Abstract
The effects of erythropoietin on osteoblasts and bone formation are controversial. Since patients with myelodysplastic syndromes often display excessively high erythropoietin levels, we aimed to analyze the effect of erythropoietin on osteoblast function in myelodysplastic syndromes and define the role of Wnt signaling in this process. Expression of osteoblast-specific genes and subsequent osteoblast mineralization was increased in mesenchymal stromal cells from healthy young donors by in vitro erythropoietin treatment. However, erythropoietin failed to increase osteoblast mineralization in old healthy donors and in patients with myelodysplasia, whereas the basal differentiation potential of the latter was already significantly reduced compared to that of age-matched controls (P<0.01). This was accompanied by a significantly reduced expression of genes of the canonical Wnt pathway. Treatment of these cells with erythropoietin further inhibited the canonical Wnt pathway. Exposure of murine cells (C2C12) to erythropoietin also produced a dose-dependent inhibition of TCF/LEF promoter activity (maximum at 500 IU/mL, −2.8-fold; P<0.01). The decreased differentiation capacity of erythropoietin-pretreated mesenchymal stromal cells from patients with myelodysplasia could be restored by activating the Wnt pathway using lithium chloride or parathyroid hormone. Its hematopoiesis-supporting capacity was reduced, while reactivation of the canonical Wnt pathway in mesenchymal stromal cells could reverse this effect. Thus, these data demonstrate that erythropoietin modulates components of the osteo-hematopoietic niche in a context-dependent manner being anabolic in young, but catabolic in mature bone cells. Targeting the Wnt pathway in patients with myelodysplastic syndromes may be an appealing strategy to promote the functional capacity of the osteo-hematopoietic niche.
Introduction
Erythropoietin is a glycoprotein mostly known for its function as a hematopoietic hormone, which is secreted from the adult kidneys in response to hypoxia. Erythropoietin receptor has been detected not only in hematopoietic cells, but also in multiple non-hematopoietic tissues, such as endothelial, skeletal muscle, and neuronal compartments, heart, kidney, pancreas and uterus, suggesting cytoprotective effects on non-erythroid cells.1 In contrast to low concentrations that are sufficient for proper erythropoiesis, other tissues appear to require relatively high concentrations of erythropoietin that are not normally reached in the circulation.2 The structure of the erythropoietin receptor in some non-hematopoietic cells is distinct from that of the receptor responsible for erythropoiesis, being a heterodimer consisting of the beta common receptor subunit (CD131) in combination with the erythropoietin receptor subunit.2,3.
The skeletal system also appears to be influenced by erythropoietin; however, the action of this glycoprotein on bone is still under debate. In most studies, erythropoietin has been shown to stimulate mesenchymal stromal cell (MSC) differentiation towards osteoblasts in vitro4–7 as well as to increase bone formation and the number of osteoblasts in vivo,8–10 especially if ephrinB2/EphB4 signaling is concomitantly activated.6 Moreover, certain observations in patients with fractures suggested accelerated fracture healing after erythropoietin treatment,11 and erythropoietin treatment of hemodialysis patients also improved the formation of bone matrix.12 Typically, the anabolic action of erythropoietin in animal models has been explained through indirect mechanisms, such as an increase in vascularization and tissue oxygenation due to an increase in hemoglobin level. Another possible indirect mechanism represents the concept of coupling hematopoiesis with bone formation through the stimulation of BMP2 secretion by hematopoietic stem and progenitor cells (HSPC).5 The impact of erythropoietin on the communication of osteoblasts and HSPC-derived osteoclasts through the ephrinB2/EphB4 signaling pathway may also explain the stimulation of the osteoblastic phenotype.6
Contradictory observations have been made by other groups,13,14 who have demonstrated bone loss after erythropoietin treatment. In particular, erythropoietin has been shown to directly stimulate osteoclastogenesis both in vitro and in vivo.15 In addition, OPG expression is increased after erythropoietin treatment, suggesting a compensatory mechanism aimed at attenuating the bone resorption.16 It is important to notice that different erythropoietin dosages can lead to opposite effects, as supraphysiological erythropoietin concentrations induced mineralization, whereas moderate concentrations suppressed bone formation and inhibited osteoblast differentiation in mice.17 It is very likely that erythropoietin may have both anabolic and catabolic actions in bone depending on experimental conditions. Moreover, it has been suggested that response to erythropoietin is more robust in younger animals than in older animals.18
Myelodysplastic syndromes (MDS) represent clonal disorders, mainly of the elderly, characterized by ineffective hematopoiesis and an increased risk of transformation into acute myeloid leukemia. The diverse interactions within the osteo-hematopoietic niche in MDS and the potential contribution of the niche to the pathogenesis of MDS have only recently been appreciated.19 Several studies have reported on the dysregulation of the Wnt pathway in MSC from MDS patients, with activation of non-canonical and suppression of canonical Wnt pathway.20,21 Moreover, the methylation status of Wnt antagonist genes has been shown to correlate with a poor prognosis in MDS.22 However, the potential connection of increased erythropoietin levels due to ineffective erythropoiesis or erythropoietin supplementation in MDS patients and a deregulated Wnt pathway has not been yet evaluated.
Keeping in mind the contradictory data about the action of erythropoietin on bone in grown vertebrates, and based on the observation that MDS patients, who are mostly elderly and often display excessive erythropoietin levels, as well as osteoporosis,23 we aimed to evaluate the influence of erythropoietin on osteoblasts derived from patients with MDS to clarify the potential association between erythropoietin levels and their effects on bone formation.
Methods
Patients
MSC were collected from young (22–49 years, both genders) and old (55–89 years, both genders) healthy donors and MDS patients (51–90 years, both genders) following Institutional Review Board approval and having obtained written informed consent. The MDS patients’ characteristics are presented in Online Supplementary Table S1). Heparinized bone marrow samples were obtained at diagnostic bone marrow puncture or during total hip arthroplasty operations.
Culture of human mesenchymal stromal cells
The culture conditions of MSC are described in detail in the Online Supplementary Materials and Methods. Cells were treated with erythropoietin alfa (10–100 IU/mL, EPREX, Janssen-Cilag). In most experiments, differentiation medium was applied for 10 days. The dosage of erythropoietin was chosen according to previous publications,3 as well as personal experience showing that 50 IU/mL erythropoietin are required to induce anabolic effects in MSC. In certain experiments, cells were treated with parathyroid hormone intermittently for 8 h three times a week (PTH, 100 ng/mL, Preotact, Nycomed) and/or lithium chloride continuously (25 mM).
Alizarin red staining
Osteoblast mineralization was assessed using Alizarin red staining. The details of the method are described in the Online Supplementary Materials and Methods.
RNA isolation, reverse transcription, and real-time polymerase chain reaction
Total RNA was isolated with the High Pure RNA Isolation kit (Roche, Mannheim, Germany) according to the manufacturer’s protocol. Five-hundred nanograms of RNA were reverse transcribed using Superscript II (Invitrogen, Darmstadt, Germany) und subsequently used for SYBR green-based real-time polymerase chain reactions (PCR) (Applied Biosystems, Carlsbad, CA, USA). The primer sequences and PCR conditions are listed in the Online Supplementary Materials and Methods.
Wnt profiler polymerase chain reaction array
Cells were differentiated with osteogenic medium for 7 days and treated with 50 IU/mL erythropoietin for 24 h. Afterwards, RNA was isolated as described above, reverse transcribed using the RT2 First Strand Kit (SABioscience) and 500 ng cDNA were subjected to the Wnt profiler PCR array (PAHS-043Z) containing 84 Wnt-related genes according to the manufacturer’s protocol (SABioscience). Genes were normalized to the mean of five house-keeping genes (β-actin, GAPDH, B2M, HPRT1, RPLP0).
TCF/LEF-reporter assay
A TCF/LEF-reporter assay (Qiagen) was done using the murine myoblast C2C12 cell line, which is commonly used to study BMP and Wnt signaling. These cells were treated with erythropoietin (10–500 IU/mL) for 24 h. Details of this method can be found in the Online Supplementary Material.
Co-culture of CD34+ cells with primary human mesenchymal stromal cells
Primary MSC were plated at a density of 1–2 × 104/cm2 in DMEM with 10% fetal calf serum and pre-treated with erythropoietin 50 IU/mL and/or lithium chloride 25 mM for 7 days. Afterwards, CD34+ cells were co-cultured with MSC for 7 days and counted using a hemocytometer. Moreover, a 4-week cobblestone area forming-cell assay was performed with or without pretreatment of the MSC layer. A detailed description of the culture conditions is given in the Online Supplementary Material.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software version 5.01 (GraphPad Software, La Jolla, CA, USA). Data are presented as mean ± standard deviation (SD). Statistical evaluations of two group comparisons were performed using a two-sided Student t-test or two-way ANOVA test. A P-value of less than 0.05 was regarded as statistically significant.
Results
Distinct effects of erythropoietin on differentiation of osteoblasts from young and old healthy donors and patients with myelodysplastic syndromes
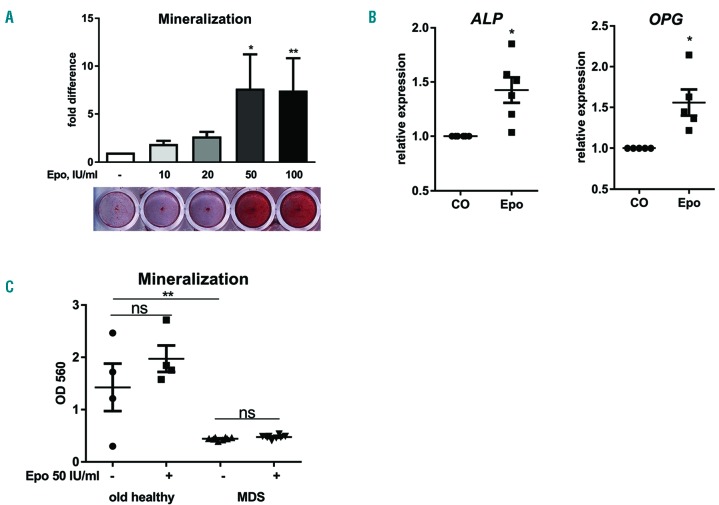
In order to determine the influence of erythropoietin on the differentiation potential of MSC towards osteoblasts, we treated human MSC from young and old healthy donors, as well as from patients with MDS, with erythropoietin. Using MSC from young donors, we could confirm previously published data3–7,24 and showed an increased mineralization upon erythropoietin treatment, with the strongest induction being seen at concentrations above 50 IU/mL (Figure 1A). Expression of ALP and OPG was also significantly increased up to 1.5-fold after treatment with 50 IU/mL erythropoietin (Figure 1B), whereas the expression of RUNX2 and OSTERIX did not change (data not shown).
Figure 1.
Different effects of erythropoietin on differentiation of osteoblasts from young and old healthy donors and patients with myelodysplastic syndromes. Human mesenchymal stromal cells from young healthy donors (A–B), MDS patients and age-matched healthy donors (C) were differentiated towards osteoblasts in the presence of various concentrations of erythropoietin (Epo). The mineralization was visualized with Alizarin red S staining and quantified after elution with cetylpyridinium chloride (A, C). Gene expression analysis of alkaline phosphatase (ALP) and osteoprotegerin (OPG) using real-time polymerase chain reaction in MSC from young healthy donors after treatment with 50 IU/mL Epo for 10 days (B). N=3–5. *P<0.05, **P<0.01, ns – not significant vs. control (CO).
In contrast, when we evaluated the effect of the same concentration of erythropoietin on MSC from old healthy donors and MDS patients, we did not observe an induction in matrix mineralization (Figure 1C). None of the erythropoietin concentrations ranging from 10 IU/mL to 100 IU/mL was able to increase the mineralization. Furthermore, the lack of effect was also independent of the MDS subtype or risk group (data not shown). In order to exclude the possibility that the lack of effect was due to the absence of erythropoietin receptor in MSC from elderly patients, we determined the level of EPOR expression and found no significant difference between the three groups (Online Supplementary Figure S1).
The basal differentiation potential of MDS-MSC was significantly reduced compared to that of MSC from age-matched healthy donors (Figure 1C), which is in line with previous reports.20
Erythropoietin inhibits canonical Wnt signaling which is already suppressed in mesenchymal stromal cells from patients with myelodysplastic syndromes
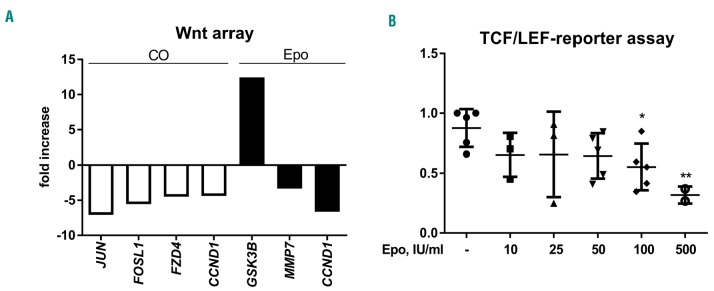
Based on previous reports showing deregulated Wnt signaling in MDS patients and the prominent role of this pathway in osteoblastic differentiation, we compared the expression of Wnt-related genes in differentiated osteoblasts from MDS patients and age-matched healthy donors using a PCR-based Wnt array. A change of at least 2-fold in gene expression between patients and the control group was considered significant. Transcripts of 18 genes out of 84 Wnt-related genes were differentially expressed between patients and controls. Four genes were validated using quantitative PCR (Figure 2A; Online Supplementary Table S2). To validate the data obtained from the PCR array, we evaluated the mRNA expression of differentially regulated genes, namely, target genes of the canonical Wnt pathway JUN, FOSL1 and CCND1 and a receptor of the canonical Wnt pathway FZD4 (Online Supplementary Figure S2). In accordance with the PCR array data, the mean relative mRNA expression of genes related to the canonical Wnt pathway was significantly downregulated in MDS patients compared to controls. Additionally, there was a tendency to an increased concentration of the Wnt inhibitor Dkk1 in the MSC culture supernatants from the patients (data not shown).
Figure 2.
Erythropoietin inhibits canonical Wnt signaling, which is already suppressed in mesenchymal stromal cells from patients with myelodysplastic syndromes. (A) Human mesenchymal stromal cells from old healthy donors and MDS patients were compared using Wnt profiler polymerase chain reaction (white bars). Afterwards, osteoblasts from MDS patients were treated with 50 IU/mL erythropoietin (Epo) for 24 h and were compared to untreated cells (black bars). All genes presented in the bar graph were significantly regulated (P<0.05). N=3. (B) Murine myoblast C2C12 cell line was transfected with the Signal TCF/LEF Reporter and treated with increasing Epo concentrations for 24 h. Luciferase activity was assayed 24 h after treatment. N=4. *P<0.05, **P<0.01 vs. control.
We then treated MDS-MSC with erythropoietin to evaluate whether it could influence the canonical Wnt pathway, even in the absence of its action on osteoblastic differentiation. Interestingly, we observed a strong upregulation of GSK3B, which is a known inhibitor of the canonical Wnt pathway, whereas MMP7 and CCND1 – target genes of the canonical Wnt pathway – were downregulated (Figure 2A; Online Supplementary Table S3). In addition to gene expression analyses, we also examined the effect of erythropoietin on Wnt promoter activity using a TCF/LEF promoter assay. Wnt promoter activity was induced in C2C12 cells using the supernatants from Wnt3a-overexpressing L-cells. After treatment with increasing concentrations of erythropoietin, the TCF/LEF promoter activity was significantly reduced at high concentrations (100 IU/mL) (Figure 2B). Hence, erythropoietin targets several components of the canonical Wnt pathway, which most likely contributes to its inhibition in MDS-MSC.
Activators of the canonical Wnt pathway can restore the attenuated osteoblast differentiation of erythropoietin-treated mesenchymal stromal cells from patients with myelodysplastic syndromes
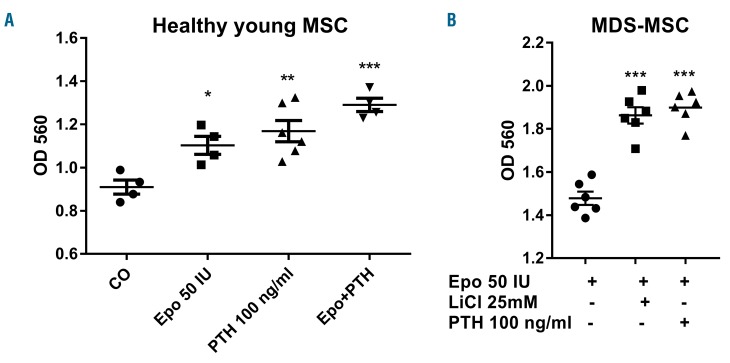
To further explore the role of a deregulated Wnt pathway in MDS-MSC after erythropoietin treatment, we evaluated osteoblast differentiation following reactivation of canonical Wnt signaling. We, therefore, used lithium chloride due to its known ability to inhibit GSK3B,25 which was significantly upregulated in erythropoietin-treated osteoblasts. In addition, intermittent parathyroid hormone treatment was used, as this hormone is applied therapeutically as a bone-forming agent in severely osteoporotic patients and exerts its effects, among other ways, through stimulating the canonical Wnt pathway.26
Parathyroid hormone further increased matrix mineralization in osteoblasts from young healthy donors treated with erythropoietin (Figure 3A). In erythropoietin-treated MDS-MSC, parathyroid hormone also improved the mineralization capacity (Figure 3B). A similar stimulatory effect was observed after concomitant treatment of erythropoietin with lithium chloride, which indicates that reactivation of the canonical Wnt pathway improves the differentiation capacity of osteoblasts from MDS patients.
Figure 3.
Activators of the canonical Wnt pathway can restore the attenuated osteoblastic differentiation of erythropoietin-treated mesenchymal stromal cells from patients with myelodysplastic syndromes. Human mesenchymal stromal cells from young healthy donors (A) and MDS patients (B) were differentiated towards osteoblasts in the presence of 50 IU/ml erythropoietin (Epo) with intermittent 100 ng/mL parathyroid hormne (PTH) or 25 mM lithium chloride (LiCl). The mineralization was visualized with Alizarin red S staining and quantified after elution with cetylpyridinium chloride. N=3–5. *P<0.05, **P<0.01, ***P<0.001 vs. control (CO).
Hematopoietic support by erythropoietin-pretreated mesenchymal stromal cells
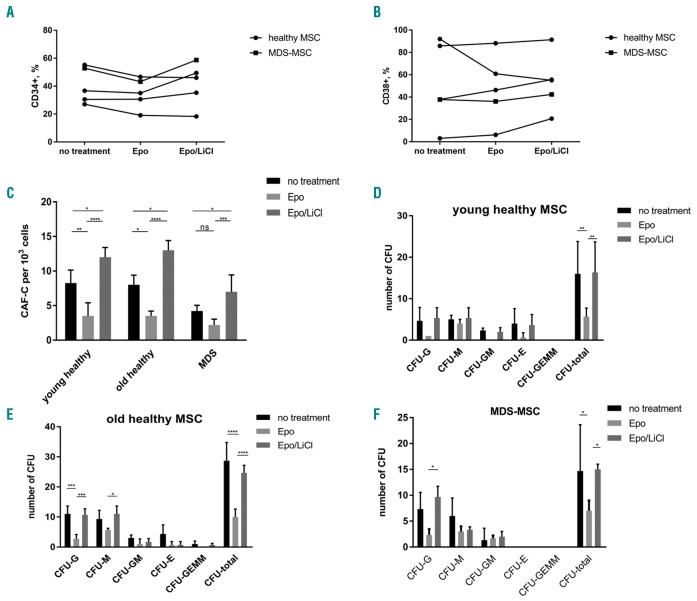
One of the main functions of MSC in the bone marrow is the support of hematopoiesis. Disturbances in its function as a consequence of excessive erythropoietin levels can also affect the support of HSPC. We, therefore, performed co-culture experiments with HSPC and MSC from healthy individuals or MDS patients pretreated with erythropoietin with or without lithium chloride, as a reactivator of the deranged canonical Wnt pathway. After 7 days of co-culture with erythropoietin-pretreated MSC, we observed a tendency for a reduction of CD34+ HSPC (Figure 4A,B), whereas CD38 expression was slightly increased or not affected in most cases suggesting a differentiation-supportive effect of erythropoietin. When the canonical Wnt pathway was reactivated in MSC using lithium chloride the numbers of CD34+ and CD38+ cells were mostly expanded and even surpassed the initial level without MSC pretreatment in some cases (Figure 4A,B). CD90 (Thy-1), which marks more immature HSPC, was not affected by MSC pre-treatment (data not shown).
Figure 4.
The hematopoietic support by erythropoietin-treated mesenchymal stromal cells is inhibited but can be restored by Wnt pathway activation. Human mesenchymal stromal cells (MSC) were pretreated with erythropoietin (Epo) 50 IU/mL and/or lithium chloride (LiCl) 25 mM for 7 days and co-cultured with freshly isolated CD34+ HSPC. After 7 days, flow cytometric analysis was performed for the non-adherent cells. (A) Number of CD34+ cells and (B) number of CD38+ cells. Each dot represents the percentage of positive-stained cells; each graph represents one donor/patient. (C) After 4 weeks of co-culture, the number of cobblestone area-forming cells (CAF-C) was determined in each well. A colony-forming unit (CFU) assay was performed in methylcellulose medium for 2 additional weeks and the colonies were classified under a microscope for HSPC co-cultured with (D) young healthy MSC, (E) old healthy MSC and (F) MDS MSC. N=3–5. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001
Next, we investigated the colony potential of pre-treated MSC in long-term co-cultures with CD34+ HSPC. The number of cobblestone area forming-cells as readout for active stromal support was significantly reduced in erythropoietin-pretreated MSC from young and old healthy donors (8.25±1.89 versus 3.5±1.9 and 8.0±1.4 versus 3.5±0.7) whereas the already diminished potential in MDS MSC was only slightly decreased (4.2±0.83 versus 2.2±0.84). Interestingly, lithium chloride completely restored the hematopoietic support capacity, which even rose above the basal level (12.0±1.4/13.0±1.5/7.0±2.4) (Figure 4C).
To study the clonogenic potential of HSPC cultured on pretreated MSC layers, a colony-forming unit assay was performed. Compared to controls, the total number of colonies was significantly reduced in co-cultures with erythropoietin-pretreated MSC from all three groups. Again, lithium chloride abrogated this effect and the number of colonies was comparable to those of untreated controls (Figure 4D–F). Moreover, significant differences could be detected for clonogenic progenitors of granulocytes and macrophages of MSC from old healthy donors and MDS patients, respectively (Figure 4E,F).
Discussion
Erythropoietin and erythropoiesis-stimulating agents are among the main treatment options for anemia in patients with low-risk MDS.27 However, the erythroid response is observed in a relatively low number of patients and often not sustained,28 which is in part due to endogenously elevated erythropoietin levels because of ineffective erythropoiesis in many patients.29 We speculated that a sustained endogenous elevation of erythropoietin levels or iatrogenic erythropoietin therapy in MDS patients might contribute to the dysregulation of the osteo-hematopoietic niche, at least partly due to its effects on MSC and osteoblasts.
In fact, we could confirm previous publications, which postulated the stimulation of osteoblastic differentiation of MSC when exposed to erythropoietin.4–7 However, we could not observe the same effect in MSC from older healthy donors or patients with MDS, although the level of EPOR expression did not differ. Of note, effective erythropoietin concentrations in our experiments were higher than previously published, which can be explained by a different source of cells, as we used primary donor material cultured in our laboratory, whereas in other papers rodent cells5–7,24 or commercially available MSC3,4 were used. Interestingly, effects were only seen when we used the α-form of erythropoietin, whereas we could not observe the biological activity in MSC using a mixture of different erythropoietin isoforms (data not shown). This raises the question whether the effect of endogenous erythropoietin on bone metabolism in patients may differ from that of exogenous erythropoietin treatment in MDS and other disorders, which requires further studies.
Further, the basal rate of osteoblast differentiation was significantly lower in MDS patients, which was associated with the downregulation of the canonical Wnt pathway.20 The genes coding for the Wnt receptor FZD4 as well as for target genes of the canonical Wnt pathway (JUN,30 FOSL1,31 CCND1) were suppressed in the MDS group. Interestingly, another study found an upregulation of JUN and CCND1 in MDS-MSC compared to the control group.21 However, in that study, patients with lymphoproliferative disorders without bone marrow involvement were selected as a control group, which may influence the results due to the known role of the Wnt pathway in lymphocyte development.32 We considered patients without hematological disorders who underwent total hip arthroplasty as a control age-matched group, which allowed us to exclude the bias due to hematological diseases.
Whereas the signaling pathways activated upon erythropoietin treatment in HSPC are relatively well known, signaling cascades in non-hematopoietic cells including osteoblasts are still a matter of debate. Activation of mTOR, AKT,4 Jak2 and PI3K3 has been shown to be involved in the anabolic action of erythropoietin in human MSC. Activation of the canonical Wnt pathway was also detected upon erythropoietin treatment in MSC during neuronal differentiation33 and kidney epithelial cells.34 Despite the inability of erythropoietin to promote the differentiation of MDS-MSC, we observed a molecular derangement in the canonical Wnt pathway following erythropoietin treatment. As such, GSK3B was strongly upregulated, whereas target genes of the canonical Wnt pathway (MMP7, CCND1) were downregulated. Supporting this observation, we demonstrated the inhibition of the canonical Wnt pathway in the C2C12 myoblast cell line upon erythropoietin treatment. However, this effect was detected only at high erythropoietin concentrations (starting from 100 IU/mL) reflecting different cellular sensitivities to erythropoietin. It is already known, that pharmacological activation of canonical Wnt signalling in MDS-MSC with a GSK3B inhibitor increases their proliferative potential and upregulates the expression of early osteoblastic genes.20 We evaluated if the same effect could be observed when reactivation of the canonical Wnt pathway is reached by compounds used in clinical practice, such as lithium salts used in bipolar disorders and, especially, parathyroid hormone, approved for the treatment of osteoporosis. Both medications in standard in vitro concentrations showed their effectiveness in restoration of osteoblastic differentiation of erythropoietin-treated MDS-MSC. Importantly, it is already known that parathyroid hormone does not further increase erythropoietin levels in vivo,5 which is relevant, based on the knowledge that excessive erythropoietin concentrations negatively influence the osteo-hematopoietic microenvironment. Interestingly, we could recently show an increased risk of osteoporosis in patients with MDS.23
Pretreatment of MSC with erythropoietin reduced the number of early CD34+ progenitors. Therefore, because MDS-MSC already have a reduced capacity to support myeloid and erythroid colony forming potential,20,35 excessive erythropoietin levels could further deteriorate hematopoiesis. In turn, reactivation of the canonical Wnt pathway in MSC can restitute or even surpass the initial number of CD34+ cells and colony potential in the co-culture. Thus, in addition to its positive influence on bone metabolism and known effectiveness in osteoporosis, activators of the canonical Wnt pathway might also improve hematopoiesis in MDS patients, potentially in those with endogenously elevated erythropoietin levels.
In conclusion, erythropoietin failed to stimulate MSC from patients with MDS to differentiate towards osteoblasts, at least in part due to the inhibition of the canonical Wnt pathway, which is intrinsically less activated in MDS-MSC compared to age-matched healthy controls, suggesting age-dependent erythropoietin resistance. Reactivation of canonical Wnt signaling does not only restore osteoblastic differentiation capacity, but also promotes hematopoiesis, which provides a potential rationale for the therapeutic use of Wnt-activators in MDS patients.
Supplementary Material
Acknowledgment
The authors would like to thank Eva Schubert, Patrick Böhme, Nicole Pacyna, Marie-Christin Mehnert, Anja Liebkopf, and Ivonne Habermann for technical assistance.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/1/61
Funding
UP and LCH are supported by a grant within the German Consortium of translational cancer research (DKTK), GE/UP/MB/LCH are supported by the Sonderforschungsbereich (SFB) 655 from the Deutsche Forschungsgemeinschaft (DFG), UP/MR are supported by a grant from the Deutsche José Carreras Leukämie-Stiftung and LCH/MR are supported by a seed grant from the CRTD. The work of EB has been supported by a Gerok rotation position within the SFB 655.
References
- 1.Ogunshola OO, Bogdanova AY. Epo and non-hematopoietic cells: what do we know? Methods Mol Biol. 2013;982:13–41. [DOI] [PubMed] [Google Scholar]
- 2.Brines M, Cerami A. The receptor that tames the innate immune response. Mol Med. 2012;18:486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rolfing JH, Baatrup A, Stiehler M, Jensen J, Lysdahl H, Bunger C. The osteogenic effect of erythropoietin on human mesenchymal stromal cells is dose-dependent and involves non-hematopoietic receptors and multiple intracellular signaling pathways. Stem Cell Rev. 2014;10(1):69–78. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Jung Y, Sun H, et al. Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem. 2012;113(1):220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiozawa Y, Jung Y, Ziegler AM, et al. Erythropoietin couples hematopoiesis with bone formation. PloS One. 2010;5(5): e10853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C, Shi C, Kim J, et al. Erythropoietin promotes bone formation through EphrinB2/EphB4 signaling. J Dent Res. 2015;94(3):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair AM, Tsai YT, Shah KM, et al. The effect of erythropoietin on autologous stem cell-mediated bone regeneration. Biomaterials. 2013;34(30):7364–7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rolfing JH, Bendtsen M, Jensen J, et al. Erythropoietin augments bone formation in a rabbit posterolateral spinal fusion model. J Orthop Res. 2012;30(7):1083–1088. [DOI] [PubMed] [Google Scholar]
- 9.Mihmanli A, Dolanmaz D, Avunduk MC, Erdemli E. Effects of recombinant human erythropoietin on mandibular distraction osteogenesis. J Oral Maxillofac Surg. 2009;67(11):2337–2343. [DOI] [PubMed] [Google Scholar]
- 10.Sun H, Jung Y, Shiozawa Y, Taichman RS, Krebsbach PH. Erythropoietin modulates the structure of bone morphogenetic protein 2-engineered cranial bone. Tissue Eng. 2012;18(19–20):2095–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakhshi H, Kazemian G, Emami M, Nemati A, Karimi Yarandi H, Safdari F. Local erythropoietin injection in tibiofibular fracture healing. Trauma Mon. 2013;17(4):386–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takenaka T, Itaya Y, Ishikawa I, Kobayashi K, Tsuchiya Y. Skeletal effects of erythropoietin in hemodialysis patients. Int Urol Nephrol. 2003;35(3):407–413. [DOI] [PubMed] [Google Scholar]
- 13.Singbrant S, Russell MR, Jovic T, et al. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood. 2011;117(21):5631–5642. [DOI] [PubMed] [Google Scholar]
- 14.Dewamitta SR, Russell MR, Nandurkar H, Walkley CR. Darbepoietin-alfa has comparable erythropoietic stimulatory effects to recombinant erythropoietin whilst preserving the bone marrow microenvironment. Haematologica. 2013;98(5):686–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiram-Bab S, Liron T, Deshet-Unger N, et al. Erythropoietin directly stimulates osteoclast precursors and induces bone loss. FASEB J. 2015;29(5):1890–1900. [DOI] [PubMed] [Google Scholar]
- 16.Deshet-Unger N, Hiram-Bab S, Haim-Ohana Y, Mittelman M, Gabet Y, Neumann D. Erythropoietin treatment in murine multiple myeloma: immune gain and bone loss. Sci Rep. 2016;6:30998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rauner M, Franke K, Murray M, et al. Increased EPO levels are associated with bone loss in mice lacking PHD2 in EPO-producing cells. J Bone Miner Res. 2016;31(10):1877–1887. [DOI] [PubMed] [Google Scholar]
- 18.McGee SJ, Havens AM, Shiozawa Y, Jung Y, Taichman RS. Effects of erythropoietin on the bone microenvironment. Growth factors. 2012;30(1):22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulycheva E, Rauner M, Medyouf H, et al. Myelodysplasia is in the niche: novel concepts and emerging therapies. Leukemia. 2015;29(2):259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlaki K, Pontikoglou CG, Demetriadou A, et al. Impaired proliferative potential of bone marrow mesenchymal stromal cells in patients with myelodysplastic syndromes is associated with abnormal WNT signaling pathway. Stem Cells Dev. 2014;23(14): 1568–1581. [DOI] [PubMed] [Google Scholar]
- 21.Falconi G, Fabiani E, Fianchi L, et al. Impairment of PI3K/AKT and WNT/beta-catenin pathways in bone marrow mesenchymal stem cells isolated from patients with myelodysplastic syndromes. Exp Hematol. 2016;44(1):75–83. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Fan R, Wang XQ, et al. Methylation of Wnt antagonist genes: a useful prognostic marker for myelodysplastic syndrome. Ann Hematol. 2013;92(2): 199–209. [DOI] [PubMed] [Google Scholar]
- 23.Weidner H, Rauner M, Trautmann F, et al. Myelodysplastic syndromes and bone loss in mice and men. Leukemia. 2017;31(4): 1003–1007. [DOI] [PubMed] [Google Scholar]
- 24.Guo L, Luo T, Fang Y, et al. Effects of erythropoietin on osteoblast proliferation and function. Clin Exp Med. 2014;14(1):69–76. [DOI] [PubMed] [Google Scholar]
- 25.Galli C, Piemontese M, Lumetti S, Manfredi E, Macaluso GM, Passeri G. GSK3b-inhibitor lithium chloride enhances activation of Wnt canonical signaling and osteoblast differentiation on hydrophilic titanium surfaces. Clin Oral Implants Res. 2013;24(8):921–927. [DOI] [PubMed] [Google Scholar]
- 26.Chandra A, Lin T, Zhu J, et al. PTH1-34 blocks radiation-induced osteoblast apoptosis by enhancing DNA repair through canonical Wnt pathway. J Biol Chem. 2015;290(1):157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hellstrom-Lindberg E, van de Loosdrecht A. Erythropoiesis stimulating agents and other growth factors in low-risk MDS. Best Pract Res Clin Haematol. 2013;26(4):401–410. [DOI] [PubMed] [Google Scholar]
- 28.Park S, Grabar S, Kelaidi C, et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: the GFM experience. Blood. 2008;111(2):574–582. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki T, Oh I, Ohmine K, et al. Distribution of serum erythropoietin levels in Japanese patients with myelodysplastic syndromes. Int J Hematol. 2015;101(1):32–36. [DOI] [PubMed] [Google Scholar]
- 30.Sassi N, Laadhar L, Allouche M, et al. The roles of canonical and non-canonical Wnt signaling in human de-differentiated articular chondrocytes. Biotech Histochem. 2014;89(1):53–65. [DOI] [PubMed] [Google Scholar]
- 31.Heo JS, Lee SY, Lee JC. Wnt/beta-catenin signaling enhances osteoblastogenic differentiation from human periodontal ligament fibroblasts. Mol Cells. 2010;30(5):449–454. [DOI] [PubMed] [Google Scholar]
- 32.Lento W, Congdon K, Voermans C, Kritzik M, Reya T. Wnt signaling in normal and malignant hematopoiesis. Cold Spring Harb Perspect Biol. 2013;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danielyan L, Schafer R, Schulz A, et al. Survival, neuron-like differentiation and functionality of mesenchymal stem cells in neurotoxic environment: the critical role of erythropoietin. Cell Death Differ. 2009;16(12):1599–1614. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Wang CC, Song SM, et al. The administration of erythropoietin attenuates kidney injury induced by ischemia/reperfusion with increased activation of Wnt/beta-catenin signaling. J Formos Med Assoc. 2015;114(5):430–437. [DOI] [PubMed] [Google Scholar]
- 35.Ferrer RA, Wobus M, List C, et al. Mesenchymal stromal cells from patients with myelodyplastic syndrome display distinct functional alterations that are modulated by lenalidomide. Haematologica. 2013;98(11):1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.