Abstract
The history of the progressive myoclonus epilepsies (PMEs) spans more than a century. However, the recent history of PMEs begins with a consensus statement published in the wake of the Marseille PME workshop in 1989 (Marseille Consensus Group, 1990). This consensus helped define the various types of PME known at the time and set the agenda for a new era of genetic research which soon lead to the discovery of many PME genes.
Prior to the Marseille meeting, and before the molecular era, there had been much confusion and controversy. Because investigators had but limited and biased experience with these rare disorders due to the uneven, skewed distribution of PMEs around the world, opinions and nosologies were based on local expertise which did not match well with the experiences of other researchers and clinicians. The three major areas of focus included: (1) the nature and limits of the concept of PME in varying scopes, which was greatly debated; (2) the description of discrete clinical entities by clinicians; and (3) the description of markers (pathological, biological, neurophysiological, etc.) which could lead to a precise diagnosis of a given PME type, with, in the best cases, a reliable correlation with clinical findings.
In this article, we shall also examine the breakthroughs achieved in the wake of the 1989 Marseille meeting and recent history in the field, following the identification of several PME genes. As in other domains, the molecular and genetic approach has challenged some established concepts and has led to the description of new PME types. However, as may already be noted, this approach has also confirmed the existence of the major, established types of PME, which can now be considered as true diseases.
Keywords: progressive myoclonus epilepsy, Lafora, Unverricht-Lundborg, Kufs
THE CONCEPT OF PROGRESSIVE MYOCLONUS EPILEPSY
The relationship between “myoclonia” and epilepsy was recognized by Prichard in 1822 (quoted by Rabot [1899]). Delasiauve had also noticed the existence of myoclonic jerks in patients with epilepsy and in his 1854 treatise on epilepsy, labelled them “petit mal moteur”. The myoclonic jerks, well described by many authors, were found in patients with various conditions, ranging from a comparatively benign, non-progressive type that would later be described as “impulsive petit mal” (Janz & Christian, 1957), to many more severe examples. Following Friedreich's “paramyoclonus multiplex” (1881), it was admitted that the jerks probably originated in the spinal cord. No clear disease entity was associated with these jerks (Friedreich, 1881).
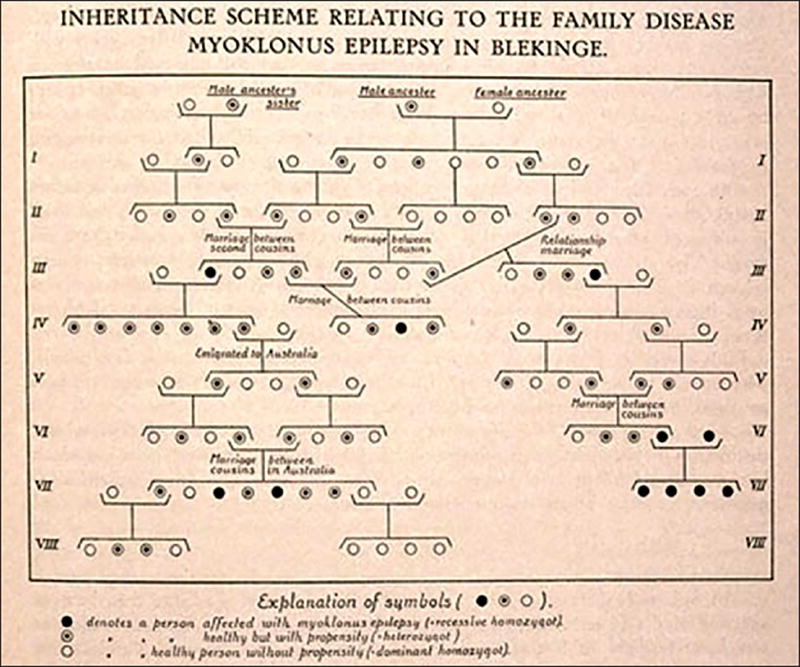
The concept of “progressive myoclonus epilepsy”(Minassian et al., 2016) was introduced by Herman Lundborg (figure 1) in 1903 (Lundborg, 1903), on the basis of several Swedish families with a common ancestor and (among other markers of “degeneration”) a particular form of epilepsy associated with progressive myoclonus and varying degrees of severity (figure 2). He acknowledged the previous reports from Estonia by Heinrich Unverricht (Unverricht, 1891) (figure 3) who had described two families with “Myoclonie” (1891) or “familiäre Myoklonie” (1895). Both authors had aptly described a fairly “pure” type of PME which did not include major symptoms other than the myoclonias and epileptic seizures. It took, however, nearly a century for this condition to be rightly recognised as “Unverricht-Lundborg” disease (ULD). Their contributions were widely read and commented upon, but failed to convince later authors that they had described a recognisable, specific condition. In order to reach a consensus, there were obviously too few cases in the patienthood of major neurologists at the time. When Lafora (figure 4) described the pathological inclusion found in the brain of a patient with a “myoclonic epilepsy”, which he also aptly described, he did not believe that his patient was different from those of Unverricht and Lundborg (Lafora, 1911).
Figure 1.
Herman Lundborg (1868–1943). Herman Lundborg wrote his dissertation in 1903 at the Karolinska Institutet, in Stockholm, about a family with the condition previously described by Unverricht, which he studied from a clinical point of view but also from a genetic perspective. His interest in genetics led him to found the notorious State Institute of Racial Biology, in Uppsala, in 1922. He came under strong criticism and disrepute due to his adherence to Nazi ideology and his advocacy of eugenics and the sterilisation of “genetically unworthy” persons.
Figure 2.
A pedigree showing recessive transmission in a family with Unverrich-Lundborg disease (From Lundborg & Runnstom [1921]).
Figure 3.
Heinrich Unverricht (1853–1912). Bust erected in 1914 at Magdeburg University. During his short tenure at Dorpat (now Tartu, Estonia), which he left because of the Russification policies of the occupying forces, Heinrich Unverricht described a family with “Myoclonie”, i.e. with the condition now named after him, “Unverricht-Lundborg disease”. He was a prolific internist who also described other conditions (polymyositis and pneumonia). His contribution is regarded as the founding description of progressive myoclonus epilepsy.
Figure 4.
Gonzalo Rodriguez Lafora (1886–1971). After studying in Spain (with Santiago Ramón Cajal), France (with Pierre Marie and Joseph Jules Dejerine), Germany (with Alois Alzheimer and Emil Kraepelin) and the USA, Gonzalo Rodriguez Lafora returned to Spain (which he had left for Mexico during the Civil War in 1938; he returned to Madrid in 1947). As a psychiatrist, he introduced the Freudian doctrine to both Spain and Argentina, but mainly dedicated his life to the care of intellectually disabled children. During his tenure as a neuropathologist at the Government Hospital for the Insane in Washington DC, he published his landmark paper on “myoclonic corpuscles”, in German.
Hunt (figure 5) contributed to the complexity of the matter by describing patients with signs of Friedreich's ataxia associated with action myoclonus and (in some cases) epilepsy (Hunt, 1921). The “Ramsay Hunt syndrome” (RHS; not to be confused with the description by the same author of the herpes infection of the geniculate ganglion, with resulting facial paresis and skin eruption) covered many clinical conditions, including ULD (Roger et al., 1968). RHS was finally discarded as a useful entity (Andermann et al., 1989a), however, at that time not for the right reasons, but because it was felt that the recent recognition of mitochondrial diseases with progressive myoclonus and seizures had cleared the way.
Figure 5.
James Ramsay Hunt (1874–1937). After studying in Philadelphia, Paris, Berlin and Vienna, James Ramsay Hunt practised and taught neurology in New York City (Cornell University and Columbia University). His name is associated with a small cutaneous zone innervated by the ganglion geniculi. His contribution to the field of PME from 1914 onward was the source of great confusion; from his area of low prevalence, he selected several unrelated cases with myoclonus (and other symptoms). The term “Ramsay Hunt Syndrome”, when applied to a neurological condition with myoclonus, was used to refer to many disparate entities. The term is no longer in use, following the delimitation of discrete PME types.
There were, however, efforts to try and introduce order to the PMEs. Van Bogaert approached the issue from a mixed neuropathological and clinical point of view, and supported the concept of PME, but failed to establish clear boundaries between the various types (Van Bogaert, 1968). In 1973, Diebold defined a nucleus of “hereditary myoclonus-epilepsy-dementia nuclear syndromes” (erbliche myoklonisch-epileptisch-dementielle Kernsyndrome), which he differentiated from the “borderline syndromes” occurring in diseases which only fit the PME definition in some cases (Diebold, 1973). Heralding the modern approach, the Montreal group also acknowledged the concept of PME and proposed a classification that was, subjectively, based on the relative frequency of these rare conditions (Berkovic et al., 1986). Before the genetic advances of the past twenty years had really had an impact, the Marseille group (Genton et al., 1990) proposed to divide the PMEs into those with known biochemical mechanisms (e.g. MERRF and sialidosis), those with a definite and reliable pathological marker (e.g. Lafora's disease, the neuronal ceroid lipofuscinoses [NCLF]), and those without any marker (the “degenerative” types, with purely clinical diagnosis and exclusion of other aetiologies: e.g. ULD and DRPLA).
CLINICAL DESCRIPTIONS AND PATHOLOGICAL MARKERS
Table 1 summarises, for the major PMEs, the progression from clinical descriptions to molecular elucidation, which is currently nearly complete. However, it appears that the process was fairly uneven. Some descriptions preceded the molecular characterisation of the condition by more than a hundred years, while in other cases, a “new” disease was described on the basis of a singular clinical, pathological or genetic feature.
Table I.
Discovery and description of the main, classical PME types: the time flow, from clinical description to diagnostic marker to genetic localisation and elucidation, by chronological order of initial clinical descriptions. In some cases, like Lafora’s disease, the discovery of a pathological marker preceded by many years the comprehensive clinical description. For a detailed history of the various PME type, refer to the relevant chapter.
| PME type | First description (year, author) |
Pre-genetic diagnotic marker (year) | Locus/gene (year) |
|---|---|---|---|
| Juvenile NCLF | 1826 Stengel, Norway 1908 Spielmeyer, Germany 1931 Sjögren, Sweden | 1963 fingerprint profiles | 1989 |
| Unverricht-Lundborg disease | 1891 Unverricht, Estonia 1905 Lundborg, Sweden | none | 1991 |
| Lafora’s disease | 1911 Lafora Spain/USA 1963 Van Heycop Ten Ham, Netherlands | 1911 «myoclonic corpuscules» | 1995 |
| Late infantle NCLF | 1913 Bielschowsky, Germany | 1963 curvilinear profiles | 199 |
| Adult NCLF | 1925 Kufs, Germany | various | 2011 |
| Sialidosis | 1978 Rapin, USA | 1978 enzyme defect | 1996 |
| MERRF | 1980 Fukuhara, Japan | 1980 ragged-red fibers in muscle | 1990 |
| DRPLA | 1982 Naito and Oyanagi, Japan | none | 1995 |
| Action myoclonus-renal failure syndrome | 1986 Andermann, Canada | None | 2008 |
NCLF: neuronal ceroidlipofuscinosis.
In the classic sequence of events, a clinical description occurred first, followed by a more or less specific biological or neurophysiological marker which helped ascertain the diagnosis. This was the case for the various forms of NCLF. The juvenile type of NCLF was described by Stengel, a general practitioner in 1826, in a geographic isolate of inland Norway (Stengel, 1826), but it took nearly a century to distinguish this and other forms of NCLF from other forms of “amaurotic idiocy”, which included non-PME disorders such as Tay-Sachs disease. While Batten had not initially distinguished these conditions from one another (Batten, 1902), in 1903 an ophthalmologist, Alfred Bielschowsky, characterised the ocular findings in the late infantile form of NCLF. The more specific pathological, ultrastructural changes associated with the infantile and juvenile types of NCLF were only described in the 1970s (Zeman et al., 1970). Although it took some time to differentiate NCLF from other types of degenerative childhood diseases, which included mental decline and retinal impairment, they were fairly well distinguished, on clinical grounds, from other types of PMEs. However, another condition with optional ophthalmological symptoms, sialidosis, was only clearly identified in the 1970s (Rapin et al., 1978).
In the case of Lafora's disease, the pathological marker, the presence of amyloid deposits in the brain, was described by Gonzalo Lafora in 1911, together with a fairly precise clinical depiction of the condition named after him. But it took half a century of controversies before a sound and precise clinical description of Lafora's disease (LD) was reached in the Netherlands (Van Heycoptenhamm & De Jager, 1963). From this point onwards, LD was for most, but not all, a clearly identifiable entity. In subsequent years, several refinements were made to the clinical description, focusing on the characteristic EEG presentation and on the occurrence of occipital lobe seizures (Roger et al., 1983; Tinuper et al., 1983).
Diagnosis was much more difficult in the absence of precise markers, when the clinician was left to speculate on patient cases purely on the basis of clinical traits. Some neurophysiological features were shared by several, clearly different conditions. As an example, the spectacular occurrence of runs of polyspikes during REM sleep which was described in several “myoclonic” and progressive conditions, such as Ramsay-Hunt syndrome (soon to become Unverricht-Lundborg disease [ULD]), was also seen in post-anoxic myoclonus, or MERRF. Indeed, based on their own experience, various authors promoted a regional type of PME, which dominated local experiences. In Finland, close to the original sites of Unverricht's and Lundborg's descriptions, the “Baltic myoclonus” was the prototype of PMEs (Koskiniemi et al., 1974; Koskiniemi, 1986). Likewise, RHS was repeatedly described in Marseille following Roger et al. (1968) and was, in the light of the Finnish publications, labelled “Mediterranean myoclonus” and considered to constitute a milder entity than the “Baltic” type and MERRF (Genton et al., 1990). An explanation had already been given for the difference in severity; in Northern Europe, phenytoin, the most prescribed anticonvulsant for epilepsies with convulsive seizures (including myoclonic seizures), had clearly contributed to an artificial aggravation of the condition (Elridge et al., 1983), in contrast to Mediterranean patients, who were more likely to be treated (or over-treated) with phenobarbital, which lacked this aggravating effect.
In the 1980s, convincing descriptions of new entities emerged, such as mitochondrial encephalopathy with ragged-red fibres (MERRF) (Fukuhara et al., 1980), and dentato-rubro-pallido-luysian atrophy (DRPLA) (Naito & Oyanagi, 1982), and it was tempting to ascribe previously unresolved cases to these new findings, thus rendering the RHS concept obsolete (Berkovic et al., 1986; Andermann et al., 1989a). The time had come to compare the experience of researchers from Europe, America and Japan; an international workshop was organised in Marseille in June 1989, which heralded the modern, genetic and molecular era in PME research.
THE GENETIC ERA
Prior to 1989, the year of the Marseille conference, it had only been possible to identify the gene for only one autosomal recessive PME (NEU1; sialidosis), using classic biochemical methods (Rapin et al., 1978). The Marseille conference coincided with momentous developments in the history of genetics. 1989 was the year when the promise of reverse genetics, identifying a disease gene by first mapping its chromosomal location, was first fulfilled with the discovery of the cystic fibrosis gene (CFTR) (Rommens et al., 1989). While CFTR was mapped using restriction length polymorphisms, that same year the discovery of microsatellite polymorphisms was also first reported (Weber & May, 1989). The microsatellite maps that rapidly followed had just the right density for homozygosity and linkage mapping of autosomal recessive Mendelian diseases, and since the vast majority of PMEs are inherited in this fashion, their genes quickly began to be identified in the years that followed.
PME gene discoveries proceeded in the approximate order in which the diseases themselves had been described, which is likely to be a reflection of the relative frequencies of the various diseases. The CLN1 (Infantile NCL) and CLN3 (Batten's disease) genes were identified in 1995 (The International Batten Disease Consortium, 1995; Vesa et al., 1995), the ULD gene in 1996 and 1997 (Pennacchio et al., 1996; Lafrenière et al., 1997; Lalioti et al., 1997; Virtaneva et al., 1997), and the LD genes between 1998 and 2003 (Minassian et al., 1998; Serratosa et al., 1999; Chan et al., 2003). The most “myoclonic” of the NCL genes, CLN2, was cloned not through reverse genetics, but by using an elegant biochemical approach, taking advantage of the realisation that most NCL are lysosomal diseases. The authors isolated lysosomal proteins and looked for a missing spot in two-dimensional gels in patients with late-infantile NCL, in order to identify CLN2, a lysosomal dipeptidyl peptidase (Sleat et al., 1997). The remaining childhood NCL genes followed in the first decade of the new millennium, again for the most part through homozygosity and linkage mapping (Nita et al., 2016).
The gene for Action Myoclonus Renal Failure Syndrome (Andermann et al., 1989b) was one of the first disease genes to be identified using the more abundant polymorphisms established in the 2000s, namely single nucleotide polymorphisms (SNPs), which made it possible to rely on very few patients in order to identify disease genes (Berkovic et al., 2008). Most recently, disease genes, including PME genes, emerged in larger numbers, through combined use of SNP mapping arrays and next-generation (whole-exome and whole-genome) sequencing. Here, identification of disease genes can be based on as few as one patient. The best example of this technical progress relates to Kufs disease (adult-onset NCL). While this disease has been known for 88 years, it was not until advanced mapping and sequencing techniques became routinely used that its genetic cause was uncovered. This turned out not to be a single gene but, to date, at least four different genetic entities (Nita et al., 2016).
Some PMEs are very rare, caused by private mutations in single families. One example of this is the PME due to mutations in PRICKLE1 (Bassuk et al., 2008). It is expected that many such PMEs will be identified, as has been the case for other diseases. Mutation for certain genes is limited to allow for viability, but may result in a specific pathology that cannot be replicated by other defects of the same protein. Other PMEs are allelic to previously known PMEs, for example, the most common form of Kufs disease is allelic to the late-infantile variant NCL, CLN6 (Arsov et al., 2011).
As recessively inherited diseases, many PMEs occur fairly frequently in pets and farm animals, due to inbreeding. This includes LD, which is widespread in certain breeds of dog (Lohi et al., 2005), and various forms of NCL in dogs and sheep. In some cases, PME genes were first discovered in animals and then translated to humans, e.g. the severest form of NCL, CLN10, with fatal neonatal disease (Tyynela et al., 2000; Siintola et al., 2006; Steinfeld et al., 2006). PME comparisons between humans and animals has also yielded fascinating insights into genome biology. For example, human ULD is a disease which is not due to the complete absence of the responsible gene (EPM1), but to drastic downregulation of the gene's expression caused by expansion of a dodecamer repeat sequence. This repeat is present in the promoter of the human EPM1 gene but not in the promoter of the orthologous genes in animals. In humans, expansion of this dodecamer leads to significant downregulation but not to the complete absence of EPM1 mRNA. No patient is reported to have, or probably exists with, a total loss of EPM1. Because of the unique genomic particularity within the promoter sequence of the EPM1 gene, ULD is, therefore, a uniquely human disease and no natural animal model of the disease has been reported. As a second example, the dog genome has a similar dodecamer repeat in the Epm2b gene, one of the genes mutated in LD. Recurrent expansion of this repeat in canine Epm2b makes LD particularly common in dogs, but this mechanism does not occur in human cases with LD (Lohi et al., 2005).
CONCLUSIONS
PMEs comprise a group of rare, heterogeneous genetic (mainly autosomal recessive) disorders, characterised by cortical myoclonus, other types of epileptic seizures, and progressive neurocognitive impairment. PMEs usually present in late childhood or adolescence, which distinguishes them from epileptic encephalopathies that start with polymorphic seizures in early infancy. However, adult-onset PMEs may be due to rare gene defects or to immune or late degenerative disorders. Recent advances in this area have clarified molecular genetic basis, biological basis, and natural history, and have also provided a rational approach to diagnosis. However, PMEs still remain uncommon disorders which are difficult to diagnose in the absence of extensive experience with such conditions, and this severely limits the number of expert groups in the field. Thus, despite the advances in molecular medicine, aetiology remains undetermined in a substantial proportion of patients. In particular, there are still huge areas in medically developing parts of the world, where the diagnosis of PME is probably overlooked. Therefore, the actual prevalence of these conditions is still debatable. The history of PMEs shows that international collaboration and sharing experience is the right way to proceed. The Marseille conference occurred at a perfectly opportune moment, serving to clarify and classify the many PME syndromes known at that time. This was the springboard from which scientists, armed with the genetic and genomic tools that were then being invented, were able to rapidly identify causative defects. It is probably safe to say that we have now identified most PME genes, but it is equally safe to expect that many others remain to be found. Each one, however unique, will fill one of the gaps in the great PME puzzle. This will enable us to better understand this severe brain disease, and to move forward towards grasping some of the mysteries of the human brain. At the same time, the emerging picture and biological insights will allow us to find ways to provide our patients with meaningful treatment.
Footnotes
DISCLOSURES
The authors have no conflict of interest to disclose.
The present manuscript is part of a Epileptic Disorders/Mariani Foundation supplement on Progressive Myoclonus Epilepsies, downloadable as a whole by visiting www.epilepticdisorders.com.
References
- Andermann F, Berkovic S, Carpenter S, Andermann E. Viewpoints on the Ramsay Hunt syndrome. 2. The Ramsay Hunt syndrome is no longer a useful diagnostic category. Mov Disord. 1989;4:13–17. doi: 10.1002/mds.870040104. [DOI] [PubMed] [Google Scholar]
- Andermann E, Andermann F, Carpenter S. Action myoclonus-renal failure syndrome: a previously unrecognised disorder unmasked by advances in nephrology. In: Fahn S, editor. Myoclonus. Advances in neurology. Vol. 43. New York: Raven Press; 1989. pp. 87–103. [PubMed] [Google Scholar]
- Arsov T, Smith KR, Damiano J. Kufs disease, the major adult form of neuronal ceroid lipofuscinosis, caused by mutations in CLN6. Am J Hum Genet. 2011;88:566–573. doi: 10.1016/j.ajhg.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassuk AG, Wallace RH, Buhr A. A homozygous mutation in human causes an autosomal-recessive progressive myoclonus epilepsy-ataxia syndrome. Am J Hum Genet. 2008;83:572–581. doi: 10.1016/j.ajhg.2008.10.003. PRICKLE1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten FE. Cerebral degeneration with symmetrical changes in the maculae in two members of a family. Trans Ophthal Soc UK. 1902;23:386–390. [Google Scholar]
- Berkovic S, Andermann F, Carpenter S, Andermann E, Wolfe LS. Progressive myoclonus epilepsies: specific causes and diagnosis. N Engl J Med. 1986;315:296–305. doi: 10.1056/NEJM198607313150506. [DOI] [PubMed] [Google Scholar]
- Berkovic SF, Dibbens LM, Oshlack A. Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis. Am J Hum Genet. 2008;82:6. doi: 10.1016/j.ajhg.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EM, Young EJ, Ianzano L. Mutations in cause progressive myoclonus epilepsy. Nat Genet. 2003;35:125–127. doi: 10.1038/ng1238. NHLRC1. [DOI] [PubMed] [Google Scholar]
- Diebold K. Die erblichen myoklonisch-epileptisch-dementiellen Kernsyndrome. Berlin: Springer; 1973. [PubMed] [Google Scholar]
- Elridge R, Livanainen M, Stern R, Koerber I, Wilder BJ. ‘Baltic’ myoclonus epilepsy: hereditary disorder of childhood made worse by phenytoin. Lancet. 1983;2:838–842. doi: 10.1016/s0140-6736(83)90749-3. [DOI] [PubMed] [Google Scholar]
- Friedreich N. Paramyoclonus multiplex. Virchows Arch. 1881:86. [Google Scholar]
- Fukuhara N, Tokiguchi S, Shirakawa K, Tsubaki T. Myoclonus epilepsy asociated with ragged-red fibers (mitochondrial abnormalities). Disease entity or a syndrome? J Neurol Sci. 1980;47:117–133. doi: 10.1016/0022-510x(80)90031-3. [DOI] [PubMed] [Google Scholar]
- Genton P, Michelucci R, Tassinari CA, Roger J. The Ramsay Hunt syndrome revisited: mediterranean myoclonus mitochondrial encephalomyopathy with ragged red fibers and Baltic myoclonus. Acta Neurol Scand. 1990;81:8–15. doi: 10.1111/j.1600-0404.1990.tb00924.x. versus. [DOI] [PubMed] [Google Scholar]
- Hunt JR. Dyssynergia cerebellaris myoclonica – primary atrophy of the dentate system: a contribution to the pathology and symptomatology of the cerebellum. Brain. 1921;44:490–538. [Google Scholar]
- Janz D, Christian W. Impulsive petit-mal. Dtsch Z Nervenheilk. 1957;176:346–386. [Google Scholar]
- Koskiniemi ML. Baltic myoclonus. In: Fahn S, Marsden CD, Van Woert M, editors. Myoclonus. Advances in neurology. Vol. 43. New York: Raven Press; 1986. pp. 57–64. [PubMed] [Google Scholar]
- Koskiniemi M, Donner M, Majuri H, Haltia M, Norio R. Progressive myoclonus epilepsy: a clinical and histopathological study. Acta Neurol Scand. 1974;50:307–332. [PubMed] [Google Scholar]
- Lafora GR. Über das Vorkommen amyloider Körperchen im Innerender Ganglienzellen. Virchows Arch Pathol Anat. 1911;205:295–303. [Google Scholar]
- Lafrenière RG, Rochefort DL, Chrétien N. Unstable insertion of the 5-prime flanking region of the cystatin B gene is the most common mutation in progressive myoclonus epilepsy type 1, EPM1. Nat Genet. 1997;15:298–302. doi: 10.1038/ng0397-298. [DOI] [PubMed] [Google Scholar]
- Lalioti MD, Scott HS, Buresi C. Dodecamer repeat expansion in cystatin B gene in progressive myoclonus epilepsy. Nature. 1997;386:847–851. doi: 10.1038/386847a0. [DOI] [PubMed] [Google Scholar]
- Lohi H, Young EJ, Fitzmaurice SN. Expanded repeat in canine epilepsy. Science. 2005;307:81. doi: 10.1126/science.1102832. [DOI] [PubMed] [Google Scholar]
- Lundborg H. Die progressive myoclonusepilepsie (Unverrichts myoklonie) Uppsala: Almqvist and Wiskell; 1903. [Google Scholar]
- Lundborg H, Runnstom J. The Swedish nation in word and picture. Stockholm: Swedish Society for race hygiene; 1921. [Google Scholar]
- Marseille Consensus Group. Classification of progressive myoclonus epilepsies and related diseases. Ann Neurol. 1990;28:113–116. doi: 10.1002/ana.410280129. [DOI] [PubMed] [Google Scholar]
- Minassian BA, Lee JR, Herbrick J-A. Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat Genet. 1998;20:171–174. doi: 10.1038/2470. [DOI] [PubMed] [Google Scholar]
- Minassian BA, Striano P, Avanzini G. Progressive Myoclonus Epilepsies: State-of-the-Art. Epileptic Disord. 2016;18(Suppl. 2):S1–S158. [Google Scholar]
- Naito H, Oyanagi S. Familial myoclonus epilepsy and choreoathetosis: hereditary dentatorubral-pallidoluysian atrophy. Neurology. 1982;32:798–807. doi: 10.1212/wnl.32.8.798. [DOI] [PubMed] [Google Scholar]
- Nita DA, Mole SE, Minassian BA. Neuronal ceroid lipofuscinoses. Epileptic Disord. 2016;18(Suppl. 2):S73–S88. doi: 10.1684/epd.2016.0844. [DOI] [PubMed] [Google Scholar]
- Pennacchio LA, Lehesjoki A-E, Stone NE. Mutations in the gene encoding cystatin B in progressive myoclonus epilepsy EPM1) Science. 1996;271:1731–1733. doi: 10.1126/science.271.5256.1731. [DOI] [PubMed] [Google Scholar]
- Rabot L. Medical thesis. Paris: 1899. De la myoclonie épileptique. [Google Scholar]
- Rapin I, Goldfisher S, Katzman R, Engel J, O’Brien JS. The cherry-red spot myoclonus syndrome. Ann Neurol. 1978;3:234–242. doi: 10.1002/ana.410030309. [DOI] [PubMed] [Google Scholar]
- Roger J, Soulayrol R, Hassoun J. La dyssynergie cérébelleuse myoclonique (syndrome de Ramsay-Hunt) Rev Neurol. 1968;119:85–106. [PubMed] [Google Scholar]
- Roger J, Pellissier JF, Bureau M, Dravet C, Revol M, Tinuper P. Le diagnostic précoce de la maladie de Lafora. Importance des manifestations paroxystiques visuelles et intérêt de la biopsie cutané. Rev Neurol. 1983;139:115–124. [PubMed] [Google Scholar]
- Rommens JM, Iannuzzi MC, Kerem B. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Serratosa JM, Gomez-Garre P, Gallardo ME. A novel protein tyrosine phosphatase gene is mutated in progressive myoclonus epilepsy of the Lafora type (EPM2) Hum Mol Genet. 1999;8:345–352. doi: 10.1093/hmg/8.2.345. [DOI] [PubMed] [Google Scholar]
- Siintola E, Partanen S, Stromme P. Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain. 2006;129:1438–1445. doi: 10.1093/brain/awl107. [DOI] [PubMed] [Google Scholar]
- Sleat DE, Donnelly RJ, Lackland H. Association of mutations in a lysosomal protein with classical late-infantile neuronal ceroid lipofuscinosis. Science. 1997;277:1802–1805. doi: 10.1126/science.277.5333.1802. [DOI] [PubMed] [Google Scholar]
- Steinfeld R, Reinhardt K, Schreiber K. Cathepsin D deficiency is associated with a human neurodegenerative disorder. Am J Hum Genet. 2006;78:988–998. doi: 10.1086/504159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel C. Beretning om et mærkeligt Sygdomstilfelde hos fire Sødskende. Account of a singular illness among four siblings in the vicinity of Røraas. Eyr (Christiana) 1, 347–352. English translation, 1982. In: Armstrong D, Koopand N, Rider JA, editors. Ceroid lipofuscinosis (Batten's disease) Amsterdam, New York, Oxford: Elsevier Biomedical Press; 1826. pp. 17–19. [Google Scholar]
- The International Batten Disease Consortium. Isolation of a novel gene underlying Batten disease, CLN3. Cell. 1995;82:949–957. doi: 10.1016/0092-8674(95)90274-0. [DOI] [PubMed] [Google Scholar]
- Tinuper P, Aguglia U, Pellissier JF, Gastaut H. Visual ictal phenomena in a case of Lafora disease proven by skin biopsy. Epilepsia. 1983;24:214–218. doi: 10.1111/j.1528-1157.1983.tb04881.x. [DOI] [PubMed] [Google Scholar]
- Tyynela J, Sohar I, Sleat DE. A mutation in the ovine cathepsin D gene causes a congenital lysosomal storage disease with profound neurodegeneration. EMBO J. 2000;19:2786–2792. doi: 10.1093/emboj/19.12.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverricht H. Die Myoclonie. Leipzig, Vienna: Franz Deuticke; 1891. [Google Scholar]
- Van Bogaert L. L’épilepsie myoclonique progressive d’Unverricht-Lundborg et le problème des encéphalopathies progressives associant épilepsies et myoclonies. Rev Neurol. 1968;119:47–57. [PubMed] [Google Scholar]
- Van Heycoptenhamm MW, De Jager H. Progressive myoclonus epilepsy with Lafora bodies. Clinical-pathological features. Epilepsia. 1963;4:95–119. doi: 10.1111/j.1528-1157.1963.tb05214.x. [DOI] [PubMed] [Google Scholar]
- Vesa J, Hellsten E, Verkruyse LA. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376:584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- Virtaneva K, D’Amato E, Miao J. Unstable minisatellite expansion causing recessively inherited myoclonus epilepsy, EPM1. Nat Genet. 1997;15:393–396. doi: 10.1038/ng0497-393. [DOI] [PubMed] [Google Scholar]
- Weber JL, May PE. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989;44:388–396. [PMC free article] [PubMed] [Google Scholar]
- Zeman W, Donahue S, Dyken P, Green J. The neuronal ceroid-lipofuscinoses (Batten-Vogt syndrome) In: Vinken PS, Bruyn GW, editors. Handbook of clinical neurology. Vol. 10. Amsterdam: Elsevier North-Holland; 1970. pp. 588–679. [Google Scholar]