Abstract
Inflammation and cancer stem cells (CSCs) are becoming increasingly recognized as components of tumorigenesis in breast cancer. In the present study, the association between inflammation and BCSC phenotype was evaluated in human breast cancer tissue. Immunohistochemical staining for cluster of differentiation (CD)24, 44, 4, 8 and 68 was performed using tissue microarray blocks containing 47 consecutive cases of invasive breast carcinoma and 10 normal breast tissue samples. The levels of inflammatory modulators and cytokines, and intratumoral or peritumoral lymphocyte infiltration, were assessed. BCSCs were defined as CD44+/CD24− tumor cells. In total, 21.3% of samples exhibited the CD44+/CD24− phenotype. This phenotype was identified to be significantly inversely associated with lymph node metastasis. In addition, the CD44+/CD24− phenotype was significantly associated with the molecular subtype of breast cancer, and was particularly increased in the basal-like subtype. Furthermore, the CD44+/CD24− phenotype was significantly associated with intratumoral inflammation and tumor-infiltrating CD4+ T cell counts. Notably, tumor-infiltrating CD4+ T cells were significantly increased in patients with the basal-like molecular subtype of breast cancer. In conclusion, the present study identified a significant association between inflammation and the CD44+/CD24− phenotype in breast cancer. These results suggest that the interaction between inflammation and CSCs may affect the tumorigenesis and progression of breast cancer. Further studies are required to clarify the role of inflammation and CSCs in breast cancer.
Keywords: inflammation, cancer stem cell, breast cancer, tumor-infiltrating lymphocyte, tumor microenvironment
Introduction
The association between inflammation and the development of cancer has been suggested for numerous years (1,2). Specifically, in breast cancer inflammation is increasingly recognized as an important component of tumorigenesis. Previous studies have reported that numerous inflammatory mediators influence breast cancer development and progression (3–7).
There is increasing evidence that cancer stem cells (CSCs) mediate tumor growth and metastasis (8). CSCs possess two main properties, the ability to self-renew and the ability to differentiate into heterogeneous lineages of cancer cells that comprise the tumor. Breast tumors cells that exhibit the properties of CSCs have been termed breast CSCs (BCSCs) (9,10). Previously, it has been suggested that inflammation may regulate BCSCs, and certain immune mediators have been reported to influence BCSC biology (11). There is an investigation into immunotherapies targeting CSCs and an initial report has demonstrated the potential of immunotherapy as a cancer treatment (12); however, the mechanisms underlying these approaches are not yet fully characterized. The majority of previous studies were pre-clinical, and the role of inflammation and CSCs in patients with breast cancer was not well defined. In the present study, the association between inflammation and the BCSC phenotype was evaluated in human breast cancer tissue. In addition, the association between BCSCs and inflammation in the progression of breast cancer was investigated.
Materials and methods
Patients and tissue microarrays (TMAs)
A total of 47 consecutive patients with primary breast cancer who had undergone surgery between May 2008 and November 2011 at Daegu Catholic University Hospital (Daegu, Korea) were included in the present study. The inclusion criteria were as follows: i) Patient had primary breast cancer; ii) patient provided informed consent; iii) patient had undergone surgery, including breast conserving surgery or mastectomy; iv) patient had a tissue sample available following surgery. All patients were female; the mean age of the patients was 55.77±13.47 years (range, 34–90 years).
All data was retrospectively analyzed. All tissue specimens had previously been formalin fixed, paraffin embedded, stained with hematoxylin and eosin, and reviewed by an experienced pathologist. The clinical information of the patients and their tumor characteristics, including tumor size, nodal status, histological grade, lymphovascular invasion status and other prognostic factors, were evaluated based on medical records, and pathological reports. Breast cancer staging was assessed according to the seventh edition of the American Joint Committee on Cancer staging manual for breast cancer (13). Histologic grade was assessed using the Nottingham grading system (14). Ethical approval for the study was obtained from the Institutional Review Board of Daegu Catholic University Hospital. Written informed consent was obtained from all patients.
TMAs were constructed using representative paraffin blocks of 47 cases of invasive breast carcinoma and 10 normal breast tissue samples obtained from the same patients, following the method described in our previous study (15).
Immunohistochemical staining
Immunohistochemical staining was performed on TMA sections, including cancer and normal tissue, using the Bond Polymer Intense Detection system (Leica Microsystems, Inc., Buffalo Grove, IL, USA) according to the manufacturer's protocol with minor modifications. The TMA blocks were cut into 5-µm-thick sections and deparaffinized with Bond Dewax solution (Leica Microsystems, Inc.). An antigen retrieval procedure was performed using Bond ER Solution (Leica Microsystems, Inc.) for 30 min at 100°C.
Endogenous peroxidase activity was quenched with hydrogen peroxide for 5 min at 25°C. Sections were then incubated for 15 min at room temperature with primary monoclonal antibodies directed against the following proteins: cluster of differentiation (CD)24 (dilution, 1:20; cat. no. SC-7034; clone C-20; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), CD44 (dilution, 1:1,000; cat. no. NBP1-47386; clone, 8E2F3; Novus Biologicals, LLC, Littleton, CO, USA), CD4 (ready-to-use dilution; cat. no. PA0368; clone 4B12; Leica Biosystems, Inc., Wetzlar, Germany), CD8 (dilution 1:200; cat. no. M7103; clone C8/144B), CD68 (dilution, 1:200; cat no. M0876; clone, PG-M1), epidermal growth factor receptor (EGFR; dilution, 1:100; cat. no. M7239; clone, EGFR.25), apoptosis regulator Bcl-2 (dilution, 1:4; cat. no. IR614; clone, 124), human epidermal growth factor receptor 2 (HER2; dilution, 1:250; cat. no. A048529-1; clone, A0485; all from Dako; Agilent Technologies, Inc.), estrogen receptor (ER; dilution, 1:100; cat. no. NCL-L-ER-6F11; clone, 6F11), progesterone receptor (PR; dilution, 1:100; cat. no. NCL-L-PGR-312; clone, 16; both from Novocastra; Leica Biosystems, Inc.), proliferation marker protein Ki-67 (dilution, 1:200; cat. no. 275R-16; clone, MM1-L; Sigma-Aldrich; Merck KGaA) and tumor antigen p53 (dilution, 1:200; cat. no. 18-0129; clone, BP53.12; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
The samples were then treated with a biotin-free polymeric horseradish peroxidase-linker antibody conjugate system (Bond Polymer Refine Detection; ready-to-use dilution; cat. no. DS9800; Leica Biosystems, Inc.). Staining was performed in a Bond-Max Automatic Slide Stainer (Leica Microsystems, Inc.). A BX50 light microscope (Olympus Corporation, Tokyo, Japan) was used to visualize staining at ×400 magnification; the stained cells were manually counted.
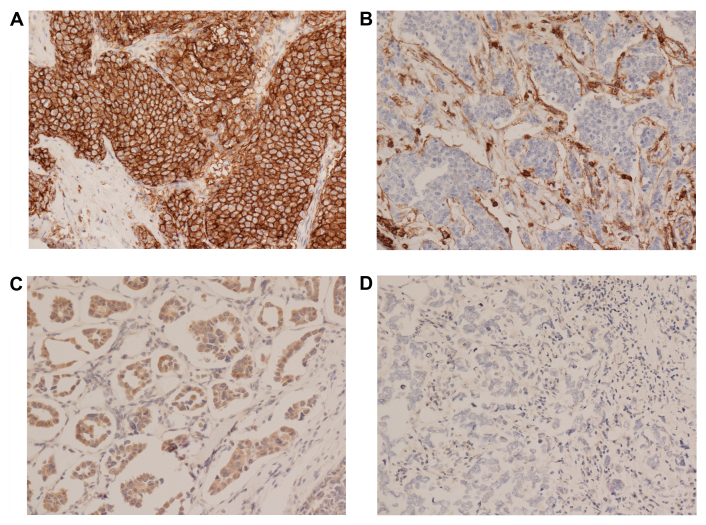
Expression levels of CD24 and CD44 were graded on staining intensity and the proportion of positively stained tumor cells. The levels of immunopositivity were semiquantitatively scored as follows: 0, No staining; 1+, minimal staining intensity, <10% of cells positively stained; 2+, moderate staining intensity, 10–50% of cells positively stained and 3+, marked, staining intensity, >50% of cells positively stained. Scores of 0 and 1 were designated as negative, and 2 and 3 as positive. Examples of this staining are illustrated in Fig. 1. BCSCs were defined as CD44+/CD24− tumor cells. For ER and PR, nuclear staining in ≥1% of tumor cells was considered positive. Cytoplasmic and membranous staining of any intensity in ≥5% of the tumor cells was considered as positive for Bcl-2. Membranous staining for HER2 with strong complete staining in 30% of the tumor cells was regarded as HER2 overexpression. p53 staining was scored positive if ≥5% of the cells were stained with a strong intensity. The Ki-67 labeling index was expressed as a percentage and was graded as high if the number of positively stained cells was ≥14%.
Figure 1.
Immunohistochemical staining for CD24 and CD44 in breast cancer tissue. The expression level was graded on the staining intensity and the proportion of positively stained tumor cells (magnification, ×400). (A) Immunopositive and (B) immunonegative CD44 expression results. (C) Immunopositive and (D) immunonegative CD24 expression results. CD, cluster of differentiation.
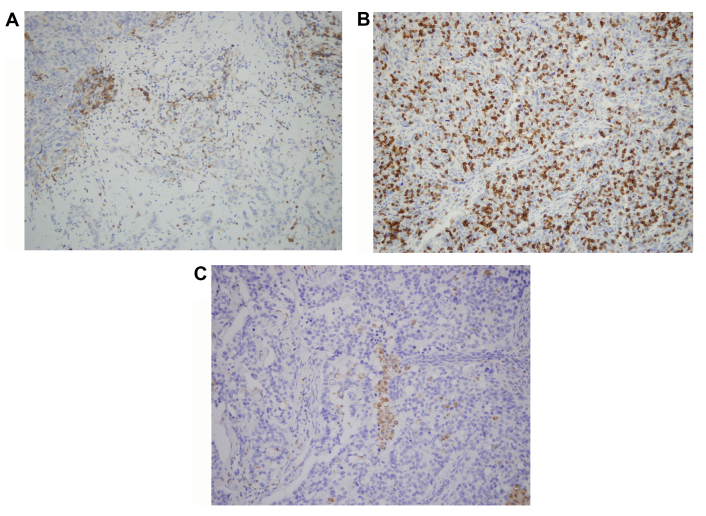
The CD4, CD8 and CD68 immunostained TMA sections were evaluated under a microscope and the number of CD4+ and CD8+ T cells and CD68+ macrophages were counted in the stroma and cancer cell nests. Examples of this staining are illustrated in Fig. 2. Intratumoral (in the tumor cell nest) or peritumoral (in the stroma around the tumor) lymphocyte infiltration was semiquantitatively graded as follows: 0, No lymphocyte infiltration; 1, mild scattered lymphocyte infiltration in either stroma or tumor cell nest; 2, moderate lymphocyte infiltration with some lymph follicle formation; 3, dense and widespread lymphocyte infiltration.
Figure 2.
Immunohistochemical staining for CD4, CD8 and CD68 in breast cancer tissue (magnification, ×400). The immunostained sections represent positive staining for (A) CD4+ T cells, (B) CD8+ T cells and (C) CD68+ macrophages. CD, cluster of differentiation.
Reverse transcription polymerase chain reaction (RT-PCR)
The levels of inflammatory modulators and cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-2, −4 and −6, interferon (IFN)-γ and nuclear factor (NF)-κB p50 were assessed by the levels of mRNA transcripts in frozen tissue using RT-PCR. Total RNA was extracted from frozen breast cancer tissues using Trizol reagent (cat. no. A33250; Invitrogen; Thermo Fisher Scientific, Inc.). Subsequent to lysing and homogenizing samples in the Trizol reagent, the samples were incubated for 5 min at room temperature. Chloroform was added to the samples, and the samples were agitated for 15 sec, then incubated for 2–3 min at room temperature. Following centrifugation for 5 min at 12,000–16,000 × g at 4°C, the RNA in the samples was precipitated by adding isopropanol. The samples were washed with in 75% ethanol then the RNA was dissolved with RNase-free water. The RNA was quantified by measuring absorbance at 260 and 280 nm.
To determine the expression of ALCAM, inflammatory modulators and cytokines, reverse transcription of the total RNA was performed. First-strand complementary (c)DNA was generated using a commercial kit (Superscript II RNase H-reverse transcriptase, cat no. 18064071; Invitrogen; Thermo Fisher Scientific, Inc.) used according to the manufacturer's protocol. For the PCR of ALCAM, TNF-α, IL-4, IFN-γ and NF-κB p50, the following primers were used: ALCAM, forward, 5′-CAAGACAACCAAGGCTGACA-3′; reverse, 5′-CGCAGACATAGTTTCCAGCA-3′; TNF-α, forwards, 5′-CCCTCAACCTCTTCTGGCTC-3′; reverse, 5′-AGGCAGCTCCTACATTGGGT−3′; IL-2, forwards, 5′-GCAACTCCTGTCTTGCATTG-3′; reverse, 5′-TGCTTTGACAAAAGGTAATCCA-3′; IL-4, forwards, 5′-ACTGCTTCCCCCTCTGTTCT-3′; reverse, 5′-TGATCGTCTTTAGCCTTTCCA-3′; IL-6, forwards, 5′-TACCCCCAGGAGAAGATTCC-3′; reverse, 5′-AAAGCTGCGCAGAATGAGAT-3′; interferon-γ, forwards, 5′-TTGGCTTTTCAGCTCTGCAT-3′; reverse, 5′-CTGTTTTAGCTGCTGGCGAC-3′; NF-kB p50, forwards, 5′-CACCTAGCTGCCAAAGAAGG-3′; reverse, 5′-TCAGCCAGCTGTTTCATGTC-3′. β-actin was used as a reference gene and the primer was as follows: Forwards, 5′-AGGGTGTGATGTGGGTATGG-3′; reverse, 5′-CAGGATCTTCATGAGGTAGTC-3′.
PCR was performed with 1 µl of cDNA and 0.4 U Taq polymerase (cat. no. #18038042; Thermo Fisher Scientific, Inc.). The thermocycler settings were as follows: An initial temperature of 94°C for 2 min, then 35 cycles of 94°C for 30 sec, 65°C for 30 sec and 72°C for 1 min. PCR products were analyzed by agarose gel electrophoresis and visualized with ethidium bromide staining.
Statistical analysis
Statistical analyses were performed using SPSS software (version 15.0; SPSS, Inc., Chicago, IL, USA). A one-sample Kolmogorov-Smirnov test was used to evaluate the distribution of continuous parameters. The association between BCSC phenotype and the number of inflammatory cells was assessed using a Student's t-test for CD8+ T cells and CD68+ macrophages and a non-parametric Mann-Whitney U test for CD4+ T cells. The association between other inflammatory modulators and the BCSC phenotype was assessed using a χ2 test for intratumoral and peritumoral inflammation, and Fisher's exact test for TNF-α, IL-4 and NF-kB p50 expression status. The association between the BCSC phenotype and the clinicopathological characteristics of the patients was analyzed using the Chi-square test for categorical data, including menopausal state, T stage, node metastasis, histologic grade, lymphovascular invasion, ER, PR, Bcl-2, p53 and EGFR expression status, HER2 overexpression status, Ki-67 index and molecular subtype. All tests were two-tailed. P<0.05 was considered to indicate a statistically significant difference.
Results
The clinicopathological characteristics of the patients included in the present study are illustrated in Table I. The mean age of the patients with breast cancer was 55.77±13.47 years (range, 34–90 years). All cases were categorized into four groups according to the immunohistochemical results for CD44 and CD24 (Table II). Of the 47 patients, 10 (21.3%) exhibited the BCSC phenotype (CD44+/CD24−). CD44 positivity was significantly higher in postmenopausal women compared with in premenopausal women (P=0.004; data not shown). Intratumoral inflammation was significantly more frequent in the CD44-negative groups (P=0.018) compared with CD44-positive groups (data not shown).
Table I.
Clinicopathological characteristics of patients with breast cancer.
| Clinicopathological characteristic | Value |
|---|---|
| Age, years [mean ± standard deviation (range)] | 55.77±13.47 (34–90) |
| Menopausal status, n (%) | |
| Premenopausal | 17 (38.6) |
| Postmenopausal | 27 (61.4) |
| Tumor size, cm [mean ± standard deviation (range)] | 1.97±1.04 (0.10–4.50) |
| Histologic grade, n (%) | |
| I | 8 (17.0) |
| II | 12 (25.5) |
| III | 27 (57.5) |
| Nodal involvement, n (%) | |
| Negative | 29 (61.7) |
| Positive | 18 (38.3) |
| Distant metastasis, n (%) | |
| Negative | 45 (95.7) |
| Positive | 2 (4.3) |
| Tumor stage, n (%) | |
| I | 18 (40.0) |
| IIA | 15 (33.3) |
| IIB | 9 (20.0) |
| IIIA | 2 (4.5) |
| IIIB | 0 (0.0) |
| IIIC | 1 (2.2) |
| IV | 0 (0.0) |
| Molecular subtype, n (%) | |
| Luminal A | 9 (22.0) |
| Luminal B | 23 (56.1) |
| HER2 | 6 (14.6) |
| Basal-like | 3 (7.3) |
| Lymphovascular invasion, n (%) | |
| Negative | 27 (57.4) |
| Positive | 20 (42.6) |
| ER expression status, n (%) | |
| Negative | 15 (31.9) |
| Positive | 32 (68.1) |
| PR expression status, n (%) | |
| Negative | 10 (21.3) |
| Positive | 37 (78.7) |
| HER2 overexpression status, n (%) | |
| Negative | 24 (57.1) |
| Positive | 18 (42.9) |
| Ki-67 index, n (%) | |
| <14% | 2 (4.3) |
| ≥14% | 44 (95.7) |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Table II.
Immunohistochemical staining results for CD44 and CD24.
| Tumor cell phenotype | No. of patients (%) |
|---|---|
| CD44+/CD24− | 10 (21.3) |
| CD44+/CD24+ | 9 (19.1) |
| CD44−/CD24+ | 22 (46.8) |
| CD44−/CD24− | 6 (12.8) |
CD, cluster of differentiation.
A CD44+/CD24− phenotype was significantly inversely associated with lymph node metastasis (P=0.038; Table III). The CD44+/CD24− phenotype was also significantly associated with the molecular subtype of breast cancer (P=0.042), being particularly more abundant in the basal-like subtype (Table III). In addition, the presence of CD44+/CD24− tumor cells was associated with intratumoral inflammation (P=0.032; Table III) and tumor-infiltrating CD4+ T cell counts (P=0.003; Table IV).
Table III.
Clinicopathological characteristics associated with a CD44+/CD24− phenotype in invasive breast cancer tissue samples.
| Clinicopathological characteristic | CD44+/CD24− positive patients (%) | P-value |
|---|---|---|
| Menopausal state | 0.057 | |
| Pre-menopausal | 5.9 | |
| Post-menopausal | 29.6 | |
| T stage | 0.366 | |
| ≤T1 | 25.9 | |
| ≥T2 | 15.0 | |
| Node metastasis | 0.038 | |
| Negative | 31.0 | |
| Positive | 5.6 | |
| Histologic grade | 0.092 | |
| 1 | 50.0 | |
| 2 | 16.7 | |
| 3 | 14.8 | |
| Lymphovascular invasion | 0.366 | |
| Negative | 25.9 | |
| Positive | 15.0 | |
| ER expression status | 0.536 | |
| Negative | 26.7 | |
| Positive | 18.8 | |
| PR expression status | 0.103 | |
| Negative | 40.0 | |
| Positive | 16.2 | |
| HER2 overexpression status | 0.347 | |
| Negative | 29.2 | |
| Positive | 16.7 | |
| Bcl-2 expression status | 0.778 | |
| Negative | 25.0 | |
| Positive | 20.5 | |
| p53 expression status | 0.609 | |
| Negative | 28.6 | |
| Positive | 20.0 | |
| Ki-67 index | 0.322 | |
| <10% | 50.0 | |
| ≥10% | 20.5 | |
| EGFR expression status | 0.579 | |
| Negative | 19.4 | |
| Positive | 27.3 | |
| Molecular subtype | 0.042 | |
| Luminal A | 44.4 | |
| Luminal B | 8.7 | |
| HER2 | 33.3 | |
| Basal-like | 66.7 | |
| Intratumoral inflammation | 0.032 | |
| Negative | 50.0 | |
| Positive | 14.3 | |
| Peritumoral inflammation | 0.156 | |
| Negative | 50.0 | |
| Positive | 18.8 |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; EGFR, epidermal growth factor receptor; Ki-67, proliferation marker protein Ki-67; Bcl-2, apoptosis regulator Bcl-2; p53, cellular tumor antigen p53.
Table IV.
Association between a CD44+/CD24− phenotype and inflammatory markers in invasive breast cancer tissue samples.
| CD44+/CD24− tumor cell phenotype | |||
|---|---|---|---|
| Inflammatory marker | Positive | Negative | P-value |
| TNF-α expression status, n | |||
| Negative | 2 | 13 | 0.362 |
| Positive | 8 | 24 | |
| IL-4 expression status, n | |||
| Negative | 4 | 18 | 0.627 |
| Positive | 6 | 19 | |
| NF-κB p50 expression status, n | |||
| Negative | 0 | 2 | 0.452 |
| Positive | 10 | 35 | |
| CD4+ T cell count, mean | 9.2 | 31.7 | 0.003 |
| CD8+ T cell count, mean | 64.0 | 120.3 | 0.110 |
| CD68+ macrophage count, mean | 25.6 | 31.9 | 0.505 |
TNF-α, tumor necrosis factor-α; IL-4, interleukin-4; NF-κB p50, nuclear factor κB p50; CD, cluster of differentiation.
Analysis of the clinicopathological significance of inflammatory mediators and inflammatory cells demonstrated that tumor-infiltrating CD8+ T cells were significantly increased in patients with basal-like subtype of breast cancer (P=0.037) compared with other molecular subtypes (data not shown).
Discussion
There is increasing evidence that inflammation and CSCs are associated with carcinogenesis in numerous tumor types (11,16–19). Recent studies have suggested an association between inflammation within the tumor microenvironment and CSCs (17,19); however, the effect of inflammation on CSCs has yet to be fully determined. Blaylock (19) reported that inflammation is essential to cancer induction through its mutagenic effects on stem cell DNA. Shigdar et al (17) demonstrated that inflammatory response and stimuli from immune cells, including cytokines, cause cancer cells to dedifferentiate into CSCs through several signaling pathways, including the NF-κB signaling pathway. In breast cancer, several studies have reported that inflammatory signaling within the tumor microenvironment affects CSCs (20–23). Particularly, IL-6 has been reported to induce epithelial-mesenchymal transition, which has been implicated in the generation of a stem cell phenotype (20,21). The main inflammatory cells in the tumor microenvironment are lymphocytes and macrophages, and the main inflammatory cytokines include TNF-α, IL-6, IL-8 and IFN-γ. Based on the results of previous in vitro studies (20–23), the association between inflammation and CSCs in human breast cancer tissue was analyzed in the present study. The results of the current study demonstrated that intratumoral inflammation and tumor-infiltrating CD4+ T cell counts are associated with CSCs in breast cancer. Typically, activated Th1 cells secrete TNF-α, IL-2, TGF-β and IFN-γ, and activated Th2 cells secrete IL-4, −5, −6, −10 and −13 (24,25). In combination with the results of previous studies, the results of the current study suggest that tumor-infiltrating lymphocytes are implicated in the generation of CSCs through their secretion of inflammatory cytokines.
It has been suggested that CSCs mediate tumor growth and metastasis (8,10). However, the prognostic significance of CSCs in breast cancer remains unclear. Previous studies have reported that BCSCs are associated with the basal-like molecular subtype of breast cancer and a poor clinical outcome (26,27). However, Mylona et al (28) revealed that BCSCs are associated with a lack of lymph node metastasis and an improved clinical outcome. Furthermore, Abraham et al (29) reported that BCSCs were not associated with the clinical outcome. Notably, consistent with these previous studies, the results of the present study demonstrated that a BCSC phenotype (CD44+/CD24−) was significantly associated with the basal-like molecular subtype of breast cancer, which confers a poor prognosis, whereas it was significantly inversely associated with lymph node metastasis. These results suggest that BCSCs may be able to initiate tumorigenesis (30), but that signaling pathways that modulate BCSCs, and interactions between BCSCs and the tumor microenvironment, may affect breast cancer progression. Further studies are required to clarify the prognostic significance of CSC phenotype in breast cancer.
It has been recognized that inflammatory mediators in the tumor microenvironment affect breast cancer development and progression (3–7,31). Previous studies have revealed that cytotoxic T lymphocytes and natural killer cells exhibit antitumor activity against breast cancer (32–34). However, numerous studies (31,35–37) have demonstrated mechanisms by which breast tumors avoid antitumor immune responses; protumorigenic inflammation in breast cancer has been reported (31). The inflammatory mediators in breast carcinogenesis include proinflammatory cytokines and chemokines, including IL-1, IL-6, IL-8, TNF-α, MCP-1, CCL5 and CXCL1/2 (3,6,7). Furthermore, previous studies have suggested that CD8+ T cells exhibit antitumor activity in breast cancer, which is dependent on the breast cancer subtype (38,39). Liu et al (38) reported that CD8+ T cell infiltration was associated with improved patient survival in basal-like, but not non-basal, triple negative breast cancer. In the current study, the clinicopathological significance of inflammatory mediators and inflammatory cells were investigated, and tumor-infiltrating CD8+ T cells were revealed to be increased in patients with the basal-like subtype of breast cancer compared with other subtypes. However, it was not possible to analyze the prognostic role of tumor-infiltrating CD8+ T cells in basal-like breast cancer in the current study. Further studies investigating the mechanism by which inflammation influences the progression of different breast cancer subtypes are warranted.
Although preliminary evidence suggests that inflammation and BCSCs are associated with breast carcinogenesis, there is limited data available. The acquisition of more clinical evidence is important for designing effective therapies and identifying improved therapeutic targets for patients with breast cancer. The present study analyzed the association between inflammation and the BCSC phenotype in human breast cancer tissue. However, the results of the current study were limited due to a relatively small sample size, and the clinical significance of these results requires further evaluation.
In conclusion, the present study identified significant associations between inflammation and the BCSC phenotype in breast cancer. The results suggest that the interaction between inflammation and BCSCs may affect tumorigenesis, in addition to the progression of breast cancer. Further studies are required to clarify the role of inflammation and BCSCs in breast cancer.
Acknowledgements
The authors wish to thank Young Chae Chang (Research Institute of Biomedical Engineering and Department of Medicine, Catholic University of Daegu School of Medicine, Daegu, Korea) and Hyun Ji Cho (Research Institute of Biomedical Engineering and Department of Medicine, Catholic University of Daegu School of Medicine, Daegu, Korea) for their technical support and interpretation of data. The present study was supported by a grant from the Daegu-Gyeongbuk Surgical Society research foundation, Korea.
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Arias JI, Aller MA, Arias J. Cancer cell: Using inflammation to invade the host. Mol Cancer. 2007;6:29. doi: 10.1186/1476-4598-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soria G, Ofri-Shahak M, Haas I, Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T, Weitzenfeld P, Meshel T, Shabtai E, Gutman M, Ben-Baruch A. Inflammatory mediators in breast cancer: Coordinated expression of TNF-α & IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition. BMC Cancer. 2011;11:130. doi: 10.1186/1471-2407-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis CE, Hughes R. Inflammation and breast cancer. Microenvironmental factors regulating macrophage function in breast tumours: Hypoxia and angiopoietin-2. Breast Cancer Res. 2007;9:209. doi: 10.1186/bcr1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 6.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: Mechanisms of action and potential targets for therapeutics. Curr Drug Targets. 2010;11:1133–1146. doi: 10.2174/138945010792006799. [DOI] [PubMed] [Google Scholar]
- 8.Korkaya H, Kim GI, Davis A, Malik F, Henry NL, Ithimakin S, Quraishi AA, Tawakkol N, D'Angelo R, Paulson AK, et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47:570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iqbal J, Chong PY, Tan PH. Breast cancer stem cells: An update. J Clin Pathol. 2013;66:485–490. doi: 10.1136/jclinpath-2012-201304. [DOI] [PubMed] [Google Scholar]
- 10.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells-perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 11.Boyle ST, Kochetkova M. Breast cancer stem cells and the immune system: Promotion, evasion and therapy. J Mammary Gland Biol Neoplasia. 2014;19:203–211. doi: 10.1007/s10911-014-9323-y. [DOI] [PubMed] [Google Scholar]
- 12.Gammaitoni L, Leuci V, Mesiano G, Giraudo L, Todorovic M, Carnevale-Schianca F, Agiletta M, Sangiolo D. Immunotherapy of cancer stem cells in solid tumors: Initial findings and future prospective. Expert Opin Biol Ther. 2014;14:1259–1270. doi: 10.1517/14712598.2014.918099. [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti FL, editors. AJCC Cancer Staging Manual, corp-author. 7th edition. Springer-Verlag; New York: 2010. pp. 347–377. [Google Scholar]
- 14.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 15.Jeong YJ, Jeong HY, Bong JG, Park SH, Oh HK. Low methylation levels of the SFRP1 gene are associated with the basal-like subtype of breast cancer. Oncol Rep. 2013;29:1946–1954. doi: 10.3892/or.2013.2335. [DOI] [PubMed] [Google Scholar]
- 16.Brabletz T. EMT and MET in metastasis: Where are the cancer stem cells? Cancer Cell. 2012;22:699–701. doi: 10.1016/j.ccr.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Shigdar S, Li Y, Bhattacharya S, O'Connor M, Pu C, Lin J, Wang T, Xiang D, Kong L, Wei MQ, et al. Inflammation and cancer stem cells. Cancer Lett. 2014;345:271–278. doi: 10.1016/j.canlet.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Vermeulen L, De Sousa Melo E, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 19.Blaylock RL. Cancer microenvironment, inflammation and cancer stem cells: A hypothesis for a paradigm change and new targets in cancer control. Surg Neurol Int. 2015;6:92. doi: 10.4103/2152-7806.157890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci USA. 2011;108:1397–1402. doi: 10.1073/pnas.1018898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katanov C, Lerrer S, Liubomirski Y, Leider-Trejo L, Meshel T, Bar J, Feniger-Barish R, Kamer I, Soria-Artzi G, Kahani H, et al. Regulation of the inflammatory profile of stromal cells in human breast cancer: Prominent roles for TNF-α and the NF-κB pathway. Stem Cell Res Ther. 2015;6:87. doi: 10.1186/s13287-015-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: Crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goto S, Sato M, Kaneko R, Itoh M, Sato S, Takeuchi S. Analysis of Th1 and Th2 cytokine production by peripheral blood mononuclear cells as a parameter of immunological dysfunction in advanced cancer patients. Cancer Immunol Immunother. 1999;48:435–442. doi: 10.1007/s002620050620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Idowu MO, Kmieciak M, Dumur C, Burton RS, Grimes MM, Powers CN, Manjili MH. CD44(+)/CD24(−/low) cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum Pathol. 2012;43:364–373. doi: 10.1016/j.humpath.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Kim MJ, Ahn SH, Son BH, Kim SB, Ahn JH, Noh WC, Gong G. Different prognostic significance of CD24 and CD44 expression in breast cancer according to hormone receptor status. Breast. 2011;20:78–85. doi: 10.1016/j.breast.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Mylona E, Giannopoulou I, Fasomytakis E, Nomikos A, Magkou C, Bakarakos P, Nakopoulou L. The clinicopathologic and prognostic significance of CD44(+)/CD24(−/low) and CD44−/CD24+ tumor cells in invasive breast carcinomas. Hum Pathol. 2008;39:1096–1102. doi: 10.1016/j.humpath.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–1159. [PubMed] [Google Scholar]
- 30.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang X, Shapiro DJ. The immune system and inflammation in breast cancer. Mol Cell Endocrinol. 2014;382:673–682. doi: 10.1016/j.mce.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tkach M, Coria L, Rosemblit C, Rivas MA, Proietti CJ, Díaz Flaqué MC, Beguelin W, Frahm I, Charreau EH, Cassataro J, et al. Targeting Stat3 induces senescence in tumor cells and elicits prophylactic and therapeutic immune responses against breast cancer growth mediated by NK cells and CD4+ T cells. J Immunol. 2012;189:1162–1172. doi: 10.4049/jimmunol.1102538. [DOI] [PubMed] [Google Scholar]
- 33.Wang B, Zaidi N, He LZ, Zhang L, Kuroiwa JM, Keler T, Steinman RM. Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice. Breast Cancer Res. 2012;14:R39. doi: 10.1186/bcr3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Gonçalves A, André P, Romagné F, Thibault G, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121:3609–3622. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsukerman P, Stern-Ginossar N, Gur C, Glasner A, Nachmani D, Bauman Y, Yamin R, Vitenshtein A, Stanietsky N, Bar-Mag T, et al. MiR-10b downregulates the stress-induced cell surface molecule MICB, a critical ligand for cancer cell recognition by natural killer cells. Cancer Res. 2012;72:5463–5472. doi: 10.1158/0008-5472.CAN-11-2671. [DOI] [PubMed] [Google Scholar]
- 36.Jiang X, Ellison SJ, Alarid ET, Shapiro DJ. Interplay between the levels of estrogen and estrogen receptor controls the level of the granzyme inhibitor, proteinase inhibitor 9 and susceptibility to immune surveillance by natural killer cells. Oncogene. 2007;26:4106–4114. doi: 10.1038/sj.onc.1210197. [DOI] [PubMed] [Google Scholar]
- 37.Bargou RC, Wagener C, Bommert K, Mapara MY, Daniel PT, Arnold W, Dietel M, Guski H, Feller A, Royer HD, Dorken B. Overexpression of the death-promoting gene bax-alpha which is downregulated in breast cancer restores sensitivity to different apoptotic stimuli and reduces tumor growth in SCID mice. J Clin Invest. 1996;97:2651–2659. doi: 10.1172/JCI118715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14:R48. doi: 10.1186/bcr3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]