Abstract
Cardiovascular disparities remain pervasive in the US. Unequal disease burden is evident among population groups based on sex, race, ethnicity, socioeconomic status (SES), educational attainment, nativity, or geography. Despite the significant declines in cardiovascular disease (CVD) mortality rates in all demographic groups over the last 50 years, large disparities remain by sex, race, ethnicity, and geography. Recent data from modeling studies, linked micromap plots, and small-area analyses also demonstrate prominent variation in CVD mortality rates across states and counties, with an especially high disease burden in the southeast US and Appalachia. Despite these continued disparities, few large-scale intervention studies have been conducted in these high-burden populations to examine the feasibility of reducing or eliminating cardiovascular disparities. To address this challenge, on June 22-23, 2017, the National Heart, Lung, and Blood Institute (NHLBI) convened experts from a broad range of biomedical, behavioral, environmental, implementation, and social science backgrounds to summarize the current state of knowledge of CVD disparities and propose intervention strategies aligned with the NHLBI mission. This report presents the themes, challenges, opportunities, available resources and recommended actions discussed at the workshop.
Keywords: Cardiovascular health, cardiovascular diseases, health disparities, community-based participatory research, implementation research, social determinants of health, community-engagement
Introduction
Disparities in cardiovascular health (CVH) and cardiovascular diseases (CVD) remain pervasive in the US and account for a large proportion of the overall health disadvantage suffered by high risk populations.1–3 Important progress in disparities has been made as shown in Figure 1. For example, the black-white disparity gap in all-cause mortality rate for all ages narrowed from 33% in 1999 to 16% in 2015 and for persons aged 65 years and older, the all-cause death rate in 2015 compared to that in 1999 declined 27% for Blacks and 17% for Whites. This decline resulted in the elimination of the black-white disparity gap in 2010 and a lower death rate in Blacks than Whites thereafter.4 For diseases of the heart, the death rate disparity in Blacks relative to Whites declined from 27.6% in 1999 to 22.2% in 2015 (Table 1).4
Figure 1. Death rates among Blacks and Whites, by age group (years) – United States, 1999–2015.
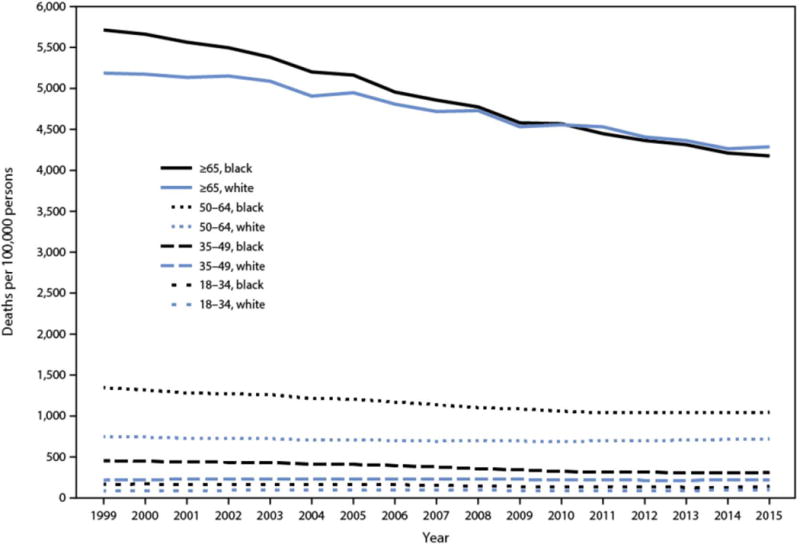
Among adults aged ≥65 years, the death rate in 2015 relative to that in 1999 declined 27% for Blacks and 17% for Whites, resulting in a crossover in death rates beginning in 2010, when Blacks had lower death rates than Whites. Reproduced from Cunningham TJ, et al. Vital Signs: Racial Disparities in Age-Specific Mortality Among Blacks or African Americans - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. May 05 2017;66(17):444-456.
TABLE 1.
Death rates per 100,000 population for all causes of death and death from diseases of the heart, percentage changes, and death rate disparities between blacks and whites, by age group — National Vital Statistics System, United States, 1999 and 2015. Reproduced from Cunningham TJ, Croft JB, Liu Y, Lu H, Eke PI, Giles WH. Vital Signs: Racial Disparities in Age-Specific Mortality Among Blacks or African Americans - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2017;66(17):444-456.
| Cause of death by age group (yrs) | Blacks | Whites | Death rate disparity relative to whites* | |||||
|---|---|---|---|---|---|---|---|---|
| 1999 rate | 2015 rate | % change (1999 to 2015) | 1999 rate | 2015 rate | % change (1999 to 2015) | 1999(%) | 2015 (%) | |
| 1. All causes | ||||||||
| All ages† | 1.135.7 | 851.9 | −25.0§ | 854.6 | 735.0 | −14.1§ | +32.9§ | +15.9§ |
| 18–34 | 167.8 | 141.5 | −15.6§ | 87.5 | 100.3 | +14. 6§ | +91.8§ | +41.1§ |
| 35–49 | 454.3 | 311.5 | −31.4§ | 218.2 | 220.3 | +1.0§ | +108.2§ | +41.4§ |
| 50–64 | 1,346.5 | 1,046.0 | −22.3§ | 746.5 | 722.4 | −3.2§ | +80.4§ | +44.8§ |
| ≥65 | 5,712.7 | 4,176.0 | −26.9§ | 5,186.0 | 4,286.9 | −17.3§ | +10.2§ | −2.6§ |
| 2. Diseases of the heart | ||||||||
| All ages | 334.3 | 205.1 | −38.7§ | 262.0 | 167.9 | −35.9§ | +27.6§ | +22.2§ |
| 18–34 | 12.5 | 10.7 | −14.5§ | 4.8 | 5.1 | +5.2 | +158.7§ | +110.3§ |
| 35–49 | 85.3 | 66.5 | −22.0§ | 37.9 | 33.3 | −12.0§ | +125.2§ | +99.7§ |
| 50–64 | 378.6 | 257.5 | −32.0§ | 193.9 | 148.1 | −23.6§ | +95.2§ | +73.9§ |
| ≥65 | 1,902.6 | 1,085.5 | −42.9§ | 1,756.7 | 1,091.8 | −37.9§ | +8.3§ | −0.6 |
Definitions:
Disparity (%) = (Black rate minus white rate) divided by white rate times 100.
“All ages” category includes infants and children. Death rates for all ages were age-standardized to the 2000 U.S. projected population.
Z-statistic significant at p<0.05 for the rate change from 1999 to 2015 or for the difference between black and white populations.
Despite this progress, Blacks younger than 65 years old and several population groups and communities continue to demonstrate marked cardiovascular disparities, and in some settings, the disparities are widening.4–12 These disparities arise from differences in major cardiovascular risk factors and causes of death and disability that are preventable1–3, 13 and are seen in all stages of the life course beginning in the intrauterine environment14, 15 and early childhood16–18 through young adulthood19–21 and old age.22–24 In addition, social determinants of health, such as low socioeconomic status (SES), stress, poor social support, depression, anxiety, and living in disadvantaged neighborhoods contribute to cardiovascular disparities.25–28
Addressing the disparate population burden of CVD remains a major clinical and public health challenge.1, 29 Accordingly, the National Heart, Lung, and Blood Institute (NHLBI) convened a workshop on June 22-23, 2017 that engaged a multidisciplinary group of experts to discuss, develop, and prioritize themes and strategies aligned with the NHLBI mission to reduce disparities using community-engagement and implementation research frameworks.30 The workshop participants included cardiovascular, social, and spatial epidemiologists; survey methodologists; community-based participatory researchers; health disparities researchers; social and behavioral science researchers; experts in the inter-sectionality of community engagement between communities and academic institutions; dissemination and implementation (D&I) science experts; health services researchers; and clinician-scientists with expertise in general internal medicine, primary care, stroke, type 2 diabetes, depression, and heart, lung, and blood diseases. For the purposes of this workshop, disparities was defined as preventable differences in the incidence, prevalence, prevention, treatment, morbidity, and mortality from CVD and related risk factors.1, 29 Inherent in this definition are preventable differences in access to health care and the quality of health care delivered.
While CVD disparities by race/ethnicity have been recognized for decades,31 geographic disparities have only recently begun to receive more attention.32, 33 For example, Roth, et al., showed a 4-fold difference in the mortality from hypertensive heart disease between counties at the 10th and 90th percentiles of the average national rate.33 Substantial county-level differences, in both relative and absolute terms, were also noted for ischemic heart disease and stroke mortality, with the largest concentration of counties with high CVD mortality extending from southeastern Oklahoma along the Mississippi River Valley to eastern Kentucky33 (Figure 2). Other studies also show large state- and county-level CVD disparities that are likely to worsen, especially in southeastern US and Appalachia. These patterns of geographic disparities in CVD are not surprising given the high levels of poverty34 and high prevalence of CVD risk factors in these regions.35
Figure 2. United States County-Level Mortality from Cardiovascular Diseases.
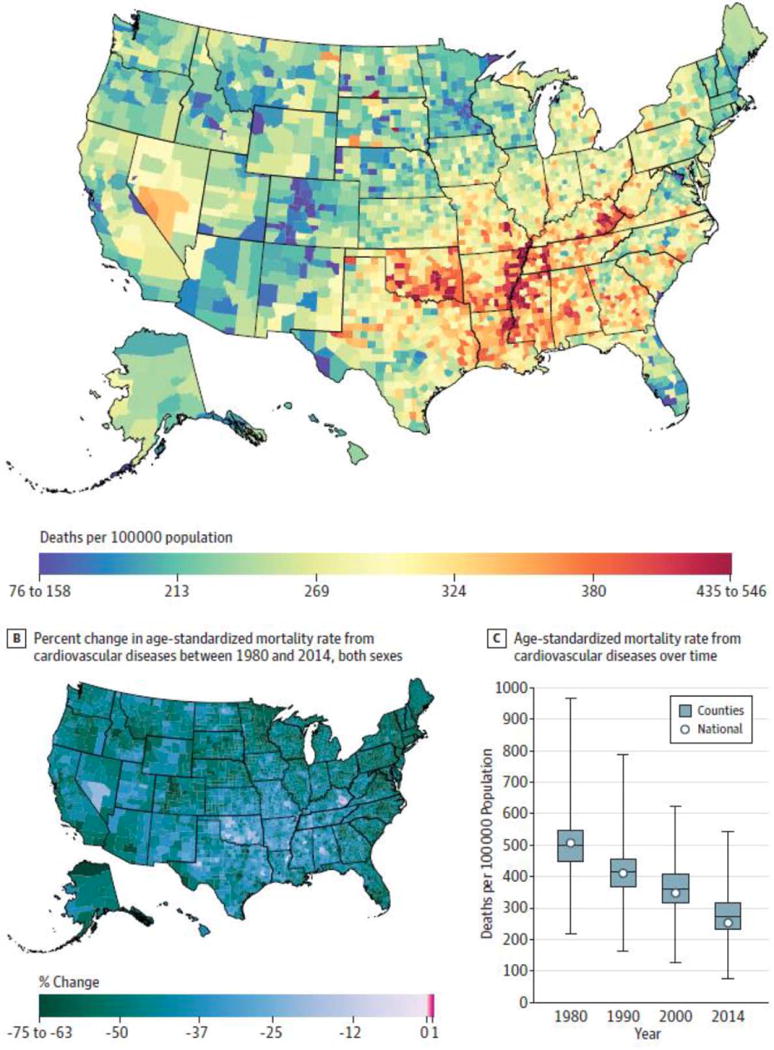
A: Age-standardized mortality rate for both sexes combined in 2014. B: Percent change in the age-standardized mortality rate for both sexes combined between 1980 and 2014. In panel A, the color scale is truncated at approximately the 1st and 99th percentiles as indicated by the range given on the scale. In panel B, the color scale is similarly truncated at the 1st percentile but not at the 99th percentile to avoid combining counties with decreases in the mortality rate and counties with increases in the mortality rate into a single group. C: Age-standardized mortality rate in 1980, 1990, 2000, and 2014. The bottom border, middle line, and top border of the boxes indicate the 25th, 50th, and 75th percentiles, respectively, across all counties; whiskers, the full range across counties; and circles, the national-level rate. Reproduced from Roth GA, et al. Trends and Patterns of Geographic Variation in Cardiovascular Mortality Among US Counties, 1980-2014. JAMA. May 16 2017;317(19):1976-1992; with permission from the American Medical Association.
Geography-based differences that contribute to CVD disparities are, among others, neighborhood characteristics, access to health-promoting resources, behavioral (e.g. diet, physical activity, and tobacco use), psychosocial (stress, depression, social support), and cultural factors (acculturation, dietary patterns), and access to quality health care.36 These geographical differences need to be understood in the context of growing economic inequality in the US and recognition that these populations are at high risk, at least in part, as a result of discrimination and economic and political disempowerment.37 The tragic explosion of the opioid epidemic bears further witness to the impact of socioeconomic challenges many of these communities face.38
The aim of the present workshop was to go beyond identifying problems. The priority was to search for and examine solutions. Although the NHLBI supported a series of large-scale multi-level intervention studies in the past, there are few recent attempts to examine translation and implementation research of evidence-based programs to reduce or eliminate CVD disparities. In this report, the workshop participants make recommendations for NHLBI to consider and emphasize (1) identification of high burden communities; (2) commitment to best practices in community engagement; (3) implementation of evidence-based interventions likely to be successful in these communities; (4) alignment of community partners and care networks; (5) incorporation of novel research methods and evaluation milestones; and (6) development of approaches for training the next generation of implementation research investigators for community-engaged health disparities research.
Identification of High Burden Communities
As noted above, the existence of significant CVD disparities across the key demographic categories in the US has been recognized for many years.3, 31, 39 The major population groups with high rates of CVD in the US conform to an all too familiar pattern, primarily including racial and ethnic minorities, especially African Americans and American Indians/Alaska Natives, and persons living in poverty.1, 31, 40 In 2015, the age-adjusted death rate from all cardiovascular conditions was 282 per 100,000 for Blacks compared with 220 per 100,000 for Whites.41 An even larger risk gradient can be identified by SES.42–44 In 2013, the mortality rate from CVD per 100,000 was 212 in large metropolitan areas compared with 259 in nonmetropolitan (rural) areas.41 Generating a framework for community-based interventions, however, requires both a comprehensive assessment of the burden of social and economic factors likely driving the disparity and the capacity to convert broad demographic descriptors, such as race and SES, into socially relevant geographic units that would self-identify as a community.1, 45 Additionally, community-based interventions should take into account the “spillover” effects of the surrounding and wider community given social interactions among individuals in surrounding communities, which can influence the effectiveness of community-based interventions.
Despite the continued growth of data resources, describing the variation of CVD disparities in some communities remains challenging. Vital statistics data and representative sample surveys, like the National Health and Nutrition Examination Survey (NHANES), the Behavioral Risk Factor Surveillance System (BRFSS), and the National Center for Health Statistics (NCHS) Linked Mortality Files and Compressed Mortality Files, provide a richly detailed description of CVD morbidity and mortality and related risk factors by age, sex, race/ethnicity, and – to a lesser extent – geographic region. State and county maps are available for cardiovascular risk factors and both non-fatal and fatal CVD events.46 A recent joint effort by the Centers for Disease Control and Prevention (CDC) and Robert Wood Johnson Foundation (RWJF) has led to creation of a rich on-line database with health and social status information for the largest 500 US cities.47 Likewise, health departments in large metropolitan areas have vital statistics and risk factor data from more homogenous neighborhoods. In Chicago, for example, data are provided on 77 “community areas”, demonstrating higher CVD burden on predominantly African Americans residing in the south and west side communities, compared to more affluent communities on the north-west and south-west sides; further detail is available on census tracts, zip codes and “neighborhoods”.48 Other useful resources recommended for NHLBI to consider are shown in Table 2A.
TABLE 2A.
High Burden Communities*
| Opportunities | Challenges | Strategies/Leverage | Partnerships | Useful Resources |
|---|---|---|---|---|
|
|
|
|
|
BRFSS: Behavioral Risk Surveillance System; CDC: Centers for Disease Control and Prevention; CVD: Cardiovascular Disease; NHANES: National Health and Nutrition Examination Survey.
Workshop participants recommended that NHLBI consider these activities
In many instances, county-level data may be uninformative for large metropolitan areas. For example, Cook County, Chicago includes both the city of Chicago (high burden) and a large number of suburban communities (low burden). On the other hand, disparities in sparsely populated rural areas can be easily identified with county maps. Moving down to the level of geographic units traditionally targeted for community interventions, however, can lead to a loss of precision since census estimates may be inaccurate in the inter-census years and the limited number of cardiovascular events to be expected within a single community may make rates unstable. The most robust approach to characterizing communities with CVD disparities might be to create a composite picture using information from broad demographic descriptors (e.g., race/ethnicity), regional or state vital statistics, as well as local survey data. It is ideal for all interventions targeted at small scale population units to begin with intensive efforts to engage the community at all levels, including efforts to validate and interpret available CVD disparity data.
Communities may also manifest disparities in access to quality health care delivery even if their actual rate of CVD is lower than that of other communities. For example, in a risk factor and health status survey in 28 communities located in 17 states, Liao et al. reported that Hispanics had the lowest likelihood of having had cholesterol or glycosylated hemoglobin checked in the preceding year, and the lowest prevalence of taking medications for hypertension.49 Influenza and pneumococcal vaccination rates in eligible adults were also lower in black, Hispanic, and Asian or Pacific Islander communities.49 In addition, the 2016 National Healthcare Quality and Disparities Report demonstrated multiple examples of worse access to quality health care especially for Blacks, Hispanics, American Indians and Alaska Natives compared to Whites; and also poor access to quality health care for poor persons (defined by the Federal Poverty Level) and those from low-income households50
Commitment to Best Practices in Community Engagement
Community-engagement research, including community-based participatory research (CBPR), involves partnerships with multiple stakeholders within a community where all are important players in identifying community problems, designing the intervention, determining how and when to intervene, what data to collect, how to implement and evaluate the intervention, interpret and disseminate the results, and put evidence-based results into practice.51–53 To reduce health disparities, a community-based approach is considered essential. Important additional principles critical for community-engaged health disparities research include: building trust among research institutions, community partners and other stakeholders; utilizing multi-prong, multi-level approaches, even though this strategy may present challenges in measuring the effect of any single intervention; tailoring interventions to the community context, which requires trade-offs between researchers’ priorities and community needs; identifying and valuing diversity within communities; and using local resources and capacities to ascertain long-term commitments.54
Several tools and resources available to support community stakeholders in efforts to reduce CVD disparities were recommended for NHLBI to consider. The RWJF “Action Cycle” for Improving a Community’s Health (Figure 3) is one example that describes key activities for all partners within a community and provides additional resources for taking action. The related County Health Rankings55, 56 is another resource that community stakeholders can use to design community needs assessment and program planning. For needs assessment, the rankings are helpful to identify health determinants such as health behaviors, clinical care settings, social and economic factors, and the physical environment. For program planning, it is imperative to identify specific health outcomes. The Health Rankings uses 2 categories: death and health status while alive. A current example of this resource being used is the community health assessment that is required by the Affordable Care Act of hospitals, local public health agencies, and Federally Qualified Healthcare Centers.57 These community assessments have led to many creative community solutions that may serve as examples for large scale interventions.58
Figure 3. The Robert Wood Johnson Foundation Action Cycle for Improving a Community’s Health.
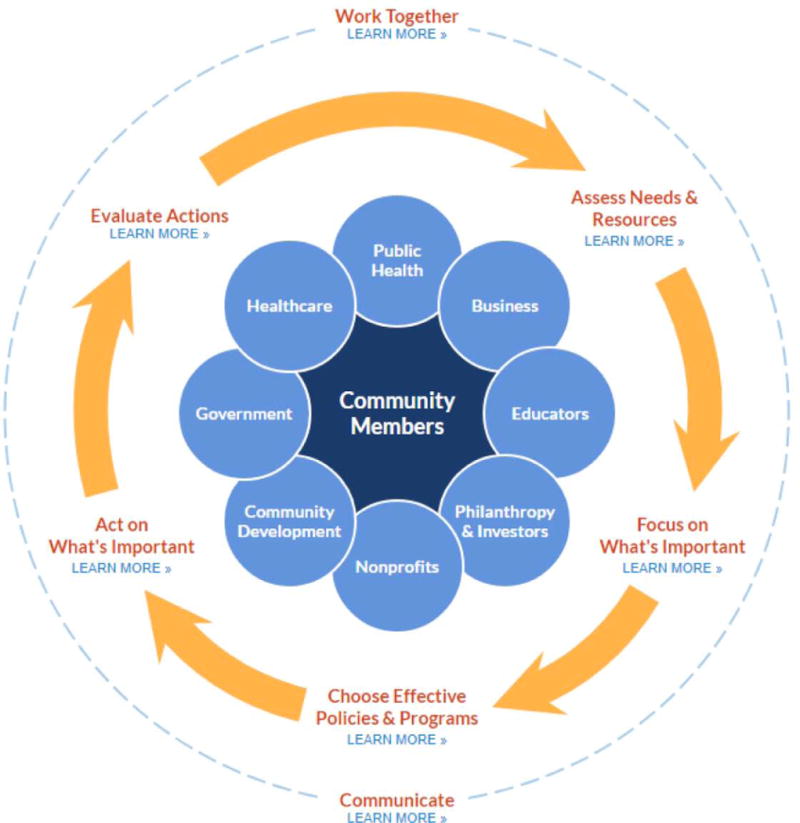
At the core of the Action Cycle are key stakeholders for taking action. Each step on the Action Cycle is considered a critical component for creating healthier communities and offers a guide that describes key activities within each step that includes suggested tools, resources, and additional reading. In this Action Cycle, “Work Together” and “Communicate” are distal because they are needed throughout the Cycle. Reproduced from The Robert Wood Johnson Foundation County Health Rankings & Roadmaps Action Center, 2017; Take Action Cycle. http://www.countyhealthrankings.org/take-action-cycle.
The workshop discussions affirmed several important opportunities and challenges for NHLBI to consider that are summarized in Table 2B. Crucial among these are strategies to build trust and sustainable partnerships, as shown in the Community-Campus Partnership for Health model.59 The workshop also stressed the importance of empowering communities to make decisions on what matters most to their health; ensuring that researchers have the cultural humility and humanity to accept these decisions; embracing the crucial role of social determinants of health; and approaching community health through a comprehensive life-course lens.60 The workshop participants recommended that the NHLBI consider strategic partnerships with other NIH Institutes and Centers, with Patient-Centered Outcomes Research Institute (PCORI), Centers for Medicare & Medicaid Services (CMS), RWJF, CDC, and other organizations with experience in funding CBPR. It is reassuring to know that recent large community-based practice network and patient-centered interventions addressing health disparities are yielding some success and important clinical and public health insights.61–64
TABLE 2B.
Engaging Community Stakeholders*
| Opportunities | Challenges | Strategies/Leverage | Partnerships | Useful Resources |
|---|---|---|---|---|
|
|
|
|
|
AHRQ: Agency for Healthcare Research and Quality; ATSDR: Agency for Toxic Substances and Disease Registry; CBPR: Community-Based Participatory Research; CTSA: Clinical and Translational Science Awards; FAQ: Frequently Asked Question; NHLBI: National Heart, Lung, and Blood Institute; NIEHS: National Institute of Environmental Health Science; PEPH: Partnerships for Environmental Public Health; RWJF: Robert Wood Johnson Foundation; PCORI: Patient-Centered Outcomes Research Institute; PRIDE: Programs to Increase Diversity Among Individuals Engaged in Health-Related Research;
Workshop participants recommended that NHLBI consider these activities.
Implementation of Evidence-Based Interventions
Many effective interventions have been developed in recent decades to enhance CVH and mitigate CVD disparities.65, 66 For example, the Franklin County Cardiovascular Health Program (FCHP), which is a comprehensive community-based integrated primary health care intervention program, targeted hypertension, cholesterol, smoking, diet and physical activity over a 40-year period and demonstrated significant reductions in CVD mortality in a low-income, rural community of Maine.67 Other examples of effective interventions include: the Dietary Approaches to Stop Hypertension (DASH) diet and self-management strategies for at-risk patients with hypertension, diabetes, and other CVD risk factors (at the individual level); peer support interventions (such as those delivered by community health workers) and barber shop/beauty parlor interventions (at the family, peer, and social network level); nurse and pharmacist-delivered care management, and provider communication skills training (at the healthcare provider level); and use of electronic medical records, tele-monitoring, and quality improvement methodology to improve patients’ blood pressure levels (at the organizational level).68 Furthermore, early-life intervention programs about healthy lifestyles and CVH that target high burden communities through schools can be effective in reducing CVD disparities in adulthood.69, 70 Importantly, active engagement of, and coordination with the healthcare system should be fostered so that safe and effective medications for the treatment and control of CVD and risk factors can be maintained.
Despite the availability of these effective interventions, substantial challenges in reducing CVD disparities in the US population persist. Important gaps in research and translation also remain. If effective evidence-based interventions are to be implemented and disseminated, it is critical that these interventions address the complex, multilevel factors that influence the presence of these disparities and that gaps in translation of these interventions into practice and policy are addressed.65, 66, 68, 71 The persisting challenges in eliminating CVD disparities may be attributed, in part, to the paucity of interventions that address social determinants of health disparities.72, 73 Indeed, the health impact pyramid described by Frieden suggests that although implementing interventions at all levels of the ecological model can achieve the maximum possible sustained public health benefit, interventions with the greatest impact on population health are those focusing on the physical and social environmental context and on socioeconomic and policy factors.74
Bridging the translation gap will require transdisciplinary research that tests the effectiveness of multi-level interventions; compares universal interventions alone to interventions targeting at-risk groups; describes the challenges to program implementation, affordability, sustainability, translation, and dissemination (for example, by tailoring interventions to context and populations); incorporates broad stakeholder engagement in design, implementation, and dissemination of evidence-based strategies; and addresses social structures and policies.71, 72, 75, 76 Table 2C describes the opportunities provided, challenges to success, and strategies for leverage, as well as important partnerships and relevant resources to unleash the potential for evidence-based interventions to eliminate CVD disparities that were recommended for NHLBI to consider.
TABLE 2C.
Evidence-Based Interventions*
| Opportunities | Challenges | Strategies/Leverage | Partnerships | Useful Resources |
|---|---|---|---|---|
|
|
|
|
|
CE: Community-engaged; HTN: hypertension; FOA: Funding Opportunity Announcement; CVD: cardiovascular disease; IS: implementation science
IC: NIH Institutes and Centers;
Workshop participants recommended that NHLBI consider these activities
Aligning Community Partners and Care Networks
The number of organizations and partners that might collaborate to eliminate CVD disparities may seem daunting. Hospital and outpatient clinics, office-based primary care practices, pharmacies, health insurance companies, community organizations, and patients have important roles to contribute to decrease CVD disparities. It is crucial to engage all the potential partners as strategic collaborators in implementation research to address the full spectrum of CVD, from prevention and risk factor reduction to diagnosis and treatment. This engagement of stakeholders is necessary to ensure that the implementation research undertaken is relevant and meaningful in the local community.77
Several approaches to engaging these strategic partners can help align the mission and goals of implementation research to eliminate CVD disparities. Health Maintenance Organizations, Accountable Care Organizations, and practice-based research networks are models for linking diverse medical clinics, hospitals, and pharmacies across large geographic areas to test, implement, and disseminate locally relevant, meaningful messages and programs to improve CVD care.78–83 National organizations working with state public health departments and their parallel county organizations as well as healthcare workforce training programs can all find alignment in community-based approaches to CVD risk reduction and treatment.84 Engaging and including patients and community members in all aspects of implementation research also aligns care with local culture and community values.85
Recently, there has been a resurgence in the desire to link medical care with public health through the creation of local and regional Communities of Solution (COS) that identify the critical links among community, public health, and primary care and call for ongoing demonstrations of COSs grounded in patient-centered care.86, 87 The COS begins by identifying the local “problem shed”, in this case, issues related to CVD disparities, and then identifies the “asset shed”, that is, those people, organizations, and health systems that can contribute to a local solution.86, 87 The COS combine the key elements of implementation research by aligning strategic partners in developing local solutions to local health problems. In addition to the use of local and regional COS in implementation research, several opportunities and challenges in efforts recommended for NHLBI to consider for eliminating CVD disparities, are identified in Table 2D.
TABLE 2D.
Aligning Networks of Care*
| Opportunities | Challenges | Strategies/Leverage | Partnerships | Useful Resources |
|---|---|---|---|---|
|
|
|
|
|
AHRQ: Agency for Healthcare Research and Quality; CVD: Cardiovascular Disease; HER: Electronic Health Record; FQHC: Federally Qualified Health Center
Workshop participants recommended that NHLBI consider these activities.
Incorporation of Novel Research Methods and Milestones
A set of core principles guided the discussion of methods and milestones to evaluate interventions. These principles included: (1) using a variety of research designs to assess the effectiveness of evidence-based interventions;88 (2) the methods that implementation research scientist use in communities with CVD disparities must be low-burden and aligned with community priorities;89 community partners must be integral co-investigators; (3) the implementation research scientist must strive for equity and fairness in the amount of scientific information produced to understand the causes and solutions to health disparities;90, 91 and (4) implementation research represents an essential stage of research for improving population health and eliminating health disparities.
Implementation research hinges upon building partnerships between communities and researchers.92 Effective and sustainable partnerships are the result of achieving mutual self-interest, often through the process of cultural exchange.93 Researchers need to be mindful that the failure of an implementation effort comes at a significantly greater cost to the community than to the academic partner. Thus, the methods used need to be responsive to and in harmony with community needs and context and ought to further the goal of creating a sustainable, community-owned practice. Additionally, milestones such as the establishment of systematic engagement of diverse community stakeholders; agreement regarding the method to conduct health needs assessment; consensus regarding community priorities; consensus regarding intervention design; selection of outcomes measures; and sustainability of the program after funding has ended should all be discussed.94–98
Evaluation of an implementation strategy to create translatable knowledge can be conducted within a single site or multiple sites (clusters) or with a randomized or non-randomized design.88, 99 For example, use of the stepped-wedge design, in which all communities or sites receive the intervention in a phased approach, can be a helpful strategy to maintain community engagement when the alternative of potential randomization to a non-intervention comparison group is viewed unfavorably.100–102 Researchers may use low-burden measures to assess the quality, quantity, speed, and extent of implementation103 in the real-world systems and find ways that are practical, feasible, and align with the needs and mission of the service provider or community. Unobtrusive measures,104 which involve data sources already being collected during service delivery, can be invaluable. Machine learning methods involving automated coding and validation of unobtrusive measures can be used for monitoring and feedback to identify service delivery challenges and how organizations can respond through rapid data-driven decisions.105
In addition to the County Health Rankings mentioned above, participants discussed other potential evaluation metrics, including, for example, the Life’s Simple Seven metrics promoted by the American Heart Association as key indicators of success of their efforts to promote the CVH of all Americans.106 Cost, the single most important factor in decisions about program adoption, also remain critically important for spread and sustainment.107 Because costs can differ substantially across organizations and communities, tools like a cost calculator can be invaluable.108 Table 2E shows other opportunities, strategies, and challenges in implementation research methods and milestones that were recommended for NHLBI consideration. Important remaining gaps include tools for implementation measurement systems109 and for calculating statistical power in research designs.
TABLE 2E.
Methods and Milestones*
| Opportunities | Challenges | Strategies/Leverage | Partnerships | Useful Resources |
|---|---|---|---|---|
|
|
|
|
|
AHRQ: Agency for Healthcare Research and Quality; CE: Community-engaged; Ce-PIM: Center for Prevention Implementation Methodology; CTSA: Clinical and Translational Science Awards; D&I: Dissemination and Implementation; IRI: Implementation Research Institute; NIDA: National Institute on Drug Abuse; NHLBI: National Heart, Lung, and Blood Institute; REDCap: Research Electronic Data Capture
Workshop participants recommended that NHLBI consider these activities
Training the Next Generation
Innovative and “out-of-the-box” training models that build upon inter-institutional collaborations could help to attract, develop, and sustain a cadre of implementation research scientist trained in CBPR to reduce CVD disparities. Such collaborations can leverage research infrastructure and investments to train a diverse workforce that is more representative of the community being targeted. For example, the inter-institutional Clinical and Translational Science Awards (CTSA) Program at Emory University and Morehouse School of Medicine (MSM) is a model of two institutions collaborating to train scholars from diverse backgrounds.110, 111 Challenges include the limited number of training slots and even fewer trained mentors. The National Institute on Minority Health and Health Disparities (NIMHD) sponsored Clinical Research Education and Career Development (CRECD) scholars could serve as a pipeline of a robust cadre of implementation research scientist focused on health disparities.112 Several CRECD scholars at MSM, the University of Puerto Rico, and across the Research Centers at Minority Institutions (RCMI) have developed successful academic careers addressing implementation research at the intersection of primary care, health care systems, population health and faith communities. These are poised to scale NHLBI implementation research training.112 Programmatic support for teams that will surround these scholars and enable them to pursue implementation research in collaboration with health care systems and community partners was considered crucial.113, 114
Research and training infrastructure such as the National Research Mentoring Network (NRMN) provides evidence-based mentor training and mentee career development to diversify biomedical research.115 Short-term training programs, modeled after the NHLBI and Office of Behavioral and Social Sciences Research (OBSSR)-supported Annual Summer Institute on Randomized Behavioral Clinical Trials116 and the NIH-supported Training Institute in Dissemination and Implementation Research in Health (TIDIRH),117 may help stimulate interest in further training and launch careers. However, more intensive and extended training will likely be necessary to build skills, especially those related to community engagement. Two such programs are currently funded in Dissemination and Implementation (D&I) research, but are not supported by NHLBI. They include the Implementation Research Institute (IRI), funded by the National Institute of Mental Health (NIMH), National Institute on Drug Abuse (NIDA), and the Department of Veterans Affairs (VA); and the Mentored Training in Dissemination and Implementation Research in Cancer (MT-DIRC), funded by the National Cancer Institute (NCI), the VA, and the Cancer Research Network. Both TIDIRH and the IRI training programs have been shown to increase D&I grant submissions and success in obtaining funding.117, 118 Unfortunately, available slots in these D&I training programs are insufficient to meet demand within the scientific and practitioner community.119 Schools of Public Health may be especially well positioned to provide the required combination of didactic and experiential training. Other opportunities and resources that could be leveraged to support training and career development in advancing implementation research for the elimination of health disparities recommended for NHLBI consideration, are shown in Table 2F.
TABLE 2F.
Research Training*
| Opportunities | Challenges | Strategies/Leverage | Partnerships | Useful Resources |
|---|---|---|---|---|
|
|
|
|
|
CTSA: Clinical and Translational Science Awards; DrPH: Doctor of Public Health degree; MPH: Master of Public Health degree; MSI: Minority Serving Institution; PRIDE: Program to Increase Diversity among Individuals Engaged in Health-Related Research; PWI: Predominantly White Institutions; RWJF: Robert Wood Johnson Foundation; UCSF: University of California San Francisco.
Workshop participants recommended that NHLBI consider these activities
An important area is training and educational opportunities for high school and middle school students to grow the pipeline of new investigators. Over the years, it has become increasingly apparent that creating a pipeline for young investigators and public health professionals that starts in middle school can be particularly productive. The CDC supports a number of initiatives including the Science Ambassadors Fellowship,120 the Science Olympiad,121 and other career paths to public health122 that could be leveraged to grow the pipeline of new racial and ethnic minority investigators.
The workshop participants made a strong recommendation for the NHLBI to consider exploring and encouraging increased use of Diversity Supplements to both engage and train a cadre of Early Stage Investigators (ESI) that are underrepresented in medicine or research (URM/URR) and are also engaged in health disparities research. It was suggested that NHLBI consider prioritizing Diversity Supplements that demonstrate how ESIs from URM/URR groups can advance their career as independent researchers. The cornerstone of the program is teaching these early career investigators how to effectively and equitably engage community partners and community stakeholders in implementation research to reduce CVD disparities.
Importance of Strategic Collaborations
Whereas the focus of this Workshop was on CVD disparities, participants recognized the substantial extent to which the social determinants of CVD disparities are shared with other health conditions. This reality underscores the potential synergy to be gained through wide collaboration. Several Institutes, Centers, and Offices at NIH have resources and programs that can be invaluable in the effort to reduce and eliminate CVD disparities. For example, NIMHD supports a research framework that incorporates multiple health determinants over the life course.123 Based on the fundamental pillars of race/ethnicity and SES, NIMHD supports research that explores how biology, behavior, the built environment, culture and community, and the role of the healthcare system, influence health outcomes. Understanding the importance of community-driven interventions can inform CVD disparities research. NIMHD supports several research activities involving community health workers, the use of mobile technology in risk reduction, and CBPR that utilizes a health behavior intervention to increase community recognition of stroke warning signs and follow up instructions engaging healthcare professionals.
Insights gained from these research activities can be leveraged in planning CBPR for reducing and eliminating CVD disparities. The workshop participants encouraged NHLBI to consider collaborating actively with NIMHD and other NIH Institutes, Centers, and Offices to address research gaps in understanding health disparities and implementation research to reduce CVD disparities. For example, how might enhancing access to health care services like portals for patients, e-referrals and telemedicine reduce cardiovascular disparities? Insights and lessons learned from several NIMHD funding opportunity announcements124 may be invaluable to NHLBI as it charts the future for health disparities research as part of its Strategic Vision.
The Prevention Research Centers (PRC) (www.cdc.gov/PRC) provide a unique opportunity for NHLBI, CDC, and Foundations to support CBPR focused on the elimination of cardiovascular disparities. The PRCs constitute a network of 26 Schools of Public Health and Schools of Medicine with preventive medicine residency programs that focus on community-based applied public health research. This research includes both investigator initiated and sponsored research that could be supported by CDC, NIH or a private foundation. Groups of PRCs have come together in a series of thematic networks in the areas of cancer prevention, obesity, physical activity and nutrition, epilepsy, workplace health promotion and healthy aging. However, there is not a specific thematic network focused on the elimination of cardiovascular disparities. Bringing together a robust group of researchers to collectively develop and implement such a research agenda could be particularly fruitful. In addition, the AHRQ Practice Based Research Networks (PBRNs) provide yet another important framework that could be used to support community based multi-sector research with a strong grounding in active community-engagement and community empowerment.
Potential Solutions, Remaining Questions, and Gaps
Tables 2A–F provide a comprehensive and detailed description of both the scope and breadth of the challenges, opportunities, and resources needed for conducting community-engaged implementation research to reduce cardiovascular disparities. Potential solutions for NHLBI to consider fall within four over-lapping domains: 1) support of true community-based, community-participatory implementation research projects; 2) strategies to advance innovative improvements in care delivery within health systems; 3) strategies that address the social determinants of health and structural changes in the built environment in communities; and 4) training a diverse workforce appropriate to the regional population and skilled in the reduction of cardiovascular disparities in strategic partnerships with community stakeholders. A detailed list of specific examples from these domains with enough granularity appropriate for different communities is beyond the scope of this workshop report; however, at the general level, these potential solutions focus on:
Supporting highly meritorious community-engaged implementation research initiatives;
Harnessing cutting-edge information sources and analytic methods to identify high-burden communities that are receptive to change;
Supporting enduring and effective community engagement policies and practices;
Developing and testing models for integration and delivery of evidence-based interventions;
Nurturing innovative efforts to align community-based organizations, public health agencies, and health care systems;
Identifying, vetting, and promoting the use of appropriate methods and metrics for study conduct and evaluation;
Implementing scalable approaches for training the needed current and future generations of the workforce; and
Supporting the development of strategic partnerships between research investigators, their institutions and centers, and community stakeholders.
While attention to the details inherent in these potential solutions could result in substantial improvement in health and health equity, many important questions and knowledge gaps remain. The best way to engage communities that may be distrustful and skeptical of research projects remains a challenge. While much progress has occurred in documenting and understanding the levels of CVD disparities by sex, race/ethnicity and geography; the impact that environment has on CVD disparities; early life exposures (including intrauterine development); the social and psychological determinants of CVD disparities; and methods to collect accurate data to evaluate if these factors have been modified, major challenges for community research still remain. Furthermore, few interventions have specifically leveraged these influences for reducing cardiovascular disparities in communities. While we have effective interventions to consider for the major CVD risk factors, most of these interventions have been evaluated in isolation. Whether these interventions alone or integrated into more comprehensive CVH promotion programs will specifically work to reduce health disparities remains less clear and untested. There is no readily available compendium of interventions, and few interventions have addressed the whole socioecological structure including patients, family, schools, community organizations, health care providers, health and social policy, and other higher organizational levels.
Successful engagement of communities, key stakeholders, and researchers in sharing resources for community-based research is also a major challenge. The research should align with the culture of the community where it is being conducted, keeping in mind the difficulties with conducting research in high-burden, low-resource communities. Often there may be inadequate and inconsistent access to the health care and public health systems within an overburdened community, particularly if many members are uninsured. A critical question that remains is how to align evidence-based research with current practice requirements without increasing the administrative burden. For example, the identification of simple, yet responsive, measures of environmental and social determinants of health that can be easily incorporated into existing community surveillance programs and electronic health records, would represent a significant advance. In addition, standardized measures of race/ethnicity, SES, education, and other social and environmental determinants of health and health care need to be incorporated into the electronic medical record. Whereas some Schools of Public Health are beginning to train implementation research scientists, the output has not been sufficient to date, in part because many competencies related to community-engagement research are difficult to teach and require substantial time and commitment from both the mentors and trainees. The success of efforts to reduce and eliminate CVD inequities would be enhanced by the development of innovative training programs and venues for sharing best practices.
There are several important themes that constitute remaining questions and gaps in cardiovascular disparities that the workshop did not have time to address. For example, dementia occurs at higher rates in racial and ethnic minority populations in the US.125–127 Although educational attainment appears to play a very significant role, place of birth and the higher prevalence of vascular disease and risk factors likely also contribute to the greater susceptibility to dementia, particularly among African Americans.125 Recent evidence from the Framingham Heart Study showing a downward trend in dementia in the last three decades strongly suggests that it is possible to improve the risk pattern leading to this devastating condition.128 Continued attention to these remaining questions and gaps in disparities research is important.
Summary and Conclusions
Despite remarkable declines in age-adjusted rates of CVD mortality in the US over the past five decades, CVD disparities remain pervasive. Few large-scale intervention studies have been conducted to examine the feasibility of reducing or eliminating these disparities. To address this challenge, on June 22-23, 2017, the NHLBI convened experts from a broad range of biomedical, behavioral, environmental, implementation, and social science backgrounds to summarize the current state of knowledge on CVD disparities and propose intervention strategies for consideration that align with the research, training, and education mission of NHLBI. As summarized in this report, workshop participants developed a set of themes that identified challenges, opportunities, and resources needed to inform and advance research to decrease CVD disparities. They also identified novel approaches for community engagement and community-participatory implementation research. As the NHLBI proceeds with implementing its Strategic Vision,30 actions to address the themes that emerged from this Workshop should support the Institute’s mission-focused strategic objectives including the reduction or elimination of cardiovascular disparities.
Acknowledgments
The authors would like to express our gratitude to our colleagues Gail Pearson, MD, ScD, and Gina Wei, MD, MPH, both from the Division of Cardiovascular Sciences, NHLBI, NIH for their valuable input on early drafts of this report. In addition, we would like to extend our appreciation to the National Institute for Minority Health and Disparities and the National Institute of Diabetes, Digestive, and Kidney Diseases, both of NIH, for their intramural support of Eliseo J. Pérez-Stable, M.D. and Anne E. Sumner, MD and their participation in this Workshop.
Non-standard Abbreviations and Acronyms
- AHRQ
Agency for Healthcare Research and Quality
- ATSDR
Agency for Toxic Substances and Disease Registry
- BRFSS
Behavioral Risk Factor Surveillance System
- CBPR
Community-based Participatory Research
- CDC
Centers for Disease Control and Prevention
- CMS
Centers for Medicare & Medicaid Services
- COS
Communities of Solution
- CRECD
Clinical Research Education and Career Development
- CTRIS
Center for Translation Research and Implementation Science
- CTSA
Clinical and Translational Science Awards
- CVD
Cardiovascular disease
- CVH
Cardiovascular health
- D&I
Dissemination and Implementation
- DASH
Dietary Approaches to Stop Hypertension
- ESI
Early Stage Investigators
- FAQ
Frequently Asked Question
- FCHP
Franklin County Cardiovascular Health Program
- FOA
Funding Opportunity Announcement
- IRI
Implementation Research Institute
- MSM
Morehouse School of Medicine
- MT-DIRC
Mentored Training in Dissemination and Implementation Research in Cancer
- NCHS
National Center for Health Statistics
- NCI
National Cancer Institute
- NHANES
National Health and Nutrition Examination Survey
- NHLBI
National Heart Lung, and Blood Institute
- NIDA
National Institute for Drug Addiction
- NIEHS
National Institute of Environmental Health Science
- NIMH
National Institute for Mental Health
- NIMHD
National Institute on Minority Health and Health Disparities
- NRMN
National Research Mentoring Network
- PBRN
Practice Based Research Networks
- PCORI
Patient-Centered Outcomes Research Institute
- PEPH
Partnerships for Environmental Public Health
- PRC
Prevention Research Centers
- PRIDE
Programs to Increase Diversity Among Individuals Engaged in Health-Related Research
- RCMI
Research Centers at Minority Institutions
- RWJF
Robert Wood Johnson Foundation
- SES
Socioeconomic status
- TIDIRH
Training Institute in Dissemination and Implementation Research in Health
- URM/URR
Underrepresented in Medicine/Underrepresented in Research
- US
United States
- VA
United States Department of Veterans Affairs
Footnotes
Conflict of Interest Disclosure: None
Publisher's Disclaimer: Disclaimer:
The views expressed in this article are those of the authors and do not necessarily represent the views of the affiliated institutions, the Centers for Disease Control and Prevention, the Health Resources and Service Administration, the National Heart, Lung and Blood Institute, the National Human Genome Research Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Minority Health and Health Disparities, the National Institutes of Health, or the United States Department of Health and Human Services.
References
- 1.National Center for Health Statistics. Health, united states, 2015: With special feature on racial and ethnic health disparities. CDC, NCHS; 2016. http://www.cdc.gov/nchs/data/hus/hus15.pdf. Accessed. [PubMed] [Google Scholar]
- 2.Woolf SH, Aron LY. The us health disadvantage relative to other high-income countries: Findings from a national research council/institute of medicine report. JAMA. 2013;309:771–772. doi: 10.1001/jama.2013.91. [DOI] [PubMed] [Google Scholar]
- 3.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the united states. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham TJ, Croft JB, Liu Y, Lu H, Eke PI, Giles WH. Vital signs: Racial disparities in age-specific mortality among blacks or african americans - united states, 1999-2015. MMWR Morb Mortal Wkly Rep. 2017;66:444–456. doi: 10.15585/mmwr.mm6617e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones K, Mansfield CJ. Premature mortality in north carolina: Progress, regress, and disparities by county and race, 2000-2010. North Carolina medical journal. 2014;75:159–168. [PubMed] [Google Scholar]
- 6.Institute of Medicine. How far have we come in reducing health disparities? Progress since 2000: Workshop summary. Washington (DC): National Academies Press; 2012. [PubMed] [Google Scholar]
- 7.Orsi JM, Margellos-Anast H, Whitman S. Black-white health disparities in the united states and chicago: A 15-year progress analysis. Am J Public Health. 2010;100:349–356. doi: 10.2105/AJPH.2009.165407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keppel KG, Pearcy JN, Heron MP. Is there progress toward eliminating racial/ethnic disparities in the leading causes of death? Public Health Rep. 2010;125:689–697. doi: 10.1177/003335491012500511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sondik EJ, Huang DT, Klein RJ, Satcher D. Progress toward the healthy people 2010 goals and objectives. Annu Rev Public Health. 2010;31:271–281. doi: 10.1146/annurev.publhealth.012809.103613. 274 p folliwng 281. [DOI] [PubMed] [Google Scholar]
- 10.Singh GK, Siahpush M, Azuine RE, Williams SD. Widening socioeconomic and racial disparities in cardiovascular disease mortality in the united states, 1969-2013. International journal of MCH and AIDS. 2015;3:106–118. [PMC free article] [PubMed] [Google Scholar]
- 11.Singh GK, Azuine RE, Siahpush M, Williams SD. Widening geographical disparities in cardiovascular disease mortality in the united states, 1969-2011. International journal of MCH and AIDS. 2015;3:134–149. [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Schumacher AE, Levitz CE, Mokdad AH, Murray CJ. Left behind: Widening disparities for males and females in us county life expectancy, 1985-2010. Popul Health Metr. 2013;11:8–11. doi: 10.1186/1478-7954-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon PW, Bastian B, Anderson RN, Collins JL, Jaffe HW. Potentially preventable deaths from the five leading causes of death–united states, 2008–2010. MMWR Morb Mortal Wkly Rep. 2014;63:369–374. [PMC free article] [PubMed] [Google Scholar]
- 14.Barker DJ. Fetal origins of cardiovascular disease. Ann Med. 1999;31(Suppl 1):3–6. [PubMed] [Google Scholar]
- 15.Dasinger JH, Alexander BT. Gender differences in developmental programming of cardiovascular diseases. Clinical science (London, England : 1979) 2016;130:337–348. doi: 10.1042/CS20150611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghuveer G, White DA, Hayman LL, Woo JG, Villafane J, Celermajer D, Ward KD, de Ferranti SD, Zachariah J. Cardiovascular consequences of childhood secondhand tobacco smoke exposure: Prevailing evidence, burden, and racial and socioeconomic disparities: A scientific statement from the american heart association. Circulation. 2016;134:e336–e359. doi: 10.1161/CIR.0000000000000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litz AM, Van Guilder GP. Increased arterial stiffness in south dakota american indian children. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2016;41:150–156. doi: 10.1139/apnm-2015-0426. [DOI] [PubMed] [Google Scholar]
- 18.Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB, Giles WH. Adverse childhood experiences and the risk of premature mortality. Am J Prev Med. 2009;37:389–396. doi: 10.1016/j.amepre.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Clark CJ, Alonso A, Spencer RA, Pencina M, Williams K, Everson-Rose SA. Predicted long-term cardiovascular risk among young adults in the national longitudinal study of adolescent health. Am J Public Health. 2014;104:e108–115. doi: 10.2105/AJPH.2014.302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kishi S, Reis JP, Venkatesh BA, Gidding SS, Armstrong AC, Jacobs DR, Jr, Sidney S, Wu CO, Cook NL, Lewis CE, Schreiner PJ, Isogawa A, Liu K, Lima JA. Race-ethnic and sex differences in left ventricular structure and function: The coronary artery risk development in young adults (cardia) study. J Am Heart Assoc. 2015;4:e001264. doi: 10.1161/JAHA.114.001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wickrama KA, Bae D, O’Neal CW. Black-white disparity in young adults' disease risk: An investigation of variation in the vulnerability of black young adults to early and later adversity. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2016;59:209–214. doi: 10.1016/j.jadohealth.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Hussein M, Waters TM, Chang CF, Bailey JE, Brown LM, Solomon DK. Impact of medicare part d on racial disparities in adherence to cardiovascular medications among the elderly. Med Care Res Rev. 2016;73:410–436. doi: 10.1177/1077558715615297. [DOI] [PubMed] [Google Scholar]
- 23.Lam CS, Carson PE, Anand IS, Rector TS, Kuskowski M, Komajda M, McKelvie RS, McMurray JJ, Zile MR, Massie BM, Kitzman DW. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: The irbesartan in heart failure with preserved ejection fraction (i-preserve) trial. Circulation Heart failure. 2012;5:571–578. doi: 10.1161/CIRCHEARTFAILURE.112.970061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen NB, Holford TR, Bracken MB, Goldstein LB, Howard G, Wang Y, Lichtman JH. Geographic variation in one-year recurrent ischemic stroke rates for elderly medicare beneficiaries in the USA. Neuroepidemiology. 2010;34:123–129. doi: 10.1159/000274804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson KM, Clark CJ, Lewis TT, Aggarwal NT, Beck T, Guo H, Lunos S, Brearley A, Mendes de Leon CF, Evans DA, Everson-Rose SA. Psychosocial distress and stroke risk in older adults. Stroke. 2013;44:367–372. doi: 10.1161/STROKEAHA.112.679159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz AJ, Kannan S, Dvonch JT, Israel BA, Allen A, 3rd, James SA, House JS, Lepkowski J. Social and physical environments and disparities in risk for cardiovascular disease: The healthy environments partnership conceptual model. Environ Health Perspect. 2005;113:1817–1825. doi: 10.1289/ehp.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayman LL, Worel JN. Reducing disparities in cardiovascular health: Social determinants matter. J Cardiovasc Nurs. 2016;31:288–290. doi: 10.1097/JCN.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW, Rosal M, Yancy CW. Social determinants of risk and outcomes for cardiovascular disease: A scientific statement from the american heart association. Circulation. 2015;132:873–898. doi: 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 29.United States Department of Health and Human Services. Introducing healthy people 2020. DHHS; Washington, DC: 2014. http://www.healthypeople.gov/2020/about/default.aspx. Accessed. [Google Scholar]
- 30.National Heart, Lung, and Blood Institute. Charting the future together: The nhlbi strategic vision. Bethesda, MD: NHLBI; 2016. [Google Scholar]
- 31.United States Department of Health and Human Services, Task Force on Black and Minority Health. Report of the secretary's task force on black & minority health (volume 4, part 2): Cardiovascular and cerebrovascular disease. Washington, DC: DHHS; 1985. [Google Scholar]
- 32.Murray CJ, Kulkarni SC, Michaud C, Tomijima N, Bulzacchelli MT, Iandiorio TJ, Ezzati M. Eight americas: Investigating mortality disparities across races, counties, and race-counties in the united states. PLoS Med. 2006;3:e260. doi: 10.1371/journal.pmed.0030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roth GA, Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Naghavi M, Mokdad AH, Murray CJL. Trends and patterns of geographic variation in cardiovascular mortality among us counties, 1980–2014. Jama. 2017;317:1976–1992. doi: 10.1001/jama.2017.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.United States Census Bureau. Small area income and poverty estimates. United States Department of Commerce; 2017. https://www.census.gov/did/www/saipe/data/interactive/saipe.html?s_appName=saipe&map_yearSelector=2014&map_geoSelector=aa_c. Accessed 4/7/2017. [Google Scholar]
- 35.Loop MS, Howard G, de Los CG, Al-Hamdan MZ, Safford MM, Levitan EB, McClure LA. Heat maps of hypertension, diabetes mellitus, and smoking in the continental united states. Circ Cardiovasc Qual Outcomes. 2017;10:e003350. doi: 10.1161/CIRCOUTCOMES.116.003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mensah GA, Goff DC, Gibbons GH. Cardiovascular mortality differences-place matters. Jama. 2017;317:1955–1957. doi: 10.1001/jama.2017.4168. [DOI] [PubMed] [Google Scholar]
- 37.Case A, Deaton A. Rising morbidity and mortality in midlife among white non-hispanic americans in the 21st century. Proc Natl Acad Sci U S A. 2015;112:15078–15083. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - united states, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 39.Cooper R, Cutler J, Desvigne-Nickens P, Fortmann SP, Friedman L, Havlik R, Hogelin G, Marler J, McGovern P, Morosco G, Mosca L, Pearson T, Stamler J, Stryer D, Thom T. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the united states: Findings of the national conference on cardiovascular disease prevention. Circulation. 2000;102:3137–3147. doi: 10.1161/01.cir.102.25.3137. [DOI] [PubMed] [Google Scholar]
- 40.Agency for Health Research and Quality. National healthcare quality and disparities report; ahrq pub. No. 15-0007. Rockville, MD: AHRQ; 2015. [Google Scholar]
- 41.Centers for Disease Control and Prevention. Cdc wonder: Compressed mortality file; mortality for 1999–2015 with icd 10 codes. 2017 https://wonder.cdc.gov/mortSQL.html. Accessed.
- 42.Brunner EJ. Social factors and cardiovascular morbidity. Neuroscience and biobehavioral reviews. 2017;74:260–268. doi: 10.1016/j.neubiorev.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sommer I, Griebler U, Mahlknecht P, Thaler K, Bouskill K, Gartlehner G, Mendis S. Socioeconomic inequalities in non-communicable diseases and their risk factors: An overview of systematic reviews. BMC public health. 2015;15:914. doi: 10.1186/s12889-015-2227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: A review of the literature. Circulation. 1993;88:1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 45.National Center for Health Statistics, National Vital Statistics System. U.S. Census populations with bridged race categories. 2017 https://www.cdc.gov/nchs/nvss/bridged_race.htm. Accessed.
- 46.Prevention CfDCa. Heart disease maps and data sources. 2015 https://www.cdc.gov/heartdisease/maps_data.htm. Accessed July 8, 2017.
- 47.Centers for Disease Control and Prevention. 500 cities: Local data for better health. 2017 https://www.cdc.gov/500cities/index.htm. Accessed.
- 48.City of Chicago. Healthy chicago 2.0 – community health assessment. 2017 https://www.cityofchicago.org/content/dam/city/depts/cdph/CDPH/Healthy%20Chicago/HealthyChicago_CHA_4102017.pdf. Accessed June 25, 2017.
- 49.Liao Y, Bang D, Cosgrove S, Dulin R, Harris Z, Taylor A, White S, Yatabe G, Liburd L, Giles W. Surveillance of health status in minority communities - racial and ethnic approaches to community health across the u.S. (reach u.S.) risk factor survey, united states, 2009. MMWR Surveill Summ. 2011;60:1–44. [PubMed] [Google Scholar]
- 50.Agency for Health Research and Quality. 2016 national healthcare quality and disparities report. Rockville, MD: 2017. (AHRQ Pub. No. 17-0001). [Google Scholar]
- 51.Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: Assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202. doi: 10.1146/annurev.publhealth.19.1.173. [DOI] [PubMed] [Google Scholar]
- 52.Altman DG. Sustaining interventions in community systems: On the relationship between researchers and communities. Health Psychol. 1995;14:526–536. doi: 10.1037//0278-6133.14.6.526. [DOI] [PubMed] [Google Scholar]
- 53.Shalowitz MU, Isacco A, Barquin N, Clark-Kauffman E, Delger P, Nelson D, Quinn A, Wagenaar KA. Community-based participatory research: A review of the literature with strategies for community engagement. J Dev Behav Pediatr. 2009;30:350–361. doi: 10.1097/DBP.0b013e3181b0ef14. [DOI] [PubMed] [Google Scholar]
- 54.Bell J, Bell J, Colmenar R, Flournoy R, McGehee M, Rubin V, Thompson M, Thompson J, Vasquez V. Reducing health disparities through a focus on communities: A policy link report 2002. 2002 http://www.policylink.org/sites/default/files/REDUCINGHEALTHDISPARITIES_FINAL.PDF. Accessed.
- 55.Remington PL, Catlin BB, Gennuso KP. The county health rankings: Rationale and methods. Popul Health Metr. 2015;13:11. doi: 10.1186/s12963-015-0044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peppard PE, Kindig DA, Dranger E, Jovaag A, Remington PL. Ranking community health status to stimulate discussion of local public health issues: The wisconsin county health rankings. Am J Public Health. 2008;98:209–212. doi: 10.2105/AJPH.2006.092981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patient protection and affordable care act, publ no. 111-148, 124 stat 727, §6301. United States Code. 2010 http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/html/PLAW-111publ148.htm. Accessed.
- 58.Janus Youth Programs. The story of village gardens. 2017 http://www.villagegardens.org/our-story/. Accessed July 7, 2017.
- 59.Community-Campus Partnerships for Health (CCPH) Board of Directors. Position statement on authentic partnerships. 2013 https://ccph.memberclicks.net/principles-of-partnership. Accessed.
- 60.Viswanathan M, Ammerman A, Eng E, Garlehner G, Lohr KN, Griffith D, Rhodes S, Samuel-Hodge C, Maty S, Lux L, Webb L, Sutton SF, Swinson T, Jackman A, Whitener L. Community-based participatory research: Assessing the evidence. Evid Rep Technol Assess (Summ) 2004:1–8. [PMC free article] [PubMed] [Google Scholar]
- 61.Cooper LA, Marsteller JA, Noronha GJ, Flynn SJ, Carson KA, Boonyasai RT, Anderson CA, Aboumatar HJ, Roter DL, Dietz KB, Miller ER, III, Prokopowicz GP, Dalcin AT, Charleston JB, Simmons M, Huizinga MM. A multi-level system quality improvement intervention to reduce racial disparities in hypertension care and control: Study protocol. Implement Sci. 2013;8:60. doi: 10.1186/1748-5908-8-60.:60-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hussain T, Franz W, Brown E, Kan A, Okoye M, Dietz K, Taylor K, Carson KA, Halbert J, Dalcin A, Anderson CA, Boonyasai RT, Albert M, Marsteller JA, Cooper LA. The role of care management as a population health intervention to address disparities and control hypertension: A quasi-experimental observational study. Ethn Dis. 2016;26:285–294. doi: 10.18865/ed.26.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boonyasai RT, Carson KA, Marsteller JA, Dietz KB, Noronha GJ, Hsu Y-J, SJ F, Charleston JM, Prokopowicz GP, Miller ER, Cooper LA. A bundled quality improvement program to standardize clinical blood pressure measurement in primary care. J Clin Hypertens (Greenwich) 2017 doi: 10.1111/jch.13166. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willems Van Dijk JA. Recommendations to advance the evidence base for spreading and scaling innovative solutions in local communities 2016. http://www.academyhealth.org/files/AH_Brief_2016_Evidence%20based_FINAL.pdf. Accessed.
- 65.Davis AM, Vinci LM, Okwuosa TM, Chase AR, Huang ES. Cardiovascular health disparities: A systematic review of health care interventions. Med Care Res Rev. 2007;64:29S–100S. doi: 10.1177/1077558707305416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clarke AR, Goddu AP, Nocon RS, Stock NW, Chyr LC, Akuoko JA, Chin MH. Thirty years of disparities intervention research: What are we doing to close racial and ethnic gaps in health care? Med Care. 2013;51:1020–1026. doi: 10.1097/MLR.0b013e3182a97ba3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Record NB, Onion DK, Prior RE, Dixon DC, Record SS, Fowler FL, Cayer GR, Amos CI, Pearson TA. Community-wide cardiovascular disease prevention programs and health outcomes in a rural county, 1970–2010. JAMA. 2015;313:147–155. doi: 10.1001/jama.2014.16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mueller M, Purnell TS, Mensah GA, Cooper LA. Reducing racial and ethnic disparities in hypertension prevention and control: What will it take to translate research into practice and policy? Am J Hypertens. 2015;28:699–716. doi: 10.1093/ajh/hpu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trevino RP, Yin Z, Hernandez A, Hale DE, Garcia OA, Mobley C. Impact of the bienestar school-based diabetes mellitus prevention program on fasting capillary glucose levels: A randomized controlled trial. Arch Pediatr Adolesc Med. 2004;158:911–917. doi: 10.1001/archpedi.158.9.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foster GD, Linder B, Baranowski T, Cooper DM, Goldberg L, Harrell JS, Kaufman F, Marcus MD, Trevino RP, Hirst K. A school-based intervention for diabetes risk reduction. N Engl J Med. 2010;363:443–453. doi: 10.1056/NEJMoa1001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Purnell TS, Calhoun EA, Golden SH, Halladay JR, Krok-Schoen JL, Appelhans BM, Cooper LA. Achieving health equity: Closing the gaps in health care disparities, interventions, and research. Health Aff (Millwood) 2016;35:1410–1415. doi: 10.1377/hlthaff.2016.0158. [DOI] [PubMed] [Google Scholar]
- 72.Thornton RL, Glover CM, Cene CW, Glik DC, Henderson JA, Williams DR. Evaluating strategies for reducing health disparities by addressing the social determinants of health. Health Aff (Millwood) 2016;35:1416–1423. doi: 10.1377/hlthaff.2015.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.National Academies of Sciences, Engineering, and Medicine. Communities in action: Pathways to health equity. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 74.Frieden TR. A framework for public health action: The health impact pyramid. Am J Public Health. 2010;100:590–595. doi: 10.2105/AJPH.2009.185652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorin SS, Badr H, Krebs P, Prabhu Das I. Multilevel interventions and racial/ethnic health disparities. J Natl Cancer Inst Monogr. 2012;2012:100–111. doi: 10.1093/jncimonographs/lgs015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cooper LA, Purnell TS, Ibe CA, Halbert JP, Bone LR, Carson KA, Hickman D, Simmons M, Vachon A, Robb I, Martin-Daniels M, Dietz KB, Golden SH, Crews DC, Hill-Briggs F, Marsteller JA, Boulware LE, Miller ER, Levine DM. Reaching for health equity and social justice in baltimore: The evolution of an academic-community partnership and conceptual framework to address hypertension disparities. Ethn Dis. 2016;26:369–378. doi: 10.18865/ed.26.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fort DG, Herr TM, Shaw PL, Gutzman KE, Starren JB. Mapping the evolving definitions of translational research. J Clin Transl Sci. 2017;1:60–66. doi: 10.1017/cts.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Westfall JM, Fagnan LJ, Handley M, Salsberg J, McGinnis P, Zittleman LK, Macaulay AC. Practice-based research is community engagement. Journal of the American Board of Family Medicine : JABFM. 2009;22:423–427. doi: 10.3122/jabfm.2009.04.090105. [DOI] [PubMed] [Google Scholar]
- 79.Westfall JM, Mold J, Fagnan L. Practice-based research–"blue highways" on the nih roadmap. JAMA. 2007;297:403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 80.Green LA, Hickner J. A short history of primary care practice-based research networks: From concept to essential research laboratories. Journal of the American Board of Family Medicine : JABFM. 2006;19:1–10. doi: 10.3122/jabfm.19.1.1. [DOI] [PubMed] [Google Scholar]
- 81.Salako A, Zhu X, MacKinney AC, Ullrich F, Mueller K. Characteristics of rural accountable care organizations (acos) - a survey of medicare acos with rural presence. Rural Policy Brief. 2015:1–4. [PubMed] [Google Scholar]
- 82.Adams AJ, Clark DR, DeLander GE, Mackinnon GE, 3rd, Malloy M, McGivney MS, Mobley C, Nuffer W, Parsons P, Smesny AL, Smith M, Ives TJ. Report of the aacp task force on patient-centered medical homes and accountable care organizations. Am J Pharm Educ. 2013;77:142. doi: 10.5688/ajpe777142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Springgate BF, Brook RH. Accountable care organizations and community empowerment. JAMA. 2011;305:1800–1801. doi: 10.1001/jama.2011.547. [DOI] [PubMed] [Google Scholar]
- 84.Frieden TR, Berwick DM. The “million hearts” initiative–preventing heart attacks and strokes. N Engl J Med. 2011;365:e27. doi: 10.1056/NEJMp1110421. [DOI] [PubMed] [Google Scholar]
- 85.Grumbach K, Vargas RA, Fleisher P, Aragon TJ, Chung L, Chawla C, Yant A, Garcia ER, Santiago A, Lang PL, Jones P, Liu W, Schmidt LA. Achieving health equity through community engagement in translating evidence to policy: The san francisco health improvement partnership, 2010–2016. Preventing chronic disease. 2017;14:E27. doi: 10.5888/pcd14.160469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Griswold KS, Lesko SE, Westfall JM. Communities of solution: Partnerships for population health. Journal of the American Board of Family Medicine : JABFM. 2013;26:232–238. doi: 10.3122/jabfm.2013.03.130102. [DOI] [PubMed] [Google Scholar]
- 87.Lesko S, Griswold K, David S, Bazemore A, Duane M, Morgan T, Westfall J, Koop C, Garrett B, Puffer J, Green L, The American Board of Family Medicine Young Leaders Advisory Group Communities of solution: The folsom report revisited. Annals of family medicine. 2012;10:250–260. doi: 10.1370/afm.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown CH, Curran G, Palinkas LA, Aarons GA, Wells KB, Jones L, Collins LM, Duan N, Mittman BS, Wallace A, Tabak RG, Ducharme L, Chambers DA, Neta G, Wiley T, Landsverk J, Cheung K, Cruden G. An overview of research and evaluation designs for dissemination and implementation. Annu Rev Public Health. 2017;38:1–22. doi: 10.1146/annurev-publhealth-031816-044215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown CH, PoVey C, Hjorth A, Gallo CG, Wilensky U, Villamar J. Computational and technical approaches to improve the implementation of prevention programs. Implementation science : IS. 2015;10(Suppl 1) [Google Scholar]
- 90.Brown CH, Mohr DC, Gallo CG, Mader C, Palinkas L, Wingood G, Prado G, Kellam SG, Pantin H, Poduska J, Gibbons R, McManus J, Ogihara M, Valente T, Wulczyn F, Czaja S, Sutcliffe G, Villamar J, Jacobs C. A computational future for preventing hiv in minority communities: How advanced technology can improve implementation of effective programs. Journal of acquired immune deficiency syndromes (1999) 2013;63(Suppl 1):S72–84. doi: 10.1097/QAI.0b013e31829372bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perrino T, Beardslee W, Bernal G, Brincks A, Cruden G, Howe G, Murry V, Pantin H, Prado G, Sandler I, Brown CH. Toward scientific equity for the prevention of depression and depressive symptoms in vulnerable youth. Prevention science : the official journal of the Society for Prevention Research. 2015;16:642–651. doi: 10.1007/s11121-014-0518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown CH, Kellam SG, Kaupert S, Muthen BO, Wang W, Muthen LK, Chamberlain P, PoVey CL, Cady R, Valente TW, Ogihara M, Prado GJ, Pantin HM, Gallo CG, Szapocznik J, Czaja SJ, McManus JW. Partnerships for the design, conduct, and analysis of effectiveness, and implementation research: Experiences of the prevention science and methodology group. Administration and policy in mental health. 2012;39:301–316. doi: 10.1007/s10488-011-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kellam SG. Developing and maintaining partnerships as the foundation of implementation and implementation science: Reflections over a half century. Administration and policy in mental health. 2012;39:317–320. doi: 10.1007/s10488-011-0402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cramer ME, Lazoritz S, Shaffer K, Palm D, Ford AL. Community advisory board members' perspectives regarding opportunities and challenges of research collaboration. West J Nurs Res. 2017:193945917697229. doi: 10.1177/0193945917697229. [DOI] [PubMed] [Google Scholar]
- 95.Taylor E, Marino D, Rasor-Greenhalgh S, Hudak S. Navigating practice and academic change in collaborative partnership with a community advisory board. J Allied Health. 2010;39:e105–110. [PubMed] [Google Scholar]
- 96.Baig K, Shaw-Ridley M, Munoz OJ. Applying geo-spatial analysis in community needs assessment: Implications for planning and prioritizing based on data. Eval Program Plann. 2016;58:42–48. doi: 10.1016/j.evalprogplan.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 97.Lebron C, Stoutenberg M, Portacio F, Zollinger TW. A community needs assessment of the physical activity and food environment in a predominantly hispanic u.S. City. Hisp Health Care Int. 2016;14:124–131. doi: 10.1177/1540415316660826. [DOI] [PubMed] [Google Scholar]
- 98.Shelton RC, Charles TA, Dunston SK, Jandorf L, Erwin DO. Advancing understanding of the sustainability of lay health advisor (lha) programs for african-american women in community settings. Translational behavioral medicine. 2017 doi: 10.1007/s13142-017-0491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Landsverk J, Brown CH, Smith JD, et al. Design and analysis in dissemination and implementation research. In: Brownson RC, Colditz GA, Proctor EK, editors. Dissemination and implementation research in health: Translating research to practice. New York: Oxford University Press; In Press. [Google Scholar]
- 100.Brown CA, Lilford RJ. The stepped wedge trial design: A systematic review. BMC Med Res Methodol. 2006;6:54. doi: 10.1186/1471-2288-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Turner EL, Prague M, Gallis JA, Li F, Murray DM. Review of recent methodological developments in group-randomized trials: Part 2-analysis. Am J Public Health. 2017;107:1078–1086. doi: 10.2105/AJPH.2017.303707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Turner EL, Li F, Gallis JA, Prague M, Murray DM. Review of recent methodological developments in group-randomized trials: Part 1-design. Am J Public Health. 2017;107:907–915. doi: 10.2105/AJPH.2017.303706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chamberlain P, Brown CH, Saldana L. Observational measure of implementation progress in community based settings: The stages of implementation completion (sic) Implementation science : IS. 2011;6:116. doi: 10.1186/1748-5908-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Webb EJ, Campbell DT, Schwartz RD, Sechrest L. Unobtrusive measures: Nonreactive research in the social sciences. Chicago: Rand McNally; 1966. [Google Scholar]
- 105.Wang D, Ogihara M, Gallo C, Villamar JA, Smith JD, Vermeer W, Cruden G, Benbow N, Brown CH. Automatic classification of communication logs into implementation stages via text analysis. 2016;11:119. doi: 10.1186/s13012-016-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van HL, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: The american heart association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 107.Ritzwoller DP, Sukhanova A, Gaglio B, Glasgow RE. Costing behavioral interventions: A practical guide to enhance translation. Ann Behav Med. 2009;37:218–227. doi: 10.1007/s12160-009-9088-5. [DOI] [PubMed] [Google Scholar]
- 108.Chamberlain P, Snowden LR, Padgett C, Saldana L, Roles J, Holmes L, Ward H, Soper J, Reid J, Landsverk J. A strategy for assessing costs of implementing new practices in the child welfare system: Adapting the english cost calculator in the united states. Administration and policy in mental health. 2011;38:24–31. doi: 10.1007/s10488-010-0318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Palinkas LA, Spear SE, Mendon SJ, Villamar J, Valente T, Chou CP, Landsverk J, Kellam SG, Brown CH. Measuring sustainment of prevention programs and initiatives: A study protocol. Implementation science : IS. 2016;11:95. doi: 10.1186/s13012-016-0467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ofili EO, Fair A, Norris K, Verbalis JG, Poland R, Bernard G, Stephens DS, Dubinett SM, Imperato-McGinley J, Dottin RP, Pulley J, West A, Brown A, Mellman TA. Models of interinstitutional partnerships between research intensive universities and minority serving institutions (msi) across the clinical translational science award (ctsa) consortium. Clin Transl Sci. 2013;6:435–443. doi: 10.1111/cts.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stephens DS, West AC, Ofili EO, Boyan BD, Blumberg HM. The atlanta clinical and translational science institute: Clinical and translational science education and training partnership. Clinical and translational science. 2011;4:143–145. doi: 10.1111/j.1752-8062.2011.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Estape E, Laurido LE, Shaheen M, Quarshie A, Frontera W, Mays MH, Harrigan R, White R., 3rd A multiinstitutional, multidisciplinary model for developing and teaching translational research in health disparities. Clinical and translational science. 2011;4:434–438. doi: 10.1111/j.1752-8062.2011.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Duncan GA, Lockett A, Villegas LR, Almodovar S, Gomez JL, Flores SC, Wilkes DS, Tigno XT. National heart, lung, and blood institute workshop summary: Enhancing opportunities for training and retention of a diverse biomedical workforce. Annals of the American Thoracic Society. 2016;13:562–567. doi: 10.1513/AnnalsATS.201509-624OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gandhi M, Johnson M. Creating more effective mentors: Mentoring the mentor. AIDS Behav. 2016;20(Suppl 2):294–303. doi: 10.1007/s10461-016-1364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.National Research Mentoring Network (NRMN) About the national research mentoring network 2017. https://nrmnet.net/. Accessed.
- 116.National Institutes of Health, Office of Behavioral and Social Sciences Research. Institute on randomized clinical trials. 2017 https://obssr.od.nih.gov/training/training-institutes/institute-on-randomized-clinical-trials/. Accessed.
- 117.Meissner HI, Glasgow RE, Vinson CA, Chambers D, Brownson RC, Green LW, Ammerman AS, Weiner BJ, Mittman B. The u.S. Training institute for dissemination and implementation research in health. Implementation science : IS. 2013;8:12. doi: 10.1186/1748-5908-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Luke DA, Baumann AA, Carothers BJ, Landsverk J, Proctor EK. Forging a link between mentoring and collaboration: A new training model for implementation science. Implementation science : IS. 2016;11:137. doi: 10.1186/s13012-016-0499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chambers DA, Proctor EK, Brownson RC, Straus SE. Mapping training needs for dissemination and implementation research: Lessons from a synthesis of existing d&i research training programs. Translational behavioral medicine. 2017;7:593–601. doi: 10.1007/s13142-016-0399-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Centers for Disease Control a0nd Prevention. Science ambassador fellowship. 2017 https://www.cdc.gov/careerpaths/scienceambassador/overview.html. Accessed.
- 121.Science Olympiad. Mission. 2017 https://www.soinc.org/about/mission. Accessed July 16, 2017.
- 122.Centers for Disease Control and Prevention. Career paths to public health (cpp) 2017 https://www.cdc.gov/careerpaths/index.html. Accessed July 16, 2017.
- 123.National Institute on Minority Health and Health Disparities. Nimhd research framework. 2017 https://www.nimhd.nih.gov/about/overview/research-framework.html. Accessed July 10, 2017.
- 124.National Institute on Minority Health and Health Disparities. Nimhd funding opportunities. 2017 https://www.nimhd.nih.gov/funding/nimhd.html. Accessed July 10, 2017.
- 125.Gilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Association between birth in a high stroke mortality state, race, and risk of dementia. JAMA Neurol. 2017;74:1056–1062. doi: 10.1001/jamaneurol.2017.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12:216–224. doi: 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, Ayonayon H, Simonsick E. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: Prospective study. Bmj. 2013;347:f7051. doi: 10.1136/bmj.f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the framingham heart study. N Engl J Med. 2016;374:523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]


