Abstract
Nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) is known to play an important role in the pathogenesis of chronic lymphocytic leukemia (CLL). Several NF-κB inhibitors were shown to successfully induce apoptosis of CLL cells in vitro. Since the microenvironment is known to be crucial for the survival of CLL cells, herein, we tested whether NF-κB inhibition may still induce apoptosis in these leukemic cells in the presence of protective stromal interaction. We used the specific NF-κB inhibitor dehydroxymethylepoxyquinomicin (DHMEQ). Microenvironmental support was mimicked by co-culturing CLL cells with bone marrow-derived stromal cell lines (HS-5 and M2-10B4). NF-κB inhibition by DHMEQ in CLL cells could be confirmed in both the monoculture and co-culture setting. In line with previous reports, NF-κB inhibition induced apoptosis in the monoculture setting by activating the intrinsic apoptotic pathway resulting in poly (ADP-ribose) polymerase (PARP)-cleavage; however, it was unable to induce apoptosis in leukemic cells co-cultured with stromal cells. Similarly, small interfering ribonucleic acid (siRNA)-mediated RELA downregulation induced apoptosis of CLL cells cultured alone, but not in the presence of supportive stromal cells. B-cell activating factor (BAFF) was identified as a microenvironmental messenger potentially protecting the leukemic cells from NF-κB inhibition-induced apoptosis. Finally, we show improved sensitivity of stroma-supported CLL cells to NF-κB inhibition when combining the NF-κB inhibitor with the SYK inhibitor R406 or the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib, agents known to inhibit the stroma-leukemia crosstalk. We conclude that NF-κB inhibitors are not promising as monotherapies in CLL, but may represent attractive therapeutic partners for ibrutinib and R406.
Introduction
Although progress has been made with the introduction of new therapeutic agents in the treatment of CLL, the disease remains mostly incurable, highlighting the need for new therapeutic targets and substances.
NF-κB is a key factor contributing to CLL pathology and has thus been suggested as a treatment target.1–4 The five subunits of NF-κB (RELA, RELB, NFκB1, NFκB2 and c-REL) reside in the cytoplasm. Once activated, they translocate into the nucleus and bind to promotor regions on the DNA, modifying gene expression.5,6 Indeed, NF-κB is constitutively activated in CLL cells and up-regulates anti-apoptotic genes (e.g., TRAF1, BCL2, BCL2L1, IAPs), increasing apoptosis resistance, a major feature of CLL cells.7,8 NF-κB signaling is also involved in increased resistance to anti-cancer therapies9 and mediates signals from crucial pathways in the crosstalk between CLL cells and their protective microenvironment, such as the B-cell receptor (BCR) pathway, the BAFF/BAFF-receptor axis, or the CD40L/CD40 axis.7,10–12 Different mouse models also highlight the major role of NF-κB in CLL biology.1 Finally, high RELA DNA-binding is associated with advanced Binet stage, shorter time to first or subsequent treatment, and overall survival.13
Several inhibitors of NF-κB have been successfully tested on CLL cells in vitro. Amongst others (e.g., parthenolide, curcumin, IMD-0354, BAY11-7082),12,14–18 the specific NF-κB inhibitor DHMEQ efficiently induced apoptosis in CLL cells in vitro.19 However, the efficacy of NF-κB inhibitors has never been tested on CLL cells cultured in the presence of their protective microenvironment, crucial for CLL cell survival. This prompted us to assess the ability of NF-κB inhibition to induce apoptosis in CLL cells co-cultured with supportive bone marrow stromal cells (BMSCs) using DHMEQ. The latter was chosen for its unique mode of action: it covalently binds to cysteine residues of NF-κB subunits, thereby inhibiting the interaction of NF-κB with its DNA-binding site.20 In addition, DHMEQ has also been shown to inhibit NF-κB translocation into the nucleus, making it a highly potent NF-κB inhibitor.21
Herein, we provide evidence that although NF-κB inhibition is highly effective at inducing apoptosis of monocultured CLL cells, NF-κB inhibition alone is not sufficient to induce apoptosis of CLL cells cultured with supportive BMSCs. Furthermore, our results suggest that in CLL treatment, NF-κB inhibitors should not be used as single agents, but rather in combination with substances that disrupt the crosstalk between CLL cells and their microenvironment.
Methods
Patients
This study was approved by the Institutional Review Board of the University Medical Center Freiburg. Blood samples were obtained from untreated CLL patients (off therapy for at least 6 months), following written informed consent.
Cell preparation and culture
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (Ficoll-Histopaque, PAA Laboratories GmbH). In PBMC samples containing fewer than 90% CD5/CD19 positive cells as measured by flow cytometry (CD5-APC, BD Pharmingen; CD19-PE-Texas Red, Southern Biotech), purification by negative selection (B cell isolation kit II, Milteny Biotec) was performed. Freshly isolated and thawed CLL cells were used explaining slight variability in the viability of untreated controls between experiments. CLL cells were treated and cultured alone or with BMSCs. BMSCs M2-10B4 (mouse) and HS-5 (human) were used, both obtained from the American Type Culture Collection (ATCC). Culture medium and treatment were replenished every other day. Reagents are listed in the Online Supplementary Materials.
BMSCs were plated (2,5 × 105 cells/mL) and CLL cells (5 × 106 cells/mL) were added the following day on confluent BMSC layers. After treatment, CLL cells were harvested by careful pipetting to rinse floating CLL cells off adherent BMSCs, taking care not to damage the BMSC layer. To distinguish between the effects of direct BMSC-CLL contact versus soluble factors-induced effects, Corning® HTS Transwell® plates were used.
Quantification of viable and apoptotic cells
Viability was measured by flow cytometry (CyAn ADP, Beckmann) after staining CLL cells with fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection kit I (BD Biosciences). Annexin V (ANX5)/propidium iodide (PI) double-negative cells were regarded as live cells, ANX5 positive/PI negative cells as early apoptotic cells, and ANX5/PI double-positive cells as late apoptotic/necrotic cells. Results were analyzed with FlowJo software (FlowJo, LLC).
NF-κB DNA-binding activity
NF-κB DNA-binding activity was measured from whole cell lysates using the TransAM® NF-κB Family Kit (Active Motif), according to the manufacturer’s instructions.
Immunoblotting
Total cell protein was extracted from CLL cells and subjected to western blotting as described previously.22 Subcellular fractionation to obtain cytosolic and nuclear protein fractions for western blotting is described in the Online Supplementary Materials. Blots were incubated with primary and secondary antibodies overnight at 4°C or for 1h at room temperature. Antibodies used are listed in the Online Supplementary Materials. Densitometric analysis was performed using LabImage 1D (Kapelan Bio-Imaging). Whole cell protein expression was normalized to β-Actin and nuclear protein expression to Histone 3.
Transfection
Freshly isolated CLL cells were transfected with 2μM RELA or non-targeting siRNA (GE Healthcare Dharmacon) using an Amaxa Human B cell Nucleofector Kit and nucleofector program U013 (Nucleofector 2b Device) following the manufacturer’s instructions (Amaxa).
Statistical Analysis
Analysis of synergistic drug effects was performed with CompuSyn (ComboSyn, Inc.). GraphPad Prism software was used for statistical analysis (version 6.0, GraphPad Software, Inc.). Data are represented as mean ± standard error of the mean (SEM). For comparisons between two parameters, a 2-tailed, paired Student’s t-test was applied; for more than two parameters, one-way ANOVA with correction for multiple comparisons with Turkey’s test was used. P<0.05 was considered statistically significant.
Results
DHMEQ induces apoptosis of primary CLL cells in monoculture but not in co-culture with bone marrow stromal cells
CLL cells cultured alone or in co-culture with protective BMSCs (HS-5 and M2-10B4) were treated with 2 or 5μg/ml of the NF-κB inhibitor DHMEQ in vitro for up to 144h (Figure 1 and Figure 2).
Figure 1.
DHMEQ reduces viability of CLL cells in monoculture but not in co-culture with stromal cells. Cell viability as measured by flow cytometry with ANX5/PI staining of CLL cells cultured in vitro (A) alone or in co-culture with M2-10B4 cells after 2, 4, and 6 days of treatment with 2μg/ml of DHMEQ, (B) alone or in co-culture with HS-5 cells after 2 days of treatment with 2μg/ml or (C) 5μg/ml of DHMEQ. (D) Cell populations as measured by flow cytometry after ANX5/PI staining of in vitro monocultured CLL cells with or without 5μg/ml of DHMEQ. Fold changes of CLL cell viability are indicated above the signs for significance. ****P<0.0001, ***P<0.001, **P<0.01 and *P<0.05. CLL: chronic lymphocytic leukemia; DHMEQ: dehydroxymethylepoxyquinomicin; ns: not significant.
Figure 2.
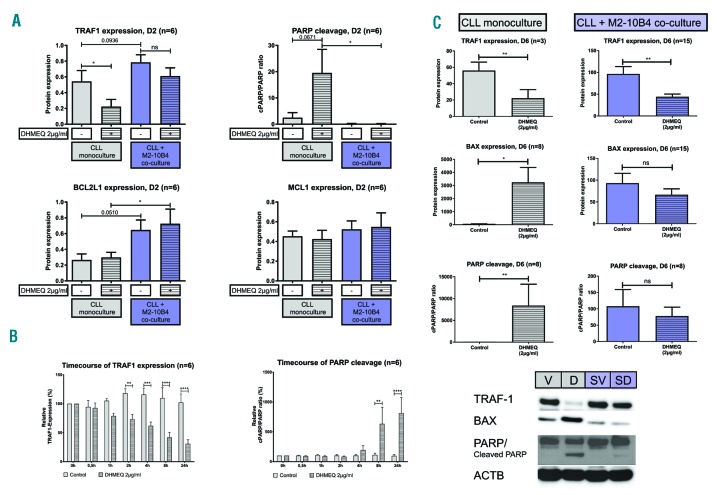
DHMEQ treatment activates the intrinsic apoptotic pathway. Densitometrically analyzed protein expression normalized to β-Actin of CLL cells cultured in vitro (A) alone or in co-culture with M2-10B4 cells after 2 days of treatment with 2μg/ml of DHMEQ, (B) alone after 0.5, 1, 2, 4, 8 and 24 hours of treatment with 2 μg/ml of DHMEQ and (C) alone or in co-culture with M2-10B4 cells after 6 days of treatment with 2μg/ml of DHMEQ shown with exemplary western blot. ****P<0.0001, ***P<0.001, **P<0.01 and *P<0.05. D: DHMEQ (dehydroxymethylepoxyquinomicin); D2: day 2; D6: day 6; SD: stroma + DHMEQ; SV: stroma + vehicle control; v: vehicle control; CLL: chronic lymphocytic leukemia; ns: not significant; ACTB: β-Actin.
DHMEQ induced apoptosis of monocultured CLL cells in a dose- and time-dependent manner (Figure 1A–C). Also, in monocultured DHMEQ-treated CLL cells, the major cell population evolved from being ANX5 single-positive after 24h, to being ANX5/PI double-positive after 48h, suggesting apoptosis induction rather than direct toxicity (Figure 1D).
Surprisingly, the viability of DHMEQ-treated CLL cells co-cultured with BMSCs remained unchanged, irrespective of DHMEQ dosage (2 or 5μg/ml for 48h) or treatment time. DHMEQ-induced apoptosis was suppressed by both BMSCs tested (HS-5, M2-10B4) (Figure 1A–C).
Of note, CLL cell viability of untreated controls after 4 and 6 days was higher than on day 2 (Figure 1A). We reasoned that the process of thawing led to apoptosis and the death of some CLL cells, which is supported by western blot analysis of PARP/cleaved PARP expression on day 0 (data not shown), leading to a comparably low viability on day 2. After cell components were processed and degraded, the percentage of live cells increased on day 4 in vehicle-treated cohorts (monoculture and co-culture).
Reduced viability in monocultured CLL cells is accompanied by activation of the intrinsic apoptotic pathway
We next analyzed whether the reduction of CLL cell viability relies on activation of the intrinsic apoptotic pathway by downregulation of the NF-κB target genes TRAF1, BCL2L1 (also known as BCL-xL), and MCL1, and increased PARP cleavage. TRAF1 is a recognized NF-κB target gene,23,24 BCL2L1 and MCL1 represent two anti-apoptotic BCL2 family members known to be regulated by NF-κB. PARP cleavage is frequently used as a surrogate marker for caspase-3 activation.
Monocultured CLL cells treated with DHMEQ (2μg/ml) for 48h showed a significant downregulation of TRAF1 expression (P=0.0194) accompanied by a notable increase of PARP cleavage (P=0.067) compared to untreated controls, while BCL2L1 and MCL1 remained unaffected (Figure 2A). Interestingly, TRAF1 downregulation occurred before the increase in PARP cleavage (Figure 2B). In contrast, in CLL cells co-cultured with M2-10B4 cells, DHMEQ treatment for 2 or 6 days did not induce changes in PARP cleavage. In fact, PARP cleavage was almost undetectable in CLL cells co-cultured with BMSCs (Figure 2A,C). Under co-culture conditions, no significant downregulation of BCL2L1 and MCL1 was detected upon treatment. Only TRAF1 expression tended to decrease (Figure 2A). Notably, BCL2L1 expression was increased in co-cultured CLL cells. Similar results were observed after 6 days of treatment with 2 μg/ml of DHMEQ. Although significant TRAF1 downregulation was seen in both monocultured and co-cultured CLL cells, PARP cleavage was only induced in monocultured cells. Additional analysis of BAX, a proapoptotic protein, showed increased expression in monocultured CLL cells after DHMEQ treatment, but no change in the co-culture setting (Figure 2C).
DNA-binding activity of all five NF-κB subunits is strongly suppressed by DHMEQ treatment in monocultured CLL cells and also in those cells co-cultured with supportive stromal cells
We next tested whether DHMEQ inhibited NF-κB activity in the various conditions by using the TransAM® NFκB Family Kit (Active Motif), a DNA-binding enzyme-linked immunosorbent assay (ELISA) which enabled us to test the DNA-binding activity of each NF-κB subunit. Additionally, western blot analyses of the expression of the different NF-κB subunits in nuclear and cytosolic extracts were performed (Figure 3).
Figure 3.
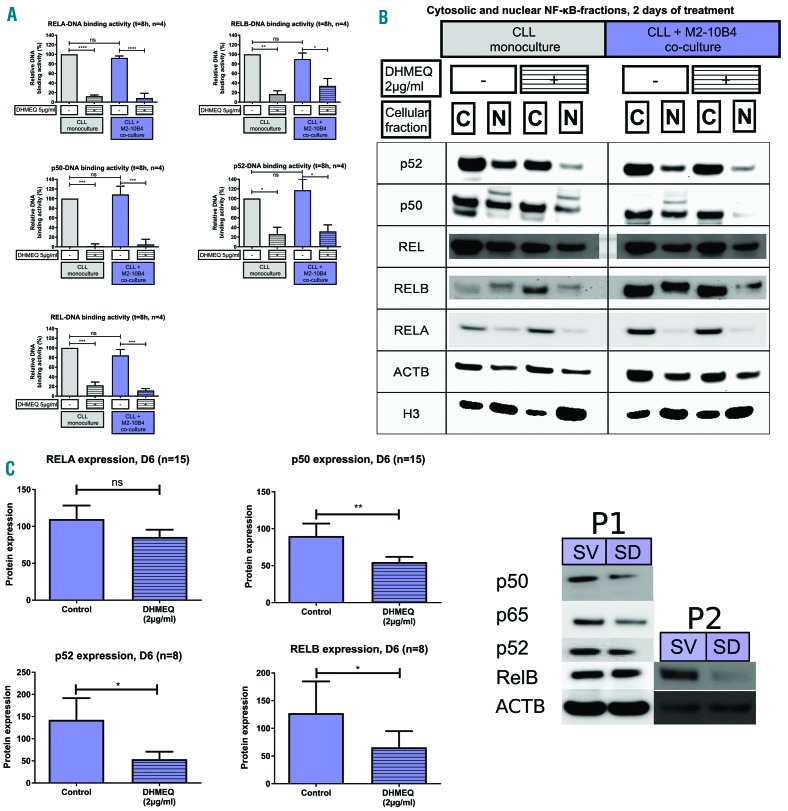
NF-κB DNA-binding activity, nuclear and cytosolic expression are significantly reduced by DHMEQ treatment in both monocultured and co-cultured CLL cells. (A) DNA-binding activity of the different NF-κB subunits in monocultured or M2-10B4 co-cultured CLL cells treated with 5μg/ml of DHMEQ or vehicle control for 8h. (B) Western blot analysis of cytosolic and nuclear NF-κB expression in monocultured or M2-10B4 co-cultured CLL cells after 2 days of treatment with 2μg/ml of DHMEQ. (C) Densitometrically analyzed NF-κB expression normalized to β-Actin of CLL cells cultured in co-culture with M2-10B4 cells after 6 days of treatment with 2μg/ml of DHMEQ shown with exemplary western blot. Since the viability of the DHMEQ-treated CLL cells under monoculture conditions was very poor, densitometric analysis was not possible in this set of experiments. ****P<0.0001, ***P<0.001, **P<0.01 and *P<0.05. ACTB: β-Actin; C: cytosolic; D6: day 6; N: nuclear; SD: stroma + DHMEQ; SV: stroma + vehicle control; P1: patient 1; P2: patient 2; CLL: chronic lymphocytic leukemia; ns: not significant.
DNA-binding activity of all five NF-κB subunits was significantly suppressed by DHMEQ treatment in monoculture in addition to CLL cells co-cultured with M2-10B4 cells (Figure 3A). DHMEQ treatment reduced nuclear translocation of all NF-κB subunits in both the monoculture and the co-culture setting (Figure 3B). Furthermore, western blot analyses of the expression of the different NF-κB subunits in whole cell lysates showed significant reduction after 6 days of treatment with 2μg/ml DHMEQ in co-culture with stromal cells (Figure 3C). Taken together, these results validate the efficiency of NF-κB inhibition by DHMEQ in both monoculture and co-culture.
siRNA-mediated knockdown of the NF-κB subunit RELA does not induce apoptosis of CLL cells in co-culture with stromal cells but appears to do so in monocultured CLL cells
To confirm the finding that NF-κB inhibition is not sufficient to induce apoptosis of CLL cells co-cultured with BMSCs, siRNA-mediated knockdowns of the NF-κB subunit RELA (also known as p65) were performed. From the different NF-κB subunits, RELA was chosen as the knockdown target as it has been shown that the high binding activity of RELA to its DNA-binding site is predictive of a short time to first treatment, time to subsequent treatment, and overall survival from the date of diagnosis.13 The knockdown efficiency in the analyzed samples was assessed by western blot, and the effect of RELA knockdown on viability of CLL cells in monoculture and co-culture with M2-10B4 cells was assessed by flow cytometric apoptosis measurements (Figure 4). In the western blot analysis, the average expression of RELA in RELA-transfected CLL cells in the monoculture setting was 79.2% as compared to 90.9% in the non-targeting transfected controls, and as such did not reach significance. In contrast, in the co-culture setting RELA expression in CLL cells was reduced to 38% in RELA-transfected cells compared to 73.2% in cells transfected with non-targeting siRNA (Figure 4A,B). However, when analyzing the apoptosis measurements, the relative reduction of RELA by about 50% had no visible effect on the viability of CLL cells co-cultured with M2-10B4 cells, whereas the less efficient knockdown in monocultured CLL cells had a marked tendency to induce apoptosis of these cells as compared to respective controls. This is in accordance with our previous data suggesting that NF-κB inhibition is not sufficient to induce apoptosis in CLL cells in the presence of supportive BMSCs.
Figure 4.
siRNA-mediated RELA downregulation seems to induce apoptosis in monocultured but not in co-cultured CLL cells. (A) Exemplary western blots of RELA in monocultured and M2-10B4 co-cultured CLL cells after transfection with non-targeting siRNA or RELA siRNA. (B) Densitometric western blot analysis illustrating the knockdown efficiency obtained in monocultured and co-cultured CLL cells 2 days after transfection. (C) CLL cell viability 3 days after transfection with non-targeting siRNA or RELA siRNA. ***P<0.001, **P<0.01 and *P<0.05. ACTB: β-Actin; D2: day 2; D3: day 3; n-t-siRNA: non-targeting small interfering ribonucleic acid; untr: untreated; CLL: chronic lymphocytic leukemia; ns: not significant.
Soluble factors and cell bound factors from the BMSCs are likely to protect CLL cells from apoptosis induction by NF-κB inhibition with DHMEQ
This discovery led us to question which factors from the BMSCs were responsible for CLL cell protection from apoptosis induction by NF-κB inhibition.
First, we used Corning® Transwell® inserts offering minuscule pores impermeable for cells, but permeable for culture medium and soluble factors for co-culture experiments. Hence, half of the CLL cells were placed in the lower compartment, in direct contact with the M2-10B4 cells, while the other half was placed in the inserts so that they could only be influenced by the BSMCs via soluble factors (Figure 5A). The viability of CLL cells from the lower compartments and from the inserts was measured separately via flow cytometry after ANX5/PI staining (Figure 5B).
Figure 5.
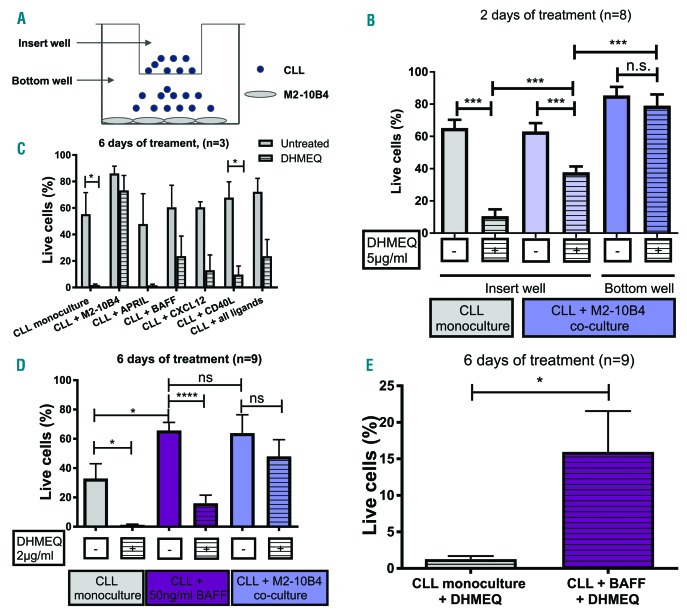
Protection from DHMEQ-induced apoptosis involves direct cell-cell interaction as well as soluble factors such as BAFF. (A) Illustration of the transwell experiment. (B) Cell viability after DHMEQ treatment of CLL cells in monoculture and co-culture, depending on their localization in the transwell experiment. (C) CLL cell viability with or without DHMEQ in monoculture or in the presence of APRIL, BAFF, CXCL12, CD40L, all ligands, or M2-10B4 cells. (D) Viability of CLL cells cultured alone, with BAFF, or with M2-10B4 cells, and treated with or without DHMEQ. (E) Additional t-test analysis of monocultured CLL cells versus BAFF-supported CLL cells, both treated with DHMEQ. ****P<0.0001, ***P<0.001 and *P<0.05. CLL: chronic lymphocytic leukemia; DHMEQ: dehydroxymethylepoxyquinomicin; BAFF: B-cell activating factor; APRIL: a proliferation-inducing ligand; CXCL12: C-X-C motif chemokine 12.
As before, the monocultured CLL cells were very sensitive to DHMEQ treatment and exhibited a strong decrease in viability, while the co-cultured CLL cells in the lower compartment with direct cell-cell contact showed virtually no apoptosis after DHMEQ treatment. In contrast, the co-cultured CLL cells in the inserts (with contact between stromal and CLL cells through soluble ligands only) showed an intermediary sensitivity to DHMEQ treatment, as their viability was significantly decreased by DHMEQ treatment while staying significantly better than the viability of DHMEQ-treated CLL cells in monoculture (Figure 5B). This indicates that the protection afforded by BMSCs from NF-κB-inhibition-induced apoptosis is partly mediated via soluble factors. However, direct contact or closer proximity of CLL cells appears to increase the protective effect of the stromal cells.
BAFF but not a proliferation-inducing ligand (APRIL), CD40L, or C-X-C motif chemokine 12 (CXCL12) can protect CLL cells from DHMEQ-induced apoptosis
To identify factors which might contribute to CLL cell protection from NF-κB-inhibition-induced apoptosis, we assessed the protective effect of four acknowledged factors involved in the crosstalk between the microenvironment and CLL cells. CLL cells were cultured alone for 144h with or without DHMEQ (2 μg/ml) and with or without one of the following: CD40L (1 μg/ml), CXCL12 (100ng/ml), BAFF (50ng/ml), and APRIL (500 μg/ml). Cell viability was measured by flow cytometry of ANX5/PI-stained CLL cells (Figure 5C,D, Online Supplementary Figure S1). While APRIL did not protect CLL cells from DHMEQ-induced apoptosis, CD40L, CXCL12 and BAFF slightly reduced DHMEQ’s proapoptotic effect. A combination of these four ligands did not result in better protection than with BAFF alone (Figure 5C, Online Supplementary Figure S1). Indeed, the viability of BAFF- and DHMEQ-treated cells was 16% as compared to 1% for solely DHMEQ-treated cells and 48% for M2-10B4 co-cultured CLL cells (Figure 5D). The decreased effectivity of DHMEQ treatment when CLL cells were stimulated with BAFF was significant when using a t-test (Figure 5E). These results suggest that, amongst other factors, BAFF could be involved in protecting CLL cells from NF-κB-inhibition-induced apoptosis.
The combination of DHMEQ with fludarabine, idelalisib, R406, or ibrutinib partly restores the DHMEQ-sensitivity of stroma-supported CLL cells
As mentioned before, NF-κB inhibition has been proposed as a promising strategy in CLL treatment. However, our results indicate that NF-κB inhibitors alone might not be as effective as previously assumed in the treatment of CLL patients. Therefore, we investigated whether DHMEQ could be effectively combined with established chemotherapeutic agents (fludarabine) or substances which have been shown to disrupt the crosstalk between the microenvironment and CLL cells (idelalisib, ibrutinib, R406). Indeed, apart from inhibiting the BCR pathway, ibrutinib, idelalisib, and R406 have been shown to disrupt at least a part of the interactions between CLL cells and their microenvironments.10,11,22 Therefore, cells in monoculture or co-culture with M2-10B4 cells were treated with or without DHMEQ and with or without ibrutinib, idelalisib, R406 or fludarabine.
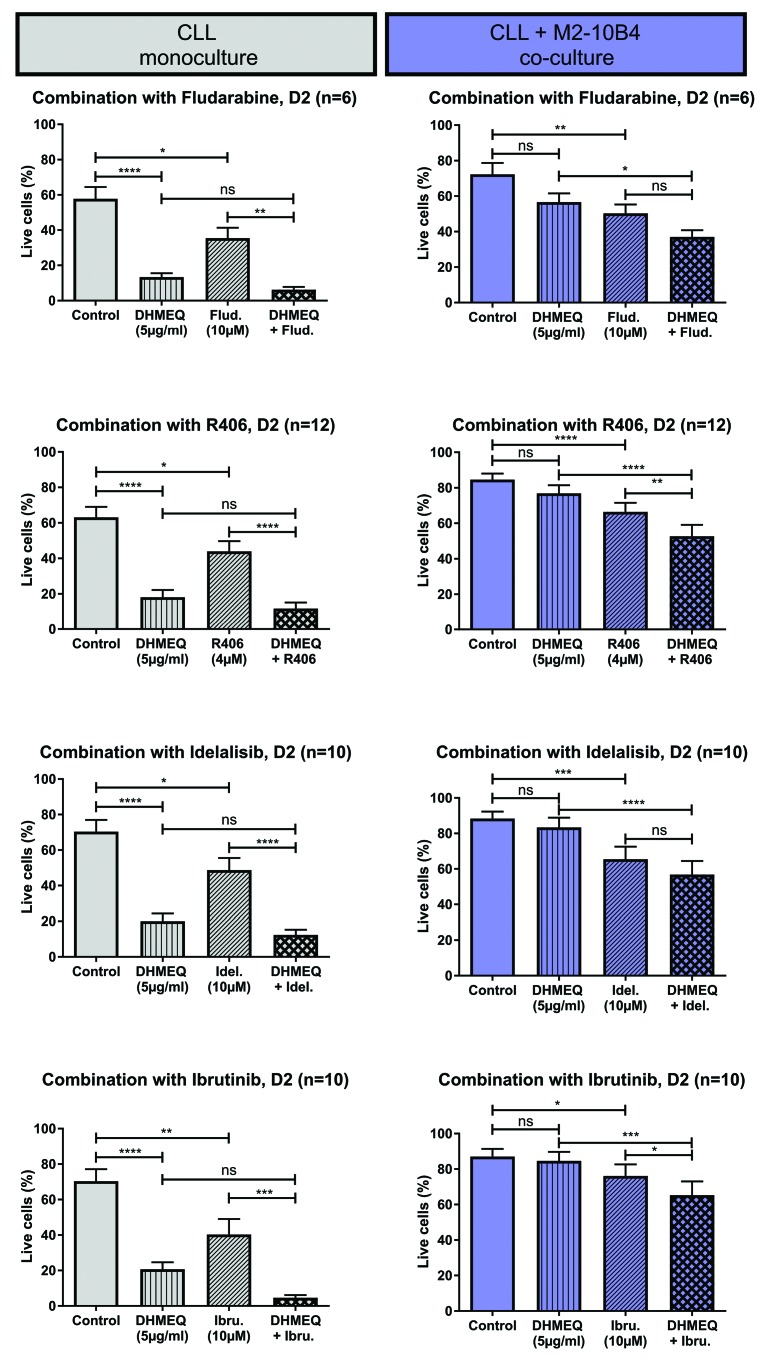
In the monoculture setting, CLL cell viability was significantly decreased by every substance used alone, including DHMEQ. In addition, combining DHMEQ with ibrutinib, R406, idelalisib, and fludarabine, respectively, led to significantly reduced CLL cell viability. In the co-culture setting, as expected, DHMEQ monotherapy did not induce a significant reduction in CLL cell viability, while fludarabine, ibrutinib, R406, and idelalisib did. However, combining DHMEQ with ibrutinib or R406, but not with fludarabine, led to a significant reduction in the viability of CLL cells cultured with stromal cells, compared to CLL cells treated with the respective single agents (Figure 6). There was no significant difference in the obtained effect when drugs were added concomitantly (DHMEQ + ibrutinib or DHMEQ + R406 added at the same time) versus sequentially (DHMEQ added 4 hours before ibrutinib or R406 and vice versa, Online Supplementary Figure S2).
Figure 6.
Combining DHMEQ with stroma crosstalk inhibiting substances partly restores DHMEQ sensitivity in stroma-supported CLL cells. Viability of CLL cells in monoculture or co-culture with M2-10B4 cells treated for 2 days with or without DHMEQ, with or without fludarabine, R406, idelalisib, or ibrutinib, or a combination of DHMEQ and one of these substances.****P<0.0001, ***P<0.001, **P<0.01 and *P<0.05. D2: day 2; Ibru: ibrutinib; Idel: idelalisib; Flud: fludarabine; CLL: chronic lymphocytic leukemia; DHMEQ: dehydroxymethylepoxyquinomicin; ns: not significant.
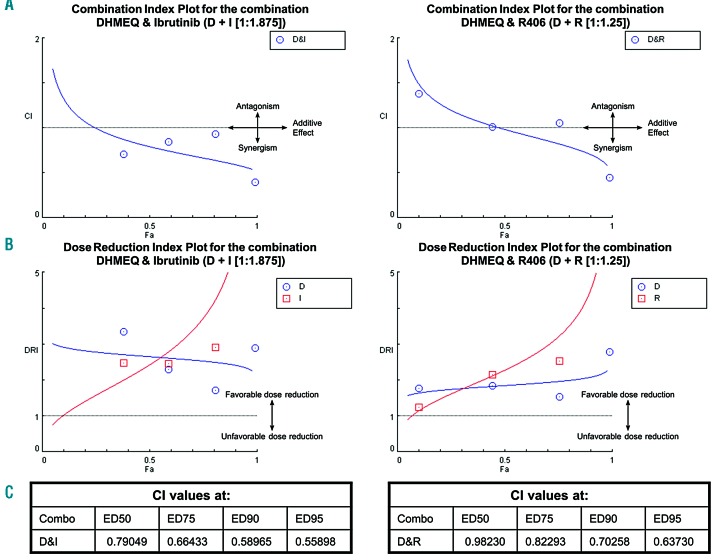
To determine whether the combined effects of DHMEQ + ibrutinib and of DHMEQ + R406 on CLL cells cultured with stromal cells are synergistic, additive, or antagonistic, combination indices were calculated (see Online Supplementary Methods).
A combination index (CI) <1, =1, and >1 indicates synergism, additive effect and antagonism, respectively. The combination of DHMEQ and ibrutinib showed moderate synergism (0.79) at the median effective dose (ED50), and frank synergism at higher effective doses (CI< 0.7 at ED75, 90 and 95, respectively) (Figure 7A,C). The combination of DHMEQ and R406, diversely, showed nearly additive effects at ED50, moderate synergism at ED75 and ED90, and frank synergism at ED95 (Figure 7A,C). Additionally, dose-reduction indices (DRIs) were calculated to investigate whether adding DHMEQ to R406 or ibrutinib treatment could lower the doses of R406 or ibrutinib needed to obtain a given effect, thus potentially decreasing side-effects. A DRI>1 indicates favorable dose-reduction. Figure 7B shows that at fractions affected (Fa) > 0.1, a favorable dose-reduction of ibrutinib or R406 can be obtained by combining these drugs with DHMEQ.
Figure 7.
Combination Index studies reveal synergistic effect for the combination of DHMEQ and ibrutinib or R406 respectively on M2-10B4 co-cultured CLL cells. Combination index (CI) studies were performed by treating CLL cells co-cultured with M2-10B4 cells with DHMEQ (titrated from 8μg/ml), ibrutinib (titrated from 15μM) or R406 (titrated from 10μM) and combinations of R406+DHMEQ (at a ratio of 1.25:1) or ibrutinib + DHMEQ (at a ratio of 1.875:1) for 48h. Drug-induced cytotoxicities were calculated using the CLL cell viabilities to generate CIs. (A) CI plots for DHMEQ+ ibrutinib (D&I) and DHMEQ+R406 (D&R). CI-values <1 indicate synergism, CI=1 indicates additive effects and CI-values >1 indicate antagonism. (B) Dose-reduction indices (DRIs) were calculated along with the CI. A DRI>1 indicates a favorable dose reduction, while a DRI<1 indicates an unfavorable dose reduction. Above a fraction affected of 0.1, all DRIs are >1, indicating that the doses of R406 or ibrutinib needed for a given effect level in single drug treatment can be reduced by adding DHMEQ. (C) The obtained CI values for the combination D&I and D&R at different effective doses (ED50, ED75, ED90, ED95) are given. Fa: fraction affected; DHMEQ: dehydroxymethylepoxyquinomicin.
Discussion
Although previous studies using NF-κB inhibitors indicated that NF-κB could be a promising therapeutic target in CLL, our data suggest that the role of the protective CLL microenvironment might have been underestimated in these reports. In this study we used the specific NF-κB inhibitor DHMEQ, which has been shown to induce apoptosis in CLL cells but not in normal B-cells,19 and has been used in vivo in mice with promising therapeutic results and no serious side effects for the treatment of rheumatoid arthritis,25 systemic lupus erythematosus,26 and most interestingly in human T-cell leukemia27,28 and multiple myeloma.29
In our study, we confirmed that DHMEQ is a potent NF-κB inhibitor blocking both nuclear translocation and DNA-binding of the NF-κB subunits, regardless of the presence of a protective microenvironment imitated by BMSCs. While NF-κB inhibition using DHMEQ led to a strong induction of the intrinsic apoptotic pathway by BAX upregulation, increased PARP cleavage, and a downregulation of TRAF1 in CLL cells cultured without BMSCs, expression of the NF-κB target genes BCL2L1 and MCL1 remained unaffected. Albeit others have observed the downregulation of BCL2L1 and/or MCL1 after NF-κB inhibition,17,19 our results are in line with those of Pickering et al. who reported that NF-κB inhibition did not alter BCL2L1, BCL2 and MCL1 expression in CLL cells in vitro.16 In the co-culture setting, DHMEQ efficiently inhibited NF-κB activity and caused downregulation of the NF-κB subunits themselves, either by DHMEQ directly,30 or by breaking the self-renewing cycle of NF-κB expression.31–36 Surprisingly, neither CLL cell survival nor the expression of BAX or cleaved PARP was influenced by DHMEQ in the co-culture setting. In the NF-κB gene-silencing performed to support the findings from pharmacological NF-κB inhibition, a loss of importance of the RELA subunit was specifically seen for the survival of CLL cells co-cultured with BMSCs. It seems that in the presence of BMSCs, NF-κB is not essential for CLL cell survival while other pathways become more relevant, thus questioning the efficacy of NF-κB inhibitors as monotherapeutic agents in CLL.
As we observed an upregulation of the proapoptotic BCL2 family member BAX upon DHMEQ treatment in the monoculture setting only, and the anti-apoptotic protein BCL2L1 was upregulated in the CLL-BMSC co-culture setting, it is possible that BCL2 family members could be key players in the NF-κB-independent resistance to apoptosis seen in CLL cells co-cultured with BSMCs. Adding BCL2 inhibitors to DHMEQ treatment might diminish the microenvironment’s protective effect and represent an attractive combination of targeted therapeutics. Fittingly, López-Guerra et al. reported strong synergism between BMS-345541 (a selective IKK inhibitor) and the pan-BCL2 inhibitor obatoclax.17 Combinations with the BCL2 inhibitors venetoclax, which received breakthrough therapy designation,37,38 and navitoclax39 could also offer good prospects of success.
Furthermore, the study herein shows that both direct cell-cell interactions between CLL cells and BMSCs as well as soluble ligands secreted by the BSMCs are involved in mediating the protective effect against NF-κB inhibition. In particular, soluble BAFF, secreted in vivo by nurse-like cells (NLC)12 and BMSCs40 was identified. CLL cells themselves have been shown to express and secrete BAFF, implying an autocrine loop.41 BAFF binds to three receptors: BCMA (B-cell maturation antigen), TACI (transmembrane activator of the calcium modulator and cyclophylin ligand interactor), and BAFF-Receptor (BR3).12,42 APRIL is known to bind to BCMA and TACI, but not to BR3.12,43,44 Since APRIL did not protect CLL cells from DHMEQ-induced apoptosis to the same extent as BAFF, a central role for the receptor BR3 can be hypothesized. A possible explanatory pathway is a recently described influence of gene expression through Histone H3 phosphorylation: the binding of BAFF to BR3 induces receptor translocation to the nucleus, where the receptor can bind IKKβ. The kinase then phosphorylates Histone H3, leading to the activation of a variety of transcription factors and cofactors.45 Since DHMEQ binds to the NF-κB subunits but not to the IKKs, a regulation of anti-apoptotic genes through BAFF may exist despite DHMEQ treatment.21 Interestingly, the BAFF inhibitor belimumab, which is currently only approved for the treatment of systemic lupus erythematosus, has recently been tested on CLL cells;46 Wild et al. report the neutralization of BAFF’s CLL-protecting effect by belimumab, sensitizing CLL cells to lysis. Combining DHMEQ with belimumab could therefore be a promising approach.
Moreover, by disrupting the interactions between the microenvironment and CLL cells through the addition of ibrutinib (a BTK inhibitor) or R406 (a SYK inhibitor), DHMEQ’s proapoptotic effect was partially restored, while the proapoptotic effects of the combined treatments were also significantly higher than those of the single drug treatments with ibrutinib or R406. Indeed, CIs for both the combination of DHMEQ + ibrutinib as well as DHMEQ + R406 indicate synergism. Dose-reduction indices for R406 and ibrutinib further imply that adding DHMEQ could lower the doses of R406 and ibrutinib needed for a given effect level, thus potentially reducing side effects. It is known that ibrutinib is able to overcome pro-survival signals derived from BCR stimulation, NLC-contact, and BAFF, among others.47,48 Ibrutinib also releases tumor cells from the tissue compartment into the peripheral blood, a drug effect that can be seen only in in vivo experiments.49 Since ibrutinib inhibits BCR signaling, potentially bypassing the NF-κB pathway in the presence of the microenvironment, and further disrupts molecular crosstalk between CLL cells and their microenvironment by redistributing CLL cells into the peripheral blood, a combination of DHMEQ and ibrutinib could possibly show an even better synergistic effect in vivo. Furthermore, BTK influences CLL cell survival by activating the NF-κB pathway. Hence, inhibiting those pathways could not only restore DHMEQ’s proapoptotic effect by disrupting interaction between CLL cells and their microenvironment, but also increase NF-κB inhibition. We and others previously identified SYK as a candidate for targeted therapy in CLL due to its enhanced expression and activity and the apoptotic effects of pharmacological SYK inhibition.22,50 Entospletinib, a selective SYK inhibitor, demonstrated promising clinical activity in patients with relapsed or refractory CLL.51 Our results suggest that combining DHMEQ and entospletinib might also be a promising therapeutic strategy.
Our study has some limitations. First, our results are based solely on experiments using the NF-κB inhibitor DHMEQ. This is because DHMEQ is quite unique, as, unlike the plethora of other NF-κB inhibitors, its exact and selective mechanism of action is well documented,20 and it disrupts the final step of the NF-κB signaling pathway, ensuring that no cross regulation via other signaling pathways downstream of the targeted signaling step is possible. To our knowledge, no other NF-κB inhibitor meets these criteria. Second, the ibrutinib concentration used in the combination experiment was relatively high (10 μM), a concentration, however, which is not uncommon for in vitro experiments.47 In vivo, the maximum concentration which is obtained with a dose of 840mg ibrutinib daily does not exceed circa 0.48 μM (210ng/ml).52 However, it has been shown that, in vivo, the primary effect obtained with the aforementioned ibrutinib concentration is the blockage of cell proliferation, while apoptosis only occurs at higher doses.53 As our primary endpoint was CLL cell apoptosis, we deliberately chose this relatively high dose.
To conclude, considering that our results strongly indicate that NF-κB inhibition alone is not able to induce apoptosis in CLL with a supportive microenvironment, we suggest that DHMEQ should be tested in combination with R406, ibrutinib, the BAFF inhibitor belimumab, potentially, or BCL2 inhibitors in subsequent studies.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/1/136
References
- 1.Pekarsky Y, Zanesi N, Aqeilan RI, Croce CM. Animal models for chronic lymphocytic leukemia. J Cell Biochem. 2007; 100(5):1109–1118. [DOI] [PubMed] [Google Scholar]
- 2.Pekarsky Y, Zanesi N, Croce CM. Molecular basis of CLL. Semin Cancer Biol. 2010;20(6):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zanesi N, Balatti V, Bottoni A M, Croce C, Pekarsky Y. Novel insights in molecular mechanisms of CLL. Curr Pharm Des. 2012;18(23):3363–3372. [DOI] [PubMed] [Google Scholar]
- 4.Gaidano G, Foà R, Dalla-Favera R. Molecular pathogenesis of chronic lymphocytic leukemia. J Clin Invest. 2012; 122(10):3432–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karin M, Cao Y, Greten FR, Li Z-W. NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–310. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal BB. Nuclear factor-κB: the enemy within. Cancer Cell. 2004;6(3):203–208. [DOI] [PubMed] [Google Scholar]
- 7.Furman RR, Asgary Z, Mascarenhas JO, Liou H-C, Schattner EJ. Modulation of NF-κB activity and apoptosis in chronic lymphocytic leukemia B cells. J Immunol. 2000; 164(4):2200–2206. [DOI] [PubMed] [Google Scholar]
- 8.Romano MF, Lamberti A, Tassone P, et al. Triggering of CD40 antigen inhibits fludarabine-induced apoptosis in B chronic lymphocytic leukemia cells. Blood. 1998; 92(3):990–995. [PubMed] [Google Scholar]
- 9.Hertlein E, Byrd JC. Signalling to drug resistance in CLL. Best Pract Res Clin Haematol. 2010;23(1):121–131. [DOI] [PubMed] [Google Scholar]
- 10.Burger JA, Gribben JG. The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: Insight into disease biology and new targeted therapies. Semin Cancer Biol. 2014;24:71–81. [DOI] [PubMed] [Google Scholar]
- 11.ten Hacken E, Burger JA. Microenvironment interactions and B-cell receptor signaling in chronic lymphocytic leukemia: implications for disease pathogenesis and treatment. Biochim Biophys Acta BBA - Mol Cell Res. 2016;1863(3): 401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endo T, Nishio M, Enzler T, et al. BAFF and APRIL support chronic lymphocytic leukemia B-cell survival through activation of the canonical NF-κB pathway. Blood. 2007;109(2):703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewamana S, Lin TT, Rowntree C, et al. Rel A is an independent biomarker of clinical outcome in chronic lymphocytic leukemia. J Clin Oncol. 2009; 27(5):763–769. [DOI] [PubMed] [Google Scholar]
- 14.Steele AJ, Jones DT, Ganeshaguru K, et al. The sesquiterpene lactone parthenolide induces selective apoptosis of B-chronic lymphocytic leukemia cells in vitro. Leukemia. 2006;20(6):1073–1079. [DOI] [PubMed] [Google Scholar]
- 15.Kanduri M, Tobin G, Åleskog A, Nilsson K, Rosenquist R. The novel NF-κB inhibitor IMD-0354 induces apoptosis in chronic lymphocytic leukemia. Blood Cancer J. 2011;1(3):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickering BM, de Mel S, Lee M, et al. Pharmacological inhibitors of NF-κB accelerate apoptosis in chronic lymphocytic leukaemia cells. Oncogene. 2006; 26(8):1166–1177. [DOI] [PubMed] [Google Scholar]
- 17.López-Guerra M, Roué G, Pérez-Galán P, et al. p65 activity and ZAP-70 status predict the sensitivity of chronic lymphocytic leukemia cells to the selective IκB kinase inhibitor BMS-345541. Clin Cancer Res. 2009;15(8):2767–2776. [DOI] [PubMed] [Google Scholar]
- 18.Hewamana S, Alghazal S, Lin TT, et al. The NF-κB subunit Rel A is associated with in vitro survival and clinical disease progression in chronic lymphocytic leukemia and represents a promising therapeutic target. Blood. 2008;111(9):4681–4689. [DOI] [PubMed] [Google Scholar]
- 19.Horie R, Watanabe M, Okamura T, et al. DHMEQ, a new NF-κB inhibitor, induces apoptosis and enhances fludarabine effects on chronic lymphocytic leukemia cells. Leukemia. 2006;20(5):800–806. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, Horie R, Takeiri M, Kozawa I, Umezawa K. Inactivation of NF-κB components by covalent binding of (−)-dehydroxymethylepoxyquinomicin to specific cysteine residues. J Med Chem. 2008; 51(18):5780–5788. [DOI] [PubMed] [Google Scholar]
- 21.Ariga A, Namekawa J, Matsumoto N, Inoue J, Umezawa K. Inhibition of tumor necrosis factor-α-induced nuclear translocation and activation of NF-κB by dehydroxymethylepoxyquinomicin. J Biol Chem. 2002;277(27):24625–24630. [DOI] [PubMed] [Google Scholar]
- 22.Buchner M, Baer C, Prinz G, et al. Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood. 2010;115(22):4497–4506. [DOI] [PubMed] [Google Scholar]
- 23.Dunn IF, Sannikova TY, Geha RS, Tsitsikov EN. Identification and characterization of two CD40-inducible enhancers in the mouse TRAF1 gene locus. Mol Immunol. 2000;37(16):961–973. [DOI] [PubMed] [Google Scholar]
- 24.Carpentier I, Beyaert R. TRAF1 is a TNF inducible regulator of NF-κB activation. FEBS Lett. 1999;460(2):246–250. [DOI] [PubMed] [Google Scholar]
- 25.Wakamatsu K, Nanki T, Miyasaka N, Umezawa K, Kubota T. Effect of a small molecule inhibitor of nuclear factor-κB nuclear translocation in a murine model of arthritis and cultured human synovial cells. Arthritis Res Ther. 2005;7(6):R1348–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu H, Bian W, Xu Y. A novel NF-κB inhibitor, DHMEQ, ameliorates pristane-induced lupus in mice. Exp Ther Med. 2014;8(1):100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohsugi T, Horie R, Kumasaka T, et al. In vivo antitumor activity of the NF-κB inhibitor dehydroxymethylepoxyquinomicin in a mouse model of adult T-cell leukemia. Carcinogenesis. 2005; 26(8):1382–1388. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe M, Ohsugi T, Shoda M, et al. Dual targeting of transformed and untransformed HTLV-1-infected T cells by DHMEQ, a potent and selective inhibitor of NF-κB, as a strategy for chemoprevention and therapy of adult T-cell leukemia. Blood. 2005;106(7):2462–2471. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe M, Dewan MZ, Okamura T, et al. A novel NF-κB inhibitor DHMEQ selectively targets constitutive NF-κB activity and induces apoptosis of multiple myeloma cells in vitro and in vivo. Int J Cancer.;114(1):32–38. [DOI] [PubMed] [Google Scholar]
- 30.Dabaghmanesh N, Matsubara A, Miyake A, et al. Transient inhibition of NF-κB by DHMEQ induces cell death of primary effusion lymphoma without HHV-8 reactivation. Cancer Sci. 2009;100(4):737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1(4): a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8(1):49–62. [DOI] [PubMed] [Google Scholar]
- 33.Bren GD, Solan NJ, Miyoshi H, Pennington KN, Pobst LJ, Paya CV. Transcription of the RelB gene is regulated by NF-κB. Oncogene. 2001;20(53):7722–7733. [DOI] [PubMed] [Google Scholar]
- 34.Neumann M, Wohlleben G, Chuvpilo S, et al. CD40, but not lipopolysaccharide and anti-IgM stimulation of primary B lymphocytes, leads to a persistent nuclear accumulation of RelB. J Immunol Baltim Md 1950;157(11):4862–4869. [PubMed] [Google Scholar]
- 35.Ten RM, Paya CV, Israël N, et al. The characterization of the promoter of the gene encoding the p50 subunit of NF-κB indicates that it participates in its own regulation. EMBO J. 1992;11(1):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lombardi L, Ciana P, Cappellini C, et al. Structural and functional characterization of the promoter regions of the NFKB2 gene. Nucleic Acids Res. 1995;23(12):2328–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruefli-Brasse A, Reed JC. Therapeutics targeting Bcl-2 in hematological malignancies. Biochem J. 2017; 474(21):3643–3657. [DOI] [PubMed] [Google Scholar]
- 38.Gentile M, Petrungaro A, Uccello G, et al. Venetoclax for the treatment of chronic lymphocytic leukemia. Expert Opin Investig Drugs. 2017;26(11):1307–1316. [DOI] [PubMed] [Google Scholar]
- 39.Kipps TJ, Eradat H, Grosicki S, et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2015;56(10):2826–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lwin T, Crespo LA, Wu A, et al. Lymphoma cell adhesion-induced expression of B cell-activating factor of the TNF family in bone marrow stromal cells protects non-Hodgkin’s B lymphoma cells from apoptosis. Leukemia. 2008; 23(1):170–177. [DOI] [PubMed] [Google Scholar]
- 41.Kern C, Cornuel J-F, Billard C, et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004; 103(2):679–688. [DOI] [PubMed] [Google Scholar]
- 42.Morrison MD, Reiley W, Zhang M, Sun S-C. An atypical tumor necrosis factor (TNF) receptor-associated factor-binding motif of B cell-activating factor belonging to the TNF family (BAFF) receptor mediates induction of the noncanonical NF-κB signaling pathway. J Biol Chem. 2005; 280(11):10018–10024. [DOI] [PubMed] [Google Scholar]
- 43.Day ES, Cachero TG, Qian F, et al. Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA. Biochemistry (Mosc). 2005;44(6):1919–1931. [DOI] [PubMed] [Google Scholar]
- 44.Haiat S, Billard C, Quiney C, Ajchenbaum-Cymbalista F, Kolb J-P. Role of BAFF and APRIL in human B-cell chronic lymphocytic leukaemia. Immunology. 2006; 118(3):281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu L, Lin-Lee Y-C, Pham LV, Tamayo AT, Yoshimura LC, Ford RJ. BAFF-R promotes cell proliferation and survival through interaction with IKKβ and NF-κB/c-Rel in the nucleus of normal and neoplastic B-lymphoid cells. Blood. 2009; 113(19):4627–4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wild J, Schmiedel BJ, Maurer A, et al. Neutralization of (Nκ-cell-derived) B-cell activating factor by Belimumab restores sensitivity of chronic lymphoid leukemia cells to direct and rituximab-induced NK lysis. Leukemia. 2015;29(8):1676–1683. [DOI] [PubMed] [Google Scholar]
- 47.Herman SEM, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011; 117(23):6287–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ponader S, Chen S-S, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119(5):1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herman SEM, Mustafa RZ, Jones J, Wong DH, Farooqui M, Wiestner A. Treatment with ibrutinib inhibits BTK- and VLA-4-dependent adhesion of chronic lymphocytic leukemia cells in vivo. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21(20): 4642–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchner M, Fuchs S, Prinz G, et al. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res. 2009;69(13):5424–5432. [DOI] [PubMed] [Google Scholar]
- 51.Sharman J, Hawkins M, Kolibaba K, et al. An open-label phase 2 trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2015;125(15):2336–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng S, Ma J, Guo A, et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia. 2014;28(3):649–657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.