Abstract
PCBs appear in school air because many school buildings were built when PCBs were still intentionally added to building materials and because PCBs are also present through inadvertent production in modern pigment. This is of concern because children are especially vulnerable to the toxic effects of PCBs. Here we report indoor and outdoor air concentrations of PCBs and OH-PCBs from two rural schools and four urban schools, the latter near a PCB-contaminated waterway of Lake Michigan in the United States. Samples (n=108) were collected as in/out pairs using polyurethane foam passive air samplers (PUF-PAS) from January 2012 to November 2015. Samples were analyzed using GC/MS-MS for all 209 PCBs and 72 OH-PCBs. Concentrations inside schools were one to two orders of magnitude higher than outdoors and ranged 0.5–194 ng/m3 (PCBs) and 4–665 pg/m3 (OH-PCBs). Congener profiles were similar within each sampling location across season but different between schools and indicated the sources as Aroclors from building materials and individual PCBs associated with modern pigment. This study is the first cohort-specific analysis to show that some children’s PCB inhalation exposure may be equal to or higher than their exposure through diet.
Graphical Abstract
Introduction
Exposure to polychlorinated biphenyls (PCBs) in schools is receiving increased attention by researchers and the public.1–12 Many schools were built when PCBs were still used in building materials, and children may be especially vulnerable to the toxic effects of PCBs.13, 14 PCBs are a group of 209 anthropogenic persistent organic pollutants that were produced in the United States as mixtures called Aroclors until 1977. Aroclors were used as electrical insulating fluids in capacitors and transformers and added as plasticizers and flame retardants to a wide variety of products such as caulk, adhesives, plastics, and carbonless copy paper.15 Aroclors are still present in many electrical devices and building materials that are currently in use, including light ballasts, sealants, and window caulking.16 PCBs are also present in modern architectural paint because they are a byproduct of pigment manufacturing.17, 18 We and others measured these non-Aroclor PCBs in paint and consumer products17–20 and the environment.21–24
Due to the ubiquitous presence of PCBs in the environment, humans are readily exposed to PCBs through diet, inhalation, and dermal contact. Diet has long-been identified as the major exposure route but inhalation may be significant for some populations, including children.4, 16, 25. Aroclor and non-Aroclor PCBs and their hydroxylated metabolites (OH-PCBs) have been measured in people around the world including children.2, 26–29 Although OH-PCBs are recognized as human28, 30 and plant31 metabolites of PCBs, they are also emerging as abiotic environmental contaminants of water,32 sediment and Aroclors,33 and outdoor air.34 Exposure of school-age children to PCBs and OH-PCBs is particularly concerning because these compounds are carcinogenic to humans,35 target the endocrine system,36, 37 and are linked to neurodevelopmental disorders.38–40 OH-PCBs may be more toxic than PCBs although fewer studies have been reported.41
In some cases the increased attention by the general public to PCBs in schools has led to lawsuits seeking remediation of PCBs.42–44 One school in Massachusetts was demolished because of excessive levels of PCBs inside.1 However, U.S. schools are not required to test for the presence of PCBs, and few research studies measuring children’s inhalation exposure to PCBs in schools have been published.1 Here we report indoor and outdoor air concentrations of PCBs and, for the first time, indoor concentrations of OH-PCBs from four urban and two rural schools in the United States. We calculate the resulting PCB inhalation exposure of children and compare to expected exposure through diet.
Methods
Sample Collection
We collected air samples inside and outside junior and senior high schools attended by adolescents enrolled in the Airborne Exposures to Semi-volatile Organic Pollutants (AESOP) Study. The AESOP Study is a twin-cohort longitudinal exposure assessment study of PCBs and other persistent pollutants among children and their mothers. Details of the AESOP Study, cohort demographics, biomonitoring methods, dietary exposure estimation, and earlier results involving home and school air sampling and blood and saliva collection has been described previously.4, 26, 28, 45–47 Polyurethane foam passive air samplers (PUF-PAS) were used to collect gas and fine particulate phase PCBs and OH-PCBs inside and outside the schools. The PUF-PAS followed the “Harner style” double domed sampler design.48 Although gas and particulate phases cannot be separated with this sampling method, studies have determined that PCBs are predominantly found in the gas phase.49
Each 24 cm diameter stainless steel top bowl and 19.5 cm diameter stainless steel bottom bowl housed a PUF disk (Tisch Environmental, Cleves, OH) that had been pre-cleaned (described in the Supporting Information). Samples were deployed in indoor/outdoor pairs at two junior and two senior high schools in East Chicago, Indiana and one junior and one senior high school in Columbus Junction, Iowa (Table S1). Columbus Junction junior and senior high schools are co-located, so only one outdoor sample was collected for every two indoor samples in Columbus Junction. Outdoor passive samplers were lost or stolen for three sequential sampling periods from East Chicago School 4, and so the outdoor paired sample was not available for analysis for three of the seven indoor samples collected at School 4. PUF-PAS were deployed and collected by trained field staff from January 2012 to November 2015. Field blanks consisting of clean PUF were shipped and returned with the samples. Deployment periods averaged 48 days and ranged 22–114 days depending on field staff’s access to the sampling sites.
An important and dominant feature of East Chicago, Indiana is the Indiana Harbor and Ship Canal (IHSC). The IHSC is a waterway serving major industries and is contaminated with Aroclor 1248 up to 35,000 ng PCBs per gram dry weight.50 The IHSC flows within 0.4–2.5 km of the schools and is a source of airborne PCBs.51 Martinez et al. determined that although the IHSC contributes nearly 7 kg of PCBs to the air annually, there are other unidentified sources of airborne PCBs in this highly industrialized community. There are no known sources of PCBs to ambient air in the Columbus Junction, Iowa community.
Sample Extraction and Instrument Analysis
A Soxhlet method that included an acid wash was developed for the co-extraction of PCBs and OH-PCBs from PUF. Acidification of the PUF prior to extraction with organic solvents was necessary to protonate and neutralize OH-PCBs.52 PUF were spiked with 10 ng 13C-labeled PCBs (Cambridge Isotope Laboratories, Inc., Andover, MA, USA) and 13C-labeled OH-PCBs (Wellington Laboratories, Guelph, ON, Canada) (Table S2), acidified with HCl, and refluxed with 250 mL acetone for 6 hours. Hexane (25 mL) was added to the extract which was then concentrated under N2, and the aqueous phase was removed after addition of hexane/methyl tert-butyl ether (MTBE, 9:1, v/v). An aqueous solution of KOH in ethanol was added, and the mixture was centrifuged to deprotonate the OH-PCBs and separate the OH-PCBs from the organic phase. The aqueous solution was then acidified with HCl, and OH-PCBs were extracted with hexane/MTBE (9:1, v/v). The OH-PCBs in hexane/MTBE fraction were derivatized to their methoxylated form (MeO-PCBs), and interferences were removed by passing the sample extracts through sulfuric acid silica gel columns. The MeO-PCB fraction was eluted from the column with dichloromethane, and then the solvent was exchanged to hexane. Of the total sample set of 108, 57 were analyzed for both sets of OH-PCB and PCB congeners. The remaining PUF were extracted for only PCBs using pressurized solvent extraction (Thermo Scientific ASE 350). PCB fractions were cleaned using the same sulfuric acid silica gel columns but eluted with hexane. Both PCB and MeO-PCB fractions were spiked with 10 ng internal standards d-PCB30 (2,4,6-trichlorobiphenyl-2′,3′,4′,5′,6′-d5, C/D/N Isotopes, Pointe-Claire, QC, Canada) and PCB 204 (2,2′,3,4,4′,5,6,6′-octachlorobiphenyl, Cambridge Isotope Laboratories, Inc.).
GC-MS/MS (Agilent 7890A GC system, Agilent 7000 Triple Quad, Agilent 7693 autosampler) in multiple reaction monitoring mode (MRM) was used for identification and quantification of 209 PCBs as 174 chromatographic peaks and 72 MeO-PCBs as 66 chromatographic peaks. Instrument operating parameters are described in the SI. Instrument blanks of hexane were analyzed before and after the calibration and after the samples to ensure no carryover.
PCBs and OH-PCBs (as MeO-PCBs) were identified in samples by comparison with the calibration standards (described in the SI) in the same MRM transition according to retention time (+/− 0.06 min). The calibration standard solution for PCBs included all 209 congeners as well as 11 labeled internal and surrogate standards. While there are 837 possible mono hydroxylated PCBs,53 the calibration standard solution contained only 72 OH-PCB congeners commercially available and 8 internal and surrogate OH-PCB standards.
Quality Control
Extraction efficiency, reproducibility, and accuracy was assessed using 13C labeled surrogate standards, replicates of method blanks, and analysis of standard reference materials (Figures S2–S3). Standard Reference Material purchased from the National Institute of Standards and Technology (NIST SRM 2585, Gaithersburg, MD, USA) was analyzed in replicates of four (Figure S1). The samples were analyzed as indoor-outdoor pairs (East Chicago) or triads (Columbus Junction) together with their associated field blank in a batch along with one method blank per batch. Results from the method blank were used to determine the limit of quantification (LOQ, SI Tables S5–S6) as the upper limit of the 99% confidence interval (average mass plus three times the standard deviation). PCB and OH-PCB masses were corrected for surrogate recoveries less than 100%.
Determining Sample Concentration
For samples collected in an outdoor environment, the sampling rate (Rs), and subsequent effective sampling volume (Veff), was calculated using a model previously described.54 The model calculates a deployment, compound, and site specific Veff based on the hourly meteorological data with wind speed as the primary component. Our model was previously calibrated for depuration compound results and validated against multiple independent studies comparing passive and active sampling results. The dynamic model removes the need for expensive depuration compounds, accounts for temperature changes, and corrects for PUF saturation (non-linear uptake). Determining sample concentration without associated meteorological data for a PUF-PAS sample is a challenging and important issue because small variations in Rs can significantly affect the estimated inhalation exposure. A few studies have estimated the indoor Rs of PCB congeners for a double-dome PUF-PAS sampler: Persoon and Hornbuckle estimated indoor sampling rates ranging 2.0–3.5 m3/d (average = 2.6 m3/d) in two separate laboratories.55 Bohlin et al. estimated indoor sampling rates ranging 0.9–1.7 m3/d (average = 1.2 m3/d) in a lecture room.56 Hazrati and Harrad estimated indoor sampling rates ranging 0.57–1.55 m3/d (average = 0.8 m3/d) in a temporarily vacant office.57 We suspect the different Rs in these studies are primarily a result of different air ventilation rates for the different room styles. According to ANSI/ASHRAE Standard 62 (Ventilation for Acceptable Indoor Air Quality) the required minimum ventilation rates for different rooms is a function of the occupancy and room size.58 The minimum ventilation rate for science laboratories (0.18 cfm/ft2) is three times higher than the minimum ventilation rate for lecture rooms and office spaces (0.06 cfm/ft2) and likely explains why Persoon and Hornbuckle estimated Rs values that were three times higher in laboratories than the other two studies conducted in a lecture room and office. For this study we assumed an Rs of 0.8 m3/d because an office best represents our sampling environment. Veff was calculated for each congener with the assumed Rs of 0.8 m3/d and an average deployment temperature of 20°C as inputs. Veff ranged 17.3–361 m3. Assuming a constant Rs and constant temperature to calculate Veff with the Herkert Model produces results nearly identical with the commonly used GAPS template.54, 59
Exposure Calculations
Dietary exposure for the AESOP children was calculated as a product of PCB concentrations in food and age- and gender-specific food ingestion rates and was published previously.4 School children’s annual inhalation exposure to PCBs was calculated as the sum of seasonal exposure during winter, spring, and autumn for each individual school. Winter was defined as the months of December, January, and February; spring was defined as the months of March, April, and May; and autumn was defined as the months of September, October, and November. Summer was not included in the calculations because the children did not attend school during the summer months. Each season’s exposure was calculated for each congener as the product of the number of days in school, the volume of air breathed, and the average concentration of the congener inside the school during that season. The number of days in school were estimated as 52, 65, and 65 for winter, spring, and autumn, respectively, for every school. The volume of air breathed was calculated as part of the model we described previously4 for each child enrolled in the AESOP study according to survey data on their activity level during each season. The volume of air breathed was calculated (Table S7) for the present study for each season after sorting into four groups: East Chicago girl (n=18), East Chicago boy (n=15), Columbus Junction girl (n=20), and Columbus Junction boy (n=23). Thus, the exposure calculations are summarized as annual exposure for girls and boys attending EC School 1, EC School 2, EC School 3, and EC School 4 and for girls and boys attending CJ School 1 and CJ School 2.
Statistics
The concentration data set was first dichotomized at the threshold of the congener-specific LOQ (Tables S5–S6): concentrations of congeners below the LOQ were treated as zero. Distribution of sum and individual congener concentrations were skewed right, and data approximated a normal distribution following logarithm transformation. Statistical analyses comparing school data were performed on the transformed data.
Cosine theta (cosθ) was calculated for combinations of sample and potential source pairs for the purpose of comparing PCB profiles,60 where cosθ = 0 describes two completely different profiles and cosθ = 1 describes identical profiles. Prior to cosine theta analysis, sample profiles were created by normalizing each congener in a sample to the sum of all congeners in that sample. Aroclor profile data was from Frame et al.61
Results and Discussion
OH-PCBs
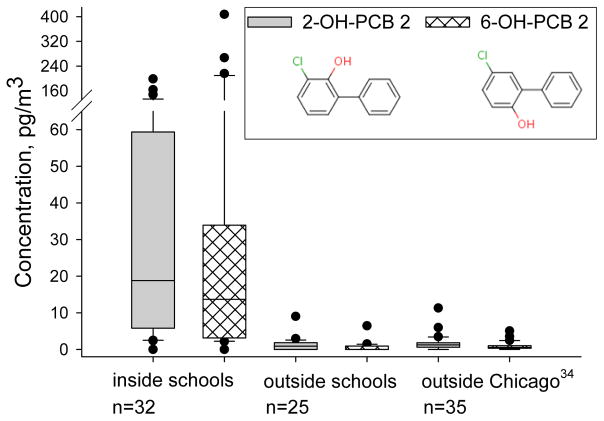
To our knowledge this is the first report of OH-PCBs in indoor air. Concentrations of ΣOH-PCBs ranged 4–665 pg/m3 indoors and from below detection to 18 pg/m3 outdoors. We detected only 11 OH-PCBs above the LOQ in the school samples. The two most common congeners, 2-OH-PCB 2 and 6-OH-PCB 2, were detected in 84% and 68% of samples, respectively. These two OH-PCBs were also the most common congeners detected in Chicago air.34 Other congeners in our OH-PCB calibration set were detected less frequently and are presented in Table S8.
Concentrations of 2-OH-PCB 2 and 6-OH-PCB2 outside of East Chicago and Columbus Junction schools are not different from our previous study of OH-PCBs in Chicago air collected with high volume air samplers,34 the only other published report of OH-PCBs in air. Indoor concentrations of these two OH-PCBs are an order of magnitude higher. We hypothesized previously that 2-OH-PCB 2 and 6-OH-PCB 2 measured in the air are a result of volatilization from Aroclors because the data did not support their presence from reaction between hydroxyl radical and PCB 2.34
PCBs
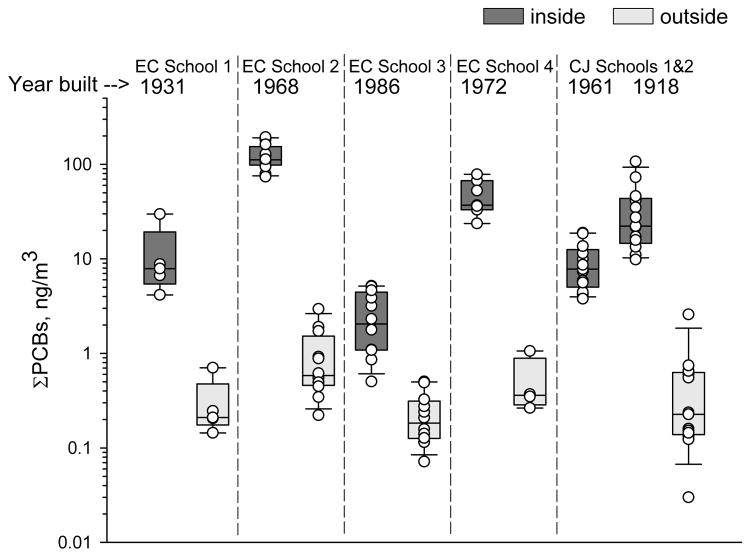
Concentrations of ΣPCBs ranged 0.5–194 ng/m3 indoors and from 0.03 to 3 ng/m3 outdoors (Tables S8–S12). Levels inside all schools were below the current US EPA’s recommended action level of 500 ng/m3 for children 12 to 15 years old. Concentrations were inside EC School 2>EC School 4>CJ School 2>CJ School 1, EC School 1>EC School 3 (Figure 2). Concentrations outdoors were not statistically significantly different across schools except that EC School 2 outdoor air was higher than EC School 3 outdoor air. Concentrations inside schools reported in this study are higher than what we reported previously4 due to higher PCB measurements at EC School 4 and CJ School 2. We did not analyze air samples from inside EC School 2 for our previous study. PCB concentrations in these schools are higher than what we previously reported for homes except for a few outliers in each community.4 Indoor concentrations were highest inside the two schools built in 1968 and 1972; years when U.S. Aroclor production was highest (Figure 2).15 The lowest concentrations were measured in the school built after PCB production in the U.S. ended in 1977 (Figure 2).15
Figure 2.
ΣPCBs inside and outside the six schools in this study ranged over three orders of magnitude. Each open circle represents a measurement. The boxes illustrate the median and 25th and 75th percentiles while the whiskers represent the 5th and 95th percentiles for each location. School construction years are listed under the school name. EC represents East Chicago and CJ represents Columbus Junction.
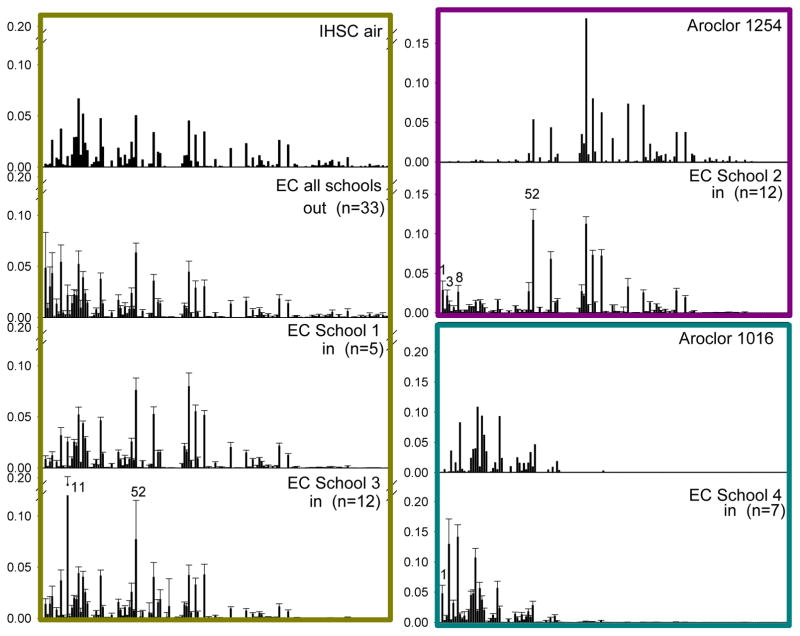
PCB congener profiles were similar within each location with very little variability across season (Figures 3–4). Therefore, average profiles inside each school were used for statistical comparison to outdoor air and IHSC. Air outside the four East Chicago schools all had similar profiles with very little variability across season and school, so the average profile of all outdoor East Chicago school samples was used for statistical comparison to IHSC and indoor air profiles.
Figure 3.
PCB congener profiles of the air above IHSC, Aroclors 1254 and 1016, and average congener profiles of East Chicago (EC) school outdoor air and East Chicago school indoor air from the four EC schools in this study. Profiles are normalized to total PCB concentration. Error bars represent the standard deviation. Profiles are grouped by color according to profile similarities as described in the text. PCBs discussed in the text are labeled in the relevant profiles.
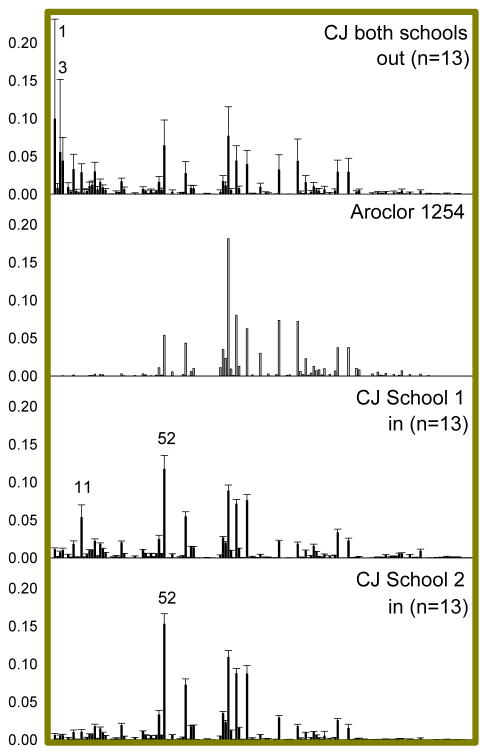
Figure 4.
PCB congener profiles of Aroclor 1254 and average congener profiles of Columbus Junction (CJ) school indoor and outdoor air from the two CJ schools in this study. Profiles are normalized to total PCB concentration. Error bars represent the standard deviation. PCBs discussed in the text are labeled in the relevant profiles.
We found evidence of common sources of PCBs in the air over the IHSC, the air outside the four East Chicago schools, and the air inside two East Chicago schools. The average PCB profile in air outside East Chicago schools is very similar to the average PCB congener profile in air collected over the water of the nearby IHSC (Figure 3, cosθ=0.92). School 1 has a very similar profile to the average outdoor air (cosθ=0.90) and the air above IHSC (cosθ=0.94). Sediment in the IHSC is contaminated with a PCB mixture dominated by Aroclor 1248. Martinez et al. have shown that the sediments of the IHSC are a major source to the overlying water and air and that there are also other sources of this PCB mixture beyond the emissions from the IHSC.62 For example, it is likely that the soils and other surfaces in the community are contaminated with this particular mixture of PCBs and were tracked into the schools. It is also possible that building materials became contaminated during construction of these schools or that long term exposure resulted in accumulation of this PCB mixture in the building which is now degassing. Indoor air at School 3 also has a profile similar to outdoor air and the air above IHSC but with the addition of a strong contribution from PCB 11 (cosθ=0.73; cosθ=0.94 excluding PCB 11). PCB 52 is also observed at higher levels in School 3 than expected from Aroclors. PCB 11 and PCB 52 are both prominent congeners in pigments. Hu and Hornbuckle found the highest proportion of PCB 11 relative to other PCBs in two yellow monoazo pigments, a red proprietary pigment, and a yellow proprietary pigment. They found the highest overall concentrations of PCB 11 in two phthalocyanine green pigments and a proprietary yellow pigment.18 Hu and Hornbuckle found PCB 52 mainly in a red proprietary pigment. Guo et al. measured PCB 11 in a variety of printed material and fabric, and those materials could be additional sources of PCB 11 to school air.17 This evidence suggests that a combination of historical contamination and materials containing modern pigment may be the main sources of PCBs inside this school.
In contrast to EC Schools 1 and 3, concentrations inside EC Schools 2 and 4 were the highest measured in this study, and their average indoor air profiles are very different from the average outdoor air profile (Figure 3). The School 2 indoor air profiles are enriched in mid- and upper-molecular weight PCBs, similar to Aroclor 1254 but also have a much higher contribution from PCB 52 (cosθ=0.85; cosθ=0.89 excluding PCB 52) and a small contribution from lower-molecular weight PCBs such as 1,3, and 8. The School 4 indoor air profiles are enriched in lower-molecular weight PCBs and are most similar to Aroclor 1016 (cosθ=0.85) with a small additional contribution from PCB 1. These profile similarities combined with the much higher concentrations detected in EC School 2 and EC School 4 suggest that the main sources in these two schools are not the same as those to outdoor air. Rather, similarity of the congener profiles from EC School 2 to Aroclor 1254 and School 4 with Aroclor 1016 suggest that Aroclor-enriched building materials are the main contributor to PCBs in the air inside these schools. Aroclor 1016 was only manufactured starting in 1971 with a simultaneous steep decline in the production of other Aroclors and was a distillation of Aroclor 1242 intended to remove the higher chlorinated biphenyls.15 Aroclor 1016 was used mostly in capacitors for fluorescent light ballasts.15 Aroclor 1254 was used in more applications including capacitors, transformers, plasticizers, adhesives, and sealants and caulking compounds.15
The congener profiles of Columbus Junction school outdoor air have the greatest variation among school sampling locations, particularly due to PCB 1 and PCB 3, which have a dominant presence in some samples but are mostly absent in others. The air outside Columbus Junction schools is not similar to background signals we previously reported in nearby Cedar Rapids, Iowa soils63 or Mississippi River sediment cores64. Indoor profiles of both Columbus Junction schools are more similar to Aroclor 1254 (CJ School 1 cosθ=0.79, CJ School 2 cosθ=0.81) than to the average outdoor profile (CJ School 1 cosθ=0.78, CJ School 2 cosθ=0.72). There is also a strong contribution from PCB 11 in CJ School 1 and PCB 52 in both CJ schools. The cosθ increases to 0.86 and 0.87 between Aroclor 1254 and CJ Schools 1 and 2, respectively, when PCBs 11 and 52 are excluded. Like EC Schools 2 and 4 in East Chicago, the higher concentration in these Columbus Junction schools and the lack of similarity between indoor and outdoor air in the suggests that the main sources in these two schools are not the same as the sources to outdoor air. The profile similarities between school indoor air and Aroclor 1254, along with the presence of PCB 11, suggest that a combination of legacy uses of Aroclors such as fluorescent light ballasts or window caulking and current use of PCB-contaminated pigments are the sources of PCBs to indoor school air in Columbus Junction.
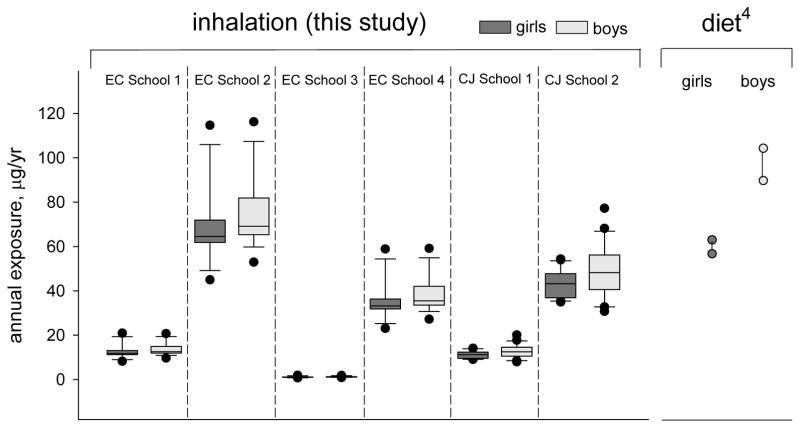
We believe this study is the first cohort-based study to demonstrate that school children’s inhalation exposure may exceed dietary exposure (Figure 5). Higher chlorinated PCBs are disproportionately taken in by ingestion while lower chlorinated PCBs are more volatile and are disproportionately taken in by inhalation. Annual indoor inhalation exposure ranged 0.7–115 μg/yr for girls and 0.9–116 μg/yr for boys. Inhalation exposure varied greatly by school due to the different air concentrations measured in the schools and varied within each school due to the different activity levels of the children in the AESOP cohort. The highest inhalation exposure was experienced by the most active children in the school with the highest air concentrations (EC School 2). Conversely, the lowest inhalation exposure was experienced by the least active children in the school with the lowest air concentrations (EC School 3). Inhalation exposures were similar between girls and boys; girls had slightly lower exposures than boys because their activity levels were on average lower than boys.
Figure 5.
Children’s inhalation exposure at the six schools varies by school, activity level, and gender. Dietary exposure for children from the same cohort ranged 66–108 ug/yr.4 Exposure was calculated individually for East Chicago (EC) girls (n=18), EC boys (n=15), Columbus Junction (CJ) girls (n=20), and CJ boys (n=23). Inhalation exposure varied by school due to the different air concentrations measured in the schools and varied within each school due to the different activity levels of the AESOP children. The highest inhalation exposure was experienced by the most active children in the school with the highest air concentrations (EC School 2), while the lowest inhalation exposure was experienced by the least active children in the school with the lowest air concentrations (EC School 3). Dietary exposure for the AESOP children was calculated as a product of PCB concentrations in food and age- and gender-specific food ingestion rates and was published previously.4
We previously reported total dietary exposure for children enrolled in the AESOP study to be 66–108 μg/yr for girls and boys in the two communities.4 The annual school inhalation exposure calculated in this study for girls and boys was as high as 115 and 116 μg/yr for girls and boys, respectively, depending on school attended. This study shows that inhalation is a significant route of PCB exposure for children in schools with PCB contamination, in some cases exceeding dietary exposure. Further, significantly higher indoor air concentrations have been reported in schools elsewhere3 suggesting that inhalation exposure is also higher than dietary exposure for those children. For example, assuming similar volume of air breathed and similar diet, a New York City school child inhaling 2920 ng/m3, as reported by Thomas et al.,5 would have an inhalation exposure of 2.5 mg/yr PCBs, or about 40 times the dietary exposure. Our conclusion that PCB inhalation exposure is as significant as dietary exposure for children is supported by a non-cohort specific analysis from Lehmann et al. that found that airborne PCBs and dietary PCBs could each account for half of a child’s total PCB exposure.16
The fate and toxicological impact of PCB inhalation in children is not well known16, although animal studies show 91% of inhaled PCB 11 is absorbed by the body and is rapidly converted to OH-PCBs and other metabolic products.65 Higher molecular weight congeners are more likely to accumulate in adipose and other tissues.66 Some PCBs are neurotoxins and may impair learning and memory, a particularly concerning effect of PCBs in schools.13, 14 The biological processes for accumulating and metabolizing PCBs in the human body is highly complex30 and prevents direct comparison between congener profiles of PCBs in school air and in the blood from children attending those schools for the purpose of source identification. Our data show that children’s inhalation exposure is dominated by concentrated indoor air and varies from school to school. The variation in concentration, congener profiles, and inhalation exposures discovered across this study’s six schools shows that indoor and outdoor measurements are needed for each school and cannot be estimated from one school to another even within the same community.
Supplementary Material
Figure 1.
Concentrations of 2-OH-PCB 2 and 6-OH-PCB 2, the most commonly detected OH-PCBs in school air as measured by PUF-PAS in this study, compared to outdoor Chicago air as measured by high volume active sampling to XAD media.34 Samples were collected January 2012 to November 2015 for this school study and January to December 2009 for the Chicago air study.
Acknowledgments
We are grateful to the school staff for their participation in our study. Professor Craig Just and David Osterberg led our community engagement efforts. Study coordinator Jeanne DeWall and field staff Barb Mendenhall and Nancy Morales organized and carried out the sampling. Professor Hans-Joachim Lehmler and Xueshu Li synthesized the diazomethane we used to derivatize samples. This study was supported by NIH P42ES013661.The authors declare no competing financial interest.
Footnotes
Supporting information. Method details, quality control data, and congener-specific summary of data.
References
- 1.Herrick RF, Stewart JH, Allen JG. Review of PCBs in US schools: a brief history, an estimate of the number of impacted schools, and an approach for evaluating indoor air samples. Environ Sci Pollut R. 2016;23(3):1975–1985. doi: 10.1007/s11356-015-4574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egsmose EL, Brauner EV, Frederiksen M, Morck TA, Siersma VD, Hansen PW, Nielsen F, Grandjean P, Knudsen LE. Associations between plasma concentrations of PCB 28 and possible indoor exposure sources in Danish school children and mothers. Environ Int. 2016;87:13–19. doi: 10.1016/j.envint.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Brauner EV, Andersen ZJ, Frederiksen M, Specht IO, Hougaard KS, Ebbehoj N, Bailey J, Giwercman A, Steenland K, Longnecker MP, Bonde JP. Health Effects of PCBs in Residences and Schools (HESPERUS): PCB - health Cohort Profile. Sci Rep-Uk. 2016;6:1–10. doi: 10.1038/srep24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ampleman MD, Martinez A, DeWall J, Rawn DFK, Hornbuckle KC, Thorne PS. Inhalation and Dietary Exposure to PCBs in Urban and Rural Cohorts via Congener-Specific Measurements. Environ Sci Technol. 2015;49(2):1156–1164. doi: 10.1021/es5048039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas K, Xue J, Williams R, Jones P, Whitaker D. EPA, U, editor. Polychlorinated Biphenyls (PCBs) in school buildings: Sources, environmental levels, and exposures. 2012. [Google Scholar]
- 6.Herrick RF, Meeker JD, Altshul L. Serum PCB levels and congener profiles among teachers in PCB-containing schools: a pilot study. Environ Health-Glob. 2011;10:1–10. doi: 10.1186/1476-069X-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrick RF, McClean MD, Meeker JD, Baxter LK, Weymouth GA. An unrecognized source of PCB contamination in schools and other buildings. Environ Health Persp. 2004;112(10):1051–1053. doi: 10.1289/ehp.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liebl B, Schettgen T, Kerscher G, Broding HC, Otto A, Angerer J, Drexler H. Evidence for increased internal exposure to lower chlorinated polychlorinated biphenyls (PCB) in pupils attending a contaminated school. Int J Hyg Envir Heal. 2004;207(4):315–324. doi: 10.1078/1438-4639-00296. [DOI] [PubMed] [Google Scholar]
- 9.Schwenk M, Gabrio T, Papke O, Wallenhorst T. Human biomonitoring of polychlorinated biphenyls and polychlorinated dibenzodioxins and dibenzofuranes in teachers working in a PCB-contaminated school. Chemosphere. 2002;47(2):229–233. doi: 10.1016/s0045-6535(01)00307-1. [DOI] [PubMed] [Google Scholar]
- 10.Hunt G, Stegeman J, Robertson L. PCBs: exposures, effects, remediation, and regulation with special emphasis on PCBs in schools. Environ Sci Pollut R. 2016;23(3):1971–1974. doi: 10.1007/s11356-015-5774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markey EJ. The ABCs of PCBs: A toxic threat to America’s schools. 2016 [Google Scholar]
- 12.Osterberg D, Scammell MK. PCBs in schools-where communities and science come together. Environ Sci Pollut R. 2016;23(3):1998–2002. doi: 10.1007/s11356-015-5009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ Health Persp. 2003;111(3):357–376. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eubig PA, Aguiar A, Schantz SL. Lead and PCBs as Risk Factors for Attention Deficit/Hyperactivity Disorder. Environ Health Persp. 2010;118(12):1654–1667. doi: 10.1289/ehp.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erickson MD, Kaley RG. Applications of polychlorinated biphenyls. Environ Sci Pollut R. 2011;18(2):135–151. doi: 10.1007/s11356-010-0392-1. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann GM, Christensen K, Maddaloni M, Phillips LJ. Evaluating Health Risks from Inhaled Polychlorinated Biphenyls: Research Needs for Addressing Uncertainty. Environ Health Persp. 2015;123(2):109–113. doi: 10.1289/ehp.1408564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo J, Capozzi SL, Kraeutler TM, Rodenburg LA. Global Distribution and Local Impacts of Inadvertently Generated Polychlorinated Biphenyls in Pigments. Environ Sci Technol. 2014;48(15):8573–8580. doi: 10.1021/es502291b. [DOI] [PubMed] [Google Scholar]
- 18.Vorkamp K. An overlooked environmental issue? A review of the inadvertent formation of PCB-11 and other PCB congeners and their occurrence in consumer products and in the environment. Sci Total Environ. 2016;541:1463–1476. doi: 10.1016/j.scitotenv.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Hu DF, Hornbuckle KC. Inadvertent Polychlorinated Biphenyls in Commercial Paint Pigments. Environ Sci Technol. 2010;44(8):2822–2827. doi: 10.1021/es902413k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anezaki K, Kannan N, Nakano T. Polychlorinated biphenyl contamination of paints containing polycyclic- and Naphthol AS-type pigments. Environ Sci Pollut R. 2015;22(19):14478–14488. doi: 10.1007/s11356-014-2985-6. [DOI] [PubMed] [Google Scholar]
- 21.Hu DF, Martinez A, Hornbuckle KC. Discovery of Non-Aroclor PCB (3,3′-Dichlorobiphenyl) in Chicago Air. Environ Sci Technol. 2008;42(21):7873–7877. doi: 10.1021/es801823r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu DF, Martinez A, Hornbuckle KC. Sedimentary records of non-Aroclor and Aroclor PCB mixtures in the Great Lakes. J Great Lakes Res. 2011;37(2):359–364. doi: 10.1016/j.jglr.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King TL, Yeats P, Hellou J, Niven S. Tracing the source of 3,3′-dichlorobiphenyl found in samples collected in and around Halifax Harbour. Mar Pollut Bull. 2002;44(7):590–596. doi: 10.1016/s0025-326x(01)00289-2. [DOI] [PubMed] [Google Scholar]
- 24.Litten S, Fowler BI, Luszniak D. Identification of a novel PCB source through analysis of 209 PCB congeners by US EPA modified method 1668. Chemosphere. 2002;46(9–10):1457–1459. doi: 10.1016/s0045-6535(01)00253-3. [DOI] [PubMed] [Google Scholar]
- 25.Carpenter DO. Exposure to and health effects of volatile PCBs. Rev Environ Health. 2015;30(2):81–92. doi: 10.1515/reveh-2014-0074. [DOI] [PubMed] [Google Scholar]
- 26.Koh WX, Hornbuckle KC, Thorne PS. Human Serum from Urban and Rural Adolescents and Their Mothers Shows Exposure to Polychlorinated Biphenyls Not Found in Commercial Mixtures. Environ Sci Technol. 2015;49(13):8105–8112. doi: 10.1021/acs.est.5b01854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinete N, Schettgen T, Bertram J, Kraus T. Occurrence and distribution of PCB metabolites in blood and their potential health effects in humans: a review. Environ Sci Pollut R. 2014;21(20):11951–11972. doi: 10.1007/s11356-014-3136-9. [DOI] [PubMed] [Google Scholar]
- 28.Koh WX, Hornbuckle KC, Marek RF, Wang K, Thorne PS. Hydroxylated polychlorinated biphenyls in human sera from adolescents and their mothers living in two US Midwestern communities. Chemosphere. 2016;147:389–395. doi: 10.1016/j.chemosphere.2015.12.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SH, Hong YS, Ha EH, Park H. Serum concentrations of PCBs and OCPs among prepubertal Korean children. Environ Sci Pollut R. 2016;23(4):3536–3547. doi: 10.1007/s11356-015-5578-0. [DOI] [PubMed] [Google Scholar]
- 30.Grimm FA, Hu DF, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman A, Robertson LW. Metabolism and metabolites of polychlorinated biphenyls. Crit Rev Toxicol. 2015;45(3):245–272. doi: 10.3109/10408444.2014.999365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma CX, Zhai GS, Wu HM, Kania-Korwel I, Lehmler HJ, Schnoor JL. Identification of a novel hydroxylated metabolite of 2,2′,3,5′,6-pentachlorobiphenyl formed in whole poplar plants. Environ Sci Pollut R. 2016;23(3):2089–2098. doi: 10.1007/s11356-015-5939-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueno D, Darling C, Alaee M, Campbell L, Pacepavicius G, Teixeira C, Muir D. Detection of hydroxylated polychlorinated biphenyls (OH-PCBs) in the abiotic environment: Surface water and precipitation from Ontario, Canada. Environ Sci Technol. 2007;41(6):1841–1848. doi: 10.1021/es061539l. [DOI] [PubMed] [Google Scholar]
- 33.Marek RF, Martinez A, Hornbuckle KC. Discovery of Hydroxylated Polychlorinated Biphenyls (OH-PCBs) in Sediment from a Lake Michigan Waterway and Original Commercial Aroclors. Environ Sci Technol. 2013;47(15):8204–8210. doi: 10.1021/es402323c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Awad AM, Martinez A, Marek RF, Hornbuckle KC. Occurrence and Distribution of Two Hydroxylated Polychlorinated Biphenyl Congeners in Chicago Air. Environ Sci Tech Let. 2016;3(2):47–51. doi: 10.1021/acs.estlett.5b00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lauby-Secretan B, Loomis D, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K, Workin IARCM. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 2013;14(4):287–288. doi: 10.1016/S1470-2045(13)70104-9. [DOI] [PubMed] [Google Scholar]
- 36.Crinnion WJ. Polychlorinated Biphenyls: Persistent Pollutants with Immunological, Neurological, and Endocrinological Consequences. Altern Med Rev. 2011;16(1):5–13. [PubMed] [Google Scholar]
- 37.Otake T, Yoshinaga J, Enomoto T, Matsuda M, Wakimoto T, Ikegami M, Suzuki E, Naruse H, Yamanaka T, Shibuya N, Yasumizu T, Kato N. Thyroid hormone status of newborns in relation to in utero exposure to PCBs and hydroxylated PCB metabolites. Environ Res. 2007;105(2):240–246. doi: 10.1016/j.envres.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 39.Park HY, Park JS, Sovcikova E, Kocan A, Linderholm L, Bergman A, Trnovec T, Hertz-Picciotto I. Exposure to Hydroxylated Polychlorinated Biphenyls (OH-PCBs) in the Prenatal Period and Subsequent Neurodevelopment in Eastern Slovakia. Environ Health Persp. 2009;117(10):1600–1606. doi: 10.1289/ehp.0900611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell MM, Woods R, Chi LH, Schmidt RJ, Pessah IN, Kostyniak PJ, LaSalle JM. Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11–q13 duplication autism spectrum disorder. Environ Mol Mutagen. 2012;53(8):589–598. doi: 10.1002/em.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhalla R, Tehrani R, Van Aken B. Toxicity of hydroxylated polychlorinated biphenyls (HO-PCBs) using the bioluminescent assay Microtox (R) Ecotoxicology. 2016;25(7):1438–1444. doi: 10.1007/s10646-016-1693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.America Unites for Kids v. Lyon, No. CV 15-2124 (C.D. Cal. Sept. 1, 2016) LEXIS 118447.
- 43.Gonzalez v. New York City Department of Education, No. 09-cv-07787 (S.D.N.Y. Sept. 9, 2009).
- 44.Worcester School Committee and Educational Association of Worcester, Inc., Case No. MUP-10-6005 (Mass. Dep’t of Labor Relations June 8, 2016).
- 45.Marek RF, Thorne PS, Wang K, DeWall J, Hornbuckle KC. PCBs and OH-PCBs in Serum from Children and Mothers in Urban and Rural US Communities. Environ Sci Technol. 2013;47(7):3353–3361. doi: 10.1021/es304455k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marek RF, Thorne PS, DeWall J, Hornbuckle KC. Variability in PCB and OH-PCB Serum Levels in Children and Their Mothers in Urban and Rural US Communities. Environ Sci Technol. 2014;48(22):13459–13467. doi: 10.1021/es502490w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh WX, Hornbuckle KC, Wang K, Thorne PS. Serum polychlorinated biphenyls and their hydroxylated metabolites are associated with demographic and behavioral factors in children and mothers. Environ Int. 2016;94:538–545. doi: 10.1016/j.envint.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaward FM, Farrar NJ, Harner T, Sweetman AJ, Jones KC. Passive air sampling of PCBs, PBDEs, and organochlorine pesticides across Europe. Environ Sci Technol. 2004;38(1):34–41. doi: 10.1021/es034705n. [DOI] [PubMed] [Google Scholar]
- 49.Harner T, Bidleman TF. Octanol-air partition coefficient for describing particle/gas partitioning of aromatic compounds in urban air. Environ Sci Technol. 1998;32(10):1494–1502. [Google Scholar]
- 50.Martinez A, Norstrom K, Wang K, Hornbuckle KC. Polychlorinated biphenyls in the surficial sediment of Indiana Harbor and Ship Canal, Lake Michigan. Environ Int. 2010;36(8):849–854. doi: 10.1016/j.envint.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinez A, Wang K, Hornbuckle KC. Fate of PCB Congeners in an Industrial Harbor of Lake Michigan. Environ Sci Technol. 2010;44(8):2803–2808. doi: 10.1021/es902911a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rayne S, Forest K. pKa values of the monohydroxylated polychlorinated biphenyls (OH-PCBs), polybrominated biphenyls (OH-PBBs), polychlorinated diphenyl ethers (OH-PCDEs), and polybrominated diphenyl ethers (OH-PBDEs) J Environ Sci Heal A. 2010;45(11):1322–1346. doi: 10.1080/10934529.2010.500885. [DOI] [PubMed] [Google Scholar]
- 53.Buser HR, Zook DR, Rappe C. Determination of Methyl Sulfone-Substituted Polychlorobiphenyls by Mass-Spectrometric Techniques with Application to Environmental-Samples. Anal Chem. 1992;64(10):1176–1183. [Google Scholar]
- 54.Herkert NJ, Martinez A, Hornbuckle KC. A Model Using Local Weather Data to Determine the Effective Sampling Volume for PCB Congeners Collected on Passive Air Samplers. Environ Sci Technol. 2016;50(13):6690–6697. doi: 10.1021/acs.est.6b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Persoon C, Hornbuckle KC. Calculation of passive sampling rates from both native PCBs and depuration compounds in indoor and outdoor environments. Chemosphere. 2009;74(7):917–923. doi: 10.1016/j.chemosphere.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bohlin P, Audy O, Skrdlikova L, Kukucka P, Vojta S, Pribylova P, Prokes R, Cupr P, Klanova J. Evaluation and guidelines for using polyurethane foam (PUF) passive air samplers in double-dome chambers to assess semi-volatile organic compounds (SVOCs) in non-industrial indoor environments. Environ Sci-Process Impacts. 2014;16(11):2617–2626. doi: 10.1039/c4em00305e. [DOI] [PubMed] [Google Scholar]
- 57.Hazrati S, Harrad S. Calibration of polyurethane foam (PUF) disk passive air samplers for quantitative measurement of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs): Factors influencing sampling rates. Chemosphere. 2007;67(3):448–455. doi: 10.1016/j.chemosphere.2006.09.091. [DOI] [PubMed] [Google Scholar]
- 58.ASHRAE. Standard 62.1–2016. Ventilation for acceptable indoor air quality. 2016 [Google Scholar]
- 59.Harner T. v1.3 Template for calculating PUF and SIP disk sample air volumes. 2016. In March 11, 2016 ed. [Google Scholar]
- 60.Morrison RD, Murphy BL. Environmental Forensics: Contaminant specific guide. Elsevier Academic Press; 2006. Polychlorinated Biphenyls. [Google Scholar]
- 61.Frame GM, Cochran JW, Bowadt SS. Complete PCB congener distributions for 17 aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. Hrc-J High Res Chrom. 1996;19(12):657–668. [Google Scholar]
- 62.Martinez A, Spak SN, Petrich NT, Hu DF, Carmichael GR, Hornbuckle KC. Atmospheric dispersion of PCB from a contaminated Lake Michigan harbor. Atmos Environ. 2015;122:791–798. doi: 10.1016/j.atmosenv.2015.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez A, Erdman NR, Rodenburg ZL, Eastling PM, Hornbuckle KC. Spatial distribution of chlordanes and PCB congeners in soil in Cedar Rapids, Iowa, USA. Environ Pollut. 2012;161:222–228. doi: 10.1016/j.envpol.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinez A, Schnoebelen DJ, Hornbuckle KC. Polychlorinated biphenyl congeners in sediment cores from the Upper Mississippi River. Chemosphere. 2016;144:1943–1949. doi: 10.1016/j.chemosphere.2015.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu X, Adamcakova-Dodd A, Thorne PS. The fate of inhaled C-14-labeled PCB11 and its metabolites in vivo. Environ Int. 2014;63:92–100. doi: 10.1016/j.envint.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson-Restrepo B, Kannan K, Rapaport DP, Rodan BD. Polybrominated diphenyl ethers and polychlorinated biphenyls in human adipose tissue from New York. Environ Sci Technol. 2005;39(14):5177–5182. doi: 10.1021/es050399x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.