Abstract
Background
Most patients with ALK- or ROS1-rearranged non-small cell lung cancer (NSCLC) are sensitive to tyrosine kinase inhibitor (TKI) therapy, but resistance invariably develops, commonly within the central nervous system (CNS). This study aimed to determine the safety, efficacy, and pharmacokinetic properties of lorlatinib, a novel, highly potent, selective, and brain-penetrant ALK/ROS1 TKI with preclinical activity against most known resistance mutations, in patients with advanced ALK- or ROS1-positive NSCLC.
Methods
In this ongoing, multicenter phase 1 study, eligible patients had advanced ALK- or ROS1-positive NSCLC. Lorlatinib was orally administered at doses ranging from 10–200 mg once daily or 35–100 mg twice daily. For some patients, tumor biopsy was performed before lorlatinib treatment to identify ALK resistance mutations. Safety was evaluated in patients who received ≥1 treatment; efficacy was evaluated in the intention-to-treat population (patients who received ≥1 dose of study treatment and were positive for either ALK or ROS1 rearrangement). The primary endpoint was dose-limiting toxicities (DLTs) during cycle 1; secondary endpoints included safety, pharmacokinetics, and overall response rate (ORR). This study is registered with ClinicalTrials.gov, NCT01970865.
Findings
Fifty-four patients were treated, including 41 with ALK-positive and 12 with ROS1-positive NSCLC. Twenty-eight patients had received ≥2 TKIs, and 39 patients had CNS metastases. The most common treatment-related adverse events among the 54 patients were hypercholesterolemia (39 [72%] of 54 patients), hypertriglyceridemia (21 [39%] of 54 patients), peripheral neuropathy (21 [39%] of 54 patients), and peripheral edema (21 [39%] of 54 patients). One DLT occurred at 200 mg (failure to deliver at least 16 of 21 prescribed total daily doses in cycle 1 because of toxicities attributable to study drug, in this case grade 2 neurocognitive adverse events comprising slowed speech and mentation and word-finding difficulty). No maximum tolerated dose was identified. The recommended phase 2 dose was selected to be 100 mg daily. Among ALK-positive patients, the ORR was 19 (46%) of 41 patients (95% CI, 31–63%); among those who had received ≥2 TKIs, the ORR was 11 (42%) of 26 patients (95% CI, 23–63%). Among ROS1-positive patients, including seven crizotinib-pretreated patients, ORR was 6 (50%) of 12 patients (95% CI, 21–79%). Responses were observed in the CNS and in patients with tumors harboring resistance mutations such as ALK G1202R.
Interpretation
In this phase 1, dose-escalation study, lorlatinib demonstrated both systemic and intracranial activity in patients with advanced ALK- or ROS1-positive NSCLC, most of whom had CNS metastases and had failed ≥2 TKIs. Therefore, lorlatinib may represent an effective therapeutic strategy for patients who have become resistant to currently available TKIs, including second-generation ALK TKIs in ALK-positive NSCLC.
Funding
Pfizer
INTRODUCTION
Chromosomal rearrangements of anaplastic lymphoma kinase (ALK) or ROS1 genes define two distinct molecular subsets of non-small cell lung cancer (NSCLC).1–3 In both cases, gene rearrangements lead to expression of constitutively activated fusion kinases that function as potent oncogenic drivers. As ALK and ROS1 are related tyrosine kinases, a number of small-molecule tyrosine kinase inhibitors (TKIs) effectively target both ALK and ROS1. These TKIs have dramatically improved outcomes for patients with advanced ALK- or ROS1-positive NSCLC, yet these cancers remain incurable.
Crizotinib, a multitargeted ALK/ROS1/MET TKI, is currently the standard first-line therapy for patients with advanced NSCLC harboring ALK- or ROS1-rearrangements.4,5 However, the majority of ALK- and ROS1-positive patients relapse on crizotinib within a few years due to the development of resistance. Several major classes of resistance mechanisms have been identified, including on-target mechanisms (eg, secondary mutations within the kinase domain) and off-target mechanisms (eg, activation of bypass signaling pathways).6
In the case of ALK-positive NSCLC, a number of more potent, second-generation ALK TKIs have been developed to overcome crizotinib resistance, including three US Food and Drug Administration (FDA)-approved second-generation ALK TKIs: ceritinib, alectinib, and brigatinib.7–9 These TKIs have been associated with a response rate of approximately 40–50% and median progression-free survival (PFS) of 7–12 months in crizotinib-resistant patients.10–12 In the crizotinib-naïve setting, these TKIs are even more efficacious. For example, in ASCEND-4, the median PFS with ceritinib in patients with newly diagnosed ALK-positive NSCLC was 16·6 months,13 while in the global ALEX study, the median PFS with alectinib in the first-line setting, as assessed by independent review, was 25·6 months.14 However, as with crizotinib, acquired resistance to second-generation ALK TKIs, given any line, inevitably develops and is mediated by on- and off-target mechanisms. Among patients relapsing on the current standard treatment of sequential first- and second-generation ALK TKIs, resistant tumors have been shown to harbor a significantly higher incidence of on-target mutations, particularly the solvent front mutation ALK G1202R. This amino acid substitution lies at the solvent-exposed region of ALK where the bulkier, charged side chain is thought to cause steric interference with the binding of most ALK TKIs.15
In the case of ROS1-positive NSCLC, the most common mechanism of resistance to crizotinib is ROS1 G2032R, analogous to ALK G1202R.16,17 Second-generation ALK TKIs with ROS1 activity (eg, ceritinib, brigatinib, and entrectinib) are ineffective against ROS1 G2032R.18 There are currently no next-generation ROS1 TKIs with established efficacy after crizotinib failure, and treatment options for crizotinib-resistant ROS1-positive patients are limited.
Lorlatinib (PF-06463922, Pfizer Oncology, Groton, CT, USA) is a novel, oral, reversible, ATP-competitive macrocyclic TKI of ALK and ROS1. This potent and highly selective third-generation inhibitor was designed to penetrate the blood–brain barrier and to overcome known ALK resistance mutations.19 In cell-line models, lorlatinib has low nanomolar potency against “wild-type” ALK and retains potency against ALK-resistant mutants, including ALK G1202R.20 Lorlatinib has also demonstrated antitumor activity across a variety of different subcutaneous xenograft tumor models of ALK-positive NSCLC and in intracranial ALK-positive tumor models. In addition, lorlatinib potently inhibits ROS1 and retains activity against ROS1 G2032R in vitro and in vivo.21 Taken together, these preclinical studies suggest that lorlatinib may represent an effective therapeutic strategy for ALK- and ROS1-positive patients who have relapsed on currently available TKIs.
Here, we report results from an ongoing, first-in-human phase 1 study of lorlatinib (NCT01970865) to determine its safety, maximum tolerated dose (MTD), and antitumor activity in patients with advanced ALK- or ROS1-positive NSCLC.
METHODS
Study Design and Participants
Eligible patients had locally advanced or metastatic NSCLC with ALK or ROS1 rearrangements. ALK positivity was established using a local FDA-approved test (ALK break-apart fluorescence in situ hybridization or immunohistochemistry). ROS1 positivity was determined locally using fluorescence in situ hybridization, reverse transcriptase–polymerase chain reaction, or next-generation sequencing (NGS). All patients were required to have an archival tissue sample collected before enrollment; de novo tumor biopsy at screening was optional. Other key eligibility criteria included age ≥18 years, Eastern Cooperative Oncology Group performance status of 0–1, and adequate end-organ function. Patients were required to have adequate bone marrow (absolute neutrophil count ≥1.5 × 109/L, platelets ≥100 × 109/L, and hemoglobin ≥9 g/dL) and adequate pancreatic (serum amylase [pancreatic isoenzyme] ≤1.5 × upper limit of normal [ULN] and serum lipase ≤1.5 × ULN), renal (serum creatinine ≤1.5 × ULN or estimated creatinine clearance ≥60 mL/min as calculated using the method standard for the institution), and liver (total serum bilirubin ≤1.5 × ULN, and aspartate aminotransferase and alanine aminotransferase ≤2.5 × ULN [≤5.0 × ULN in the event of liver metastases] function for study eligibility. Patients who had received prior ALK/ROS1 TKIs or had asymptomatic untreated or treated central nervous system (CNS) metastases were eligible.
Patients with spinal cord compression; active and clinically significant bacterial, fungal, or viral infection (including hepatitis B, hepatitis C, human immunodeficiency virus, acquired immunodeficiency syndrome [AIDS], or AIDS-related illness); clinically significant cardiovascular disease; predisposing characteristics for acute pancreatitis; history of extensive, disseminated, bilateral, or presence of grade 3–4 interstitial fibrosis or interstitial lung disease; severe acute or chronic psychiatric conditions; acute malignancy (other than NSCLC, non-melanoma skin cancer, in situ cervical cancer, papillary thyroid cancer, ductal carcinoma in situ of the breast, or localized and presumed cured prostate cancer) within the last 3 years; active inflammatory gastrointestinal disease, chronic diarrhea, symptomatic diverticular disease, previous gastric resection or lap band; or abnormal left ventricular ejection fraction (LVEF) were not eligible for inclusion. Patients with major surgery within 4 weeks of study entry, radiation therapy within 2 weeks of study entry (except palliative to relieve bone pain, which must have been completed at least 48 hours before study entry), systemic anticancer therapy completed within a minimum of five half-lives of study entry, prior therapy with an antibody or drug specifically targeting T-cell costimulation or immune checkpoint pathways, previous high-dose chemotherapy requiring stem cell rescue, or prior irradiation to >25% of the bone marrow were not eligible for study entry.
The protocol was approved by the institutional review board or independent ethics committee at each site and complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. All patients provided written informed consent before participation. Enrollment and study sites are shown on p 2 of the appendix. A starting lorlatinib dose of 10 mg once daily (QD), administered orally, was selected based on preclinical toxicology studies. We planned to evaluate lorlatinib at escalating doses of 10, 25, 50, 75, 100, 150, 200, 250, 300, and 400 mg QD. Dose escalation was guided by a modified continual reassessment method with a minimum of three patients per dose level and a target dose-limiting toxicity (DLT) rate ≤0·33 (see appendix p 3 for the definition of DLT). The MTD was defined as the dose of study drug that would be closest to but not higher than a 33% probability of a DLT. The protocol allowed for evaluation of different dose schedules (eg, twice-daily [BID] dosing) based on emerging safety and exposure data; thus, we also evaluated BID doses of 35, 75, and 100 mg.
Cycles were 21 days long. Treatment continued until investigator-assessed disease progression or clinical deterioration, unacceptable toxicity, withdrawal of consent, or death. Patients were allowed to continue treatment beyond progression at the investigator’s discretion. Intrapatient dose escalation was permitted if: 1) cycle 1 had been completed without any DLT; 2) the patient’s maximum drug-related toxicity during prior cycles was grade ≤2; and 3) three patients at the next higher dose level had completed cycle 1 without any DLT. Radiographic assessments (computed tomography [CT], magnetic resonance imaging [MRI], or equivalent) were conducted at screening, on cycle 3, day 1 (C3D1), and every 6 weeks until cycle 25, after which scans were conducted every 12 weeks and at the end of treatment.
Dose holds and dose reductions were permitted per investigator discretion and recommended in the event of specific treatment-related hematologic and nonhematologic toxicities, which included pancreatitis, pneumonitis, QTc prolongation, lipid elevations, CNS effects, and LVEF dysfunction. Patients who did not recover from treatment-related toxicity within 42 days were discontinued. Once a dose was reduced for a given patient, all subsequent doses were administered at that dose level, unless further dose reduction was required. Dose reescalation was allowed per investigator discretion for appropriate patient management.
Pharmacokinetic (PK) samples were collected for lorlatinib after single- and multiple-dose administration for all dosing cohorts evaluated in the study. The lorlatinib PK parameters were calculated using nominal collection times and quality-controlled, non–quality-assured bioanalytical data and estimated using noncompartmental analysis (Phoenix WinNonlin version 6·3; Pharsight Corporation, Mountain View, CA, USA).
Procedures
Hematologic and lipid assessments were conducted at screening, C1D1, C1D15, day 1 of each subsequent cycle, and end of treatment. Coagulation and urinalysis assessments took place at screening, C1D1, and end of treatment. Pregnancy tests were conducted at screening, day –7 (lead-in PK analysis), C1D1, day 1 of each subsequent cycle, and end of treatment. Twelve-lead electrocardiograms were conducted at screening, day –7 (lead-in PK analysis), C1D1, C1D8, C1D15, day 1 of each subsequent cycle, and end of treatment. LVEF assessments (echocardiogram or multigated acquisition scans) were conducted at screening, before dosing at C2D12, before dosing at C3D1, before dosing at C5D1, every two cycles thereafter until approximately 18 months, and then every 4 cycles thereafter. Starting at cycle 25, all laboratory testing occurred every other cycle.
Safety evaluations were performed at baseline, every week for the first cycle, and every 3 weeks thereafter. Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v4·03. DLTs were evaluated during the first cycle. All patients underwent baseline tumor imaging, including CT scans of the chest, abdomen, and pelvis, and brain imaging by MRI. Restaging scans were obtained at 6-week intervals and judged by investigators according to Response Evaluation Criteria in Solid Tumors (RECIST) v1·1.22 For patients with brain metastases, intracranial response was assessed using modified RECIST v1·123; no specific criteria were established for determining disease progression following TKI-treatment, or surgery/radiotherapy for patients with CNS disease.
ALK mutation status was assessed in formalin-fixed, paraffin-embedded tumor tissue samples by using either local sequencing platforms (eg, Massachusetts General Hospital SNaPshot NGS24 or FoundationOne®, Foundation Medicine, Cambridge, MA, USA) or a central customized NGS assay on the Ion Torrent PGM platform at MolecularMD Corporation (Portland, OR, USA). All NGS assays were validated in accordance with the Clinical Laboratory Improvement Amendments.
Outcomes
The primary objective was to assess the safety and tolerability of lorlatinib at increasing doses to determine the MTD and select the recommended phase 2 dose (RP2D) in the safety population. Key secondary objectives were to evaluate overall and intracranial antitumor activity, characterize the PK profile of lorlatinib, and analyze tumor and blood-based molecular markers of drug sensitivity in the intention-to-treat population (ITT).
The primary endpoint was DLTs occurring in cycle 1 as assessed by the investigator. Secondary endpoints were AEs, laboratory abnormalities, LVEF, vital signs (heart rate and blood pressure), total Mini-Mental State Examination score, PK parameters, patient-reported outcomes and global quality of life, QTc interval, disease control rate at 12 weeks by RECIST v1·1, objective tumor response, PFS, overall survival at 12 and 18 months, duration of response (DOR), time to tumor response, response to prior systemic therapies, and molecular profiling. All endpoints not presented here are still being evaluated and will be reported in future publications.
Statistical Analysis
As a dose-finding study, no hypothesis was pre-established and no power calculation was done; due to the dynamic nature of dose escalation schema, the sample size was not defined prior to study initiation.
Safety data were summarized for all patients who received at least one dose of lorlatinib. Efficacy data are reported for the ITT population, which was initially defined as patients who had received at least one dose of lorlatinib; following the inclusion of one patient with unconfirmed ALK/ROS1 status, the definition was amended to include that patients were also positive for ALK or ROS1 gene rearrangements; all amendments to the statistical analysis plan are included on pp 501–504 of the appendix. Intracranial efficacy was determined for patients in the ITT population with brain metastases. The antitumor activity of lorlatinib was summarized in terms of objective response rate (ORR), intracranial ORR, DOR, and PFS. The Kaplan-Meier method was used to estimate DOR and PFS. The data cutoff date was August 15, 2016. All analyses were performed using SAS version 9·2 (SAS Institute, Inc., Cary, NC, USA) with the exception of the unpaired t test for DOR, which was performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA). This study is registered with ClinicalTrials.gov, number NCT01970865.
Role of the Funding Source
This study was designed jointly by investigators and representatives of Pfizer. Pfizer collected and analyzed the data. Molecular analysis of tumor specimens was conducted in Dr Alice Shaw’s laboratory and funded by an RO1 grant from the National Institutes of Health. The first author wrote the first draft of the manuscript, had full access to all the data, and had final responsibility for the decision to submit the manuscript. All authors (ATS, EF, TMB, BB, AN, SP-V, JFG, MJ, JD, LPJ, JSC, JC, J-FM, AA, and BJS), including Pfizer representatives, had access to the raw data, were involved in data analysis, interpretation, and manuscript preparation, made the decision to publish the manuscript, and vouch for the completeness and accuracy of the data and analyses.
RESULTS
Patients
A total of 55 patients at five study sites were enrolled between 22 January 2014 and 10 July 2015, and 54 patients were treated; one patient was omitted from the safety and efficacy analyses because they did not receive ≥1 dose of study treatment (Table 1). Among patients with documented ALK/ROS1 positivity, 41 (77%) of 53 patients had ALK-positive NSCLC, while 12 (23%) had ROS1-positive NSCLC; one patient had an unconfirmed ALK/ROS1 status (ALK FISH analysis was negative with 6% split signals; ROS1 testing was not performed) and was omitted from the ITT population. One ALK-positive and five ROS1-positive patients were ALK/ROS1 TKI-naïve. Twenty (38%) and 27 (51%) of 53 patients had received one or at least two prior ALK/ROS1 TKIs, respectively (see appendix p 4). Brain metastases were present at baseline in 39 (72%) of 54 patients in the safety population, 12 of whom had received no prior brain radiation therapy. The patient with unconfirmed ALK/ROS1 status received two cycles of lorlatinib (35 mg BID) before progressing at the first tumor assessment, after which the patient received crizotinib and nivolumab before dying due to the disease under study.
Table 1.
Patient baseline characteristics
| Characteristic | Lorlatinib (N=54) |
|---|---|
| Age, years | |
| Mean | 51·9 |
| Standard deviation | 12·8 |
| Sex | |
| Male | 22 (41) |
| Female | 32 (59) |
| Race* | |
| White | 42 (78) |
| Asian | 7 (13) |
| Other | 5 (9) |
| Histology | |
| Adenocarcinoma | 51 (94) |
| Other | 3 (6) |
| ECOG performance status† | |
| 0 | 20 (38) |
| 1 | 31 (59) |
| >1 | 2 (4) |
| Brain metastases | |
| Present | 39 (72) |
| Absent | 15 (28) |
| ALK/ROS1 status | |
| ALK-positive | 41 (76) |
| ROS1-positive | 12 (22) |
| Unconfirmed‡ | 1 (2) |
| Prior ALK/ROS1 TKI | |
| 0 | 6 (11) |
| 1 | 20 (37) |
| ≥2 | 28 (52) |
Data are n (%) unless stated otherwise.
Race was determined by the investigators.
An ECOG performance status of 0 denotes full activity and increasing numbers denote increasing impairment in daily living abilities. One patient was excluded from the ECOG analysis because of unconfirmed ALK/ROS1 status at screening. Baseline ECOG performance status was determined on day 1 before dosing.
This patient was considered negative for ALK rearrangement (only 6% split signals) and was not included in the efficacy analysis.
ECOG=Eastern Cooperative Oncology Group; TKI=tyrosine kinase inhibitor.
Adverse Events
Patients were initially treated at doses ranging from 10 to 200 mg QD (appendix p 11). One DLT occurred during cycle 1 in a patient with brain metastases receiving 200 mg QD who failed to receive 16 of the 21 planned lorlatinib doses because of grade 2 CNS effects (slowed speech and mentation and word-finding difficulty). These side effects resolved 48 hours after discontinuation of lorlatinib. After lower QD dose cohorts were expanded and safety was assessed at those dose levels, enrollment into exploratory BID dosing cohorts began. All three patients in the 35-mg BID cohort tolerated the study drug but discontinued at the time of first tumor assessment because of disease progression. Both the 75- and 100-mg BID doses were less well tolerated, with all four patients in the 100-mg BID cohort requiring dose reduction. Overall, no DLTs were observed in these cohorts, and an MTD was not established. Based on the safety profile seen across all doses, the expected plasma coverage over the lorlatinib concentrations predicted to inhibit ALK G1202R, and ease of administration, the RP2D was selected to be 100 mg QD.
Table 2 summarizes treatment-related AEs of any grade occurring in ≥10% of treated patients, while p 5 of the appendix shows treatment-related AEs occurring in ≥10% of patients treated at the RP2D of 100 mg daily. The most common AEs among the 54 patients were hypercholesterolemia (39 [72%] of 54 patients), hypertriglyceridemia (21[39%] of 54 patients), peripheral neuropathy (21[39%] of 54 patients), peripheral edema (21 [39%] of 54 patients), cognitive effects (13 [24%] of 54 patients), speech effects (10 [19%] of 54 patients), increased weight (9 [17%] of 54 patients), mood effects (8 [15%] of 54 patients), and fatigue (8 [15%] of 54 patients). Cognitive, speech, and mood effects were generally transient and reversible. Gastrointestinal side effects were uncommon and predominantly grade 1. There were no cases of interstitial lung disease or pneumonitis. Eighteen (33%) and 13 (24%) of 54 patients, respectively, temporarily discontinued lorlatinib or required at least one dose reduction because of treatment-related AEs. In the RP2D (100 mg QD) cohort, no patients required a dose reduction, and the most common reasons for treatment discontinuation at the RP2D (100 mg) were hypercholesterolemia (n=2/17) and increased lipase (n=2/17). No patients permanently discontinued lorlatinib because of treatment-related AEs, and there were no treatment-related deaths; a listing of all causes of death is available on p 6 of the appendix.
Table 2.
Treatment-related adverse events reported in ≥10% of patients (N=54)
| Adverse events, n (%) | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | Total* |
|---|---|---|---|---|---|
| Hypercholesterolemia† | 32 (59) | 5 (9) | 2 (4) | 0 | 39 (72) |
| Hypertriglyceridemia†‡ | 18 (33) | 3 (6) | 0 | 0 | 21 (39) |
| Peripheral edema | 21 (39) | 0 | 0 | 0 | 21 (39) |
| Peripheral neuropathy† | 21 (39) | 0 | 0 | 0 | 21 (39) |
| Cognitive effects† | 12 (22) | 1 (2) | 0 | 0 | 13 (24) |
| Speech effects† | 10 (19) | 0 | 0 | 0 | 10 (19) |
| Lipase increasedठ| 7 (13) | 2 (4) | 0 | 0 | 9 (17) |
| Weight increased | 6 (11) | 3 (6) | 0 | 0 | 9 (17) |
| Fatigue | 8 (15) | 0 | 0 | 0 | 8 (15) |
| Mood effects† | 8 (15) | 0 | 0 | 0 | 8 (15) |
| Amylase increasedठ| 7 (13) | 0 | 0 | 0 | 7 (13) |
| AST increased | 6 (11) | 1 (2) | 0 | 0 | 7 (13) |
| Constipation | 7 (13) | 0 | 0 | 0 | 7 (13) |
| Tinnitus | 7 (13) | 0 | 0 | 0 | 7 (13) |
| Vision disorder† | 7 (13) | 0 | 0 | 0 | 7 (13) |
| Edema | 6 (11) | 0 | 0 | 0 | 6 (11) |
| Nausea | 6 (11) | 0 | 0 | 0 | 6 (11) |
No grade 5 treatment-related adverse events were reported.
Clustered term comprising adverse events that represent similar clinical symptoms/syndromes.
There was no direct correlation between the occurrence of hypertriglyceridemia (20/54 patients, 37%) and elevations in lipase (7/54, 13%) or amylase (6/54, 11%) when considering AE data alone. Two patients with increased amylase and lipase also had hypertriglyceridemia; two other patients with increased lipase had hypertriglyceridemia.
No cases of pancreatitis were observed.
AST=aspartate transaminase.
Pharmacokinetics
Lorlatinib was rapidly absorbed with peak plasma concentrations occurring 1–2 hours after dosing (appendix p 7). Lorlatinib plasma concentrations showed a biexponential decline with a terminal elimination half-life of 19·0–28·8 hours. Among most patients treated at the RP2D, lorlatinib exposure exceeded the predicted effective concentrations required for inhibition of wild-type and mutant ALK, including ALK G1202R (appendix p 12). Pilot evaluation of the effect of food on the 100-mg single dose indicated minimal changes in maximum plasma concentration or area under the concentration–time curve in the fed versus fasted state.
Four patients underwent lumbar puncture for cerebrospinal fluid (CSF) sampling. Three patients were receiving lorlatinib at 100 mg QD at the time of lumbar puncture; a fourth patient had their dose reduced to 75 mg QD and had held study drug for 8 days before lumbar puncture. Blood samples for PK analysis were collected at approximately the same time as the CSF samples. As shown on page 8 of the appendix, the mean (standard deviation) ratio of CSF/plasma concentrations was 0·75 (0·16). Thus, the mean CSF concentrations of lorlatinib correspond to 75% of unbound plasma concentrations.
Efficacy
Systemic Response
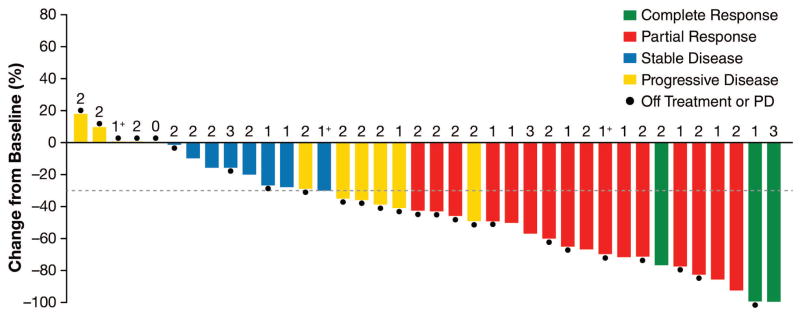
Of patients with ALK-positive NSCLC treated with lorlatinib, three (7%) of 41 patients achieved a confirmed complete response, 16 (39%) of 41 patients achieved a confirmed partial response, and eight (20%) of 41 patients had stable disease (Figure 1A, appendix p 9). The ORR was 19 (46%) of 41 patients (95% confidence interval [CI], 31–63%). Eleven (27%) of 41 patients had progressive disease on first restaging scans. Of the 19 patients with confirmed responses, eight had received one prior ALK TKI, while 11 had received two or more distinct ALK TKIs. The ORR was 8 (57%) of 14 patients (95% CI, 29–82%) among patients who had received one prior ALK TKI and 11 (42%) of 26 patients (95% CI, 23–63%) among those who had received two or more ALK TKIs. With the exception of the 10-mg QD and 35-mg BID dose cohorts, confirmed responses were observed at all doses and schedules. Responses to lorlatinib were frequently rapid, with a median time to first response of 1·3 months (range, 1·2–5·6). At data cutoff, eight of the 19 responses (42%) were still ongoing. The estimated median DOR was 12·4 months (95% CI, 6·5–not reached [NR]) among responding patients and 11·7 months (95% CI, 2·7–NR) among those previously treated with two or more ALK TKIs. Median duration of treatment among all ALK-positive patients was 9·3 months (95% CI, 6·8–NR; appendix p 13).
Figure 1. Tumor responses to lorlatinib in ALK-positive NSCLC.
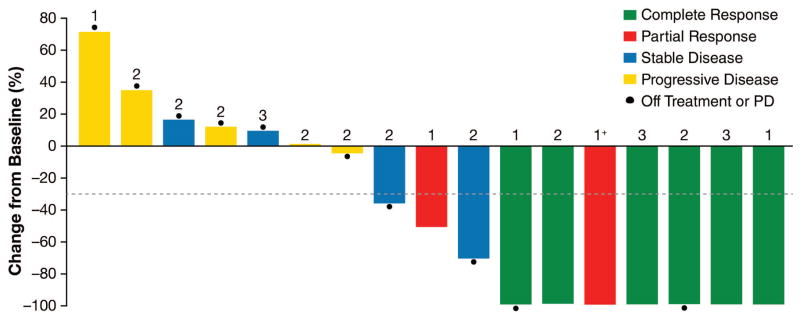
(A) Best confirmed tumor responses of 41 ALK-positive patients treated with lorlatinib across all dose levels based on investigator assessment. The bars indicate best percent change in target tumor burden from baseline. The number above each bar indicates the number of different ALK TKIs each patient received prior to lorlatinib; 1+ indicates that the patient received one prior ALK TKI and that TKI was a second-generation TKI (not crizotinib). Filled circles indicate ongoing treatment. Data for three patients are not included: two with objective progression in whom not all target lesions were assessed and one who was not assessed on treatment. (B) Best intracranial tumor response of ALK-positive patients who had evaluable central nervous system metastases at baseline according to investigator assessment. The bars indicate best percent change in intracranial lesions from baseline. The numbers are as described above. Two patients who were not evaluable on treatment were not included. ALK=anaplastic lymphoma kinase; NSCLC=non-small cell lung cancer; TKI=tyrosine kinase inhibitor.
Among patients with ROS1-positive NSCLC, six (50%) of 12 patients (95% CI, 21–79%) achieved a confirmed partial response (appendix pp 9 and 14). Two patients had received prior crizotinib, while the remaining four patients were crizotinib-naïve but had received platinum doublet chemotherapy. Similar to the ALK cohort, median time to first response was 1·4 months (range, 1·2–2·7) and median DOR was 12·0 months (95% CI, 5·7–NR). Median duration of treatment among all ROS1-positive patients was 16·6 months (95% CI, 2·7–23·3).
Intracranial Response
Twenty-four patients had measurable CNS target lesions at baseline according to modified RECIST v1·1,21 19 with ALK-positive NSCLC and five with ROS1-positive NSCLC. At the time of data cutoff, seven (29%) of 24 patients had a confirmed complete response and four (17%) of 24 patients had a confirmed partial response, for an intracranial ORR of 11 (46%) of 24 patients (95% CI, 26–67%). Of the 19 ALK-positive patients, intracranial ORR was eight (42%) of 19 patients (95% CI, 20–67%) (Figure 1B). Among ROS1-positive patients, three (60%) of five patients had intracranial responses, including two who had failed prior crizotinib.
Of the 32 ALK-positive patients with measurable or nonmeasurable CNS disease at baseline, ten had a confirmed complete or partial intracranial response. Five of the ten responding patients had failed two or more different ALK TKIs (including a second-generation TKI), four had failed crizotinib only, and one had failed ceritinib only. In addition, four of the ten responding patients had never received radiation therapy to the brain. These findings suggest that lorlatinib has CNS activity independent of prior brain radiotherapy and in patients previously treated with second-generation TKIs.
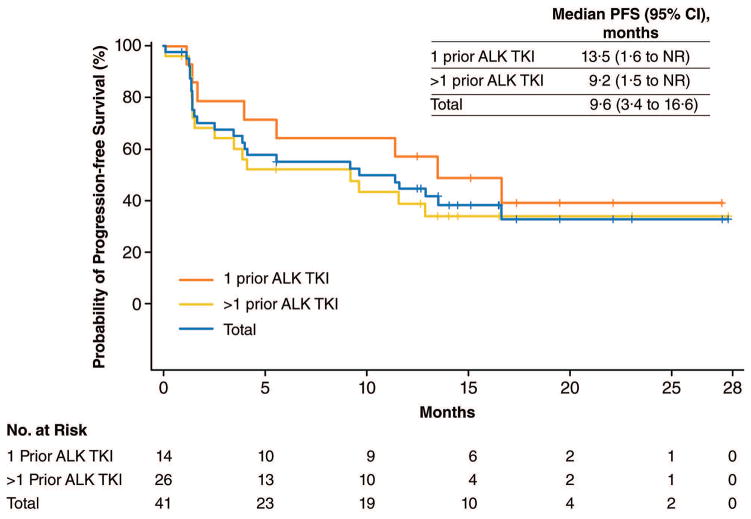
Progression-free survival
Median follow-up of all 53 ALK- and ROS1-positive patients was 17·4 months (95% CI, 16·1–22·1; interquartile range, 15·6–23·2). The estimated median PFS among these patients was 9·6 months (95% CI, 3·9–13·5), with 19 (36%) of 53 patients censored. Of the 41 ALK-positive patients, the estimated median PFS was identical at 9·6 months (95% CI, 3·4–16·6; Figure 2). Median PFS was 13·5 months (95% CI, 1·6–NR) for the 14 ALK-positive patients treated with one prior ALK TKI, while median PFS was 9·2 months (95% CI, 1·5–NR) for the 26 patients treated with two or more distinct ALK TKIs. Among the 12 patients with ROS1-positive NSCLC, including seven who had previously received crizotinib, median PFS was 7·0 months (95% CI, 1·4–13·9).
Figure 2. Progression-free survival of patients with ALK-positive NSCLC.
Shown are Kaplan-Meier estimates of progression-free survival in patients with advanced, ALK-positive NSCLC treated with lorlatinib. Among all 41 ALK-positive patients, median progression-free survival was 9·6 months (blue). In the subset of 14 patients who had received one prior ALK TKI, median progression-free survival was 13·5 months (orange). In the subset of 26 patients who had received two or more ALK TKIs (including at least one second-generation TKI), median progression-free survival was 9·2 months (yellow). One ALK-positive patient who had received no prior ALK TKIs is included in the total but is not shown separately. Vertical lines on the survival curves indicate censoring of data. ALK=anaplastic lymphoma kinase; NSCLC=non-small cell lung cancer; TKI=tyrosine kinase inhibitor.
Molecular Determinants of Response in ALK-positive NSCLC
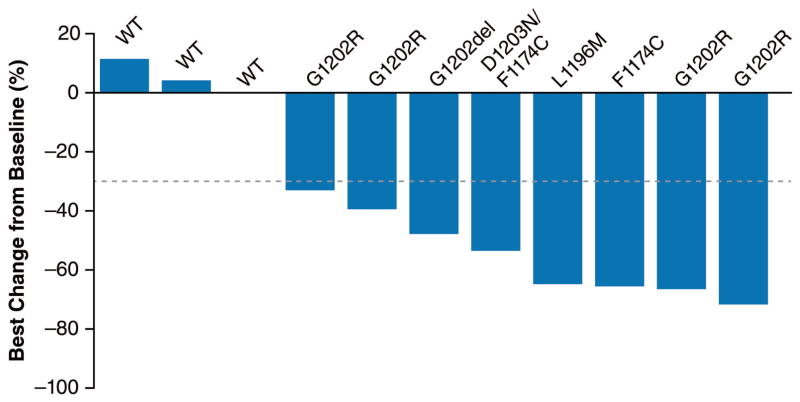
Previous studies have demonstrated that most crizotinib-resistant tumors remain sensitive to second-generation ALK TKIs, including those lacking a secondary ALK resistance mutation.10 However, in preclinical studies, tumors that have become resistant to second-generation ALK TKIs may or may not be sensitive to further ALK inhibition, depending on whether an ALK resistance mutation is present.13 To determine whether ALK mutation status correlates with lorlatinib response in patients who failed a second-generation ALK inhibitor, we conducted an additional retrospective analysis of 12 patients who had failed at least one second-generation ALK TKI and who had undergone a biopsy on the last TKI received. Eight of the 12 samples were found to harbor known resistance mutations in the ALK kinase domain. In the remaining four cases, no genetic mutation in ALK was detected; while other genetic alterations were identified (appendix p 10), the molecular basis of resistance in these four cases remains to be determined.
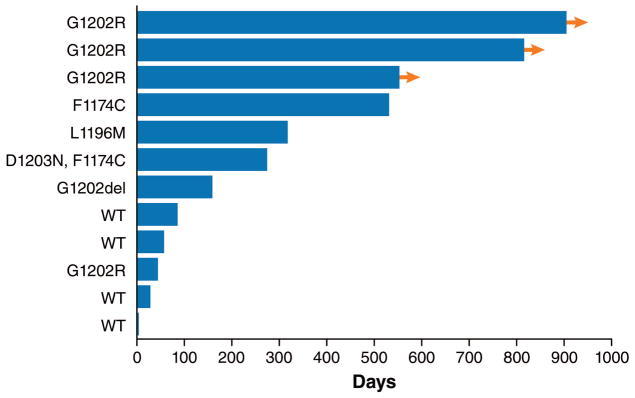
The best systemic tumor responses in this cohort of ALK-positive patients are summarized in Figure 3A. Tumor regression was observed among all patients with lung cancers harboring ALK mutations, including five tumors with ALK G1202R or G1202del. In patients without detectable ALK resistance mutations, none demonstrated tumor regression. Consistent with this data, the duration of treatment with lorlatinib was longer among patients with ALK resistance mutations compared to those without (mean 448 vs 42 days, respectively, p=0·027 by unpaired t test; Figure 3B). These findings suggest that the activity of lorlatinib may be higher in second-generation ALK TKI-treated tumors that have acquired on-target ALK resistance mutations, compared to those tumors without an ALK mutation.
Figure 3. Response to lorlatinib and correlation with ALK resistance mutations in patients treated with ≥2 ALK TKIs.
(A) Waterfall plot of 12 ALK-positive patients who had received ≥2 prior ALK TKIs and who underwent repeat biopsy before study enrollment. These biopsies may have been collected as de novo samples for the study or may have been collected outside of this study. All biopsies were taken from extracranial sites of disease. For ten samples from Massachusetts General Hospital (MGH), sequencing was performed using local sequencing platforms and these data were stored, summarized, and validated by MGH; for the remaining two samples, next-generation sequencing was performed in a central laboratory and additionally summarized and validated by MGH (see Methods). For each patient, the ALK resistance mutation is shown above the bar corresponding to the patient’s best percent change in systemic (ie, extracranial) target lesions according to Response Evaluation Criteria in Solid Tumors v1·1. WT (wildtype) indicates that no ALK mutation was identified in the resistant specimen. One patient whose resistant tumor showed no ALK mutation on biopsy was not evaluable because of rapid progression and is not shown here. (B) The duration of treatment in days for the same group of ALK-positive patients. ALK mutation status is indicated to the left of each bar. Arrows indicate patients who were still receiving lorlatinib at the time of data cutoff. ALK=anaplastic lymphoma kinase; TKI=tyrosine kinase inhibitor.
DISCUSSION
In this first-in-human, dose-escalation study, lorlatinib, a highly potent, selective, and brain-penetrant third-generation ALK/ROS1 inhibitor, demonstrated antitumor activity in patients with previously treated ALK- or ROS1-positive NSCLC.
At present, standard treatment of advanced ALK-positive NSCLC consists of first-line crizotinib,5 followed by a more potent, second-generation ALK TKI, such as ceritinib, alectinib, or brigatinib.10–12 Previous work has shown that most crizotinib-resistant tumors remain ALK dependent10 and that more potent inhibition of the target is sufficient to overcome crizotinib resistance. In this study, lorlatinib, which is more potent than second-generation ALK TKIs in biochemical and cellular assays,15,21 was highly active in patients previously treated with first- and second-generation ALK TKIs, with a confirmed response rate of 11 (42%) of 26 patients and a median DOR of 11·7 months. Thus, even after failure of a second-generation ALK TKI, a significant proportion of ALK-positive patients remain ALK-dependent and derive benefit from more potent ALK inhibition.
Recent studies have identified multiple different mechanisms of resistance to second-generation ALK TKIs.15,25 In contrast to crizotinib-resistant tumors in which 20–25% acquire secondary ALK mutations, about 50% of tumors that have become resistant to second-generation TKIs have secondary ALK mutations, with ALK G1202R predominating. Preclinical studies have demonstrated that lorlatinib retains activity against all single ALK resistance mutants.21 In this study, we observed tumor regressions in all eight patients with documented ALK resistance mutations in pre-lorlatinib tumor specimens, including four patients with ALK G1202R, suggesting that these tumors remained ALK dependent. In contrast, we observed no tumor regression in four patients whose tumors did not harbor detectable ALK resistance mutations. This finding is distinctly different than what has been observed in the setting of crizotinib resistance and suggests that, after failure of a second-generation TKI, some tumors may lose ALK dependency. A limitation in our study is the small number of patients who underwent de novo biopsies prior to lorlatinib. Larger studies are needed to determine whether ALK resistance mutations detected at the time of relapse on a second-generation inhibitor can serve as a molecular predictor of response to lorlatinib.
The CNS is a common and often refractory site of relapse for patients with ALK-positive NSCLC.26 Lorlatinib was originally designed to have pan-inhibitory activity against ALK as well as maximal penetration into the CNS.19 The latter was achieved in part by minimizing p-glycoprotein 1-mediated efflux, which can lead to poor blood–brain barrier penetration.27 Consistent with preclinical studies, lorlatinib was highly active in the CNS, inducing intracranial responses in eight (42%) of 19 ALK-positive patients with baseline measurable CNS disease, over one-half of whom had failed two or more prior ALK TKIs. Lorlatinib also induced intracranial responses in three (60%) of five ROS1-positive patients with baseline measurable CNS disease, two of whom had failed prior crizotinib. Based on PK analyses of paired blood and CSF samples in four patients, we found that the average ratio of CSF/plasma (unbound) concentrations of lorlatinib was 0·75, far exceeding the 0·03 ratio reported with crizotinib.28 Overall, these results suggest that lorlatinib can effectively cross the blood–brain barrier to induce significant and durable CNS responses. As it is not possible to completely exclude the potential confounding effects of prior radiotherapy for brain lesions, this represents a possible limitation of this study. Future studies of lorlatinib with larger sample sizes may be able to more definitively address this question.
Lorlatinib was generally well tolerated in this study, with predominantly grade 1 or 2 AEs. No patients treated at the RP2D required dose reductions. Several side effects seen with lorlatinib have been reported with other ALK TKIs, including peripheral edema, weight gain, and constipation. However, some side effects appear to be unique to lorlatinib. First, hypercholesterolemia and hypertriglyceridemia were the most common AEs. The mechanism by which lorlatinib causes dysregulation of lipid metabolism is unknown, but these AEs were readily managed with pharmacologic therapy (eg, statins and fibrates). Second, about one-quarter of patients treated with lorlatinib reported mild neurocognitive side effects, including difficulty multitasking, slowing of speech, and short-term memory deficits. Eight (15%) of 54 patients also reported emotional lability. Both the neurocognitive and mood side effects were reversible with dose interruption and improved or resolved with dose reduction. As alectinib, which is also highly brain penetrant, has not been reported to cause similar neurologic side effects,29,30 it seems unlikely that inhibition of ALK by lorlatinib is mediating these side effects. Whether inhibition of ROS1 or a different kinase could account for the CNS effects of lorlatinib remains to be determined.
In conclusion, this study refines the treatment paradigm for patients with advanced ALK-positive NSCLC and highlights the potential utility of developing increasingly potent and pan-inhibitory TKIs to overcome drug resistance in oncogene-driven cancers. In patients for whom crizotinib is no longer effective, more potent target inhibition can induce durable responses in most cases. After failure of a second-generation ALK TKI, lorlatinib is effective in almost one-half of cases, likely corresponding to those tumors with on-target resistance mechanisms and continued ALK dependency. While patients can derive significant benefit from sequential ALK TKIs, the optimal sequencing of ALK-targeted agents remains to be determined. Two recent studies suggest that upfront treatment with a second-generation ALK TKI may be superior to sequential first- and second-generation TKIs.13,31 Based on its efficacy in the resistant setting, lorlatinib is currently in phase 3 testing to determine whether upfront treatment with a third-generation, pan-inhibitory TKI can further improve clinical outcomes for patients with advanced ALK-positive NSCLC.
Supplementary Material
Acknowledgments
We thank the participating patients and their families, as well as the investigators, subinvestigators, research nurses, study coordinators, and operations staff. We would also like to thank Karen Klamerus for her support of the study. This study was supported by Pfizer Inc., by grants from the National Cancer Institute (5R01CA164273 to Dr. Shaw) and the National Foundation for Cancer Research (to Dr. Shaw), by Be a Piece of the Solution, and by LungStrong. Meghan Sullivan of inScience Communications, Springer Healthcare, provided editorial support funded by Pfizer, Inc.
Footnotes
Contributions
ATS, EF, TMB, BB, LPJ, JSC, JC, J-FM, AA, and BJS contributed towards the study conception and design. ATS, EF, TMB, BB, JFG, and BJS contributed towards patient recruitment. All authors contributed towards data analysis and interpretation; JFG assisted with pharmacokinetic and midazolam assessment, JD contributed neurological assessments. All authors contributed to the drafting of the manuscript and approved the final version for submission.
Declaration of Interests
ATS has received fees for consulting/advisory board roles from Ariad, Blueprint Medicines, Daiichi Sankyo, EMD Serono, Genentech/Roche, Ignyta, KSQ, Loxo, Novartis, Pfizer, and Taiho, honoraria from Foundation Medicine, Novartis, Pfizer, and Genentech/Roche, and her institution has received research funding from Pfizer, Novartis, and Genentech/Roche. EF has received fees for consulting or advisory roles from Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Pfizer, and Genentech/Roche, and fees for serving on speaker bureaus from AstraZeneca, Bristol-Myers Squibb, and Novartis. TMB’s institution has received research funding from AbbVie, AstraZeneca, Calithera Biosciences, Daiichi Sankyo, Deciphera, Eli Lilly, Genentech/Roche, GlaxoSmithKline, Ignyta, ImmunoGen, Incyte, Kolltan Pharmaceuticals, Leap Therapeutics, MabVax, MedImmune, Medpacto Inc., Merck, Merrimack, Millennium, Mirati Therapeutics, Novartis, Peleton, Pfizer, Principia Biopharma, and Stemline Therapeutics. BB has received research funding from Pfizer. JFG has received personal fees from Boehringer Ingelheim, Bristol-Myers Squibb, Clovis, Genentech/Roche, Incyte, Loxo, Merck, Novartis, and Theravance, and travel expenses from Affymetrix. MJ reports that her institution has received research funding from AbbVie, Adaptimmune, Apexigen, Array BioPharma, AstraZeneca, BerGenBio, Checkpoint Therapeutics, Eli Lilly, EMD Serono, Genentech/Roche, Genmab, Janssen, Kadmon, Mirati Therapeutics, Merrimack, Novartis, OncoMed, Pfizer, Regeneron, Stemcentrix, and Tarveda, and fees for consulting/advisory board roles from Boehringer Ingelheim, Celgene, and Genentech/Roche. AA, JC, J-FM, and LPJ are employees of and own stock in Pfizer. JSC is an employee of InVentiv Health and works as a contractor for Pfizer. BJS has received fees for serving on advisory boards for and honoraria from AstraZeneca, Bristol-Myers Squibb, Genentech/Roche, Merck, Novartis, and Pfizer, and his institution has received clinical trial support from Pfizer. No other potential conflict of interest relevant to this article was reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bergethon K, Shaw AT, Ignatius Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–70. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–71. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 6.Lovly CM, Shaw AT. Molecular pathways: resistance to kinase inhibitors and implications for therapeutic strategies. Clin Cancer Res. 2014;20:2249–56. doi: 10.1158/1078-0432.CCR-13-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang WS, Liu S, Zou D, et al. Discovery of brigatinib (AP26113), a phosphine oxide-containing, potent, orally active inhibitor of anaplastic lymphoma kinase. J Med Chem. 2016;59:4948–64. doi: 10.1021/acs.jmedchem.6b00306. [DOI] [PubMed] [Google Scholar]
- 8.Marsilje TH, Pei W, Chen B, et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulf onyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem. 2013;56:5675–90. doi: 10.1021/jm400402q. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19:679–90. doi: 10.1016/j.ccr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–97. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017 May 5; doi: 10.1200/JCO.2016.71.5904. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2016;34:661–8. doi: 10.1200/jco.2015.63.9443. [DOI] [PubMed] [Google Scholar]
- 13.Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–29. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 14.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017 Jun 6; doi: 10.1056/NEJMoa1704795. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–33. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awad MM, Katayama R, McTigue M, et al. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med. 2013;368:2395–401. doi: 10.1056/NEJMoa1215530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gainor JF, Friboulet L, Yoda S, et al. Frequency and spectrum of ROS1 resistance mutations in ROS1-positive lung cancer patients progressing on crizotinib. J Clin Oncol Precision Oncol. 2017 in press. [Google Scholar]
- 18.Katayama R, Kobayashi Y, Friboulet L, et al. Cabozantinib overcomes crizotinib resistance in ROS1 fusion-positive cancer. Clin Cancer Res. 2015;21:166–74. doi: 10.1158/1078-0432.CCR-14-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(m etheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57:4720–44. doi: 10.1021/jm500261q. [DOI] [PubMed] [Google Scholar]
- 20.Zou HY, Li Q, Engstrom LD, et al. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc Natl Acad Sci U S A. 2015;112:3493–8. doi: 10.1073/pnas.1420785112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell. 2015;28:70–81. doi: 10.1016/j.ccell.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–95. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20:1479–84. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 25.Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4:662–73. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881–8. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schinkel AH. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev. 1999;36:179–94. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- 28.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29:e443–5. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 29.Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15:1119–28. doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 30.Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013;14:590–8. doi: 10.1016/S1470-2045(13)70142-6. [DOI] [PubMed] [Google Scholar]
- 31.Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.