Abstract
Acute pancreatitis is a complex disorder involving both premature intracellular protease activation and inflammatory cell invasion. An initiating event is the intracellular activation of trypsinogen by cathepsin B (CTSB), which can be induced directly via G protein–coupled receptors on acinar cells or through inflammatory cells. Here, we studied CTSB regulation by another lysosomal hydrolase, cathepsin D (CTSD), using mice with a complete (CTSD−/−) or pancreas-specific conditional CTSD knockout (KO) (CTSDf/f/p48Cre/+). We induced acute pancreatitis by repeated caerulein injections and isolated acinar and bone marrow cells for ex vivo studies. Supramaximal caerulein stimulation induced subcellular redistribution of CTSD from the lysosomal to the zymogen-containing subcellular compartment of acinar cells and activation of CTSD, CTSB, and trypsinogen. Of note, the CTSD KO greatly reduced CTSB and trypsinogen activation in acinar cells, and CTSD directly activated CTSB but not trypsinogen in vitro. During pancreatitis in pancreas-specific CTSDf/f/p48Cre/+ animals, markers of severity were reduced only at 1 h, whereas in the complete KO, this effect also included the late disease phase (8 h), indicating an important effect of extra-acinar CTSD on course of the disease. CTSD−/− leukocytes exhibited reduced cytokine release after lipopolysaccharide (LPS) stimulation, and CTSD KO also reduced caspase-3 activation and apoptosis in acinar cells stimulated with the intestinal hormone cholecystokinin. In summary, CTSD is expressed in pancreatic acinar and inflammatory cells, undergoes subcellular redistribution and activation during experimental pancreatitis, and regulates disease severity by potently activating CTSB. Its impact is only minimal and transient in the early, acinar cell–dependent phase of pancreatitis and much greater in the later, inflammatory cell–dependent phase of the disease.
Keywords: cathepsin B (CTSB), cell biology, enzyme processing, pancreas, protease, acute pancreatitis, cathepsin D (CTSD)
Introduction
Acute pancreatitis is a complex disorder and its pathogenesis involves both premature intracellular protease activation (1–3) as well as the invasion of inflammatory cells (3, 4). Under physiological conditions digestive proteases are secreted as inactive precursor zymogens and activated only in the duodenum by the brush border enzyme enteropeptidase (PRSS7, also known as enterokinase). Trypsin is believed to play a critical role in acute pancreatitis as, once activated, it can activate multiple other proteases in a cascade-like fashion.
Trypsinogen itself is activated via limited proteolysis by the lysosomal enzyme cathepsin B (CTSB)3 or by autoactivation. The role of CTSB in the activation of trypsinogen has been confirmed for in vitro, ex vivo, and in vivo settings (1, 5, 6).
Cathepsin D (CTSD) is a lysosomal aspartic protease that is almost ubiquitously expressed (7). Like other cathepsins it is synthesized as an inactive prepro-form that, after conversion to the proenzyme in the endolysosomal compartment, is processed to its mature form. Active CTSD exists in a two-chain form consisting of a disulfide bridge–linked amino-terminal light chain (14 kDa) and a carboxy-terminal heavy chain (34 kDa) (7, 8). Unlike CTSB or cathepsin L (CTSL), cathepsin D is not a secretory protein under physiological conditions (9). CTSD is involved in multiple cellular functions such as protein degradation and cell death, and has been linked to the development of cancer and neurodegenerative disorders (10–12). Recessively inherited homozygous deficiency of CTSD in humans is causing the lethal early-onset neuronal ceroid lipofuscinosis type 10, which is recapitulated by the constitutive gene deletion in mice (13).
In terms of interactions between cysteine and aspartic proteases, CTSB and CTSL have been reported to be involved in the processing of CTSD (14–16). In view of the prominent role of CTSB and CTSL in regulating trypsinogen activation and disease severity in experimental pancreatitis we have here investigated the role of CTSD. To this end we used an experimental model for acute pancreatitis in two different genetically engineered mouse strains with either a complete knockout (CTSD−/−) or a pancreas-specific knockout (CTSDf/f/p48Cre/+). Our data indicate that CTSD is a potent activator of CTSB, mediates its effect on the severity of pancreatitis through activation of CTSB, and does so largely via its effects on inflammatory cells.
Results
CTSD expression in the pancreas and intracellular activation upon supramaximal cholecystokinin (CCK) stimulation in isolated acini
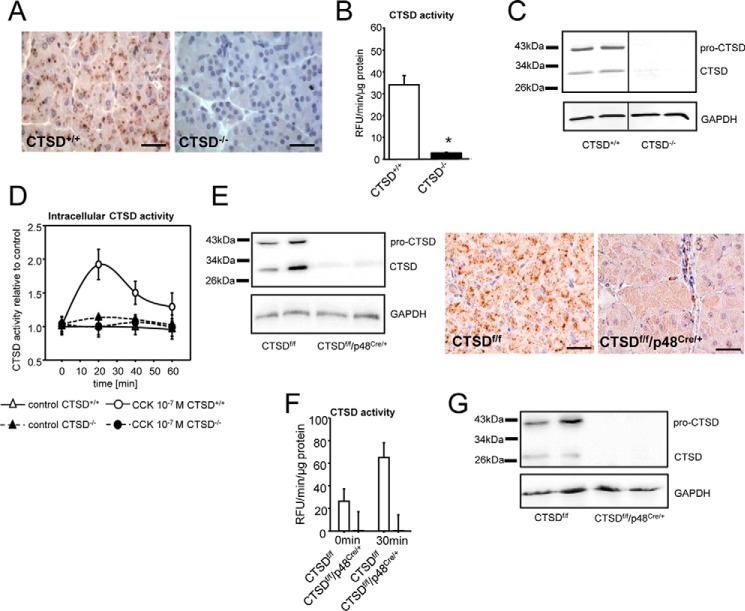
Immunohistochemistry of C57BL/6 pancreatic tissues showed CTSD localized in the basolateral part of acini whereas CTSD−/− pancreata displayed no CTSD expression (Fig. 1A). Measurements of enzymatic activities in acinar cell homogenates using a specific fluorogenic CTSD substrate confirmed the presence of CTSD in acinar cells (Fig. 1B). The CTSD antibody detected two distinct bands with a size of about 30 kDa and 43 kDa, corresponding to mature (∼ 33 kDa) and pro-CTSD (∼ 46 kDa) whereas knockout tissues showed no CTSD expression (Fig. 1C). In preliminary experiments we tested the antibody for detection of purified recombinant CTSD enzyme (human CTSD from spleen) that showed the same band at 30 kDa. Upon supramaximal stimulation of isolated acini with cholecystokinin, an ex vivo model of acute pancreatitis, intracellular CTSD activity increased rapidly with a maximum at 20 min and a decline thereafter. No CTSD activity was observed in CTSD-deficient acini (CTSD−/−). Unstimulated acinar cells showed no relevant changes in intracellular CTSD activity during ex vivo culturing (Fig. 1D).
Figure 1.
A–C, cathepsin D is expressed in pancreatic tissue but is absent in CTSD−/− mice (complete knockout) shown by immunohistochemistry (A), enzyme activity in acini (B), and Western blot from pancreas homogenates. C, the vertical line indicates gel splice. D, CTSD is intracellularly activated in isolated acinar cells upon supramaximal CCK stimulation but not in knockout animals. E, pancreas homogenates of CTSDf/f/p48Cre/+ mice show only weak expression of CTSD, and positive CTSD staining is detected only outside of acinar cells. F, absence of CTSD activity in acini of CTSDf/f/p48Cre/+ mice (conditional knockout). G, lack of CTSD expression in acinar cells of CTSDf/f/p48Cre/+ mice shown by Western blot. *, p < 0.05. Data points show mean ± S.E. of five or more experiments in each group and at each time point. Asterisks indicate differences significant at p < 0.05. Scale bar denotes 50 μm.
There was a residual expression of active CTSD in CTSDf/f/p48Cre/+ mice. In these animals p48 (Ptf1a) promoter implements CTSD deletion in acinar but not in ductal or endocrine cells or resident macrophages which explains the presence of a weak signal (Fig. 1E). Determination of CTSD activity by fluorometry in CTSDf/f/p48Cre/+ acinar cells demonstrated absence of CTSD compared with CTSD floxed acini, and CTSD activity remained undetectable after supramaximal cholecystokinin stimulation (Fig. 1F). Protein expression analysis by Western blotting of CTSDf/f/p48Cre/+ acinar cells confirmed absence of CTSD (Fig. 1G), which was in line with the results from fluorometry.
Trypsinogen and cathepsin B activation depends on cathepsin D
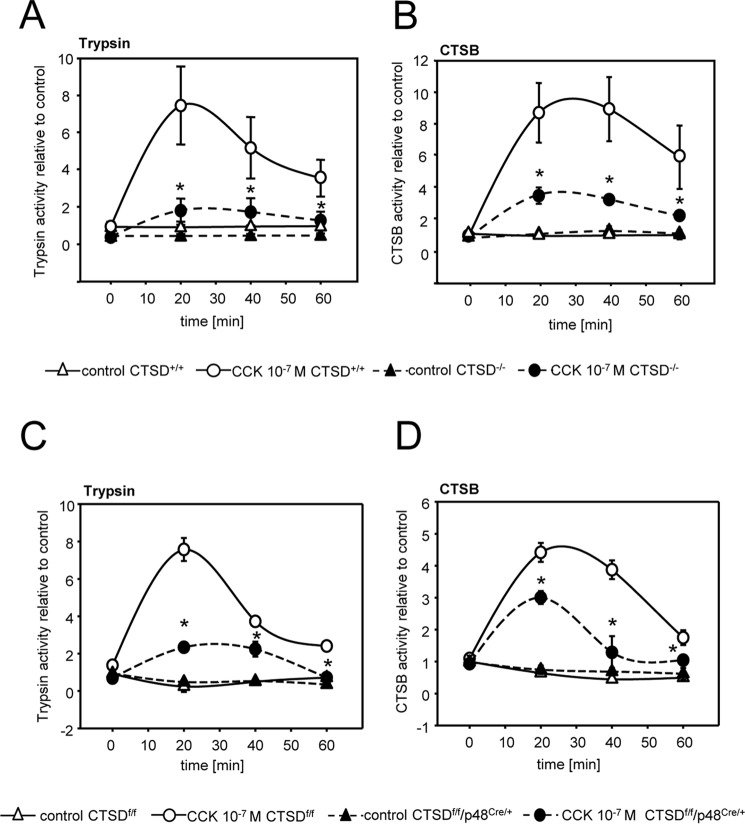
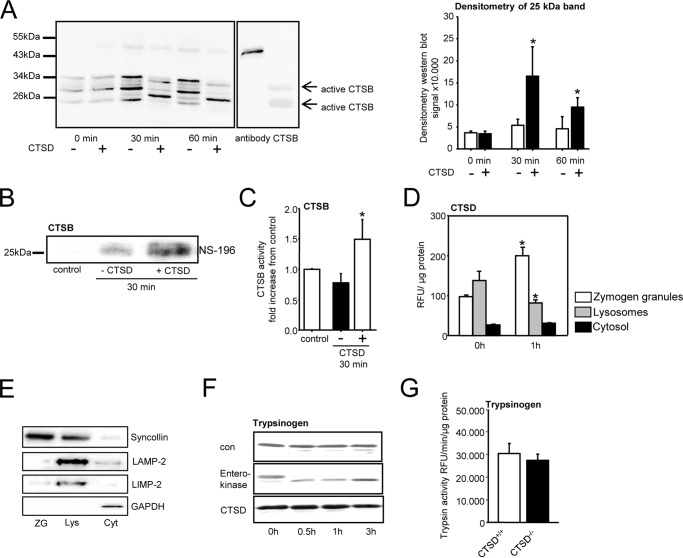
To study whether intracellular protease activation is dependent on CTSD we isolated acinar cells from CTSD−/− mice and stimulated them with CCK for up to 60 min. Deletion of CTSD prevented activation of trypsinogen by around 75% (Fig. 2A) and of CTSB to a similar extent (Fig. 2B). These observations were confirmed in acini from the pancreas-specific CTSD knockout (CTSDf/f/p48Cre/+ mice) (Fig. 2, C and D). To further elucidate the role of CTSD in CTSB activation we immunoprecipitated CTSB from wildtype mouse pancreas and added recombinant, active human CTSD to the samples (10 μU). Western blotting of untreated CTSB immunoprecipitates showed a band pattern with a faint signal at ∼ 25 kDa, which became much more prominent after addition of CTSD. The molecular size of active cathepsin B is around 25 kDa (17) so that the increase in intensity of this band indicates activation of CTSB by CTSD (Fig. 3A). Densitometry of the 25-kDa band showed a strong increase of intensity after 30 min and a still significant increase after 60 min of incubation with CTSD. Any cross-reaction to CTSD was ruled out by in vitro experiments in which the CTSB antibody detected no recombinant CTSD (Fig. S1A). When probing for CTSD, recombinant CTSD enzyme and the coadministered CTSD to the immunoprecipitated fractions were detected showing a heavier band at 30 kDa (Fig. S1B). Besides bands for active cathepsin B at 34 kDa and 25 kDa there were additional bands at ∼ 30 kDa and 24 kDa in the untreated pro-CTSB fractions, which disappeared upon CTSD incubation, suggesting either unspecific immunoprecipitation products or a slight, additional degradative effect of CTSD. To confirm CTSB activation we co-incubated immunoprecipitates with the biotin-coupled substrate NS-196, an epoxysuccinyl-based CTSB specific reagent that binds only to the active site of processed CTSB but not its inactive precursor thus allowing a highly sensitive and selective detection of active CTSB (18). Subsequent incubation with CTSD led to an increase of bound NS-196 indicating an increase in active CTSB (Fig. 3B). Further evidence for the activation of CTSB by CTSD activity was obtained by fluorometric analyses using the CTSB substrate AMC-Arg2 and its addition to CTSB immunoprecipitates. Again we found an increase of CTSB activity after co-incubation with CTSD (Fig. 3C).
Figure 2.
Intracellular protease activation in isolated acinar cells in response to CCK. A and B, reduced trypsin (A) and cathepsin B (B) activation in isolated acinar cells of CTSD−/− mice upon supramaximal CCK stimulation. C and D, reduced trypsin (C) and cathepsin B (D) activation in isolated acinar cells of CTSDf/f/p48Cre/+ mice upon supramaximal CCK stimulation. *, p < 0.05. Data points show mean ± S.E. of five or more experiments in each group and at each time point. Asterisks indicate differences significant at p < 0.05.
Figure 3.
Procathepsin B is the unprocessed pro-form. A, addition of active CTSD enzyme to immunoprecipitated CTSB, using an antibody that detects both pro- and active CTSB, shows processing of bands leading to a band that corresponds in size to active CTSB. Densitometry of the 25-kDa band for active CTSB shows a strong increase in the CTSD-treated fractions. B, detection of active CTSB using a biotinylated CTSB inhibitor (NS-196) in immunoprecipitates with a CTSB antibody indicates activation of CTSB by CTSD. C, enzymatic CTSB activity is increased in CTSB immunoprecipitates after co-incubation with CTSD (results shown as -fold increase). D, under resting conditions CTSD activity is detectable in the zymogen and lysosomal fraction and is redistributed to the zymogen fraction of pancreatic tissue homogenates after 1 h of supramaximal caerulein stimulation. E, Western blots of fractions show the zymogen (ZG) marker syncollin distributed to the zymogen granule fraction, the lysosomal markers LAMP-2 and LIMP-2 to be lysosomal (Lys), and the cytosolic marker GAPDH was confined to cytosolic fraction (Cyt). F, in vitro activation of trypsinogen is achieved by enteropeptidase but not by CTSD, indicating that cathepsin D does not directly induce trypsinogen activation. G, trypsinogen content, measured as trypsin activity (Arg-110–Ile-Pro-Arg) after activation with enteropeptidase is identical in CTSD−/− mice and their controls. *, p < 0.05. Data points show mean ± S.E. of five or more experiments in each group and at each time point. Asterisks indicate differences significant at p < 0.05.
Subcellular distribution of CTSD activity was found to be similar to that of CTSB. While in the resting state CTSD was localized in both the lysosomal and the zymogen-containing compartment, a shift of CTSD activity to the zymogen-containing fraction was found 1 h after the first caerulein injection, which parallels that known for CTSB (Fig. 3D) (19). Fig. 3E demonstrates the distribution of marker proteins in subcellular fractions under resting conditions. The zymogen marker syncollin was predominantly recovered in the secretory vesicle fraction (zymogens); the lysosomal markers LAMP-2 and LIMP-2 were found in the lysosomal fraction and GAPDH in the cytosolic compartment. To clarify whether CTSD activates trypsinogen directly we co-incubated CTSD with trypsinogen in vitro and detected no cleavage of bands on Western blot analysis over an incubation period of 3 h. In contrast, enteropeptidase, an activator of trypsinogen cleaved trypsinogen readily after 30 min (Fig. 3F). The deletion of CTSD had no effect on trypsinogen expression because the pancreata of C57BL/6 wildtype and CTSD−/− mice contained similar amounts of enteropeptidase-activatable trypsin activity (Fig. 3G).
Deletion of cathepsin D alters the severity of acute experimental pancreatitis depending on the type of knockout
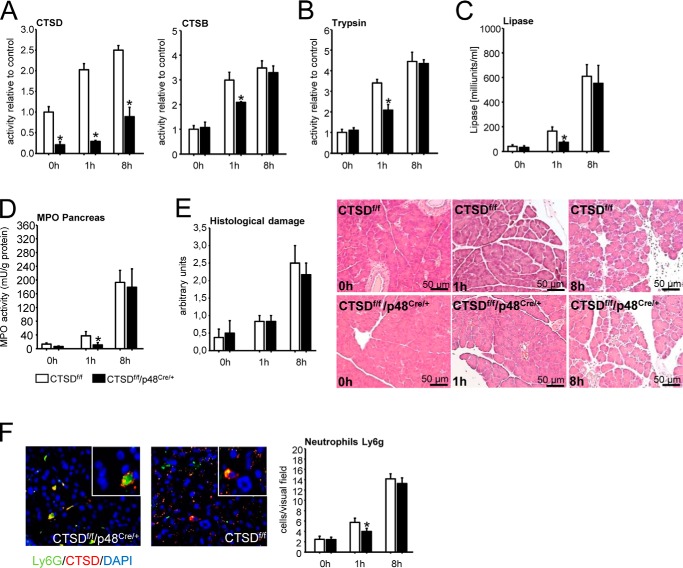
To explore the significance of CTSD in regulating the severity of acute pancreatitis we used the caerulein-induced model of acute pancreatitis in both CTSDf/f/p48Cre/+ mice and CTSD−/− mice and their corresponding littermate controls (CTSDf/f and C57BL/6 mice). As would be predicted from ex vivo experiments in acinar cells (Fig. 2) the severity of pancreatitis was reduced in CTSDf/f/p48Cre/+ mice at an early time point (1 h) in parallel with a reduction in CTSD and CTSB activation (Fig. 4A) as well as activation of trypsin (Fig. 4B). Indicating reduced disease severity CTSDf/f/p48Cre/+ mice displayed reduced serum lipase at 1 h of pancreatitis (Fig. 4C) and less activity of myeloperoxidase (MPO), a neutrophil enzyme, in pancreatic homogenates (Fig. 4D), indicating less infiltration of neutrophils into the pancreas. Total histological damage remained unaffected at either early (1 h) or late (8 h) time point in pancreatitis (Fig. 4E). At 8 h the acinar cell–specific deletion of CTSD (CTSDf/f/p48Cre/+ mice) had no effect on either CTSB (Fig. 4A), trypsin activation (Fig. 4B), or any parameter of disease severity (Fig. 4, C–E). The residual presence and progressive increase in CTSD activity in the pancreas of CTSDf/f/p48Cre/+ was attributable to resident and infiltrating inflammatory cells (Fig. 4F). Although numbers of neutrophils differed between CTSDf/f/p48Cre/+ and controls at 1 h, there was no difference at 8 h, comparable with the MPO activities.
Figure 4.
Caerulein induced acute pancreatitis in CTSDf/f/p48Cre/+ mice and their controls (CTSDf/f). A, CTSD activation is strongly impaired in conditional knockout mice and increases slowly only after 8 h, an effect attributed to infiltrating CTSDf/f inflammatory cells (see F). CTSB activity is decreased at 1 h but parallels controls after 8 h. B, trypsin activation in CTSDf/f/p48Cre/+ mice is decreased at 1 h and similar to controls at 8 h. C, lipase activity of CTSDf/f/p48Cre/+ mice is decreased at 1 h but similar to controls at 8 h. D, reduced myeloperoxidase (MPO) activity in conditional CTSD mice at 1 h but not at 8 h. E, histological damage is slightly increased after 1 h and strongly increased after 8 h in both CTSDf/f/p48Cre/+ and control mice and was of comparable extent. F, cathepsin D staining in pancreata of CTSDf/f/p48Cre/+ mice 8 h after induction of acute pancreatitis shows CTSD expression in infiltrating neutrophils. The number of Ly6g-positive cells were counted for a total of 10 different view fields. Data points show mean ± S.E. of five or more experiments in each group and at each time point. Asterisks indicate differences significant at p < 0.05.
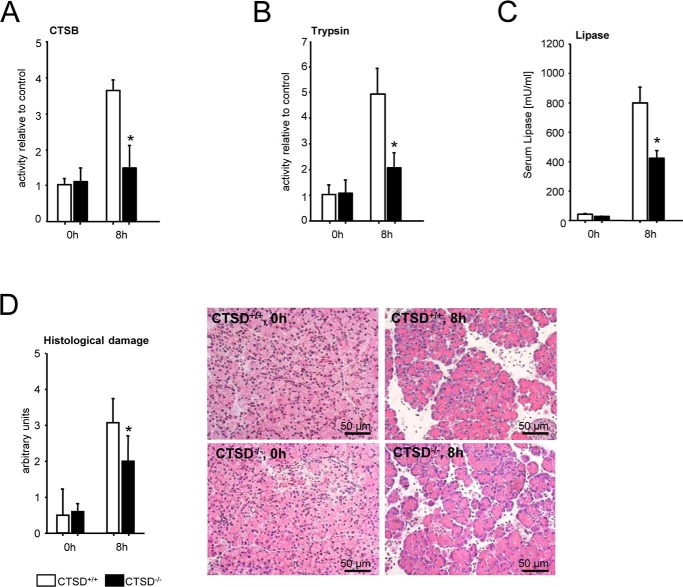
Results were quite different when we used the mouse model with a complete CTSD knockout (CTSD−/−). In these experiments we used CTSD−/− and wildtype controls (C57BL/6) at an age of 14 days to avoid the effects of intestinal atrophy and loss of lymphoid cells in older animals as reported by Saftig and co-workers (20) and focused on the late (8 h) phase of pancreatitis. Unlike the acinar cell deletion of CTSD the complete absence of CTSD in all cells including inflammatory cells greatly reduced not only the activation of CTSB (Fig. 5A) and of trypsin (Fig. 5B), but also the disease severity at 8 h as indicated by a reduced serum lipase (Fig. 5C) and cumulate histological damage (Fig. 5D). Taken together these data suggest that the CTSD, CTSB, trypsin cascade in acinar cells is of minor and only transient importance for the ultimate severity of pancreatitis and the role of CTSD in pancreatitis predominantly involves nonacinar cells.
Figure 5.
Caerulein-induced acute pancreatitis in CTSD−/− mice (complete knockout) and littermate controls. A–D, unlike in the pancreas-specific knockout, a reduction in intrapancreatic CTSB (A) and trypsin (B) activation remained significant at 8 h. Indicators of severity such as serum lipase (C) and histological damage (D) were also reduced in late pancreatitis. *, p < 0.05. Data points show mean ± S.E. of five or more experiments in each group and at each time point. Asterisks indicate differences significant at p < 0.05.
CTSD−/− bone marrow cells show a reduced cytokine release after lipopolysaccharide (LPS) stimulation
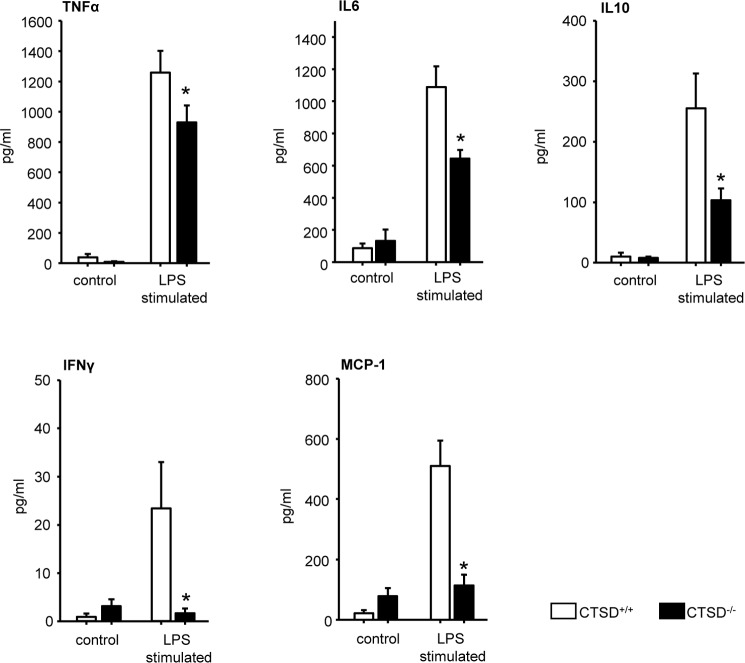
Based on our observation that in a late time point severity of pancreatitis was reduced in CTSD−/− but not in CTSDf/f/p48Cre/+ mice and that acinar cells from both animal strains showed similar responses in terms of CTSB and trypsin activation upon CCK stimulation, we concluded that the effect on disease severity could be mediated by resident or infiltrating inflammatory cells. We therefore isolated immune cells from bone marrow of CTSD−/− mice and subjected them to lipopolysaccharide stimulation in vitro. Quantitative measurements of the cytokines TNF-α, IL6, IL10, IFN-γ, and MCP-1 (monocyte chemoattractant protein-1) in the supernatants of stimulated immune cells showed significantly lower cytokine production in CTSD−/− bone marrow cells after 6 h LPS stimulation (Fig. 6). These results indicate that the cytokine production of inflammatory cells is greatly impaired after deletion of CTSD.
Figure 6.
Isolated and LPS stimulated bone marrow cells from CTSD−/− and corresponding wildtype littermate controls. Reduced secretion of cytokines TNF-α, IL6, IL10, IFNγ, and MCP in CTSD−/− mice. *, p < 0.05. Data points show mean ± S.E. of five or more experiments in each group and at each time point. Asterisks indicate differences significant at p < 0.05.
CTSD deletion reduces apoptosis during pancreatitis in two different knockout models
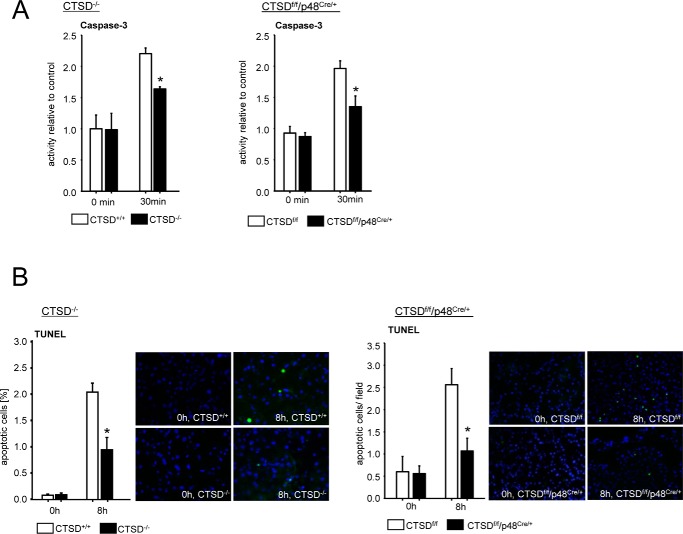
Cell death in acute pancreatitis can involve either necrosis or apoptosis of acinar cells, and the latter has been attributed to a milder phenotype. We therefore investigated whether a deletion of CTSD would lead to a shift from necrosis to apoptosis to account for the reduced disease severity at 8 h. Our results in pancreatic acinar cells indicate that the opposite does, in fact, occur and both the acinar cells of CTSD−/− as well as of CTSDf/f p48Cre/+ animals show reduced caspase-3 activation after supramaximal CCK stimulation (Fig. 7A). In vivo, after 8 h of pancreatitis, the number of TUNEL-positive, apoptotic cells was reduced to 50% in both CTSD-deleted animal strains, indicating that acinar cell CTSD is critical for pancreatic tissue apoptosis to proceed and independent of CTSD in inflammatory cells (Fig. 7B).
Figure 7.
Apoptosis in isolated acinar cells and caerulein induced acute pancreatitis after CTSD depletion. A, deletion of CTSD reduced caspase-3 activation in isolated and CCK-stimulated acinar cells of CTSDf/f/p48Cre/+ and CTSD−/− mice compared with controls. B, reduced apoptosis shown by TUNEL assay in vivo in CTSDf/f/p48Cre/+ and CTSD−/− mice. *, p < 0.05. Data points show mean ± S.E. of five or more experiments in each group and at each time point. Asterisks indicate differences significant at p < 0.05.
Discussion
Evidence in favor of a protease-activation–based pathogenesis of pancreatitis includes data from a whole variety of experimental disease models (21–23) as well as human studies, which have identified germline mutations in protease and antiprotease genes as by far the most common genetic risk factors for pancreatitis (24–26).
One of the underlying mechanisms for premature trypsinogen activation is the transactivation via the lysosomal enzyme CTSB, which represents the most effective intracellular trypsinogen activator known so far. Direct CTSB-mediated trypsinogen activation was demonstrated by in vitro and in vivo experimental studies (6, 19, 27). Conversely, pharmacological inhibition or genetic deletion of CTSB led to a reduction of intracellular trypsin activation and a reduction in the severity of pancreatitis in mice (1, 28).
A precondition for CTSB-mediated trypsinogen activation would be the presence of both classes of enzymes in the same compartment (29). Different underlying mechanisms for such a colocalization have been discussed and include missorting of lysosomal enzymes into secretory vesicles by defective function of the mannose 6-phosphate receptor–dependent sorting mechanism (30), fusion of zymogen granules with lysosomes via crinophagy (31) or colocalization via impaired autophagy with inefficient lysosomal clearance (32), or by aberrant endocytosis from the cell surface (33, 34).
Cathepsin L, another widely expressed cysteine proteinase (35), exerts antagonistic effects to CTSB as it cleaves trypsinogen and trypsin resulting in their inactivation. Yet, deletion of CTSL led to a milder disease course, which was explained by a shift toward an apoptosis-predominant cellular injury (2).
The results of the present study allow for several conclusions. CTSD is expressed in the exocrine pancreas, redistributed like CTSB and CTSL to a zymogen-containing subcellular compartment, and its deletion leads to milder pancreatitis. However, CTSD acts entirely through CTSB and cannot activate trypsin directly. Unlike CTSL, the deletion of CTSD leads to less, rather than more, apoptosis. Moreover, the effect of CTSD deletion in the early, acinar cell–dependent phase of pancreatitis is only minimal and transient, whereas it is prominent in the later, inflammatory cell–dependent phase of the disease.
CTSD is an aspartic protease of the pepsin superfamily and as an endopeptidase its major function is to catalyze the hydrolysis of peptide bonds in proteins (8, 36). In addition CTSD has been implicated in the processing of pro-CTSB and other proteolytic enzymes primarily in cells after malignant transformation (37). Isolated pro-CTSB from rat microsomal fraction was activated by CTSD in vitro under acidic conditions and was blocked by the aspartic protease inhibitor pepstatin A (38, 39).
Our results indicate that this CTSD-mediated activation of CTSB represents the predominant activation mechanism for CTSB, applies equally to nonmalignant cells and in vivo settings, and does affect the severity of pancreatitis. It is not, however, the only mechanism through which CTSB can be activated because Quraishi et al. (40) observed an autocatalytic activation of pro-CTSB to intermediate and mature CTSB which could be inhibited by cystatin C. Our study further confirms that supramaximal stimulation of acinar cells leads to the same redistribution of the aspartic protease CTSD as previously shown for the cysteine proteases CTSB and CTSL (2, 6). The reason for this parallel processing of different classes of lysosomal enzymes is their mutual dependence on the mannose 6-phospate receptor–dependent intracellular sorting mechanism (7, 30). The presence of CTSD in the secretory compartment was previously demonstrated in rats, which were subjected to caerulein stimulation, showing large vacuoles containing digestive enzymes (41) and underlining the spatial proximity of CTSD to digestive enzymes and CTSB.
A CTSB activation by CTSD in the lysosomal compartment under resting conditions where they physiologically colocalize is probably prevented by intracellular inhibitors (42) or by the intravesicular pH which would require an acidic optimum for CTSD activity (43).
The difference in improving the severity of pancreatitis between the complete CTSD−/− and the pancreas-specific knockout indicates that the initial acinar cell effect on CTSB-mediated trypsinogen activation is only minor, transient, and less than critical for the ultimate disease severity, a point previously made for a specific trypsin 7 knockout (44). The fact that a complete CTSD−/− knockout has a much less transient effect on disease severity, which also remains significant after 8 h of pancreatitis suggests that this role of CTSD involves nonacinar cell types. Our studies on bone marrow cells indicate that the most likely target cell population is inflammatory cells and the underlying mechanisms are their greatly impaired production of pro- (and anti-) inflammatory cytokines. Although they can still infiltrate the pancreas to some extent they no longer produce much local or systemic inflammation. Potential cellular players, which have previously been identified as promoting acute pancreatitis, are neutrophil granulocytes and macrophages, mediated by an effect of TNF-α (3).
Future studies need to specify which types of inflammatory cells are mostly affected and responsible for this effect. Our results do not conflict with observations by Mehanna et al. (45), who could not find differences in severity of pancreatitis and CTSB expression in a conditional CTSD knockout harboring a loxP-flanked allele of CTSD with a Spink3-cre transgene and acinar cell specificity (CDf/f;Spink3cre/+). Our data would suggest that this kind of approach can only affect the early disease phase, whereas for a less transient effect deletion of CTSD from inflammatory cells is required. The authors furthermore assumed a delayed degradation of CTSB in case of CTSD deficiency based on their observations that CTSB expression was increased in stimulated CDf/f;Spink3cre/+ mice. Our results support this hypothesis, as Western blot analysis of immunoprecipitated CTSB incubated with CTSD showed a disappearance of CTSB bands at 60 min, suggesting that initial CTSB activation could be followed by later CTSB degradation. As CTSD mediates its action in pancreatitis via CTSB further studies need to investigate how this applies to inflammatory cells and whether these events evolve in a vesicular compartment (19) or in the cytosol (46).
In terms of cell death mechanisms, necrosis and apoptosis have both been reported during pancreatitis, and cathepsins have been implicated in their regulation (47–49) using different approaches. CTSB and CTSL have both been found to either impair (2) or promote apoptosis (19, 46) and also for CTSD conflicting evidence has been presented (50–52). For pancreatic acinar cells at least our answer is quite unequivocal that absence of CTSD reduces the degree of apoptosis either in vitro after supramaximal CCK stimulation or in vivo during experimental pancreatitis.
We conclude that the aspartic protease CTSD is expressed in the pancreas and undergoes subcellular redistribution following supramaximal CCK stimulation in parallel with the cysteine proteases CTSB and CTSL. Its effect on cellular damage of acinar cells is mediated via CTSB for which it is a potent activator and not via direct activation of trypsinogen. The acinar cell effect of CTSD deletion is only transient in terms of how it influences the ultimate disease severity, whereas the deletion of CTSD from inflammatory cells impairs their capacity for cytokine production and has a much more sustained effect in the course of pancreatitis.
Experimental procedures
Materials
Caerulein was obtained from Sigma. Collagenase from Clostridium histolyticum (EC.3.4.24.3) was purchased from SERVA Electrophoresis (lot no. 14007, Heidelberg, Germany). Recombinant human cathepsin D was obtained from Calbiochem (EMD Biosciences) and cathepsin B from Sigma. Trypsinogen from bovine pancreas and enteropeptidase from porcine intestine were from Sigma. Fluorogenic substrates for cathepsin D were obtained from OncoImmunin, Inc. (CaDiLux®, Gaithersburg, MD) and from Enzo Life Sciences (Lörrach, Germany). Substrates for trypsin (R110-(CBZ-IPR)2) and caspase-3 (R110-DEVD) were from Life Technologies. Substrates for chymotrypsin (Suc-AAPF-AMC) and cathepsin B (AMC-Arg2) were purchased from Bachem AG (Bubendorf, Switzerland). Lipase was quantified using a kit from Roche Hitachi (Grenzach-Wyhlen, Germany). The FragELTM DNA fragmentation detection kit (catalogue no. QIA39–1EA) was obtained from Merck Millipore (Darmstadt, Germany) and CBA mouse inflammation kit was from BD Biosciences. Ly6g antibody was obtained from Abcam (Cambridge, MA). NS-196 was provided by N. Schaschke.
For Western blot analysis the following antibodies were used: anti-cathepsin D (catalogue no. sc-6486, Santa Cruz Biotechnology, Dallas, TX), anti-cathepsin B (R&D Systems, Minneapolis, MN and Cell Signaling Technology Europe, Leiden, The Netherlands), anti-GAPDH (Meridian Life Science Inc., Memphis, TN) and trypsinogen (EMD Biosciences).
Induction of acute pancreatitis
Cathepsin D−/− (20) and CTSDf/f mice were generated as previously reported (47). Wildtype C67BL/6 mice were obtained from Charles River Laboratories (Sulzfeld, Germany). CTSDf/f were crossbred with p48-Cre (Ptf1aCre) mice, with an acinar cell–specific expression site (53, 54) to generate conditional CTSDf/f/p48Cre/+ mice. All mice were maintained in our animal facility. Pancreatitis was induced by 8 hourly injections of caerulein (50 μg/kg/body weight) (55). All animals used were maintained according to institutional guidelines and animal facility protocols. Approval by the Institutional Animal Care and Use Committee was obtained for all experiments including induction of pancreatitis.
For histological evaluation the pancreas was fixed in 4.5% formaldehyde for paraffin embedding. The main part of the pancreas as well as lungs were frozen in liquid nitrogen and stored at −80 °C for later analysis (3). Serum was sampled and stored at −20 °C.
Isolation of acinar cells
Acinar cells were prepared by collagenase digestion as previously described (56). After a resting period of 30 min in a 37 °C water bath acinar cells were stimulated in Dulbecco's modified Eagle's medium containing 2% BSA and 10 mm 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES) using 0.1 μm cholecystokinin. In vivo measurement of protease activation in living acinar cells was performed in a cell medium system at pH 7.4 containing 24.5 mm HEPES, 96 mm NaCl, 11.5 mm glucose, 6 mm KCl, 1 mm MgCl2 6H2O, 0.5 mm CaCl2 2H2O, 2.5 mm NaH2PO4 H2O, 5 mm sodium fumarate, 5 mm sodium glutamate, 5 mm sodium pyruvate, and 1% BSA and DMEM. Trypsin activity was measured by adding 10 μm R110-IPR substrate, CTSD activity by using 350 nm CaDiLux® substrate and for CTSB 20 μm AMC-Arg2 was used. Necrosis was quantitated by propidium-iodide exclusion (19, 57). All measurements were done in triplicate in a 96-well microtiter plate.
Subcellular fractions
Subcellular fractions were performed from pancreatic tissue immediately after sacrifice of mice as described previously (19) using a modified protocol from Saluja et al. (6). Briefly, after cutting and homogenization of tissue final fractionation was performed by two strokes of Douncer size A followed by two strokes of Douncer size B. In serial density centrifugation steps at 4 °C the postnuclear supernatant was removed followed by isolation of the zymogen granule–enriched fraction at 470 × g for 15 min. Preparation of a lysosome-enriched fraction was done at 12,200 × g for 12 min. The remaining supernatant was cleaned up by centrifugation at 20,800 × g for 10 min and was used as a cytoplasmic fraction. Successful fractionation was controlled using marker proteins for the zymogen fraction (syncollin), lysosomes (LAMP-2, LIMP-2) and cytosol (GAPDH).
Biochemical assays
Serum lipase activities were measured by photometric assays (Roche Hitachi) as kinetic over 30 min with an absorbance at 570 nm and standardized using purified enzymes (Sigma). Protease activity was determined from either whole pancreatic or acinar cell homogenates. Trypsin and chymotrypsin activity were measured as enzyme kinetic over 1 h at 37 °C in a buffer containing 100 mm Tris (pH 8.0) and 5 mm CaCl2 at an excitation wavelength of 485 nm and an emission wavelength of 520 nm using their specific fluorogenic substrates. Trypsinogen content was determined by measuring whole trypsin activity after complete activation by enteropeptidase (40 milliunits/milliliter for 30 min). CTSB activity was measured in 100 mm sodium acetate and 5 mm CaCl2 containing 10 mm DTT at pH 5.5 and at pH 4.0 for CTSD activity. Caspase-3 activity was determined in PBS at neutral pH condition. The enzymatic activities of samples were corrected for protein content using the MicroBCA Kit (Pierce Biotechnology, Rockford, IL).
Myeloperoxidase activity measurement was performed as described previously (58). Briefly, pancreatic tissue was homogenized on ice in 20 mm potassium phosphate buffer (pH 7.4) and centrifuged at 20,000 × g at 4 °C for 10 min. The pellet was resuspended in 50 mm potassium phosphate buffer (pH 6.0) containing 0.5% cetyltrimethylammonium bromide. The suspension was frozen and thawed in cycles, sonicated twice, and centrifuged at 20,000 × g at 4 °C for 10 min. Myeloperoxidase activity was assayed in 50 mm potassium phosphate buffer (pH 6) containing 0.53 mm o-dianisidine and 0.15 mm H2O2. The initial increase in absorbance was measured at room temperature with a SpectraMax Spectrophotometer (Molecular Devices, Sunnyvale, CA). The results are expressed in units of MPO activity on the basis of 1 unit being able to oxidize 1 μmol H2O2 per minute per milligram pancreatic protein (56).
Measurement of cytokine release in bone marrow cells
After removal of the femur epiphyses Ca2+- and Mg2+-free Hanks' balanced salt solution (HBSS) (Life Technologies) was forced through the bone with a syringe. The resulting cell suspension was passed over a 70-μm sterile nylon filter (BD Falcon) for removal of cell aggregates and other debris (59). After washing for three times with PBS, neutrophil fraction was activated with 1 μg/ml lipopolysaccharide (Sigma). Measurements were done in triplicate in a 96-well microtiter plate. Cytokines TNF-α, IL6, IL10, IFN-γ, and MCP-1 were measured in the supernatants by FACS analysis with the CBA mouse-inflammation kit according to the manufacturer's instructions (BD Biosciences).
Western blotting, NS-196 blotting, and immunoprecipitation
Tissues were homogenized and lysed in a buffer containing 25 mm HEPES, 75 mm NaCl, 0.5%, Triton X-100, 5% glycerin, and 1 mm EDTA in the presence of 1 mm PMSF, 5 mm Na4P2O7, 10 mm NaF, and 1 μg/ml aprotinin. Protein content was determined using the MicroBCA Kit (Pierce Biotechnology). 20 μg of protein was loaded on polyacrylamide gel and transferred to a nitrocellulose membrane as described previously (3, 19).
For in vitro proteolytic cleavage of trypsinogen either enteropeptidase (10 milliunits) or cathepsin D enzyme (10 milliunits) were given to 40 μg/ml bovine trypsinogen. Aliquots were removed at 0 min, 30 min, 1 h, and 3 h. Reaction was stopped by adding immediately loading buffer containing 10% β-mercaptoethanol, boiled at 96 °C for 5 min and freezing to −20 °C.
For detection of activated CTSB we used NS-196, that binds to the catalytic center of CTSB, as described previously (19, 60). Briefly, 200 μg of total protein was incubated with 1 μm NS-196 for 5 min on ice. Labeling of NS-196 was done in NET (150 mm NaCl, 5 mm EDTA, 50 mm Tris-HCl, and 0.05% Triton X-100)-gelatin containing streptavidin-conjugated peroxidase (1:15,000) for 45 min at room temperature.
For immunoprecipitation lysates were precleared with protein A-Sepharose beads (GE Healthcare). Supernatant was incubated with 2 mg cathepsin B antibody overnight at 4 °C and protein A–Sepharose was added for 1 h. The beads were washed four times in wash buffer and precipitates were subjected to immunoblot analysis with CTSB-antibody. To one fraction active human recombinant CTSD (10 μU) was added and aliquots were taken at 30 and 60 min.
Protein samples were diluted in loading buffer containing 10% β-mercaptoethanol, boiled at 96 °C for 5 min and loaded on SDS gels for electrophoresis. Transfer was made on nitrocellulose membranes. Blocking was achieved in NET containing 2% gelatin. Incubation with specific primary antibodies was followed by enhanced chemiluminescence (ECL) detection using ECL peroxidase (HRP)-linked secondary antibodies (GE Healthcare). Bands were detected by Fusion-FX system for chemiluminescence (Vilber Lourmat, Collegién, France).
Histology and TUNEL assay
Paraffin sections were used for hematoxylin and eosin staining. For assessment of damage a modified score adapted from Niederau et al. (61) was used. The scoring was based on the extent of necrosis, vacuolization of acinar cells, and invasion of inflammatory cells into the pancreas. A minimum of five images from each of five animals was investigated. For assessment of apoptosis paraffin sections were labeled using the FragELTM DNA fragmentation detection kit (Merck Millipore) according to the manufacturer's instructions. Apoptotic cells were quantified by Image J software and numbers of apoptotic cells were given as percent of total cells.
Statistical analysis
All data are expressed as mean ± S.E. from at least five animals in each group. Statistical analyses were performed by SigmaPlot (Systat Software GmbH, Erkrath, Germany) using the Student's t test for independent samples or analysis of variances for samples without normality. Differences were considered significant at a level of p < 0.05.
Author contributions
A. A. A., D. S. J., and M. S. were involved in the acquisition, analysis, and interpretation of the data and drafting of the manuscript. F. U. W. and T. R. provided technical support and critical revision of the manuscript for important intellectual content. T. R. generated the pancreas-specific CTSD knockout animals. A. A. A., D. S. J., J. M., and M. M. L., conceived, designed, and supervised the study, drafted the manuscript, and obtained funding.
Supplementary Material
Acknowledgments
We thank Kathrin Gladrow and Norina Loth for technical assistance, Norbert Schaschke, Hochschule Aalen, Germany, for providing NS-196, and Walter Halangk and Thomas Wartmann, both of University of Magdeburg, for helpful criticism and discussions.
This work was supported by Deutsche Forschungsgemeinschaft Grant DFG AG 203/2–1 (to A. A. A.) with additional support from DFG GRK1947(A3) and the PePPP Project Grant ESF/14-BM-A55–0045/16 funded by the European Union. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Fig. S1.
- CTSB
- cathepsin B
- AMC
- 7-amino-4-methylcoumarin
- CCK
- cholecystokinin
- CTSD
- cathepsin D
- CTSL
- cathepsin L
- LAMP-2
- lysosomal associated membrane protein 2
- LIMP-2
- lysosomal integral membrane protein 2
- LPS
- lipopolysaccharide
- MCP-1
- monocyte chemoattractant protein-1
- MPO
- myeloperoxidase
References
- 1. Halangk W., Lerch M. M., Brandt-Nedelev B., Roth W., Ruthenbuerger M., Reinheckel T., Domschke W., Lippert H., Peters C., and Deussing J. (2000) Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J. Clin. Invest. 106, 773–781 10.1172/JCI9411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wartmann T., Mayerle J., Kähne T., Sahin-Tóth M., Ruthenbürger M., Matthias R., Kruse A., Reinheckel T., Peters C., Weiss F. U., Sendler M., Lippert H., Schulz H. U., Aghdassi A., Dummer A., Teller S., Halangk W., and Lerch M. M. (2010) Cathepsin L inactivates human trypsinogen, whereas cathepsin L-deletion reduces the severity of pancreatitis in mice. Gastroenterology 138, 726–737 10.1053/j.gastro.2009.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sendler M., Dummer A., Weiss F. U., Krüger B., Wartmann T., Scharffetter-Kochanek K., van Rooijen N., Malla S. R., Aghdassi A., Halangk W., Lerch M. M., and Mayerle J. (2013) Tumour necrosis factor alpha secretion induces protease activation and acinar cell necrosis in acute experimental pancreatitis in mice. Gut 62, 430–439 10.1136/gutjnl-2011-300771 [DOI] [PubMed] [Google Scholar]
- 4. Mayerle J., Schnekenburger J., Krüger B., Kellermann J., Ruthenbürger M., Weiss F. U., Nalli A., Domschke W., and Lerch M. M. (2005) Extracellular cleavage of E-cadherin by leukocyte elastase during acute experimental pancreatitis in rats. Gastroenterology 129, 1251–1267 10.1053/j.gastro.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 5. Greenbaum L. M., Hirshkowitz A., and Shoichet I. (1959) The activation of trypsinogen by cathepsin B. J. Biol. Chem. 234, 2885–2890 [PubMed] [Google Scholar]
- 6. Saluja A. K., Donovan E. A., Yamanaka K., Yamaguchi Y., Hofbauer B., and Steer M. L. (1997) Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology 113, 304–310 10.1016/S0016-5085(97)70108-2 [DOI] [PubMed] [Google Scholar]
- 7. Zaidi N., Maurer A., Nieke S., and Kalbacher H. (2008) Cathepsin D: A cellular roadmap. Biochem. Biophys. Res. Commun. 376, 5–9 10.1016/j.bbrc.2008.08.099 [DOI] [PubMed] [Google Scholar]
- 8. Conner G. E. (2002) Cathepsin D. in Handbook of Proteolytic Enzyme (Barrett A. J., Rawlings N. D., and Woessner J. F., eds.), pp. 746–751, Elsevier Academic Press, London [Google Scholar]
- 9. Menzel K., Hausmann M., Obermeier F., Schreiter K., Dunger N., Bataille F., Falk W., Scholmerich J., Herfarth H., and Rogler G. (2006) Cathepsins B, L and D in inflammatory bowel disease macrophages and potential therapeutic effects of cathepsin inhibition in vivo. Clin. Exp. Immunol. 146, 169–180 10.1111/j.1365-2249.2006.03188.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benes P., Vetvicka V., and Fusek M. (2008) Cathepsin D—many functions of one aspartic protease. Crit. Rev. Oncol. Hematol. 68, 12–28 10.1016/j.critrevonc.2008.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liaudet-Coopman E., Beaujouin M., Derocq D., Garcia M., Glondu-Lassis M., Laurent-Matha V., Prébois C., Rochefort H., and Vignon F. (2006) Cathepsin D: Newly discovered functions of a long-standing aspartic protease in cancer and apoptosis. Cancer Lett. 237, 167–179 10.1016/j.canlet.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 12. Zhou W., Scott S. A., Shelton S. B., and Crutcher K. A. (2006) Cathepsin D-mediated proteolysis of apolipoprotein E: Possible role in Alzheimer's disease. Neuroscience 143, 689–701 10.1016/j.neuroscience.2006.08.019 [DOI] [PubMed] [Google Scholar]
- 13. Ketterer S., Gomez-Auli A., Hillebrand L. E., Petrera A., Ketscher A., and Reinheckel T. (2017) Inherited diseases caused by mutations in cathepsin protease genes. FEBS J. 284, 1437–1454 10.1111/febs.13980 [DOI] [PubMed] [Google Scholar]
- 14. Laurent-Matha V., Derocq D., Prébois C., Katunuma N., and Liaudet-Coopman E. (2006) Processing of human cathepsin D is independent of its catalytic function and auto-activation: Involvement of cathepsins L and B. J. Biochem. 139, 363–371 10.1093/jb/mvj037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wille A., Gerber A., Heimburg A., Reisenauer A., Peters C., Saftig P., Reinheckel T., Welte T., and Bühling F. (2004) Cathepsin L is involved in cathepsin D processing and regulation of apoptosis in A549 human lung epithelial cells. Biol. Chem. 385, 665–670 10.1515/BC.2004.082 [DOI] [PubMed] [Google Scholar]
- 16. Zheng X., Chu F., Mirkin B. L., Sudha T., Mousa S. A., and Rebbaa A. (2008) Role of the proteolytic hierarchy between cathepsin L, cathepsin D and caspase-3 in regulation of cellular susceptibility to apoptosis and autophagy. Biochim. Biophys. Acta 1783, 2294–2300 10.1016/j.bbamcr.2008.07.027 [DOI] [PubMed] [Google Scholar]
- 17. Kern U., Wischnewski V., Biniossek M. L., Schilling O., and Reinheckel T. (2015) Lysosomal protein turnover contributes to the acquisition of TGFbeta-1 induced invasive properties of mammary cancer cells. Mol. Cancer 14, 39 10.1186/s12943-015-0313-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schaschke N., Assfalg-Machleidt I., Lassleben T., Sommerhoff C. P., Moroder L., and Machleidt W. (2000) Epoxysuccinyl peptide-derived affinity labels for cathepsin B. FEBS Lett. 482, 91–96 10.1016/S0014-5793(00)02047-0 [DOI] [PubMed] [Google Scholar]
- 19. Sendler M., Maertin S., John D., Persike M., Weiss F. U., Krüger B., Wartmann T., Wagh P., Halangk W., Schaschke N., Mayerle J., and Lerch M. M. (2016) Cathepsin B activity initiates apoptosis via digestive protease activation in pancreatic acinar cells and experimental pancreatitis. J. Biol. Chem. 291, 14717–14731 10.1074/jbc.M116.718999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saftig P., Hetman M., Schmahl W., Weber K., Heine L., Mossmann H., Köster A., Hess B., Evers M., von Figura K., and et al. (1995) Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J. 14, 3599–3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt J., Fernández-del Castillo C., Rattner D. W., Lewandrowski K., Compton C. C., and Warshaw A. L. (1992) Trypsinogen-activation peptides in experimental rat pancreatitis: Prognostic implications and histopathologic correlates. Gastroenterology 103, 1009–1016 10.1016/0016-5085(92)90036-X [DOI] [PubMed] [Google Scholar]
- 22. Lerch M. M., Saluja A. K., Dawra R., Saluja M., and Steer M. L. (1993) The effect of chloroquine administration on two experimental models of acute pancreatitis. Gastroenterology 104, 1768–1779 10.1016/0016-5085(93)90658-Y [DOI] [PubMed] [Google Scholar]
- 23. Aghdassi A. A., Mayerle J., Christochowitz S., Weiss F. U., Sendler M., and Lerch M. M. (2011) Animal models for investigating chronic pancreatitis. Fibrogenesis Tissue Repair 4, 26 10.1186/1755-1536-4-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whitcomb D. C., Gorry M. C., Preston R. A., Furey W., Sossenheimer M. J., Ulrich C. D., Martin S. P., Gates L. K. Jr., Amann S. T., Toskes P. P., Liddle R., McGrath K., Uomo G., Post J. C., and Ehrlich G. D. (1996) Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat. Genet. 14, 141–145 10.1038/ng1096-141 [DOI] [PubMed] [Google Scholar]
- 25. Keim V., Bauer N., Teich N., Simon P., Lerch M. M., and Mössner J. (2001) Clinical characterization of patients with hereditary pancreatitis and mutations in the cationic trypsinogen gene. Am. J. Med. 111, 622–626 10.1016/S0002-9343(01)00958-5 [DOI] [PubMed] [Google Scholar]
- 26. Witt H., Sahin-Tóth M., Landt O., Chen J. M., Kähne T., Drenth J. P., Kukor Z., Szepessy E., Halangk W., Dahm S., Rohde K., Schulz H. U., Le Maréchal C., Akar N., Ammann R. W., et al. (2006) A degradation-sensitive anionic trypsinogen (PRSS2) variant protects against chronic pancreatitis. Nat. Genet. 38, 668–673 10.1038/ng1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kukor Z., Mayerle J., Krüger B., Tóth M., Steed P. M., Halangk W., Lerch M. M., and Sahin-Tóth M. (2002) Presence of cathepsin B in the human pancreatic secretory pathway and its role in trypsinogen activation during hereditary pancreatitis. J. Biol. Chem. 277, 21389–21396 10.1074/jbc.M200878200 [DOI] [PubMed] [Google Scholar]
- 28. Van Acker G. J., Saluja A. K., Bhagat L., Singh V. P., Song A. M., and Steer M. L. (2002) Cathepsin B inhibition prevents trypsinogen activation and reduces pancreatitis severity. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G794–G800 10.1152/ajpgi.00363.2001 [DOI] [PubMed] [Google Scholar]
- 29. Hofbauer B., Saluja A. K., Lerch M. M., Bhagat L., Bhatia M., Lee H. S., Frossard J. L., Adler G., and Steer M. L. (1998) Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am. J. Physiol. 275, G352–G362 [DOI] [PubMed] [Google Scholar]
- 30. Meister T., Niehues R., Hahn D., Domschke W., Sendler M., Lerch M. M., and Schnekenburger J. (2010) Missorting of cathepsin B into the secretory compartment of CI-MPR/IGFII-deficient mice does not induce spontaneous trypsinogen activation but leads to enhanced trypsin activity during experimental pancreatitis—without affecting disease severity. J. Physiol. Pharmacol. 61, 565–575 [PubMed] [Google Scholar]
- 31. Koike H., Steer M. L., and Meldolesi J. (1982) Pancreatic effects of ethionine: Blockade of exocytosis and appearance of crinophagy and autophagy precede cellular necrosis. Am. J. Physiol. 242, G297–G307 [DOI] [PubMed] [Google Scholar]
- 32. Mareninova O. A., Hermann K., French S. W., O'Konski M. S., Pandol S. J., Webster P., Erickson A. H., Katunuma N., Gorelick F. S., Gukovsky I., and Gukovskaya A. S. (2009) Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J. Clin. Invest. 119, 3340–3355 10.1172/JCI38674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sherwood M. W., Prior I. A., Voronina S. G., Barrow S. L., Woodsmith J. D., Gerasimenko O. V., Petersen O. H., and Tepikin A. V. (2007) Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc. Natl. Acad. Sci. U.S.A. 104, 5674–5679 10.1073/pnas.0700951104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lerch M. M., Saluja A. K., Rünzi M., Dawra R., and Steer M. L. (1995) Luminal endocytosis and intracellular targeting by acinar cells during early biliary pancreatitis in the opossum. J. Clin. Invest. 95, 2222–2231 10.1172/JCI117912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kirschke H., and Barrett A. J. (1985) Cathepsin L—a lysosomal cysteine proteinase. Prog. Clin. Biol. Res. 180, 61–69 [PubMed] [Google Scholar]
- 36. Fusek M., and Vetvicka V. (2005) Dual role of cathepsin D: Ligand and protease. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 149, 43–50 10.5507/bp.2005.003 [DOI] [PubMed] [Google Scholar]
- 37. van der Stappen J. W., Williams A. C., Maciewicz R. A., and Paraskeva C. (1996) Activation of cathepsin B, secreted by a colorectal cancer cell line requires low pH and is mediated by cathepsin D. Int. J. Cancer 67, 547–554 10.1002/(SICI)1097-0215(19960807)67:4%3C547::AID-IJC14%3E3.0.CO%3B2-4 [DOI] [PubMed] [Google Scholar]
- 38. Kawabata T., Nishimura Y., Higaki M., and Kato K. (1993) Purification and processing of rat liver procathepsin B. J. Biochem. 113, 389–394 10.1093/oxfordjournals.jbchem.a124056 [DOI] [PubMed] [Google Scholar]
- 39. Nishimura Y., Kawabata T., and Kato K. (1988) Identification of latent procathepsins B and L in microsomal lumen: Characterization of enzymatic activation and proteolytic processing in vitro. Arch. Biochem. Biophys. 261, 64–71 10.1016/0003-9861(88)90104-X [DOI] [PubMed] [Google Scholar]
- 40. Quraishi O., and Storer A. C. (2001) Identification of internal autoproteolytic cleavage sites within the prosegments of recombinant procathepsin B and procathepsin S. Contribution of a plausible unimolecular autoproteolytic event for the processing of zymogens belonging to the papain family. J. Biol. Chem. 276, 8118–8124 10.1074/jbc.M005851200 [DOI] [PubMed] [Google Scholar]
- 41. Watanabe O., Baccino F. M., Steer M. L., and Meldolesi J. (1984) Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: Early morphological changes during development of experimental pancreatitis. Am. J. Physiol. 246, G457–G467 [DOI] [PubMed] [Google Scholar]
- 42. Polajnar M., Zavašnik-Bergant T., Škerget K., Vizovišek M., Vidmar R., Fonović M., Kopitar-Jerala N., Petrovič U., Navarro S., Ventura S., and Žerovnik E. (2014) Human stefin B role in cell's response to misfolded proteins and autophagy. PLoS One 9, e102500 10.1371/journal.pone.0102500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen C. S., Chen W. N., Zhou M., Arttamangkul S., and Haugland R. P. (2000) Probing the cathepsin D using a BODIPY FL-pepstatin A: Applications in fluorescence polarization and microscopy. J. Biochem. Biophys. Methods 42, 137–151 10.1016/S0165-022X(00)00048-8 [DOI] [PubMed] [Google Scholar]
- 44. Sah R. P., Dudeja V., Dawra R. K., and Saluja A. K. (2013) Cerulein-induced chronic pancreatitis does not require intra-acinar activation of trypsinogen in mice. Gastroenterology 144, 1076–1085.e2 10.1053/j.gastro.2013.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mehanna S., Suzuki C., Shibata M., Sunabori T., Imanaka T., Araki K., Yamamura K., Uchiyama Y., and Ohmuraya M. (2016) Cathepsin D in pancreatic acinar cells is implicated in cathepsin B and L degradation, but not in autophagic activity. Biochem. Biophys. Res. Commun. 469, 405–411 10.1016/j.bbrc.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 46. Talukdar R., Sareen A., Zhu H., Yuan Z., Dixit A., Cheema H., George J., Barlass U., Sah R., Garg S. K., Banerjee S., Garg P., Dudeja V., Dawra R., and Saluja A. K. (2016) Release of cathepsin B in cytosol causes cell death in acute pancreatitis. Gastroenterology 151, 747–758.e5 10.1053/j.gastro.2016.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ketscher A., Ketterer S., Dollwet-Mack S., Reif U., and Reinheckel T. (2016) Neuroectoderm-specific deletion of cathepsin D in mice models human inherited neuronal ceroid lipofuscinosis type 10. Biochimie (Paris) 122, 219–226 10.1016/j.biochi.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 48. Conus S., and Simon H. U. (2008) Cathepsins: Key modulators of cell death and inflammatory responses. Biochem. Pharmacol. 76, 1374–1382 10.1016/j.bcp.2008.07.041 [DOI] [PubMed] [Google Scholar]
- 49. Reiser J., Adair B., and Reinheckel T. (2010) Specialized roles for cysteine cathepsins in health and disease. J. Clin. Invest. 120, 3421–3431 10.1172/JCI42918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu G. S., Saftig P., Peters C., and El-Deiry W. S. (1998) Potential role for cathepsin D in p53-dependent tumor suppression and chemosensitivity. Oncogene 16, 2177–2183 10.1038/sj.onc.1201755 [DOI] [PubMed] [Google Scholar]
- 51. Haendeler J., Popp R., Goy C., Tischler V., Zeiher A. M., and Dimmeler S. (2005) Cathepsin D and H2O2 stimulate degradation of thioredoxin-1: Implication for endothelial cell apoptosis. J. Biol. Chem. 280, 42945–42951 10.1074/jbc.M506985200 [DOI] [PubMed] [Google Scholar]
- 52. Bröker L. E., Kruyt F. A., and Giaccone G. (2005) Cell death independent of caspases: A review. Clin. Cancer Res. 11, 3155–3162 10.1158/1078-0432.CCR-04-2223 [DOI] [PubMed] [Google Scholar]
- 53. Magnuson M. A., and Osipovich A. B. (2013) Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab. 18, 9–20 10.1016/j.cmet.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kawaguchi Y., Cooper B., Gannon M., Ray M., MacDonald R. J., and Wright C. V. (2002) The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat. Genet. 32, 128–134 10.1038/ng959 [DOI] [PubMed] [Google Scholar]
- 55. Lerch M. M., Lutz M. P., Weidenbach H., Müller-Pillasch F., Gress T. M., Leser J., and Adler G. (1997) Dissociation and reassembly of adherens junctions during experimental acute pancreatitis. Gastroenterology 113, 1355–1366 10.1053/gast.1997.v113.pm9322531 [DOI] [PubMed] [Google Scholar]
- 56. Halangk W., Krüger B., Krüger M., Stürzebecher J., Albrecht E., Lippert H., and Lerch M. M. (2002) Trypsin activity is not involved in premature, intrapancreatic trypsinogen activation. Am. J. Physiol. Gastrointest. Liver Physiol. 282, G367–G374 10.1152/ajpgi.00315.2001 [DOI] [PubMed] [Google Scholar]
- 57. Mooren F. C., Turi S., Gunzel D., Schlue W. R., Domschke W., Singh J., and Lerch M. M. (2001) Calcium-magnesium interactions in pancreatic acinar cells. FASEB J. 15, 659–672 10.1096/fj.00-0172com [DOI] [PubMed] [Google Scholar]
- 58. Schwaiger T., van den Brandt C., Fitzner B., Zaatreh S., Kraatz F., Dummer A., Nizze H., Evert M., Bröker B. M., Brunner-Weinzierl M. C., Wartmann T., Salem T., Lerch M. M., Jaster R., and Mayerle J. (2014) Autoimmune pancreatitis in MRL/Mp mice is a T cell-mediated disease responsive to cyclosporine A and rapamycin treatment. Gut 63, 494–505 10.1136/gutjnl-2012-303635 [DOI] [PubMed] [Google Scholar]
- 59. Essin K., Salanova B., Kettritz R., Sausbier M., Luft F. C., Kraus D., Bohn E., Autenrieth I. B., Peschel A., Ruth P., and Gollasch M. (2007) Large-conductance calcium-activated potassium channel activity is absent in human and mouse neutrophils and is not required for innate immunity. Am. J. Physiol. Cell Physiol. 293, C45–C54 10.1152/ajpcell.00450.2006 [DOI] [PubMed] [Google Scholar]
- 60. Balaji K. N., Schaschke N., Machleidt W., Catalfamo M., and Henkart P. A. (2002) Surface cathepsin B protects cytotoxic lymphocytes from self-destruction after degranulation. J. Exp. Med. 196, 493–503 10.1084/jem.20011836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Niederau C., Ferrell L. D., and Grendell J. H. (1985) Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology 88, 1192–1204 10.1016/S0016-5085(85)80079-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.