Abstract
The dmrt1 (doublesex and mab-3 related transcription factor 1) gene is a key regulator of sex determination and/or gonadal sex differentiation across metazoan animals. This is unusual given that sex determination genes are typically not well conserved. The mechanisms by which zebrafish sex is determined have remained elusive due to the lack of sex chromosomes and the complex polygenic nature of sex determination in domesticated strains. To investigate the role of dmrt1 in zebrafish sex determination and gonad development, we isolated mutations disrupting this gene. We found that the majority of dmrt1 mutant fish develop as fertile females suggesting a complete male-to-female sex reversal in mutant animals that would have otherwise developed as males. A small percentage of mutant animals became males, but were sterile and displayed testicular dysgenesis. Therefore zebrafish dmrt1 functions in male sex determination and testis development. Mutant males had aberrant gonadal development at the onset of gonadal sex-differentiation, displaying reduced oocyte apoptosis followed by development of intersex gonads and failed testis morphogenesis and spermatogenesis. By contrast, female ovaries developed normally. We found that Dmrt1 is necessary for normal transcriptional regulation of the amh (anti-Müllerian hormone) and foxl2 (forkhead box L2) genes, which are thought to be important for male or female sexual development respectively. Interestingly, we identified one dmrt1 mutant allele that cooperates with a linked segregation distorter locus to generate an apparent XY sex determination mechanism. We conclude that dmrt1 is dispensable for ovary development but necessary for testis development in zebrafish, and that dmrt1 promotes male development by transcriptionally regulating male and female genes as has been described in other animals. Furthermore, the strong sex-ratio bias caused by dmrt1 reduction-of-function points to potential mechanisms through which sex chromosomes may evolve.
Keywords: Zebrafish, Sex-determination, Testis, Gonad, Spermatogenesis, Dmrt1
1. Introduction
While sex-determination and sex-differentiation are ancient and highly conserved biological processes, the mechanisms by which they are controlled vary extensively among animal species. The factors underlying the decision to develop as one sex over the other are triggered by diverse cues among different species, such as temperature, social dynamics, sex chromosomes, and the concerted effects of multiple sex-associated genes. Despite this diversity, many genes amongst vertebrates share a conserved role in regulating sexual development, for example Sox9 (SRY-box 9) and FoxL2 (Forkhead box L2) (Trukhina et al., 2013). Interestingly, the only known unifying feature across metazoan sexual development is dependence on proteins with a doublesex and mab-3 related zinc finger-like DNA-binding domain (DM domain) (Matson and Zarkower, 2012; Picard et al., 2015). Furthermore, vertebrates, which include mammals, birds, amphibians, and teleosts, all appear to require a homolog of doublesex and mab-3 related transcription factor 1 (dmrt1) to regulate sexual development (Matson and Zarkower, 2012). While Dmrt1 most commonly regulates male sexual development, its precise position within the sex-determining genetic hierarchy differs across species.
The first DM domain encoding gene was discovered in D. melanogaster and is called doublesex (dsx). D. melanogaster have XY chromosomal sex determination, whereby XX animals are female and XY animals are male. Dsx is an autosomal gene, but is differentially spliced between males and females resulting in DsxMand DsxF proteins in males and females, respectively (Salz and Erickson, 2010). These two transcription factor proteins have a common DNA binding domain, but different C-terminal domains. In males, DsxM activates the expression of male differentiation genes and represses female differentiation genes, whereas, in females, DsxF activates female differentiation genes and represses male differentiation genes (Verhulst and van de Zande, 2015). Thus, Dsx is the downstream effector of the sex determination cascade in Drosophila, regulating the differentiation of sex-specific cell types.
In mammals, Dmrt1 is an autosomal gene that is essential for development of the testis, where it is required for proper differentiation of male germ cells and to maintain male sexual cell fates of somatic gonad cells (Matson et al., 2011). Mammals have XY sex determination with a dominant male determining gene on the Y chromosome called Sry, which is responsible for initiating male sex-determination (Eggers et al., 2014). In mice, Dmrt1, is not required for sex-determination during embryogenesis. However, once this choice is made, Dmrt1 maintains lifelong testis fate in males while inhibiting ovary-promoting pathways. This is facilitated through the direct activation of male determining genes and repression of female determining genes (Murphy et al., 2010). Disruption of Dmrt1 in XY mice post-sex determination causes loss of testis-determining gene expression, acquired expression of ovary determining genes, and transdifferentiation of testis cell types to ovary cell types (Matson et al., 2011). Furthermore, in humans mutation or deletion of Dmrt1 causes male-to-female sex reversal and gonadal dysgenesis, as well as increased risk for testicular germ cell tumor (TGCT) (Kanetsky et al., 2011; Kratz et al., 2011; Krentz et al., 2009). The XY sex reversal seen in humans lacking one functional copy of Dmrt1 suggests that the gene may have a more direct role in sex-determination in humans than that observed in mice.
In non-mammalian animals, dmrt1 sometimes resides on sex chromosomes and has a direct role in determining sex. For example, in the Japanese rice fish, medaka, two paralogs of the dmrt1 gene exist, one of which is the male determining gene called DMY/Dmrt1by. DMY resides on the Y chromosome and is sufficient to drive male sex determination and loss of DMY function in XY medaka causes male-to-female sex reversal (Matsuda et al., 2007, 2002; Nanda et al., 2002). Medaka also have an autosomal copy of dmrt1 which is expressed in testes later than DMY/Dmrt1by but is essential for testis development and male sexual fate (Kobayashi et al., 2004; Masuyama et al., 2012). Similarly, in chickens, dmrt1 is a male determining gene residing on the Z sex chromosome. Birds have ZZ/ZW chromosomal sex determination, whereby ZZ animals are male and ZW individuals are female. ZZ birds have two copies and thus higher expression levels of dmrt1 compared to ZW individuals. The higher dosage of dmrt1 is thought to drive testis development in ZZ birds, whereas ZW birds lack sufficient dmrt1 to induce male fate and develop ovaries (Ayers et al., 2013). This hypothesis is supported by data demonstrating that dmrt1 is both necessary and sufficient for testis determination in chickens (Lambeth et al., 2014; Smith et al., 2009). Xenopus laevis also have ZW chromosomal sex determination with a DMRT1-related gene on the W chromosome, called DM-W, which encodes a truncated protein that is thought to dominantly inhibit the function of the autosomally located DMRT1, thus leading to female fate determination in ZW individuals. ZZ individuals do not have DM-W and therefore develop as males (Yoshimoto and Ito, 2011). Thus, dmrt1 has an essential role in sex-determination and/or gonadal sex-differentiation across vertebrates.
Despite its importance as a research model organism, the mechanism by which sex is determined in zebrafish has remained elusive. Sex chromosomes have been found in wild zebrafish and in laboratory strains recently derived from wild fish (Anderson et al., 2012). However, sex chromosomes are absent in common laboratory strains derived from domesticated populations (i.e. pet shops), with the chromosome homologous to the wild sex-chromosome no longer segregating with a particular sex. Domestic strains generally have complex sex determination that is primarily genetically controlled via polygenic sex determination (PSD) with moderate environmental effects (Liew and Orbán, 2014). Genomic analyses have revealed several sex-associated loci demonstrating that the combined effect of multiple genes controls sex-determination, though the specific genes remain unknown (Anderson et al., 2012; Bradley et al., 2011; Howe et al., 2013; Luzio et al., 2015b). The mechanisms underlying PSD have not been studied in any species making the zebrafish an excellent model to understand this mode of sex determination.
As is generally the case in vertebrates, the first organ to become sexually dimorphic in zebrafish is the gonad. The early zebrafish gonad is bipotential: capable of developing into either an ovary or a testis. Irrespective of the final sexual fate of the gonad, this bipotential gonad contains early-stage oocytes and is commonly referred to as a “juvenile ovary” (Takahashi, 1977). Animals that undergo this method of sexual development are called undifferentiated gonochorists (Yamamoto, 1969). Between 20 and 30 days post fertilization (dpf), the gonads of some animals begin to transition from the bipotential state to a testis. This process is characterized by oocyte apoptosis, increased stromal tissue, followed by cavitation to form early testis tubules and eventual differentiation of sperm (Maack and Segner, 2003; Takahashi, 1977; Uchida et al., 2002). In animals that will develop as females, oogenesis continues to progress to form more advanced-stage follicles. Studies suggest that genes with ovary or testis-enriched expression, such as cyp19a1a and sox9a, are expressed in the juvenile ovary and but then become dimorphically expressed during sex differentiation (Rodríguez-Marí et al., 2005; Siegfried and Nüsslein-Volhard, 2008). Therefore, the zebrafish juvenile gonad is poised to develop as either an ovary or testis under the influence of as-yet-unknown genetic factors.
In some fish species, the germ cells have a major influence on sex determination. In zebrafish and medaka the germ cells are required for ovary fate determination as loss of germ cells leads to development of a testis and male organismal fate, regardless of the genetic sex of the animal (Kurokawa et al., 2007; Siegfried and Nüsslein-Volhard, 2008; Slanchev et al., 2005). However, germ cells are not necessary for ovary development in all fish; goldfish and loach both develop ovary tissue in the absence of germ cells (Fujimoto et al., 2010; Goto et al., 2012). In medaka, increased germ cell numbers can drive ovary fate and cause feminization of XY animals, suggesting a potential active role of germ cells in sex determination (Morinaga et al., 2007; Nakamura et al., 2012). In zebrafish, early germ cell numbers (counted prior to histologically apparent sex-differentiation) correlate with sex. Individuals with more germ cells were more likely to develop as females than males (Tzung et al., 2015). However, it is not clear whether this is a causative or consequential effect of sex determination, as the timing of sex determination in zebrafish is not well defined. Resolution of this issue will require a better understanding of the genes that regulate sex determination in zebrafish.
Here we test the role of dmrt1 in male sex determination and male gonadal development in zebrafish. Using loss-of-function and reduction-of-function mutants we show that dmrt1 has a role in male sex determination and/or maintenance of male sex, as the majority of mutants develop as females. Surprisingly, we found that male sex can be determined in the absence of dmrt1, however these males have abnormal testis development and eventually lose germ cells. Germ cell loss combined with female-biased sexual fate is a unique phenotype in zebrafish as aberrant germ cell development typically results in male-biased sex ratios due to the necessity of germ cells in supporting ovary differentiation. This suggests that dmrt1 is necessary for the maintenance of male-specified germ cells. By contrast, dmrt1 mutant females are fertile with normally developed ovaries indicating a sex-specific role in testis development. We find that testes derived from dmrt1 mutant fish fail to express the anti-Müllerian hormone (amh) gene, a key testis-expressed gene, and over-express the ovary-associated gene foxl2. Therefore, zebrafish dmrt1 shares similar roles in male sexual development as other organisms in regulating sex determination and testis differentiation.
2. Material and methods
All primer sequences are listed in Supplementary Table 1.
2.1. Zebrafish husbandry
Zebrafish were maintained by standard conditions. Institutional IACUC approval was attained prior carrying out all animal procedures.
2.2. Generation of dmrt1 mutations
Four mutations disrupting the dmrt1 gene were found: dmrt1t32242, dmrt1ZM00173004, dmrt1uc27 and dmrt1uc67. For simplicity, we refer to these alleles based on the affect each mutation has on the encoded protein. We refer to dmrt1t32242 and dmrt1ZM00173004 as dmrt1S10X and dmrt1E3ins, respectively. The dmrt1uc27and dmrt1uc67 alleles result from deletions of seven (dmrt1uc27) and nine (dmrt1uc67) base pairs, but both cause the same protein truncation. We therefore refer to these alleles collectively as dmrt1C41X.
The dmrt1t32242 mutation was isolated by screening DNA libraries isolated from ENU mutagenized fish of the Tübingen strain (i.e. TILLING). Exons one and two of the Dmrt1 locus were amplified by semi-nested PCR using primers KS38 and KS37 for the first reaction and KS36 and KS37 for the second reaction. This amplicon was sequenced using primer KS68 (exon1) and KS61 (exon2). One live female founder was found carrying this mutation and was crossed to the WIK strain. The dmr1ZM00173004 allele is a retroviral insertion that was generated and purchased from Znomics.
The dmrt1uc27and dmrt1uc67 mutations were generated using TALEN mediated mutagenesis. TALENs were designed to target the first coding exon of dmrt1 and were assembled using The Golden Gate cloning method (Cermak et al., 2011) as described (Dahlem et al., 2012) using the Golden Gate TALEN and TAL Effector Kit (Addgene) and the backbone plasmids pCS2TAL3DD and pCS2TAL3RR. The DNA sequences that were targeted are as follows: Left TALEN: 5′-GAAGGGCCACAAACGC-3′; Left TALEN: 5′- CTGCCAGTGTCAGAAA-3′. TALEN RNAs were synthesized by in vitro transcription using the mMESSAGE MACHINE kit (Ambion). The TALEN pair were then co-injected at the one-cell stage at 50–100 pg each TALEN/embryo. Founders were identified by screening sperm DNA by high resolution melt (HRM) analysis (Dahlem et al., 2012), using Light Scanner Master Mix (BioFire Defense), a CFX-96 real-time PCR machine and Precision Melt Analysis software (BioRad). Primer sequences used in HRM analysis were BWD934 and BWD935, with a wild-type amplicon size of 100 bp and deletion amplicon size of 93 bp (dmrt1uc27) and 91 bp (dmrt1uc67).
2.3. In situ hybridizations
Whole mount in situ hybridizations (ISH) were performed as previously described (Siegfried and Nüsslein-Volhard, 2008). To make probes for dmrt1 ISH, the C-terminal region of dmrt1 cDNA was isolated by RT-PCR using primers KS73 and KS74 and sub-cloned into pGEMT-Easy (Promega). For ISH on sections, tissue was fixed overnight in 4% paraformaldehyde (PFA), and either embedded in paraffin (Fig. 1) or in OCT and processed for cryosections (Fig. 7 and supplemental Fig. 2). Paraffin embedded tissue sections were deparaffinized and rehydrated and ISH processing was carried out using the InsituPro VSi automated in situ system (Intavis). Detection was carried out using an alkaline phosphatase conjugated anti-Digoxigenin antibody and visualized with nitro-blue tetrazolium chloride (NBT) and 5-bromo-4-chloro-3′indolyphosphate p-toluidine salt (BCIP). In situ hybridizations performed on cryosections were conducted according to Smith, 2008 and visualized using BM purple (Smith et al., 2008). Sense controls were run for all experiments and exhibited no staining (data not shown).
Fig. 1.
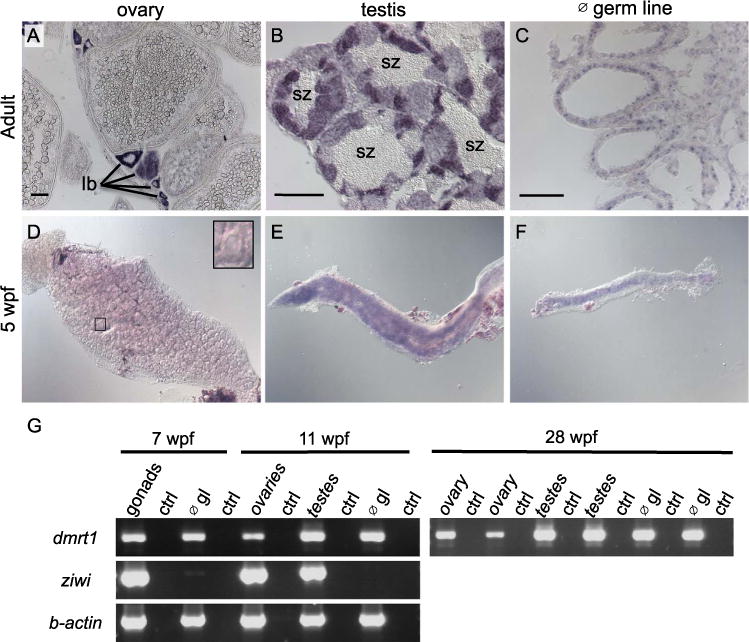
Dmrt1 expression in zebrafish gonads. A-C) Expression of dmrt1 by in situ hybridization on sections of adult gonads. A) In adult ovaries, dmrt1 expression was observed primarily in stage Ib oocytes; staging according to Selman et al. (Selman et al., 1993). B) Adult testis had dmrt1 expression in nearly all stages of spermatogenesis, except spermatozoa (sz). C) In testis devoid of germ cells (∅ germ line (gl)), dmrt1 was detected primarily in cells lining the testis tubules, indicative of Sertoli cell expression. D-F) Whole mount in situ hybridization on gonads from 5 weeks post fertilization (wpf) fish. D) Dmrt1 was not detected in gonads with ovarian morphology as judged by presence of many early stage oocytes (inset shows an early stage oocyte). E-F) Dmrt1 expression was detected in gonads that morphologically resembled testes (E) and in gonads devoid of germ cells (F). G) RT-PCR on gonadal tissue at 7 wpf, 11 wpf and 28 wpf. At 7 wpf, gonads could not easily be distinguished as ovary or testis during tissue harvesting, therefore, all gonads were mixed at this stage. By 11 wpf, dissected gonads were chosen that resembled either ovaries or testis as judged by morphology during dissection. RT-PCR was also performed on gonads that lacked germ cells (∅ gl) to detect expression in somatic tissues. RT-PCR for the ziwi transcript demonstrated that germ cells were present in intact gonads but absent from germ cell depleted gonads (∅ gl) at all ages (shown for 7 wpf and 11 wpf). Dmrt1 expression was detected by RT-PCR in all samples. Control RT-PCR samples (ctrl) were samples where no reverse-transcriptase was added to the reaction. For 28 wpf samples, two independent samples were run for each: each ovary sample was derived from a single individual while each testes sample contained testes from 4 or more individuals. RT-PCR results for these 28 wpf samples for ziwi and b-actin were previously published (Siegfried and Nüsslein-Volhard, 2008). scale bars =50 μm.
Fig. 7.
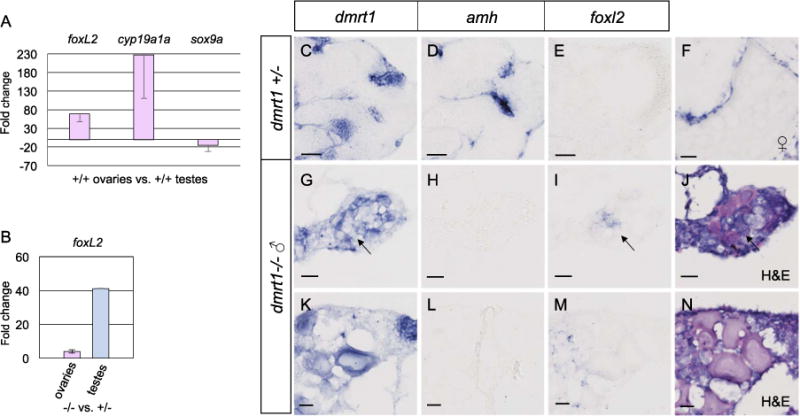
Foxl2 is antagonized by dmrt1 in zebrafish gonads. A-B) Quantitative RT-PCR of adult ovaries and testes from adult fish. A) Comparison of adult wild-type ovaries to testes showed that foxl2 expression was 68 fold higher in ovaries compared to testis, therefore exhibited ovary-enriched expression. The expected upregulation of the granulosa cell marker, cyp19a1a, and diminished expression of the Sertoli cell marker sox9a were also observed in these samples. B) Comparison of adult dmrt1E3ins mutant ovaries to wild-type ovaries (left) and mutant testes to wild-type testes (right) revealed a 4-fold increase in foxl2 expression in ovaries and a 41-fold increase in foxl2 in testes in dmrt1 mutants compared to wild-type. C-N) In situ hybridization on adult dmrt1C41X +/− and −/− gonad cryosections. C-E are serial sections of the same testis. G-I are serial sections of the same gonad and J is an H & E stain of panel I. K-M are serial sections of the same gonad and N is an H & E stain of M. Headings designate the probe used. C-E) Dmrt1 heterozygous testes showed expected localization of dmrt1 (C) and amh (D) in germ cells and Sertoli cells, respectively, whereas foxl2 (E) was not detected. F) In wild-type ovaries, foxl2 transcript localized to the somatic cells of pre-vitellogenic and vitellogenic oocytes, a vitellogenic oocyte is shown. G-N) Two dmrt1 male mutant gonads are shown. G-J) A testis-like gonad with scant residual oocytes. K-N) An intersex gonad containing pre-vitellogenic oocytes. G, K) Dmrt1 was expressed in residual oocytes, but was not detected in all germ cells (arrow). H, L) Amh was not detected. I, M) Misexpression of foxl2 was found interstitially, in presumptive gonial cells but not in the follicles, as supported by H & E staining (J, N). Detection of dmrt1 transcript in homozygous mutants is attributed to the dmrt1C41X allele producing mRNA. Scale bars=20 μm.
2.4. Antibody labeling
Immunohistochemistry was performed on 5 μm sections from 4% PFA fixed, paraffin embedded 3 wpf zebrafish torsos. Antigen retrieval was done with pH 6.4 sodium citrate buffer. Cleaved Caspase-3 primary antibody (Cell Signaling #9661) was used at 1:300 concentration and incubated at 4 degrees overnight. Cleaved Caspase-3 labeling was done for 16 gonads of each genotype (8 fish per genotype) and quantified by bright field microscopy. Percentage of apoptosis was calculated as the proportion of Cleaved Caspase-3 positive cells among total number of cells within the gonad in a given section, which was averaged over 5 sections of 5 μm each. An unpaired t-test was done to calculate the P value.
2.5. Sexing and genotyping fish to detect mutations
Fish were sexed based on morphologically apparent dimorphic features, such as color pattern and presence or absence of male-specific breeding tubercles, the latter of which gives pectoral fins of males a refractile appearance when viewed from above (McMillan et al., 2015). In some cases, fish were also sexed by dissection and visual examination of gonads. To genotype all alleles, the affected region of the locus was first amplified by PCR. For the dmrt1S10Xmutation, PCR products were sequenced with primer KS68 to identify the mutant allele. The retroviral insertion allele, dmrt1E3ins, was detected by nested PCR reactions using one primer that annealed within the retroviral insertion and the other in the dmrt1 gene (KS425 and KS426, followed by KS427 and KS428). The presence of a wild-type product was detected by a nested PCR reaction using first KW1 and KW2 primers followed by KW1 and KW3. The dmrt1C41X deletion allele was PCR amplified and either sequenced or heteroduplexed and heterozygotes detected on a high-resolution agarose gel using the primers BWD934 and BWD935. The latter approach was used to genotype crosses of homozygous mutant females to heterozygous male carriers.
2.6. Histological analysis of gonads
Fish were euthanized in tricaine solution prior to dissection of whole trunks. For adults, abdomens were opened and gonads grossly examined to determine sex prior to fixation. All trunks were fixed overnight in Bouin’s solution and embedded in paraffin prior to sectioning. Sections of 5 μm were stained using Harris hematoxylin and Eosin Y, according to standard protocols.
2.7. Ablation of germ cells
Wild-type zebrafish embryos were injected at the 1-cell stage with a morpholino targeting the deadend (dnd) gene, as described (Weidinger et al., 2003). Wild-type fish were injected in parallel to dmrt1 mutants and used to confirm germ cell loss.
2.8. RNA extraction and cDNA synthesis for RTPCR
Whole gonads were dissected from fish and collected directly into TRI Reagent. Ovaries were collected singly while testes were pooled from 3 to 5 males, depending on size. Tissues were homogenized with a motorized pestle and total RNA extraction was performed according to the manufacturer’s protocol. First-strand cDNA synthesis was reverse-transcribed from total RNA using AMV Reverse Transcriptase. All RTPCR gene-specific primers are listed in Supplementary Table 1. All primers were designed such that amplification from genomic DNA would give a larger band than that expected from cDNA amplification.
2.9. Quantitative RT-qPCR
Three biological replicates of gonads were collected for each sex and genotype, and RNA extraction and cDNA synthesis were conducted as described above. The ThermoFisher QuantStudio 12K Flex Real-Time PCR System was used for transcript quantification. Samples were tested in triplicate for each gene, and resultant CT values were averaged. Primer efficiencies and gene expression were calculated according to Pfaffl (2001). Data presented here were compared against the ubiquitous gene rpl13a. Primers for rpl13a RT-qPCR were as in Tang et al. (2007). All RT-qPCR gene-specific primers are listed in Supplementary Table 1 and were designed such that products could not be generated from genomic DNA.
3. Results
3.1. Expression of dmrt1 in developing and adult gonads
Dmrt1 is expressed in germ cells and somatic cells of the adult testes. Germ cell expression of dmrt1 in ovaries and testis of adult zebrafish was previously reported (Guo et al., 2005). In adult ovaries and testes, we also observed germ line expression in developing spermatocytes and oocytes by in situ hybridization (ISH) (Fig. 1A and B). Due to the intense expression in the spermatocytes, we could not determine whether or not dmrt1 was also expressed in somatic cells of the testes. By RT-PCR, dmrt1 transcript was detected in testes devoid of germ cells demonstrating that dmrt1 was expressed in somatic cells and that somatic cell-expression of dmrt1 was not dependent on germ cells (Fig. 1G). We next performed ISH on adult testes devoid of germ cells to better visualize expression in somatic tissues. Dmrt1 was expressed in cells within the testicular tubules where Sertoli cells reside (Fig. 1C). Thus, in zebrafish adults, dmrt1 is expressed in the germ line in both ovaries and testes and in the Sertoli cells of the testes.
To ask if dmrt1 is expressed during gonadal sex-differentiation, we performed RT-PCR and ISH on gonads isolated from juvenile fish. By ISH, 5 wpf, gonads that resembled ovaries typically had no detectable dmrt1 expression whereas those with strong expression typically resembled testes indicating that dmrt1 expression levels are sexually dimorphic during early stages of gonadal sex-differentiation (Fig. 1D and E). In addition, we detected dmrt1 expression in gonads of 5 wpf fish for which germ cells had been depleted, indicating that somatic cells expressed dmrt1 at this stage (Fig. 1F) Using RT-PCR, we found that dmrt1 is expressed in gonads at 7 weeks post fertilization (wpf) and 11 wpf in normal gonads and gonads devoid of germ cells (Fig. 1G). These ages represent later stages of gonadal differentiation (see Fig. 5). We conclude that dmrt1 is expressed in testicular somatic cells from 5 wpf through 11 wpf. We cannot determine if dmrt1 is also expressed in germ cells in testes at these stages. In conclusion, during gonadal development, dmrt1 expression was detected in testicular somatic tissues and correlated with developing testis during and subsequent to sex-differentiation.
Fig. 5.
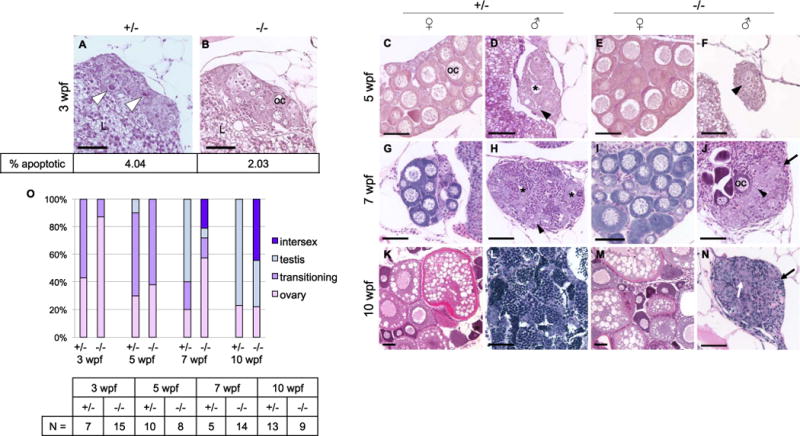
Dmrt1 is required for normal testis development and gonadal sex-differentiation. A-N) H & E staining of dmrt1E3ins heterozygous and homozygous mutant siblings throughout development. Specimens are progeny from a dmrt1 homozygous female and heterozygous male of from the Pair 1 family of Fig. 3B. These crosses consistently produced progeny with a strong female bias in mutants. A-B) At 3 wpf, mutant gonads typically had fewer apoptotic cells compared to heterozygous siblings (white arrowhead). Immunohistochemistry to detect Cleaved caspase 3 positive cells revealed half the quantity of apoptotic cells in mutant gonads compared to heterozygotes (2.03% vs. 4.04%, respectively, P value < 0.0001; N=16 gonads). C-F) 5 wpf heterozygotes progressed to early ovary (C) and testes (D) stages, as indicated by developing oocytes in females (oc), and loss of oocytes combined with lumen formation (asterisk) in males. Mutant gonads were either apparently normal ovaries (E) or testes with no lumens that were typically reduced in size compared to heterozygotes (F). G-J) At 7 wpf heterozygotes were fully committed female (G) and male (H) gonads, whereas mutants that were not clearly female formed either intersex gonads (J) (marked by dying oocytes alongside clustering spermatogonia (arrowhead)), or presumptive testes (not shown). The latter were never observed to form tubules or lumens, and had severely reduced spermatocytes. K-N) By 10 wpf heterozygotes were maturing with ova (K) or spermatids (L). The mutant males (N) were marked by increased stroma (black arrows), and lack of spermatogenesis, although clusters of germ cells were apparent (white arrow). E, I, M) All mutant females developed normally with no detectable phenotype. O) Quantification of the number of gonads in each histological category shown in panels A-N. The number of individuals, “N”, analyzed for each age group is shown in the table. Categories of gonad development observed in each age group reveal delayed transition to testes fate in mutants. Ovary=comprised mainly of oocytes of various stages; transitioning=one or more of the following: apoptotic cells, lumen formation, loss of oocytes, gonial cell clustering; intersex=containing maturing oocytes in addition to testis-like gonial clusters and stroma; testis=containing clusters of spermatogonia (or spermatocytes), having no oocytes, lumen if heterozygous. Apoptotic cells=white arrowhead; spermatogonia=black arrowhead; lumen=black asterisk; oocyte=oc; stroma=black arrow; germ cell cluster=white arrow; liver=L. Scale bar=50 μm.
3.2. Isolation of dmrt1 mutants
To assess the function of dmrt1 in zebrafish sex determination and gonadal development, we isolated and analyzed mutations affecting this gene (Fig. 2). All mutations occur within the first exon of dmrt1, which is present in every known splice form of this gene (Fig. 2B) (Guo et al., 2005; Schulz et al., 2007). The dmrt1 gene encodes a transcription factor and all mutations are predicted to truncate the DNA binding domain of the encoded protein (Fig. 2B). The dmrt1t32242 mutation is a C to A transversion resulting in a premature termination codon at codon 10, dmrt1ZM00173004 is a retroviral insertion following the 8th nucleotide of the coding sequence, leading to disruption of the gene product after the 2nd codon and dmrt1uc27and dmrt1uc67 are seven and nine nucleotide deletions, respectively, that are both predicted to cause a premature termination at codon 41. For simplicity we refer to these mutations as dmrt1S10X, dmrt1E3ins and dmrt1C41X, respectively, as this nomenclature indicates the effect each allele has on the resulting protein. While all mutations were predicted to severely disrupt protein function, the dmrt1S10X mutants exhibited a weaker phenotype, suggesting that it is a hypomorphic allele (described below).
Fig. 2.
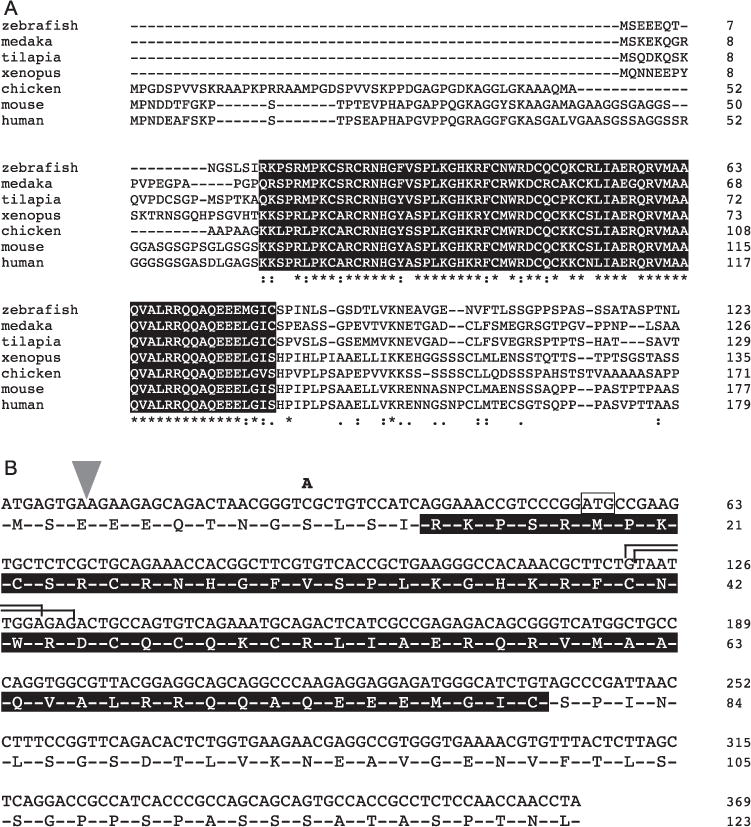
Mutations disrupting the dmrt1 locus are predicted to disrupt the conserved DM domain. A) The DM domain, boxed in black, of Dmrt1 is highly conserved across species. B) The four mutations disrupting zebrafish dmrt1 are shown: a retroviral insertion after the 8th nucleotide of the coding sequence (wedge); a cytosine to adenine generating a nonsense mutation at codon 10; two TALEN-mediated deletions of nucleotides 122–128 (top bracket) and 123 - 131 (bottom bracket) of the coding sequence, both resulting in a premature stop at codon 41. A putative alternative ATG that may be used for translation initiation in the S10X allele is boxed. All sequences shown correspond to exons 1 and 2 of zebrafish dmrt1.
3.3. dmrt1 is required for normal sex determination
All dmrt1 mutant alleles exhibited skewed sex-ratios with an overwhelming female bias among homozygous mutants (Fig. 3A–C). The dmrt1S10X allele was studied in two mixed genetic backgrounds, Tue/WIK/TLF and Tue/WIK. Progeny derived from multiple heterozygous parents were raised together then genotyped and assayed for sexual phenotype as adults. In both backgrounds, between 80% and 90% of the dmrt1S10X homozygotes were female in contrast their wild-type counterparts, which were 69% and 63% female (Fig. 3A).
Fig. 3.
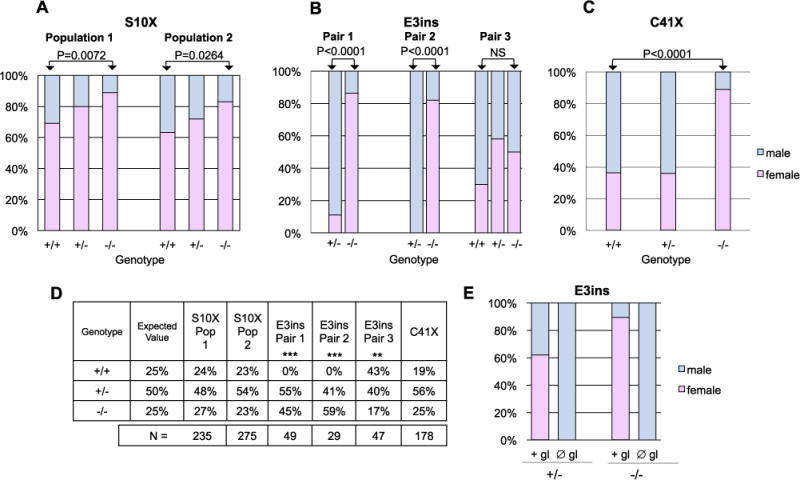
Dmrt1 mutants exhibit female-biased sex ratios. A-E) All data are from progeny derived from heterozygous incrosses, except for (E), which are progeny from homozygous mutant females crossed to heterozygous males. A-C) Charts show the sex ratios of a given genotype. All homozygous mutants, with the exception of Pair 3 in B, displayed significantly female biased sex ratios compared to wild-type siblings based on Fisher’s Exact Test (NS=not significant). A) Progeny of multiple crosses were pooled prior to raising. Sex-ratios of two populations of mixed backgrounds carrying dmrt1S10X are shown; population 1 is a mixed Tue/Wik background; population 2 is a mixed Tue/Wik/TLF background. B) Sex-ratios of progeny derived from three independent pairs carrying dmrt1E3ins. C) Sex ratios of progeny derived from multiple pairs carrying dmrt1C41X. D) Genotypic ratios of populations represented in A-C. Only segregation of the dmrt1E3ins allele deviated significantly from expected Mendelian ratios (Χ2 goodness of fit test, ** P < 0.02; *** is P < 0.002). E) Sex ratios of progeny derived from crossing dmrt1E3ins −/− females to +/− males. Embryos from each cross were split into two groups and either injected with a morpholino oligonucleotide targeting the dead end gene to deplete the germ line (∅ gl) or were not injected (+ gl). Sex was scored in adult fish. The female bias in homozygous dmrt1 mutants was completely reversed by depletion of the germline (N =32 and 31 for + gl and ∅ gl, respectively).
A similar female bias was observed in fish homozygous for the dmrt1E3ins allele (Fig. 3B). Three pairs of dmrt1E3ins heterozygotes were incrossed and their progeny were genotyped and sexed as adults. Homozygous dmrt1E3ins mutants from parental pairs 1 and 2 had severely female-biased sex ratios, having fewer than 20% males (Fig. 3B). The heterozygous siblings were largely male (89% and 100%) in these two populations. Surprisingly, no genetically wild-type progeny were present in these populations. Further analysis of this non-Mendelian segregation pattern suggests that this is due to a segregation distorter locus, or “selfish genetic element” (described in supplemental data). This non-Mendelian segregation pattern was observed only for this mutant allele and is therefore not likely to be a direct result of dmrt1 loss of function. The dmrt1 mutant progeny from Pair 3 had a larger percentage of males compared to other populations analyzed and did not display a significant female bias compared to wild-type siblings (Fig. 3B). However, in subsequent generations of this family, the dmrt1 mutants displayed a significant female bias (Supplementary Table 2).
The, dmrt1C41X, alleles were derived and maintained in an AB background and showed similar female biased sex-ratios as the other two alleles when homozygous (Fig. 3C). Combined clutches derived from heterozygous dmrt1C41X incrosses were 89% female as homozygotes, whereas only 37% of heterozygotes and 36% of wild-type fish were female (Fig. 3C). In summary, each of the dmrt1 mutant alleles resulted in similar female-biased sex ratios in homozygous populations, typically having less than 20% males.
To ask whether the female bias was due to sex-specific lethality of dmrt1 mutants, we analyzed the genotypic ratios of the heterozygous incrosses (Fig. 3D). Both the dmrt1S10X and dmrt1C41X alleles segregated following expected Mendelian genotypic ratios, therefore the sex-bias was not caused by sex-specific lethality of dmrt1 mutants (Fig. 3D). The dmrt1E3insallele exhibited non-Mendelian segregation. However, the non-Mendelian segregation pattern was detected as early as 4–6 h post fertilization (Supplementary Table 3B), weeks prior to histological signs of sex differentiation. Thus, sex-specific lethality is unlikely to have caused the sex-bias in this allele.
The female-biased sex ratios seen in dmrt1 homozygous mutants suggest that loss of dmrt1 function disrupts male sex determination in zebrafish. Interestingly, A small percentage of males developed in the absence of dmrt1 function demonstrating that dmrt1 is not required for male sex determination in zebrafish.
As germ cell loss results in all male populations in zebrafish, we asked if the female-biased sex ratios seen in dmrt1 mutants would be affected in fish devoid of germ cells. To this end, we depleted germ cells in dmrt1 mutants and compared sex-ratios to un-manipulated siblings. Interestingly, depletion of germ cells in dmrt1 mutants resulted in a complete reversal of the female sex-bias such that 100% of homozygotes were male (Fig. 3E). We observed this effect with the dmrt1S10X (data not shown) and dmrt1E3ins (Fig. 3E) alleles, the dmrt1C41X allele was not tested. Therefore, germ cells are required for ovary fate even in the absence of dmrt1.
3.4. dmrt1 has a sex-specific role in testis development
Expression of dmrt1 was detected in early stage oocytes (Fig. 1A) but homozygous dmrt1 mutant females appeared normal and were fertile. Homozygous females of all alleles reached sexual maturity by three months of age, laid consistently large clutches and exhibited no apparent morphological defects. They presented histologically normal ovaries, and fertility assays resulted in 75% or higher fertilization rates, similar to wild-type females (data not shown). Furthermore, homozygous mutant females gave rise to viable and fertile offspring. We conclude that dmrt1 does not have a major role in ovary development or function.
Although dmrt1 mutants were primarily female, ~10% developed as phenotypic males, which facilitated the study of dmrt1 function in zebrafish testis development. Adult dmrt1 mutant males had normal secondary sex-characteristics but were sterile. To determine the cause of sterility, we compared the structure of mutant and wild-type gonads by histology (Fig. 4). Wild-type adult zebrafish testes are paired organs forming elongated structures, internally organized into tubules. These tubules are formed by seminiferous epithelium containing cysts of spermatogonia and spermatocytes surrounded by somatic support cells, called Sertoli cells (SCs). Cysts are arranged around a lumen containing spermatozoa that is connected to the efferent ducts. The interstitial regions between the tubules contain the steroid-producing Leydig cells, connective tissue, and blood vessels (Fig. 4A) (Leal et al., 2009).
Fig. 4.
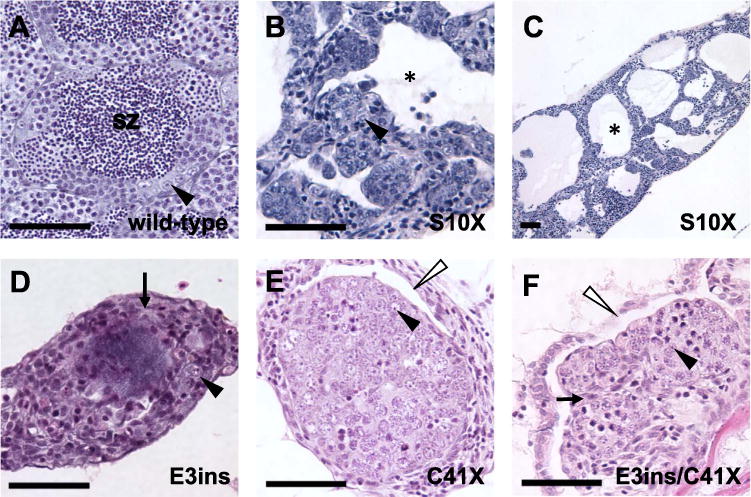
Adult homozygous dmrt1 mutants have disorganized testes with reduced germ cells. A-F) H & E staining of adult male gonads. A) Wild-type adult testes are organized into tubules, with cysts of differentiating germ cells surrounding lumens containing spermatozoa (sz). B and C) Dmrt1S10X mutants form enlarged testis tubules with reduced numbers of germ cells and no spermatozoa. Tubules have large lumens devoid of germ cells (e.g. black asterisk). D) dmrt1E3ins and E) dmrt1C41X mutant testes exhibit similar phenotypes, often displaying increased stroma (e.g. arrows in D, F), with few germ cells and retention of ovarian cavities (white arrowheads). Neither genotype organizes testis tubules nor forms lumens. F) A transheterozygous dmrt1E3ins/C41X mutant looks highly similar to both dmrt1E3insand dmrt1C41Xhomozygotes. Spermatogonia=black arrowhead; lumen filled with spermatozoa=sz; lumen lacking spermatozoa=black asterisk; stroma=arrow; ovarian cavities=white arrowhead. Scale bar=50 μm.
In comparison to wild-type testes, the testes of adult dmrt1 mutant males exhibited abnormal organization and aberrant spermatogenesis. Both dmrt1E3insand dmrt1C41Xmutant males had hypoplastic testes that lacked organized testis tubules entirely (Fig. 4D and E). Testes of six-month-old wild-type males contained germ cells representing all stages of spermatogenesis. By contrast, few germ cells were present in mutant siblings, which typically appeared to be undifferentiated (i.e. pre-meiotic) and occasionally a few oocytes were present. By seven months the testes of most mutant males were devoid of germ cells. Interestingly, structures that histologically resembled ovarian cavities, which initially form in association with the juvenile ovary in all zebrafish but regress in males (Takahashi, 1977), persisted in mutant testes through adulthood, indicating partial feminization of the gonadal ducts (Fig. 4E and F). The predicted disruption in the encoded protein by these two alleles and the similarity of the mutant phenotypes suggests that both are null alleles.
Dmrt1S10X mutant testes presented a less severe phenotype than either of the null alleles. Adult mutant males typically had severely enlarged, fluid-filled testis tubules (Fig. 4C). These were either devoid of germ cells or contained single or small clusters of spermatogonial-like cells, as seen by histology (Fig. 4B) and in situ hybridization for the vasa transcript (not shown). Some areas of the testis appeared disorganized and contained small clusters of germ cells (Fig. 4B). Occasionally, meiotic germ cells were seen, but mature sperm were never observed. We interpreted this phenotype to be the result of aberrant testis tubule morphogenesis and germ cell development. Because testes tubules were formed in dmrt1S10X homozygous mutants, but not in the apparent null mutants, this allele might represent a reduction rather than a loss of function of dmrt1. To test this hypothesis, we assessed the phenotype of fish trans-heterozygous for dmrtS10X/dmrt1E3ins. Testes from trans-heterozygous fish most closely resembled those of dmrt1E3ins mutants in that they lacked tubules, and primarily contained stroma, and few to no germ cells (Fig. 4F). We conclude that dmrtS10X is a hypomorphic allele. We postulate that a truncated protein is produced through initiation of translation at a downstream ATG codon present at codon 19 of the transcript (Fig. 2B), resulting in production of a protein with partial function.
3.5. dmrt1 mutants display aberrant male sexual differentiation
Juvenile zebrafish gonads are bipotential, but form an immature ovary until approximately 3–4 wpf when a differentiation event occurs. At this point, morphological gonadal sex-differentiation is apparent by histology. In females, oocytes will persist and support female sexual fate. Otherwise, an apoptotic event degrades the oocytes and the now male-fated gonad will have an increase in somatic stromal cells and gonial cells will begin differentiation as spermatocytes.
To determine the age at which dmrt1 loss of function interferes with testes development we made crosses of dmrt1E3ins homozygous females to heterozygous males and raised the offspring to 3, 5, 7, and 10 wpf to analyze gonad development (Fig. 5). This crossing strategy was used as this pairing was expected to produce a larger number of homozygous mutants than heterozygous crosses (50% mutant vs. 25% mutant with a heterozygote incross). Given that heterozygous males showed normal testis morphology and fertility, they were used for comparison in lieu of genotypic wild-type fish. Gonads were observed for each time point by histology.
No gross histological variation was visible between mutants and heterozygotes at 3 wpf, before the occurrence of gonadal sex differentiation (Fig. 5A and B). However, 3 wpf mutants largely lacked evidence of apoptosis by histology, which is a hallmark of the transition to male sexual fate (Fig. 5 A and B). The average proportion of apoptotic cells was quantified in 3 wpf heterozygous and mutant gonads by immunohistochemistry with anti-Cleaved Caspase-3 antibodies. In transitioning heterozygotes, an average of 4.04% of germ cells were positive for Cleaved Caspase-3, whereas mutant gonads typically presented half this quantity (Fig. 5 A and B). By 5 wpf male heterozygotes were typically in a state of transition between immature ovary and testis-like, displaying apoptosis, germ cell clustering, and lumen formation (Fig. 5D and O). A small percentage of males had clear spermatocytes and were therefore classified as having testes (Fig. 5O). Among mutant fish, many had transitioning gonads that showed decreased oocytes, increased stroma, and no clear lumen formation (Fig. 5F and O). At this stage there was no apparent spermatocyte development in mutant gonads and therefore none were classified as having testes (Fig. 5O). At 7 wpf and 10 wpf, all presumed male mutants displayed abnormal testes (Fig. 5J and N). However, in many mutants, scattered pre-vitellogenic oocytes were visible alongside testis tissue, whereas heterozygous gonads were clearly either ovary or testis, with a few still transitioning at 7 wpf (Fig. 5 G–J). Mutants with oocyte-containing testis-like gonads were categorized as intersex (Fig. 5O). Intersex gonads were considered distinct from bipotential juvenile ovaries in that they exhibited hallmarks of male specification, such as cord-like clustering of germ cells and stromal ingrowth in addition to oocytes. By 10 wpf some mutant animals appeared to have clearly determined testes, however these were small and had reduced numbers of undifferentiated germ cells in comparison to wild-type testes, and that were interspersed with stromal cells (Fig. 5N). Overall these observations show that lack of dmrt1 in developing males results in delayed transition from the bipotential gonad to a committed testes fate, persistence of oocytes, and an inability to support differentiation of male germ cells or tubular organization of testicular somatic cells.
3.6. Loss of dmrt1 causes misregulation of sex-associated genes
To determine how loss of dmrt1 contributes to the failure of the testis-promoting program in the developing gonad, we looked at expression of germ cell, testis-, and ovary-expressed genes in adult zebrafish using RT-PCR. Comparison of young adult (3 month old; mo) and advanced adult (7 mo) gonads using the germ cell marker deadend (dnd) revealed loss of germ cells during adulthood in dmrt1 homozygous mutants over time (Fig. 6A), consistent with our histological analysis. Expression of germ cell markers and ovary-specific cyp19a1a were normal in mutant ovaries, and the latter was not observed to be upregulated in mutant testes (Fig. 6A).
Fig. 6.
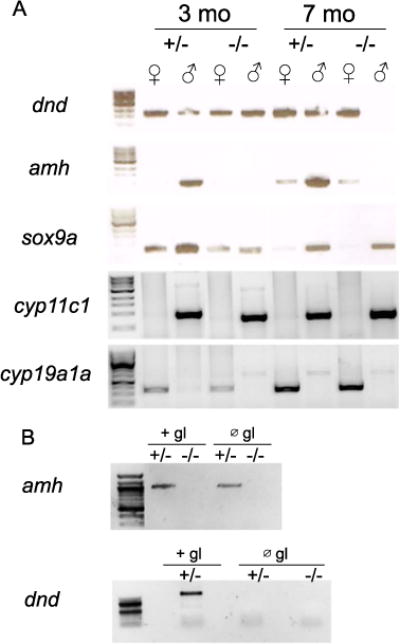
Expression of anti-Müllerian hormone (amh) is dependent on dmrt1 in adult testes. A) RT-PCR on RNA derived from ovaries (♀) and testes (♂) from 3 month old (3 mo) and 7 month old (7 mo) adults showing standard markers of gonad cell lineages: dnd=germline; amh and sox9a=Sertoli cells; cyp11c1=Leydig cells; cyp19a1a (aromatase)=granulosa cells. Gonads from heterozygous (+/−) and homozygous (−/−) dmrt1E3ins mutants were analyzed. Amh expression was undetectable from 3 months onward in dmrt1 mutant testes. Expression of the germ cell-specific gene dnd was retained in 3 month old mutant testis, but was lost by 7 months. All other gonad markers were expressed as expected. B) Depletion of the germline (∅ gl), mediated by injection of a morpholino targeting the dnd gene, demonstrated that loss of amh expression resulted from its dependence on dmrt1 and not lack of germ cells. Depletion of the germ line was confirmed by the absence dnd expression, a germ cell-specific gene. All primers were designed such that amplification from genomic DNA would give a larger band than that expected from cDNA amplification.
To investigate if loss of dmrt1 affected testis differentiation and/or androgen synthesis, we assayed the expression of the somatic testis-expressed genes sox9a, amh, and cyp11c1 (cytochrome P450, family 11, subfamily C, polypeptide 1) by RT-PCR using isolated gonads from adult fish. In wild-type adult testes, Sox9a and amh are normally expressed in Sertoli cells whereas cyp11c1 is expressed in Leydig cells (Chiang et al., 2001; Rodríguez-Marí et al., 2005; Wang and Orban, 2007). In dmrt1 mutant testes, Sox9a expression appeared normal, whereas amh was not detected (Fig. 6A). By contrast, amh expression was detected equally in wild-type and mutant ovaries. Finally, cyp11c1 was detected in both wild-type and dmrt1 mutant testes indicating that dmrt1 may not be required for androgen synthesis. This is supported by the observation that dmrt1 mutant males exhibited normal secondary sex characteristics, such as male pigmentation and presence of breeding tubercles (not shown). However, dmrt1E3ins mutants were unable to induce female spawning (not shown), suggesting that dmrt1 may have a role in sex-specific behaviors.
To ask whether the loss of amh expression was merely a result of germ cell loss in the dmrt1 mutants, we depleted germ cells in offspring derived from dmrt1E3ins ♂ +/− × ♀ −/− crosses, and analyzed amh expression in adult testes (Fig. 6B). Germ cell depletion was done by injection of a morpholino oligonucleotide targeting the dead end (dnd) gene, which is essential for PGC survival during embryogenesis (Weidinger et al., 2003). Expression of amh was normal in heterozygous males lacking germ cells, but was undetectable in dmrt1 mutant males, consistent with our results above and indicating that amh expression in Sertoli cells is dependent on dmrt1 even in the absence of germ cells.
In mammals and tilapia, dmrt1 has been shown to have an antagonistic relationship with ovary-promoting genes, specifically foxl2 (Li et al., 2013; Matson et al., 2011). We first asked whether foxl2 expression is ovary-specific, in zebrafish, as in mammals. Initial RT-PCR showed that foxl2 was expressed in the adult gonads of both sexes (data not shown). To test whether foxl2 expression levels differed between sexes, RT-qPCR was conducted on wild-type adult ovaries and testes (Fig. 7A). Expression of foxl2 was 68 fold higher in ovaries compared to testes. As a control, we also analyzed two genes known to be ovary-specific and testis-enriched, cyp19a1a and sox9a, respectively. Both exhibited the expected ovary- or testis-enriched expression in our samples. Therefore, foxl2 expression is ovary-enriched in zebrafish.
Next we investigated whether dmrt1 is involved in the repression of foxl2 in zebrafish, as it is in mammals. RT-qPCR comparing homozygous adult mutant ovaries to ovaries from heterozygous siblings revealed an average 4.5-fold increase in foxl2 expression resulting from loss of dmrt1 (Fig. 7B). Mutant testes displayed a 41-fold increase in foxl2 expression relative to heterozygotes. Analysis of localization of amh and foxl2 transcripts in adult zebrafish gonads by in situ hybridization supports these findings (Fig. 7 C–N). As expected, amh expression was detected in Sertoli cells of the heterozygous gonad, though no transcript was detected in dmrt1 mutant gonads (Fig. 7D, H, L). In wild-type ovaries, foxl2 expression was limited to the cells surrounding some pre-vitellogenic and vitellogenic oocytes (Fig. 7D, supplemental Fig. 2). Testes from dmrt1+/− fish did not express detectable levels of foxl2 whereas gonads from phenotypically male dmrt1 mutants displayed foxl2 misexpression (Fig. 7E, I, M). H & E staining of these sections confirmed that the foxl2 expression was not merely attributable to oocytes or ovarian structures (Fig. 7J, N). Although, the mutant male gonads contained some immature oocytes, foxl2 expressing cells were present in some regions of the gonad that did not contain oocytes. These results indicate that foxl2 is relieved from repression in the absence of dmrt1 expression in male gonads, thus the interaction and roles of these sex-associated genes may be conserved in mammals and zebrafish.
4. Discussion
Here we have reported the first study of dmrt1 function in zebrafish. Through our analysis of dmrt1 mutants, we have shown that loss of dmrt1 disrupts normal male sexual development from the onset of histological gonadal sex differentiation. Dmrt1 mutant males exhibited sterility, disorganized testes, and aberrant spermatogenesis before ultimate germ cell depletion, whereas females developed normally and were fertile. Notably, we observed a strong female bias among dmrt1 mutants suggesting an important role for this gene in zebrafish male sex determination. We found that amh expression was dependent on dmrt1 in somatic cells of the testis, but not in the ovary. Furthermore, foxl2 repression in males required dmrt1 function, as in other animals. Our results place zebrafish dmrt1 in a role similar to that of other vertebrates in that it specifies male sexual fate. As amh expression is dependent on dmrt1 in male mutants, dmrt1 may carry out this role, in part, through activation of amh expression.
4.1. dmrt1 has a sex-specific role in testis development
Loss of dmrt1 resulted in male infertility while females were apparently normal. Although dmrt1 is expressed in ovaries and testes, it has been shown to have much stronger expression in adult testes (Guo et al., 2005). In juvenile fish, we did not detect dmrt1 expression in ovaries by in situ hybridization, whereas testes exhibited clear expression. Therefore, dmrt1 expression was greatly reduced or absent in developing ovaries compared to testes, suggesting a male-specific role in gonadal sex differentiation.
In all stages analyzed, ovaries from dmrt1 mutant fish appeared normal and mutant females were fertile even though dmrt1 is expressed in early-stage oocytes in adults. By contrast, males exhibited aberrant testis development at the earliest histological stages of sex differentiation and were sterile. Therefore dmrt1 is essential for male development but not for female reproductive function. However, it is possible that subtle phenotypes are present in dmrt1 mutant females that do not lead to infertility.
The phenotype we report links loss of dmrt1 with reduced male-specific apoptosis during the transition from the juvenile ovary to testis, failure to form testis tubules, and complete failure of spermatogenesis. While male sterility was universally observed with all mutant alleles, tubule dysgenesis was less severe in dmrt1S10X testes despite the early termination codon. We hypothesize that a protein product is made from this mutant allele by translation initiation at a downstream methionine at codon 19, leading to a hypomorphic allele. The other two alleles, which are predicted null, displayed complete failure to form testes tubules in addition to aberrant spermatogenesis.
It has been observed that testis size and spermatogenic output is limited by the total number of Sertoli cells (Leal et al., 2009). In dmrt1 mutant testes, we detected expression of sox9a, suggesting that Sertoli cells are present in dmrt1 mutants. However, these cells fail to express amh indicating that they are not normal. Thus, dmrt1 is not essential for Sertoli cells to develop, but is necessary for them to carry out at least some of their normal functions, which includes orchestrating testes morphogenesis.
In mouse, dmrt1 has been shown to have a germ cell autonomous role in spermatogenesis as well as a non-autonomous role in the Sertoli cells to promote germ cell development (Agbor et al., 2012; Kim et al., 2007). We found that zebrafish dmrt1 is expressed in Sertoli cells and germ cells as was reported in mice. It is therefore possible that the germ cell defects we observed in dmrt1 mutant testes are due to loss of dmrt1 function in both of these cell types. Tissue-specific analysis of dmrt1 loss in zebrafish testes will be necessary to dissect its precise role in these two cell types.
4.2. dmrt1 in zebrafish sex determination
As primary sex determination is not well defined in zebrafish, it is difficult to determine whether zebrafish dmrt1 functions in primary sex determination, promotion of sex-differentiation, maintenance of sex determination, or a combination of these. We observed defects in testis-specific apoptosis in dmrt1 mutants, which is the earliest described sex-specific differentiation event in zebrafish. These data suggest that dmrt1 is an early regulator of sex-specification and a candidate gene for controlling primary sex determination. Laboratory zebrafish strains have polygenic sex determination and several studies have identified multiple different genomic loci that contribute to sexual fate (Anderson et al., 2012; Bradley et al., 2011; Howe et al., 2013; Liew et al., 2012; Luzio et al., 2015a; Wilson et al., 2014). Bradley et al. identified a genomic region linked to sex that includes the dmrt1 gene, accounting for 7% of the trait variance. Analysis of the dmrt1 locus between males and females identified several differences including a SNP that lies within a conserved regulatory motif located in the 3′UTR of dmrt1 (Bradley et al., 2011). Based on data from Medaka, the female-associated SNP is predicted to result in decreased expression of dmrt1 and is therefore a good candidate for the causative variant at this sex-associated region (Bradley et al., 2011; Herpin et al., 2009). Here we have shown that reduction or loss of dmrt1 function strongly correlates with female sex determination. Thus, our data support the notion that polymorphisms abrogating dmrt1 function may affect sex determination in natural populations and laboratory strains of zebra-fish.
Current data on zebrafish sex differentiation indicate that there is a germ cell signal secreted by oocytes that promotes ovarian fate determination. During normal development, at late larval to early juvenile stages, zebrafish pass through a “juvenile ovary” stage of development, during which time all animals produce immature oocytes (Takahashi, 1977). The juvenile ovary expresses genes that later become either ovary or testis-specific and is therefore apparently bipotential (Rodríguez-Marí et al., 2005). When germ cells, and more specifically meiotic oocytes, are absent during the bipotential stage, ovarian fate cannot be maintained and testes develop, resulting in adoption of male organismal fate (Rodríguez-Marí et al., 2010; Siegfried and Nüsslein-Volhard, 2008). Furthermore, there is a strong correlation between the number of germ cells an individual has during gonadal development and their eventual sexual fate (X. G. Wang et al., 2007a, 2007b; Tzung et al., 2015). These data suggest that a threshold level of oocytes is required for ovarian fate and therefore female sex-determination. It is postulated that fish with more germ cells produce more of an ovary-promoting signal from the oocytes of the juvenile ovary and therefore assume female sexual fate while those with fewer oocytes become males. It is not yet clear if zebrafish sex determination involves genetic regulation of germ cell numbers, which in turn regulates testis vs. ovary fate, or if germ cell numbers are naturally variable irrespective of sex determination signals. Regardless, the role of oocytes in promoting female development continues into adulthood, as adult females that are depleted of oocytes readily sex-reverse and become males with functional testes (Dranow et al., 2016, 2013).
It was possible that the main role of the female-promoting oocyte signal during primary sex determination was to down-regulate Dmrt1 function, thus allowing for female development as a default. If this were the case, then dmrt1 mutants would have been capable of female development even in the absence of the ability to produce oocytes. However, we instead found that germ cells were still necessary for specification of female sexual fate even in the absence of dmrt1 (Fig. 3E). Therefore Dmrt1 likely suppresses female fate through countering the female-promoting oocyte signal. This suggests that this signal does not function solely to inhibit male development, but more likely is involved in actively promoting female-specific gene expression in the early somatic gonad. Unfortunately, the female-promoting oocyte signal has yet to be identified making it difficult to further dissect these interactions.
The inhibitory effect that dmrt1 exerts on the female-promoting germ cell signal, may stem from a role in promoting germ cell apoptosis. The earliest histological difference between zebrafish males and females is that of elevated apoptosis in male-specified gonads as they transition from the juvenile ovary stage to developing testes (Takahashi, 1977; Uchida et al., 2002). This apoptotic wave clears juvenile oocytes in gonads that adopt testis fate. We found that the occurrence of apoptosis in dmrt1 mutant gonads at 3 wpf was half that of heterozygotes, suggesting that dmrt1 is necessary for this male-specific wave of programmed oocyte death. By 7 wpf, when wild-type siblings contained either an obvious ovary or testis, the majority of dmrt1 mutant gonads appeared to be either a morphologically normal ovary or an intersex gonad, the latter of which contained a mixture of oogenic and spermatogenic germ cells. A third, more rare mutant category had gonads that appeared testis-like, albeit abnormal. These data suggest that one function of Dmrt1 is to down regulate the ovary-promoting oocyte signal by promoting the clearance of early stage oocytes during the juvenile ovary-to-testis transition of gonadal sex differentiation.
4.3. Why so many females?
We suggest a model to explain the high frequency of females observed in dmrt1 mutants based on the developmental progression of the mutant phenotype (Fig. 8). The failure to clear juvenile oocytes, via oocyte apoptosis, during the juvenile ovary to testis transition results in intersex gonads in juvenile mutant fish. We hypothesize that these intersex gonads continue to produce ovary-promoting signals from remaining oocytes. In addition, the testis portion of intersex gonads fails to develop properly, due to a requirement of dmrt1 in testis development. We propose that these intersex gonads fail to fully commit to testis fate and eventually adopt ovary fate resulting in development into normal adult females. Thus, the most severe mutant phenotype is development of a normal female after failed juvenile ovary to testis transition. A small percentage of dmrt1 mutants commit to male fate, but display aberrant testis development due to the lack of dmrt1 function. This model is supported by the proportion of intersex gonads observed at 7 and 10 wpf relative to the female sex bias observed in the adult mutant populations. The data shown for proportions of intersex individuals in Fig. 5 was derived from progeny of multiple crosses of dmrtE3ins homozygous females to heterozygous males, from which we consistently observed female-biased sex ratios of 80% or more females (not shown), as was typical in progeny derived from heterozygous incrosses (Fig. 3B, Supplemental Table 2). In summary, dmrt1 may be necessary to promote male development by initiating degradation of female structures and facilitating commitment to testis identity, and thus likely functions through similar mechanisms as in other animals, by repressing female development and promoting male development.
Fig. 8.
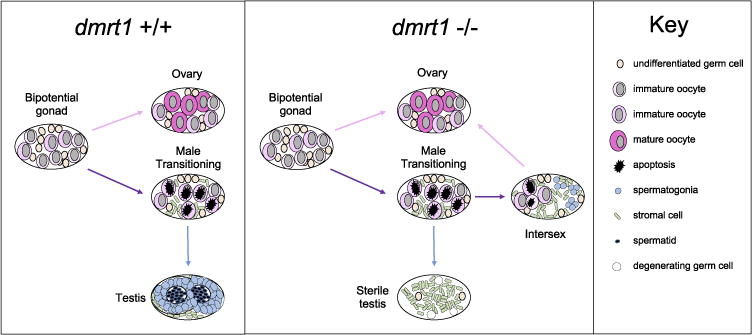
A model for the requirement of dmrt1 in zebrafish testis development. Wild-type juvenile zebrafish gonads are bipotential with immature oocytes until a sexual differentiation event occurs between 3 and 4 weeks post fertilization. Determined females will develop normal ovaries with oogonia that proceed through oocyte maturation. Fish that will become male undergo a transitional stage, during which a wave of apoptosis depletes oocytes. Undifferentiated germ cells will undergo a male-specific program and develop into spermatogonia and ultimately become organized within the testes tubules. In the absence of dmrt1, male transitioning gonads retain ovarian tissues, and have reduced apoptosis. Testis lumen formation, which forms the testis tubules, is also not initiated. A majority of the dmrt1 −/− transitioning fish retain maturing oocytes alongside nascent spermatogonia in a state we term intersex. In this model, these intersex fish revert to ovary development and become normal females, accounting for the biased sex ratios we report. A small subset of dmrt1 −/− fish will become male, although they are sterile and never develop normal testis structures, ultimately losing all germ cells. Female dmrt1 −/− fish develop normal ovaries.
Evidence of uninhibited ovarian development in dmrt1 mutants was also visible in the persistence of an ovarian cavity in males, which forms the female reproductive ducts. This cavity forms in association with the bipotential gonad and, in males, regresses during sex differentiation (Takahashi, 1977; Uchida et al., 2002). We observed perdurance of an ovarian cavity in dmrt1 mutant male zebrafish into adulthood (Fig. 1 E and F). Therefore, despite eventual degeneration of oocytes in these mutants, at least one female-specific tissue remains. It is tempting to speculate that the degeneration of the ovarian cavity in zebrafish mimics that of degeneration of the female Müllerian ducts in mammals. In mammals, Amh is secreted from the testis and signals degeneration of the Müllerian ducts in males. We observed retention of the female reproductive ducts in dmrt1 mutants and loss of amh expression by the testes. It would be interesting to test if the degeneration of the female reproductive ducts is dependent on Amh signaling in zebrafish as it is in mammals.
4.4. Transcriptional regulation of sex-enriched genes by Dmrt1 may be key to sexual differentiation
The persistence of oocytes and ovarian cavities in presumed dmrt1 mutant males implies suppressive functions of dmrt1 in zebrafish sex-differentiation, specifically in regard to ovary development. We hypothesize that dmrt1 carries out this role by suppressing expression of genes regulating ovary fate and differentiation. In zebrafish, cyp19a1a, which encodes Aromatase that converts androgens to estrogens, is expressed in somatic cells of the ovaries but not in testes (Rodríguez-Marí et al., 2005). Testicular dmrt1 expression opposes cyp19a1a in Nile tilapia as well as cyp19a1 in chicken (Lambeth et al., 2014; Li et al., 2013). We did not detect upregulation of cyp19a1a in zebrafish dmrt1 mutant testes, however we only tested adult animals that had already committed to a testis fate. Because estrogen treatment is sufficient to fully sex-reverse zebrafish, we predict that overproduction of cyp19a1a would lead to complete feminization. It will therefore be important to determine if cyp19a1a is dysregulated during sex-differentiation in dmrt1 mutants, which may help define the mechanism that causes the female-bias in dmrt1 mutants.
In tilapia and mouse, FoxL2 is a female-expressed antagonist of Dmrt1 expression and regulates female sex-promoting genes such as Cyp19a1 (Georges et al., 2014; Wang et al., 2007a, 2007b). In mammals, Dmrt1 directly suppresses FoxL2 expression in testes and FoxL2 is necessary for suppression of Dmrt1 expression in ovaries (Matson et al., 2011; Uhlenhaut et al., 2009). Here we have shown that zebrafish foxl2 has ovary-enriched expression, as it does in mammals, and that Dmrt1 is necessary for its down-regulation. Thus, the antagonistic relationship between FoxL2 and Dmrt1 during sex determination may be conserved in all vertebrates. Although no previous studies have directly tested the role of foxl2 in zebrafish sex differentiation, foxl2 disruption in Tilapia has been shown to cause masculinization of XX animals (Li et al., 2013; Wang et al., 2007a, 2007b). Furthermore, depletion of foxl3 in medaka, a gene that is related to foxl2, was shown to cause de-novo sperm development in committed ovaries (Nishimura et al., 2015). The interaction of foxl genes with known zebrafish sex regulators begs further exploration.
We found that two Sertoli cell-expressed genes, sox9a and amh have different transcriptional dependencies on Dmrt1. In mice, the maintenance of Sox9 expression in testes is dependent on Dmrt1, and overexpression of Dmrt1 in XX gonadal cells leads to activation of Sox9a expression (Lindeman et al., 2015; Matson et al., 2011; Zhao et al., 2015). Similarly, in chickens, Sox9a expression is greatly reduced in Dmrt1 knockdowns and overexpression of Dmrt1 induced both Sox9 and Amh expression (Lambeth et al., 2014; Smith et al., 2009). In contrast to mice and chickens, we found that maintenance of sox9a expression was not dependent on dmrt1 in the adult testis in zebrafish. However, the expression of amh in Sertoli cells was dependent on Dmrt1. These results position dmrt1 either downstream or in parallel to that of sox9a and upstream of amh in the zebrafish sex differentiation gene regulatory network. However, it is also possible that Dmrt1 exerts some influence on sox9a expression despite not being absolutely required.
The loss of amh expression in dmrt1 mutant zebrafish suggests a potential mechanism through which dmrt1 regulates male sex determination. The function of amh has not been reported in zebrafish, however, several studies provide evidence that Amh signaling is integral to sex determination in various fish species. Amh or its receptor is the master regulator of sex determination in some fish; the Y-chromosome amh duplication, amhy, in Odontesthes hatcheri and Type II Amh Receptor, amhr2, in three Takifugu species are determinants of male sex (Hattori et al., 2012; Kamiya et al., 2012). A duplicated copy of amh, called amhy, is also the likely male determining gene on the Y chromosome in Nile Tilapia (Oreochromis niloticus) (Li et al., 2015). The female bias reported here in zebrafish dmrt1 mutants, is due to failed initiation of and commitment to testis fate during gonadal sex-differentiation, and is coupled to loss of amh expression. As amh has a predominant role in sex-determination in some species, it is possible that Dmrt1 acts through amh transcriptional regulation, at least in part, to specify or maintain testis determination in zebrafish.
4.5. Generation of an artificial sex chromosome through a combination of dmrt1 loss and chromosome drive
In some families carrying the dmrt1E3insallele, we discovered lines for which the dmrt1+/−to dmrt1−/−sex ratios were nearly 1:1, with males being dmrt1+/−and females being dmrt1−/−. Therefore, the chromosome harboring the dmrt1E3ins allele behaved as a Y sex chromosome. This appears to be due to the combined effect of loss of the dmrt1+/+ genotype in the population together with the sex-ratio differences caused by the dmrt1 mutation. However, the cause of the extreme male bias in heterozygotes is not clear. Our analysis of the loss of the dmr1+/+ genotype suggests that the chromosome harboring the dmrt1E3insallele contains a segregation distorter locus (Supplemental Data). As this segregation distortion is only apparent in some families carrying this allele and not apparent in lines carrying other dmrt1 mutations, we conclude that the segregation distortion is not due to loss of dmrt1 function, but rather the affect of either the retroviral insertion itself or another linked locus.
Segregation distorters have been suggested to drive the rapid evolution of sex determination mechanisms, oftentimes due to sex chromosome drive (Kozielska et al., 2010). Sex chromosome drive leads to sex ratio bias, which can reduce fitness. Acquisition of an alternative sex-determination mechanism can restore sex-ratios and displace the previous method of sex determination (Kozielska et al., 2010). In our fish, we began with a system that lacked sex chromosomes. The association of dmrt1E3ins with a strong chromosomal driver led to adoption of an apparent XY sex determination system. Therefore, the combination of a linked segregation distorter with an allele or mutation that alters sex ratios may represent one mechanism by which sex-chromosomes can evolve.
5. Conclusions
In summary, we have shown that dmrt1 functions in male sexual fate in zebrafish and is essential for normal development of testis tubules and spermatogenesis. These results complement recent findings for female-promoting pathways, such as NF-κB and Wnt (Pradhan et al., 2012; Sreenivasan et al., 2014). Additionally, we provide support for previous reports linking the chromosome 5 sub-region to sex-determination in zebrafish (Bradley et al., 2011). We have shown some conservation of the role and relationships of dmrt1 from other animals and present novel findings that implicate a unique role for dmrt1 in zebrafish juvenile ovary-to-testis transformation and male germ cell differentiation and maintenance. Furthermore, our analysis of the segregation distortion effect co-segregating with the dmrt1E3ins reveals a novel mechanism through which sex chromosomes may evolve.
Supplementary Material
Acknowledgments
We would like to thank Christiane Nüsslein-Volhard (MPI for Developmental Biology) for generous early support of this work. We also thank Brigitte Walderich (MPI) and Maria Geisler (MPI) for support in generating the mutagenesis libraries in which the dmrt1t32242allele was isolated. Adeeba Nahrin (UMB) and Nuria Cerdá-Esteban (MPI) both provided technical support for this study. We thank David Grunwald (U. of Utah) and Simon Chan (U.C. Davis) for plasmid constructs necessary for TALEN assembly. K.W. was supported by a fellowship from the UMass Boston-DF/HCC U54 Partnership NIH/NCI award 1U54CA156734. A.O. was a participant in the Biology Undergraduate Scholars Program (BUSP) and BUSP-Honors Research, which are supported by NIH-IMSD grant GM56765, HHMI grant 52005892, the UC Davis College of Biological Sciences, and the UC Davis Office of the Chancellor & Provost. B.W.D. was supported by National Science Foundation (IOS-09296376).
Appendix A. Supplementary information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ydbio.2016.12.008.
References
- Agbor VA, Tao S, Lei N, Heckert LL. A Wt1-Dmrt1 transgene restores DMRT1 to sertoli cells of Dmrt1-/- testes: * a novel model of DMRT1-deficient germ cells. Biol Reprod. 2012;88 doi: 10.1095/biolreprod.112.103135. http://dx.doi.org/10.1095/biolreprod.112.103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Marí A, Braasch I, Amores A, Hohenlohe P, Batzel P, Postlethwait JH. Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040701. http://dx.doi.org/10.1371/journal.pone.0040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers KL, Smith CA, Lambeth LS. The molecular genetics of avian sex determination and its manipulation. Genesis. 2013;51:325–336. doi: 10.1002/dvg.22382. http://dx.doi.org/10.1002/dvg.22382. [DOI] [PubMed] [Google Scholar]
- Bradley KM, Breyer JP, Melville DB, Broman KW, Knapik EW, Smith JR. An SNP-Based Linkage Map for Zebrafish Reveals Sex Determination Loci. G3: Genes|Genomes|Genetics. 2011;1:3–9. doi: 10.1534/g3.111.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, Baller JA, Somia NV, Bogdanove AJ, Voytas DF. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39:e82. doi: 10.1093/nar/gkr218. http://dx.doi.org/10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang EF, Pai CI, Wyatt M, Yan YL, Postlethwait J, Chung B. Two sox9 genes on duplicated zebrafish chromosomes: expression of similar transcription activators in distinct sites. Dev Biol. 2001;231:149–163. doi: 10.1006/dbio.2000.0129. http://dx.doi.org/10.1006/dbio.2000.0129. [DOI] [PubMed] [Google Scholar]
- Dahlem TJ, Hoshijima K, Jurynec MJ, Gunther D, Starker CG, Locke AS, Weis AM, Voytas DF, Grunwald DJ. Simple methods for generating and detecting. PLoS Genet. 2012;8:e1002861. doi: 10.1371/journal.pgen.1002861. http://dx.doi.org/10.1371/journal.pgen.1002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranow DB, Hu K, Bird AM, Lawry ST, Adams MT, Sanchez A, Amatruda JF, Draper BW. Bmp15 Is an oocyte-produced signal required for maintenance of the adult female sexual phenotype in Zebrafish. PLoS Genet. 2016;12:e1006323. doi: 10.1371/journal.pgen.1006323. http://dx.doi.org/10.1371/journal.pgen.1006323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranow DB, Tucker RP, Draper BW. Germ cells are required to maintain a stable sexual phenotype in adult zebrafish. Dev Biol. 2013;376:43–50. doi: 10.1016/j.ydbio.2013.01.016. http://dx.doi.org/10.1016/j.ydbio.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Eggers S, Ohnesorg T, Sinclair A. Genetic regulation of mammalian gonad development. Nat Rev Endocrinol. 2014;10:673–683. doi: 10.1038/nrendo.2014.163. http://dx.doi.org/10.1038/nrendo.2014.163. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Nishimura T, Goto-Kazeto R, Kawakami Y, Yamaha E, Arai K. Sexual dimorphism of gonadal structure and gene expression in germ cell-deficient loach, a teleost fish. Proc Natl Acad Sci USA. 2010;107:17211–17216. doi: 10.1073/pnas.1007032107. http://dx.doi.org/10.1073/pnas.1007032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges A, L’Hôte D, Todeschini AL, Auguste A, Legois B, Zider A, Veitia RA. The transcription factor FOXL2 mobilizes estrogen signaling to maintain the identity of ovarian granulosa cells. Elife. 2014;3:e04207. doi: 10.7554/eLife.04207. http://dx.doi.org/10.7554/eLife.04207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto R, Saito T, Takeda T, Fujimoto T, Takagi M, Arai K, Yamaha E. Germ cells are not the primary factor for sexual fate determination in goldfish. Dev Biol. 2012;370:98–109. doi: 10.1016/j.ydbio.2012.07.010. http://dx.doi.org/10.1016/j.ydbio.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Guo Y, Cheng H, Huang X, Gao S, Yu H, Zhou R. Gene structure, multiple alternative splicing, and expression in gonads of zebrafish Dmrt1. Biochem Biophys Res Commun. 2005 doi: 10.1016/j.bbrc.2005.03.066. http://dx.doi.org/10.1016/j.bbrc.2005.03.066. [DOI] [PubMed]
- Hattori RS, Murai Y, Oura M, Masuda S, Majhi SK, Sakamoto T, Fernandino JI, Somoza GM, Yokota M, Strussmann CA. A Y-linked anti-Mullerian hormone duplication takes over a critical role in sex determination. Proc Natl Acad Sci. 2012;109:2955–2959. doi: 10.1073/pnas.1018392109. http://dx.doi.org/10.1073/pnas.1018392109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpin A, Nakamura S, Wagner TU, Tanaka M, Schartl M. A highly conserved cis-regulatory motif directs differential gonadal synexpression of Dmrt1 transcripts during gonad development. Nucleic Acids Res. 2009 doi: 10.1093/nar/gkn1065. http://dx.doi.org/10.1093/nar/gkn1065. [DOI] [PMC free article] [PubMed]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assunção JA, Zhou Y, Gu Y, Yen J, Vogel J-H, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Eliott D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Mortimer B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper JD, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Ürün Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberländer M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SMJ, Enright A, Geisler R, Plasterk RHA, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nüsslein-Volhard C, Hubbard TJP, Roest Crollius H, Rogers J, Stemple DL. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. http://dx.doi.org/10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T, Kai W, Tasumi S, Oka A, Matsunaga T, Mizuno N, Fujita M, Suetake H, Suzuki S, Hosoya S, Tohari S, Brenner S, Miyadai T, Venkatesh B, Suzuki Y, Kikuchi K. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu) PLoS Genet. 2012;8:e1002798. doi: 10.1371/journal.pgen.1002798. http://dx.doi.org/10.1371/journal.pgen.1002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsky PA, Mitra N, Vardhanabhuti S, Vaughn DJ, Li M, Ciosek SL, Letrero R, D’Andrea K, Vaddi M, Doody DR, Weaver J, Chen C, Starr JR, Håkonarson H, Rader DJ, Godwin AK, Reilly MP, Schwartz SM, Nathanson KL. A second independent locus within DMRT1 is associated with testicular germ cell tumor susceptibility. Hum Mol Genet. 2011;20:3109–3117. doi: 10.1093/hmg/ddr207. http://dx.doi.org/10.1093/hmg/ddr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Bardwell VJ, Zarkower D. Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation. Dev Biol. 2007;307:314–327. doi: 10.1016/j.ydbio.2007.04.046. http://dx.doi.org/10.1016/j.ydbio.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Matsuda M, Kajiura-Kobayashi H, Susuki A, Saito N, Nakamoto M, Shibata N, Nagahama Y. Two DM domain genes, DMY DMRT1, involved in testicular differentiation and development in the medaka, Oryzias latipes. Dev Dyn. 2004;231:518–526. doi: 10.1002/dvdy.20158. http://dx.doi.org/10.1002/dvdy.20158. [DOI] [PubMed] [Google Scholar]
- Kozielska M, Weissing FJ, Beukeboom LW, Pen I. Segregation distortion and the evolution of sex-determining mechanisms. Heredity (Edinb) 2010;104:100–112. doi: 10.1038/hdy.2009.104. http://dx.doi.org/10.1038/hdy.2009.104. [DOI] [PubMed] [Google Scholar]
- Kratz CP, Han SS, Rosenberg PS, Berndt SI, Burdett L, Yeager M, Korde LA, Mai PL, Pfeiffer R, Greene MH. Variants in or near KITLG, BAK1, DMRT1, and TERT-CLPTM1L predispose to familial testicular germ cell tumour. J Med Genet. 2011;48:473–476. doi: 10.1136/jmedgenet-2011-100001. http://dx.doi.org/10.1136/jmedgenet-2011-100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentz AD, Murphy MW, Kim S, Cook MS, Capel B, Zhu R, Matin A, Sarver AL, Parker KL, Griswold MD, Looijenga LHJ, Bardwell VJ, Zarkower D. The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc Natl Acad Sci USA. 2009;106:22323–22328. doi: 10.1073/pnas.0905431106. http://dx.doi.org/10.1073/pnas.0905431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa H, Saito D, Nakamura S, Katoh-Fukui Y, Ohta K, Baba T, Morohashi K, Tanaka M. Germ cells are essential for sexual dimorphism in the medaka gonad. Proc Natl Acad Sci USA. 2007;104:16958–16963. doi: 10.1073/pnas.0609932104. http://dx.doi.org/10.1073/pnas.0609932104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth LS, Raymond CS, Roeszler KN, Kuroiwa A, Nakata T, Zarkower D, Smith CA. Over-expression of DMRT1 induces the male pathway in embryonic chicken gonads. Dev Biol. 2014 doi: 10.1016/j.ydbio.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal MC, Cardoso ER, Nóbrega RH, Batlouni SR, Bogerd J, França LR, Schulz RW. Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol Reprod. 2009;81:177–187. doi: 10.1095/biolreprod.109.076299. http://dx.doi.org/10.1095/biolreprod.109.076299. [DOI] [PubMed] [Google Scholar]
- Li MH, Yang HH, Li MR, Sun YL, Jiang XL, Xie QP, Wang TR, Shi HJ, Sun LN, Zhou LY, Wang DS. Antagonistic roles of Dmrt1 and Foxl2 in sex differentiation via estrogen production in tilapia as demonstrated by TALENs. Endocrinology. 2013;154:4814–4825. doi: 10.1210/en.2013-1451. http://dx.doi.org/10.1210/en.2013-1451. [DOI] [PubMed] [Google Scholar]
- Li M, Sun Y, Zhao J, Shi H, Zeng S, Ye K, Jiang D, Zhou L, Sun L, Tao W, Nagahama Y, Kocher TD, Wang D. A tandem duplicate of Anti-Müllerian Hormone with a Missense SNP on the Y chromosome is essential for male sex determination in Nile Tilapia, Oreochromis niloticus. PLoS Genet. 2015;11:e1005678. doi: 10.1371/journal.pgen.1005678. http://dx.doi.org/10.1371/journal.pgen.1005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew WC, Bartfai R, Lim Z, Sreenivasan R, Siegfried KR, Orban L. Polygenic sex determination system in zebrafish. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034397. http://dx.doi.org/10.1371/journal.pone.0034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew WC, Orbán L. Zebrafish sex: a complicated affair. Brief Funct Genom. 2014;13:172–187. http://dx.doi.org/10.1093/bfgp/elt041. [Google Scholar]
- Lindeman RE, Gearhart MD, Minkina A, Krentz AD, Bardwell VJ, Zarkower D. Sexual cell-fate reprogramming in the ovary by DMRT1. Curr Biol. 2015;25:764–771. doi: 10.1016/j.cub.2015.01.034. http://dx.doi.org/10.1016/j.cub.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio A, Coimbra AM, Benito C, Fontaínhas-Fernandes AA, Matos M. Screening and identification of potential sex-associated sequences in Danio rerio. Mol Reprod Dev. 2015a;82:756–764. doi: 10.1002/mrd.22508. http://dx.doi.org/10.1002/mrd.22508. [DOI] [PubMed] [Google Scholar]
- Luzio A, Coimbra AM, Benito C, Fontaínhas-Fernandes AA, Matos M. Screening and identification of potential sex-associated sequences in Danio rerio. Mol Reprod Dev. 2015b doi: 10.1002/mrd.22508. http://dx.doi.org/10.1002/mrd.22508. [DOI] [PubMed]
- Maack G, Segner H. Morphological development of the gonads in zebrafish. J Fish Biol. 2003 http://dx.doi.org/10.1046/j.1095-8649.2003.00074.x.
- Masuyama H, Yamada M, Kamei Y, Fujiwara-Ishikawa T, Todo T, Nagahama Y, Matsuda M. Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosom Res. 2012;20:163–176. doi: 10.1007/s10577-011-9264-x. http://dx.doi.org/10.1007/s10577-011-9264-x. [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature. 2011;476:101–104. doi: 10.1038/nature10239. http://dx.doi.org/10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CK, Zarkower D. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat Rev Genet. 2012 doi: 10.1038/nrg3161. http://dx.doi.org/10.1038/nrg3161. [DOI] [PMC free article] [PubMed]
- Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, Hori H, Hamaguchi S, Sakaizumi M. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002 doi: 10.1038/nature751. http://dx.doi.org/10.1038/nature751. [DOI] [PubMed]
- Matsuda M, Shinomiya A, Kinoshita M, Suzuki A, Kobayashi T, Paul-Prasanth B, Lau E, Hamaguchi S, Sakaizumi M, Nagahama Y. DMY gene induces male development in genetically female (XX) medaka fish. Proc Natl Acad Sci USA. 2007;104:3865–3870. doi: 10.1073/pnas.0611707104. http://dx.doi.org/10.1073/pnas.0611707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan SC, Géraudie J, Akimenko MA. Pectoral fin breeding tubercle clusters: a method to determine zebrafish sex. Zebrafish. 2015;12:121–123. doi: 10.1089/zeb.2014.1060. http://dx.doi.org/10.1089/zeb.2014.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga C, Saito D, Nakamura S, Sasaki T, Asakawa S, Shimizu N, Mitani H, Furutani-Seiki M, Tanaka M, Kondoh H. The hotei mutation of medaka in the anti-Mullerian hormone receptor causes the dysregulation of germ cell and sexual development. Proc Natl Acad Sci USA. 2007;104:9691–9696. doi: 10.1073/pnas.0611379104. http://dx.doi.org/10.1073/pnas.0611379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MW, Sarver AL, Rice D, Hatzi K, Ye K, Melnick A, Heckert L, Zarkower D, Bardwell VJ. Genome-wide analysis of DNA binding and transcriptional regulation by the mammalian doublesex homolog DMRT1 in the juvenile testis: editorial comment. Proc Natl Acad Sci USA. 2010;107:13360–13365. doi: 10.1073/pnas.1006243107. http://dx.doi.org/10.1016/j.juro.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Watakabe I, Nishimura T, Picard JY, Toyoda A, Taniguchi Y, di Clemente N, Tanaka M. Hyperproliferation of mitotically active germ cells due to defective anti-Mullerian hormone signaling mediates sex reversal in medaka. Development. 2012;139:2283–2287. doi: 10.1242/dev.076307. http://dx.doi.org/10.1242/dev.076307. [DOI] [PubMed] [Google Scholar]
- Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, Shimizu A, Shan Z, Haaf T, Shimizu N, Shima A, Schmid M, Schartl M. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc Natl Acad Sci USA. 2002 doi: 10.1073/pnas.182314699. http://dx.doi.org/10.1073/pnas.182314699. [DOI] [PMC free article] [PubMed]
- Nishimura T, Sato T, Yamamoto Y, Watakabe I, Ohkawa Y, Suyama M, Kobayashi S, Tanaka M. Sex determination foxl3 is a germ cell-intrinsic factor involved in sperm-egg fate decision in medaka. Science. 2015;349:328–331. doi: 10.1126/science.aaa2657. http://dx.doi.org/10.1126/science.aaa2657. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard MAL, Cosseau C, Mouahid G, Duval D, Grunau C, Toulza È, Allienne JF, Boissier J. The roles of Dmrt (Double sex/male-abnormal-3 related transcription factor) genes in sex determination and differentiation mechanisms: ubiquity and diversity across the animal kingdom. C R Biol. 2015;338:451–462. doi: 10.1016/j.crvi.2015.04.010. http://dx.doi.org/10.1016/j.crvi.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Pradhan A, Khalaf H, Ochsner SA, Sreenivasan R, Koskinen J, Karlsson M, Karlsson J, McKenna NJ, Orbán L, Olsson PE. Activation of NF-κB protein prevents the transition from juvenile ovary to testis and promotes ovarian development in Zebrafish. J Biol Chem. 2012;287:37926–37938. doi: 10.1074/jbc.M112.386284. http://dx.doi.org/10.1074/jbc.M112.386284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Marí A, Cañestro C, Bremiller RA, Nguyen-Johnson A, Asakawa K, Kawakami K, Postlethwait JH. Sex reversal in zebrafish fancl mutants is caused by Tp53-mediated germ cell apoptosis. PLoS Genet. 2010;6:e1001034. doi: 10.1371/journal.pgen.1001034. http://dx.doi.org/10.1371/journal.pgen.1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Marí A, Yan YL, BreMiller RA, Wilson C, Cañestro C, Postlethwait JH. Characterization and expression pattern of zebrafish anti-Müllerian hormone (amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expr Patterns. 2005 doi: 10.1016/j.modgep.2005.02.008. http://dx.doi.org/10.1016/j.modgep.2005.02.008. [DOI] [PubMed]
- Salz HK, Erickson JW. Sex determination in Drosophila: The View From the Top. Fly, Austin. 2010 doi: 10.4161/fly.4.1.11277. http://dx.doi.org/10.4161/fly.4.1.11277. [DOI] [PMC free article] [PubMed]
- Schulz RW, Bogerd J, Male R, Ball J, Fenske M, Olsen LC, Tyler CR. Estrogen-induced alterations in amh and dmrt1 expression signal for disruption in male sexual development in the Zebrafish estrogen-induced alterations in amh and dmrt1 expression signal for disruption in male sexual development in the Zebrafish. Environ Sci Technol. 2007;41:6305–6310. doi: 10.1021/es070785+. http://dx.doi.org/10.1021/es070785. [DOI] [PubMed] [Google Scholar]
- Selman K, Wallace RA, Sarka A, Qi X. Stages of oocyte development in the zebrafish, Brachydanio rerio. J Morphol. 1993;218:203–222. doi: 10.1002/jmor.1052180209. [DOI] [PubMed] [Google Scholar]
- Siegfried KR, Nüsslein-Volhard C. Germ line control of female sex determination in Zebrafish. Dev Biol. 2008;324:277–287. doi: 10.1016/j.ydbio.2008.09.025. http://dx.doi.org/10.1016/j.ydbio.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Slanchev K, Stebler J, de la Cueva-Méndez G, Raz E. Development without germ cells: the role of the germ line in Zebrafish sex differentiation. Proc Natl Acad Sci USA. 2005 doi: 10.1073/pnas.0407475102. http://dx.doi.org/10.1073/pnas.0407475102. [DOI] [PMC free article] [PubMed]
- Smith A, Zhang J, Guay D, Quint E, Johnson A, Akimenko MA. Gene expression analysis on sections of zebrafish regenerating fins reveals limitations in the whole-mount in situ hybridization method. Dev Dyn. 2008;237:417–425. doi: 10.1002/dvdy.21417. http://dx.doi.org/10.1002/dvdy.21417. [DOI] [PubMed] [Google Scholar]
- Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ, Sinclair AH. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature. 2009;461:267–271. doi: 10.1038/nature08298. http://dx.doi.org/10.1038/nature08298. [DOI] [PubMed] [Google Scholar]
- Sreenivasan R, Jiang J, Wang X, Bártfai R, Kwan HY, Christoffels A, Orbán L. Gonad differentiation in zebrafish is regulated by the canonical Wnt signaling pathway. Biol Reprod. 2014;90:45. doi: 10.1095/biolreprod.113.110874. http://dx.doi.org/10.1095/biolreprod.113.110874. [DOI] [PubMed] [Google Scholar]
- Takahashi H. Juvenile hermaphroditism in the Zebrafish, Brachydanio rerio. Bull Fac Fish (Hokkaido Univ) 1977;28:57–65. [Google Scholar]
- Tang R, Dodd A, Lai D, McNabb WC, Love DR. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim Biophys Sin (Shanghai) 2007;39:384–390. doi: 10.1111/j.1745-7270.2007.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trukhina AV, Lukina NA, Wackerow-Kouzova ND, Smirnov AF. The variety of vertebrate mechanisms of sex determination. Biomed Res Int. 2013;2013:587460. doi: 10.1155/2013/587460. http://dx.doi.org/10.1155/2013/587460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzung KW, Goto R, Saju JM, Sreenivasan R, Saito T, Arai K, Yamaha E, Hossain MS, Calvert MEK, Orbán L. Early depletion of primordial germ cells in Zebrafish promotes testis formation. Stem Cell Rep. 2015;4:61–73. doi: 10.1016/j.stemcr.2014.10.011. http://dx.doi.org/10.1016/j.stemcr.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida D, Yamashita M, Kitano T, Iguchi T. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol. 2002 doi: 10.1242/jeb.205.6.711. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schütz G, Cooney AJ, Lovell-Badge R, Treier M. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. http://dx.doi.org/10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Verhulst EC, van de Zande L. Double nexus–Doublesex is the connecting element in sex determination. Brief Funct Genom. 2015:1–11. doi: 10.1093/bfgp/elv005. http://dx.doi.org/10.1093/bfgp/elv005. [DOI] [PMC free article] [PubMed]
- Wang DS, Kobayashi T, Zhou LY, Paul-Prasanth B, Ijiri S, Sakai F, Okubo K, Morohashi K, Nagahama Y. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with ad4 binding protein/steroidogenic factor 1. Mol Endocrinol. 2007;21:712–725. doi: 10.1210/me.2006-0248. http://dx.doi.org/10.1210/me.2006-0248. [DOI] [PubMed] [Google Scholar]
- Wang XG, Bartfai R, Sleptsova-Freidrich I, Orban L. The timing and extent of “juvenile ovary” phase are highly variable during zebrafish testis differentiation. J Fish Biol. 2007b;70:33–44. http://dx.doi.org/10.1111/j.1095-8649.2007.01363.x. [Google Scholar]
- Wang XG, Orban L. Anti-Müllerian hormone and 11 beta-hydroxylase show reciprocal expression to that of aromatase in the transforming gonad of zebrafish males. Dev Dyn. 2007a;236:1329–1338. doi: 10.1002/dvdy.21129. http://dx.doi.org/10.1002/dvdy.21129. [DOI] [PubMed] [Google Scholar]
- Weidinger G, Stebler J, Slanchev K, Dumstrei K, Wise C, Lovell-Badge R, Thisse C, Thisse B, Raz E. dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol. 2003;13:1429–1434. doi: 10.1016/s0960-9822(03)00537-2. [DOI] [PubMed] [Google Scholar]
- Wilson CA, High SK, McCluskey BM, Amores A, Yan Y-L, Titus TA, Anderson JL, Batzel P, Carvan MJ, Schartl M, Postlethwait JH. Wild sex in Zebrafish: loss of the natural sex determinant in domesticated strains. Genetics. 2014 doi: 10.1534/genetics.114.169284. http://dx.doi.org/10.1534/genetics.114.169284. [DOI] [PMC free article] [PubMed]
- Yamamoto T. Sex differentiation. In: Hoar WS, Randall DJ, editors. Fish Physiology Vol III Reproduction and Growth, Bioluminescence, Pigments, and Poisons. New York: 1969. pp. 117–175. [Google Scholar]
- Yoshimoto S, Ito M. A ZZ/ZW-type sex determination in xenopus laevis. FEBS J. 2011 doi: 10.1111/j.1742-4658.2011.08031.x. http://dx.doi.org/10.1111/j.1742-4658.2011.08031.x. [DOI] [PubMed]
- Zhao L, Svingen T, Ng ET, Koopman P. Female-to-male sex reversal in mice caused by transgenic overexpression of Dmrt1. Development. 2015;142:1083–1088. doi: 10.1242/dev.122184. http://dx.doi.org/10.1242/dev.122184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


