Abstract
Type I IFN production and signaling in macrophages play critical roles in innate immune responses. High salt (i.e. high concentrations of NaCl) has been proposed to be an important environmental factor that influences immune responses in multiple ways. However, it remains unknown whether high salt regulates type I IFN production and signaling in macrophages. Here, we demonstrated that high salt promoted IFNβ production and its signaling in both human and mouse macrophages, and consequentially primed macrophages for strengthened immune sensing and signaling when challenged with viruses or viral nucleic acid analogues. Using both pharmacological inhibitors and RNA interference we showed that these effects of high salt on IFNβ signaling were mediated by the p38 MAPK/ATF2/AP1 signaling pathway. Consistently, high salt increased resistance to vesicle stomatitis virus (VSV) infection in vitro. In vivo data indicated that a high-salt diet protected mice from lethal VSV infection. Taken together, these results identify high salt as a crucial regulator of type I IFN production and signaling, shedding important new light on the regulation of innate immune responses.
Keywords: interferon, macrophage, p38 MAPK, viral immunology, virus, High salt, High salt, type I interferon, macrophage, virus, p38 MAPK, high salt, type I interferon
Introduction
Type I interferon production and signaling are maintained in a feed-forward manner and play essential roles in host immune responses including defense against viral infections and reaction to vaccines. Type I interferons sequentially engage interferon α/β receptor (IFNAR),4 activate transcription factor complex termed as IFN-stimulated gene factor 3 (ISGF3), and then induce expression of hundreds of interferon-stimulated genes (ISGs). ISGs themselves are also critical signaling molecules controlling type I interferon transcription (1). Type I interferons confer target cells in an antiviral state through ISGs that enhance innate capabilities to sense viruses and that directly inhibit viral infections by affecting virus life cycle (2, 3). In addition, type I interferons promote the survival and function of immune cells including natural killer cells, T cells, and B cells to effectively restrict virus propagation and remove them from host cells (4, 5). Furthermore, induction of type I interferons is a major effector mechanism for vaccine adjuvants and adjuvant candidates such as polyinosinic:polycytidylic acid (poly(I·C)), because type I interferons potentiate dendritic cell activation and function including antigen cross-presentation (6–9). Therefore, regulation of type I interferon production and signaling is an important means to control host immune responses.
Macrophages are instrumental in innate immune response by mediating type I interferon production and signaling. They serve as sentinel cells that encounter and respond to pathogens at the earliest time (10, 11). These cells are sufficiently equipped with immune signaling molecules and capable of producing type I interferons to activate expression of ISGs (1). When either the production or downstream signaling of type I interferons is impaired in macrophages, mice are highly susceptible to viral infections (5, 12, 13). Importantly, type I interferons secreted from macrophages not only protect macrophages themselves, but also their neighboring cells from viral infections (1).
High salt (increased sodium chloride) directly and markedly affects a variety of immune response and may regulate type I interferon production and signaling in macrophages. Salt is readily available and overconsumed in modern societies (14), leading to accumulation of NaCl in tissues (15, 16). The high-salt condition mounts inflammation (17, 18) and enhances bacterial killing (19) through shifting macrophage activation into the classically activated phenotype (M1). Earlier reports have also demonstrated that high salt aggravates experimental autoimmune encephalomyelitis by promoting Th17 cells (20, 21). Additionally, high salt dampens the immune suppressive functions of Treg cells, and thereby exacerbates both xenogeneic graft versus host diseases (22) and allograft rejection (23). A recent study has demonstrated that high-salt formulation of aluminum-based adjuvant strongly promotes cancer vaccine-induced immune responses and antitumor effects due to the improvement of antigen cross-presentation in dendritic cells (24). Despite the close relationships between high salt and immune responses, it remains unknown whether high salt regulates type I interferon production and signaling in macrophages.
In this study we investigated the impact of high salt on type I interferon production and signaling as well as on host defense against viral infections. We first determined the expression profile of ISGs in both human and mouse macrophages treated with high salt. We then identified interferon β (IFNβ) as the mediator of the effects of high salt on ISGs. We next explored the influence of high salt on immune sensing and signaling of mouse macrophages responding to viruses or viral nucleic acids. We further uncovered the molecular mechanisms by which high salt regulated type I interferon production and signaling in macrophages. Finally, we studied the roles of high salt in resistance to vesicular stomatitis virus (VSV) infection both in vitro and in vivo.
Results
High salt promotes type I interferon signaling through up-regulating IFNB1 in macrophages
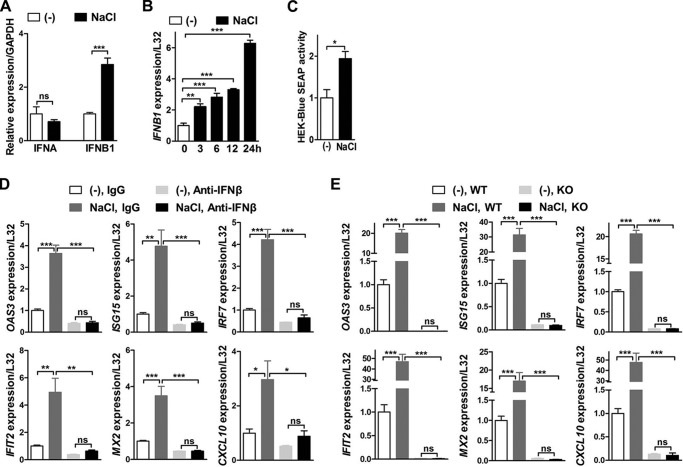
Macrophages were treated with additional NaCl to explore the influence of high salt on type I interferon signaling. Gene ontology analysis of RNA-Seq results in human monocytes-derived macrophages demonstrated that the type I interferon signaling pathway and its associated responses to virus were among the most remarkably promoted biological processes by high salt (Fig. S1). Consistently, expression of dozens of ISGs was significantly increased by high salt (Fig. 1A). The up-regulated expression was further validated by qRT-PCR (Fig. 1B). Some of these ISGs including interferon-induced protein 44-like (IFI44L) and interferon α-inducible protein 27 (IFI27) rose by several 10-folds in high-salt–treated macrophages compared with those in untreated macrophages (Fig. 1, A and B). Results of qRT-PCR in mouse bone marrow-derived macrophages (BMDMs) showed that high salt increased transcription of ISGs both time and dose dependently (Fig. 1, C and D). These ISGs included 2′,5′-oligoadenylate synthetase 3 (Oas3), interferon-stimulated gene 15 (Isg15), interferon regulatory factor 7 (Irf7), interferon-induced protein with tetratricopeptide repeats 2 (Ifit2), MX dynamin-like GTPase 2 (Mx2), and C-X-C motif chemokine ligand 10 (Cxcl10). Furthermore, sodium gluconate, but not the same osmolarity of mannitol or urea, raised expression of ISGs (Fig. 1E). These data together indicated that increased sodium, but not elevation of osmolarity, was sufficient to up-regulate ISGs in macrophages.
Figure 1.
High salt promotes type I interferon signaling in macrophages. A, RNA-Seq analysis of ISGs in human monocyte-derived macrophages treated with an additional 50 mm NaCl. The heat map represents fold-changes of mRNA levels in NaCl-treated macrophages relative to those in untreated macrophages. B, qRT-PCR analysis of ISGs in human macrophages treated with or without additional 50 mm NaCl. C, qRT-PCR analysis of ISGs in mouse bone BMDMs treated with an additional 50 mm NaCl for the indicated time periods. D, qRT-PCR analysis of ISGs in BMDMs treated with different concentrations of additional NaCl. E, qRT-PCR analysis of ISGs in mouse BMDMs treated with sodium gluconate (Na-Glu, 50 mm), mannitol (100 mm), or urea (100 mm). Cells were treated for 24 h except for those specifically indicated. Representative results of three independent experiments are shown. Student's t test was used in B and E, and one-way ANOVA followed by Tukey's multiple comparisons test was used in C for statistical analysis. ns, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Both interferon α (IFNα) and interferon β (IFNβ) are engaged in type I interferon signaling (1). We next addressed which interferon mediated the effects of high salt on ISGs in macrophages. Results of qRT-PCR showed that high salt up-regulated gene expression of IFNB1, but not IFNA, in both human monocyte-derived macrophages and mouse BMDMs (Fig. 2, A and B, and Fig. S2A). Importantly, the expression Ifnb1 was increased much earlier than that of ISGs by high salt in mouse BMDMs (Figs. 1C and 2B). Furthermore, type I IFN bioactivity assay indicated that much more IFNβ was produced in high-salt–treated BMDMs than untreated BMDMs (Fig. 2C). IFNβ neutralizing antibodies abolished high-salt–induced up-regulation of ISGs in BMDMs; whereas IFNα neutralizing antibodies did not affect this up-regulation (Fig. 2D and Fig. S2B). In addition, deficiency of interferon α and β receptor subunit 1 (IFNAR1) (Fig. S3A) consistently abolished this up-regulation of ISGs (Fig. 2E), but did not affect the high-salt–induced increase of Ifnb1 expression (Fig. S3B). These results together suggested a pivotal role of IFNβ in high-salt–induced expression of ISGs. Taken together, high salt enhanced type I interferon signaling through up-regulation of IFNβ in macrophages.
Figure 2.
IFNβ mediates the impact of high salt on ISGs in macrophages. A, qRT-PCR analysis of IFNA and IFNB1 in human monocyte-derived macrophages treated with or without additional 50 mm NaCl. B, qRT-PCR analysis of IFNB1 in mouse BMDMs treated with an additional 50 mm NaCl for the indicated time periods. C, type I IFN bioactivity assay of conditioned media from mouse BMDMs using HEK-BlueTM IFNα/β cell line-based secreted alkaline phosphatase (SEAP) detection. BMDMs were treated with or without additional 50 mm NaCl for 12 h and the conditioned media were used for the assay. D, qRT-PCR analysis of ISGs in mouse BMDMs treated with or without an additional 50 mm NaCl and in the presence of either immunoglobulin G (IgG) or IFNβ neutralizing antibodies (Anti-IFNβ, 200 units/ml). E, qRT-PCR analysis of ISGs in BMDMs isolated from either wildtype (WT) or IFNAR1 knockout (KO) mice and treated with or without an additional 50 mm NaCl. Cells were treated for 24 h except for those specifically indicated. Representative results of three independent experiments are shown. Student's t test was used in A and C, and one-way ANOVA followed by Turkey's multiple comparisons was used in B, D, and E for statistical analysis. ns, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
High salt primes macrophage responses to virus and viral nucleic acids
ISGs play crucial roles in sensing viral pathogen-associated molecular patterns and transmitting host pattern recognition receptors-activated signaling (2). Therefore, we next explored whether high salt primes macrophage responses to virus and viral nucleic acids through up-regulating these ISGs.
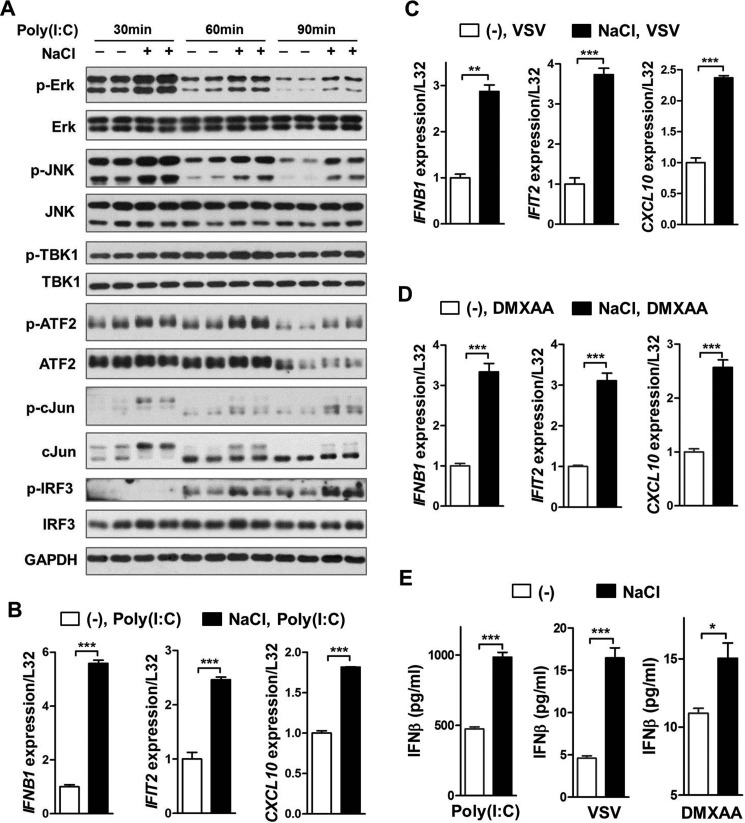
Mouse BMDMs were first treated with additional 50 mm NaCl for 16 h, washed with phosphate-buffered solution (PBS), and then stimulated with virus or viral nucleic acid analogues. Western blotting results demonstrated that high-salt pre-treatment was sufficient to significantly promote phosphorylation of Erk, JNK, TBK1, ATF2, c-Jun, and IRF3, all of which are critical players in controlling virus-induced IFNβ production, in mouse BMDMs treated with double-strand RNA analogue poly(I·C) (Fig. 3A). Consequently, high-salt pre-treatment markedly potentiated poly(I·C)-induced expression of Ifnb1 and subsequent ISGs including Ifit2 and Cxcl10 (Fig. 3B). Similarly, high-salt pre-treatment potentiated expression of Ifnb1 and ISGs in BMDMs treated with VSV or cytosolic DNA sensing pathway activator 5,6-dimethylxanthenone 4-acetic acid (DMXAA) (Fig. 3, C and D). Consistently, high-salt pre-treatment substantially augmented the secretion of IFNβ from macrophages treated with poly(I·C), VSV, or DMXAA (Fig. 3E). In addition, high-salt pre-treatment significantly enhanced the level of p-STAT1 (Tyr-701), but not of p-STAT1 (Ser-727), in BMDMs treated with poly(I·C) (Fig. S4, A and B), consistent with previous observations that IFNβ-induced engagement of IFNAR canonically promotes STAT1 phosphorylation at tyrosine 701, but not at serine 727 (25). These results collectively suggested that high salt primed macrophages to launch a more profound type I interferon production and signaling responding to viruses or viral nucleic acids.
Figure 3.
High-salt pre-treatment enhances virus- and viral nucleic acid analogue-induced IFNβ production and type I interferon signaling in macrophages. A, Western blotting analysis of molecules in the signaling pathway that controls Ifnb1 gene expression in mouse BMDMs. The cells were pretreated with or without an additional 50 mm NaCl for 16 h, washed twice with PBS, and then treated with 100 μg/ml of poly(I·C) in the absence of additional NaCl for the indicated time periods. B–D, qRT-PCR analysis of Ifnb1 and ISGs in mouse BMDMs. The cells were treated the same as in A except that a 2-h treatment of poly(I·C) was carried out in B, VSV (m.o.i. = 0.1) was used for the last 1.5 h of treatment in C, and 50 μg/ml of DMXAA was used for the last hour of treatment in D. E, ELISA of IFNβ in the supernatant of mouse BMDMs. The cells were treated the same as in A except that poly(I·C), VSV, and DMXAA were, respectively, used for the last 2.5, 1.5, and 1.5 h of treatment. Representative results of three independent experiments are shown. Student's t test was used for statistical analysis. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
p38 MAPK mediates high-salt–induced up-regulation of IFNβ and type I interferon signaling in macrophages
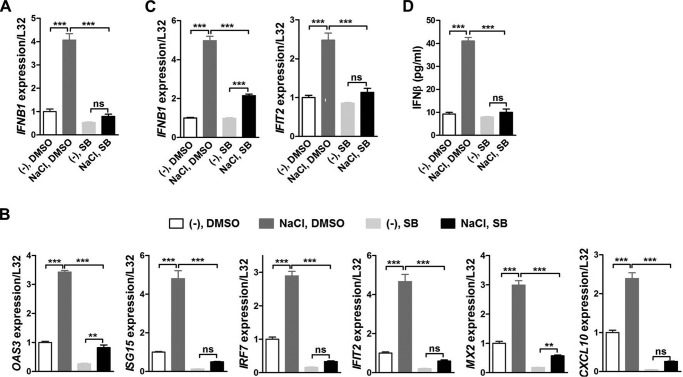
We next tried to delineate the underlying mechanisms by which high salt promoted IFNβ production and type I interferon signaling. Similar to our previous results (17), p38 phosphorylation was enhanced by additional NaCl treatment in BMDMs (Fig. S5A). Thereby, we further used both the p38 selective inhibitor SB203580 (abbreviated as SB) and RNA interference to explore its potential role in mediating the effects of high salt on type I interferon signaling in macrophages. Results of qRT-PCR illustrated that SB completely abrogated high-salt–induced up-regulation of Ifnb1 gene expression in BMDMs (Fig. 4A). Consequently, the induction of ISGs by high salt was markedly attenuated by SB treatment, and as a result SB significantly reduced expression of ISGs in high-salt–treated BMDMs (Fig. 4B). Similarly, knockdown of p38 ameliorated high-salt–induced up-regulation of ISGs (Fig. S5, B and C). In BMDMs challenged with both poly(I·C) and high salt, SB remarkably suppressed expression of Ifnb1 and Ifit2, an ISG (Fig. 4C). Moreover, the induction of IFNβ secretion by high salt was abolished by SB in poly(I·C)-challenged BMDMs (Fig. 4D). Putting these results together, p38 MAPK mediated high-salt–promoted IFNB1 and type I interferon signaling in macrophages either with or without poly(I·C) treatment. In addition, p-p38 was increased by additional 50 mm Na-gluconate, but not by the same osmolarity of mannitol or urea in mouse BMDMs (Fig. S5D), indicating that p38 phosphorylation was promoted specifically by high concentrations of sodium in mouse macrophages.
Figure 4.
p38 MAPK mediates the impact of high salt on IFNβ production and type I interferon signaling in macrophages. qRT-PCR analysis of Ifnb1 (A) and ISGs (B) in mouse BMDMs treated with DMSO or SB203580 (SB) and without stimulation by poly(I·C). The cells were pretreated with 10 μm SB or DMSO for 1 h, and then treated with or without an additional 50 mm NaCl for 24 h in the continued presence of 5 μm SB or DMSO. C, qRT-PCR analysis of Ifnb1 and Ifit2 in mouse BMDMs treated with DMSO or SB and with stimulation by poly(I·C). The cells were pretreated with 10 μm SB or DMSO for 1 h, treated with or without an additional 50 mm NaCl for 16 h in the continued presence of 5 μm SB or DMSO, washed twice with PBS, and finally treated with 100 μg/ml of poly(I·C) for 2 h. D, ELISA of IFNβ in the supernatant of mouse BMDMs treated with DMSO or SB and with stimulation by poly(I·C). The cells were treated the same as in C. Representative results of three independent experiments are shown. One-way ANOVA followed by Turkey's multiple comparisons was used for statistical analysis. ns, not significant; **, p < 0.01; ***, p < 0.001.
Phosphorylation of JNK was not elevated by high salt alone (Fig. S5E). Although high salt increased phosphorylation of Erk (Fig. S5F), inhibition of Erk by PD98059 did not affect high-salt–induced up-regulation of ISGs (Fig. S5G). These results together suggested that p38 but not JNK or Erk mediated the effects of high salt on IFNβ production and type I interferon signaling in macrophages.
ATF2/AP1 mediates high-salt–induced up-regulation of IFNβ and type I interferon signaling in macrophages
Transcription factors ATF2 and c-Jun constitute a heterodimer AP1 that plays an essential role in regulating transcription of IFNB1 (26, 27). In addition, p38 directly phosphorylates ATF2 and promotes its transcriptional activity (28). Therefore, we explored whether ATF2/AP1 was part of the mechanisms regarding how high salt induced expression of IFNB1 and ISGs.
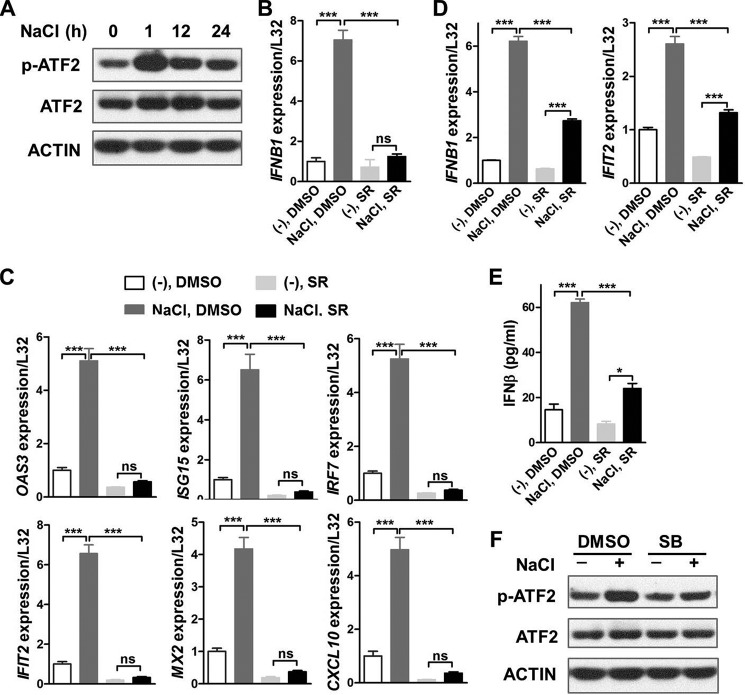
ATF2 phosphorylation, but not its total protein level, was significantly up-regulated by high salt in BMDMs (Fig. 5A). Results of qRT-PCR demonstrated that SR11302, an AP1 selective inhibitor, markedly reduced mRNA levels of Ifnb1 and ISGs in high-salt–treated mouse BMDMs (Fig. 5, B and C). Likewise, gene expression of Ifnb1 and Ifit2 were substantially inhibited by SR in BMDMs treated with both high salt and poly(I·C) (Fig. 5D). Moreover, the induction of IFNβ secretion by high salt was notably alleviated by SR in poly(I·C)-challenged BMDMs (Fig. 5E). Finally, both SB and p38 knockdown significantly reduced high-salt–induced phosphorylation of ATF2 in BMDMs (Fig. 5F and Fig. S6), suggesting that high salt promoted ATF2 phosphorylation through p38 activation. Taken together, a p38/ATF2/AP1 signaling axis mediated the impact of high salt on IFNB1 and type I interferon signaling in macrophages.
Figure 5.
AP1 mediates the impact of high salt on IFNβ production and type I interferon signaling in macrophages. A, Western blotting analysis of ATF2 in mouse BMDMs treated with or without an additional 50 mm NaCl for the indicated time periods. qRT-PCR analysis of Ifnb1 (B) and ISGs (C) in mouse BMDMs treated with DMSO or SR11302 (SR) and without stimulation by poly(I·C). The cells were pretreated with 10 μm SR or DMSO for 1 h, and then treated with or without an additional 50 mm NaCl in the continued presence of 5 μm SR or DMSO for 24 h. D, qRT-PCR analysis of Ifnb1 and Ifit2 in mouse BMDMs treated with DMSO or SR and with stimulation by poly(I·C). The cells were pretreated with 10 μm SR or DMSO for 1 h, treated with or without additional 50 mm NaCl in the continued presence of 5 μm SR for 16 h, washed twice with PBS, and finally treated with 100 μg/ml of poly(I·C) for 2 h. E, ELISA of IFNβ in the supernatant of mouse BMDMs treated with DMSO or SR and with stimulation by poly(I·C). The cells were treated the same as in D. F, Western blotting analysis of ATF2 in mouse BMDMs treated with DMSO or SB. The cells were pretreated with 10 μm SB or DMSO for 1 h, and then treated with or without an additional 50 mm NaCl for 1 h. Representative results of three independent experiments are shown. One-way ANOVA followed by Turkey's multiple comparisons was used for statistical analysis. ns, not significant; *, p < 0.05; ***, p < 0.001.
High salt augments defense against VSV infection in vitro
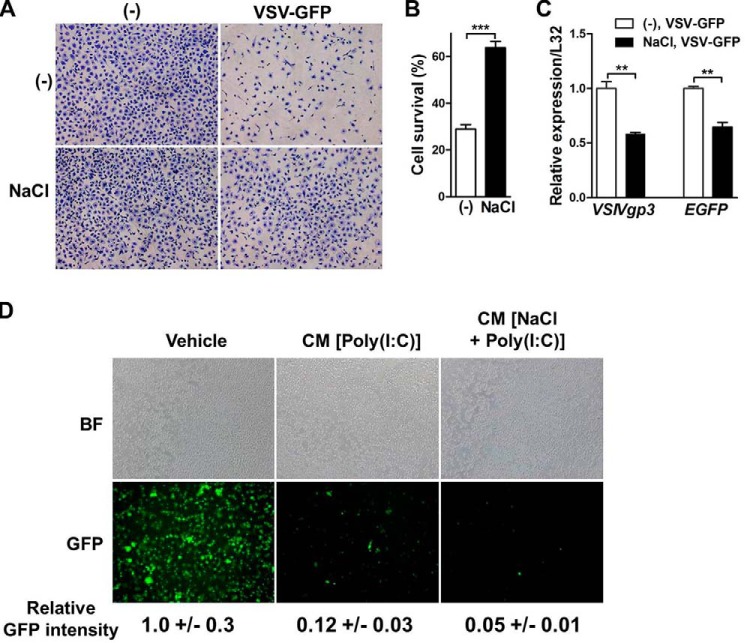
Given the importance of IFNβ and ISGs in host defense (29), we then tested whether high salt would strengthen the ability of cells to fight against virus infection. We first used cell lysis assay to detect survival of mouse BMDMs infected with vesicle stomatitis virus coexpressing GFP (VSV-GFP). Pre-treatment with high salt notably improved cell survival in VSV-GFP-infected BMDMs (Fig. 6, A and B). Moreover, mRNA levels of viral gene VSIVgp3 and EGFP were significantly lower in high-salt–treated BMDMs than those in untreated BMDMs, indicating less replication of VSV (Fig. 6C). Viral replication was then directly measured in L929 cells. Conditioned media from poly(I·C)-stimulated BMDMs restrained VSV replication in L929 cells, and this restraint was further potentiated by high-salt treatment in BMDMs (Fig. 6D).
Figure 6.
High salt enhances the defense against VSV infection in vitro. A, cell lysis assay of mouse BMDMs infected with or without vesicular stomatitis virus coexpressing GFP (VSV-GFP). The cells were pretreated with or without an additional 50 mm NaCl for 16 h, infected with or without VSV-GFP (m.o.i. = 1) for 3 h, then cultured in VSV-GFP-free media for 24 h, and finally stained with crystal violet. B, quantification of cell lysis assay. Cell survival is presented as percentages of cell numbers in the VSV-GFP-infected group relative to those without infection (−). C, qRT-PCR analysis of VSIVgp3 and EGFP in mouse BMDMs. The cells were pretreated with or without an additional 50 mm NaCl for 16 h, washed twice with PBS, and then infected with VSV-GFP (m.o.i. = 0.1) for 6 h. D, analysis of viral replication in L929 cells. L929 cells were treated with RPMI 1640 media (Vehicle), conditioned media from mouse BMDMs stimulated by poly(I·C) (CM (poly(I·C))), or conditioned media from mouse BMDMs stimulated by additional NaCl and poly(I:C) (CM (NaCl + poly(I·C))) for 2 h, and then infected with VSV-GFP (m.o.i. = 1). BF, bright field. Representative results of three independent experiments are shown. Student's t test was used for statistical analysis. **, p < 0.01; ***, p < 0.001.
High salt protects mice from lethal VSV infection
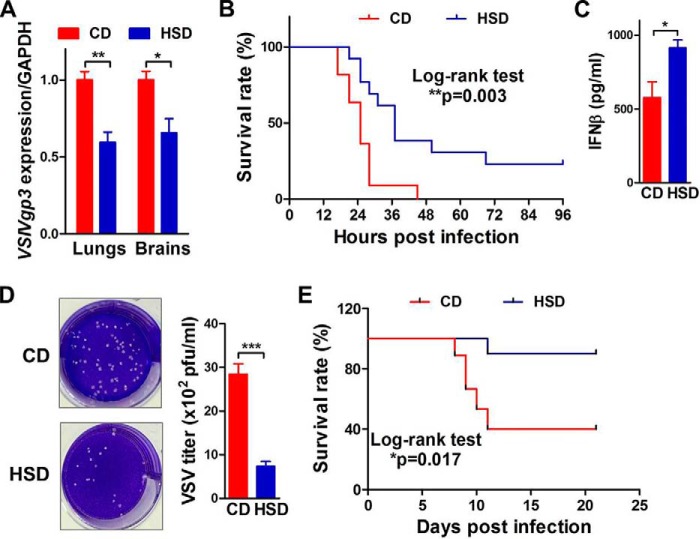
To investigate the effect of high salt on host defense against viral infections in vivo, mice were fed a high-salt diet (HSD) or chow diet (CD) and then infected with VSV. In mice with intraperitoneal injection of VSV, qRT-PCR results demonstrated that HSD significantly decreased the VSIVgp3 mRNA level in mouse lungs and brains, suggesting decreased viral load in these two tissues (Fig. 7A). HSD did not affect the expression of VSIVgp3 in other tissues such as livers, spleens, kidneys, or lymph nodes (Fig. S7). Monitoring the survival revealed that mice fed with HSD had a much lower mortality rate than those fed with CD (Fig. 7B). These results suggested that HSD improved mouse survival by suppressing VSV replication, in particular tissues such as lungs and brains.
Figure 7.
High-salt diet protects mice from lethal VSV infection. A, qRT-PCR analysis of VSIVgp3 in lungs and brains of mice fed with CD or HSD for 5 weeks and infected with VSV (1.5 × 107 pfu) intraperitoneally for 15 h. n = 9:9. B, survival curves of mice after intraperitoneal VSV infection. n = 11:12. C, ELISA of IFNβ in mouse sera after intrabronchial VSV (7.0 × 107 pfu) infection for 3 h. n = 9:9. D, plaque assay of viral load in mouse lungs after intrabronchial VSV infection for 5 days. n = 9:9. E, survival curves of mice after intrabronchial VSV infection. n = 9:10. Student's t test was used in A, C, and D; and Log-rank test was used in B and E for statistical analysis. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
We further studied the effects of HSD on pulmonary VSV infection in mice. In mice with intrabronchial injection of VSV, HSD feeding resulted in significantly higher IFNβ protein levels in the sera (Fig. 7C). Plaque assay showed that VSV titers were remarkably lower in lungs from HSD-fed mice compared with CD-fed mice (Fig. 7D). Moreover, HSD strikingly lowered the mortality rate of mice with intrabronchial VSV infection (Fig. 7E). In summary, high salt alleviated viral load and improved survival in mice with either systemic or pulmonary VSV infection.
Discussion
Although recent studies have highlighted the important regulatory roles of high salt in macrophages, the impact of high salt on type I interferon production and signaling have not been explored in these cells. In this study, we demonstrated that high salt promoted the production of IFNβ and expression of ISGs in macrophages. We identified a p38/ATF2/AP1 axis that mediated these effects of high salt. Consequently, high salt enhanced the defense against viral infections in cell culture and in mice.
Our data support that NaCl is an important regulator of innate immunity against viral infections in that it regulates type I interferon production and signaling in macrophages. We for the first time demonstrated that high salt promoted expression of ISGs through increased production of IFNβ in macrophages. Importantly, high salt primed macrophages to initiate a strengthened response to viruses or viral nucleic acid analogues, leading to enhanced defense against VSV infections. A previous study (19) has shown that high salt accelerates the elimination of intracellular bacteria and parasites. On the other hand, high salt has been demonstrated to skew a proinflammatory phenotype of macrophages and aggravate inflammatory diseases (17), to promote Th17 (20, 21) or follicular helper T cell phenotype (30) and worsen autoimmune diseases, and to diminish the immune suppressive Treg phenotype and exacerbate xenogeneic graft versus host diseases (22) and allograft rejection (23). Putting the results of the current study together with available literature, a more complete picture emerges to reflect the roles of high salt in immunoregulation, up-regulation of immune response. Resultantly, high salt helps to fight pathogens including viruses, bacteria, and parasites, while exacerbating inflammatory diseases, autoimmune diseases, and alloimmunity-related diseases such as graft versus host diseases and allograft rejection.
Regulation of type I interferon production and signaling by high salt may also have important implications in development of vaccine adjuvants. High salt has been recently shown to improve antigen cross-presentation in dendritic cells and consequently promote host immune responses to aluminum-based vaccine adjuvant (24). Intriguingly, earlier results have revealed that type I interferon signaling is required for immune responses induced by chitosan, a prominent candidate for vaccine adjuvants (6). Chitosan induces IFNβ that in turn directly targets dendritic cells and promotes their maturation and activation, and subsequently enhances host adaptive immunity (6). It is plausible that IFNβ signaling at least partially mediates the high-salt–strengthened vaccine immune responses. Macrophage-derived IFNβ may be part of the mechanisms, and it would be interesting to further determine whether high salt boosts type I interferon production and signaling in dendritic cells to bolster vaccine immune responses.
We have identified a p38/ATF2/AP1 axis that mediates the intracellular impact of high salt on type I interferon production and signaling, although future work is warranted to delineate the mechanisms how extracellular stimulus of increased sodium is transmitted across the plasma membrane. Mammalian cells survive hypertonicity stress including high salt with adaptation (31). The early responses of such adaptation are characterized by change of cytoskeletal architecture and cell shrinkage, which contribute to increases of intracellular ionic strength and DNA damage (31). In this study, we revealed that high salt up-regulated IFNβ and its downstream expression of ISGs via p38 MAPK activation, which was previously accounted for by change of cytoskeletal architecture (32), indicating that change of cytoskeletal architecture is involved in the transmembrane signal transmission of increased sodium.
In summary, high salt up-regulates IFNβ production and signaling, and thereby strengthens immune responses in macrophages through a p38/ATF2/AP1 axis. Accordingly, high salt enhances resistance to VSV infection both in vitro and in vivo. These results identify high salt as a novel regulator of type I interferon production and signaling in macrophages, providing a fundamental rationale for utilizing high salt as a maneuver to boost host innate immunity.
Experimental procedures
Ethics statement
All experimental protocols have been approved by the Institutional Review and Ethics Board of Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine. Mouse procedures were done strictly in compliance with NIH Guide for the Care and Use of Laboratory Animals. Blood was obtained from healthy donors who signed informed consent documents.
Animals
IFNAR1 knockout mice (33) and C57BL/6 mice were fed ad libitum under 12/12-h light/dark cycle in specific pathogen-free facility. Three-month-old mice were randomized into chow diet group (CD group) and high-salt diet group (HSD group). Mice in the CD group were fed tap water and normal diet, whereas those in the HSD group were fed tap water containing 1% NaCl and diet containing 4% NaCl as previously described (20, 21).
Human and mouse macrophages
Human and mouse macrophages were derived from human peripheral blood monocytes and mouse bone marrow progenitors, respectively (17). Mouse bone marrow-derived macrophages were treated with an additional 50 mm NaCl in the culture medium.
Chemicals and neutralizing antibodies
SB203580 (1402, Tocris), PD98059 (P215, Sigma), SR11302 (2476, Tocris), IFNα neutralizing antibody (32100-1, PBL Assay Science), and IFNβ neutralizing antibody (32400-1, PBL Assay Science) were used.
RNA sequencing and qRT-PCR
Total RNA was extracted using a TRIzol kit and gene expression was evaluated using RNA sequencing or qRT-PCR (17). The original data (GSE68482) of RNA sequencing were uploaded to the Gene Expression Omnibus. For qRT-PCR, 1 μg of RNA was reversely transcribed into cDNA and specific primers were designed to amplify target genes. PCR was done on an ABI7900 machine using SYBR Green mixture. Relative values of gene expression were normalized to L32 or GAPDH.
Western blotting and ELISA
Total protein was extracted from macrophages and subjected to SDS-PAGE separation and immunoblotting. The results were visualized with chemiluminescence. These primary antibodies were used: p-Erk1/2 (4376s), Erk1/2 (4695), p-JNK (9255), JNK (9252), p-TBK1 (5483), TBK1 (3504), p-ATF2 (9221), ATF2 (9226), p-cJun (9164), c-Jun (9165), p-IRF3 (4947), p-STAT1(Tyr-701) (9167), p-STAT1(Ser-727) (9177), and STAT1 (9172) from Cell Signaling Technology, and IRF3 (ab68481) from Abcam. Enzyme-linked immunosorbent assay (ELISA) was used to measure the IFNβ protein level in mouse sera and macrophage culture media according to the manufacturer's protocol (42400-1, PBL Assay Science).
Type I IFN bioactivity assay
HEK-BlueTM IFNα/β cells (InvivoGen) were seeded in 96-well plates and cultured for 24 h. Conditioned media from mouse BMDMs treated with high salt or not were added to each well. The cells were allowed to incubate for another 24 h. Secreted alkaline phosphatase activity was determined using QUANTI-BlueTM reagents (InvivoGen) according to the manufacturer's protocol. The optical density was measured at 655 nm.
RNA interference
Culture media were changed to Opti-MEM 2 h before transfection. BMDMs were transfected with scramble siRNA or the combination of p38α siRNA (sc-29434) and p38β siRNA (sc-39117) (Santa Cruz Biotechnology) using Lipofectamine RNA iMAX reagent (Invitrogen).
Cell lysis assay
BMDMs were infected with VSV and then cultured in virus-free media for 24 h. At the end of the culture, dead cells were washed off with PBS and the living cells were fixed with methanol and stained with 1% crystal violet for 15 min. Images were captured for cell counting.
VSV propagation, titer measurement, and mouse infection
293FT cells were seeded in the T75 flasks and grown to 60% confluence for VSV propagation. The cells were infected with 0.01 plaque-forming units (pfu) of VSV per cell and allowed to culture for 24 h before viral particles were harvested. Viral particles were concentrated using ultracentrifugation and quantified using plaque assay. Mice were infected with VSV via intraperitoneal or intrabronchial injections.
Plaque assay of viral load
Mouse lungs were homogenized in PBS and the supernatant was collected for viral load quantification. Vero cells were infected with the virus-containing supernatant for 2 h after reaching confluence, and then washed twice with PBS. The cells were then cultured in media containing 1.5% methylcellulose for 3–4 days. Crystal violet staining was used to visualize plaques in which cells were lysed by viruses.
Statistics
All statistics were analyzed using Prism software (GraphPad). Data were presented as mean ± S.D. Unpaired Student's t test was used for comparisons of two independent groups. One-way ANOVA followed by Turkey's test was used for multiple comparisons. Mouse survival curves were compared using Log-rank (Mantel-Cox) test. For all comparisons, p value of less than 0.05 was considered significant.
Author contributions
S. Z. D. and S. S. conceived the study; S. Z. D. and W. C. Z. designed experiments and wrote the manuscript; W. C. Z., L. J. D., and X. J. Z. performed experiments and analyzed the data. X. Q. C., C. S., B. Y. C., X. N. S., C. L., Y. Y. Z., and Y. L. performed experiments. H. X., Q. L., X. J., Z. Z., and S. S. coordinated the study. All authors reviewed the results and approved the manuscript.
Supplementary Material
This work was supported by the National Natural Science Foundation of China Grants 81725003, 31671181, 31371153, and 91339110 and the Shanghai Summit & Plateau Discipline Developing Projects. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S7.
- IFNAR
- interferon α/β receptor
- ISG
- interferon-stimulated gene
- VSV
- vesicular stomatitis virus
- BMDM
- bone marrow-derived macrophage
- qRT
- quantitative RT
- IRF
- interferon regulatory factor
- CD
- chow diet
- HSD
- high-salt diet
- ANOVA
- analysis of variance
- DMXAA
- 5,6-dimethylxanthenone 4-acetic acid.
References
- 1. Ivashkiv L. B., and Donlin L. T. (2014) Regulation of type I interferon responses. Nat. Rev. Immunol. 14, 36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider W. M., Chevillotte M. D., and Rice C. M. (2014) Interferon-stimulated genes: a complex web of host defenses. Annu. Rev. Immunol. 32, 513–545 10.1146/annurev-immunol-032713-120231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu S. Y., Sanchez D. J., and Cheng G. (2011) New developments in the induction and antiviral effectors of type I interferon. Curr. Opin. Immunol. 23, 57–64 10.1016/j.coi.2010.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karupiah G., Buller R. M., Van Rooijen N., Duarte C. J., and Chen J. (1996) Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J. Virol. 70, 8301–8309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Züst R., Toh Y. X., Valdés I., Cerny D., Heinrich J., Hermida L., Marcos E., Guillén G., Kalinke U., Shi P. Y., and Fink K. (2014) Type I interferon signals in macrophages and dendritic cells control dengue virus infection: implications for a new mouse model to test dengue vaccines. J. Virol. 88, 7276–7285 10.1128/JVI.03827-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carroll E. C., Jin L., Mori A., Muñoz-Wolf N., Oleszycka E., Moran H. B. T., Mansouri S., McEntee C. P., Lambe E., Agger E. M., Andersen P., Cunningham C., Hertzog P., Fitzgerald K. A., Bowie A. G., and Lavelle E. C. (2016) The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity 44, 597–608 10.1016/j.immuni.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hafner A. M., Corthésy B., and Merkle H. P. (2013) Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant. Adv. Drug Deliv. Rev. 65, 1386–1399 10.1016/j.addr.2013.05.013 [DOI] [PubMed] [Google Scholar]
- 8. Chang C. J., Sun B., and Robertsen B. (2015) Adjuvant activity of fish type I interferon shown in a virus DNA vaccination model. Vaccine 33, 2442–2448 10.1016/j.vaccine.2015.03.093 [DOI] [PubMed] [Google Scholar]
- 9. Martínez-Gil L., Goff P. H., Hai R., García-Sastre A., Shaw M. L., and Palese P. (2013) A Sendai virus-derived RNA agonist of RIG-I as a virus vaccine adjuvant. J. Virol. 87, 1290–1300 10.1128/JVI.02338-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schiwon M., Weisheit C., Franken L., Gutweiler S., Dixit A., Meyer-Schwesinger C., Pohl J. M., Maurice N. J., Thiebes S., Lorenz K., Quast T., Fuhrmann M., Baumgarten G., Lohse M. J., Opdenakker G., et al. (2014) Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 156, 456–468 10.1016/j.cell.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lehmann M. H., Price P. J., Brandmüller C., and Sutter G. (2015) Modified vaccinia virus Ankara but not vaccinia virus induces chemokine expression in cells of the monocyte/macrophage lineage. Virol. J. 12, 21 10.1186/s12985-015-0252-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Long L., Deng Y., Yao F., Guan D., Feng Y., Jiang H., Li X., Hu P., Lu X., Wang H., Li J., Gao X., and Xie D. (2014) Recruitment of phosphatase PP2A by RACK1 adaptor protein deactivates transcription factor IRF3 and limits type I interferon signaling. Immunity 40, 515–529 10.1016/j.immuni.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 13. Wang Q., Liu X., Cui Y., Tang Y., Chen W., Li S., Yu H., Pan Y., and Wang C. (2014) The E3 ubiquitin ligase AMFR and INSIG1 bridge the activation of TBK1 kinase by modifying the adaptor STING. Immunity 41, 919–933 10.1016/j.immuni.2014.11.011 [DOI] [PubMed] [Google Scholar]
- 14. Powles J., Fahimi S., Micha R., Khatibzadeh S., Shi P., Ezzati M., Engell R. E., Lim S. S., Danaei G., Mozaffarian D., Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. (2013) Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 3, e003733 10.1136/bmjopen-2013-003733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wiig H., Schröder A., Neuhofer W., Jantsch J., Kopp C., Karlsen T. V., Boschmann M., Goss J., Bry M., Rakova N., Dahlmann A., Brenner S., Tenstad O., Nurmi H., Mervaala E., et al. (2013) Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J. Clin. Investig. 123, 2803–2815 10.1172/JCI60113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao Y., Gao P., Sun F., Li Q., Chen J., Yu H., Li L., Wei X., He H., Lu Z., Wei X., Wang B., Cui Y., Xiong S., Shang Q., Xu A., Huang Y., Liu D., and Zhu Z. (2016) Sodium intake regulates glucose homeostasis through the PPARδ/adiponectin-mediated SGLT2 pathway. Cell Metab. 23, 699–711 10.1016/j.cmet.2016.02.019 [DOI] [PubMed] [Google Scholar]
- 17. Zhang W. C., Zheng X. J., Du L. J., Sun J. Y., Shen Z. X., Shi C., Sun S., Zhang Z., Chen X. Q., Qin M., Liu X., Tao J., Jia L., Fan H. Y., Zhou B., et al. (2015) High salt primes a specific activation state of macrophages, M(Na). Cell Res. 25, 893–910 10.1038/cr.2015.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Binger K. J., Gebhardt M., Heinig M., Rintisch C., Schroeder A., Neuhofer W., Hilgers K., Manzel A., Schwartz C., Kleinewietfeld M., Voelkl J., Schatz V., Linker R. A., Lang F., Voehringer D., et al. (2015) High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J. Clin. Investig. 125, 4223–4238 10.1172/JCI80919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jantsch J., Schatz V., Friedrich D., Schröder A., Kopp C., Siegert I., Maronna A., Wendelborn D., Linz P., Binger K. J., Gebhardt M., Heinig M., Neubert P., Fischer F., Teufel S., et al. (2015) Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab. 21, 493–501 10.1016/j.cmet.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kleinewietfeld M., Manzel A., Titze J., Kvakan H., Yosef N., Linker R. A., Muller D. N., and Hafler D. A. (2013) Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496, 518–522 10.1038/nature11868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu C., Yosef N., Thalhamer T., Zhu C., Xiao S., Kishi Y., Regev A., and Kuchroo V. K. (2013) Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496, 513–517 10.1038/nature11984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hernandez A. L., Kitz A., Wu C., Lowther D. E., Rodriguez D. M., Vudattu N., Deng S., Herold K. C., Kuchroo V. K., Kleinewietfeld M., and Hafler D. A. (2015) Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J. Clin. Investig. 125, 4212–4222 10.1172/JCI81151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Safa K., Ohori S., Borges T. J., Uehara M., Batal I., Shimizu T., Magee C. N., Belizaire R., Abdi R., Wu C., Chandraker A., and Riella L. V. (2015) Salt accelerates allograft rejection through serum- and glucocorticoid-regulated kinase-1-dependent inhibition of regulatory T cells. J. Am. Soc. Nephrol. 26, 2341–2347 10.1681/ASN.2014090914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo M., Shao B., Yu J. Y., Liu T., Liang X., Lu L., Ye T. H., He Z. Y., Xiao H. Y., and Wei X. W. (2017) Simultaneous enhancement of cellular and humoral immunity by the high salt formulation of Al(OH)3 adjuvant. Cell Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bonjardim C. A., Ferreira P. C., and Kroon E. G. (2009) Interferons: signaling, antiviral and viral evasion. Immunol. Lett. 122, 1–11 10.1016/j.imlet.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Honda K., Takaoka A., and Taniguchi T. (2006) Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25, 349–360 10.1016/j.immuni.2006.08.009 [DOI] [PubMed] [Google Scholar]
- 27. Hornung V. (2014) SnapShot: nucleic acid immune sensors, part 1. Immunity 41, 868 10.1016/j.immuni.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 28. Ouwens D. M., de Ruiter N. D., van der Zon G. C., Carter A. P., Schouten J., van der Burgt C., Kooistra K., Bos J. L., Maassen J. A., and van Dam H. (2002) Growth factors can activate ATF2 via a two-step mechanism: phosphorylation of Thr71 through the Ras-MEK-ERK pathway and of Thr69 through RalGDS-Src-p38. EMBO J. 21, 3782–3793 10.1093/emboj/cdf361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McNab F., Mayer-Barber K., Sher A., Wack A., and O'Garra A. (2015) Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 10.1038/nri3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu H., Huang X., Qiu H., Zhao M., Liao W., Yuan S., Xie Y., Dai Y., Chang C., Yoshimura A., and Lu Q. (2016) High salt promotes autoimmunity by TET2-induced DNA demethylation and driving the differentiation of Tfh cells. Sci. Rep. 6, 28065 10.1038/srep28065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alfieri R. R., and Petronini P. G. (2007) Hyperosmotic stress response: comparison with other cellular stresses. Pflugers Arch. 454, 173–185 10.1007/s00424-006-0195-x [DOI] [PubMed] [Google Scholar]
- 32. Uhlik M. T., Abell A. N., Johnson N. L., Sun W., Cuevas B. D., Lobel-Rice K. E., Horne E. A., Dell'Acqua M. L., and Johnson G. L. (2003) Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat. Cell Biol. 5, 1104–1110 10.1038/ncb1071 [DOI] [PubMed] [Google Scholar]
- 33. Yang J., Yang C., Guo N., Zhu K., Luo K., Zhang N., Zhao H., Cui Y., Chen L., Wang H., Gu J., Ge B., Qin C. F., and Leng Q. (2015) Type I interferons triggered through the Toll-like receptor 3-TRIF pathway control coxsackievirus A16 infection in young mice. J. Virol. 89, 10860–10867 10.1128/JVI.01627-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.