Abstract
Bacteria produce chemical signals (pheromones) to coordinate behaviors across a population in a process termed quorum sensing (QS). QS systems comprising peptide pheromones and their corresponding Rgg receptors are widespread among Firmicutes and may be useful targets for manipulating microbial behaviors, like suppressing virulence. The Rgg2/3 QS circuit of the human pathogen Streptococcus pyogenes controls genes affecting resistance to host lysozyme in response to short hydrophobic pheromones (SHPs). Considering that artificial activation of a QS pathway may be as useful in the objective of manipulating bacteria as inhibiting it, we sought to identify small-molecule inducers of the Rgg2/3 QS system. We report the identification of a small molecule, P516-0475, that specifically induced expression of Rgg2/3-regulated genes in the presence of SHP pheromones at concentrations lower than typically required for QS induction. In searching for the mode of action of P516-0475, we discovered that an S. pyogenes mutant deficient in pepO, a neprilysin-like metalloendopeptidase that degrades SHP pheromones, was unresponsive to the compound. P516-0475 directly inhibited recombinant PepO in vitro as an uncompetitive inhibitor. We conclude that this compound induces QS by stabilizing SHP pheromones in culture. Our study indicates the usefulness of cell-based screens that modulate pathway activities to identify unanticipated therapeutic targets contributing to QS signaling.
Keywords: gene regulation, gram-positive bacteria, peptidase, pheromone, quorum sensing, antivirulence, neprilysin, quorum quenching
Introduction
Bacterial cell–cell communication (quorum sensing; QS)3 relies on secreted chemicals that act as autocrine and paracrine signals to facilitate the coordination of gene expression across bacterial populations. This social regulation of behavior provides benefits to bacteria in a variety of ways, for instance in cooperative sharing of resources (secreted enzymes (1, 2)), in coordinating horizontal gene transfer (conjugation and natural transformation, (3, 4)), and in defending the community (biofilm development, (5)). Across bacterial taxonomies, QS pathways are commonly classified by the chemical nature of the signals, here termed pheromones, and the types of receptor proteins that directly bind to pheromones.
Among species of Streptococcus, a widespread QS system is one that relies on short, linear peptides as pheromones that bind directly to a receptor family termed Rgg (named after the prototype called regulator of glucosyl transferase genes) (6). Rgg proteins are located within the cytoplasm and act as transcription factors whose activities are allosterically controlled by direct binding of peptide pheromones (7). Multiple paralogs of Rgg-like proteins are commonly encoded within individual genomes and are characterized by an N-terminal, helix-turn-helix DNA-binding domain, and a C-terminal helical-repeat domain whose structure resembles tetratricopeptide repeats that are common in nature (8). In Streptococcus pyogenes, four Rgg paralogs have been identified and designated Rgg1–Rgg4 (9). Rgg1, more commonly referred to as RopB, is activated during stationary phase and induces expression of several genes associated with S. pyogenes virulence, including the secreted cysteine protease SpeB (10). The inducing factor of RopB was recently discovered (37). Rgg4, now called ComR, is a transcriptional activator that binds to the pheromone termed XIP (comX-inducing peptide) and induces expression of a large regulon of genes associated with natural competence (13–15). Finally, Rgg2 and Rgg3 work together to control expression of transcripts encoding several genes, including two peptide pheromones, SHP2 and SHP3 (Fig. 1). Activation of Rgg2 and Rgg3 is associated with biofilm development in some strains of S. pyogenes, as well as enhanced lysozyme resistance and antibiotic sensitivity (16).
Figure 1.
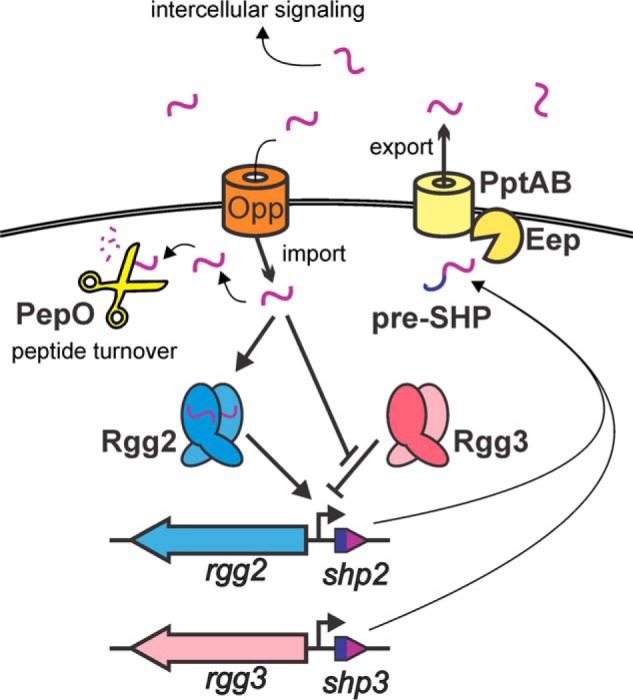
Streptococcus pyogenes Rgg2 and Rgg3 quorum-sensing circuit. The regulatory circuit begins with production of small hydrophobic peptides (pre-SHP) that are exported and processed by the PptAB ABC-type transporter and Eep intramembrane protease, respectively. Extracellular SHPs may diffuse to other nearby cells (paracrine signaling) or be auto-stimulatory (autocrine signaling). SHPs are internalized by the oligopeptide importer Opp and subsequently interact with their receptors, the transcriptional regulators Rgg2 and Rgg3. When not bound to SHP, Rgg3 binds DNA and represses transcription of the shp gene promoters. However, Rgg3 releases DNA when engaged with SHP, derepressing gene transcription. Rgg2 works oppositely, acting as a transcriptional activator in response to bound SHP and causes an increase to SHP pheromone production. The endopeptidase PepO degrades SHP peptides and therefore can down-regulate the quorum-sensing pathway.
The Rgg2/3 regulatory pathway has provided a powerful model system to identify genetic factors that influence the quorum sensing process and to discover small molecules that interfere with signaling. SHP2 (DILIIVVG) and SHP3 (DIIIIVGG) are products of ribosomally generated prepeptides that are secreted and processed by an ABC-type transporter, PptAB, and a membrane-associated metalloprotease Eep, respectively (17). Following the secretion and processing steps that generate the mature pheromones, the SHP peptides can be isolated from culture supernatants, which indicates their ability to serve as intercellular signals (9). For bacteria to respond to SHP pheromones, the peptides must be transported across the cellular membrane by an oligopeptide permease (Opp) into the cytoplasm, where they directly engage the Rgg receptors (16). It has been determined empirically that SHP2 and SHP3 are functionally equivalent in their abilities to bind Rgg2 or Rgg3 (18). Rgg3 is a transcriptional repressor and binds to DNA at sites proximal to promoters from which shp2 and shp3 are expressed (19). When SHP2 or SHP3 bind to Rgg3, the receptor undergoes a conformational change that causes the release of DNA and derepression of the shp promoters. Rgg2 can then bind to the same DNA element previously occupied by Rgg3, but for transcription to be activated, Rgg2 must form a complex with SHP2 or SHP3 (19, 20) (Fig. 1). Thus, Rgg2 and Rgg3 appear to maintain tight control of transcription of the shp2 and shp3 promoters, repressing them in the absence of pheromone and robustly inducing them when pheromones engage the Rgg2/3 receptors.
The likelihood that a pheromone elicits a response in a cell is directly related to the length of time a signal is maintained in a biological system. The rate of turnover of a pheromone is likely to influence a variety of signaling attributes including temporal and spatial distributions and intensities of response. Recently, we ascribed rapid SHP2 and SHP3 turnover in bacterial cultures to be a function of the metalloendopeptidase PepO (21). PepO is a member of the Neprilysin peptidase family (22), whose orthologous members are found widely from bacteria to mammals and are known to process or degrade biologically active peptides (23). In cultures of S. pyogenes grown in a chemically defined medium (CDM), deletion of pepO allowed for the accumulation of SHP2/3 pheromones to a concentration that stimulated the Rgg2/3 QS pathway (21). PepO expression is controlled by the CovRS signal-transduction pathway, a critical regulator of virulence activity in S. pyogenes that is responsive to cell-membrane stress, particularly the antimicrobial peptide LL-37 (24–26). Stimulation of CovRS by an LL-37–derived peptide increased levels of PepO that led to degradation of pheromones and diminished Rgg2/3 activity. Thus, PepO is a mediator of the Rgg2/3 signaling pathway in S. pyogenes, able to switch off QS activity.
The Rgg2/3 pathway is susceptible to small molecule modulators. Previously, we screened a library of 1,200 Food and Drug Administration–approved drug compounds to identify molecules that specifically blocked activity of the Rgg2/3 system. Cyclosporine A, a fungal secondary metabolite with immunosuppressive properties, was found to be a competitive inhibitor of SHP2/3 binding to Rgg2 and Rgg3. Cyclosporine A and the non-immunosuppressive analog Valspodar could inhibit Rgg2/3-dependent shp transcription and block biofilm formation (27). Here, we report our first efforts to probe components of the Rgg2/3 QS pathway by screening a 10,000-compound library for molecules capable of positively modulating pheromone signaling. Compounds with agonist activity were tested for transcriptional activation and promotion of lysozyme resistance. One compound, P516-0475, could mediate activation of the Rgg2/3 pathway but required the presence of SHP2 or SHP3 for activity. We determined that P516-0475 is an inhibitor of the endopeptidase PepO.
Results
Identification of quorum-sensing inducers from a bacterial cell-based high-throughput screen
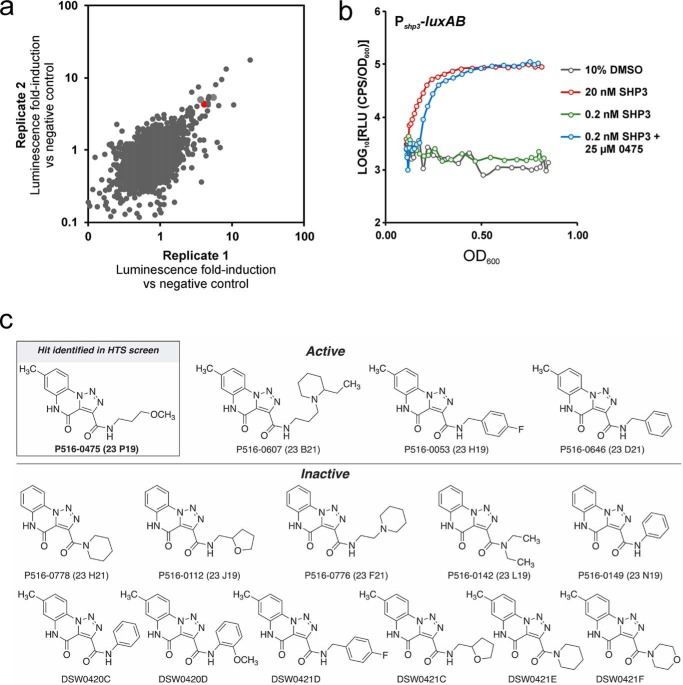
The Rgg2/3 quorum-sensing pathway of S. pyogenes strain NZ131, grown in a CDM, is robustly stimulated with exogenous addition of the synthetic peptide pheromone SHP3 (DIIIIVGG) provided at a concentration of 20 nm. Stimulation can be readily observed in isogenic strain JCC157 harboring a transcriptional reporter in which the shp3 promoter (responsive to Rgg2/3 regulation) controls expression of bacterial luciferase genes luxAB; bioluminescence is recorded during culturing (9). Application of non-inducing concentrations of SHP3 (0.2 nm) or a solvent vehicle (DMSO) fails to activate the quorum-sensing pathway (Fig. 2b). To help elucidate underlying mechanisms of pheromone signaling, the goal of this study was to identify small-molecule compounds capable of stimulating the Rgg2/3 pathway under non-inducing conditions (when 0.2 nm SHP was present). To identify agonistic compounds, we employed a high-throughput cell-based screen, in which wild-type reporter strain JCC157 was grown to early exponential phase and transferred to an assay well containing 0.2 nm SHP3 and 25 μm 10,000 compounds present in the library. All 10,000 compounds were tested individually, in duplicate, for their ability to induce luciferase expression (Fig. 2a and Table S1). Thirteen compounds were initially identified as potential agonists by this screening platform, each displaying 5-fold or greater induction of luminescence activity above levels seen in uninduced cultures. To verify the activities of the positive hits, each of the 13 compounds were either obtained independently from ChemDiv or were synthesized in-house (see “Experimental procedures” and supporting information). Of these, only three chemically unrelated compounds (P516-0475, D487-0075, and L929-0707) displayed the anticipated agonistic activity, and compound P516-0475 (henceforth referred to as “0475”) was selected for further characterization.
Figure 2.
P516-0475 identification and validation. a, a 10,000-compound library was screened in duplicate for agonists of the Rgg2/3 quorum-sensing pathway using a cell-based bioluminescent reporter assay in S. pyogenes strain JCC157. Luminescence induction by each compound compared with uninduced cells were plotted as replicates on each axis. The red data point is compound 0475. b, a time course of luciferase activity of strain JCC157 treated with vehicle, SHP pheromone, or compound 0475. c, compound structures of agonist hit P516-0475 and its analogs represented in the screened library (P516 compounds) or synthesized de novo (DSW compounds). In parentheses are the plate and well identifiers for results of the HTS screen available in Table S1.
A titration of 0475 was used to identify the minimal amount of the compound needed to stimulate a response in strain JCC157. Although no luminescence activity was observed when 1.6 μm 0475 was provided to cultures (data not shown), at 6.25 μm, luminescence was stimulated but displayed a delayed onset until the culture cell density had increased (Fig. 3a). Once stimulated, luminescence intensity reached levels near that obtained from higher concentrations of 0475. This “all-or-nothing” type of response suggested to us that a threshold amount of pheromone had been reached in cultures and triggered the positive feedback loop that leads to self-sustaining production of SHP2/3 pheromones. We also tested the effect of 0475 on cultures that were not supplemented with 0.2 nm SHP3 and found that as low as 6.25 μm was able to stimulate luminescence in a similar fashion (Fig. 3b). These results favored the likelihood that 0475 activity required the presence of a SHP peptide, but to test the possibility that the compound worked in the absence of pheromone, we tested the effects of 0475 on a strain unable to produce endogenous SHP2 or SHP3. The reporter strain BNL170 contains mutations in the shp2 and shp3 genes (referred to here as Δshp) that disable translation of either peptide (28). Although exogenous provision of SHP3 was able to induce luciferase in BNL170, when 0475 was provided to this strain without the provision of pheromone, the reporter was not expressed even when the compound was provided at concentrations up to 100 μm (Fig. 3c). Therefore, 0475 fails to induce Rgg2/3 signaling on its own and is unlikely to function directly as an Rgg2/3 agonist. Likewise, an intact Rgg QS was required for 0475 to function, because deletion of rgg2 led to no response (Fig. 3d).
Figure 3.
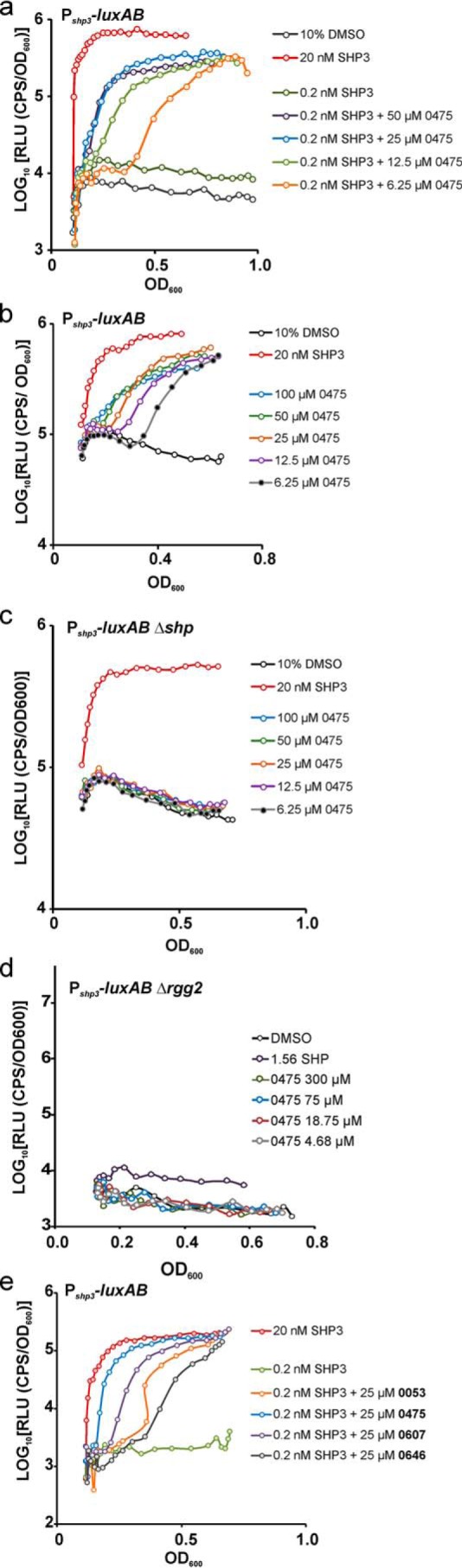
P516-0475 and analog activities require subthreshold concentrations of SHP. a, bioluminescent reporter strain JCC157 contains chromosomally integrated Pshp3-luxAB and was treated with vehicle, inducing and subinducing concentrations of SHP3 or subinducing concentrations of SHP3 with a range of 0475 concentrations. Relative luciferase activity is plotted as function of culture density. b, JCC157 (Pshp3-luxAB) treated with 0475 without exogenously added SHP. c, strain BNL170, isogenic to JCC157 but containing null mutations in shp2 and shp3 (termed Δshp), was treated with a range of concentrations of 0475. d, strain JCC166, isogenic to JCC157 but containing a deletion of rgg2, was treated with 0475. e, JCC157 (Pshp3-luxAB) was treated with analogs of compound 0475 (see Fig. 2 for analog structures).
The 10,000-compound library contains eight additional chemical analogs of 0475 with a core component of 1,2,3-triazolo[1,5-α]quinoxaline (Fig. 2c). Luminescence results from the high-throughput screen indicated that the analogs did not meet the minimal criteria (5-fold induction of luminescence) to be considered hits. Nevertheless, each analog was retested at a single concentration (25 μm) for their ability to stimulate Rgg2/3 signaling in wild-type strain JCC157 in the presence of non-inducing concentrations of SHP3 over longer periods of culturing time than used for the high-throughput screen described above. Three analogs (−0053, −0607, and −0646) stimulated Pshp3-luxAB expression, reaching a maximum luminescence levels in culture like that seen with SHP3 and 0475 (Fig. 3e and Fig. S2b). However, as seen with low concentrations of 0475, there was a clear delay in the response time to these compounds, indicating a diminished activity compared with 0475. Despite the low relative activity, their ability to eventually stimulate luminescence indicates that the criteria of the primary screen to identify agonistic compounds was overly stringent in terms of stimulation kinetics. The five remaining 0475 analogs displayed very low or no stimulatory activity by this cell-based assay (Fig. S1a). Finally, to initiate a structure–activity relationship study, a series of 0475 analogs were synthesized to investigate the effect of changing the identity of the amide substituent on the activity of the lead compound. The amines were chosen to vary the position and orientation of the ether, as well as the rigidity and lipophilicity of the amide substituent. However, in each case, none of the synthesized compounds displayed activity in the luminescence bioassay (Fig. S1b); thus, the selected alterations were apparently non-productive.
Activation of Rgg signaling by 0475 is restricted to the Rgg2/3 pathway
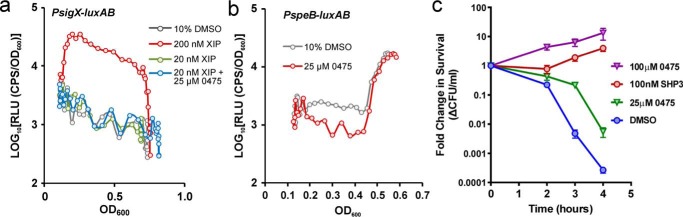
S. pyogenes contains two additional Rgg-mediated regulatory pathways, ComR and RopB, that are independent from the regulatory mechanisms of Rgg2/3 and do not respond to SHP2/3 pheromones (14, 29). To determine whether 0475 can stimulate these other Rgg pathways, we employed cell-based luciferase reporters to evaluate ComR and RopB activities. These reporters indicate transcriptional activation of the ComR target gene sigX (PsigX-luxAB; MW354) or the RopB target speB (PspeB-luxAB; MW185) (27, 14). Activation of the ComR pathway can be achieved by addition of the synthetic peptide XIP (SigX-inducing peptide; SAVDWWRL). Treatment with cell-free culture supernatants from high-cell-density cultures leads to transcriptional activation of speB (11, 12, 37). We hypothesized that if 0475 is a general agonist of Rgg pathways, stimulation of cultures harboring ComR and RopB transcriptional reporters with 0475 would cause luciferase induction. Each reporter culture was treated with 25 μm 0475 or a vehicle control and luciferase activity was monitored over time. For strain MW354, containing PsigX-luxAB, a high level of luminescence was observed when 20 nm XIP pheromone was added to cultures but not when 0475 was added alone or in the presence of a substimulatory concentration of XIP (Fig. 4a). Similarly, cultures of strain MW185, containing PspeB-luxAB, displayed induction of luciferase at higher cell densities, but supplementation with 0475 did not alter the density at which cells induced luminescence (Fig. 4b). These results show that 0475 was unable to mediate activation of other Rgg-based quorum-sensing pathways in S. pyogenes.
Figure 4.
P516-0475 does not modulate other Rgg pathways in S. pyogenes but does stimulate Rgg2/3-dependent lysozyme resistance. a, S. pyogenes strain MW354, which contains a ComR-dependent luciferase reporter (PsigX-luxAB), was treated with vehicle, inducing and subinducing concentrations of XIP or subinducing concentrations of XIP with 0475. Relative luciferase activities were plotted as a function of cell density. b, S. pyogenes strain MW185, which contains a RopB-dependent luciferase reporter (PspeB-luxAB), was treated with vehicle or with 0475. c, cultures of wild-type S. pyogenes strain JCC157 were treated with vehicle, SHP3, or 0475 and incubated for 45 min. 5 mg/ml lysozyme was then added to each culture, and at time points of 0, 2, 3, and 4 h, samples of viable cells were plated for enumeration of CFUs.
Because 0475 activation occurs only through Rgg2/3, we tested the ability of the compound to exert positive regulatory effects on a phenotype associated with this quorum-sensing pathway. Previous work determined that Rgg2/3 induction leads to lysozyme resistance in several S. pyogenes serotypes (16). Thus, we examined whether 0475 could promote bacterial survival upon lysozyme challenge. Cultures grown to the exponential phase were treated with 20 nm SHP3 or with 25 μΜ 0475 and then exposed to 5 mg/ml lysozyme. Cell viability was monitored over time by enumeration of colony-forming units (CFUs) on solid medium. As seen previously, after 4 h of exposure to lysozyme, cultures treated with 20 nm SHP3 contained ∼1,000 times more viable cells than cultures treated with a vehicle control (Fig. 4c). In cultures where 100 μm 0475 was added, cell viability was comparable with that of 20 nm SHP3-activated cells. These results demonstrate that 0475 promotes the expression of Rgg2/3 target genes that are important for survival by assaults on the cell wall.
Compound 0475 inhibits the peptide protease PepO
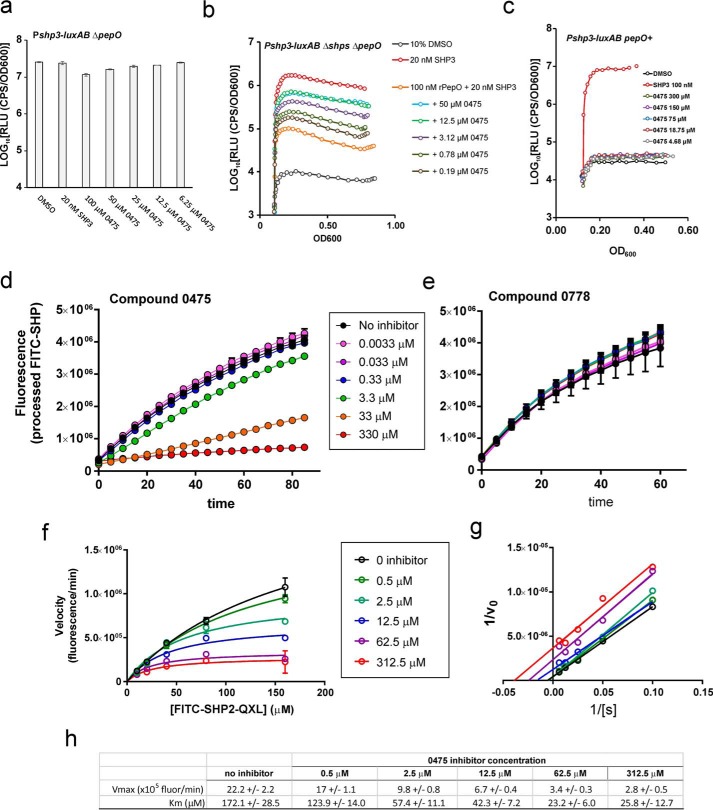
Because 0475 was unable to stimulate the Rgg2/3 pathway in the absence of pheromones and because the compound, when provided at low concentrations, had a delayed and all-or-nothing effect on induction, we considered the possibility that 0475 implemented its stimulatory effect by enhancing the stability SHP pheromones. Recently, we characterized the ability of endopeptidase PepO to degrade SHP peptides, both in bacterial culture and in vitro (21). An S. pyogenes pepO mutant displays auto-inducing activity of the Rgg2/3 pathway in laboratory conditions where the wild-type parent strain remains unstimulated unless pheromones are provided exogenously (21). Considering the appreciable role PepO contributes in SHP turnover, we speculated that 0475 may inhibit PepO degradation of SHP peptides. To test this hypothesis, we investigated the ability of 0475 to modulate Pshp-luxAB expression in a strain in which pepO was deleted (RW38). We expected that if 0475 exerted its activity on a target other than PepO, then luminescence activity would be further enhanced in the pepO-deletion mutant. However, providing a range of 0475 concentrations to the pepO mutant found in every case that the maximum luminescence achieved was unaffected, indicating that 0475 was no longer a quorum-sensing agonist toward this strain (Fig. 5a). Because these results support the possibility that the compound targets the endopeptidase, we tested this notion directly by employing recombinant S. pyogenes PepO (rPepO) and assessed the activity of the enzyme in vitro following treatment with 0475 (21). Because PepO inactivates SHP signaling by hydrolysis of the peptide, we assessed the ability of 0475 to interfere with SHP3 degradation by rPepO. A range of concentrations of 0475 were added to reactions with rPepO prior to addition of synthetic SHP3. Reaction products were then provided to cultures of reporter strain RW47 (Δshp2 Δshp3 ΔpepO), and Pshp3-luxAB transcriptional activity was assessed by bioluminescence levels. As expected, incubation of SHP3 with rPepO decreased luciferase activation substantially compared with reactions in which rPepO was omitted (Fig. 5b). The addition of 0475 increased the amount of active SHP3 remaining in reactions and did so in a dose-dependent relationship. The addition of the inhibitor to a strain in which pepO was constitutively expressed and likely produces much higher amounts of PepO than wild type prevented 0475 from having an effect (Fig. 5c), probably by overwhelming 0475.
Figure 5.
P516-0475 inhibits endopeptidase PepO activity. a, S. pyogenes strain RW38, which contains a deletion of pepO and a Pshp3-luxAB luciferase reporter, was treated with a range of concentrations of compound 0475. Maximum relative luciferase activities are displayed. b, purified rPepO was combined with a range of concentrations of compound 0475 in CDM, followed by addition of SHP3. The reaction products were added to cultures of RW47, which contains deletions of pepO and both shp genes and the luciferase reporter Pshp3-luxAB, and relative luciferase activity was monitored during growth of the cultures. c, treatment of strain RW29 (NZ131, containing Pshp-luxAB and over-expressing pepO from a plasmid) with SHP or 0475. d and e, rPepO was combined with compounds 0475 or 0778 in a magnesium buffer. 100 μm FAM-SHP2-QXL peptide was added to reactions, and fluorescence emission (produced upon peptide cleavage) was measured over time. The reactions were conducted in triplicate with standard error indicated as bars. f, kinetics of rPepO activity and inhibition of compound 0475 were determined by initial reaction velocities (V0) of FAM-SHP2-QXL peptide processing as a function of substrate concentration. g, double-reciprocal plot of kinetic data of f with linear regression analysis. h, reaction statistics of Michaelis–Menten analysis of data in f.
To monitor directly the proteolytic activity of rPepO and inhibition by 0475, we utilized a fluorescein-labeled SHP peptide conjugated to a QXL quencher. Hydrolysis of the peptide dissociates the quencher from fluorescein and enables visibility of the fluorescence emission. Samples containing 10 nm rPepO with a range of concentrations of 0475 were combined prior to addition of the fluorescent FAM-SHP2-QXL peptide. Because concentrations of 0475 increased above 3.3 μm, a dose-dependent decrease in fluorescence was observed. In comparison, when a similar gradient of the inactive compound 0778 was combined with rPepO, SHP2 degradation was not inhibited. A PepO ortholog from Streptococcus gordonii was also purified and tested by this assay and shown to be inhibited by 0475 (Fig. S2b). Together, these results demonstrate that 0475 is capable of inhibiting rPepO proteolytic activity of SHP from at least two bacterial species.
Inhibition of rPepO processing of FAM-SHP2-QXL by 0475 was repeated with a range of substrate and inhibitor concentrations to help inform the mechanism by which inhibition was occurring (Fig. 5f). The Michaelis–Menten rate constants (Km) and maximum velocities (Vmax) for rPepO were determined in the presence and absence of 0475 inhibitor (Fig. 5h). From these plots, the concentration of 0475 providing half-maximal inhibition (IC50) was estimated to be ∼10 μm. Notably, as the concentration of 0475 was increased in the reactions, both the Vmax and Km values decreased proportionally. These results suggest a mechanism of uncompetitive competition, where the inhibitor binds the enzyme–substrate complex only after the substrate is bound.
Discussion
Quorum sensing provides an ability to unify populations of bacteria through the coordination of gene expression and behavior of individuals in the community. Social coordination of behaviors benefits communities through shared labor in resource acquisition, defense mechanisms, and stress response. Because QS systems are responsive to extracellular pheromones, there is a strong potential to harness or manipulate bacterial behavior through modulation of sensory systems through exogenous application of chemical agonists and antagonists. Quorum-sensing modulators also provide an opportunity to identify or validate functional components in bacterial signaling pathways. Here, it was our intention to identify small molecules that displayed a capacity to either substitute for or potentiate the stimulatory activity of SHP2/3 pheromones in S. pyogenes. We anticipated two general mechanisms for the provocation of the Rgg2/3 pathway. In one scenario, we anticipated identifying molecules that would directly bind to Rgg2 and/or Rgg3 and serve as agonistic ligands in place of SHP peptides or that would bind allosterically to favor Rgg-SHP–binding energetics, perhaps by decreasing dissociation rates. It was for this reason, to not overlook small molecule potentiators of the native ligands, that we included subinducing amounts of SHP pheromone in the screening strategy. In a second scenario, we reasoned that small molecule inducers could raise the steady-state concentration of pheromones in a culture (or within a cell) by increasing production of pheromones or by slowing down their turnover. Helpful in both identifying agonistic molecules and determining their sites of action was the luminescence reporter that provided sensitivity in monitoring activation of the Rgg2/3 signaling pathway (9). For the compound featured in this report, 0475, we quickly dismissed the possibility that it acted as a direct agonist on Rgg2 or Rgg3 because activity required the presence of a SHP peptide. However, distinguishing between possibilities that 0475 acted either as a SHP potentiator or an agent that could increase SHP concentrations was not straightforward using transcriptional reporters. However, we interpreted observations of threshold-like responses to low levels of the compound (Fig. 3, a and b) as consistent with an ability to raise levels of pheromone available to or within cells. With the discovery that the endopeptidase PepO degrades SHP2/3 signals in S. pyogenes cultures, it presented an obvious candidate as the target for 0475.
PepO, a member of the M13 neprolysin family of peptidases, is best studied in Lactococcus lactis, but similar homologs are found among many bacterial phyla. Identified roles for PepO have included uses in catabolic nutritional acquisition and recycling of endogenous proteins within the cell but also roles in pathogenesis (30–32). Human neprolysin is expressed in a wide range of tissues and organs, including the brain, heart, pulmonary, renal, and gastrointestinal systems and can process several peptide substrates involved in paracrine and hormone signaling. Inhibitors of neprilysin have been developed mainly for treating heart failure, as a benefit in blocking the role of neprilysin in angiotensin peptide activities in cardiovascular function (33). Processing and turnover of signaling peptides is therefore a common biological function for this family of endopeptidases, and inhibition of these enzymes can have beneficial applications. It remains unclear how blocking SHP2/3 turnover by inhibition of PepO in S. pyogenes could have application, as studies continue to investigate physiological roles of the Rgg2/3 quorum sensing pathway. However, other uses for inhibitors of PepO may be found as useful in other bacteria. For instance, because PepO of Streptococcus pneumoniae was proposed to bind human complement factor C1, plasminogen, and fibronectin, perhaps PepO inhibition might block these interactions and associated cell adherence and invasion (32, 34).
The mechanism by which 0475 inhibits PepO activity and the pathway by which the compound can access cytoplasmic PepO will require further investigation to optimize compound attributes. Enzyme kinetic analysis in the presence of inhibitor suggested that 0475 acts as an uncompetitive inhibitor and indicates the compound binds to the enzyme–substrate complex but not to unbound enzyme alone. Efforts to map interaction sites between 0475 and PepO could help guide enzyme structure-led compound design. However, in vitro analysis of inhibitor activity utilizing recombinant PepO does not consider the ability of inhibitors to enter cells where PepO is thought to reside. Our prior studies indicated that PepO activity was retained within the bacterial cytoplasm, which is consistent with the sequence of the protein not containing any sorting motifs that would enable delivery to the membrane or be secreted from the cell (21). The use of a cell-based screening strategy was fortuitous in identifying a compound readily able to traverse the cytoplasmic membrane. Some analogs of the compound retained an ability to induce the quorum-sensing pathway, although at lower potential levels, but designed analogs generated in house were not active. The difference in potentiating ability between the analogs suggests that the amide substituent plays an important role in inhibiting PepO activity. The Lewis basic nature of the methyl ether of 0475 could be a critical attribute in maximal activity, because it may be involved in binding a metal or accepting a hydrogen bond. However, designed modifications altering the lipophilicity and overall rigidity of the molecule should consider effects on traversing the cytoplasmic membrane. Additional structure–activity relationship studies of 0475 will help identify optimal activity groups to enhance inhibition potency.
Our efforts contribute to a long-term strategic plan to identify methodologies for manipulating bacterial behaviors without an intent to disrupt or diminish bacterial growth. Signaling pathways like QS circuits present ideal targets for chemical modulators because pheromones are themselves chemical entities that transmit between cells. QS pathways are typically not critical for bacterial viability but do provide a means to enhance fitness for a population, depending on environmental conditions. Relatively few QS networks are understood to a sufficient level to predict how their modulation would affect outcomes of bacterial behavior in complex systems, for instance during infection of an animal host. Identifying means to antagonize or stimulate QS networks are both worthwhile pursuits, particularly in establishing relationships between pathway components even if their use as therapeutics are not immediate. Positive and negative modulators are sure to help in the process of determining roles of signaling pathways during complex interactions with a host or with other microbes. The PepO inhibitor 0475 presents a new opportunity to interfere with social behavior of bacteria. It will be intriguing to test whether PepO is utilized by other bacteria to modulate cell-cell signaling, whether other enzymes of the large neprilysin family—commonly found in mammalian hosts of bacterial pathogens—affect QS, and whether inhibitors of these peptidases can be used to modulate bacterial communication.
Experimental procedures
Bacterial strains and culturing conditions
Bacterial strains used in this study are described in Table 1. S. pyogenes was grown in Todd Hewitt broth or CDM supplemented with 1% glucose at 37 °C unless stated otherwise (35). Luciferase assays included the following antibiotics when required: erythromycin (1 μg/ml) and kanamycin (100 μg/ml).
Table 1.
Strains used in this study
| Strain | Description | Reference |
|---|---|---|
| MW185 | NZ131 PspeB-luxAB ErmR int | Ref. 27 |
| MW354 | NZ131 PsigX-luxAB ErmR comRWT | Ref. 14 |
| RW47 | NZ131 Pshp3-luxAB ErmR ΔpepO:KanR shp2GGG shp3GGG | Ref. 21 |
| RW29 | NZ131 Pshp3-luxAB ErmR (pLZ12-PrecA-pepO) | Ref. 21 |
| RW38 | NZ131 Pshp3-luxAB ErmR ΔpepO:KanR | Ref. 21 |
| JCC166 | NZ131 Pshp3-luxAB ErmR Δrgg2 | Ref. 9 |
| JCC157 | NZ131 Pshp3-luxAB ErmR | Ref. 9 |
| BNL170 | NZ131 Pshp3-luxAB ErmR shp2GGG shp3GGG | Ref. 28 |
Chemical reagents
Quorum-sensing peptide pheromone SHP3 (DIIIIVGG) was synthesized and provided as a desiccated material by AbClonal (Woburn, MA). The peptide was dissolved in DMSO to prepare a 1 mm stock solution and was diluted into growth medium as appropriate. The 10,000-compound chemical was provided by the High-Throughput Screening Facility at the University of Illinois at Chicago and comprised a selection of chemicals supplied by ChemDiv (San Diego, CA). All compounds were reconstituted in DMSO and prepared as 10 mm stock solutions. Larger quantities of compound 0475 and its analogs were synthesized in-house in the Department of Chemistry, University of Illinois at Chicago. A series of P516-0475 analogs were synthesized starting from commercially available 2-nitro-4-toluidine (see the supporting information). Diazotization followed by nucleophilic aromatic substitution with sodium azide produced azide 1, which was reacted with sodium enolate 2 in a [3 + 2] cycloaddition reaction to form triazole 3 as a single regioisomer. Palladium-catalyzed hydrogenation followed by base-mediated hydrolysis of both ethyl ester groups produced acid 4. Conversion to the acid chloride by treatment with thionyl chloride provided an electrophilic intermediate that was reacted with a series of primary and secondary amines to investigate the effect of changing the identity of the amide substituent on the activity of the lead compound 5.
High-throughput screen
The high-throughput chemical screen was conducted in Greiner 96-well flat-bottomed polystyrol plates containing CDM with 10% DMSO, 0.2 or 20 nm SHP3, and 25 μm sample compound. Bacterial cultures of a Pshp3-luxAB luciferase reporter (JCC157) were grown as described, and a volume of 180 μl was added to each well containing 1 of the 10,000 compounds of the ChemDiv library. Eight wells per plate were reserved for the negative control containing 0.2 nm SHP3, and eight wells received 20 nm SHP3 as a positive control. Each compound was tested in duplicate on independent plates. The plates were incubated at room temperature for 30 min following the combination of all components. 1 μl of decanal, which provides the substrate for luciferase as a vapor, was placed in wells prior to measuring luminescence using a Tecan Infinite F200 Pro plate reader. The mean values and standard deviations of the positive and negative controls were used to determine the Z-factor [1-(3 × standard deviation #1 + 3 - standard deviation #2)/(positive mean − negative mean)] for each plate; all plates in this study displayed a Z-factor between 0.5 and 1.0 (Ref. 36 and Table S1).
Luciferase transcriptional assay
White-walled, clear-bottomed, 96-well plates were used for postscreening bioassays. Plate lids were treated with a surfactant to inhibit condensation during incubation, and the luciferase substrate, 1% decanal suspended in mineral oil, was injected into the spaces separating the assay wells and provided an ongoing source of substrate in a vaporous form (18). Into each well was placed 10 μl of CDM containing synthetic peptide pheromones at a final concentration of 0.2 or 20 nm SHP3 dissolved in 10% DMSO and 10 μl of the tested compound at indicated concentrations (μm). S. pyogenes cultures (using appropriate mutant strains as indicated in the text) were grown in CDM at 37 °C to an A600 of 0.1, and 180 μl of the culture was added to each well of the prepared 96-well plate. The plate was incubated at 37 °C with linear shaking in a Biotek Synergy 2 plate reader, and the A600 and luminescence (LUM) was measured once every 10 min for 6 h. Relative luminescence activity was calculated as LUM values divided by A600, reported as relative light units (RLUs), and is typically graphed as a function of A600. Alternatively, maximum RLU is reported as the peak relative luminescence counts in an experiment or at a set time point. All experiments were conducted a minimum of three times as independent biological replicates.
Lysozyme challenge
Lysozyme-sensitivity assays were performed as described previously (16). Briefly, the cells were grown at 37 °C in CDM to OD 0.1, upon which vehicle, 100 nm SHP3, 25 μm 0475, or 100 μm 0475 were added to the cultures. The cultures were incubated for 45 min at 37 °C, upon which they were diluted to OD 0.1, and lysozyme was added to a final concentration of 5 mg/ml. Samples were removed once per hour to enumerate viable CFUs after lysozyme challenge by plating onto Todd Hewitt broth agar and incubating overnight at 37 °C. Each experiment was performed in duplicate, and the results are representative of at least three biological replicates.
Cell-based peptide degradation assays
An assay to indicate the ability of recombinant PepO to degrade SHP3 pheromone was performed as described previously (21). Samples containing 100 nm rPepO, 20 nm SHP3 in the presence or absence of various concentrations of 0475 were incubated in CDM with 10% DMSO for 5 h at 20 °C. 20 μl of the reactions were added to 180 μl of an exponentially growing culture of S. pyogenes (RW47, A600 = 0.1) in a 96-well flat clear bottom plate. LUM and growth (A600) of these cultures were measured continuously for 6 h at 37 °C in a Biotek plate reader. Maximum RLU values were determined and compared with the positive control (receiving no inhibitory compound) and to the negative control, receiving no rPepO.
PepO enzyme kinetic assay
To determine the rate of SHP processing by PepO, a fluorescent SHP2-C8 peptide with quencher (5-FAM-DIIIIVGGK-QXL520; AnaSpec) was used. 10 nm recombinant PepO (rPepO) was combined with a range of concentrations of fluorescent peptide substrate and of compounds 0475 and 0778 in 20-μl reactions containing buffer (0.0015 mm MgSO4 and 10 mm Tris-HCl, pH 7). To reactions containing inhibitors, PepO and the inhibitor were preincubated for 30 min at 20 °C, after which substrate was added, and fluorescence was measured every 5 min in a Biotek Synergy 2 plate reader using excitation at 485 nm and emission at 528 nm. The results were adjusted for FAM-SHP2-QXL background and plotted as relative fluorescent units versus time to determine reaction velocities. The reactions were conducted in triplicate, and the data were analyzed for Vmax and Km values calculated using GraphPad Prism with a Michaelis–Menten model of enzyme kinetics.
Author contributions
T. G. P. M. and M. J. F. designed the research; K. R. managed HTS setup; D.-S. W. synthesized all 0475 compound analogs; T. G. P. M. and A. G. performed the experiments and analyzed the data; M. J. F. and K. R. provided data analysis for HTS and protein kinetics; T. G. P. M. wrote the original draft; and M. J. F. and T. G. D. wrote, reviewed, and edited the paper.
Supplementary Material
Acknowledgments
We thank Reid Wilkening for preparation of S. pyogenes and S. gordonii rPepO and Dr. Laura Bloem and Dr. Jason Hickock of the UICentre for valuable project discussions and the gift of FAM-SHP2-QXL peptide.
This work was supported by National Institutes of Health Grants AI091779 and AI125452, the Burroughs Wellcome Fund PATH award, and the Chicago Biomedical Consortium with support from the Searle Funds at the Chicago Community Trust. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Table S1 and Figs. S1 and S2.
- QS
- quorum sensing
- SHP
- short hydrophobic pheromone
- CDM
- chemically defined medium
- CFU
- colony-forming unit
- LUM
- luminescence
- RLU
- relative light unit.
References
- 1. Dandekar A. A., Chugani S., and Greenberg E. P. (2012) Bacterial quorum sensing, metabolic incentives to cooperate. Science 338, 264–266 10.1126/science.1227289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goo E., Majerczyk C. D., An J. H., Chandler J. R., Seo Y. S, Ham H., Lim J. Y., Kim H., Lee B., Jang M. S., Greenberg E. P., and Hwang I. (2012) Bacterial quorum sensing, cooperativity, and anticipation of stationary-phase stress. Proc. Natl. Acad. Sci. U.S.A. 109, 19775–19780 10.1073/pnas.1218092109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnston C., Martin B., Fichant G., Polard P., and Claverys J. (2014) Bacterial transformation: distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 12, 181–196 10.1038/nrmicro3199 [DOI] [PubMed] [Google Scholar]
- 4. Chen Y., Bandyopadhyay A., Kozlowicz B. K., Haemig H. A., Tai A., Hu W. S., et al. (2017) Mechanisms of peptide sex pheromone regulation of conjugation in Enterococcus faecalis. Microbiologyopen 6, 10.1002/mbo3.492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parsek M. R., and Greenberg E. P. (2005) Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 13, 27–33 10.1016/j.tim.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 6. Sulavik M. C., Tardif G., and Clewell D. B. (1992) Identification of a gene, rgg, which regulates expression of glucosyltransferase and influences the spp. phenotype of Streptococcus gordonii Challis. J. Bacteriol. 174, 3577–3586 10.1128/jb.174.11.3577-3586.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fleuchot B., Gitton C., Guillot A., Vidic J., Nicolas P., Besset C., Fontaine L., Hols P., Leblond-Bourget N., Monnet V., and Gardan R. (2011) Rgg proteins associated with internalized small hydrophobic peptides: a new quorum sensing mechanism in streptococci. Mol. Microbiol. 80, 1102–1119 10.1111/j.1365-2958.2011.07633.x [DOI] [PubMed] [Google Scholar]
- 8. Blatch G. L., and Lässle M. (1999) The tetratricopeptide repeat: a structural motif mediating protein–protein interactions. Bioessays 21, 932–939 10.1002/(SICI)1521-1878(199911)21:11%3C932::AID-BIES5%3E3.0.CO%3B2-N [DOI] [PubMed] [Google Scholar]
- 9. Chang J. C., LaSarre B., Jimenez J. C., Aggarwal C., and Federle M. J. (2011) Two group A streptococcal peptide pheromones act through opposing Rgg regulators to control biofilm development. PLoS Pathogens 7, e1002190 10.1371/journal.ppat.1002190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neely M. N., Lyon W. R., Runft D. L., and Caparon M. (2003) Role of RopB in growth phase expression of the SpeB cysteine protease of Streptococcus pyogenes. J. Bacteriol. 185, 5166–5174 10.1128/JB.185.17.5166-5174.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shelburne S. A. 3rd, Olsen R. J., Makthal N., Brown N. G., Sahasrabhojane P., Watkins E. M., Palzkill T., Musser J. M., and Kumaraswami M. (2011) An amino-terminal signal peptide of Vfr protein negatively influences RopB-dependent SpeB expression and attenuates virulence in Streptococcus pyogenes. Mol. Microbiol. 82, 1481–1495 10.1111/j.1365-2958.2011.07902.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Makthal N., Gavagan M., Do H., Olsen R. J., Musser J. M., and Kumaraswami M. (2016) Structural and functional analysis of RopB: a major virulence regulator in Streptococcus pyogenes. Mol. Microbiol. 99, 1119–1133 10.1111/mmi.13294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mashburn-Warren L., Morrison D. A., and Federle M. J. (2010) A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol. Microbiol. 78, 589–606 10.1111/j.1365-2958.2010.07361.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mashburn-Warren L., Morrison D. A., and Federle M. J. (2012) The cryptic competence pathway in Streptococcus pyogenes is controlled by a peptide pheromone. J Bacteriol. 194, 4589–4600 10.1128/JB.00830-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shanker E., Morrison D. A., Talagas A., Nessler S., Federle M. J., and Prehna G. (2016) Pheromone recognition and selectivity by ComR proteins among Streptococcus species. PLoS Pathogens 12, e1005979 10.1371/journal.ppat.1005979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang J. C., Jimenez J. C., and Federle M. J. (2015) Induction of a quorum sensing pathway by environmental signals enhances group A streptococcal resistance to lysozyme. Mol. Microbiol. 97, 1097–1113 10.1111/mmi.13088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang J. C., Federle M. J. (2016) PptAB exports Rgg quorum-sensing peptides in Streptococcus. PLoS One 11, e0168461 10.1371/journal.pone.0168461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. LaSarre B., Chang J. C., and Federle M. J. (2013) Redundant group A Streptococcus signaling peptides exhibit unique activation potentials. J. Bacteriol. 195, 4310–4318 10.1128/JB.00684-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lasarre B., Aggarwal C., and Federle M. J. (2013) Antagonistic Rgg regulators mediate quorum sensing via competitive DNA binding in Streptococcus pyogenes. mBio 3, e00333–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parashar V., Aggarwal C., Federle M. J., and Neiditch M. B. (2015) Rgg protein structure-function and inhibition by cyclic peptide compounds. Proc. Natl. Acad. Sci. U.S.A. 112, 5177–5182 10.1073/pnas.1500357112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilkening R. V., Chang J. C., and Federle M. J. (2016) PepO, a CovRS-controlled endopeptidase, disrupts Streptococcus pyogenes quorum sensing. Mol. Microbiol. 99, 71–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rawlings N. D., and Barrett A. J. (1995) Evolutionary families of metallopeptidases. Methods Enzymol. 248, 183–228 10.1016/0076-6879(95)48015-3 [DOI] [PubMed] [Google Scholar]
- 23. Bland N. D., Pinney J. W., Thomas J. E., Turner A. J., and Isaac R. E. (2008) Bioinformatic analysis of the neprilysin (M13) family of peptidases reveals complex evolutionary and functional relationships. BMC Evol. Biol. 8, 16 10.1186/1471-2148-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dalton T. L., and Scott J. R. (2004) CovS inactivates CovR and is required for growth under conditions of general stress in Streptococcus pyogenes. J. Bacteriol. 186, 3928–3937 10.1128/JB.186.12.3928-3937.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gryllos I., Tran-Winkler H. J., Cheng M. F., Chung H., Bolcome R. 3rd, Lu W., Lehrer R. I., and Wessels M. R. (2008) Induction of group A Streptococcus virulence by a human antimicrobial peptide. Proc. Natl. Acad. Sci. U.S.A. 105, 16755–16760 10.1073/pnas.0803815105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Velarde J. J., Ashbaugh M., and Wessels M. R. (2014) The human antimicrobial peptide LL-37 binds directly to CsrS, a sensor histidine kinase of group A Streptococcus, to activate expression of virulence factors. J. Biol. Chem. 289, 36315–36324 10.1074/jbc.M114.605394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aggarwal C., Jimenez J. C., Lee H., Chlipala G. E., Ratia K., and Federle M. J. (2015) Identification of quorum-sensing inhibitors disrupting signaling between Rgg and short hydrophobic peptides in streptococci. mBio 6, e00393–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cook L. C., LaSarre B., and Federle M. J. (2013) Interspecies communication among commensal and pathogenic streptococci. mBio 4, e00382–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lyon W. R., Gibson C. M., and Caparon M. G. (1998) A role for trigger factor and an rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 17, 6263–6275 10.1093/emboj/17.21.6263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang N., and Portis A. R. (1999) Mechanism of light regulation of Rubisco: a specific role for the larger Rubisco activase isoform involving reductive activation by thioredoxin-F. Proc. Natl. Acad. Sci. U.S.A. 96, 9438–9443 10.1073/pnas.96.16.9438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ansai T., Yu W., Urnowey S., Barik S., and Takehara T. (2003) Construction of a pepO gene deficient mutant of Porphyromonas gingivalis: potential role of endopeptidase O in the invasion of host cells. Oral Microbiol. Immunol. 18, 398–400 10.1046/j.0902-0055.2003.00080.x [DOI] [PubMed] [Google Scholar]
- 32. Agarwal V., Sroka M., Fulde M., Bergmann S., Riesbeck K., and Blom A. M. (2014) Binding of Streptococcus pneumoniae endopeptidase O (PepO) to complement component C1q modulates the complement attack and promotes host cell adherence. J. Biol. Chem. 289, 15833–15844 10.1074/jbc.M113.530212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bayes-Genis A., Barallat J., and Richards A. M. (2016) A test in context: neprilysin. J. Am. Coll. Cardiol. 68, 639–653 10.1016/j.jacc.2016.04.060 [DOI] [PubMed] [Google Scholar]
- 34. Agarwal V., Kuchipudi A., Fulde M., Riesbeck K., Bergmann S., and Blom A. M. (2013) Streptococcus pneumoniae endopeptidase O (PepO) is a multifunctional plasminogen- and fibronectin-binding protein, facilitating evasion of innate immunity and invasion of host cells. J. Biol. Chem. 288, 6849–6863 10.1074/jbc.M112.405530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van de Rijn I., and Kessler R. E. (1980) Growth characteristics of group A streptococci in a new chemically defined medium. Infect. Immun. 27, 444–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang J. H., Chung T. D., and Oldenburg K. R. (1999) A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen 4, 67–73 10.1177/108705719900400206 [DOI] [PubMed] [Google Scholar]
- 37. Do H., Makthal N., VanderWal A. R., Rettel M., Savitski M. M., Peschek N., Papenfort K., Olsen R. J., Musser J. M., and Kumaraswami M. (2017) Leaderless secreted peptide signaling molecule alters global gene expression and increases virulence of a human bacterial pathogen. Proc. Natl. Acad. Sci. U.S.A. 114, E8498–E8507 10.1073/pnas.1705972114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.