The third-generation tyrosine kinase inhibitor (TKI) ponatinib exerts a strong anti-neoplastic effect in all stages of chronic myeloid leukemia (CML) as well as in Philadelphia(Ph)-positive acute lymphoblastic leukemia (ALL). It is capable of suppressing the kinase activity of BCR-ABL1 carrying any single mutation in the tyrosine kinase domain (TKD), including the gate-keeper mutation T315I.1 Nevertheless, resistance to ponatinib can evolve in sub-clones carrying BCR-ABL1 variants with two or more mutations on the same allele, if the IC50 values for this TKI exceed the maximum achievable effective plasma levels.2,3 These so-called compound mutations (CMs) represent a powerful mechanism of resistance to all currently available TKIs.2 Our data indicate that individual CMs confer high resistance to ponatinib, thus precluding clinical use of this particular TKI. However, several CMs only display low to intermediate resistance to ponatinib, suggesting that successful suppression of the mutant subclones is feasible, provided that the appropriate dosing regimen is applied.
The occurrence of CMs in CML has been principally linked to sequential treatment with different TKIs,3 and is far more frequently observed in Ph-positive ALL.4 Although CMs have been suggested not to confer primary/secondary resistance to ponatinib in the chronic phase of CML,5 they still pose a major problem in advanced stages of CML and Ph-positive ALL.2 The identification of their responsiveness to ponatinib is therefore of paramount importance for subsequent clinical management. Therapy with ponatinib may, however, be associated with serious side effects,6 and reports on apparently dose-dependent severe adverse events provided the basis for reduction of the recommended daily dose from 45 mg to 30 or even 15 mg.7 These modifications in the dosing regimen imply that the achievable levels of effective average plasma concentrations (efCave) of ponatinib decrease from 28 nM to 23 and 10 nM, respectively.8 In this regard, stringent analysis of the in vitro responses of CMs to ponatinib could permit assessment of the required effective dose in the clinical setting. This notion is supported by exemplary courses of three patients displaying the CM F317L/E459K who were treated within the Ponatinib Ph-positive ALL and CML Evaluation (PACE) trial, a phase 2 clinical study with ponatinib in heavily pretreated patients with resistant or intolerant Ph-positive leukemia. Two patients who had received 42 and 45 mg daily (efCave ~26 and 28 nM) achieved durable major molecular response (MMR) in 168 and 87 days, respectively, whereas the other patient with this CM, who had received an average daily dose of 32 mg (corresponding to an efCave of ~24 nM), needed 583 days to reach MMR, and was withdrawn from the study 82 days after having achieved this.5 The latter observation is in line with an inadequately low dose of ponatinib, but it is necessary to consider the possibility that the slow response may have been attributable to a mutation-independent mechanism.
To assess the anticipated responses to ponatinib based on in vitro testing, we introduced a panel of 28 BCR-ABL1 CMs as well as various single mutations as controls into Ba/F3 cells (Table 1). This cellular background was selected because the majority of published heat maps indicating the TKI responses of individual BCR-ABL1 mutations had been established in this model.1,2,9 Our recently published observations indicate that the in vitro data on TKI resistance of some mutations presented in earlier reports may be overestimated.10 This is likely attributable to the testing of cells inadvertently carrying multiple insertions of mutant BCR-ABL1 cassettes in the genome.10 We have therefore established a transposon-based approach to rapid and efficient transfection of mutant BCR-ABL1 constructs into cells, and implemented a flow cytometry-assisted selection method facilitating targeted enrichment of cells carrying single gene construct insertions in the genome. This protocol was demonstrated to provide BCR-ABL1 expression levels similar to those observed in patient specimens and to permit unbiased testing of TKI sensitivity in vitro.10
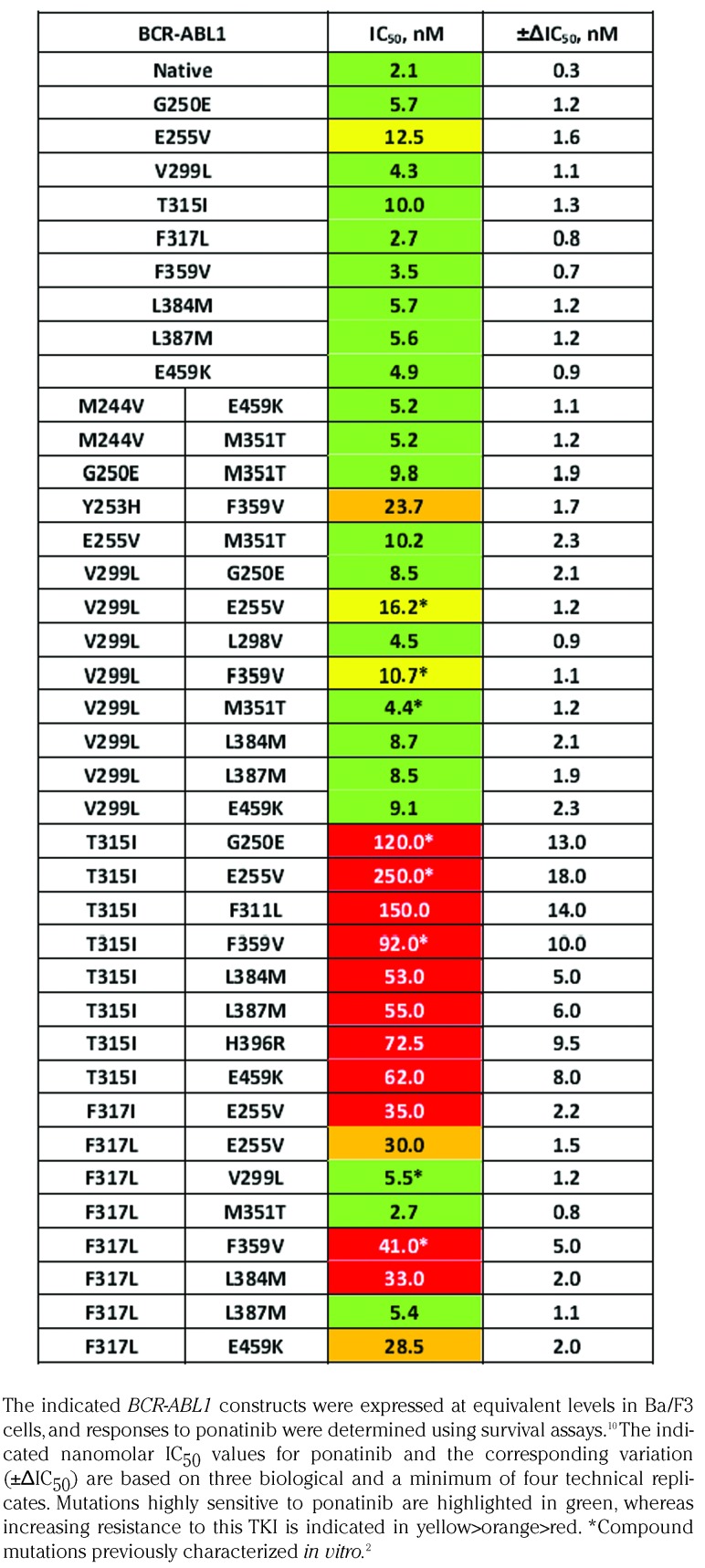
Table 1.
In vitro responses of native and mutant BCR-ABL1 to ponatinib.
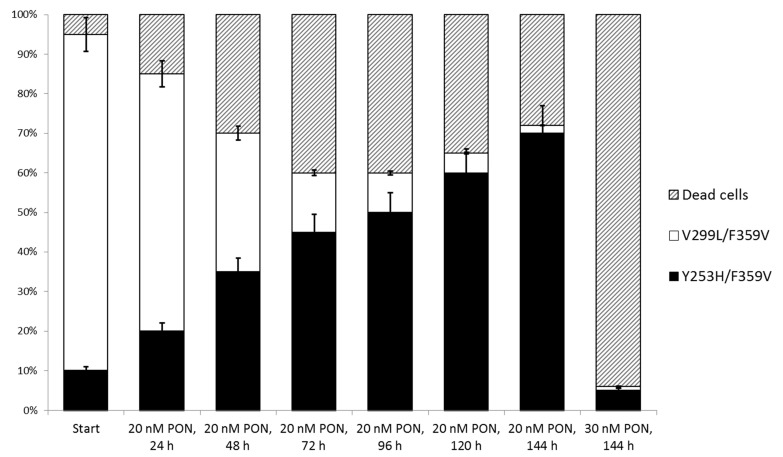
The IC50 values of ponatinib were determined by employing a widely used in vitro proliferation assay.10 Most CMs involving sites with no previous evidence for implication in resistance to ponatinib displayed IC50 values below 10 nM. This efCave is readily achievable with the 15 mg daily dose of ponatinib,8 thus suggesting high sensitivity to treatment with this TKI. This finding supports the notion that CMs do not necessarily confer resistance to ponatinib, unless specific sites including both T315 and F317 in particular are affected.5 CMs revealing elevated resistance to ponatinib in vitro almost invariably included mutations at these two sites. In fact, the only CM constellation within the tested panel bearing neither T315I nor F317L and displaying reduced sensitivity to ponatinib was Y253H/F359V, with an IC50 value of 23.7 ± 1.7 nM (Table 1). This in vitro assessment is supported by a clinical observation made within the PACE trial: a patient with CML had presented with a V299L/F359V prior to therapy with ponatinib, a constellation displaying an IC50 for this TKI in the range of 10 nM (Table 1). However, the patient showed signs of progressive disease under an average daily ponatinib dose of 26 mg, corresponding to an efCave of ~20 nM.2 Mutational analysis at this time revealed the Y253H/F359V which displays an IC50 value for ponatinib beyond the efCave apparently achieved in this patient. It is conceivable, therefore, that the actual dose of ponatinib may not have been sufficient to suppress the kinase activity of BCR-ABL1Y253H/F359V, thus resulting in disease progression. Competitive in vitro co-culture experiments have demonstrated that Ba/F3 cells expressing BCR-ABL1Y253H/F359V have a proliferative advantage over Ba/F3 carrying BCR-ABL1V299L/F359V in the presence of 20 nM ponatinib, whereas a significant decrease in survival of both cell lines is observed at a 30 nM concentration of ponatinib (Figure 1). This example highlights the potential relevance of selecting the appropriate dose of ponatinib by considering the IC50 of mutations identified prior to the initiation of treatment or during therapy. It is necessary to consider, however, that the prevalence of mutant subclones is elevated in advanced disease stages, and higher doses of ponatinib may be generally required in this instance in order to prevent clonal selection and expansion, even in the absence of mutations amenable to detection by currently available approaches.
Figure 1.
Competitive co-culture of BaF3 cells expressing BCR-ABL1Y253H/F359V and BCR-ABL1V299L/F359V. BaF3 cells expressing BCR-ABL1Y253H/F359V (black columns) and BCR-ABL1V299L/F359V (white columns) were mixed at a 1:9 ratio and incubated in the presence of 20 or 30 nM ponatinib. Clonal evolution was monitored by fluorescence of the co-expressed fluorescent proteins ZsGreen for BCR-ABL1Y253H/F359V and TdTomato for BCR-ABL1V299L/F359V. The results were confirmed by Sanger sequencing of the BCR-ABL1 TKD using gDNA and cDNA isolated and prepared at the indicated time points. The proportion of dead cells was calculated using Annexin/PI staining (striped columns). The indicated error bars are based on two biological and three technical replicates.
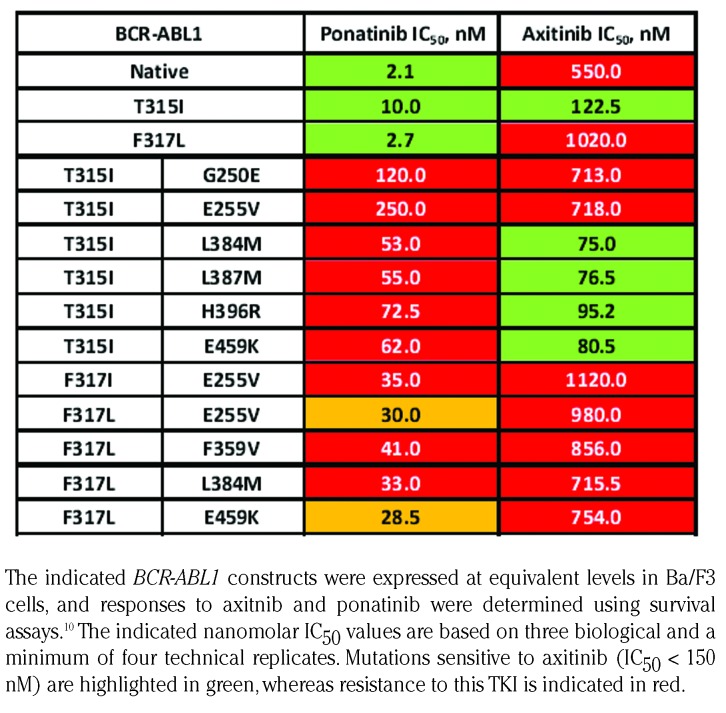
In agreement with earlier reports, CMs involving the T315I mutation displayed the highest IC50 values for ponatinib, clearly exceeding the clinically achievable plasma concentration of the drug.2,11 These mutational constellations included combinations of T315I with the P-loop mutations G250E and E255V as well as T315I/F359V, which arise during treatment with ponatinib, as has been described earlier.2,3 Our analysis of these CMs also indicated high levels of resistance to ponatinib, but the IC50 values determined were lower than reported previously,2 conceivably due to the technical reasons as outlined above. In addition to constellations already shown to confer resistance to ponatinib, we identified CMs involving T315I and residues at sites displaying very distinct functional properties, which also showed IC50 values for ponatinib clearly above the efCave of 28 nM. The additional sites affected included the south lobe mutation E459K and the A-loop mutations L384M, L387M and H396R. Similar to A-loop mutations, it has been suggested that the E459K mutation destabilizes the inactive conformation of the ABL1 TKD.12 This functional property may play a critical role, as ponatinib is a type II TKI which binds primarily to the inactive conformation of the BCR-ABL1 TKD13 and as such requires considerably higher concentrations of the drug in order to inhibit the kinase activity. The CM T315I/L384M was suggested and confirmed in vitro to confer resistance to ponatinib at the clinically achievable plasma levels, but this constellation has not been reported to date in patients with Ph-positive leukemia. By contrast, the compound mutations T315I/L387M, T315I/H396R and T315I/E459K have been observed in the clinical setting,2 but susceptibility to ponatinib has only been characterized by in vitro analysis for T315I/H396R.2 Intriguingly, the CM T315I/H396R has shown sensitivity to axitinib,14 a Food and Drug Administration (FDA)-approved inhibitor of the vascular endothelial growth factor receptor (VEGFR), which also displays activity against the BCR-ABL1T315I mutant.14,15 Likewise, we demonstrated that other CMs combining T315I with A-loop mutations, including T315I/L384M, T315I/L387M, and T315I/E459K, respond to axitinib with IC50 values <100 nM (Table 2), suggesting clinical sensitivity to this drug.14 However, axitinib at clinically achievable concentrations was not effective against ponatinib-resistant CMs affecting the F317 position (Table 2).
Table 2.
In vitro responses of selected BCR-ABL1 CMs to ponatinib and axitinib.
Therapy with ponatinib has been associated with considerable cardiovascular toxicity and other potentially serious side effects which appear to be dose-related.6,7 However, as highlighted by the data presented, current strategies that aim at decreasing the dose of ponatinib should carefully consider the presence and type of mutations in the BCR-ABL1 TKD in order to enable effective treatment. It would therefore be highly desirable to implement testing of the effective plasma drug concentrations and monitor the kinetics of mutant subclones covering, in addition, compound mutations16 in routine diagnostic surveillance so as to provide a basis for optimized clinical management of patients treated with ponatinib. Nevertheless, despite all the evidence regarding the important role of mutations in limiting the efficacy of TKI treatment, other mechanisms of resistance which remain undetermined by current experimental and diagnostic evidence may operate in mutant BCR-ABL1 cells, and can affect clinical responses to therapy.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
Funding: this work was supported by the Austrian Science Fund (FWF), SFB Grants F4705-B20 (TL) and F4704-B20 (PV). The authors declare no conflict of interests.
References
- 1.O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16(5):401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zabriskie MS, Eide CA, Tantravahi SK, et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell. 2014;26(3):428–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah NP, Skaggs BJ, Branford S, et al. Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency. J Clin Invest. 2007;117(9):2562–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pritchard JR, Schmitt MW, Hodgson JG, et al. Analysis of the subclonal origins of compound mutations in patients with refractory Ph+ malignancies treated with ponatinib. Blood. 2016;128(22):1061. [Google Scholar]
- 5.Deininger MW, Hodgson JG, Shah NP, et al. Compound mutations in BCR-ABL1 are not major drivers of primary or secondary resistance to ponatinib in CP-CML patients. Blood. 2016;127(6):703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldemeyer L, Dugan M, Edwards J, Akard L. Long-term side effects of tyrosine kinase inhibitors in chronic myeloid leukemia. Curr Hematol Malig Rep. 2016;11(2):71–79. [DOI] [PubMed] [Google Scholar]
- 7.Cortes JE, Kim D-W, Pinilla-Ibarz J, et al. Long-term follow-up of ponatinib efficacy and safety in the phase 2 PACE trial. Blood. 2014; 124(21):3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012; 367(22):2075–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gozgit JM, Schrock A, Chen T-H, Clackson T, Rivera VM. Comprehensive analysis of the in vitro potency of ponatinib, and all other approved BCR-ABL tyrosine kinase inhibitors (TKIs), against a panel of single and compound BCR-ABL mutants. Blood. 2013; 122(21):3992. [Google Scholar]
- 10.Byrgazov K, Lucini CB, Berkowitsch B, et al. Transposon-mediated generation of BCR-ABL1-expressing transgenic cell lines for unbiased sensitivity testing of tyrosine kinase inhibitors. Oncotarget. 2016; 7(47):78083–78094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khorashad JS, Kelley TW, Szankasi P, et al. BCR-ABL1 compound mutations in tyrosine kinase inhibitor-resistant CML: frequency and clonal relationships. Blood. 2013;121(3):489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D, Kim DW, Cho BS, et al. Structural modeling of V299L and E459K Bcr-Abl mutation, and sequential therapy of tyrosine kinase inhibitors for the compound mutations. Leuk Res. 2009;33(9):1260–1265. [DOI] [PubMed] [Google Scholar]
- 13.Zhou T, Commodore L, Huang WS, et al. Structural mechanism of the Pan-BCR-ABL inhibitor ponatinib (AP24534): lessons for overcoming kinase inhibitor resistance. Chem Biol Drug Des. 2011; 77(1):1–11. [DOI] [PubMed] [Google Scholar]
- 14.Zabriskie MS, Eide CA, Yan D, et al. Extreme mutational selectivity of axitinib limits its potential use as a targeted therapeutic for BCR-ABL1-positive leukemia. Leukemia. 2016;30(6):1418–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pemovska T, Johnson E, Kontro M, et al. Axitinib effectively inhibits BCR-ABL1(T315I) with a distinct binding conformation. Nature. 2015;519(7541):102–105. [DOI] [PubMed] [Google Scholar]
- 16.Kastner R, Zopf A, Preuner S, et al. Rapid identification of compound mutations in patients with Philadelphia-positive leukaemias by long-range next generation sequencing. Eur J Cancer. 2014;50(4):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.