Abstract
Purpose of review
New treatment approaches to weight loss and weight loss maintenance in humans are critical. Given its potential role in stimulating energy expenditure, brown adipose tissue (BAT) activation has become a trending topic as an anti-obesity treatment.
Recent findings
Most studies on BAT stimulation have been conducted in rodents and used cold stimulation. To date, few human trials exist that tested the effect of cold exposure on BAT. Those studies show that BAT contributes a small amount to overall energy metabolism which is unlikely to cause weight loss. Nonetheless, improvements in glucose metabolism have been demonstrated in humans. While new pharmacological approaches demonstrate some contribution of BAT to overall energy expenditure, the potential cardiovascular risk (increased heart rate and blood pressure to sustain the extra energy expenditure) may preclude their use.
Summary
There is no convincing evidence yet to indicate that BAT may be a viable pharmaceutical target for body weight loss or even weight loss maintenance. More research is needed to confirm the relevance of BAT and beige tissue to whole-body energy metabolism in humans.
Keywords: energy balance, brown adipose tissue, BAT, beige, brite, thermogenesis, cold exposure, cold acclimation, cold-induced thermogenesis, CIT, diet-induced thermogenesis, DIT, uncoupling protein 1, UCP1, β3-adrenoreceptor agonists, capsinoids, capsaicin
Introduction
Obesity has reached epidemic proportions around the world and poses a severe threat to global public health. Driven by a chronic energy imbalance between excess energy (food) intake and/or reduced energy expenditure (physical activity), obesity in the United States reached 35.0% of men and 40.4% of women in 2013–2014 [1] with the yearly cost of obesity to the US public health system estimated to be $190.2 billion or nearly 21% of annual medical spending [2]. As a result, new treatment approaches to induce weight loss and improve weight loss maintenance are of growing importance. Over the past few decades, pharmaceutical companies have mostly developed drugs targeting food intake and have neglected the other side of the energy balance equation, i.e. energy expenditure, thus leaving a whole new area of research open to targeting energy expenditure as an anti-obesity treatment option.
To understand how new treatment approaches may potentially contribute to weight loss or weight loss maintenance via increased energy expenditure, it is important to understand the three major components of energy expenditure. Specifically, the variability in daily energy requirements is related to the variability in the energy expended in its three major components: (1) basal metabolic rate (BMR), (2) thermic effect of food (or diet-induced thermogenesis), and (3) the energy cost of physical activity including both volitional exercise and spontaneous physical activity. BMR is closely related to body size and accounts for approximately 60–70% of daily energy expenditure in sedentary adults [3] with up to 75% of the variance in BMR being determined by fat-free mass [4, 5] and to a lesser extent by fat mass, sex, and age. The thermic effect of food accounts for approximately 5–15% of daily energy expenditure [6–8], and consists of the increase in energy expenditure associated with chewing, digestion, absorption, and storage of food in response to a single meal. Activity thermogenesis is the most variable component of daily energy expenditure and is related to the energy necessary to produce skeletal muscle contraction for motion or for spontaneous physical activity such as fidgeting. Indeed, while increasing activity levels seems an effective way to enhance energy expenditure, willingness to follow the normal physical activity recommendations is highly variable and the evidence of structured exercise as a tool for weight loss is far from convincing. As a consequence, the search for a safe and efficacious way to increase metabolic rate has become increasingly important not only to help weight loss but also to counteract the metabolic adaptation occurring with caloric restriction and weight loss [9–11].
The Adipose Organ
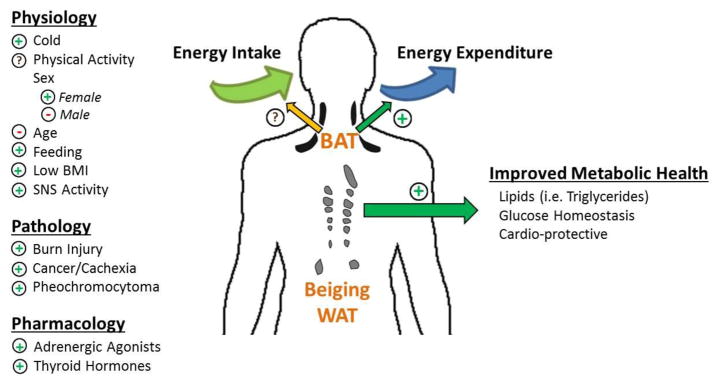
Both white adipose tissue (WAT) and brown adipose tissue (BAT) make up the adipose organ. WAT is the primary site of energy storage and of release of hormones and cytokines that modulate whole-body metabolism and insulin resistance [12–15]. Conversely, BAT is a tissue designed for maintaining body temperature significantly higher than ambient temperatures through heat production primarily via non-shivering thermogenesis. Mediated by the expression of the tissue-specific uncoupling protein 1 (UCP1) within the abundant mitochondria (contributing to a brown appearance), BAT functions to facilitate adaptive thermogenesis, or the uncoupling of ATP production and substrate oxidation. Brown adipocytes are thus able to quickly oxidize their own fat stores and circulating substrates, thus producing heat and increasing metabolic rate [16, 17]. The exact amount (volume) of active BAT in adult humans, however, remains highly variable (see below and Figure 1).
Figure 1. Mediators of BAT (or Beige) Amount/Activity and the Impact on Metabolism.
There are several physiological, pathological, and pharmacological conditions that are known to mediate the amount of BAT and its activity (on the left). Specifically, “+” and “−” indicates that BAT is “stimulated” or “depressed” by the following condition or stimuli, respectively. On the right are the potential effects of BAT on energy intake, energy expenditure, and cardiometabolic health.
BAT Activation vs. BAT Induction
The rediscovery of BAT in adults more than a decade ago has sparked a resurgence in the targeting/activation of BAT or BAT induction (or beiging) in WAT as a means to promote adaptive thermogenesis and energy expenditure [18].
BAT activation
Two facets of adaptive thermogenesis via BAT activation have been proposed, that is cold-induced thermogenesis (CIT) and diet-induced thermogenesis (DIT). First, CIT is triggered mostly by sympathetic nervous system and thyroid activation [19]. Recent CIT studies in adult humans reignited interest in BAT activation to increase energy expenditure and improve weight control [20–24]. As summarized in Figure 1, there are many physiological and pathological factors which can impact the amount and activity of BAT. Specifically, BAT amount and activity was reported to be 4-fold higher in lean individuals compared to overweight/obese individuals [23], thus suggesting a role of BAT in weight control. BAT mass was also inversely associated with outdoor temperature at the time of PET/CT scans, as well as age and BMI with lesser BAT mass present in males [20]. Furthermore, cold exposure increases both BAT activity [22, 23] and glucose uptake [24]. Nonetheless, while the role of BAT in CIT has been extensively studied in rodents, it requires further investigation to support a definitive role in humans.
The thermic effect of food (TEF) in response to a single meal and DIT in response to overfeeding have been shown to be triggered by sympathetic activation (short-term) and thyroid hormones (long-term) and seem partially related to a dissipation of the excess energy intake in the form of heat (rather than storage) and thus protects against weight gain [25]. However, recent data in humans indicate that unlike CIT, DIT is probably not mediated by BAT [26]. Additionally, we recently reported that BAT probably does not mediate the metabolic adaptation following 8 weeks of overfeeding in men [27]. Together, these studies do not support a role of BAT in modulating DIT and do not lend support to an important role for BAT in the regulation of human body weight as previously suggested by Kozak [28].
BAT induction
In recent years, a large number of rodent studies have emerged showing UCP1 positive cells in WAT with very similar properties as BAT cells [29–31]. The action of browning WAT into BAT-like adipocytes, or beige (“brite”) cells, has been further postulated to protect against obesity via body fat reduction and related complications [32–35, 31, 36, 37]. Similar to BAT adipocytes, beige adipocytes have the ability to take on a BAT-like thermogenic phenotype in response to various stimuli such as cold, endocrine factors or chemical compounds. Specifically, a high UCP1 expression and increased energy consumption similar to brown adipocytes has been reported during cold exposure [33, 38, 31, 39, 36, 40, 37, 41]. A compensatory browning has been shown to occur in the WAT of mice when constitutive BAT is scarce, thereby demonstrating the potential role of recruitable BAT in restoring BAT-mediated thermogenesis [42].
Although cold challenges have not proven to consistently mediate browning of white fat depots in humans, chronically elevated sympathetic activation has been reported to produce brown fat morphology and function in human white adipose tissue. Specifically, exercise has been shown to induce browning of the subcutaneous fat in mice, yet browning of WAT has not been reproducible in the subcutaneous human fat [43, 44]. Browning has also been observed in the subcutaneous depot in response to severe burn injury [45]. Finally, browning has been shown to occur in mice in response to cancer cachexia [46, 47].
Contributions of BAT to Energy Metabolism, Glucose Metabolism, and Cardiometabolic Regulation
Over the past several years, BAT activation has sparked a potential newer role in regulating metabolic risk factors for cardiovascular diseases such as lipoprotein cholesterol, glucose uptake and metabolism, and potentially conferring cardioprotective effects. Findings from mouse models demonstrate that cold-activated BAT can effectively clear plasma triglycerides [48–50] and may even mitigate hypercholesterinemia and suppress the formation of atherosclerotic plaque [51, 52, 49]. One study demonstrated a retardation of atherosclerotic plaque growth with only 8 weeks of cold exposure [53]; however, this may have been the result of rapid exposure instead of gradual acclimation. While evidence of a cardioprotective effect in humans has yet to be shown, a recent study in humans found that the main substrate used by activated BAT is fatty acids derived from intracellular triglycerides thus decreasing triglyceride storage [54].
The role of BAT activation and its contribution to the regulation of glucose metabolism in humans has also given us additional insight into the potentially underappreciated role of BAT in human metabolic health. Indeed, human studies have shown improved glucose uptake in activated BAT [20, 22–24, 55] and cold-induced BAT activation increases glucose uptake and improves whole-body glucose disposal and insulin sensitivity [54, 56–63]. One study in healthy humans using indirect calorimetry and stable isotopes showed that cold exposure resulted in an increase in resting metabolic rate of 14% in subjects who had detectable BAT levels, and that this increase was fueled by both plasma-derived glucose (30%) and free fatty acid oxidation (70%) [56]. Similarly, individuals with obesity or type 2 diabetes experienced an improvement in insulin sensitivity following a sequential cold stimulation protocol. Specifically, a short-term 10-day cold acclimation resulted in enhanced BAT activity and improved whole-body insulin sensitivity (43%) in overweight men with type 2 diabetes [57]. Recruitment of active BAT in obese individuals was also reported following short-term cold exposure for up to 6 hours per day for 10 days [64]; however, no increase in energy expenditure was observed potentially due to smaller amounts of BAT activation. Another study of cold acclimation (sleeping in a 19 °C room with light clothing for a month) did not alter CIT but was accompanied by an enhancement in postprandial insulin sensitivity [65].
To test the role of BAT in the direct regulation of glucose homeostasis, Stanford and colleagues transplanted BAT from male donor mice into the visceral cavity of age- and sex-matched recipient mice and observed improved glucose tolerance and insulin sensitivity, lower body weight, decreased fat mass, as well as a complete reversal of high-fat diet-induced insulin resistance in recipient mice [66]. While the molecular mechanisms underlying the improvements in insulin sensitivity in both mice and humans are unknown, these cardiometabolic benefits offer an alternative way to improve overall health beyond increases in energy expenditure. Regardless, the lack of observable weight loss despite increased energy expenditure with BAT activation is troubling. In humans, while decreases in body fat mass have been observed, total body weight was unchanged in obese individuals [63]. A few intervention-based studies have reported weight loss with increased BAT activity in obese individuals, one following a conventional lifestyle modification program [67] and one following laproscopic adjustable banding [68].
BAT Activation via Pharmacological Approaches
Long term studies of adult humans are needed to weigh the potential risks and benefits of pharmacologic interventions aimed at activating BAT or beige adipose tissue. Among pharmacological agents tested to date, β3-adrenoreceptor agonists are well-known to induce UCP1 expression and thermogenesis in vivo in rodents and in vitro in isolated brown adipocytes [69–72]. Additionally, β3-adrenoreceptor agonists have also been shown to enhance glucose metabolic activity of BAT in rodents [73–75]. In humans, early efforts to increase BAT activity with the use of β3-adrenoreceptor agonists have failed in clinical trials due to their β1- and β2-adrenoreceptor-mediated cardiovascular and muscular events [76, 77]. Indeed, a recent study showed that mirabegron (Myrbetriq®), a selective β3-adrenoreceptor agonist approved for treatment of overactive bladder, elicited BAT activation in healthy male subjects with a single oral dose of 200 mg and increased resting metabolic rate by 203 kcal/day, or +13% [78]. Though a 203 kcal/day increase in energy expenditure was postulated to lead to an eventual weight loss of ~5kg in one year and 10kg by three years [79], the actual amount of weight loss would be less since the 203 kcal/day estimate was the peak energy expenditure and not sustainable throughout the day. Additionally, administration of mirabegron was accompanied by increased heart rate and systolic blood pressure, lending itself to potentially increased cardiovascular side effects.
Studies in rodent have also demonstrated that non-pungent capsaicin analogues (capsinoids) may be a potential therapeutic approach to obesity management [80]. Capsinoids may increase BAT thermogenesis through the activation of TRPV1 and the sympathetic nervous system, and potentially decrease body fat. In humans, a similar thermogenic effect of capsinoids has been observed [81–83]. Specifically, a 6-week treatment with oral capsinoids increased resting energy expenditure in those individuals with previously low or undetectable BAT activity, yet no loss of body weight or fat mass was observed [83]. Conversely, Galgani and colleagues reported that acute oral capsinoids doses (1 to 13 mg) did not influence energy expenditure, blood pressure, or axillary temperature for ≥2 hours [84]. Alternatively, obese patients with type 2 diabetes treated with liraglutide (a GLP-1 analogue) demonstrated an increase in energy expenditure, possibly the result of stimulated BAT thermogenesis and browning of WAT as observed in mice [85]. Whether GLP-1 agonists mediated weight loss seen in humans also involves altered BAT activity is still a matter of investigation. Conversely, chenodeoxycholic acid (CDCA), a common bile acid that can be formulated to treat choleserolemia, has already been demonstrated to increase BAT activity in mice and humans [86, 87]. Other potential pharmacotherapies targeting BAT activation or browning of WAT are bone morphogenic proteins (BMPs), specifically BMP7 and BMP8b. These BMPs are important for BAT development and thermogenesis, and the browning of WAT [88–90]. The increased sympathetic outflow to BAT via BMP8b is postulated to result in weight loss in mice [90].
A Perspective Outlook
While there is excitement about the potential for BAT activation or beiging of WAT to increase energy expenditure and thereby induce weight loss, there is still much human research to be conducted to confidently conclude about the relative impact of BAT to overall energy expenditure and weight control. One exciting aspect of BAT activation in humans is the potential for improved glucose metabolism and increased cholesterol clearance observed in initial trials of overweight and obese subjects [57, 64]. Unfortunately, BAT activation via CIT in humans has not elicited any decreases in body weight despite small increases in energy expenditure and potentially decreases in fat mass [63]. Indeed, the estimated contribution of BAT to whole-body energy expenditure in cold-exposed adults is relatively small and has been suggested to be irrelevant to whole-body energy turnover and therefore obesity management [91]. While the consistent lack of weight loss in response to increase BAT activation is troubling, the limited duration of these studies may offer an explanation as to lack of observable weight loss. Another explanation is that maximal activation of BAT for extended periods of time is not an easy feat, especially in humans. Indeed, exposing people to severe and prolonged cold exposure may be impractical compared to the classic rodent model. Importantly, it is likely that longer bouts and more severe cold exposure may elicit a compensatory increase in appetite and food intake in humans, thus rendering the use of BAT activation inefficacious in the treatment of obesity [92, 93].
While a 10–20% increase in energy expenditure has been reported [23, 24], other studies have suggested the contribution of BAT to resting metabolic rate is more of the order of 1–7% [94–97]. Our own speculations and calculations provide numbers of approximately 100 kcal/day of energy expenditure for fully stimulated BAT. For instance, Rothwell and Stock suggested that a hypothetical amount of 40–50 grams of BAT could account up to 15–20% of energy expenditure [98]. However, such amount of BAT, if maximally stimulated (assuming a 100x increased oxidative activity from inactivated state) could account for only approximately 125 kcal/day. Similarly, Virtanen et al. reported an average of 63 grams of BAT and a glucose uptake by BAT of 12.2 μmol/100g/min [24]. Such numbers translate to 126 kcal/day in resting metabolic rate assuming that glucose represents only 10% of BAT metabolism. Finally, Ouellet et al. reported a mean total BAT volume of 168 grams (varying from 31 to 329 grams) and a glucose uptake by BAT of 10.8 μmol/100g/min, thus representing roughly 8–100 kcal/day [60]. Therefore, such minor amounts of energy expenditure dissipated by fully activated BAT (approximately 100 Kcal/day) does not make it a good target for weight loss since larger gap in energy (intake minus expenditure) are required to induce significant weight loss (usually a minimum of 500 kcal/day is desired). However a 100 kcal/day makes it a potential realistic contributor to better weight loss maintenance by counteracting some of the metabolic adaptation induced by weight loss.
Even if BAT activation via pharmacological therapies has taken center stage in an effort to manage obesity, such approach may be associated with more risks than benefit. While increased BAT activation and therefore increased energy expenditure has been observed in some human studies, the cardiovascular risks associated with such treatments (like β3-adrenoreceptor agonists) cannot be ignored since any intervention aimed at increasing energy expenditure will likely increase heart rate and potentially blood pressure.
Conclusions
Despite the ensuing explosion of pre-clinical investigations and identification of an extensive list of potential molecular targets for BAT recruitment and activation, our understanding of human BAT physiology remains limited, particularly regarding interventions which might hold therapeutic promise. In order to translate what has been learned from rodent models, we need to better understand how both BAT volume and its metabolic activity contribute to overall energy expenditure in humans.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Kara L. Marlatt and Eric Ravussin declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–91. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31(1):219–30. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78(6):1568–78. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogardus C, Lillioja S, Ravussin E, Abbott W, Zawadzki JK, Young A, et al. Familial dependence of the resting metabolic rate. N Engl J Med. 1986;315(2):96–100. doi: 10.1056/NEJM198607103150205. [DOI] [PubMed] [Google Scholar]
- 5.Tataranni PA, Ravussin E. Variability in metabolic rate: biological sites of regulation. Int J Obes Relat Metab Disord. 1995;19(Suppl 4):S102–6. [PubMed] [Google Scholar]
- 6.Donahoo WT, Levine JA, Melanson EL. Variability in energy expenditure and its components. Curr Opin Clin Nutr Metab Care. 2004;7(6):599–605. doi: 10.1097/00075197-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Tataranni PA, Larson DE, Snitker S, Ravussin E. Thermic effect of food in humans: methods and results from use of a respiratory chamber. Am J Clin Nutr. 1995;61(5):1013–9. doi: 10.1093/ajcn/61.4.1013. [DOI] [PubMed] [Google Scholar]
- 8.Weststrate JA. Resting metabolic rate and diet-induced thermogenesis: a methodological reappraisal. Am J Clin Nutr. 1993;58(5):592–601. doi: 10.1093/ajcn/58.5.592. [DOI] [PubMed] [Google Scholar]
- 9.Doucet E, St-Pierre S, Almeras N, Despres JP, Bouchard C, Tremblay A. Evidence for the existence of adaptive thermogenesis during weight loss. Br J Nutr. 2001;85(6):715–23. doi: 10.1079/bjn2001348. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88(4):906–12. doi: 10.1093/ajcn/88.4.906. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34(Suppl 1):S47–55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aldhahi W, Hamdy O. Adipokines, inflammation, and the endothelium in diabetes. Curr Diab Rep. 2003;3(4):293–8. doi: 10.1007/s11892-003-0020-2. [DOI] [PubMed] [Google Scholar]
- 13.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4(4):263–73. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 2006;64(4):355–65. doi: 10.1111/j.1365-2265.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 15.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444(7121):847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaton GM, Wagenvoord RJ, Kemp A, Jr, Nicholls DG. Brown-adipose-tissue mitochondria: photoaffinity labelling of the regulatory site of energy dissipation. Eur J Biochem. 1978;82(2):515–21. doi: 10.1111/j.1432-1033.1978.tb12045.x. [DOI] [PubMed] [Google Scholar]
- 17.Ricquier D, Kader JC. Mitochondrial protein alteration in active brown fat: a soidum dodecyl sulfate-polyacrylamide gel electrophoretic study. Biochem Biophys Res Commun. 1976;73(3):577–83. doi: 10.1016/0006-291x(76)90849-4. [DOI] [PubMed] [Google Scholar]
- 18.Tam CS, Lecoultre V, Ravussin E. Brown adipose tissue: mechanisms and potential therapeutic targets. Circulation. 2012;125(22):2782–91. doi: 10.1161/CIRCULATIONAHA.111.042929. [DOI] [PubMed] [Google Scholar]
- 19.Silva JE, Bianco SD. Thyroid-adrenergic interactions: physiological and clinical implications. Thyroid. 2008;18(2):157–65. doi: 10.1089/thy.2007.0252. [DOI] [PubMed] [Google Scholar]
- 20.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293(2):E444–52. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 22.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–31. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 24.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 25.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281(5726):31–5. doi: 10.1002/j.1550-8528.1997.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 26•.Peterson CM, Lecoultre V, Frost EA, Simmons J, Redman LM, Ravussin E. The thermogenic responses to overfeeding and cold are differentially regulated. Obesity (Silver Spring) 2016;24(1):96–101. doi: 10.1002/oby.21233. This study reported that BAT activity seem to mediate cold-induced thermogenesis but not dietary-induced thermogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson CM, Orooji M, Johnson DN, Naraghi-Pour M, Ravussin E. Brown adipose tissue does not seem to mediate metabolic adaptation to overfeeding in men. Obesity (Silver Spring) 2017;25(3):502–5. doi: 10.1002/oby.21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010;11(4):263–7. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298(6):E1244–53. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 30.Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14(3):324–38. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiefer FW. Browning and thermogenic programing of adipose tissue. Best Pract Res Clin Endocrinol Metab. 2016;30(4):479–85. doi: 10.1016/j.beem.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Barquissau V, Beuzelin D, Pisani DF, Beranger GE, Mairal A, Montagner A, et al. White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Mol Metab. 2016;5(5):352–65. doi: 10.1016/j.molmet.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10(1):24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 34.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 35.Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010;11(4):257–62. doi: 10.1016/j.cmet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285(10):7153–64. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121(1):96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishibashi J, Seale P. Medicine. Beige can be slimming. Science. 2010;328(5982):1113–4. doi: 10.1126/science.1190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SH, Plutzky J. Brown Fat and Browning for the Treatment of Obesity and Related Metabolic Disorders. Diabetes Metab J. 2016;40(1):12–21. doi: 10.4093/dmj.2016.40.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez-Gurmaches J, Hung CM, Guertin DA. Emerging Complexities in Adipocyte Origins and Identity. Trends Cell Biol. 2016;26(5):313–26. doi: 10.1016/j.tcb.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–76. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495(7441):379–83. doi: 10.1038/nature11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes. 2015;64(7):2361–8. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sidossis LS, Porter C, Saraf MK, Borsheim E, Radhakrishnan RS, Chao T, et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell Metab. 2015;22(2):219–27. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513(7516):100–4. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20(3):433–47. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17(2):200–5. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 49.Laurila PP, Soronen J, Kooijman S, Forsstrom S, Boon MR, Surakka I, et al. USF1 deficiency activates brown adipose tissue and improves cardiometabolic health. Sci Transl Med. 2016;8(323):323ra13. doi: 10.1126/scitranslmed.aad0015. [DOI] [PubMed] [Google Scholar]
- 50.Schlein C, Talukdar S, Heine M, Fischer AW, Krott LM, Nilsson SK, et al. FGF21 Lowers Plasma Triglycerides by Accelerating Lipoprotein Catabolism in White and Brown Adipose Tissues. Cell Metab. 2016;23(3):441–53. doi: 10.1016/j.cmet.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Berbee JF, Boon MR, Khedoe PP, Bartelt A, Schlein C, Worthmann A, et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun. 2015;6:6356. doi: 10.1038/ncomms7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoeke G, Kooijman S, Boon MR, Rensen PC, Berbee JF. Role of Brown Fat in Lipoprotein Metabolism and Atherosclerosis. Circ Res. 2016;118(1):173–82. doi: 10.1161/CIRCRESAHA.115.306647. [DOI] [PubMed] [Google Scholar]
- 53.Dong M, Yang X, Lim S, Cao Z, Honek J, Lu H, et al. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metab. 2013;18(1):118–29. doi: 10.1016/j.cmet.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blondin DP, Labbe SM, Noll C, Kunach M, Phoenix S, Guerin B, et al. Selective Impairment of Glucose but Not Fatty Acid or Oxidative Metabolism in Brown Adipose Tissue of Subjects With Type 2 Diabetes. Diabetes. 2015;64(7):2388–97. doi: 10.2337/db14-1651. [DOI] [PubMed] [Google Scholar]
- 55.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23(9):3113–20. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 56•.Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63(12):4089–99. doi: 10.2337/db14-0746. This study in healthy humans utilized indirect calorimetry and stable isotopes and reported that cold exposure increased resting metabolism by 14% in subjects who had detectable BAT levels, and was fueled primarily by plasma-derived glucose (30%) and free fatty acid oxidation (70%) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med. 2015;21(8):863–5. doi: 10.1038/nm.3891. A short-term cold acclimation protocol for 10 days in humans significantly enhanced BAT activity and improved whole-body insulin sensitivity by 43% in overweight men with type 2 diabetes, primarily due to increased insulin-stimulated glucose disposal. [DOI] [PubMed] [Google Scholar]
- 58.Matsushita M, Yoneshiro T, Aita S, Kameya T, Sugie H, Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes (Lond) 2014;38(6):812–7. doi: 10.1038/ijo.2013.206. [DOI] [PubMed] [Google Scholar]
- 59.Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14(2):272–9. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 60.Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122(2):545–52. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123(8):3395–403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS One. 2011;6(2):e17247. doi: 10.1371/journal.pone.0017247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123(8):3404–8. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Hanssen MJ, van der Lans AA, Brans B, Hoeks J, Jardon KM, Schaart G, et al. Short-term Cold Acclimation Recruits Brown Adipose Tissue in Obese Humans. Diabetes. 2016;65(5):1179–89. doi: 10.2337/db15-1372. Short-term cold exposure for up to 6 hours per day for 10 days elicited recruitment of active BAT in obese individuals. No increase in energy expenditure was observed, however, potentially due to smaller amounts of BAT activation. [DOI] [PubMed] [Google Scholar]
- 65•.Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63(11):3686–98. doi: 10.2337/db14-0513. This study reported BAT acclimation via sleeping in a cold (19 °C) room with light clothing for a month did not alter cold-induced thermogenesis but was accompanied an enhancement in postprandial insulin sensitivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123(1):215–23. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orava J, Nuutila P, Noponen T, Parkkola R, Viljanen T, Enerback S, et al. Blunted metabolic responses to cold and insulin stimulation in brown adipose tissue of obese humans. Obesity (Silver Spring) 2013;21(11):2279–87. doi: 10.1002/oby.20456. [DOI] [PubMed] [Google Scholar]
- 68.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Hoeks J, Schrauwen P, et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2012;97(7):E1229–33. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- 69.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 70.Cao W, Medvedev AV, Daniel KW, Collins S. beta-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. J Biol Chem. 2001;276(29):27077–82. doi: 10.1074/jbc.M101049200. [DOI] [PubMed] [Google Scholar]
- 71.Collins S, Daniel KW, Petro AE, Surwit RS. Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology. 1997;138(1):405–13. doi: 10.1210/endo.138.1.4829. [DOI] [PubMed] [Google Scholar]
- 72.Geloen A, Collet AJ, Guay G, Bukowiecki LJ. Beta-adrenergic stimulation of brown adipocyte proliferation. Am J Physiol. 1988;254(1 Pt 1):C175–82. doi: 10.1152/ajpcell.1988.254.1.C175. [DOI] [PubMed] [Google Scholar]
- 73.Mirbolooki MR, Constantinescu CC, Pan ML, Mukherjee J. Quantitative assessment of brown adipose tissue metabolic activity and volume using 18F-FDG PET/CT and β3-adrenergic receptor activation. EJNMMI Res. 2011;1(1):30. doi: 10.1186/2191-219X-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mirbolooki MR, Schade KN, Constantinescu CC, Pan ML, Mukherjee J. Enhancement of 18F-fluorodeoxyglucose metabolism in rat brain frontal cortex using a beta3 adrenoceptor agonist. Synapse. 2015;69(2):96–8. doi: 10.1002/syn.21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mirbolooki MR, Upadhyay SK, Constantinescu CC, Pan ML, Mukherjee J. Adrenergic pathway activation enhances brown adipose tissue metabolism: a [(1)(8)F]FDG PET/CT study in mice. Nucl Med Biol. 2014;41(1):10–6. doi: 10.1016/j.nucmedbio.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arch JR. Challenges in beta(3)-Adrenoceptor Agonist Drug Development. Ther Adv Endocrinol Metab. 2011;2(2):59–64. doi: 10.1177/2042018811398517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A. 2012;109(25):10001–5. doi: 10.1073/pnas.1207911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78•.Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elia E, Kessler SH, Kahn PA, et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab. 2015;21(1):33–8. doi: 10.1016/j.cmet.2014.12.009. This study demonstrated that 200 mg mirabegron, a selective β3-adrenoreceptor agonist, elicited BAT activation in healthy male subjects and increased resting metabolic rate (+203 kcal/day, or +13%) albeit an increase in heart rate and systolic blood pressure was observed thus lending to potentially increase cardiovascular risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826–37. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baskaran P, Krishnan V, Ren J, Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol. 2016;173(15):2369–89. doi: 10.1111/bph.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saito M, Yoneshiro T. Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans. Curr Opin Lipidol. 2013;24(1):71–7. doi: 10.1097/MOL.0b013e32835a4f40. [DOI] [PubMed] [Google Scholar]
- 82.Whiting S, Derbyshire E, Tiwari BK. Capsaicinoids and capsinoids. A potential role for weight management? A systematic review of the evidence. Appetite. 2012;59(2):341–8. doi: 10.1016/j.appet.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 83.Yoneshiro T, Aita S, Kawai Y, Iwanaga T, Saito M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am J Clin Nutr. 2012;95(4):845–50. doi: 10.3945/ajcn.111.018606. [DOI] [PubMed] [Google Scholar]
- 84.Galgani JE, Ryan DH, Ravussin E. Effect of capsinoids on energy metabolism in human subjects. Br J Nutr. 2010;103(1):38–42. doi: 10.1017/S0007114509991358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63(10):3346–58. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 86.Broeders EP, Nascimento EB, Havekes B, Brans B, Roumans KH, Tailleux A, et al. The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab. 2015;22(3):418–26. doi: 10.1016/j.cmet.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Teodoro JS, Zouhar P, Flachs P, Bardova K, Janovska P, Gomes AP, et al. Enhancement of brown fat thermogenesis using chenodeoxycholic acid in mice. Int J Obes (Lond) 2014;38(8):1027–34. doi: 10.1038/ijo.2013.230. [DOI] [PubMed] [Google Scholar]
- 88.Modica S, Wolfrum C. Bone morphogenic proteins signaling in adipogenesis and energy homeostasis. Biochim Biophys Acta. 2013;1831(5):915–23. doi: 10.1016/j.bbalip.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 89.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454(7207):1000–4. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vazquez MJ, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149(4):871–85. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jensen MD. Brown adipose tissue--not as hot as we thought. J Physiol. 2015;593(3):489. doi: 10.1113/jphysiol.2014.287979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carey AL, Formosa MF, Van Every B, Bertovic D, Eikelis N, Lambert GW, et al. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia. 2013;56(1):147–55. doi: 10.1007/s00125-012-2748-1. [DOI] [PubMed] [Google Scholar]
- 93.Ravussin Y, Xiao C, Gavrilova O, Reitman ML. Effect of intermittent cold exposure on brown fat activation, obesity, and energy homeostasis in mice. PLoS One. 2014;9(1):e85876. doi: 10.1371/journal.pone.0085876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carey AL, Kingwell BA. Brown adipose tissue in humans: therapeutic potential to combat obesity. Pharmacol Ther. 2013;140(1):26–33. doi: 10.1016/j.pharmthera.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 95.Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med. 2013;54(4):523–31. doi: 10.2967/jnumed.112.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Porter C, Chondronikola M, Sidossis LS. The Therapeutic Potential of Brown Adipocytes in Humans. Front Endocrinol (Lausanne) 2015;6:156. doi: 10.3389/fendo.2015.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Marken Lichtenbelt WD, Schrauwen P. Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301(2):R285–96. doi: 10.1152/ajpregu.00652.2010. [DOI] [PubMed] [Google Scholar]
- 98.Rothwell NJ, Stock MJ. Luxuskonsumption, diet-induced thermogenesis and brown fat: the case in favour. Clin Sci (Lond) 1983;64(1):19–23. doi: 10.1042/cs0640019. [DOI] [PubMed] [Google Scholar]