Dysfunctional DNA-damage response and consequent genomic instability play a pivotal role in the initiation and progression of both solid and hematologic tumors. Preservation of DNA integrity is, in fact, a key cellular function, hence several mechanisms that repair the damaged DNA need to be studied. Recent studies have focused on key players that are able to improve the DNA repair and thus may act as targets for new therapeutic approaches.
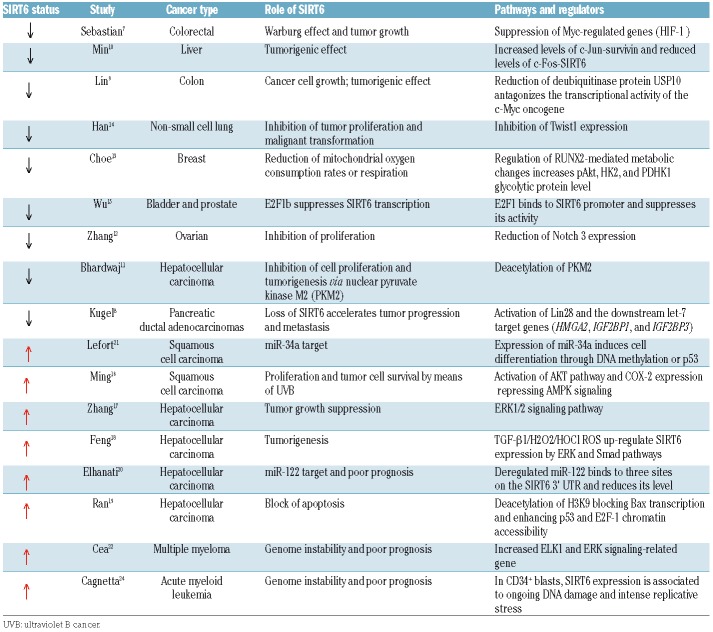
Several data have been obtained on overexpression and hyperactivity of Sirtuins (SIRTs), a family of proteins with deacylase or mono-adenosine diphosphate (ADP)-ribosyltransferase activities that degrade nicotinamide adenine dinucleotide (NAD+) enzymes to enable their biological processes1 and promote longevity.2 In mammalian cells, the Sirtuin family is composed of seven members that show different subcellular localization and functions (transcription, metabolism, fat mobilization, DNA repair, stress responses, apoptosis, tumorigenesis and aging),3,4 and conserve the catalytic domain and the NAD+ binding site.5 In cancer and aging-associated pathways, SIRT6 is crucial since it prevents genomic instability, maintains telomere integrity, and regulates metabolic homeostasis and DNA repair.6 SIRT6 can be considered a double-edged sword in cancer because of its dual role of both tumor suppressor and oncogene (Table 1). In healthy conditions, SIRT6 either acts as a gatekeeper of DNA repair mechanisms or regulates cell survival and proliferation. Following the DNA damage, SIRT6 triggers the apoptotic process, hence it is down-regulated in several cancers. However, in other cancers, it is up-regulated, corroborating the idea that it can also act as oncogene.
Table 1.
SIRT6 expression and its role in cancer.
SIRT6 as a tumor suppressor
Studies in colorectal, breast, ovarian, hepatocellular, lung, and other tumors correlate the reduction of SIRT6 expression with tumor progression and poor clinical outcome. In the presence of DNA-damage, SIRT6 promotes apoptotic cell death, ensuring damaged cells do not proliferate. Sebastian et al.7 demonstrated in vivo that SIRT6 deficiency favors tumor growth and invasiveness. They also showed that SIRT6 is involved in the Warburg effect, a glycolytic metabolic shift important for supporting rapid tumor growth. SIRT6 promotes both in vitro and in vivo tumor suppression through repression of hypoxia-inducible factor 1-alpha (HIF-1α) that inhibits glycolytic metabolism in cancer cells.7 Interestingly, in mouse and human pancreatic ductal adenocarcinoma (PDAC), the SIRT6 knockdown is due to repression of Myc-target oncofetal protein Lin28b that negatively regulates the let-7 family of miRNAs.8 In detail, loss of SIRT6 triggers activation of Lin28 promoter, Myc recruitment, and consequent activation of Lin28b, the downstream let-7 target genes (HMGA2, IGF2BP1) and IGF2BP3 that accelerate the PDAC progression and metastasis.8 In human colon cancer, Lin et al.9 discovered the crosstalk between UPS10 and SIRT6 that regulates cell-cycle progression and proliferation, and showed that the dysregulated USP10 function promotes tumorigenesis through SIRT6 degradation. Lin et al. also showed an important reduction in USP10 (a deubiquitinase protein) and SIRT6 expression. Indeed, the downregulation of USP10 triggers SIRT6 instability and negatively controls the transcriptional activity of the c-Myc oncogene that inhibits cell-cycle progression, cancer cell growth, and tumor formation.9 In liver cancer, the SIRT6 suppression is regulated by the c-Jun/c-Fos pathway:10 c-Fos induces SIRT6 transcription and represses survivin by reducing histone H3K9 acetylation and NF-κB activation. The increase in SIRT6 impairs cancer development by targeting the anti-apoptotic activity of survivin. Min et al.10 identified in human dysplastic liver nodules a specific expression pattern characterized by increased c-Jun-survivin and reduced c-Fos-SIRT6 level. In hepatocellular carcinoma (HCC), Bhardwaj et al.11 found that SIRT6 acts as a tumor suppressor because it deacetylates nuclear pyruvate kinase M2 (PKM2) inhibiting cell proliferation and tumorigenesis via PKM2. In ovarian cancer, Zhang et al.12 showed that SIRT6 is downregulated at mRNA and protein levels in tumor cells compared to normal cells. Moreover, SIRT6 reduces the expression of neurogenic locus notch homolog protein 3 (Notch3) while the Notch3 overexpression antagonists SIRT6 exert an effect on the ovarian cell proliferation; SIRT6 thus inhibits the proliferation of ovarian tumor cells through regulation of Notch3.12 In breast cancer, the repression of SIRT6, mediated by runt-related transcription factor 2 (RUNX2), regulates metabolic pathways and promotes tumor development.13 More specifically, Choe et al.13 showed that RUNX2 downregulates the SIRT6 expression at both mRNA and protein levels, and that endogenous SIRT6 expression is lower in the tumor breast tissue and cell lines expressing high levels of RUNX2 regulating the metabolic pathways. In addition, Han et al.14 demonstrated in non-small cell lung cancer (NSCLC) that SIRT6 inhibits Twist1 expression. Twist1 is a member of basic helix-loop-helix transcription factor family that promotes tumor proliferation and malignant transformation. Thus, SIRT6 is able to inhibit tumor cell proliferation through Twist1 suppression. Finally, the overexpression of E2F transcription factor 1 (E2F-1) in bladder and prostate cancer induces the downregulation of SIRT6 that closely correlates with cancer progression and poor prognosis.15
SIRT6 as a tumor promoter
In contrast to these studies, several papers show that overexpression of SIRT6 in solid and in hematologic tumors can promote oncogenic activity. Ming et al.16 demonstrated the oncogenic role of up-regulated SIRT6 in human skin squamous cell carcinoma (SCC): in skin keratinocytes, SIRT6 is increased upon exposure to ultraviolet B (UVB) light through the activation of the AKT pathway and promotes the cyclooxygenase 2 (COX-2) expression that represses the AMP-activated protein kinase (AMPK) signaling and increases proliferation and cell survival.16 Zhang et al.17 demonstrated that SIRT6 overexpression in HCC suppresses tumor growth by blocking extracellular signal-regulated kinases (ERK) 1/2 signaling pathway. In addition, Feng et al.18 and Ran et al.19 showed that SIRT6 plays an oncogenic role in HCC. In particular, the overexpression of SIRT6 is required for induction of transforming growth factor (TGF)-β1 and H2O2/HOCl reactive oxygen species (ROS) that mediate tumorigenesis. TGF-β1 upregulates the SIRT6 expression inducing the activation of ERK and Smad pathways, and altering the effect of these proteins on cellular senescence.18 Ran et al.19 demonstrated an oncogenic effect of SIRT6 via chromatin remodeling. At molecular level, SIRT6 induces deacetylation of H3K9 that blocks Bcl-2-associated X protein (Bax) transcription. As a consequence, it enhances p53 and E2F-1 chromatin accessibility thus inhibiting apoptosis. Elhanati et al.20 and Lefor et al.21 correlated SIRT6 regulation to two microRNAs (miR-) in two different cancers. At basal conditions, SIRT6 and miR-122 negatively regulate each other in HCC. SIRT6 down-regulates miR-122 by deacetylating H3K56 in the promoter region. The miR-122 binds SIRT6 3′ UTR and reduces its levels, while the loss of the negative correlation between SIRT6 and miR-122 expression is significantly associated with better prognosis.20 In addition, miR-34a plays a key role during the differentiation process of HCC and SIRT6 represents one of its targets. SIRT6 downregulation induces differentiation effects mediated by miR-34a.21
The role of SIRT6 is not well known in hematologic malignancies. In multiple myeloma (MM), SIRT6 is highly expressed as adaptive response to genomic stability, and its overexpression is associated to proliferation and poor prognosis.22 Cea et al.22 demonstrated in vitro and in a human MM xenograft model that SIRT6 down-regulates the expression of ERK signaling-related genes and suppresses the activity of ETS-domain transcription factor (ELK1), increasing DNA repair level via Chk1 (a critical messenger of the genome integrity checkpoints involved in the evolution of human cancer23), and conferring resistance to DNA-damaging agents. In this scenario, the paper by Cagnetta et al.24 studies the biological relevance and the genomic instability and poor prognosis associated with the mRNA upregulation of SIRT6 in the acute myeloid leukemia (AML) cells compared with low SIRT6 levels detected in normal CD34+ hematopoietic progenitors. SIRT6 participates in DNA double-strand break repair by deacetylation of C-terminal binding protein (CtBP), interacting protein (CtIP), poli ADP-ribosio polimerase-1 (PARP-1) and DNA-protein kinase (PK) complex. Indeed, AML cells are able to recruit SIRT6 in DNA-damaged sites and to promote deacetylation by means of DNA-PKs and CtIP. On the contrary, downregulation of SIRT6 expression both in vitro and in a murine xenograft model of human AML promotes genomic instability that sensitizes AML cells to daunorubicin (DNR) and cytarabine (ARA-C). Importantly, the results from Cagnetta et al. suggest an innovative chemotherapy that may selectively target AML cells enhancing their sensitivity to DNA-damage agents (DDAs).24
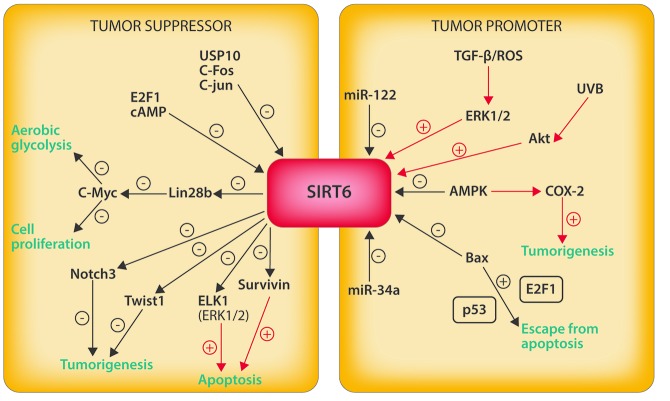
In conclusion, SIRT6 fulfills a controversial role in the pathogenesis of several cancers (Figure 1). It is clear that SIRT6 plays a crucial role in the regulation of tumorigenesis through its implication in different biological pathways where it can act as tumor suppressor or oncogene. The pleiotropism of SIRT6 means that studies directed toward understanding the cellular mechanisms through which the Sirtuin impacts cancer are difficult to carry forward but tremendously exciting. As Cagnetta et al. suggest,24 it is important that SIRT6 be included in the prospective clinical trials as a novel strategy of anti-tumor therapy.
Figure 1.
SIRT6 in cancer acts as tumor suppressor and tumor promoter in different cellular pathways.
Supplementary Material
References
- 1.Gertler AA, Cohen HY. SIRT6, a protein with many faces. Biogerontology. 2013;14(6):629–639. [DOI] [PubMed] [Google Scholar]
- 2.Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126(2):257–268. [DOI] [PubMed] [Google Scholar]
- 3.Rajendran R, Garva R, Krstic-Demonacos M, Demonacos C. Sirtuins: molecular traffic lights in the crossroad of oxidative stress, chromatin remodeling, and transcription. J Biomed Biotechnol. 2011;2011: 368276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Onofrio N, Vitiello M, Casale R, Servillo L, Giovane A, Balestrieri ML. Sirtuins in vascular diseases: emerging roles and therapeutic potential. Biochim Biophys Acta. 2015;1852(7):1311–1322. [DOI] [PubMed] [Google Scholar]
- 6.Lerrer B, Gertler AA, Cohen HY. The complex role of SIRT6 in carcinogenesis. Carcinogenesis. 2016;37(2):108–118. [DOI] [PubMed] [Google Scholar]
- 7.Sebastian C, Zwaans BMM, Silberman DM, et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151(6):1185–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kugel S, Sebastián C, Fitamant J, et al. SIRT6 suppresses pancreatic cancer through control of Lin28b. Cell. 2016;165(6):1401–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Z, Yang H, Tan C, et al. USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. Cell Rep. 2013;5(6):1639–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Min L, Ji Y, Bakiri L, et al. Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nat Cell Biol. 2012;14(11):1203–1211. [DOI] [PubMed] [Google Scholar]
- 11.Bhardwaj A, Das S. SIRT6 deacetylates PKM2 to suppress its nuclear localization and oncogenic functions. Proc Natl Acad Sci USA. 2016;113(5):E538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Yin XJ, Xu CJ, et al. The histone deacetylase SIRT6 inhibits ovarian cancer cell proliferation via down-regulation of Notch 3 expression. Eur Rev Med Pharmacol Sci. 2015;19(5):818–824. [PubMed] [Google Scholar]
- 13.Choe M, Brusgard JL, Chumsri S, et al. The RUNX2 Transcription Factor Negatively Regulates SIRT6 Expression to Alter Glucose Metabolism in Breast Cancer Cells. J Cell Biochem. 2015;116(10): 2210–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han Z, Liu L, Liu Y, Li S. Sirtuin SIRT6 suppresses cell proliferation through inhibition of Twist1 expression in non-small cell lung cancer. Int J Clin Exp Pathol. 2014;7(8):4774–4781. [PMC free article] [PubMed] [Google Scholar]
- 15.Wu M, Seto E, Zhang J. E2F1 enhances glycolysis through suppressing Sirt6 transcription in cancer cells. Oncotarget. 2015;6(13):11252–11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ming M, Han W, Zhao B, et al. SIRT6 promotes COX-2 expression and acts as an oncogene in skin cancer. Cancer Res. 2014;74(20):5925–5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang ZG, Qin CY. Sirt6 suppresses hepatocellular carcinoma cell growth via inhibiting the extracellular signal-regulated kinase signaling pathway. Mol Med Rep. 2014;9(3):882–888. [DOI] [PubMed] [Google Scholar]
- 18.Feng XX, Luo J, Liu M, et al. Sirtuin 6 promotes transforming growth factor-β1/H2O2/HOCl-mediated enhancement of hepatocellular carcinoma cell tumorigenicity by suppressing cellular senescence. Cancer Sci. 2015;106(5)559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ran LK, Chen Y, Zhang ZZ, et al. SIRT6 overexpression potentiates apoptosis evasion in hepatocellular carcinoma via BCL2-associated X protein-dependent apoptotic pathway. Clin Cancer Res. 2016;22(13):3372–3382. [DOI] [PubMed] [Google Scholar]
- 20.Elhanati S, Ben-Hamo R, Kanfi Y, et al. Reciprocal regulation between SIRT6 and miR-122 controls liver metabolism and predicts hepatocarcinoma prognosis. Cell Rep. 2016;14(2):234–242. [DOI] [PubMed] [Google Scholar]
- 21.Lefort K, Brooks Y, Ostano P, et al. A miR-34a-SIRT6 axis in the squamous cell differentiation network. EMBO J. 2013;32(16):2248–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cea M, Cagnetta A, Adamia S, et al. Evidence for a role of the histone deacetylase SIRT6 in DNA damage response of multiple myeloma cells. Blood. 2016;127(9):1138–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3(5):421–429. [DOI] [PubMed] [Google Scholar]
- 24.Cagnetta A, Soncini D, Orecchioni S, et al. Depletion of SIRT6 enzymatic activity increases acute myeloid leukemia cells vulnerability to DNA-damaging agents. Haematologica. 2018;103(1):80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.