Abstract
Purpose of review
Irritable bowel syndrome (IBS) is the most common functional gastrointestinal disorder encountered by the pediatrician and consultant. The primary focus of this review is to provide an update on beneficial nutritional interventions for managing this patient population with discussion on gut microbiome effects.
Recent findings
A common complaint amongst the pediatric population is IBS-related recurrent abdominal pain. The prevalence of IBS is estimated to range between 6%–14% and is defined by the Rome III criteria for functional gastrointestinal disorders. Recent studies highlight the role of nutritional interventions in mitigating symptoms of IBS. While eliminating foods which aggravate IBS gastrointestinal symptoms has become a main nutritional approach for acute management of IBS, recent literature reflects how this may impact the gut microbiome and potentially have long-term implications.
Summary
There are emerging studies suggesting IBS symptomatic improvement with different dietary interventions in the pediatric population, but most of what is known at this time has been extrapolated from the adult literature.
Keywords: Irritable bowel syndrome, Nutritional interventions, FODMAP, Gut microbiota, Probiotics
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder (FGID) characterized by chronic or recurrent gastrointestinal (GI) symptoms not attributed to an underlying structural or biochemical disorder 1. Defined by the Rome III criteria for FGID, IBS is estimated to affect up to 20% of school age children2 and accounts for nearly 30% of all consultations to Pediatric Gastroenterologists with costly workups approximating $6000 per child 3. IBS is defined as abdominal pain occurring at least once a week for a minimum of 2 months prior to diagnosis. The abdominal pain or discomfort is associated with two or more of the following occurring at least 25% of the time: symptomatic improvement with defecation, onset associated with changes in stool appearance and/or onset associated with change in stool frequency without an underlying organic etiology 2,4. There are three clinical subtypes of IBS which include: diarrhea predominant (IBS-D), constipation predominant (IBS-C) and alternating diarrhea and constipation termed IBSA2.
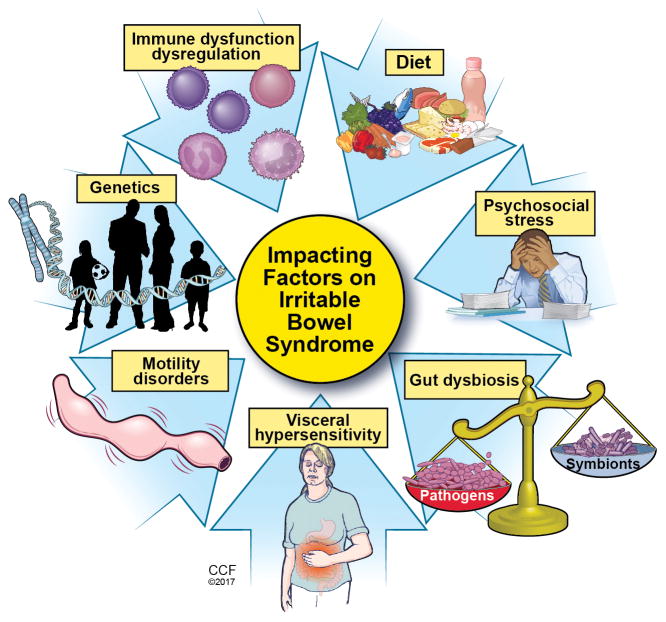
Although etiology of FGID is uncertain, it is believed multifactorial including: visceral hypersensitivity, abnormal motility, low grade mucosal inflammation, psychosocial stressors, abnormal/altered gut microbiome, and/or environmental triggers such as diet (Figure 1). IBS is also considered a brain-gut disorder, hypothesized as an element of dysregulation between the enteric nervous system and central nervous system resulting in altered gut sensation, motility and potential immune dysfunction5.
Figure 1.
Impacting factors on Irritable Bowel Syndrome
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2017. All Rights Reserved.
Diagnosing IBS
Patient History
Most children present with recurrent periumbilical abdominal pain, which is classically relieved following defecation. Associated symptoms include bloating, incomplete evacuation, and fecal urgency. A detailed history and physical exam is essential to making a correct diagnosis of IBS. Ruling out red flag symptoms including nighttime symptoms, recurrent fever, hematochezia, arthralgia, weight loss, poor growth, delayed puberty, severe vomiting or diarrhea, or steatorrhea is important as these would suggest an underlying organic disorder requiring further evaluation. As an underlying history of anxiety and emotional stress may precipitate symptoms, eliciting a detailed psychological history to uncover areas of stress that may be aggravating symptoms including bullying, school workload or upcoming tests, parental discord or financial difficulties should be included6. Functional abdominal pain significantly affects patient quality of life, ability to perform activities of daily living and has a negative impact on social involvement and can affect school performance given frequent absenteeism.
Clinical Evaluation
Baseline laboratories to rule out more potentially serious disorders should be obtained. This includes serology to screen for celiac disease; inflammatory markers (erythrocyte sedimentation rate and C-reactive protein (CRP)) to assess for inflammatory bowel disease (IBD); complete metabolic panel to evaluate liver function and nutritional status; and complete blood count as anemia may indicate blood loss in the setting of IBD. Depending on patient presentation, stool studies may be warranted to rule out infectious causes of diarrhea. Difficult cases where diagnosis is unclear may warrant endoscopy or imaging2.
Medical Management of IBS
Currently standard medical management incorporates dietary advice, reassurance, and education6. This review focuses on the role of nutritional interventions for managing IBS and potential effects on the gut microbiome.
Nutritional Interventions for IBS
Low FODMAP Diet
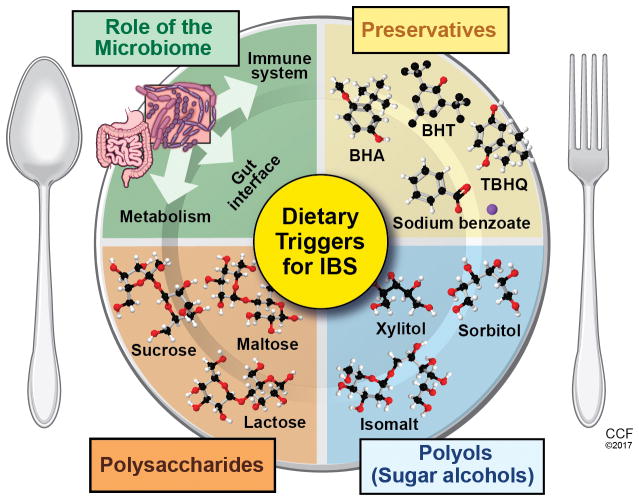
Limiting Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols (FODMAP) (Figure 2) is a dietary approach utilized to ameliorate IBS-associated symptoms. Diet implementation involves two phases: strict reduction of all dietary FODMAP followed by a gradual reintroduction based on GI symptom analysis (Table 1). In sensitive people, FODMAP are proposed to stimulate mechanoreceptors in response to luminal distention induced by: an osmotic effect and increased luminal water due to malabsorption of lactose, fructose and polyols; and luminal gases (carbon dioxide and hydrogen) from oligosaccharide fermentation. FODMAP intolerance leads to abdominal bloating, pain and altered motility7. While emerging evidence supports altered FODMAP diet in improving IBS symptoms in adults, skepticism remains due to limited studies comparing low FODMAP with the Western diet. Halmos et al 8 conducted a randomized, controlled, single-blind, cross-over trial conducted consisting of 30 IBS patients and 8 healthy controls. Subjects were randomized to a Western or FODMAP diet for a period of 21 days, then crossed over to the alternative diet which was preceded by a 21 day washout period. In contrast to the Western diet, IBS patients following a low FODMAP diet had less overall GI symptoms (e.g., bloating, pain, flatulence, stool consistency). While data regarding FODMAP diet for IBS in pediatrics is limited, it is promising. School aged children (aged 7–17 years) following a low FODMAP diet for 2-days exhibited less frequency and severity of abdominal pain episodes and total composite GI score compared to a Western diet9.
Figure 2.
Dietary Triggers for Irritable Bowel Syndrome
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2017. All Rights Reserved.
Table 1.
Foods High and Low in FODMAP
| Type of Food (FODMAP Group) | High in FODMAP | Low in FODMAP |
|---|---|---|
| Milk, yogurt, cheese (Lactose and Galactans) | Milk from cows, goats or sheep; creamed soup or sauces made with milk, evaporated milk, sweetened condensed milk, soy milk, dried milk solids, whipping cream, custard, ice cream, sherbert; Cow’s milk yogurt, soy yogurt; Cottage cheese, ricotta cheese, mascarpone cheese |
8 oz of almond, hazelnut, hemp, rice, lactose-free cow’s milk, lactose-free kefir, coconut milk (4 oz), butter, half-and-half, cream cheese, sour cream, lactose-free ice cream, gelato, sorbet made from allowed ingredients; Greek yogurt, coconut milk yogurt, lactose-free yogurt (1 cup); 1 oz of Swiss cheese, cheddar cheese, brie, blue cheese, mozzarella, camembert parmesan, feta, lactose free cottage cheese (1/2 cup) or < 2 Tbsp ricotta or regular cottage cheese |
| Fruit, (Fructose, Polyols) | Apples, apple juice or cider, applesauce, avocado, pears, mango, cherries, raspberries*, blackberries, watermelon, nectarines*, white peaches, apricots*, plums*, peaches*, prunes, mango, papaya, persimmon, orange juice; canned fruit in heavy syrup |
Banana, blueberries, boysenberry, cranberry, strawberries, cantaloupe, honeydew, grapefruit, lemon, lime, grapes, kiwi, pineapple, rhubarb, tangelos, mandarin, orange, star fruit, passion fruit, rhubarb Limit intake to one low -FODMAP fruit per meal |
| Vegetables ((Fructose, Fructans, Polyols) | Asparagus, cabbage, onions, shallot, leek, garlic, cauliflower, mushrooms, green peppers, broccoli, Brussels sprouts, fennel, okra, sugar snap peas, beets, dandelion leaves, kale, radicchio, peas, snow peas, sweet corn, pumpkin, tomato paste |
Alfalfa, artichoke, bamboo shoots, bean shoots, bok choy, carrot, celery, cucumber, endive, ginger, green beans, lettuce, parsnip, white potato, red bell pepper, spinach, chives, summer squash, sweet potato, tomato, turnip, yam, zucchini |
| Grains (Fructans) | Wheat, rye, barley in large quantities such as bread, crackers, cookies, couscous, pasta, cakes and baked goods, sweet biscuits, pastry, bread crumbs, semolina, bulgur, spelt |
Brown rice, white rice, oats, oat bran, quinoa, gluten-free bread, cereals, pastas, crackers without honey, polenta, tapioca, buckwheat, corn tortillas, corn chips |
| Legumes (Galactans) | Baked beans, black beans, chickpeas (hummus), kidney beans, lentils, edamame (soy), lima beans, soy burgers, textured vegetable protein |
Tofu, peanuts <1/3 cup green peas |
| Nuts and Seeds (Fructans, Galactans) | Pistachios, soy nuts | 10 – 15 or 1 – 2 Tbsp of: almonds, macadamia, pecans, peanuts, pine nuts, walnuts, pumpkin seeds, sesame seeds, sunflower seeds |
| Sweeteners (Fructose, Polyols) | Honey, agave, high fructose corn syrup, sorbitol, mannitol, xylitol, maltitol, fructose |
Sugar (sucrose), glucose, pure maple syrup, aspartame, corn syrup solids, brown sugar, sucralose, stevia |
| Additives (Fructans) | Inulin, FOS (fructo-oligosaccharides), chicory root |
|
| Miscellaneous (Fructose, fructans) | Onion powder, garlic powder, dehydrated onion, whey protein concentrate (unless lactose-free), molasses |
Baker’s yeast, baking powder, Coconut water (8 oz), whey protein isolate, chocolate, guar gum, jam/jelly (1 ½ Tbsp.—avoid that made with high fructose corn syrup), vinegar (except apple cider), maltodextrin, pectin, coffee (1 cup per day), tea (1 cup), sucrose-sweetened soft drink, salt, pepper, soy sauce |
| Alcohol | Rosé wine, margaritas, piña coladas, sours, sweet martinis, rum |
Wine (4 oz), beer (12 oz), vodka, gin, sherry |
| Meats and High Protein | Beef, chicken, fish (seafood), lamb, pork, turkey, eggs |
|
| Fats | Peanut or almond butter (2 Tbsp), coconut (2 Tbsp), oil (any type), Olives (9), margarine, mayonnaise |
There is concern with following restrictive diets in the pediatric population as this can lead to food aversions, eating disorders as well as imbalanced nutrient intake that may compromise growth and development. Children under the age of 10 years have a reduced capacity to absorb fructose, and yet this age group typically consumes large amounts in the form of fruit and/or fruit juices as well as high-fructose corn syrup. Limiting this ingredient alone or in combination with other culprits may be an effective approach to alleviate IBS symptoms in pediatrics10.
Specific Carbohydrate Diet (SCD) in patients with IBS and inflammatory bowel disease
A diet high in refined sugars and processed foods, triggering an abnormal immune response is postulated to have contributed to the rise in pediatric IBD over the past 60 years11. Some studies have demonstrated the protective effects of a diet high in fruits, vegetables and fiber12.
The Specific Carbohydrate Diet (SCD) claims to assist with drug-free remission in IBD patients by limiting carbohydrate consumption to only monosaccharides, thus most polysaccharides (linear or branch-chained multiple sugars or starches) and disaccharides are eliminated. The premise of SCD is that in IBD alterations in mucosal integrity and digestive enzymes compromise polysaccharide digestion which results in small intestinal inflammation and GI symptoms such as bloating due to excessive fermentation and gas production. By limiting the diet to monosaccharides, polysaccharide digestion is minimized13.
Currently, there are no large scale studies reporting the use of SCD in the pediatric population. A case series performed by Suskind et al followed 7 pediatric patients with Crohn’s disease (CD) who were not on immunosuppressive agents over a 3 month period13. After following a strict SCD these subjects showed symptom improvement as demonstrated on the pediatric CD activity index and significant improvement in laboratory parameters, including albumin, CRP, hematocrit and stool calprotectin. In a retrospective chart review conducted at a single academic center, Obih et al analyzed the effect of the SCD in their IBD population (20 with CD and 6 with ulcerative colitis (UC)). Patients adhered to a SCD for between 3–48 months. At the end of the intervention 12 subjects had reduced inflammatory markers as well as exhibited clinical improvement. Some patients were able to discontinue medications and maintain control of their disease on the SCD alone14. There are challenges posed by the SCD as it is very carbohydrate restrictive and difficult for many to maintain, therefore the diet is sometimes liberalized to expand dietary options. However, adding new foods may increase the risk for potentially worsening disease activity. More studies are needed to evaluate liberalizing the diet without causing adverse clinical outcomes.
Mediterranean Diet
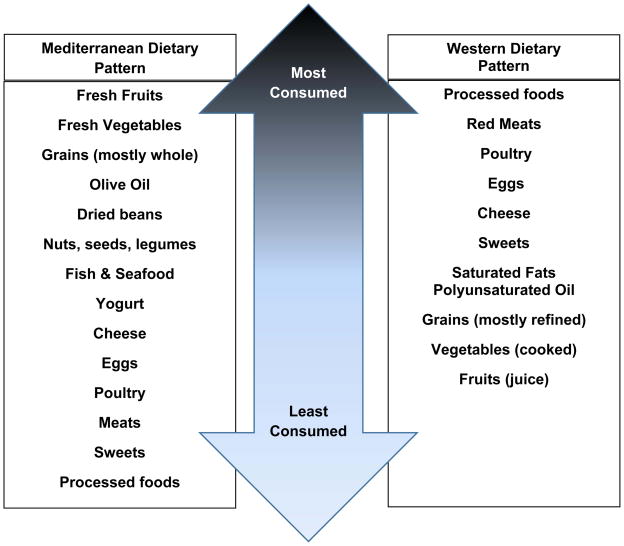
Rather than focusing on individual nutrients or food properties, another dietary approach for relieving FGID is to alter dietary food patterns. The Mediterranean Diet (MD) contains foods and eating habits typical of people residing along the Mediterranean Diet Sea (Figure 3)15. Foods are consumed more frequently (up to six times per day) in smaller quantities and include fiber-rich foods such as fresh fruits, vegetables, whole grains, nuts and legumes; milk or dairy products; and low consumption of meat or meat-based products. Thus, saturated fat is limited with the majority of dietary fat as monounsaturated (olive oil) and omega-3 fatty acids (fish). In contrast, the Western Diet (Figure 3) is low in fiber and high in simple sugars, processed meats and saturated fats. Evaluations of the effect of the MD on incidence of chronic diseases in adults (e.g., malignancy, type 2 diabetes, metabolic and cardiovascular disease) find populations that adopt this eating pattern have reductions in all-cause mortality16–18. Recently Zito et al prospectively analyzed the relationship of upper and lower GI symptoms with adherence to the MD in a group of 1134 subjects residing in southern Italy 19. Based on Rome III questionnaire, subjects were grouped as those with IBS, functional dyspepsia, or no symptoms (controls). Subjects were then further classified into age groups: 17–24, 25–34, 35–49, 50–64, and >64 years old. An age appropriate food frequency questionnaire was used to assess MD adherence and scored as very low, intermediate and optimal adherence. Of interest, the younger subjects, age groups 17–24, had lower MD adherence scores which associated with higher incidence of IBS and functional dyspepsia, particularly amongst females; lower MD adherence scores in this age grouping of males associated with functional dyspepsia. Analyzing the age 25–34 year age group found that only males and females with IBS had lower MD adherence scores compared to controls. The older age groupings reported higher MD adherence despite presence of GI symptoms. As the aging process comes with many other factors that can influence GI symptoms, it is not surprising that the older age groups continued with GI symptoms despite MD adherence. Young people are more apt to eat convenience foods with higher fat, which have delayed gastric emptying and may aggravate GI hypersensitivity. As the MD is more liberalized in allowed foods compared to previously described diets, these data suggest the MD may be a feasible option in this patient population and with proper education adherence may improve. However, more prospective studies comparing different dietary interventions including the MD are required in the full age range of the pediatric patient population.
Figure 3.
Foods Consumed in Mediterranean versus Western Dietary Pattern
The gut microbiota and IBS
Appreciation for the role the gut microbiota plays in maintaining intestinal homeostasis is growing. While many factors influence gut microbiota composition, it’s response to diet is astounding. As fermentable soluble fiber is the preferred food source for the gut microbiota, not surprisingly the low-fiber containing Western diet negatively alters gut microbial patterns20. Animal and human ex vivo models investigating dietary emulsifiers, including carboxymethylcellulose, polysorbate-80 and maltodextrin, negatively impact gut microbial balance as well as induce metabolic syndrome and low-grade inflammation leading to colitis21. Given these findings, the gut microbiota has been implicated in the pathogenesis of IBS 22.
Chumpitazi et al evaluated the gut microbiota and symptomatic (pain) response in children with IBS when randomized to a low and high FODMAP diet for 2 days9. Patients were categorized as responders, non-responders, and placebo-responders. An abundance of baseline taxa known as carbohydrate fermenters (Bacteroides, Ruminoccus, Faecalibacterium prausnitzii) was found in the responder group. Authors concluded patients with IBS with saccharolytic enriched microbiota may benefit most from a low FODMAP diet. It is important to only limit aggravating FODMAP rather than all FODMAP as the latter could have negative long-term outcomes on the microbiome and theoretically intestinal integrity. This was evidenced in a study that found a low FODMAP diet, while improving IBS symptoms, also depleted levels of butyrate-producing bacteria from Clostridium cluster XIVa and mucus-associated bacteria23.
Probiotics and IBS
Challenges with using modification of gut microbiota to alleviate IBS related GI symptoms surrounds the fact that the exact etiology of IBS as well as a “normal” gut microbiota pattern are undefined. Nonetheless, investigations for probiotic use in alleviating IBS symptoms for abdominal pain and discomfort, bloating and flatulence, bowel habits, gut motility and quality of life have been conducted. A recent meta-analysis with a total of 1793 patients included randomized controlled trials comparing probiotics with a placebo for altering IBS symptoms24. Overall the responder rate for abdominal pain, global symptom, and general symptoms scores were improved in the probiotic groups compared to placebo. Study limitations include utilization of various IBS symptom scoring systems as well as various strains, doses and duration of probiotic treatments across the studies, making it challenging to fully appreciate probiotic effects. A systematic review published in 2014 reviewed the effect different probiotic strains had in relation to FGID in children and adolescents25. This review concluded that in children with IBS the use of Lactobacillus GG, L. reuteri DSM17 939 and VSL#3 helped reduce the intensity of abdominal pain. Overall, probiotics may be helpful in the IBS population, but further studies are required to determine specific species, strains and dose required to produce optimal results.
Conclusion
Dietary manipulation is becoming common practice for functional abdominal pain and emerging studies suggest symptomatic improvement with the above mentioned dietary interventions in pediatrics, but most of what is currently known has been extrapolated from the adult literature. Evidence to recommend initiating a low FODMAP diet as a primary intervention in pediatrics is growing, however, more long-term prospective studies are required to determine efficacy and any potential associated risks with this dietary approach in pediatric patients with IBS. While the initial phase of strict FODMAP limitations has been found to be helpful in reducing IBS symptoms, sustainability of these effects following reintroduction of FODMAPS is less robust. Therefore, as evidence-based practice guidelines for managing FGID with diet in pediatrics are not currently available, using a sensible approach is suggested. Diagnosis a FGID and managing symptoms is preferred over subjecting children to excessive hydrogen breath testing for diagnosis and thus labeling of specific food intolerances. As to be mindful about the importance of optimal nutrition to promote growth and development, a balanced dietary approach is advised. This includes eating regular meals and snacks that comprise a variety of foods from all food groups avoiding excessive amounts and limiting processed foods, such as with the MD dietary pattern. Involving a dietitian familiar with IBS to obtain a thorough nutrition history so that IBS trigger foods can be identified and an age-appropriate meal plan can be developed and monitored is suggested. If IBS symptoms are persistent and an eating disorder or aversion is not suspected, a low FODMAP diet may be implemented under expert supervision (physician plus dietitian) for a limited time (≤ 6 weeks) followed by careful reintroduction of high-FODMAP foods.
Key Points.
More prospective, high quality, randomized studies are warranted to determine the efficacy, utility and impact on growth and development with these nutritional interventions in pediatric patients with irritable bowel syndrome and other FGID.
Long-term follow-up is required to determine the safety and effectiveness of probiotics in the pediatric IBS population.
Dietary interventions and/or elimination diets should be implemented with caution in the pediatric population in order to not compromise growth and development nor create food aversions or eating disorders. Nutritional consultation with a registered dietitian is highly recommended.
Acknowledgments
Financial support and sponsorship: K99AA023266- GAMC
Footnotes
Conflicts of interest: The authors do not have any conflicts of interest.
References and recommended reading
- 1*.Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, Van Tilburg M. Functional Disorders: children and adolescents. Gastroenterology. 2016;150(6):1456–1468. e2. doi: 10.1053/j.gastro.2016.02.015. This review provides updates regarding process in which a functional gut disorder is diagnosed and introduces the Rome IV criteria. This could provide clarity for clinicians and researchers. [DOI] [PubMed] [Google Scholar]
- 2.Sandhu BK, Paul SP. Irritable bowel syndrome in children: Pathogenesis, diagnosis and evidence-based treatment. World J Gastroenterol. 2014;20:6013–6023. doi: 10.3748/wjg.v20.i20.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhroove G, Chogle A, Saps M. A million dollar work up for abdominal pain: is it worth it? J Pediatr Gastroenterol Nutr. 2010;5:579–583. doi: 10.1097/MPG.0b013e3181de0639. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka Y, Kanazawa M, Fukudo S, Drossman DA. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17(2):131–139. doi: 10.5056/jnm.2011.17.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri M. Peripheral Mechanisms in Irritable Bowel Syndrome. N Engl J Med. 2012;367(17):1626–1635. doi: 10.1056/NEJMra1207068. [DOI] [PubMed] [Google Scholar]
- 6.Morcos A, Dinan T, Quigley EMM. Irritable bowel syndrome: Role of food in pathogenesis and management. J Dig Dis. 2009;10(4):237–246. doi: 10.1111/j.1751-2980.2009.00392.x. [DOI] [PubMed] [Google Scholar]
- 7.Ong DK, Mitchell SB, Barrett JS, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25(8):1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 8.Halmos EP, Power VA, Shepaherd SJ, et al. A diet low in FODMAP reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 9**.Chumpitazi BP, Cope JL, Hollister EB, et al. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with irritable bowel syndrome. Aliment Pharmacol Ther. 2015;42:418–427. doi: 10.1111/apt.13286. This study reports improved abdominal pain score episodes in children following a low FODMAP diet compared to a typical American diet. Of special interest is that low FODMAP diet responders gut microbiota were enriched at baseline in taxa with saccharolytic metabolic capacity. This observation may provide insight as to who will have the best outcomes when following a low FODMAP therefore preventing unnecessary dietary restrictions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabritz J, Muhlbauer M, Domagk D, et al. Significance of hydrogen breath tests in children with suspected carbohydrate malabsorption. BMC Pediatr. 2014;14:59. doi: 10.1186/1471-2431-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am J Gastroenterol. 2011;106(4):563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 12.Ananthakrishnan AN, Khalili H, Konijeti GG, et al. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013;145(5):970–977. doi: 10.1053/j.gastro.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suskind DL, Wahbeh G, Gregory N, Vendettuoli H, Christie D. Nutritional therapy in pediatric Crohn disease: the specific carbohydrate diet. J Pediatr Gastroenterol Nutr. 2014;58(1):87–91. doi: 10.1097/MPG.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 14*.Obih C, Wahbeh G, Lee D, et al. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition. 2016;32(4):418–425. doi: 10.1016/j.nut.2015.08.025. This is retrospective review of the impact of the specific carbohydrate diet on clinical outcomes and laboratory studies in pediatric patients with Crohn’s disease or ulcerative colitis. Duration of the diet from 3–48 months resulted in decreases in disease severity scores for both forms of IBD and suggest this diet can be integrated into a tertiary care center. [DOI] [PubMed] [Google Scholar]
- 15.Willett WC, Sacks F, Trichopoulou A, et al. Mediterranean diet pyramid: A cultural model for healthy eating. Am J Clin Nutr. 1995;61(6 SUPPL) doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 16.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Del Chierico F, Vernocchi P, Dallapiccola B, Putignani L. Mediterranean diet and health: Food effects on gut microbiota and disease control. Int J Mol Sci. 2014;15(7):11678–11699. doi: 10.3390/ijms150711678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro-Quezada I, Román-Viñas B, Serra-Majem L. The mediterranean diet and nutritional adequacy: A review. Nutrients. 2014;6(1):231–248. doi: 10.3390/nu6010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Zito FP, Polese B, Vozzella L, et al. Good adherence to mediterranean diet can prevent gastrointestinal symptoms: A survey from Southern Italy. World J Gastrointest Pharmacol Ther. 2016;7(4):564–571. doi: 10.4292/wjgpt.v7.i4.564. This prospective study compared the adherance to a mediterranean diet to the onset of functional gut disorders utilizing an age-appropriate questionnaire. In younger individuals, nonadherance to a mediterranean diet was associated with an increase in GI symptoms. However in older indivuals GI symptoms did not correlate with mediterranean diet adherance. Results suggest that modifying dietary patterns rather than individual foods may be better suited for younger indiviuals with functional gut disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Cresci GA, Bawden E. The gut microbiome: what we do and don’t know. Nutr Clin Pract. 2015 Dec;30(6):734–46. doi: 10.1177/0884533615609899. This thorough review presents information known about how the microbiome is shaped and changed throughout the lifecycle and by environmental factors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene epression ex vivo potentiating intestinal inflammation. Gut. 2017;0:1–14. doi: 10.1136/gutjnl-2016-313099. Using a human intestinal microbial ecosystem model to maintain a complex stable human microbiota, several dietary emulsifers were tested to determine their effects on microbiota and promoting inflammation. Emulsifiers increased the proinflammatory potential of microbiota by increasing levels of bioactive flagellin. When bacteria were transplanted into germ-free mice, low-grade inflammation associated phenotypes occurred suggesting direct effects of emulsifiers on microbiota to drive metabolic-disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saulnier DM, Riehle K, Mistretta T-A, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141(5):1782–1791. doi: 10.1053/j.gastro.2011.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64:93–100. doi: 10.1136/gutjnl-2014-307264. This study identified that a low FODMAP diet improved IBS related GI symptoms compared to a standard Australian diet. However, the low FODMAP diet altered the fecal gut microbiota structure, decreasing total bacterial abundance. The Australian diet increased relative abundance of butyrate-producing bacteria and mucus associated bacteria. This observation may have negative implications if a low FODMAP diet is followed long-term. [DOI] [PubMed] [Google Scholar]
- 25*.Didari Tina, Mozaffari Shilan, Nikfar Shekoufeh, Abdollahi Mohammad. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21:3072–3084. doi: 10.3748/wjg.v21.i10.3072. This study including nearly 1800 patients investigated the efficacy of probiotics in all subtypes of irritable bowel syndrome. Despite limitations of analysis due to variance between studies with scales used to evaluate GI symptoms and probiotic strains, duration and mode of delivery, overall probiotic use may influence IBS symptoms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korterink JJ, Ockeloen L, Benninga MA, Tabbers MM, Hilbink M, Deckers-Kocken JM. Probiotics for childhood functional gastrointestinal disorders: a systematic review and meta-analysis. Acta Paediatr. 2014;103(4):365–372. doi: 10.1111/apa.12513. [DOI] [PubMed] [Google Scholar]