengerich PF. Annu Rev Pharmacol Toxicol. 2005;45:27–49. doi: 10.1146/annurev.pharmtox.45.120403.100010. [DOI] [PubMed] [Google Scholar]
- 20.Turesky RJ, Le Marchand L. Chem Res Toxicol. 2011;24:1169–1214. doi: 10.1021/tx200135s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murata M, Tamura A, Tada M, Kawanishi S. Free Radical Biol Med. 2001;30:765–773. doi: 10.1016/s0891-5849(01)00463-4. [DOI] [PubMed] [Google Scholar]
- 22.Ohnishi S, Murata M, Kawanishi S. Jpn J Cancer Res. 2002;93:736–743. doi: 10.1111/j.1349-7006.2002.tb01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naiman K, Martínková M, Schmeiser HH, Frei E, Stiborová M. Mutat Res, Genet Toxicol Environ Mutagen. 2011;726:160–168. doi: 10.1016/j.mrgentox.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Murata M, Yoshiki Y, Tada M, Kawanishi S. Int J Cancer. 2002;102:311–317. doi: 10.1002/ijc.10717. [DOI] [PubMed] [Google Scholar]
- 25.Wang K, Guengerich PF. Chem Res Toxicol. 2013;26:993–1004. doi: 10.1021/tx400139p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang CK, Vaze A, Rusling JF. Lab Chip. 2012;12:281–286. doi: 10.1039/c1lc20833k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan S, Hvastkovs EG, Bajrami B, Choudhary D, Schenkman JB, Rusling JF. Anal Chem. 2008;80:5279–5285. doi: 10.1021/ac800763r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lvov Y. In: Protein Architecture: Interfacing Molecular Assemblies and Immobilization Biotechnology. Lvov Y, Möhwald H, editors. Marcel Dekker; New York: 2000. pp. 125–167. [Google Scholar]
- 29.Wasalathanthri D, Faria RC, Malla S, Joshi AA, Schenkman JB, Rusling JF. Analyst. 2013;138:171–178. doi: 10.1039/c2an35993f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan S, Wasalathanthri D, Zhao L, Schenkman JB, Rusling JF. J Am Chem Soc. 2011;133:1459–1465. doi: 10.1021/ja108637s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadlubar FF, Miller JA, Miller EC. Cancer Res. 1977;37:805–814. [PubMed] [Google Scholar]
- 32.Culp SJ, Roberts DW, Talaska G, Lang NP, Fu PP, Lay JO, Teitel CH, Snawder JE, Von Tungeln LS, Kadlubar FF. Mutat Res, Fundam Mol Mech Mutagen. 1997;378:97–112. doi: 10.1016/s0027-5107(97)00101-2. [DOI] [PubMed] [Google Scholar]
- 33.Feng Z, Hu W, Rom WN, Beland FA, Tang MS. Carcinogenesis. 2002;23:1721–1727. doi: 10.1093/carcin/23.10.1721. [DOI] [PubMed] [Google Scholar]
- 34.Beranek DT, White GL, Heflich RH, Beland FA. Proc Natl Acad Sci U S A. 1982;79:5175–5178. doi: 10.1073/pnas.79.17.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naiman K, Dračínský M, Hodek P, Martínková M, Schmeiser HH, Frei E, Stiborová M. Toxicol Sci. 2012;127:348–359. doi: 10.1093/toxsci/kfs104. [DOI] [PubMed] [Google Scholar]
- 36.Kadlubar FF, Unruh LE, Beland FA, Straub KM, Evans FE. Carcinogenesis. 1980;1:139–150. doi: 10.1093/carcin/1.2.139. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi N, Fischer V, Schreiber J, Mason RP. Free Radical Res Commun. 1988;4:351–358. doi: 10.3109/10715768809066903. [DOI] [PubMed] [Google Scholar]
- 38.Fischer V, Mason RP. Chem-Biol Interact. 1986;57:129–142. doi: 10.1016/0009-2797(86)90033-5. [DOI] [PubMed] [Google Scholar]
- 39.Makena PS, Chung K. Environ Mol Mutagen. 2007;48:404–413. doi: 10.1002/em.20288. [DOI] [PubMed] [Google Scholar]
- 40.Turesky RJ, Le Marchand L. Chem Res Toxicol. 2011;24:1169–1214. doi: 10.1021/tx200135s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stiborová M, Naiman K, Martínková M, Martínek V, Svobodová M, Schmeiser HH, Frei E. Interdiscip Toxicol. 2009;2:24–27. doi: 10.2478/v10102-009-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohkuma Y, Kawanishi S. Arch Biochem Biophys. 2001;389:49–56. doi: 10.1006/abbi.2001.2302. [DOI] [PubMed] [Google Scholar]
- 43.Chen F, Oikawa S, Hiraku Y, Murata M, Yamashita N, Kawanishi S. Cancer Lett. 1998;126:67–74. doi: 10.1016/s0304-3835(97)00533-8. [DOI] [PubMed] [Google Scholar]
- 44.Stiborova M, Mikšanová M, Šulc M, Rýdlová H, Schmeiser HH, Frei E. Int J Cancer. 2005;116:667–678. doi: 10.1002/ijc.21122. [DOI] [PubMed] [Google Scholar]
- 45.Robbiano L, Carrozzino R, Bacigalupo M, Corbu C, Brambilla G. Toxicology. 2002;179:115–128. doi: 10.1016/s0300-483x(02)00354-2. [DOI] [PubMed] [Google Scholar]
- 46.Plewa MJ, Wagner ED, Yu TW, Anderson D. Environ Mol Mutagen. 1995;26:171. doi: 10.1002/em.2850260211. [DOI] [PubMed] [Google Scholar]
- 47.Olive PL, Banáth JP, Durand RE. J Natl Cancer Inst. 1990;82:779–783. doi: 10.1093/jnci/82.9.779. [DOI] [PubMed] [Google Scholar]
- 48.Gold LS. The Carcinogenic Potency Database. http://potency.berkeley.edu.
- 49.Peto R, Pike MC, Bernstein L, Gold LS, Ames BN. Environ Health Perspect. 1984;58:1–8. doi: 10.1289/ehp.84581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naiman K, Dracínská H, Martínková M, Šulc M, Dračínský M, Kejíková L, Hodek P, Hudecček J, Liberda J, Schmeiser HH, Frei E, Stiborová M. Chem Res Toxicol. 2008;21:1610–1621. doi: 10.1021/tx8001127. [DOI] [PubMed] [Google Scholar]
- 51.Anderson KE, Hammons GJ, Kadlubar FF, Potter JD, Kaderlik KR, Ilett KF, Minchin RF, Teitel CH, Chou HC, Martin MV. Carcinogenesis. 1997;18:1085–1092. doi: 10.1093/carcin/18.5.1085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.