Abstract
Despite promising preliminary results in treating fibromyalgia (FM) pain, no neuromodulation technique has been adopted in clinical practice because of limited efficacy, low response rate, or poor tolerability. This phase II open-label trial aims to define a methodology for a clinically effective treatment of pain in FM by establishing treatment protocols and screening procedures to maximize efficacy and response rate. High-definition transcranial direct current stimulation (HD-tDCS) provides targeted subthreshold brain stimulation, combining tolerability with specificity. We aimed to establish the number of HD-tDCS sessions required to achieve a 50% FM pain reduction, and to characterize the biometrics of the response, including brain network activation pain scores of contact heat-evoked potentials. We report a clinically significant benefit of a 50% pain reduction in half (n = 7) of the patients (N = 14), with responders and nonresponders alike benefiting from a cumulative effect of treatment, reflected in significant pain reduction (P = .035) as well as improved quality of life (P = .001) over time. We also report an aggregate 6-week response rate of 50% of patients and estimate 15 as the median number of HD-tDCS sessions to reach clinically meaningful outcomes. The methodology for a pivotal FM neuromodulation clinical trial with individualized treatment is thus supported.
Keywords: Fibromyalgia, pain, noninvasive brain stimulation, high-definition transcranial direct current stimulation, motor cortex
Fibromyalgia (FM) is a chronic pain syndrome that affects most of the musculoskeletal system; symptoms include diffuse pain, fatigue, and emotional distress.48 The estimated prevalence of this disorder in the general population ranges between 2 and 5%,2,46 with a higher incidence among females.27 The pathophysiologic mechanisms accounting for the diffuse signs and symptoms are not yet fully understood, but current evidence suggests that alterations in nociceptive pathways and modifications in sensory processing seem to play a key role in both the initiation and the maintenance of pain in this condition. These pernicious alterations seem to be caused mainly by maladaptive plasticity in brain areas involved in these processes,10 which is a common finding in chronic pain syndromes.
Different noninvasive brain stimulation (NIBS) techniques have been tested extensively in chronic pain syndromes given their ability to modify brain activity, targeting mainly the primary motor cortex (M1) as an entryway to modulating the aberrant activity of the circuit in charge of pain processing.9 Several studies5,14,24,30,37,42 have shown that stimulation of this brain area can induce significant analgesic effects in FM, mainly through modification in sensory processing of pain by thalamic inhibitory networks. Nonetheless, the results are inconsistent and some studies have achieved only marginal benefits. This variability in clinical efficacy may be associated with differences in trial design and stimulation parameters; therefore, optimization and standardization of the treatment framework used in FM may lead to significant improvements in clinical efficacy. For example, the cost of transcranial direct current stimulation (tDCS) is low, it is well tolerated, and broadly deployable, which has made it one of the most frequently used techniques, but its main drawback is that it produces diffuse brain current flow. On the other hand, high-definition tDCS using the 4 × 1 montage (4 × 1 HD-tDCS) allows noninvasive focal application of low-intensity direct current,12 which is believed to enhance the clinical effects of this therapeutic tool.7,29,41 Previous results using just a single session of HD-tDCS with a 4 × 1 electrode configuration over M14,44 demonstrated an incremental reduction of experimental and FM pain, and exceptionally long neuroplasticity changes,22 which together support cumulative analgesic effects with repeated sessions.42
Therefore, we set out to evaluate the optimal stimulation parameters and criteria for patient selection and evaluation of clinical response in patients with FM receiving 4 × 1 HD-tDCS for pain management. This effort was driven mainly by the critical relevance of obtaining as much information as possible on clinical responses in early study phases to design protocols with high response rates, high efficacy, and limited side effects; which is a prerequisite for the development of pivotal phase III efforts in the field of NIBS. The primary aim of this phase II open-label trial was to establish the mean number of 4 × 1 HD-tDCS sessions needed to achieve a clinically meaningful response, defined as >50% decrease in pain, quantified by a visual analog scale (VAS). In addition, we assessed biomarkers of response, including an electroencephalography (EEG)/ event-related potential (ERP) analysis of brain reorganization, known as brain network activation (BNA).33,36,39 The exploratory aims were to test screening procedures to predict response and individualize treatment.
Methods
Study Design and Overview
The study was conducted in the Neuromodulation Center at Spaulding Rehabilitation Hospital, Harvard Medical School. It was approved by the local institutional review board and conducted in compliance with the Declaration of Helsinki (1964).
The phase II study consisted of an open-label single arm, in which enrolled patients were asked to remain in treatment until a clinically meaningful reduction in pain was achieved for a maximum of 6 weeks of treatment. A clinically meaningful response was defined as a pain intensity reduction of 50% or more compared with VAS baseline measures obtained 1 week before visit 2 using a daily pain diary.
After potential participants were identified, they underwent a detailed telephone screening and were scheduled for a first study visit to the treatment center, at which written informed consent was obtained. On the participants’ first visit, baseline measurements were collected, including the Fibromyalgia Impact Questionnaire (FIQ) and sensory assessments (detailed later); participants underwent further screening using the 2010 American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia47 and were considered enrolled patients after screening.
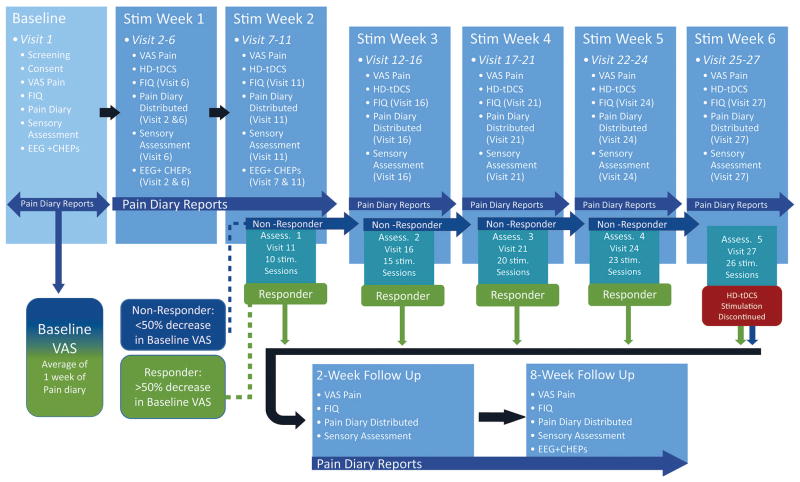
All enrolled patients were scheduled to complete 10 HD-tDCS sessions in a period of 2 weeks (visits 2–11), after which they completed their first response assessment (stimulation week 2, assessment 1, visit 11; Fig 1). If patients met the criteria for clinical response, and therefore were deemed responders, after any response assessment, subsequent stimulation sessions were discontinued, and patients were asked to complete 2 follow-up assessment visits. Patients who did not meet the criteria for a clinical response (nonresponders) received 5 additional HD-tDCS sessions during the third week of stimulation (visits 12–16), after which a second response assessment was conducted (stimulation week 3, assessment 2, visit 16; Fig 1). For nonresponders, the same procedure, 5 additional HD-tDCS sessions, was repeated during the fourth week of stimulation with a subsequent response assessment (stimulation week 4, assessment 3, visit 21; Fig 1). If patients continued to be nonresponders after visit 21, they received 3 additional HD-tDCS sessions during the fifth week with a fourth response assessment (stimulation week 5, assessment 4, visit 24; Fig 1). After visit 24, nonresponders were scheduled for 3 additional HD-tDCS sessions during the sixth week, when a final response assessment was made (stimulation week 6, assessment 5, visit 27; Fig 1), and at this point, regardless of response, HD-tDCS stimulation was discontinued and patients were scheduled for a 2-week and 8-week follow-up visit.
Figure 1.
Study overview illustrating patient visits and response assessment (Assess.) time points. Baseline VAS measures were recorded in a pain diary 1 week before visit 2 (7 days between visits 1 and 2) and HD-tDCS stimulation (Stim) was started on visit 2. Patients reported daily pain scores in pain diaries for every day they remained enrolled in the trial, starting from visit 1. Pain diaries were distributed and discussed on visits 2, 6, 11, 16, 21, 24, and 27 (depending on the patient’s response), as well as on both follow-up visits. Five response assessments were performed on visits 11, 16, 21, 24, and 27 (depending on the patient’s response). During the response assessments, if patients responded (achieved >50% decrease in VAS compared with baseline VAS), HD-tDCS was discontinued and they were scheduled for follow-up visits; if patients were nonresponders (<50% decrease in VAS compared with baseline VAS), they received additional HD-tDCS stimulation sessions (up to a total of 26 sessions). Follow-up visits were performed 2 and 8 weeks after HD-tDCS was discontinued.
Selection Criteria
Enrolled patients met the following criteria: 1) between 18 and 85 years of age; 2) a formal diagnosis of FM made by a practicing physician; 3) existing pain for more than 3 months with an average of at least 4 on a 0 to 10 VAS; and 4) pain resistant to common analgesics and medications for chronic pain. Exclusion criteria included 1) positive test for pregnancy; 2) tDCS contradictions, including metallic implants in the head; 3) drug or alcohol abuse problems; 4) carbamazepine use within the last 6 months before enrollment; 5) severe depression; 6) a history of epilepsy, stroke, moderate to severe traumatic brain injury, unexplained fainting spells, or severe migraines; and 7) a history of neurosurgery. Patients taking central nervous system–active medications were enrolled if dosages had been stable for at least 2 months. Patients were encouraged to continue taking their usual medication for the duration of the trial.
Intervention: HD-tDCS
All patients were scheduled to receive active anodal HD-tDCS using the montage previously described.43,44 A conventional tDCS device (Model 1224-B; Soterix Medical Inc, New York, NY) connected to a 4 × 1 Multichannel Stimulation Adaptor (Model 4x1-C2; Soterix Medical Inc) was used to supply and divide 2 mA of current across 5 stimulating electrodes for 20 minutes. Direct current was delivered using Ag/AgCl sintered ring electrodes, held in place by specially designed plastic casings embedded in a 64-channel EEG recording cap. The plastic casings were also used to house EEG recording electrodes during data acquisition and before and after HD-tDCS treatment (see EEG Recordings section).
The anode was placed over C3 according to the International 10/10 EEG system, corresponding to the approximate location of the left M1. Four return cathodal electrodes were placed approximately 5 cm radially from C3, corresponding roughly to locations Cz, T7, P3, and F3 (Fig 2). The preparation procedure consisted of exposing the scalp by separating the hair underlying each electrode and adding approximately 1.5 mL of highly conductive gel (Signa Gel; Parker Laboratories, Fairfield, NJ). Contact quality and impedance levels were verified for each electrode before each stimulation session began.
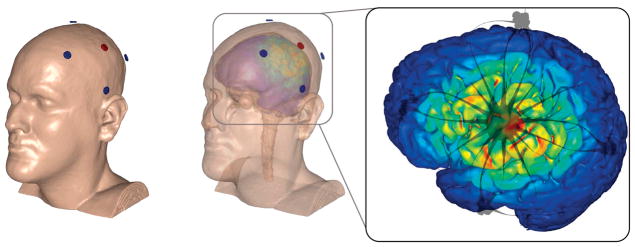
Figure 2.
(Left) Finite element model of a 4 × 1 HD-tDCS montage over M1 on the scalp. (Middle) Finite element model showing underlying cortical electric field magnitude during HD-tDCS over M1. (Right) Anodal stimulation over left M1 was modeled with a wide radius (~75 mm) 4 × 1 ring. Electric field peaks of .42 V/m were predicted on the motor strip as a result of 2-mA stimulation. Streamline images (gray) demonstrate the direction of current crossing the skull.
Clinical Outcomes
All patients sat in a comfortable chair with back and arm support during all stages of data collection.
VAS for Pain
Pain levels were obtained on all visits and quantified using a VAS, which consisted of a gradient color-coded scale ranging from 0 (green; corresponding to complete absence of pain) to 10 (red; corresponding to worst imaginable pain) in intervals of .5. In addition, immediately before and after each HD-tDCS session, patients were asked to rate their current overall level of pain on the aforementioned VAS from 0 to 10. The VAS, collected on days of the response assessments (Fig 1), was used to gauge patients’ responses compared with baseline VAS (see Pain Diary section).
Pain Diary
For each day enrolled in the study, starting from visit 1, patients were asked to record their daily pain intensity as well as any medication they took using a diary. Patients recorded this information on a scaled-down version of the color-coded VAS. The diary also included spaces for medication reporting. Baseline VAS pain levels were calculated for each patient by using the average of 1 week of pain diary reports, collected every day between visits 1 and 2, before their first HD-tDCS stimulation session. During response assessments, if patients’ post-HD-tDCS VAS reduced by more than 50% compared with the baseline VAS, they were deemed to have achieved a clinical response, whereas those who did not were deemed nonresponders and continued HD-tDCS.
FIQ
This questionnaire was used as a quality-of-life assessment in patients with FM and was administered on visits 6, 11, 16, 21, 24, and 27 (according to the patient’s response) and on both follow-up visits. It consists of 10 questions that evaluate activities of daily living and pain, divided into 3 main categories: function, overall impact, and symptoms. The total maximal score achievable was 100.
Beck Depression Inventory
This screening tool was administered on visit 1 for an assessment of depression and consisted of 21 items measuring emotional, behavioral, and somatic symptoms. The score for each item ranged from 0 to 3 with total scores of 10 to 18 indicating mild, 19 to 29 indicating moderate, and greater than 30 indicating severe depression.3
Quantitative Sensory Assessment
Semmes-Weinstein Monofilaments
While patients kept their eyes closed, calibrated esthesiometers (Touch-Test Sensory Evaluators; North Coast Medical Inc, Morgan Hill, CA) were applied in increasing filament thickness. Filaments were applied to 4 different paired body areas (tender points): the thenar eminence, an area 2 cm distal to the lateral epicondyle, above the medial border of the scapular spine, and the occiput area. The value at which patients first perceived the sensory stimuli (mechanical detection threshold) and value at which the sensation became painful (pain threshold) were registered and recorded.
Pain Pressure Threshold
Blunt pressure was delivered to the 4 aforementioned paired body areas using the standard 1-cm2 hard rubber nozzle of an algometer (Commander Algometer; JTECH Medical, Salt Lake City, UT). Patients were asked to keep their eyes closed while increasing pressure was applied. They reported the moment at which the sensation transitioned from pressure to pain. At this moment, pressure was relieved and the applied pressure was recorded. The average of 3 trials for each point was used. The points selected for mechanical and pressure pain detection correspond to the areas showing the highest correlation with overall pain threshold, as shown by Petzke and colleagues31 and Tastekin and colleagues.40 The thenar eminence was used as a control for both measurements. Both pain pressure threshold (PPT) and Semmes-Weinstein monofilament (SWM) measurements were completed at baseline and before and after HD-tDCS on visits 6, 11, 16, 21, 24, and 27 (according to the patients’ responses), as well as on both follow-up visits.
Neurophysiologic Outcomes: Contact Heat-Evoked Potentials
Heat Stimulation
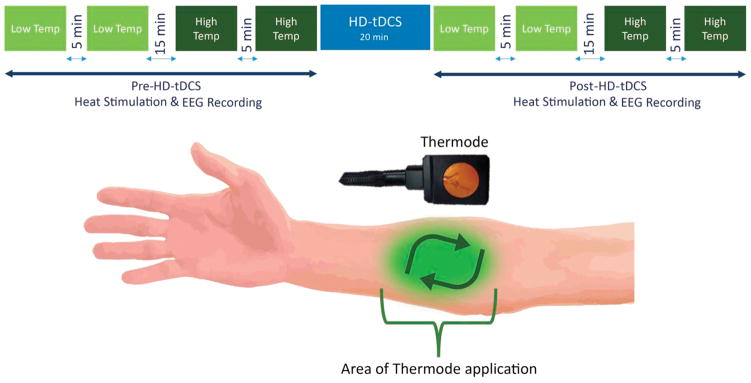
Contact heat stimuli were delivered to the right dominant proximal volar forearm using a round thermode of 572.5 mm2 (PATHWAY; Medoc Ltd, Ramat-Yishai, Israel) in 4-block heat stimulation sessions to produce contact heat-evoked potentials (CHEPs) (Fig 3, top). Each CHEPs stimulus trial began with the thermode applied to the skin, followed by triggering of the heat stimulus, and ended with a beep, which prompted patients to rate the pain produced by the heat stimulus. This thermode application was repeated in a clockwise manner on the designated area of the forearm (Fig 3, bottom). On visit 1, patients underwent a temperature-determination protocol, in which they received 3 different heat stimuli of the same temperature, ranging from 38°C to 52°C. Patients were instructed to rate each stimulus on a numeric rating scale (NRS), with 0 indicating the absence of pain and 10 indicating the worst imaginable pain. The 2 temperatures that patients rated as 3 and 6 on the NRS were chosen as the low and high temperature, respectively, for the pre-HD-tDCS and post-HD-tDCS EEG recordings (Fig 3, top). The first 2 blocks of heat stimuli used the low temperature (NRS rating of 3) determined for each individual patient and were separated by a 5-minute interval. The following 2 blocks of heat stimuli used the high temperature (NRS rating of 6) determined for each individual patient and were also separated by a 5-minute break. The low-temperature and high-temperature blocks were further separated by a 15-minute interval. There were 20 stimuli (trials) per block and the interstimulus interval ranged from 8 to 13 seconds. After the 4-block heat stimulation session, the HD-tDCS intervention was applied, then the 4-block heat stimulation session was repeated (Fig 3, top). This 4-block low-temperature and high-temperature regime was administered before and after HD-tDCS on visits 2, 6, 7, and 11 and once at the 8-week follow-up (when only the pre-HD-tDCS aspect was done; Fig 3, top).
Figure 3.
(Top) The low temperature (Temp) heat stimuli were applied first, with 2 blocks, each consisting of 20 stimuli. These 2 low-temperature blocks were separated by a 5-minute break, followed by the 2 high-temperature heat stimuli also separated by a 5-minute break. Both the low-temperature and high-temperature blocks were separated by a 15-minute break. HD-tDCS treatment was then administered and the heat stimulation procedure was repeated (post-HD-tDCS). (Bottom) During the heat stimulation blocks, the thermode was placed on the indicated area of the forearm for each stimulus. After the heat stimulus was applied, the thermode was moved slightly in a clockwise manner and the process was repeated for the entirety of the block.
EEG Recordings
CHEPs were recorded using a high-density 64-channel BioSemi ActiveTwo system (BioSemi Instrumentation, Amsterdam, Netherlands), according to the extended 10/10 International system with the reference to the nose tip. Data were acquired during the pre-HD-tDCS and post-HD-tDCS periods, concurrent with the heat stimulation application by the thermode (Fig 3, top). Electrooculogram electrodes to monitor eye movements were placed below and above the right eye and at the right and left external canthi. Data were sampled at 512 Hz and offline analysis included filtering the data with a .5-Hz to 30-Hz band-pass finite impulse response filter and continuous data epoching with a 200-millisecond prestimulus window to 1000-millisecond poststimulus window. At least 25 artifact-free traces per train were averaged for CHEPs analysis.
BNA Analysis
BNA analysis was performed on the CHEPs data recorded at each electrode location by clustering the basic time-frequency characteristics of the ERPs (ie, waveforms at specific location, amplitude, frequency, and timing [latency]), and finding the relations between the clusters. The BNA analysis involves 2 independent processes: a group-level pattern recognition process used to generate the characteristic group’s network (reference brain network model [RBNM]) and a single patient-level similarity evaluation process in which a single patient is compared with the RBNM and the degree of similarity is measured by the BNA score. The group-level process does not consist of averaging across patients, which masks variability within the group, but is rather based on identifying the largest common denominator of activation across patients, yet still preserving the interpatient variability of individual patients. A detailed description of the methodology is given elsewhere.33–35,39
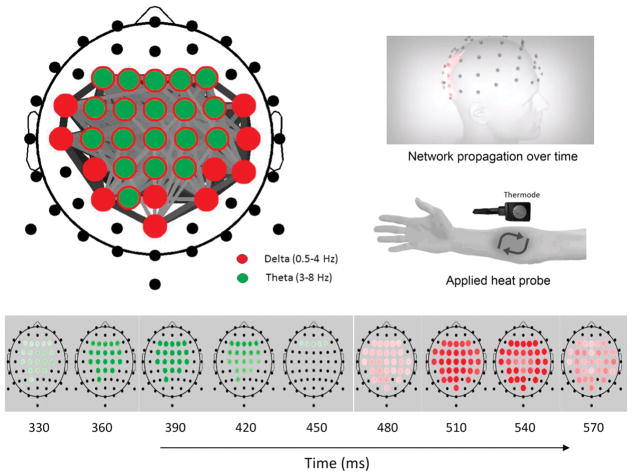
In this study, the BNA score of individual patients was calculated against an RBNM (N = 70, 33 female, mean age = 32 years, SD = ±7.86, range = 20–45 years) that was generated based on CHEPs elicited to 52°C (visit 1 and visit 2 datasets) collected from healthy participants at Ohio Clinical Trials, Inc. The RBNM was characterized with central theta-delta activations and represented the response to pain of healthy individuals (Fig 4, top left). This network has been validated in a previous study36 in which we showed a similar central theta-delta network for CHEPs at 52°C. The dynamics of the network activity can be revealed in a frame-by-frame presentation (Fig 4, bottom), with a heat probe applied, activating pain-related network activity (Fig 4, top right).
Figure 4.
(Top left) RBNM for pain (52°C) showing central theta-delta activation. Red circles represent delta frequency nodes, green circles represent theta frequency nodes, and gray lines represent connectivity between the nodes. Line width and darkness denote connectivity strength. (Top right) Models depicting mapped network propagation over time and area of applied head probe. (Bottom) Time frames of the network showing activity development over time, starting ~330 milliseconds after the heat stimulus.
Results
A total of 20 participants (17 female, 3 male, mean age = 49.50 years, SD = ±11.96, range = 29–68 years) provided written informed consent and were recruited from the Boston area through online publicity, newspaper advertisements, and local support groups over a period of 10 months. The recruitment and data collection were completed between March 2013 and July 2014. Of the 20 participants, 14 were included in the data analysis because they received at least 1 HD-tDCS treatment after visit 1. Patients were classified as responders or nonresponders according to whether they achieved a significant clinical response or not. Patients who discontinued treatment at any other time point were considered dropouts. At the end of the fifth assessment (week 6 of treatment) there were 7 responders, 6 dropouts, and 1 patient who remained in treatment without showing a response (Table 1). These 6 dropouts and 1 patient who remained in treatment without showing a response were collectively considered nonresponders. The median time to response in these 7 responders was 15 visits. Some dropout occurred early on. For example, 1 patient failed to return after a single HD-tDCS session, in which the individual reported a decrease in VAS pain score from 8 to 6. It is not certain what the outcome for this and other dropouts could have been if they had adhered to the treatment regime.
Table 1.
Baseline Characteristics of the Study Patients Divided Into Responders and Nonresponders
| Responders | NonResponders | |
|---|---|---|
| Number | 7 | 7 |
| Sex (number of females) | 7 | 5 |
| Age (SD) | 50.14 (±12.47) | 46.57 (±12.23) |
| VAS baseline (SD) | 5.69 (±1.48) | 6.01 (±1.74) |
| BDI (SD) | 14.14 (±8.41) | 13 (±8.35) |
| FIQ baseline (SD) | 52.36 (±24.35) | 55.07 (±12.90) |
| SWMs mechanical (SD) | 3.24 (±.46) | 3.88 (±1.73) |
| SWMs pain (SD) | 5.73 (±.54) | 5.37 (±2.06) |
| PPT kg (SD) | 2.41 (±1.13) | 1.52 (±0.83) |
| Number of tDCS sessions (SD) | 16.29 (±5.31) | 14.29 (±8.34) |
| Minimum–maximum | 10–24 | 1–25 |
NOTE. Responders include all patients whose VAS decreased by >50% compared with the baseline VAS, and nonresponders include 6 dropouts and 1 patient who remained in treatment without showing a response to HD-tDCS treatment. The means and standard deviations (SD) are shown unless otherwise stated.
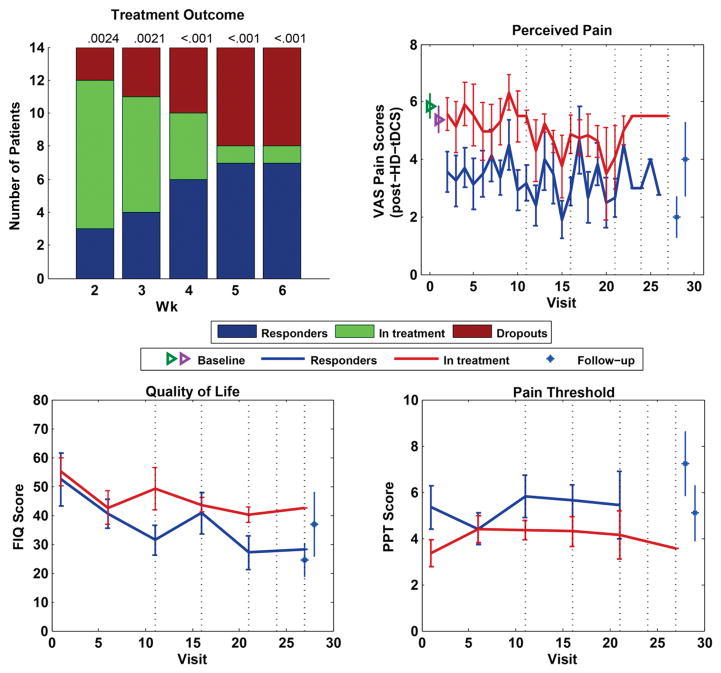
To estimate the odds of obtaining the observed number of responders by chance we performed a Monte Carlo simulation of this assessment regime as follows. We drew 1, 2, 3, 4, and 5 independent samples from normal distributions with means and standard deviations matching the 14 patients. Means were set to the VAS baseline scores and standard deviations were determined from daily diary entries 1 week preceding treatment (between visits 1 and 2). On the diary entries, VAS scores were not correlated across subsequent days (r = .017, P > .1). These samples were compared with each patient’s baseline scores as in the actual experiment and considered successful if they decreased below 50% of baseline VAS pain scores for any of the multiple assessment points. The number of successfully treated patients with such randomly generated outcomes was on average .75, 1.39, 1.92, 2.38, and 2.78. The likelihood of observing 3, 4, 6, 7, and 7, as in the experiment, is small (P < .0021; see P values, Fig 5, top left).
Figure 5.
In the first 4 weeks there were 5 days of treatment, in the last 2 weeks only 3, such that assessment days where on the 11th, 16th, 21st, 24th, and 27th visit. (Top left) Treatment outcome with Monte Carlo simulation performed at all assessment points corresponding to 2, 3, 4, 5, and 6 weeks after treatment commenced or visits 11, 16, 21, 24, and 27, respectively. (Top right) VAS progression over visits for responders and patients in treatment, including follow-up visits, pain diary baseline (green), and visit 1 baseline (magenta). (Bottom left) Patient quality of life, assessed by FIQ, progression over visits, comparing responders and patients in treatment. (Bottom right) PPT measures over visits comparing responders and patients in treatment.
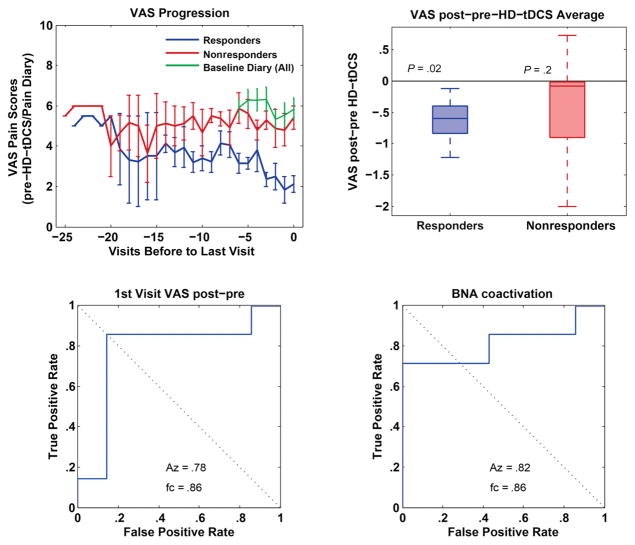
To determine if there was a cumulative effect of treatment, we analyzed the progression of VAS over time for responders and nonresponders (Fig 5, top right). We used a multilevel mixed-effects model to account for repeated measures in each patient in the presence of missing data (because no data were available after patients chose to discontinue treatment as well as occasional missed treatment days). Treatment response was a fixed across-group factor and time was a random within-subject factor, with patient ID being a nested variable in the binary treatment response variable (we used MATLAB’s anovan function [The Mathworks, Inc., Natick, MA]). This analysis confirmed that VAS changes with time (P = .035) and that there is a difference in VAS between the responders and nonresponders (P = .030). However, there is no interaction between time and response (P = .97), suggesting that the cumulative effect is comparable in both groups. To visualize this cumulative effect, we also displayed VAS scores aligned to the last day of treatment (Fig 6. top left). By design, a decrease in VAS on the last day of treatment is expected. However, Fig 6 (top left) seems to suggest that VAS scores trend lower in responders a few days before the day of discontinuation of successful HD-tDCS treatment.
Figure 6.
(Top left) Because there were no interactions between time and response, to visualize cumulative effects of treatment between responders and nonresponders, we displayed VAS scores aligned to the last day of treatment. (Top right) Determination of an acute effect between responders and nonresponders by averaging VAS scores before and after HD-tDCS treatment, across all sessions. Here, responders show a significant acute decrease in VAS compared with nonresponders. (Bottom left) ROC analysis examining the acute effect observed on the first treatment day, showing that it is a reasonable predictor of treatment success. (Bottom right) ROC analysis on the BNA scores from the pretreatment session showing that responsiveness can be predicted before the commencement of treatment. All pairwise comparisons between responders and nonresponders used the Wilcoxon rank sum test with N = 14 measures.
To see if the benefits captured by the VAS are paralleled by improvements in quality of life, we analyzed the FIQ scores at the 5 assessment points as secondary outcome measure (Fig 5, bottom left). There is a reduction of the FIQ with time (P = .001), but scores do not differ between responders and nonresponders (P = .18). A further secondary outcome measure, PPT, did not show an effect between responders and nonresponders or a trend in time (P = .36, P = .45). Similarly, the SWMs, for both mechanical and pain, did not show an effect between responders and nonresponders or a trend in time (P > .20). The Beck Depression Inventory (BDI) also showed no effect between responders and nonresponders (P = .83).
To determine if the effects persisted after treatment, patients were asked to return to 2 follow-ups (* in Fig 5, top right). All responders returned to the 2-week follow-up and their VAS scores remained significantly lower than their baseline values (−3.7 ± 2.3, P = .0062, n = 7). At the 8-week follow-up, the improvement in pain was no longer statistically different from baseline (−1.9 ± 3.7 P = .26, n = 6).
To assess whether treatment success can be predicted, we analyzed the BNA scores of the first pretreatment session of responders and nonresponders. Responders started with higher BNA scores than nonresponders, reflecting higher similarity to the healthy network reference, whereas nonresponders showed low similarity if any to healthy patients’ pain-related network. A receiver operating characteristic (ROC) analysis on the BNA scores from the pretreatment session shows that responsiveness can be predicted before the commencement of treatment (Fig 6, bottom left; P = .05 in a Wilcoxon rank sum test).
To determine if there is also an acute effect that explains the difference between responders and nonresponders, we analyzed the difference in VAS scores provided immediately before and after HD-tDCS treatment and averaged across all sessions. Responders show a significant acute decrease in VAS (Fig 6, top right; P = .02). The acute effect observed on the first treatment day is also a reasonable predictor of treatment success (Fig 6, bottom left; P = .04 in a Wilcoxon rank sum test). A separate analysis shows that baseline VAS scores do not differ significantly between responders and nonresponders (P = .26).
These data suggest that there is an acute effect in responders and that responders and nonresponders alike benefit from a cumulative effect of treatment. These combined effects result in a clinically significant benefit of a 50% reduction in perceived pain in a statistically significant number of patients, and these patients can be identified relatively accurately in advance.
Discussion
Primary Outcomes
Our overarching goal is to validate a clinically efficacious HD-tDCS protocol to treat FM-related pain that incorporates response rate, efficacy, and tolerability features that support adoption. Neuromodulation can in principle be optimized by dose, repetition (sessions), adjunct treatments, and patient selection (inclusion). Here, dose was fixed to the 4 × 1 montage of HD-tDCS over M1 with 2 mA of current delivery, selected for few side effects,25 extended neuroplasticity,22 and anatomic specificity,12 and promising pilot results.4,44
Our study was based on the notion of a monotonic dose response, following the rationale of increasing the number of HD-tDCS treatment sessions until a clinical response was achieved. The number of treatment sessions is a critical factor in determining tDCS effectiveness for pain relief,42 presumably leveraging well-established cumulative changes in excitability and plasticity with repeated tDCS sessions.6,15,21,26
In our analysis, we took a conservative approach calling all dropouts, nonresponders; however, 6 of 7 nonresponders were dropouts with an uncertain outcome had they remained in treatment, with only 1 patient who adhered to the 6-week treatment regime without reaching the treatment goal of a 50% reduction in VAS. Using this conservative approach, we show a successful reduction of VAS pain by more than 50% in 7 of 14 patients with FM, at above chance levels. We estimate 15 stimulation sessions as the median number of HD-tDCS sessions required to produce at least a 50% decrease levels in perceived pain.
These findings further emphasize the need for optimization and standardization of treatment parameters, given their critical influence on clinical efficacy. Recent studies have addressed the variability in results of NIBS, especially tDCS, for the treatment of pain, hypothesizing that the limited and marginal clinical results are directly related to the lack of coupling between neurophysiologic responses in the areas stimulated and translated improvements in signs and symptoms. Contrary to this theory, we believe that the lack of clinical efficacy reported in several studies is mainly associated with a lack of consistency in clinical trial design and stimulation parameters, so that the treatment framework is not optimized to achieve the best clinical response. VAS benefits were paralleled by FIQ, which is a practically more meaningful measure assessing broader quality of life.
Biomarkers, Response Predictors
Although necessary for clinical response, an extensive treatment protocol (weeks) increases the burden to both patients and caregivers; therefore, methods to predict responsiveness would enhance clinical effectiveness and avoid unnecessary burdens for those patients who are more likely to not obtain a clinical benefit from the protocol. Predicting likeliness of response may also encourage patients to persist in treatment and decrease the rate of dropouts. We observed a 30% attrition of screened patients (before the first HD-tDCS) and 42% dropout on enrolled patients over the course of the trial, despite minimal side effects and a trend in nonresponder dropouts toward reduced VAS. As indicated by patients who decided to end their participation in the trial, this dropout rate could be attributed in part to several aspects of the study design, mainly required duration of participation, daily visits to the study center, and length of study visits. Other reasons that could be considered among the causes of the high dropout rate are that patients perceived the treatment as ineffective and baseline pain levels were too high to commit to daily visits to the center.
Biomarkers included CHEPs as a measurement of pain-related central sensitization. Because pain is a multidimensional phenomenon,1,16 it is necessary to represent pain perception in a multivariate manner38 and not just by relying on a single dimension (eg, CHEPs amplitudes). Therefore, in this study we used an ERP analysis tool, BNA,33–35,39 to assess the reorganization of brain networks, after HD-tDCS stimulation.
In the post hoc analysis, CHEPs before first treatment (BNA score) were predictive of response to treatment. This measurement and its association with responsiveness is of paramount importance, given that it can be interpreted as a marker of central sensitization, suggesting that differences in the baseline characteristics of brain adaptation to pain determine the pattern of response to this treatment approach. Changes in pain score after the first treatment session (VAS) were also reasonably predictive of treatment responsiveness. Although this analysis was not blinded to outcome, given the logistical burden of extended treatment, these predictive measures warrant inclusion as planned comparisons in a future phase III trial.
Mechanisms
The analgesic effects produced by stimulating M128,41 have made it a main target point of NIBS for pain syndromes like FM. NIBS techniques including repetitive transcranial magnetic stimulation5,24,30 and tDCS14,37,42 over M1 have been shown to have significant analgesic effects in FM, possibly through modification of the sensory components of pain by thalamic inhibitory networks.
The BNA scores in this study were computed against an RBNM (the group’s characteristic brain pattern) consisting of central theta-delta coactivations. The low-frequency brain network activity characterizing the RBNM might correspond to the somatosensory and affective components of pain perception.45 However, it has been previously shown that tDCS over M1 had an effect mainly on sensory aspects of pain through modulation of M1-thalamic inhibitory connections.32 Previous findings19,38 have also suggested that the amplitude of evoked responses at the theta frequencies are correlated with the individual’s sensitivity to pain and can potentially predict pain in humans. Furthermore, theta oscillations in the thalamocortical loop including primary somatosensory cortex (SI) could serve as a biomarker for pain.8,23 Because SI receives projections from the ventral posterolateral thalamus, the resemblance of the activations of responders to the theta-delta pain network of healthy control individuals may reflect the effect of pain on thalamocortical activations in humans.23
FM is characterized by a lack of inhibitory control over somatosensory processing.44 Accordingly, we speculate that those patients who exhibit a distinct and pronounced response to electrical modulation of the M1 have more abnormal plastic modifications in the circuits in charge of the sensory processing component of pain, characterized by a high level of similarity to the pain network of healthy control individuals, whereas those patients who have little or no response to this treatment technique may have more pronounced alterations in affective-emotional networks in charge of modulation of the unpleasant sensations derived from pain, resulting in a low level of similarity to this pain network. Therefore, it would be possible to hypothesize that M1 stimulation would elicit prominent analgesic effects in patients with FM whose pain is mostly caused by a deregulation in the modulatory output exerted by this cortical region. Not surprisingly, responders benefit more from acute treatment as well. Hence, the fact that the activation pattern of responders in this study resembles the pain activation pattern of healthy control individuals may indicate that this pattern may potentially serve as a BNA biomarker for the prediction of treatment success.
Side Effects
Treatment-related adverse events were mostly mild and transient. The most common side effects reported included a tingling sensation over the scalp (73.33% of patients), mild headaches (40%), mild pain in the stimulation area (40%), and skin redness in the stimulation area (26.66%). The frequency and severity of adverse events did not increase with the number of sessions, even for those patients who received up to 26 stimulation sessions.
Limitations and Conclusions
We find a decreasing trend in the VAS and FIQ over time, which does not differ between responders and nonresponders. This may be the result of patients remaining in treatment if they see an improvement but otherwise dropping out. However, this possible confounder does not rule out a cumulative effect of treatment (Fig 6, top left) nor does it explain the relatively large number of responders we observed above the expected chance levels.
Patients were not asked to stop current medications during the study period, but they were asked to report if they had any changes in medication dosage or type. None of our patients reported an increase in their usual analgesic medication dosage or any new medications added to their regimen. Nonetheless, given the uncontrolled nature of this study, it is not possible to rule out any interference of the patient’s concurrent medications and the HD-tDCS treatment.
To judge chance levels of response, we assumed that fluctuations in VAS over 6 weeks are comparable with fluctuations over 1 week preceding the trial. It is possible that fluctuations over that longer timescale are larger and thus that we underestimated the numbers of responders we may have observed by chance. However, in the per-treatment week, VAS values were negatively correlated in time (other than subsequent days). That means that VAS values tended to rebound after 2 or more days if they were high or low on any given day, and thus, that there was no apparent drift of VAS at least in the time frame of 1 week.
The relatively small sample size (N = 14) can be considered another limitation for the study, which could in turn lead to a reduction in statistical power. Nonetheless, given that our main focus was not to test the efficacy of tDCS against sham but instead to establish a stimulation protocol that maximizes efficacy and response rate, and because of the constraints imposed by the study design, the selected patient pool was sufficient to test our hypothesis. Further studies should aim to test this stimulation protocol in larger populations.
Placebo response rates in patients diagnosed with FM as reported in randomized clinical trials have been shown to be substantial,18 although not as significant as those reported here. According to Häuser et al, 18 in randomized controlled trials assessing pain response in FM, the placebo effect is expected to be approximately 18 to 30%. Several studies have addressed the efficacy of tDCS and HD-tDCS for pain control in FM, showing that tDCS is statistically more effective than sham stimulation, but have not directly addressed the magnitude of a placebo effect in these groups.11,42,44 In this trial, we assume that our selected population with FM is comparable with that in previous trials assessing the efficacy of NIBS for FM pain, and therefore, the intervention will be similarly effective. Therefore, an important assumption for this study is that active tDCS is superior to placebo tDCS. Such an assumption is also commonly used, for instance, when an active treatment is compared with another active treatment in a noninferiority design. It is clear that in any clinical trial involving testing of a treatment protocol, the result of such treatment reflects a combination of both the direct effect of the intervention and a placebo effect. Our trial was not designed to test this; instead, our main aim was to gather information regarding HD-tDCS treatment in a real-life scenario to develop a stimulation protocol that enhanced response rate and efficacy.
To further analyze if our results were mainly driven by a placebo response, we examined the results of placebo-controlled studies on pain that looked into predictors for placebo response. Most of these analyses were conducted on neuropathic pain13,20 but 1 trial17 also assessed placebo responders in FM. All these studies show that baseline pain levels are associated with a higher placebo response. It is important to underscore that, in our analyses, baseline pain levels were not associated with response to treatment. Other predictors that were found in some of these studies only, and thus not consistent across studies, were younger age, longer study duration, higher ratio of patients on active treatment to placebo, study year, and patients in study sites with faster recruitment.17 The only predictor considered here that would be relevant to our study is the study duration; however, we found that response to the first session of stimulation was associated with response to multiple sessions, and thus, speaking against that, response to consecutive sessions could be associated with a placebo response only.
The improvements in VAS and FIQ over time may be the result of a placebo effect. This study, by design, did not control for placebo, nor did it aim to compare active stimulation with placebo or establish possible predictors of placebo effect. Instead, we focused on dose-response optimization, consistent with a phase II study. Nonetheless, we believe that the decrease in pain levels observed in those who responded to the stimulation protocol was not entirely the result of a placebo effect but mainly a direct benefit of the treatment. We determined that the median time to reach a clinically meaningful outcome was 15 treatment days (3 weeks) and that 7 of 8 patients who adhered to the 6-week treatment protocol benefited from treatment, with benefits persisting for at least 2 weeks after treatment. With these encouraging results, a future phase III study should aim to increase adherence to the 6 weeks of treatment to rule out that the observed benefits were a result of selective attrition. Only after that may a phase III efficacy study (double-blind sham-controlled) be warranted.
We did not exhaust the list of potential predictors that could be associated with response to HD-tDCS. Further studies should address, for instance, the effects of HD-tDCS stimulation protocols on neuropsychological markers, including attention, in patients with FM.
Perspective.
In this article, an optimized protocol for the treatment of fibromyalgia pain with targeted subthreshold brain stimulation using high-definition transcranial direct current stimulation is outlined.
Acknowledgments
This research was supported in part by grants from Elminda, Ltd, Coulter Foundation (grant 75969-00-02), the National Institutes of Health (NIH grant 5U54CA132378-08, NIH/NSF grant 5R01MH092926-05) and the United States Department of Defense (grant FA9550-13-1-0073). The City University of New York owns patents on High-Definition tDCS with M.B., L.C.P., and A.D. as inventor. M.B., L.C.P., and A.D. have equity in So-terix Medical Inc.
We want to thank Renata Brandao, Carolina Perez, Fernanda Fiorillo, and Flavia Orange for their help with data collection and conducting the study.
Footnotes
Online Registration: Registered in Clinicaltrials.gov under registry number NCT01842009.
References
- 1.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Bannwarth B, Blotman F, Roue-Le Lay K, Caubere JP, Andre E, Taieb C. Fibromyalgia syndrome in the general population of France: a prevalence study. Joint Bone Spine. 2009;76:184–187. doi: 10.1016/j.jbspin.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Beck AT, Steer RA, Brown GK. BDI-II, Beck Depression Inventory: Manual. 2. San Antonio, TX.; Boston: Psychological Corp.; Harcourt Brace; 1996. [Google Scholar]
- 4.Borckardt JJ, Bikson M, Frohman H, Reeves ST, Datta A, Bansal V, Madan A, Barth K, George MS. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J Pain. 2012;13:112–120. doi: 10.1016/j.jpain.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Boyer L, Dousset A, Roussel P, Dossetto N, Cammilleri S, Piano V, Khalfa S, Mundler O, Donnet A, Guedj E. rTMS in fibromyalgia: a randomized trial evaluating QoL and its brain metabolic substrate. Neurology. 2014;82:1231–1238. doi: 10.1212/WNL.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 6.Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cano T, Morales-Quezada JL, Bikson M, Fregni F. Methods to focalize noninvasive electrical brain stimulation: principles and future clinical development for the treatment of pain. Expert Rev Neurother. 2013;13:465–467. doi: 10.1586/ern.13.41. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso-Cruz H, Sameshima K, Lima D, Galhardo V. Dynamics of circadian thalamocortical flow of information during a peripheral neuropathic pain condition. Front Integr Neurosci. 2011;5:43. doi: 10.3389/fnint.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo Saavedra L, Mendonca M, Fregni F. Role of the primary motor cortex in the maintenance and treatment of pain in fibromyalgia. Med Hypotheses. 2014;83:332–336. doi: 10.1016/j.mehy.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Diers M, Koeppe C, Yilmaz P, Thieme K, Markela-Lerenc J, Schiltenwolf M, van Ackern K, Flor H. Pain ratings and somatosensory evoked responses to repetitive intramuscular and intracutaneous stimulation in fibromyalgia syndrome. J Clin Neurophysiol. 2008;25:153–160. doi: 10.1097/WNP.0b013e31817759c5. [DOI] [PubMed] [Google Scholar]
- 11.Donnell A, DNT, Lawrence M, Gupta V, Zieba T, Truong DQ, Bikson M, Datta A, Bellile E, DaSilva AF. High-definition and non-invasive brain modulation of pain and motor dysfunction in chronic TMD. Brain Stimul. 2015;8:1085–1092. doi: 10.1016/j.brs.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M. Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage. 2013;74:266–275. doi: 10.1016/j.neuroimage.2013.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman R, Emir B, Parsons B. Predictors of placebo response in peripheral neuropathic pain: insights from pre-gabalin clinical trials. J Pain Res. 2015;8:257–268. doi: 10.2147/JPR.S78303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, Bravo R, Rigonatti SP, Freedman SD, Nitsche MA, Pascual-Leone A, Boggio PS. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54:3988–3998. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- 15.Fricke K, Seeber AA, Thirugnanasambandam N, Paulus W, Nitsche MA, Rothwell JC. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2011;105:1141–1149. doi: 10.1152/jn.00608.2009. [DOI] [PubMed] [Google Scholar]
- 16.Friebel U, Eickhoff SB, Lotze M. Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain. Neuroimage. 2011;58:1070–1080. doi: 10.1016/j.neuroimage.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser W, Bartram-Wunn E, Bartram C, Reinecke H, Tolle T. Systematic review: placebo response in drug trials of fibromyalgia syndrome and painful peripheral diabetic neuropathy-magnitude and patient-related predictors. Pain. 2011;152:1709–1717. doi: 10.1016/j.pain.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 18.Hauser W, Sarzi-Puttini P, Tolle TR, Wolfe F. Placebo and nocebo responses in randomised controlled trials of drugs applying for approval for fibromyalgia syndrome treatment: systematic review and meta-analysis. Clin Exp Rheumatol. 2012;30:78–87. [PubMed] [Google Scholar]
- 19.Iannetti GD, Zambreanu L, Cruccu G, Tracey I. Operculoinsular cortex encodes pain intensity at the earliest stages of cortical processing as indicated by amplitude of laser-evoked potentials in humans. Neuroscience. 2005;131:199–208. doi: 10.1016/j.neuroscience.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 20.Irizarry MC, Webb DJ, Ali Z, Chizh BA, Gold M, Kinrade FJ, Meisner PD, Blum D, Silver MT, Weil JG. Predictors of placebo response in pooled lamotrigine neuropathic pain clinical trials. Clin J Pain. 2009;25:469–476. doi: 10.1097/AJP.0b013e31819ddded. [DOI] [PubMed] [Google Scholar]
- 21.Knotkova H, Nitsche MA, Cruciani RA. Putative physiological mechanisms underlying tDCS analgesic effects. Front Hum Neurosci. 2013;7:628. doi: 10.3389/fnhum.2013.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo HI, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, Nitsche MA. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimul. 2013;6:644–648. doi: 10.1016/j.brs.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Leblanc BW, Lii TR, Silverman AE, Alleyne RT, Saab CY. Cortical theta is increased while thalamocortical coherence is decreased in rat models of acute and chronic pain. Pain. 2014;155:773–782. doi: 10.1016/j.pain.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Mhalla A, Baudic S, Ciampi de Andrade D, Gautron M, Perrot S, Teixeira MJ, Attal N, Bouhassira D. Long-term maintenance of the analgesic effects of transcranial magnetic stimulation in fibromyalgia. Pain. 2011;152:1478–1485. doi: 10.1016/j.pain.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 25.Minhas P, Bansal V, Patel J, Ho JS, Diaz J, Datta A, Bikson M. Electrodes for high-definition transcutaneous DC stimulation for applications in drug delivery and electrotherapy, including tDCS. J Neurosci Methods. 2010;190:188–197. doi: 10.1016/j.jneumeth.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, Nitsche MA. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013;6:424–432. doi: 10.1016/j.brs.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Neumann L, Buskila D. Epidemiology of fibromyalgia. Curr Pain Headache Rep. 2003;7:362–368. doi: 10.1007/s11916-003-0035-z. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen JP, Keravel Y, Feve A, Uchiyama T, Cesaro P, Le Guerinel C, Pollin B. Treatment of deafferentation pain by chronic stimulation of the motor cortex: report of a series of 20 cases. Acta Neurochir Suppl. 1997;68:54–60. doi: 10.1007/978-3-7091-6513-3_10. [DOI] [PubMed] [Google Scholar]
- 29.O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2014:CD008208. doi: 10.1002/14651858.CD008208.pub3. [DOI] [PubMed] [Google Scholar]
- 30.Passard A, Attal N, Benadhira R, Brasseur L, Saba G, Sichere P, Perrot S, Januel D, Bouhassira D. Effects of unilateral repetitive transcranial magnetic stimulation of the motor cortex on chronic widespread pain in fibromyalgia. Brain. 2007;130:2661–2670. doi: 10.1093/brain/awm189. [DOI] [PubMed] [Google Scholar]
- 31.Petzke F, Khine A, Williams D, Groner K, Clauw DJ, Gracely RH. Dolorimetry performed at 3 paired tender points highly predicts overall tenderness. J Rheumatol. 2001;28:2568–2569. [PubMed] [Google Scholar]
- 32.Polania R, Paulus W, Nitsche MA. Modulating corticostriatal and thalamocortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp. 2012;33:2499–2508. doi: 10.1002/hbm.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reches A, Kerem D, Gal N, Laufer I, Shani-Hershkovitch R, Dickman D, Geva AB. A novel ERP pattern analysis method for revealing invariant reference brain network models. Functional Neurology, Rehabilitation, and Ergonomics. 2013;3:295–317. [Google Scholar]
- 34.Reches A, Laufer I, Ziv K, Cukierman G, McEvoy K, Ettinger M, Knight RT, Gazzaley A, Geva AB. Network dynamics predict improvement in working memory performance following donepezil administration in healthy young adults. Neuroimage. 2013;88C:228–241. doi: 10.1016/j.neuroimage.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reches A, Levy-Cooperman N, Laufer I, Shani-Hershkovitch R, Ziv K, Kerem D, Gal N, Stern Y, Cukierman G, Romach MK, Sellers EM, Geva AB. Brain network activation (BNA) reveals scopolamine-induced impairment of visual working memory. J Mol Neurosci. 2014;54:59–70. doi: 10.1007/s12031-014-0250-6. [DOI] [PubMed] [Google Scholar]
- 36.Reches A, Shram MJ, Dickman D, Laufer I, Shani-Hershkovitch R, Stern Y, Weiss M, Nir RR, Yarnitsky D, Geva AB. A novel electroencephalography-based tool for objective assessment of network dynamics activated by nociceptive stimuli. Eur J Pain. 2015 doi: 10.1002/ejp.716. [DOI] [PubMed] [Google Scholar]
- 37.Riberto M, Marcon Alfieri F, Monteiro de Benedetto Pacheco K, Dini Leite V, Nemoto Kaihami H, Fregni F, Rizzo Battistella L. Efficacy of transcranial direct current stimulation coupled with a multidisciplinary rehabilitation program for the treatment of fibromyalgia. Open Rheumatol J. 2011;5:45–50. doi: 10.2174/1874312901105010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulz E, Tiemann L, Schuster T, Gross J, Ploner M. Neurophysiological coding of traits and states in the perception of pain. Cereb Cortex. 2011;21:2408–2414. doi: 10.1093/cercor/bhr027. [DOI] [PubMed] [Google Scholar]
- 39.Shahaf G, Reches A, Pinchuk N, Fisher T, Ben Bashat G, Kanter A, Tauber I, Kerem D, Laufer I, Aharon-Peretz J, Pratt H, Geva AB. Introducing a novel approach of network oriented analysis of ERPs, demonstrated on adult attention deficit hyperactivity disorder. Clin Neurophysiol. 2012;123:1568–1580. doi: 10.1016/j.clinph.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Tastekin N, Uzunca K, Sut N, Birtane M, Mercimek OB. Discriminative value of tender points in fibromyalgia syndrome. Pain Med. 2010;11:466–471. doi: 10.1111/j.1526-4637.2009.00784.x. [DOI] [PubMed] [Google Scholar]
- 41.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortexstimulationfor the treatment of central pain. Acta Neurochir Suppl (Wien) 1991;52:137–139. doi: 10.1007/978-3-7091-9160-6_37. [DOI] [PubMed] [Google Scholar]
- 42.Valle A, Roizenblatt S, Botte S, Zaghi S, Riberto M, Tufik S, Boggio PS, Fregni F. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J Pain Manag. 2009;2:353–361. [PMC free article] [PubMed] [Google Scholar]
- 43.Villamar MF, Volz MS, Bikson M, Datta A, Dasilva AF, Fregni F. Technique and considerations in the use of 4x1 ring high-definition transcranial direct current stimulation (HD-tDCS) J Vis Exp. 2013:e50309. doi: 10.3791/50309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villamar MF, Wivatvongvana P, Patumanond J, Bikson M, Truong DQ, Datta A, Fregni F. Focal modulation of the primary motor cortex in fibromyalgia using 4x1-ring high-definition transcranial direct current stimulation (HD-tDCS): immediate and delayed analgesic effects of cathodal and anodal stimulation. J Pain. 2013;14:371–383. doi: 10.1016/j.jpain.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Walton KD, Dubois M, Llinás RR. Abnormal thalamocortical activity in patients with complex regional pain syndrome (CRPS) type I. Pain. 2010;150:41–51. doi: 10.1016/j.pain.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 46.White KP, Harth M. Classification, epidemiology, and natural history of fibromyalgia. Curr Pain Headache Rep. 2001;5:320–329. doi: 10.1007/s11916-001-0021-2. [DOI] [PubMed] [Google Scholar]
- 47.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 48.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]