Abstract
Platelets, non-nucleated blood components first described over 130 years ago, are recognized as the primary cell regulating hemostasis and thrombosis. The vascular importance of platelets has been attributed to their essential role in thrombosis, mediating myocardial infarction, stroke, and venous thromboembolism (VTE). Increasing knowledge regarding the platelets’ role in the vasculature has led to many advances in understanding not only how platelets interact with the vessel wall but how they convey changes in the environment to other circulating cells. In addition to their well-described hemostatic function, platelets are active participants in the immune response to microbial organisms and foreign substances. Although incompletely understood, the immune role of platelets is a delicate balance between its pathogenic response and its regulation of thrombotic and hemostatic functions. Platelets mediate complex vascular homeostasis via specific receptors and/or granule release, RNA transfer, and mitochondrial secretion that subsequently regulates hemostasis and thrombosis, infection, and innate and adaptive immunity.
Keywords: platelets, communication, immunity, infection, thrombosis
Introduction
After erythrocytes, platelets are the most prevalent blood component, and they vary in size from 2–5 µm1. Once released by their megakaryocytic precursor, platelets enter the bloodstream and circulate for 7–10 days2, 3. Platelets are complex cells that contain three distinct types of granules: α-granules, dense or δ-granules, and lysosomes4, 5. Platelet α-granules contain various proteins, chemokines, cytokines and growth factors that are assembled in platelets by megakaryocytes and are necessary for normal platelet functionality. Platelet δ-granules contain small molecules such as ADP, serotonin, polyphosphates, glutamate, histamine and calcium that are necessary for hemostasis4, 6. Platelet lysosomes contain enzymes such as glycohydrolases and enzymes that degrade glycoproteins, glycolipids and glycosaminoglycans4, 7. Platelets do not have a nucleus but contain prepackaged proteins and various forms of RNA from their precursor cells. Found throughout the vasculature, platelets respond to signals from the endothelium, circulating cells, or other blood components. In this review, we will focus on the complex roles of platelets in thrombosis and immunity facilitated by their various interactions with the endothelium and circulating immune cells.
Established mechanisms for hemostasis and thrombosis
It is well established that in primary hemostasis, platelets adhere to the damaged vessel wall at the site of injury2, 3, 8. This process occurs through multiple signaling cascades and is highly dependent on glycoproteins (GPs) expressed on the platelet surface2, 3, 8. The inhibitory function of the intact endothelium (specifically, the production of prostacyclin and nitric oxide as well as expression of CD39) is decreased upon vessel damage, and extracellular matrix proteins such as collagen are exposed to the circulation2, 3.
Platelet adhesion, or initial binding of platelets to the damaged site, differs depending on the level of shear stress in the circulation. At high shear stress, circulating plasma-derived von Willebrand factor (vWF) rapidly unfolds and deposits on the exposed subendothelial collagen at the injury site. The unfolding of vWF exposes multiple binding sites for GP1b (CD42), a member of the GP1b-V-IX receptor complex2, 3, 8. This process decreases platelet velocity allowing for low shear stress and GPVI-collagen interactions2, 3, 8. Under low shear stress, platelets adhere predominantly to collagen through their GP1a/2a integrins. This phase of adhesion leads to platelet activation as well as a number of distinct platelet physiological and cytoskeletal changes, including a shift from a resting discoid shape to an activated state with numerous pseudopodia (Figure 1)2, 3, 8.
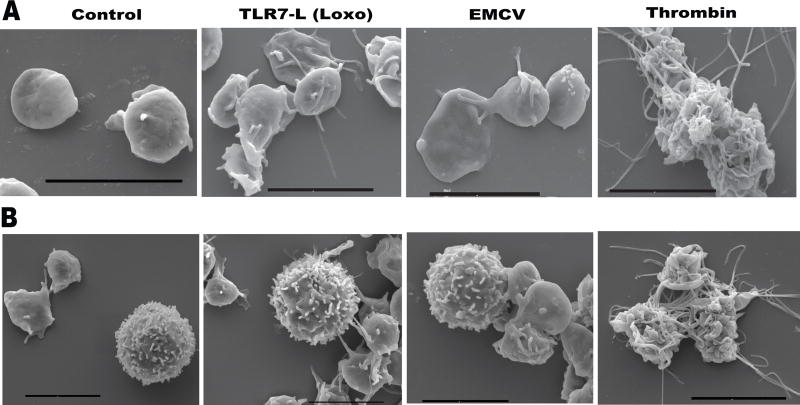
Figure 1. Differences in platelet thrombotic vs. immune activation visualized by scanning electron microscopy.
Isolated A. human platelets or B. human platelets mixed with leukocytes, were treated with immune (Loxoribine-Loxo, Encephalomyocarditis virus-EMCV) or thrombotic agonists. Panels demonstrate differing levels of activation when platelets are activated via immune vs. thrombotic pathways.
The initial adhesion and activation of platelets is followed by recruitment of additional platelets from the circulation and formation of three-dimensional aggregates through a number of molecular interactions. The recruitment of other platelets, their activation, and platelet aggregation is mediated by platelet generation of thromboxane A2 (TxA2) and release of ADP from their δ-granules. Platelet-platelet interactions during thrombi formation are further mediated and stabilized by the platelet fibrinogen receptor GP2b/3a2, 3, 8.
Depending on the level of the injury, blood can leak into the tissue and expose platelets to tissue factor expressed by cells that comprise the vessel. The presence of tissue factor leads to thrombin generation which mediates firm platelet aggregation. Once formed, the primary hemostatic plug retracts and becomes firmly anchored at the site of injury, preventing re-bleeding. Clot stabilization is described as secondary hemostasis and is characterized by consolidation of the platelet mass through actin/myosin-mediated platelet retraction. At this stage, thrombin, in addition to activating platelets, converts fibrinogen to fibrin leading to the formation of a network of fibrin fibers. In certain settings, normal hemostasis is overwhelmed by pathological factors leading to uncontrolled clot formation and potential vessel occlusion. As previously discussed, uncontrolled clot formation is defined as thrombosis and, in the artery, is primarily mediated by platelets. Arterial thrombosis is a central cause of myocardial infarction (MI) and stroke (embolic or in situ), while venous clot formation may lead to deep vein thrombosis and/or venous thromboembolism (VTE).
In response to blood borne pathogens and consequent tissue damage, platelets are also involved in a coordinated intravascular coagulation response recently termed “immunothrombosis”. During this process, platelets and immune cells form a physical barrier of confinement preventing dissemination of pathogens and potentially leading to activation of the innate and adaptive branches of the immune system6, 9. Interestingly, platelets mediate the crosstalk between the hemostatic and the immune system utilizing similar pathways (Table 1). Consistently, various viral or bacterial infections have been found to contribute to the risk of thrombosis that manifests as arterial thrombosis or VTE and may contribute to atherosclerosis10–12. Thus, although immunothrombosis may be an efficient way of assisting the immune system, it may significantly contribute to the overall risk of cardiovascular disease.
Table 1.
Platelet-derived mediators linking thrombosis, infection and immunity
| Category of platelet mediator |
Platelet receptor/ protein/ molecule |
Vascular or /circulating cell interaction |
Setting | Functions | Ref. |
|---|---|---|---|---|---|
| Integrins | GP1b (Cd42) | vWF; endothelial cells; leukocytes; bacteria | High shear stress / infection | unfolded vWF deposited on collagen; endothelial P-selectin; binds thrombin limiting leukocyte recruitment; binds complement C3 on bacteria to enhance adaptive immunity. | 15, 18, 87 |
| GP1a/2a | subendothelial collagen | low shear stress | collagen | 8 | |
| GP2b/3a (CD41) | other platelets through Fgn; T-cells; bacteria | hemostasis; infection; immunity | platelet aggregation; endothelial ICAM1 or αVβ3 leading to firm adhesion; aggregation with activated T-cytolytic and T-helper cells; aggregation around bacteria; increase after H1N1 infection | 8, 57,81, 105 | |
| α-granule proteins | PF4 (CXCL4) | T-cells; monocytes; RBCs; bacteria | infection; atherosclerosis | limit Th17 expansion and differentiation; monocyte recruitment (heterodimer with RANTES); inhibits TGFβ signaling; binds gram-negative bacteria increasing opsonization; kill plasmodium in RBCs | 60, 61, 83, 84, 107–109 |
| RANTES (CCL5) | immunity; atherogenesis | infection; chronic inflammation | T-cell activation and differentiation; monocyte/macrophage adhesion and recruitment; | 29, 53, 60 | |
| TGFβ | tumor cells (TGFβR) | metastasis | reduce NK antitumor activity; contribute to induction of invasive epithelial mesenchymal transition to metastasis | 65, 72, 73 | |
| β-defensin | neutrophils | S. aureus alpha toxin | netosis | 48 | |
| δ-granule molecules | Serotonin (5HT) | endothelial cells; T-cells | hemostasis/thrombosis; adaptive immunity | constricts injured blood vessels; enhances platelet aggregation to minimize blood loss; T-cell activation and differentiation; endothelial cell proliferation | 27, 28 |
| ADP | platelet P2Y12; P2Y1 | hemostasis | platelet recruitment, activation and aggregation during clot formation; exposure of P-selectin (P2Y12, P2Y1)and PS and thrombin generation (P2Y12) | 132 | |
| surface protein expression | P-selectin | leukocyte PSGL1 (neutrophils, monocytes, DC) endothelial PSGL1 metastatic cells PSGL1 | infection; other platelets | platelet-neutrophil and platelet-monocyte HAGs; interactions of leukocytes with the thrombi; platelet-DC interactions; increase as a result of TLR7 stimulation; metastatic PSGL1 adhesion | 5, 6, 15, 62, 71 |
| PSGL1 | endothelial P-selectin | high shear stress | platelet-endothelial interactions for thrombus formation in small venules | 18 | |
| CD40 | leukocyte CD154 | inflammation/ Infection/ immunity | Surface expression as a result of TLR7platelet-neutrophil tethering to the endothelium; platelet-DC leading to T-cell antigen presentation | 5, 40 | |
| CD154 | endothelial CD40 | inflammation/ infection | increase in endothelial expression of E-selectin, VCAM1 and ICAM1, as well as secretion of MCP1 and IL-8 | 21 | |
| synthesized/ secreted | IL-1β | endothelial IL-1R associated with αVβ3 in the presence of Fgn | infection; inflammation | increases endothelial permeability by secreting NO | 133 |
| TxA2 | thromboxane A2 Receptor | hemostasis; | platelet recruitment, activation and aggregation during clot formation | 5 |
abbreviations: Fgn-fibrinogen, vWF-von Willebrand factor; RBC-red blood cell; NK-natural killer cell; PS-phosphatidylserine; PSGL1-P-selectin glycoprotein ligand 1; DC-dendritic cells; NO-nitric oxide; TxA2-thromboxane A2; HAG- heterotypic aggregates;
Contemporary mechanisms for hemostasis and thrombosis
Recent in vivo studies have provided a more complex view of hemostasis and thrombosis. This new model suggests that the hemostatic plug is characterized by a stringent architecture, with a distinct core and outer shell13, 14. Fibrin deposition is distinctly localized at the base of the core in the extravascular space before complete hemostasis is achieved. A platelet activation gradient is also established, with the inner core of the plug composed of tightly packed, activated platelets which are degranulated and P-selectin positive, while the outer shell is composed of less activated loosely packed platelets that do not express P-selectin13, 14. The outer shell, however, is stable and permeable to plasma solutes. Consistent with the activation-gradient of platelet distribution, there is a distinct distribution of platelet agonists throughout the thrombus. The core of the plug contains a high concentration of thrombin and, as the plug becomes more porous, a gradient of ADP and TxA2 develops13, 14.
The porous outer shell of the thrombus may allow for recruitment of leukocytes necessary for injury repair or pathogen elimination. At the site of injury in veins (low shear stress), in addition to platelet activation and thrombus formation, thrombin plays a central role in leukocyte recruitment to the hemostatic plug. This process is mediated predominantly by activated platelets and not endothelial cells15. Activation/cleavage of the platelet thrombin receptor PAR4 promotes leukocyte recruitment and migration15 and platelet P-selectin enables leukocyte interaction with the thrombus15, 16. Thrombin also mediates fibrin generation which limits leukocyte migration in the clot by forming a physical barrier15. Finally, platelet GP1b can bind thrombin depleting its effect and limiting leukocyte trafficking into the thrombus15.
Under lower shear stress conditions either in veins or in arteries (ischemia reperfusion), platelet adhesive interactions can also lead to formation of a CXCL7 chemotactic gradient within the thrombus body. This gradient guides intravascular migration of leukocytes through the thrombi to the sites of injury via their CXCR1/2 receptors16. This suggests that platelets, by mediating hemostasis, can also facilitate leukocyte recruitment to the clot enabling close contact of the immune system with potential pathogens that may have penetrated the skin barrier. Further studies are necessary to elucidate how platelet distribution changes in vivo in pathogen-associated venous thrombi.
Platelets and endothelial cells
The interaction between platelets and endothelial cells has been previously and extensively reviewed17, 18. Platelet rolling over intact endothelium is observed predominantly in veins and increases with endothelial activation in response to inflammatory stimulus or infection. Under low shear stress in veins, platelet-endothelial interactions are mediated by platelet-GP1bα and endothelial-vWF18. Under higher shear stress in small venules, endothelial P-selectin and platelet PSGL1 or GP1bα mediate platelet rolling18. In the absence of additional signals, platelets disengage and return to the circulation. When the endothelium is under stress, firm adhesion of platelets will occur through endothelial ICAM1 (or through endothelial αVβ3) and platelet GP2b/3a in a fibrinogen-dependent manner. Endothelial stress is manifested during inflammation or infection by an increase in endothelial surface expression of P-selectin, E-selectin, VCAM and ICAM-119. Endothelial cells also regulate platelet activation and thrombus size by secreting thrombo-regulators such as nitric oxide (NO) and prostacyclins, and removing ADP/ATP through CD39-ectonucleotidase. NO can also be secreted by platelets and can inhibit additional platelet recruitment, reduce P-selectin expression and promote disaggregation20.
In addition to integrin/selectin surface expression, platelets can also affect the endothelium by increasing their surface expression of CD154 (CD40L) or by secreting cytokines such as IL-1β. Seconds after thrombin stimulation of human platelets in vitro, CD154 surface expression occurs. Platelet CD154, in turn, can lead to expression of endothelial E-selectin, VCAM1 and ICAM1, as well as secretion of MCP1 and IL-8 from endothelial cells21. In addition to surface protein expression, platelets can synthesize proteins from stored RNA templates22, 23. One of these proteins, the cytokine IL-1β, is synthesized in response to inflammatory stimuli22 (Figure 2). Released IL-1β can increase endothelial permeability, as well as recruitment and attachment of leukocytes to the endothelium24, 25. This leads to platelet alteration of the inflammatory cell transmigration to damaged or infected tissue. Interestingly, IL-1β increases the binding potential of platelets to collagen and fibrinogen and, in the presence of collagen, promotes aggregation26.
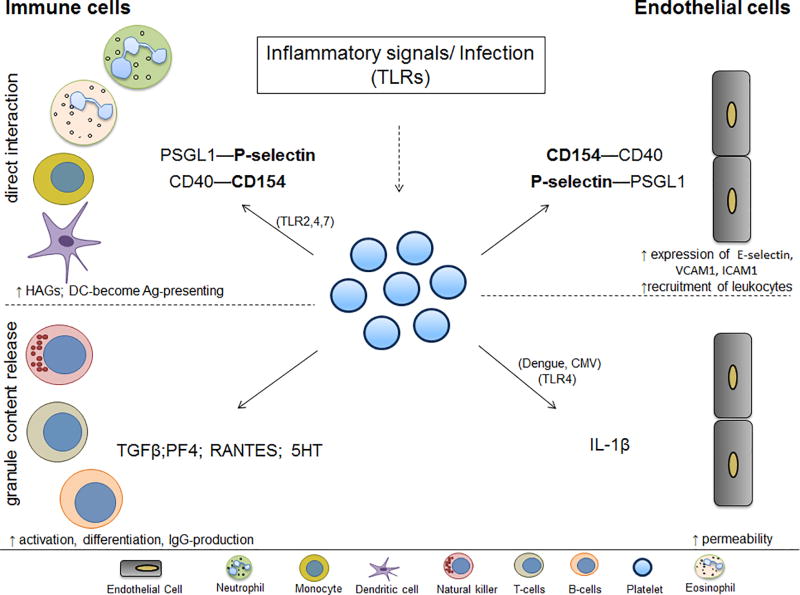
Figure 2. Platelet-mediated interactions with vascular or circulating cells.
Platelets interact with endothelial and immune cells in the circulation, orchestrating a response to microbes, inflammatory stimuli and vessel damage. Through their TLRs (or inflammatory signals) platelets can change their surface expression and release their granule content thereby engaging different immune cells. Platelets form HAGs and initiate innate immune responses in the presence of TLR agonists and viruses such as EMCV, CVB, dengue, flu, HIV. Platelets can interact with DC through their P-selectin, activate them to become Ag-presenting through their CD154. By releasing α- or δ-granule content which leads to IgG (IgG1, IgG2, IgG3) production and control of T-cell function, platelets engage the adaptive immune response. Similarly, platelets are able to activate the endothelium, make it more permeable and mediate leukocyte trafficking to the inflamed endothelium. Proteins in bold represent changes of expression on the platelet surface. Continuous lines represent direct binding; dotted lines represent interaction through secretion. Abbreviations: HAG-heterotypic aggregates; Ag-antigen; 5-HT-serotonin; PF4-platelet factor 4; CMV- cytomegalovirus; EMCV-encephalomyocarditis virus; CVB-coxsackievirus B;
Receptors in platelet-immune cell interactions
To understand the role of platelets in thrombosis in diverse clinical settings, their role in immunity should be addressed. Under certain infectious conditions or inflammatory stimuli, platelets directly interact with circulating leukocytes by changing surface expression of P-selectin or CD405. Platelet P-selectin and CD40 are recognized by leukocyte PSGL1 and CD154, respectively, leading to formation of platelet-leukocyte aggregates. Platelets form these aggregates predominantly with circulating monocytes or neutrophils, thereby contributing to the innate immune response5. Platelets also contribute to adaptive immunity by interacting with and activating dendritic cells (DCs) through the CD40-CD154 axis. Platelet-mediated activation of DCs causes the DCs to present antigen to T-cells5. In addition, platelet activation can also lead to the release of δ-granule content and secretion of molecules such as serotonin and RANTES (CCL5) that are also known to mediate T-cell activation and differentiation27–29. Notably, platelets contain the mRNA transcripts for all Toll-like receptors (TLR1-TLR10)4, 30, 31. TLRs are pathogen-associated molecular pattern recognition receptors that are key regulators in the initiation of the innate immune response to foreign organisms.
Platelets express functional TLR432–34, TLR235–37 and TLR938, 39, and these TLRs can initiate thrombotic responses of varying intensities (Table 2). Platelet-TLR4 and -TLR2 can also mediate interactions with neutrophils33, 36. Additionally, platelets express functional TLR740 and TLR341, 42; however, these TLRs do not induce prothrombotic responses (aggregation mediated by thrombin40), but instead alter platelet-leukocyte interactions. Stimulation of TLR7 leads to the platelet surface expression of P-selectin and CD154, suggesting select α-granule release40. Stimulation of TLR3 does not lead to P-selectin or CD154 surface expression but, at high agonist concentration (possibly equivalent to late stages of infection), potentiates arachidonic acid-, ADP- or collagen-mediated aggregation41, 42. Interestingly, analysis of platelets from 1864 people shows that women have significantly higher expression of all TLRs as compared to men30. TLR expression in platelets from women associated with an increase of plasma P-selectin while, in men, TLR expression associated with an increase in IL-6, TNFRII and soluble ICAM-130.
Table 2.
Platelet TLRs and their contribution to thrombosis and immunity
| TLRs | Agonist | Interaction with cells |
Result | Thrombosis | Platelet TLR- contribution to netosis |
Organ netosis |
Ref. |
|---|---|---|---|---|---|---|---|
| TLR2 | Pam3CK4; oxPCCD36 | neutrophils | Platelet-neutrophil HAGs through P-selectin; activation of GP2b/3a hyperreactivity and the prothrombotic state in the setting of hyperlipidemia | + | + 136 |
? | 35, 36 |
| TLR4 | LPS | neutrophils, monocytes | Platelets reduce the time of netosis in vivo; platelets aggregate in TLR4/MyD88- and cGMP/PKG-dependent pathway | + | + | + | 33, 134 |
| TLR3 | pIC; pAU | Activation of NF-kappaB, PI3K-Akt and ERK1/2 pathways in platelets; at high agonist concentrations may potentiate platelet aggregation; liver netosis (pIC) | __ | ? | + | 41, 42 | |
| TLR7 | Loxoribine; R838 | neutrophils | Modest increase of P-selectin and CD40 through AKT and p38-MAPK; no effect on platelet aggregation; liver netosis (R838) | __ | ? | + | 40, 135 |
| TLR9 | CpG; Carboxy-alkylpyrrole protein adducts (CAPA) | Platelet clumping in presence of collagen (CpG); platelet activation, granule secretion, and aggregation in vitro and thrombosis in vivo via the TLR9/MyD88 pathway (CAPA) | __ | ? | ? | 38, 39 |
TLR3 may potentiate platelet aggregation at very high concentrations (50 µg/µl); Mitochondrial DNA can mediate netosis through TLR9.
Abbreviations: HAG- heterotypic aggregates; LPS-lipopolysaccharides; pIC- poly(I:C); pAU –poly(A:U);
Importantly, platelet immune activation differs from hemostatic, thrombin-mediated platelet responses. Scanning electron micrographs of human platelets alone and in the presence of leukocytes, illustrate a distinct morphological shape change dependent on their function (Figure 1)40. Platelet hemostatic function demonstrated by stimulation with thrombin causes complete disappearance of the platelet’s discoid shape and conversion into long intertwined “spaghetti-like” pseudopodia (Figure 1). Platelet immune function demonstrated by stimulation with TLR7 agonist or TLR7-activating virus leads to smaller platelet groups in which single platelets can be identified with fewer pseudopodia connecting to other platelets or to leukocytes (Figure 1). This suggests that platelets undergo distinct forms of activation depending on the stimulatory signal and their functional role. Different levels of activation appear necessary for the proper formation of either the hemostatic plug or recruitment of leukocytes (see “Contemporary mechanisms for hemostasis and thrombosis”).
Platelets and neutrophils
The platelet interaction with neutrophils is central to initiating the immune response (Figure 2). In humans, neutrophils are the most abundant blood leukocyte and, together with monocytes, are the major initiators of the innate immune response. Platelets form heterotypic aggregates with neutrophils as a function of TLR7, TLR2, and TLR4 activation in the circulation33, 36, 40. Platelet-neutrophil interactions are mediated by the platelet P-selectin/neutrophil PSGL1 axis. Neutrophils can also phagocytose activated platelets in vivo with internalization mediated by phosphatidylserine platelet surface expression43. Interestingly, as a result of TLR7 stimulation, platelet components (as measured by CD41) are internalized by neutrophils40. It is currently unclear whether platelet microparticles44 or the entire platelet is internalized as a function of TLR7-stimulation40.
Platelet-neutrophil interactions are also important for neutrophil extracellular trap (NET) formation, a process termed netosis whereby pathogens are captured and neutralized45–47. Staphylococcus aureus alpha toxin for instance mediates secretion of β-defensin 1 from platelets which can lead to NET formation48. In the setting of lipopolysaccharide (LPS) activation of TLR4, platelets contribute to netosis by reducing the time required for NET formation33 (Table 2). Although various TLRs are implicated in organ netosis, the role of platelet TLRs, with the exception of TLR4, has not been elucidated (Table 2).
Platelets and monocytes
Platelets can interact with monocytes as a consequence of inflammatory stimuli or infection (Figure 2). Platelet-monocyte aggregates may be a more sensitive method for assessing platelet activation as compared to P-selectin expression49. Circulating platelet-monocyte aggregates are elevated in patients with acute myocardial infarction50, 51 and, in older patients, platelet-monocyte aggregates are associated with vascular thromboembolism52. Murine studies have shed light on the complex interaction between platelets and monocytes. During chronic inflammation, simultaneous activation of platelets and neutrophils leads to secretion of RANTES and human neutrophil peptide 1 (HNP1), respectively53. These proteins form heterodimers responsible for monocyte/macrophage adhesion and recruitment in a model of myocardial infarction53. Thrombin-activated human platelets also express the chemokine receptor CX3CR1 leading to the formation of platelet-monocyte complexes through the CX3CL1 ligand on monocytes54.
Platelets and eosinophils
Platelets are described to affect eosinophil function as well. Eosinophils are granulocytes that compose about 2% of all leukocytes and it has been proposed that they may contribute to the innate immune response to helminth parasite (worms) infection. Pathogenically, eosinophil function has been attributed to the profound damage they cause in an allergic asthma. Direct interaction between platelets and eosinophils in the circulation during helminth infection in vivo has not been described. However, co-incubation experiments of platelets and eosinophils (for 48h) show that platelets prolong eosinophil life by secreting granulocyte-macrophage colony-stimulating factor (GM-CSF)55. This suggests that platelets may enable eosinophils in fighting helminth infection. However, by delaying eosinophil apoptosis platelets may also contribute to asthma-mediated tissue damage. A murine model of allergic inflammation has suggested that platelets through their P-selectin recruit eosinophils to the lungs56 and consequently may propagate tissue damage. Allergen sensitized mice had an increase of platelet-eosinophil aggregates in the circulation and in the lung tissue and aggregate interactions were mediated through platelet P-selectin and eosinophil PSGL156.
Platelets, B-cells and T-cells
When interacting with lymphocytes, platelets are more selective and bind preferentially to larger, activated cells57. In the presence of LPS or ADP, aggregation between platelets and B-cells is almost unaffected. However, when platelets are co-incubated with B-cells for three days in vitro, B-cells increase their production of IgG1, IgG2 and IgG3, suggesting that platelet content can contribute to B-cell function and alter adaptive immunity58.
T-cell activation more robustly increases platelet aggregation via both T-cytolytic and T-helper cells57 (Figure 2). This process is mediated by platelet GP2b/3a, CD154, and lymphocyte CD11b57. CD154 is expressed under various stimulants (such as TLR7 activation40) and is capable of enhancing T-cell immunity to viral challenge. Evidence suggests that, by interacting with lymphocytes, platelets may facilitate the recruitment of these cells to an injured vessel at a site of inflammation or infection59, and this is a central step in T-cell trafficking. Activated platelets may mediate T-cell functions further by releasing a number of factors, such as platelet factor 4 (PF4, CXCL4)-RANTES or serotonin27, 28, 60. The release of these factors impacts T-cell function in various ways. PF4, for instance, is necessary for the limitation of Th17 expansion and differentiation61. Serotonin, the largest peripheral circulation reservoir of which is stored in platelet δ-granules, can push naïve T-cells to activate and proliferate27, 28.
Platelets and dendritic cells
As previously mentioned, platelets interact with and influence another important cell in adaptive immunity, the DC. Platelets, through the CD40-CD154 axis, can induce DCs to present antigen to T-cells5. Additionally, platelet-DC interactions can be mediated by the P-selectin/PSGL1 axis, followed by firm adhesion through platelet junctional adhesion molecule C (JAM-C) with MAC-1 on DCs62 (Figure 2). Interestingly, this recruitment and activation of DCs is followed by phagocytosis of platelets by DCs62. The mechanism by which DCs change after platelet phagocytosis is still largely unknown. It is possible also that serotonin originating from phagocytosed platelets may be utilized by DCs and released as needed for T-cell proliferation and differentiation.
Platelets and natural killer cells
The interaction between platelets and natural killer (NK) cells is the least understood interaction within the circulation (Figure 2). In murine studies, tumors coated with platelets become impermeable to NK cell destruction63 with fibrinogen involvement64. It has been proposed that circulating metastatic cells attract platelets and influence them to release their granule content. As a result, human NK cells lose their cytotoxicity and their ability to produce IFNγ, possibly through the downregulation of NKG2DL mediated by platelet-TGFβ release65. However, the baseline communication between platelets and NK cells remains unknown as does the effect on this interaction during infection or autoimmune disease.
Platelet-leukocyte interactions in atherogenesis
The role of platelet-leukocyte interactions during inflammation has been determined to be contributory in murine models of atherosclerosis. It is now established that activated platelets can increase endothelial atherogenic potential and lead to elevated platelet-leukocyte aggregate formations in the circulation. Platelets may further contribute to atherogenesis through the delivery of proinflammatory factors to leukocytes and endothelial cells66. The platelet-derived factors implicated in this setting are surface P-selectin and the platelet-derived chemokines RANTES and PF466. PF4/RANTES heterodimer disruption reduces atherosclerosis in ApoE-deficient mice by attenuating monocyte recruitment60. Murine models lacking P-selectin show that this adhesion receptor is a key mediator of leukocyte recruitment into lesions and promotes their advancement in ApoE-deficient mice67. In this case, both platelet and endothelial P-selectin contribute to lesion development; however, endothelial P-selectin plays a crucial role in atherosclerotic lesion growth68. Similarly, murine models lacking CD40 in ApoE-deficient mice demonstrate that platelet CD40 promotes atherosclerosis by activation and recruitment of leukocytes to endothelial cells69. When platelets lacking CD40 are injected in the circulation of mice, expression of VCAM1, PECAM, VE-Cadherin and P-selectin are decreased on endothelial cells. This decrease in adhesion molecule expression is accompanied by less advanced atherosclerotic plaques with reduced levels of macrophages and neutrophils69. Increased expression of platelet CD154, in turn, contributes to accelerated atherosclerosis by increasing platelet-platelet interactions and inflammatory response through mediation of platelet-endothelium and platelet-leukocyte interactions70. Additionally, platelet CD154 suppresses Treg cell recruitment leading to accelerated atherosclerosis70.
Platelets and metastatic cells in the circulation
Platelets contribute to the increased thrombotic risk of cancer patients. High platelet count is associated with decreased survival in various malignancies and thrombocytopenia, in turn, is associated with decreased metastatic potential of tumors. Entry of metastatic cancer cells into the blood stream leads to platelet activation. Metastatic cells express PSGL1 and secrete high levels of tissue factor71. Platelet-cancer cell interactions and cancer-induced thrombi formation in the vasculature may inhibit recognition and elimination by the immune system. Additionally, the thrombi formation can lead to tethering of the cancer cell to the endothelium, promoting survival and invasive potential. More recent studies in mice have proposed that, as a consequence of interaction with tumor cells, platelet signals recruit granulocytes to the tumor, thereby, guiding the formation of an early prometastatic microenvironment. Depletion of platelets by cell-specific depleting antibodies inhibits granulocyte recruitment to metastatic cells in lungs and depletion of granulocytes leads to fewer metastases and reduced metastatic progression72.
Platelet-derived TGF-β is an important factor contributing to metastasis. In vitro studies have shown that thrombin-mediated platelet-derived TGF-β can alter NK cell antitumor reactivity65. In vivo studies eliminating TGF-β specifically from platelets have proposed that this cytokine, when originating from platelets, is solely responsible for inducing an invasive epithelial mesenchymal transition to metastasis73. These studies utilized the PF4Cre murine model which, at the time, was believed to eliminate TGF-β only from the megakaryocyte/platelet lineage73. We have since learned that a subset of epithelial cells can express PF474 in addition to activated CD4+ and CD8+ T-cells61. These studies suggest that other tissue-resident cells or epithelial cells themselves can contribute to the invasive potential of cancer cells mediated by TGF-β. The role of the platelet-mediated increase in metastatic potential through TGF-β is further complicated because thrombin stimulation also leads to the release of PF4 from platelet α-granules61. PF4, in turn, inhibits TGF-β signaling in a SMAD2/3 dependent manner61. Regardless of TGF-β signaling, metastatic cancer cells entering the blood stream hijack the hemostatic function of platelets by releasing tissue factor and, consequently, generate thrombin and initiate thrombus formation and neutrophil recruitment. The formed thrombi can attach to the endothelium and promote viability and metastatic potential of the cancer cell.
Platelets in bacterial infections
During bacterial infections, platelets actively mediate the host response through interactions with circulating leukocytes. In addition to PF4 and RANTES, platelet α-granules contain various antimicrobial compounds such as platelet connective tissue activating peptide 3 (CTAP-3), platelet basic protein, thymosin β-4 (Tβ-4), and fibrinopeptide (A and B)75, 76. These platelet-derived antimicrobial compounds are known to target various bacterial organisms (Figure 3)4, 77, 78. Our understanding of how platelets mediate these interactions in the circulation is still developing.
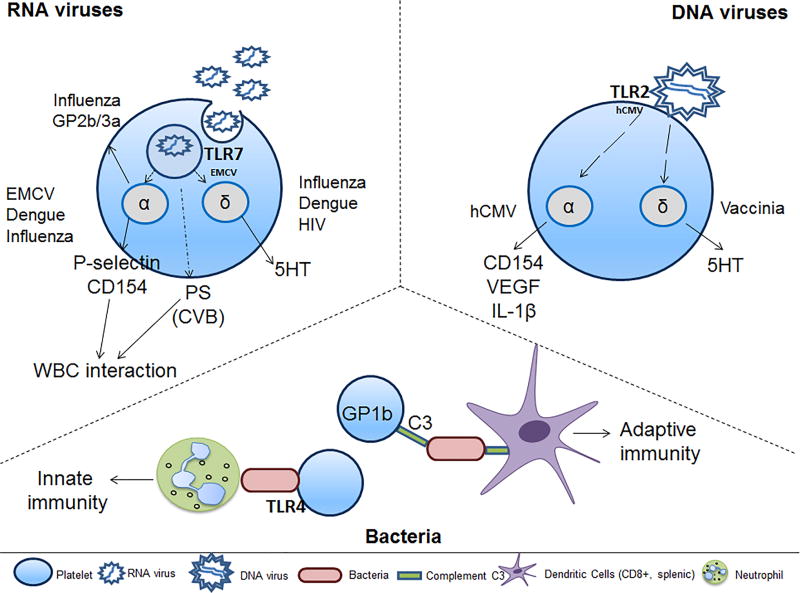
Figure 3. Platelet and circulating cell interactions during infection initiate the innate or adaptive immune response.
Platelets achieve cell-to-cell communication during bacterial or viral infection either by direct interaction with WBCs through surface expression of platelet proteins or through indirect protein release from their α- or δ-granules. EMCV-activated platelets interact with neutrophils in a TLR7-dependent manner. CVB-activated platelets bind to neutrophils in a PS-dependent manner. Dengue and influenza increase microparticle release; dengue-mediated microparticles contain IL-1β. HCMV-activated platelets interact with neutrophils, monocytes, B-cells, T-cells and DCs, suggesting activation of innate and adaptive immune responses. Vaccinia-bound platelets have reduced aggregation potential in the presence of ADP, collagen, or thrombin. The specific pathways by which platelets respond to Herpes simplex virus (HSV)1 or HSV2 are currently unknown. During bacterial infection, platelet interactions with complement C3 opsonized bacteria through GP1b (CD42) lead to slowing of bacterial clearance. DCs recognize the platelet-bacterial complexes, thereby, inducing adaptive immunity. These platelet-bacterial interactions are true for Gram-positive or Gram-negative bacteria. Abbreviations: WBC-white blood cells; 5HT-serotononin; VEGF-vascular endothelial growth factor; PS-phosphatidylserine; C3-complement C3; EMCV-encephalomyocarditis virus; hCMV-human cytomegalovirus; CVB-coxsackievirus B; HIV-human immunodeficiency virus;
Platelets can interact with various strains of bacteria including the Staphylococci family, Neisseria gonorrhea, Porphyromonas gingivalis, and Helicobacter pylori77, 79, 80. Platelets adhere and aggregate around bacteria, typically utilizing their GP2b/3a, FcγRIIa, and IgG receptors in conjunction with fibrinogen or fibronectin81, 82. After platelets interact with bacteria, they release antimicrobial compounds contained in their granules. S. Aureus alpha toxin mediates release of β-defensin from platelets and, consequently, β-defensin induces NET formation48. Released PF4 can bind to Gram-negative bacteria, leading to the exposure of PF4 heparin-like epitopes. Exposed PF4-epitopes, in turn, increase antibody binding to the bacterial surface leading to the opsonization and possibly phagocytosis of bacteria by neutrophils83, 84. This mechanism is particularly important since some Gram-negative bacteria, such as Yersinia pestis, are poorly recognized by the mammalian TLR485, 86. In vivo studies using another Gram-negative bacteria, Porphyromonas gingivalis, show that, during infection, platelets interact with neutrophils forming heterotypic aggregates in a TLR2-dependent manner and TLR2 can also promote platelet aggregation36. This study provides evidence that, by recognizing bacterial components, platelets can activate platelet thrombotic and/or inflammatory pathways.
Studies using Lysteria monocytogenes have recently shed light on the role of platelets in inducing adaptive immunity during bacterial infection. Platelet binding to intravascular bacteria leads to slowing of the cytotoxic clearance of the pathogen, keeping bacteria available for induction of an adaptive immune response by splenic CD8+ T-cells87. This platelet-mediated process is dependent on the opsonization of bacteria by complement C3, subsequent platelet binding by GP1b, and the capture of the bacteria-platelet aggregates by cells of the immune system87. Platelet-bacteria interactions in the presence of C3 lead to different kinetics of clearance between free (fast clearance) and platelet-bound (slow clearance) bacteria. This observation is true for Gram-positive strains such as L. monocytogenes, Bacillus subtilis, Enterococcus faecalis and Staphylococcus epidermidis, and for Gram-negative strains such as Escherichia coli, Salmonella typhimurium and Klebsiella pneumoniae87. This study suggests a sophisticated role for platelets in balancing clearance of bacteria and induction of adaptive immunity.
Platelets in viral infections
Platelets interact with various types of viruses. Viral infections are often associated with thrombocytopenia and in some cases thrombosis. Viruses can be simply classified as those with a DNA or RNA genome. RNA viruses are further subdivided into double-stranded and single-stranded viruses. DNA viruses from the herpes viral family such as herpes simplex virus type 1 (HSV1), cytomegalovirus (CMV) and vaccinia, have been found associated with platelets, but it is unclear if they can be internalized by platelets88–90. RNA viruses such as HIV, hepatitis C virus (HCV), dengue, influenza, coxsackievirus B (CVB) and encephalomyocarditis virus (EMCV) are much smaller in size and can be internalized by platelets40, 91–95. Here, we have focused on the interaction of virally-activated platelets with circulating cells.
In general, platelet function during viral infection can be beneficial and/or detrimental to the host depending on the length of time post infection. Human CMV (hCMV) binds to platelet-TLR2, resulting in release of CD154, IL-1β, and VEGF (Figure 3). hCMV-activated platelets do not show increased aggregation or adhesive potential but rapidly form platelet-neutrophil heterotypic aggregates. In addition to their interactions with neutrophils, hCMV-platelets also exhibit increased interaction with monocytes, B-cells, T-cells and DCs, suggesting a multifactorial interaction with the innate and adaptive immune systems and induction of proangiogenic responses88. In vitro studies utilizing vaccinia virus show that ADP-, collagen-, or thrombin-mediated aggregation in isolated platelets is inhibited by the virus but there is an increase in serotonin release90 (Figure 3). In vivo studies, however, show that infection with vaccinia leads to fatal intravascular coagulation at 24h post infection96. In vitro studies using HSV1-infected human umbilical vein endothelial cells (HUVEC) show platelet binding due to increased thrombin generation from the damaged cells97. These observations suggest that other circulating or vascular cells can contribute to the overall prothrombotic balance.
RNA virus infections exhibit more diverse platelet responses. Many RNA viruses cause various degrees of thrombocytopenia at different stages of infection. In the setting of murine EMCV infection, platelets interact with neutrophils to form heterotypic aggregates as early as 1h post infection and this leads to a sudden drop in platelet count. This drop also occurs at 24h post infection. Elimination of platelets before EMCV infection leads to reduced survival similar to mice deficient in TLR740.
In humans, infection with dengue, an RNA virus, is characterized by profound hemorrhagic fever and thrombocytopenia. In a rhesus macaque model, dengue viral infection leads to platelet-monocyte interactions at 24h post infection while platelet-neutrophil interactions peak at 3d post infection98. In this study, blood contained monocytes that had engulfed platelets containing dengue virus98. Increased permeability of the endothelium during dengue infection is also facilitated by platelets. Dengue infection mediates assembly of the NLPR3 inflammasome and activation of Caspase-1 in platelets. Caspase-1, in turn, alters secretion of IL-1β-rich microparticles99. Platelet-monocyte interactions are also increased in people infected with HIV1 or influenza100, 101 and are influenced by inflammatory stimuli in a COX-dependent manner102.
During infection with CVB, platelets form aggregates with the neutrophil population and directly improve the outcome of CVB-induced myocarditis95. CVB is internalized by platelets causing phosphatidylserine exposure on the platelet surface without affecting apoptosis95. The virus cannot replicate in platelets and platelet depletion causes an increased viral load in heart tissue as well as increased myocarditis95.
Influenza infection illustrates the potential for dysregulation of the hemostatic-inflammatory balance. In patients infected with influenza H1N1 virus, there is an elevation of platelet-monocyte aggregates and a two-fold increase in platelet aggregation potential as measured by PAC1 incorporation103 (Figure 3). As the infection progresses, coagulation potential increases, as evidenced by the elevation of macrovesicle tissue factor activity104. In addition, in non-survivor influenza-infected patients, there is a trend toward lowered platelet and increased white blood cell counts104. H1N1 activates human platelets independently through both FcγRIIA signaling and through thrombin generation105, while H1N1–platelet interaction increases the expression of the active form of the fibrinogen receptor GP2b/3a, arachidonic acid metabolism, and microparticle release105. Studies utilizing a lethal dose of the H1N1/PR8 strain in C57BL/6J mice suggest that, in critical non-survivor settings, platelet degranulation and activation contribute to lung inflammation and reduced survival106. In summary, platelets contribute to the overall procoagulant and inflammatory states during influenza infection, but it is unclear if they are necessary for overall immunity and vascular health.
Platelets in Plasmodium parasite (malaria) infection
There are six plasmodium species that can infect humans and lead to development of malaria. The role of platelets in Plasmodium parasite infection changes as the infection progresses. Studies using non-inflammatory malaria with P. falciparum or P. chabaudi show that platelets kill the parasite inside of the red blood cells (RBCs) by secreting PF4107. PF4, in turn, interacts with the Duffy antigen on RBCs through an unknown mechanism and mediates survival of infection108.
Although these studies suggest a protective role during early infection, a model of cerebral malaria using P. berghei ANKA (PbA) iRBCs clone 2.34 concludes that platelets play multifactorial roles depending on the pathogenic stage of infection109. Platelets in this case were activated throughout the first 3 days of infection as measured by an increase in circulating PF4. This early activation of platelets is protective possibly due to a platelet-dependent reduction in parasite burden and leukocyte recruitment through an increase of the IL-1β pool109. As infection progresses, however, platelet activation, possibly mediated by the overall immune response and tissue factor release, leads to microvascular thrombosis as found in the postmortem brains of patients with cerebral malaria4,110.
Platelets and RNA transfer
The ability of platelets to communicate with other vascular cells through receptor signaling, direct protein interactions, or released granule content has long been understood. More recently, other methods of platelet vascular communication have been described including RNA transfer. Bidirectional RNA transmission is illustrated by transfer of platelet-derived transcripts to leukocytes or endothelial cells and by the uptake of leukocyte- or vascular-derived transcripts into platelets111 (Figure 4). Co-culture of platelet-like particles (PLPs) with either a HUVEC cell line or a human monocytic cell line (THP-1) demonstrates functional transfer of platelet-derived mRNA transcripts111. Endogenous analysis of THP-1 cells post PLP incubation reveals an increased expression of α, γ, and ε globin genes as a consequence of RNA transfer111. Confirmation of this phenomenon in vivo utilizing mouse infusion experiments demonstrates that platelets carrying TLR2 mRNA can transfer their transcripts to mice with TLR2-deficient blood mononuclear leukocytes111.
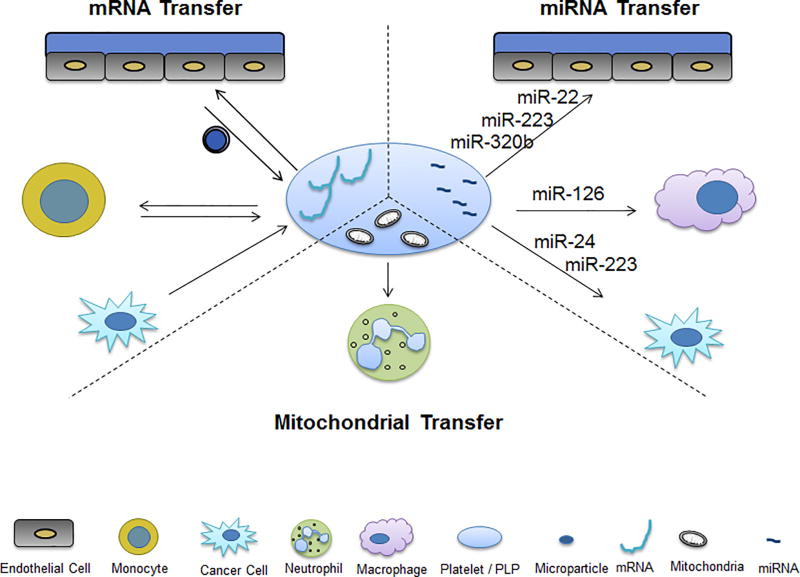
Figure 4. Platelet vascular and circulating cellular communication by non-protein-dependent mechanisms.
Platelets and platelet-like particles (PLPs) have been shown to communicate with vascular cells in several protein-independent manners, specifically through mitochondrial and RNA transfer. RNA can be transferred from platelets to endothelial cells, monocytes, macrophages, and cancer cells. Inversely, RNA can be taken up by platelets from endothelial cells, monocytes, and cancer cells. Mitochondrial transfer from platelets to neutrophils can also occur. Microparticles mediate a significant number of these communication pathways.
Analysis of human platelets post co-incubation with HUVECs and THP-1 cells reveals that platelets can also take up transcripts from surrounding cells. There are distinct changes in expression of platelet transcripts including those associated with platelet-vascular adhesion and apoptosis112. Platelet RNA uptake has been confirmed by the presence of TLR2 in TLR2-negative platelets post infusion of TLR2-positive leukocytes (peripheral blood mononuclear cells, PBMCs) into TLR2 knockout mice112. Interestingly, platelet RNA uptake can occur in the presence or absence of thrombin stimulation112.
Platelet microparticles, miRNA and vascular inflammation
In addition to the hemostatic and inflammatory responses described, platelet activation also leads to microparticle formation. This is a particularly important observation since platelets are a major source of microparticles in the circulation4. Proteins that mediate platelet function related to hemostasis or immunity are also found in platelet microparticles. This includes adhesion molecules such as P-selectin, chemokines such as RANTES, and cytokines such as IL-1β, among others99, 113. Platelet microparticles can promote monocyte recruitment to the inflamed endothelium of the atherosclerotic plaque113. Increased circulating platelet microparticles have been associated with atherosclerosis development in diabetic patients. Infections such as influenza or dengue also lead to increased platelet microparticle release99. During vascular injury, platelet microparticles can increase the regenerative potential of endothelial progenitor cells by enhancing their recruitment, differentiation and release of proangiogenic factors114.
In addition to proteins, platelet microparticles contain various forms of RNA including micro RNA (miRNA), which are small non-coding RNAs that function in post-transcriptional regulation of gene expression. Platelets may affect surrounding cells by transferring miRNA115,116. The platelet miRNAs, miR-223 or miR-320b, are present in platelet-derived microparticles post thrombin stimulation and can transfer into endothelial cells117, 115 (Figure 4). Functionally, it has been shown that miR-320b transfer into endothelial cells can decrease ICAM1 expression115. Similarly, miR-126-3p, highly expressed in platelets, can be taken up and enriched in macrophages, resulting in an increased phagocytic phenotype116. Platelet microparticle-derived miR-223 transfer to cancer cells may play a role in cancer progression. This miRNA transfer leads to downregulation of the tumor suppressor EPB4L13 and a concurrent increase in tumor cell invasion118. Alternatively, transfer of platelet-derived miR-24 to solid tumor cells results in disrupted mitochondrial function, leading to tumor cell death and proliferation decline119. These studies support the hypothesis that platelet RNA can affect function of vascular or circulating cells through RNA transfer.
In support of the relevance of miRNA transfer, is the observation of decreased miRNA expression only in platelets at the site of injury and not in the peripheral circulation115. While the differential expression of miRNAs in platelet-derived microparticles is interesting, it is crucial to establish how these microparticle miRNAs affect the function of recipient cells.
Platelets and mitochondrial transfer
As both protein and RNA communication between platelets and the vasculature can occur through microparticle production, there is the possibility that other platelet contents may transfer. Platelets contain mitochondria (averaging four mitochondria/platelet) that are both functional and have been shown to impact platelet activation and subsequent thrombosis44, 120, 121. Platelet-derived mitochondria may play a role in communication as platelets release functional mitochondria when stimulated with numerous agonists via both platelet microparticles and as extracellular free organelles44. These mitochondria may impact overall vascular health and level of inflammation44. Extracellular mitochondria have been implicated as damage-associated molecular patterns (DAMPs)44, 122–125. Additionally, mitochondria can act as a substrate for sPLA2-IIA, a phospholipase known to be induced during inflammatory settings44. In its interaction with extracellular mitochondria, sPLA2-IIA leads to mitochondrial dysfunction and subsequent mitochondrial content release (arachidonic acid, lysophospholipids and mitochondrial DNA), all of which reinforce a proinflammatory environment. sPLA2-IIA has also been implicated in the internalization of platelet microparticles by neutrophils44 (Figure 4). Released mitochondria interact and are internalized by neutrophils, where mitochondria-neutrophil interactions appear to impact neutrophil functionality (increased rolling and interaction with vascular walls, increased induction of NETs), morphology (development of pseudopodia), and substance release (leukotrienes)44. Finally, extracellular mitochondria expression is linked to a number of proinflammatory settings. Mouse studies show that sPLA2-IIA-dependent release of mitochondrial factors leads to increased proinflammatory cytokine release and decreased body temperature44. Human studies reveal increased levels of extracellular mitochondria in the synovial fluid of multiple inflammatory autoimmune pathologies such as rheumatoid arthritis and osteoarthritis44, and these levels correlate with the known elevation in platelet microparticles44, 126–128. These observations suggest that extracellularly released platelet mitochondria may function as a potent communicator of vascular inflammation.
Antiplatelet therapies and complex platelet function
In addition to continual use of established platelet inhibitors, there has been great progress in the development of antiplatelet/antithrombotic therapies for the treatment of various thrombotic pathologies. Cyclooxygenase (COX) in platelets, responsible for TxA2 generation and three dimensional clot formation, has been targeted in the regulation of platelet activity. Platelets contain predominantly COX1 while endothelial cells can contain COX1 and COX2. Aspirin is an irreversible inhibitor of COX activity, and as platelets do not regenerate COX protein, this drug is an effective inhibitor of platelet aggregation potential. Aspirin has provided beneficial effective secondary prevention of arterial thromboembolic events.35 Inhibition of the prothrombotic activity of platelets by aspirin is also considered to be beneficial in infectious diseases such as influenza. Relevant to the role of platelets in cancer, it is suggested that aspirin may be beneficial in preventing the platelet microthrombi formation around cancer cells in the circulation that may aid metastasis. A recent meta-analysis study concludes that long term aspirin use is associated with reduced risk of overall cancer129.
A range of P2Y12 inhibitors are also extensively used in patients with cardiovascular syndromes. In vitro blocking of P2Y12 by Cangrelor reduces platelet deposition on the injured endothelium and delays vessel sealing, thereby leading to prolonged escape of plasma-born molecules into the microvaculature130. The results observed with inhibition of GP2b/3a signaling are more complex. While still, on occasion, used intravenously to inhibit platelet function in the setting of acute unstable cardiac ischemia14, oral formulations were associated with increased morbidity and mortality131. Still unknown is whether inhibiting P2Y12 or GP2b/3a will alter the immune responding functions of the platelet.
Interestingly, the thrombin inhibitor, hirudin, which binds and inhibits thrombin and prevents fibrin generation, does not block the sealing of vessel injury130. This observation suggests that the core and the shell of clots can form without fibrin but the stability and retraction may be affected. Finally, TLR agonists/antagonists may be useful in promoting platelet immune function and survival of infection possibly depending on the specific receptor. This is consistent with the observation that elimination of platelets before infection with the TLR7-activating virus, EMCV, leads to decreased survival of mice40. As therapeutics designed to target platelets progress, the type of injury, mechanism/type of infection, and timing of infection must be considered.
Conclusion
Platelets exhibit complex interactions with circulating cells and the vessel wall during a broad range of inflammatory processes that manifest in many types of disease. However, there is overlap between the platelets’ hemostatic and inflammatory roles. For example, platelets may engage leukocytes and the immune system by utilizing proteins involved in hemostatic plug formation. More recent data suggest that these interactions are regulated by processes beyond traditionally-defined receptors, e.g. RNA or mitochondrial transfer to other vascular or circulating cells. These complex and sometimes contradictory interactions appear to directly affect disease. During certain stages of infection, platelets initiate activation of both innate and adaptive immunity which is beneficial to the host. However, uncontrolled endothelial damage and inflammation as a result of viral infection progression can lead to adverse prothrombotic responses and increased cardiovascular risk. Thus, platelets appear to have broad and multifaceted functions firmly grounded in their vascular cell interactions. Maintaining these complicated and distinct states of activation is imperative to regulating vascular homeostasis and health. As our understanding of the role of platelets in the vasculature and circulation grows, we have the potential to design better platelet-targeted treatments for patients who present with a wide range of vascular, immune, and oncological diseases.
Acknowledgments
We apologize to all whose work was not cited due to limited space and limited number of citations.
Sources of Funding:
This work was supported by AHA Award 16SDG30450001 (to M.K.) and U54 HL12311, NIH Common Fund Award UH3 TR000921, and AHA Award 16SFRN31740000 (to J.E.F.).
Nonstandard Abbreviations and Acronyms
- VTE
venous thromboembolism
- GP
glycoprotein GP2b/3a
- vWF
von Willebrand factor
- TxA2
thromboxane
- MI
myocardial infarction
- P-selectin
platelet selectin
- E-selectin
endothelial selectin
- PSGL1
P-Selectin glycoprotein ligand 1
- PAR4
protease-activated receptor-4
- PF4
platelet factor 4 (also CXCL4)
- CXCL7
Chemokine (C-X-C motif) ligand 7
- CXCR1/2
C-X-C Chemokine Receptor Type 1 or 2
- CX3CR1
C-X3-C Motif Chemokine Receptor 1
- ICAM1
Intercellular Adhesion Molecule 1
- VCAM1
vascular cell adhesion molecule 1
- PECAM
Platelet endothelial cell adhesion molecule (also CD31)
- VE-cadherin
vascular endothelial cadherin
- NO
nitric oxide
- IL-1β
Interleukin 1 beta
- IL-8
Interleukin 8
- IL-6
Interleukin 6
- MCP-1
Monocyte chemotactic protein 1 (also CCL2)
- TLR
toll-like receptor
- RANTES
regulated on activation, normal T cell expressed and secreted (also CCL5)
- DCs
dendritic cells
- NK
natural killer cells
- TNFRII
Tumor necrosis factor receptor 2
- HNP1
human neutrophil peptide 1
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- LPS
Lipopolysaccharide
- IgG
Immunoglobulin G
- JAM-C
junctional adhesion molecule C
- MAC-1
Macrophage-1 antigen (also integrin αMβ2 or CD11b/CD18)
- IFNγ
interferon gamma
- TGFβ
Transforming growth factor beta
- NKG2DL
natural killer G2D ligand
- ApoE
apolipoprotein E
- CTAP-3
connective tissue activating peptide 3
- Tβ-4
thymosin β-4
- FcγRIIa
Fc fragment of IgG receptor IIa
- NET
neutrophil extracellular trap
- hCMV
human cytomegalovirus
- HSV
herpes simplex virus
- CVB
coxsackievirus B
- EMCV
encephalomyocarditis virus
- HCV
hepatitis C virus
- VEGF
vascular endothelial growth factor
- HUVEC
human umbilical vein endothelial cells
- COX
Cyclooxygenase
- PAC-1
antibody clone PAC-1 recognizing an epitope on GP2b/3a and measures platelet activation
- PLP
platelet like particles
- PBMCs
peripheral blood mononuclear cells
- sPLA2-IIA
Serum secretory phospholipase A2-IIa
- P2Y12
purinergic receptor 2Y12
Footnotes
Disclosures
None
References
- 1.Michelson AD. Platelets. London ; Waltham, MA: Academic Press; 2013. [Google Scholar]
- 2.Ghoshal K, Bhattacharyya M. Overview of platelet physiology: Its hemostatic and nonhemostatic role in disease pathogenesis. ScientificWorldJournal. 2014;2014:781857. doi: 10.1155/2014/781857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drelich DA, Bray PF. The traditional role of platelets in hemostasis. In: Kerrigan S, Moran N, editors. The non-thrombotic role of platelets in health and disease. Rijeka: InTech; 2015. Ch. 02. [Google Scholar]
- 4.Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123:2759–2767. doi: 10.1182/blood-2013-11-462432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 6.Koupenova M, Kehrel BE, Corkrey HA, Freedman JE. Thrombosis and platelets: An update. Eur Heart J. 2017;38:785–791. doi: 10.1093/eurheartj/ehw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciferri S, Emiliani C, Guglielmini G, Orlacchio A, Nenci GG, Gresele P. Platelets release their lysosomal content in vivo in humans upon activation. Thromb Haemost. 2000;83:157–164. [PubMed] [Google Scholar]
- 8.Jurk K, Kehrel BE. Platelets: Physiology and biochemistry. Semin Thromb Hemost. 2005;31:381–392. doi: 10.1055/s-2005-916671. [DOI] [PubMed] [Google Scholar]
- 9.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 10.Tichelaar YI, Kluin-Nelemans HJ, Meijer K. Infections and inflammatory diseases as risk factors for venous thrombosis. A systematic review. Thromb Haemost. 2012;107:827–837. doi: 10.1160/TH11-09-0611. [DOI] [PubMed] [Google Scholar]
- 11.Bunce PE, High SM, Nadjafi M, Stanley K, Liles WC, Christian MD. Pandemic h1n1 influenza infection and vascular thrombosis. Clin Infect Dis. 2011;52:e14–17. doi: 10.1093/cid/ciq125. [DOI] [PubMed] [Google Scholar]
- 12.Campbell LA, Rosenfeld ME. Infection and atherosclerosis development. Arch Med Res. 2015;46:339–350. doi: 10.1016/j.arcmed.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, Voronov R, Diamond SL, Brass LF. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121:1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomaiuolo M, Brass LF, Stalker TJ. Regulation of platelet activation and coagulation and its role in vascular injury and arterial thrombosis. Interv Cardiol Clin. 2017;6:1–12. doi: 10.1016/j.iccl.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan ZS, Zarpellon A, Alwis I, Yuan Y, McFadyen J, Ghasemzadeh M, Schoenwaelder SM, Ruggeri ZM, Jackson SP. Thrombin-dependent intravascular leukocyte trafficking regulated by fibrin and the platelet receptors gpib and par4. Nat Commun. 2015;6:7835. doi: 10.1038/ncomms8835. [DOI] [PubMed] [Google Scholar]
- 16.Ghasemzadeh M, Kaplan ZS, Alwis I, Schoenwaelder SM, Ashworth KJ, Westein E, Hosseini E, Salem HH, Slattery R, McColl SR, Hickey MJ, Ruggeri ZM, Yuan Y, Jackson SP. The cxcr1/2 ligand nap-2 promotes directed intravascular leukocyte migration through platelet thrombi. Blood. 2013;121:4555–4566. doi: 10.1182/blood-2012-09-459636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etulain J, Schattner M. Glycobiology of platelet-endothelial cell interactions. Glycobiology. 2014;24:1252–1259. doi: 10.1093/glycob/cwu056. [DOI] [PubMed] [Google Scholar]
- 18.Wagner DD, Frenette PS. The vessel wall and its interactions. Blood. 2008;111:5271–5281. doi: 10.1182/blood-2008-01-078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khlgatian M, Nassar H, Chou HH, Gibson FC, 3rd, Genco CA. Fimbria-dependent activation of cell adhesion molecule expression in porphyromonas gingivalis-infected endothelial cells. Infect Immun. 2002;70:257–267. doi: 10.1128/IAI.70.1.257-267.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gkaliagkousi E, Ritter J, Ferro A. Platelet-derived nitric oxide signaling and regulation. Circ Res. 2007;101:654–662. doi: 10.1161/CIRCRESAHA.107.158410. [DOI] [PubMed] [Google Scholar]
- 21.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. Cd40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 22.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, Tolley E, Kraiss LW, McIntyre TM, Zimmerman GA, Weyrich AS. Escaping the nuclear confines: Signal-dependent pre-mrna splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 25.Puhlmann M, Weinreich DM, Farma JM, Carroll NM, Turner EM, Alexander HR., Jr Interleukin-1beta induced vascular permeability is dependent on induction of endothelial tissue factor (tf) activity. J Transl Med. 2005;3:37. doi: 10.1186/1479-5876-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaulieu LM, Lin E, Mick E, Koupenova M, Weinberg EO, Kramer CD, Genco CA, Tanriverdi K, Larson MG, Benjamin EJ, Freedman JE. Interleukin 1 receptor 1 and interleukin 1beta regulate megakaryocyte maturation, platelet activation, and transcript profile during inflammation in mice and humans. Arterioscler Thromb Vasc Biol. 2014;34:552–564. doi: 10.1161/ATVBAHA.113.302700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leon-Ponte M, Ahern GP, O'Connell PJ. Serotonin provides an accessory signal to enhance t-cell activation by signaling through the 5-ht7 receptor. Blood. 2007;109:3139–3146. doi: 10.1182/blood-2006-10-052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connell PJ, Wang X, Leon-Ponte M, Griffiths C, Pingle SC, Ahern GP. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and t cells. Blood. 2006;107:1010–1017. doi: 10.1182/blood-2005-07-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawford A, Angelosanto JM, Nadwodny KL, Blackburn SD, Wherry EJ. A role for the chemokine rantes in regulating cd8 t cell responses during chronic viral infection. PLoS Pathog. 2011;7:e1002098. doi: 10.1371/journal.ppat.1002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koupenova M, Mick E, Mikhalev E, Benjamin EJ, Tanriverdi K, Freedman JE. Sex differences in platelet toll-like receptors and their association with cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2015;35:1030–1037. doi: 10.1161/ATVBAHA.114.304954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cognasse F, Nguyen KA, Damien P, McNicol A, Pozzetto B, Hamzeh-Cognasse H, Garraud O. The inflammatory role of platelets via their tlrs and siglec receptors. Front Immunol. 2015;6:83. doi: 10.3389/fimmu.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nocella C, Carnevale R, Bartimoccia S, Novo M, Cangemi R, Pastori D, Calvieri C, Pignatelli P, Violi F. Lipopolysaccharide as trigger of platelet aggregation via eicosanoid over-production. Thromb Haemost. 2017;117:1558–1570. doi: 10.1160/TH16-11-0857. [DOI] [PubMed] [Google Scholar]
- 33.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet tlr4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 34.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional toll-like receptor-4. Blood. 2005;106:2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 35.Biswas S, Zimman A, Gao D, Byzova T, Podrez E. Tlr2 plays a key role in platelet hyperreactivity and accelerated thrombosis associated with hyperlipidemia. Circ Res. 2017 doi: 10.1161/CIRCRESAHA.117.311069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, Hayashi C, Genco CA, Iafrati M, Freedman JE. Stimulation of toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res. 2009;104:346–354. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falker K, Klarstrom-Engstrom K, Bengtsson T, Lindahl TL, Grenegard M. The toll-like receptor 2/1 (tlr2/1) complex initiates human platelet activation via the src/syk/lat/plcgamma2 signalling cascade. Cell Signal. 2014;26:279–286. doi: 10.1016/j.cellsig.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Panigrahi S, Ma Y, Hong L, Gao D, West XZ, Salomon RG, Byzova TV, Podrez EA. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circ Res. 2013;112:103–112. doi: 10.1161/CIRCRESAHA.112.274241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thon JN, Peters CG, Machlus KR, Aslam R, Rowley J, Macleod H, Devine MT, Fuchs TA, Weyrich AS, Semple JW, Flaumenhaft R, Italiano JE., Jr T granules in human platelets function in tlr9 organization and signaling. J Cell Biol. 2012;198:561–574. doi: 10.1083/jcb.201111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koupenova M, Vitseva O, MacKay CR, Beaulieu LM, Benjamin EJ, Mick E, Kurt-Jones EA, Ravid K, Freedman JE. Platelet-tlr7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood. 2014;124:791–802. doi: 10.1182/blood-2013-11-536003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D'Atri LP, Etulain J, Rivadeneyra L, Lapponi MJ, Centurion M, Cheng K, Yin H, Schattner M. Expression and functionality of toll-like receptor 3 in the megakaryocytic lineage. J Thromb Haemost. 2015;13:839–850. doi: 10.1111/jth.12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anabel AS, Eduardo PC, Pedro Antonio HC, Carlos SM, Juana NM, Honorio TA, Nicolas VS, Sergio Roberto AR. Human platelets express toll-like receptor 3 and respond to poly i:C. Hum Immunol. 2014;75:1244–1251. doi: 10.1016/j.humimm.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Maugeri N, Rovere-Querini P, Evangelista V, Covino C, Capobianco A, Bertilaccio MT, Piccoli A, Totani L, Cianflone D, Maseri A, Manfredi AA. Neutrophils phagocytose activated platelets in vivo: A phosphatidylserine, p-selectin, and {beta}2 integrin-dependent cell clearance program. Blood. 2009;113:5254–5265. doi: 10.1182/blood-2008-09-180794. [DOI] [PubMed] [Google Scholar]
- 44.Boudreau LH, Duchez AC, Cloutier N, Soulet D, Martin N, Bollinger J, Pare A, Rousseau M, Naika GS, Levesque T, Laflamme C, Marcoux G, Lambeau G, Farndale RW, Pouliot M, Hamzeh-Cognasse H, Cognasse F, Garraud O, Nigrovic PA, Guderley H, Lacroix S, Thibault L, Semple JW, Gelb MH, Boilard E. Platelets release mitochondria serving as substrate for bactericidal group iia-secreted phospholipase a2 to promote inflammation. Blood. 2014;124:2173–2183. doi: 10.1182/blood-2014-05-573543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenne CN, Kubes P. Virus-induced nets--critical component of host defense or pathogenic mediator? PLoS Pathog. 2015;11:e1004546. doi: 10.1371/journal.ppat.1004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinod K, Wagner DD. Thrombosis: Tangled up in nets. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaplan MJ, Radic M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J Immunol. 2012;189:2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kraemer BF, Campbell RA, Schwertz H, Cody MJ, Franks Z, Tolley ND, Kahr WH, Lindemann S, Seizer P, Yost CC, Zimmerman GA, Weyrich AS. Novel anti-bacterial activities of beta-defensin 1 in human platelets: Suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011;7:e1002355. doi: 10.1371/journal.ppat.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface p-selectin: Studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104:1533–1537. doi: 10.1161/hc3801.095588. [DOI] [PubMed] [Google Scholar]
- 50.Sarma J, Laan CA, Alam S, Jha A, Fox KA, Dransfield I. Increased platelet binding to circulating monocytes in acute coronary syndromes. Circulation. 2002;105:2166–2171. doi: 10.1161/01.cir.0000015700.27754.6f. [DOI] [PubMed] [Google Scholar]
- 51.Furman MI, Barnard MR, Krueger LA, Fox ML, Shilale EA, Lessard DM, Marchese P, Frelinger AL, 3rd, Goldberg RJ, Michelson AD. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. 2001;38:1002–1006. doi: 10.1016/s0735-1097(01)01485-1. [DOI] [PubMed] [Google Scholar]
- 52.Shih L, Kaplan D, Kraiss LW, Casper TC, Pendleton RC, Peters CL, Supiano MA, Zimmerman GA, Weyrich AS, Rondina MT. Platelet-monocyte aggregates and c-reactive protein are associated with vte in older surgical patients. Sci Rep. 2016;6:27478. doi: 10.1038/srep27478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alard JE, Ortega-Gomez A, Wichapong K, Bongiovanni D, Horckmans M, Megens RT, Leoni G, Ferraro B, Rossaint J, Paulin N, Ng J, Ippel H, Suylen D, Hinkel R, Blanchet X, Gaillard F, D'Amico M, von Hundelshausen P, Zarbock A, Scheiermann C, Hackeng TM, Steffens S, Kupatt C, Nicolaes GA, Weber C, Soehnlein O. Recruitment of classical monocytes can be inhibited by disturbing heteromers of neutrophil hnp1 and platelet ccl5. Sci Transl Med. 2015;7:317ra196. doi: 10.1126/scitranslmed.aad5330. [DOI] [PubMed] [Google Scholar]
- 54.Postea O, Vasina EM, Cauwenberghs S, Projahn D, Liehn EA, Lievens D, Theelen W, Kramp BK, Butoi ED, Soehnlein O, Heemskerk JW, Ludwig A, Weber C, Koenen RR. Contribution of platelet cx(3)cr1 to platelet-monocyte complex formation and vascular recruitment during hyperlipidemia. Arterioscler Thromb Vasc Biol. 2012;32:1186–1193. doi: 10.1161/ATVBAHA.111.243485. [DOI] [PubMed] [Google Scholar]
- 55.Raiden S, Schettini J, Salamone G, Trevani A, Vermeulen M, Gamberale R, Giordano M, Geffner J. Human platelets produce granulocyte-macrophage colony-stimulating factor and delay eosinophil apoptosis. Lab Invest. 2003;83:589–598. doi: 10.1097/01.lab.0000062851.71286.47. [DOI] [PubMed] [Google Scholar]
- 56.Pitchford SC, Momi S, Giannini S, Casali L, Spina D, Page CP, Gresele P. Platelet p-selectin is required for pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation. Blood. 2005;105:2074–2081. doi: 10.1182/blood-2004-06-2282. [DOI] [PubMed] [Google Scholar]
- 57.Li N, Ji Q, Hjemdahl P. Platelet-lymphocyte conjugation differs between lymphocyte subpopulations. J Thromb Haemost. 2006;4:874–881. doi: 10.1111/j.1538-7836.2006.01817.x. [DOI] [PubMed] [Google Scholar]
- 58.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Chavarin P, Cogne M, Richard Y, Garraud O. Human platelets can activate peripheral blood b cells and increase production of immunoglobulins. Exp Hematol. 2007;35:1376–1387. doi: 10.1016/j.exphem.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 59.Hu H, Zhu L, Huang Z, Ji Q, Chatterjee M, Zhang W, Li N. Platelets enhance lymphocyte adhesion and infiltration into arterial thrombus. Thromb Haemost. 2010;104:1184–1192. doi: 10.1160/TH10-05-0308. [DOI] [PubMed] [Google Scholar]
- 60.Koenen RR, von Hundelshausen P, Nesmelova IV, Zernecke A, Liehn EA, Sarabi A, Kramp BK, Piccinini AM, Paludan SR, Kowalska MA, Kungl AJ, Hackeng TM, Mayo KH, Weber C. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat Med. 2009;15:97–103. doi: 10.1038/nm.1898. [DOI] [PubMed] [Google Scholar]
- 61.Shi G, Field DJ, Ko KA, Ture S, Srivastava K, Levy S, Kowalska MA, Poncz M, Fowell DJ, Morrell CN. Platelet factor 4 limits th17 differentiation and cardiac allograft rejection. J Clin Invest. 2014;124:543–552. doi: 10.1172/JCI71858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Langer HF, Daub K, Braun G, Schonberger T, May AE, Schaller M, Stein GM, Stellos K, Bueltmann A, Siegel-Axel D, Wendel HP, Aebert H, Roecken M, Seizer P, Santoso S, Wesselborg S, Brossart P, Gawaz M. Platelets recruit human dendritic cells via mac-1/jam-c interaction and modulate dendritic cell function in vitro. Arterioscler Thromb Vasc Biol. 2007;27:1463–1470. doi: 10.1161/ATVBAHA.107.141515. [DOI] [PubMed] [Google Scholar]
- 63.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–1300. [PubMed] [Google Scholar]
- 64.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirouskova M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 65.Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates nkg2d thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69:7775–7783. doi: 10.1158/0008-5472.CAN-09-2123. [DOI] [PubMed] [Google Scholar]
- 66.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein e. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 67.Dong ZM, Brown AA, Wagner DD. Prominent role of p-selectin in the development of advanced atherosclerosis in apoe-deficient mice. Circulation. 2000;101:2290–2295. doi: 10.1161/01.cir.101.19.2290. [DOI] [PubMed] [Google Scholar]
- 68.Burger PC, Wagner DD. Platelet p-selectin facilitates atherosclerotic lesion development. Blood. 2003;101:2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 69.Gerdes N, Seijkens T, Lievens D, Kuijpers MJ, Winkels H, Projahn D, Hartwig H, Beckers L, Megens RT, Boon L, Noelle RJ, Soehnlein O, Heemskerk JW, Weber C, Lutgens E. Platelet cd40 exacerbates atherosclerosis by transcellular activation of endothelial cells and leukocytes. Arterioscler Thromb Vasc Biol. 2016;36:482–490. doi: 10.1161/ATVBAHA.115.307074. [DOI] [PubMed] [Google Scholar]
- 70.Lievens D, Zernecke A, Seijkens T, Soehnlein O, Beckers L, Munnix IC, Wijnands E, Goossens P, van Kruchten R, Thevissen L, Boon L, Flavell RA, Noelle RJ, Gerdes N, Biessen EA, Daemen MJ, Heemskerk JW, Weber C, Lutgens E. Platelet cd40l mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010;116:4317–4327. doi: 10.1182/blood-2010-01-261206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mueller BM, Reisfeld RA, Edgington TS, Ruf W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc Natl Acad Sci U S A. 1992;89:11832–11836. doi: 10.1073/pnas.89.24.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A. 2014;111:E3053–3061. doi: 10.1073/pnas.1411082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pertuy F, Aguilar A, Strassel C, Eckly A, Freund JN, Duluc I, Gachet C, Lanza F, Leon C. Broader expression of the mouse platelet factor 4-cre transgene beyond the megakaryocyte lineage. J Thromb Haemost. 2015;13:115–125. doi: 10.1111/jth.12784. [DOI] [PubMed] [Google Scholar]
- 75.Blair P, Flaumenhaft R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009;23:177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang YQ, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun. 2002;70:6524–6533. doi: 10.1128/IAI.70.12.6524-6533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yeaman MR. Platelets in defense against bacterial pathogens. Cell Mol Life Sci. 2010;67:525–544. doi: 10.1007/s00018-009-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Assinger A. Platelets and infection - an emerging role of platelets in viral infection. Front Immunol. 2014;5:649. doi: 10.3389/fimmu.2014.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fitzgerald JR, Foster TJ, Cox D. The interaction of bacterial pathogens with platelets. Nat Rev Microbiol. 2006;4:445–457. doi: 10.1038/nrmicro1425. [DOI] [PubMed] [Google Scholar]
- 80.Hamzeh-Cognasse H, Damien P, Chabert A, Pozzetto B, Cognasse F, Garraud O. Platelets and infections - complex interactions with bacteria. Front Immunol. 2015;6:82. doi: 10.3389/fimmu.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fitzgerald JR, Loughman A, Keane F, Brennan M, Knobel M, Higgins J, Visai L, Speziale P, Cox D, Foster TJ. Fibronectin-binding proteins of staphylococcus aureus mediate activation of human platelets via fibrinogen and fibronectin bridges to integrin gpiib/iiia and igg binding to the fcgammariia receptor. Mol Microbiol. 2006;59:212–230. doi: 10.1111/j.1365-2958.2005.04922.x. [DOI] [PubMed] [Google Scholar]
- 82.Loughman A, Fitzgerald JR, Brennan MP, Higgins J, Downer R, Cox D, Foster TJ. Roles for fibrinogen, immunoglobulin and complement in platelet activation promoted by staphylococcus aureus clumping factor a. Mol Microbiol. 2005;57:804–818. doi: 10.1111/j.1365-2958.2005.04731.x. [DOI] [PubMed] [Google Scholar]
- 83.Krauel K, Weber C, Brandt S, Zahringer U, Mamat U, Greinacher A, Hammerschmidt S. Platelet factor 4 binding to lipid a of gram-negative bacteria exposes pf4/heparin-like epitopes. Blood. 2012;120:3345–3352. doi: 10.1182/blood-2012-06-434985. [DOI] [PubMed] [Google Scholar]
- 84.Krauel K, Potschke C, Weber C, Kessler W, Furll B, Ittermann T, Maier S, Hammerschmidt S, Broker BM, Greinacher A. Platelet factor 4 binds to bacteria, [corrected] inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia. Blood. 2011;117:1370–1378. doi: 10.1182/blood-2010-08-301424. [DOI] [PubMed] [Google Scholar]
- 85.Dziarski R. Deadly plague versus mild-mannered tlr4. Nat Immunol. 2006;7:1017–1019. doi: 10.1038/ni1006-1017. [DOI] [PubMed] [Google Scholar]
- 86.Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, Akira S, Cotter RJ, Goguen JD, Lien E. Virulence factors of yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006;7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- 87.Broadley SP, Plaumann A, Coletti R, Lehmann C, Wanisch A, Seidlmeier A, Esser K, Luo S, Ramer PC, Massberg S, Busch DH, van Lookeren Campagne M, Verschoor A. Dual-track clearance of circulating bacteria balances rapid restoration of blood sterility with induction of adaptive immunity. Cell Host Microbe. 2016;20:36–48. doi: 10.1016/j.chom.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 88.Assinger A, Kral JB, Yaiw KC, Schrottmaier WC, Kurzejamska E, Wang Y, Mohammad AA, Religa P, Rahbar A, Schabbauer G, Butler LM, Soderberg-Naucler C. Human cytomegalovirus-platelet interaction triggers toll-like receptor 2-dependent proinflammatory and proangiogenic responses. Arterioscler Thromb Vasc Biol. 2014;34:801–809. doi: 10.1161/ATVBAHA.114.303287. [DOI] [PubMed] [Google Scholar]
- 89.Forghani B, Schmidt NJ. Association of herpes simplex virus with platelets of experimentally infected mice. Arch Virol. 1983;76:269–274. doi: 10.1007/BF01311111. [DOI] [PubMed] [Google Scholar]
- 90.Bik T, Sarov I, Livne A. Interaction between vaccinia virus and human blood platelets. Blood. 1982;59:482–487. [PubMed] [Google Scholar]
- 91.Youssefian T, Drouin A, Masse JM, Guichard J, Cramer EM. Host defense role of platelets: Engulfment of hiv and staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029. doi: 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 92.Danon D, Jerushalmy Z, De Vries A. Incorporation of influenza virus in human blood platelets in vitro. Electron microscopical observation. Virology. 1959;9:719–722. doi: 10.1016/0042-6822(59)90168-0. [DOI] [PubMed] [Google Scholar]
- 93.de Almeida AJ, Campos-de-Magalhaes M, Brandao-Mello CE, de Oliveira RV, do Espirito-Santo MP, Yoshida CF, Lampe E. Detection of hepatitis c virus in platelets: Evaluating its relationship to antiviral therapy outcome. Hepatogastroenterology. 2009;56:429–436. [PubMed] [Google Scholar]
- 94.Noisakran S, Gibbons RV, Songprakhon P, Jairungsri A, Ajariyakhajorn C, Nisalak A, Jarman RG, Malasit P, Chokephaibulkit K, Perng GC. Detection of dengue virus in platelets isolated from dengue patients. Southeast Asian J Trop Med Public Health. 2009;40:253–262. [PubMed] [Google Scholar]
- 95.Negrotto S, Jaquenod de Giusti C, Rivadeneyra L, Ure AE, Mena HA, Schattner M, Gomez RM. Platelets interact with coxsackieviruses b and have a critical role in the pathogenesis of virus-induced myocarditis. J Thromb Haemost. 2015;13:271–282. doi: 10.1111/jth.12782. [DOI] [PubMed] [Google Scholar]
- 96.Sottnek HM, Campbell WG, Jr, Cassel WA. The pathogenesis of vaccinia virus toxicity. Ii. An electron microscopic study. Lab Invest. 1975;33:522–532. [PubMed] [Google Scholar]
- 97.Visser MR, Tracy PB, Vercellotti GM, Goodman JL, White JG, Jacob HS. Enhanced thrombin generation and platelet binding on herpes simplex virus-infected endothelium. Proc Natl Acad Sci U S A. 1988;85:8227–8230. doi: 10.1073/pnas.85.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Onlamoon N, Noisakran S, Hsiao HM, Duncan A, Villinger F, Ansari AA, Perng GC. Dengue virus-induced hemorrhage in a nonhuman primate model. Blood. 2010;115:1823–1834. doi: 10.1182/blood-2009-09-242990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hottz ED, Lopes JF, Freitas C, Valls-de-Souza R, Oliveira MF, Bozza MT, Da Poian AT, Weyrich AS, Zimmerman GA, Bozza FA, Bozza PT. Platelets mediate increased endothelium permeability in dengue through nlrp3-inflammasome activation. Blood. 2013;122:3405–3414. doi: 10.1182/blood-2013-05-504449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rondina MT, Brewster B, Grissom CK, Zimmerman GA, Kastendieck DH, Harris ES, Weyrich AS. In vivo platelet activation in critically ill patients with primary 2009 influenza a(h1n1) Chest. 2012;141:1490–1495. doi: 10.1378/chest.11-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh MV, Davidson DC, Kiebala M, Maggirwar SB. Detection of circulating platelet-monocyte complexes in persons infected with human immunodeficiency virus type-1. J Virol Methods. 2012;181:170–176. doi: 10.1016/j.jviromet.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Passacquale G, Vamadevan P, Pereira L, Hamid C, Corrigall V, Ferro A. Monocyte-platelet interaction induces a pro-inflammatory phenotype in circulating monocytes. PLoS One. 2011;6:e25595. doi: 10.1371/journal.pone.0025595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rondina MT, Schwertz H, Harris ES, Kraemer BF, Campbell RA, Mackman N, Grissom CK, Weyrich AS, Zimmerman GA. The septic milieu triggers expression of spliced tissue factor mrna in human platelets. J Thromb Haemost. 2011;9:748–758. doi: 10.1111/j.1538-7836.2011.04208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rondina MT, Tatsumi K, Bastarache JA, Mackman N. Microvesicle tissue factor activity and interleukin-8 levels are associated with mortality in patients with influenza a/h1n1 infection. Crit Care Med. 2016;44:e574–578. doi: 10.1097/CCM.0000000000001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boilard E, Pare G, Rousseau M, Cloutier N, Dubuc I, Levesque T, Borgeat P, Flamand L. Influenza virus h1n1 activates platelets through fcgammariia signaling and thrombin generation. Blood. 2014;123:2854–2863. doi: 10.1182/blood-2013-07-515536. [DOI] [PubMed] [Google Scholar]
- 106.Le VB, Schneider JG, Boergeling Y, Berri F, Ducatez M, Guerin JL, Adrian I, Errazuriz-Cerda E, Frasquilho S, Antunes L, Lina B, Bordet JC, Jandrot-Perrus M, Ludwig S, Riteau B. Platelet activation and aggregation promote lung inflammation and influenza virus pathogenesis. Am J Respir Crit Care Med. 2015;191:804–819. doi: 10.1164/rccm.201406-1031OC. [DOI] [PubMed] [Google Scholar]
- 107.McMorran BJ, Marshall VM, de Graaf C, Drysdale KE, Shabbar M, Smyth GK, Corbin JE, Alexander WS, Foote SJ. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 2009;323:797–800. doi: 10.1126/science.1166296. [DOI] [PubMed] [Google Scholar]
- 108.McMorran BJ, Wieczorski L, Drysdale KE, Chan JA, Huang HM, Smith C, Mitiku C, Beeson JG, Burgio G, Foote SJ. Platelet factor 4 and duffy antigen required for platelet killing of plasmodium falciparum. Science. 2012;338:1348–1351. doi: 10.1126/science.1228892. [DOI] [PubMed] [Google Scholar]
- 109.Aggrey AA, Srivastava K, Ture S, Field DJ, Morrell CN. Platelet induction of the acute-phase response is protective in murine experimental cerebral malaria. J Immunol. 2013;190:4685–4691. doi: 10.4049/jimmunol.1202672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grau GE, Mackenzie CD, Carr RA, Redard M, Pizzolato G, Allasia C, Cataldo C, Taylor TE, Molyneux ME. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J Infect Dis. 2003;187:461–466. doi: 10.1086/367960. [DOI] [PubMed] [Google Scholar]
- 111.Risitano A, Beaulieu LM, Vitseva O, Freedman JE. Platelets and platelet-like particles mediate intercellular rna transfer. Blood. 2012;119:6288–6295. doi: 10.1182/blood-2011-12-396440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clancy L, Beaulieu LM, Tanriverdi K, Freedman JE. The role of rna uptake in platelet heterogeneity. Thromb Haemost. 2017;117:948–961. doi: 10.1160/TH16-11-0873. [DOI] [PubMed] [Google Scholar]
- 113.Mause SF, von Hundelshausen P, Zernecke A, Koenen RR, Weber C. Platelet microparticles: A transcellular delivery system for rantes promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. 2005;25:1512–1518. doi: 10.1161/01.ATV.0000170133.43608.37. [DOI] [PubMed] [Google Scholar]
- 114.Mause SF, Ritzel E, Liehn EA, Hristov M, Bidzhekov K, Muller-Newen G, Soehnlein O, Weber C. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation. 2010;122:495–506. doi: 10.1161/CIRCULATIONAHA.109.909473. [DOI] [PubMed] [Google Scholar]
- 115.Gidlof O, van der Brug M, Ohman J, Gilje P, Olde B, Wahlestedt C, Erlinge D. Platelets activated during myocardial infarction release functional mirna, which can be taken up by endothelial cells and regulate icam1 expression. Blood. 2013;121:3908–3917. S3901–3926. doi: 10.1182/blood-2012-10-461798. [DOI] [PubMed] [Google Scholar]
- 116.Laffont B, Corduan A, Rousseau M, Duchez AC, Lee CH, Boilard E, Provost P. Platelet microparticles reprogram macrophage gene expression and function. Thromb Haemost. 2015;115 doi: 10.1160/TH15-05-0389. [DOI] [PubMed] [Google Scholar]
- 117.Laffont B, Corduan A, Ple H, Duchez AC, Cloutier N, Boilard E, Provost P. Activated platelets can deliver mrna regulatory ago2*microrna complexes to endothelial cells via microparticles. Blood. 2013;122:253–261. doi: 10.1182/blood-2013-03-492801. [DOI] [PubMed] [Google Scholar]
- 118.Liang H, Yan X, Pan Y, Wang Y, Wang N, Li L, Liu Y, Chen X, Zhang CY, Gu H, Zen K. Microrna-223 delivered by platelet-derived microvesicles promotes lung cancer cell invasion via targeting tumor suppressor epb41l3. Mol Cancer. 2015;14:58. doi: 10.1186/s12943-015-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Michael JV, Wurtzel JGT, Mao GF, Rao AK, Kolpakov MA, Sabri A, Hoffman NE, Rajan S, Tomar D, Madesh M, Nieman MT, Yu J, Edelstein LC, Rowley JW, Weyrich AS, Goldfinger LE. Platelet microparticles infiltrating solid tumors transfer mirnas that suppress tumor growth. Blood. 2017 doi: 10.1182/blood-2016-11-751099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jobe SM, Wilson KM, Leo L, Raimondi A, Molkentin JD, Lentz SR, Di Paola J. Critical role for the mitochondrial permeability transition pore and cyclophilin d in platelet activation and thrombosis. Blood. 2008;111:1257–1265. doi: 10.1182/blood-2007-05-092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu F, Gamez G, Myers DR, Clemmons W, Lam WA, Jobe SM. Mitochondrially mediated integrin alphaiibbeta3 protein inactivation limits thrombus growth. J Biol Chem. 2013;288:30672–30681. doi: 10.1074/jbc.M113.472688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial damps cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marques PE, Amaral SS, Pires DA, Nogueira LL, Soriani FM, Lima BH, Lopes GA, Russo RC, Avila TV, Melgaco JG, Oliveira AG, Pinto MA, Lima CX, De Paula AM, Cara DC, Leite MF, Teixeira MM, Menezes GB. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology. 2012;56:1971–1982. doi: 10.1002/hep.25801. [DOI] [PubMed] [Google Scholar]
- 125.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cloutier N, Tan S, Boudreau LH, Cramb C, Subbaiah R, Lahey L, Albert A, Shnayder R, Gobezie R, Nigrovic PA, Farndale RW, Robinson WH, Brisson A, Lee DM, Boilard E. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: The microparticle-associated immune complexes. EMBO Mol Med. 2013;5:235–249. doi: 10.1002/emmm.201201846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gyorgy B, Szabo TG, Turiak L, Wright M, Herczeg P, Ledeczi Z, Kittel A, Polgar A, Toth K, Derfalvi B, Zelenak G, Borocz I, Carr B, Nagy G, Vekey K, Gay S, Falus A, Buzas EI. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS One. 2012;7:e49726. doi: 10.1371/journal.pone.0049726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mehta RS, Nishihara R, Cao Y, Song M, Mima K, Qian ZR, Nowak JA, Kosumi K, Hamada T, Masugi Y, Bullman S, Drew DA, Kostic AD, Fung TT, Garrett WS, Huttenhower C, Wu K, Meyerhardt JA, Zhang X, Willett WC, Giovannucci EL, Fuchs CS, Chan AT, Ogino S. Association of dietary patterns with risk of colorectal cancer subtypes classified by fusobacterium nucleatum in tumor tissue. JAMA Oncol. 2017;3:921–927. doi: 10.1001/jamaoncol.2016.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Welsh JD, Muthard RW, Stalker TJ, Taliaferro JP, Diamond SL, Brass LF. A systems approach to hemostasis: 4. How hemostatic thrombi limit the loss of plasma-borne molecules from the microvasculature. Blood. 2016;127:1598–1605. doi: 10.1182/blood-2015-09-672188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leebeek FW, Boersma E, Cannon CP, van de Werf FJ, Simoons ML. Oral glycoprotein iib/iiia receptor inhibitors in patients with cardiovascular disease: Why were the results so unfavourable. Eur Heart J. 2002;23:444–457. doi: 10.1053/euhj.2001.2751. [DOI] [PubMed] [Google Scholar]
- 132.Leon C, Ravanat C, Freund M, Cazenave JP, Gachet C. Differential involvement of the p2y1 and p2y12 receptors in platelet procoagulant activity. Arterioscler Thromb Vasc Biol. 2003;23:1941–1947. doi: 10.1161/01.ATV.0000092127.16125.E6. [DOI] [PubMed] [Google Scholar]
- 133.Sahni A, Sahni SK, Francis CW. Endothelial cell activation by il-1beta in the presence of fibrinogen requires alphavbeta3. Arterioscler Thromb Vasc Biol. 2005;25:2222–2227. doi: 10.1161/01.ATV.0000183605.27125.6f. [DOI] [PubMed] [Google Scholar]
- 134.Zhang G, Han J, Welch EJ, Ye RD, Voyno-Yasenetskaya TA, Malik AB, Du X, Li Z. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via tlr4/myd88 and the cgmp-dependent protein kinase pathway. J Immunol. 2009;182:7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jenne CN, Wong CH, Zemp FJ, McDonald B, Rahman MM, Forsyth PA, McFadden G, Kubes P. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013;13:169–180. doi: 10.1016/j.chom.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 136.Carestia A, Kaufman T, Rivadeneyra L, Landoni VI, Pozner RG, Negrotto S, D’Atri LP, Gomez RM, Schattner M. Mediators and molecular pathways involved in the regulation of neutrophil extracellular trap formation mediated by activated platelets. J Leukoc Biol. 2016;99:153–162. doi: 10.1189/jlb.3A0415-161R. [DOI] [PubMed] [Google Scholar]