Abstract
Humans prefer to live within their thermal comfort or neutral zone, which they create by making shelters, wearing clothing, and more recently, by regulating their ambient temperature. This allows humans to maintain a constant core temperature with minimal energy expenditure, a trait that is conserved across all endotherms, including mammals and birds. Although this primordial drive leads us to seek thermal comfort, we house our experimental subjects, laboratory mice (Mus musculus), under thermal stress conditions. Here we discuss how housing mice below their thermoneutral zone limits our ability to model and study human diseases. Using examples from cardiovascular physiology, metabolic disorders, infections, and tumor immunology, we point out that certain phenotypes observed under thermal stress conditions disappear when mice are housed at thermoneutrality, whereas others emerge that are more consistent with human biology. Thus, we propose that warming the mouse might allow for more predictive modeling of human diseases and therapies.
Introduction
Endotherms, such as mammals and birds, use heat liberated during cellular metabolism to maintain a stable internal temperature1,2. This maintenance of a constant core temperature, which is close to the optimum for enzymatic reactions, allows mammals and birds to be active in diverse environments3,4. For example, while the activity level of ectotherms, such as reptiles and amphibians, drops during the cooler temperatures of the night, mammals are able to avoid this drop in nocturnal activity, permitting them to forage for food and look for mates at night. In addition, endothermy is better able to support the growth of developing embryos, which are less tolerant of thermal fluctuations in the environment. However, these adaptive advantages afforded by a stable core temperature come at a price – the higher energetic demands of endotherms. For example, the metabolic rate per unit mass of endotherms is ~5-10-fold higher than that of ectotherms, necessitating greater investment in looking for food and in the storage of nutrients2-5. Since a stable core temperature is critical for the survival of endotherms, endothermic animals go to great lengths to defend their core temperature in colder environments, a trait that has profound effects on their metabolic, cardiovascular, and immunologic responses. However, when defense of the core temperature is not possible, such as during food scarcity or seasonal cold, many endotherms, including mice, will abandon homeothermy and engage in daily torpor or seasonal hibernation to conserve energy6,7.
Over the last two decades, the laboratory mouse, Mus musculus, has emerged as a preferred model system for studies of metabolism, immunity, and cardiovascular physiology, and for modeling human disease8-10. In part, this has been fueled by the conservation of genes between mice and humans, and the ease with which mouse genes can be manipulated to study their function. The underlying assumption has been that investigations in mice will provide fundamental insights into human biology. While this has largely been the case, the thermal physiology of the mouse is quite different from that of humans, which might limit the direct translation of these preclinical findings11. For example, like other small mammals, the mouse has a large surface area and a small body mass, which makes it vulnerable to fluctuations in its ambient housing temperature (Ta), especially when the Ta is lower than its thermoneutral zone10-15. Because a primordial drive in all mammals is to defend their core temperature, the mouse employs various adaptations to maintain thermal homeostasis in colder environments. This has the net effect of promoting tradeoffs between somatic maintenance programs, such as those mediating storage of nutrients or protecting against pathogens or tumors. As a consequence, mechanistic studies performed in mice that are housed at temperatures below their thermoneutral zone might not directly apply to humans, who primarily live in their thermal comfort or neutral zone8,9,12.
In this review, we provide a framework for understanding how Ta affects metabolic, immune, and cardiovascular phenotypes in mice, and the importance of Ta on modeling of human diseases in these small rodents. We use examples from diet-induced obesity, insulin resistance and atherosclerosis, infection and immunity, and cancer biology to highlight how housing of mice at different Ta’s alters the phenotypic expression of disease. These findings lead us and others to propose that Ta might be an additional variable that can be exploited to enhance the modeling of human diseases in laboratory mice8,9,12,13,16.
Thermal physiology and thermoneutral zone
Mammals employ heat conservation and heat generation mechanisms to maintain thermal homeostasis, which is reflected in their constant core temperature5. For example, exposure to environmental cold activates a number of behavioral heat-conserving mechanisms, including vasoconstriction, piloerection, hunched posture (which minimizes surface area), and huddling. When these heat conserving adaptations prove to be insufficient for defense against the cold, mammals increase their metabolic rate (also known as energy expenditure) to generate heat from involuntary muscle contractions (shivering thermogenesis) and uncoupled respiration in brown adipocytes (non-shivering thermogenesis). The converse occurs when mammals are confronted with environmental warmth. Behavioral adaptations, such as vasodilatation, increase passive heat loss, whereas panting, licking, and sweating (in humans) increase active heat loss through evaporative cooling. Between these metabolic adaptations for environmental cold and warmth lies the thermoneutral zone, which is operationally defined as the nadir in the metabolic rate (Figure 1a)5,10-12. When the Ta is within the thermoneutral zone, basal metabolic rate generates sufficient heat to maintain a constant core temperature at 37-38°C (Figure 1a). For young (~3-month-old) C57BL/6J mice, the thermoneutral zone lies between 29-31°C8,10,12,13, which is similar to the thermoneutral zone of a naked human (~28°C)17-21. However, the thermoneutral or comfort zone of clothed humans is around 20-22°C, which is often the temperature of the vivarium in which mice are housed. This substantially cooler Ta places significant thermal stress on mice, resulting in activation of non-shivering thermogenesis in brown adipose tissue to maintain thermal homeostasis. As a consequence, the metabolic rate and food intake of mice housed at Ta of 20°C is ~100% higher than those housed at 30°C (Figure 2a, b), both parameters increase by another ~100% when mice are housed at Ta of 4-5°C22. As discussed in detail below, chronic housing of mice in thermally stressed conditions (Ta of 20-22°C) has profound effects on many physiological phenotypes and their intrinsic capacity to adapt to environmental challenges (Table 1).
Figure 1. Scholander plots of energy expenditure at different ambient temperatures for animals.
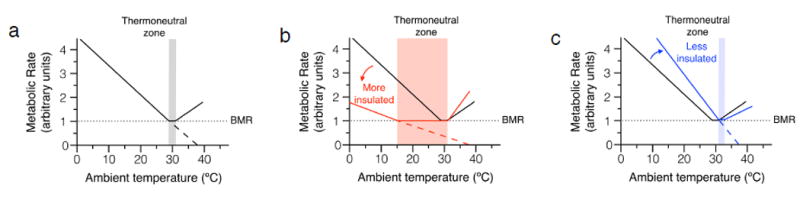
For mammals and birds, changes in ambient temperature below the thermoneutral zone result in a linear increase in metabolic rate (oxygen consumption). (a) For mice, the thermoneutral zone lies between 29-31°C (depicted in by the grey zone). The black line depicts changes in metabolic rate as the ambient temperature drops below the thermoneutral zone of mice. Note that the metabolic rate is ~2-fold higher at Ta of 20°C than at Ta of 30°C. The slope of this line is directly proportional to the thermal conductance of the animal. (b) Animals with a lower thermal conductance (more insulated by fur and subcutaneous fat), such as artic animals, have a larger thermoneutral zone and a smaller rise in metabolic rate at lower temperatures (red line). (c) Conversely, animals with higher thermal conductance (less insulated), such as equatorial animals or nude mice, have a rightward shift in their thermoneutral zone and a larger increase in metabolic rate at lower temperatures (blue line). Although less well studied, temperatures higher than the thermoneutral zone also result in an increase in metabolic rate, reflecting energy required to dissipate heat. Within a given species, the thermoneutral zone changes during the life of a species. A number of factors can alter the thermoneutral zone, including age, muscle mass, locomotor activity, pregnancy, lactation, and insulation. Dotted line represents basal metabolic rate (BMR). Although hypothetical, when BMR is zero, heat loss is zero and the core or defended temperature is equal to Ta. Thus, when dashed black (a), red (b), and blue (c) lines cross the x-axis at zero, the Ta is the defended or core temperature of the animal.
Figure 2. Effects of ambient temperature on oxygen consumption and food intake in mice.
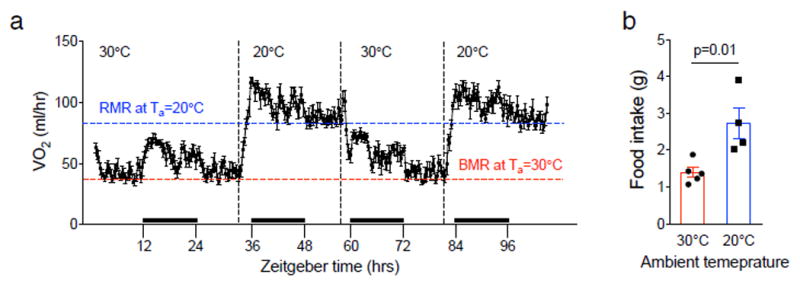
(a) Changes in oxygen consumption (energy expenditure) in C57BL/6J female mice housed at different ambient temperatures. Note that oxygen consumption changes in real time as the ambient temperature is changed between 20 and 30°C (n=5). Red and blue dashed lines mark basal metabolic rate (BMR) of C57BL/6J female mice housed at Ta=30°C and the resting metabolic rate (RMR) at Ta=20°C, respectively. RMR at Ta=20°C is ~2-fold higher than the BMR at Ta=30°C. Black bars on x-axis denote the night cycle. (b) Cumulative food intake by C57BL/6J female mice housed at different ambient temperatures (n=5).
Table 1. Physiologic characteristics and disease susceptibility of mice housed at different ambient temperatures.
Data is compiled from various listed references in which comparative analyses were performed at two different ambient temperatures.
| Ambient temperature (Ta) | 30°C | 20-22°C | Ref. |
|---|---|---|---|
|
| |||
| Core temperature | 37-38°C | 37-38°C | |
|
| |||
| Cardiovascular | |||
|
| |||
| Oxygen consumption (VO2) | ~40 ml/hr | ~80 ml/hr (~2x rate of mice at Ta of 30°C) | 26, Figure 2 |
|
| |||
| Heart rate | ~300 bpm | ~550-600 bpm (~2x rate of mice at Ta of 30°C) | 26-28 |
|
| |||
| Blood pressure (mean arterial pressure) | ~75 mmHg | ~105 mmHg | 26 |
|
| |||
| Metabolic disorders | |||
|
| |||
| Obesity | Adipose tissue inflammation does not contribute to insulin resistance. | Adipose tissue inflammation contributes to insulin resistance. | 53 |
|
| |||
| Atherosclerosis | Increased atherogenic plaque burden and inflammation in aorta of Apoe-/- mice. | Reduced disease in Apoe-/- mice as compared those at Ta of 30°C. | 53, 56 |
| Development of atherogenic lesions in aortic roots C57BL/6J mice on western diet. | C57BL/6J mice are resistant to atherogenesis on western diet. | ||
|
| |||
| Inflammation | |||
|
| |||
| Infection | Improved survival following infection (viral, bacterial, protozoal). | Impaired survival following infection (viral, bacterial, protozoal). | 59-66 |
| Febrile response following LPS injection. | Transient hypothermia following LPS injection. | ||
|
| |||
| Cancer | Reduced tumor growth due to anti-tumor CD8+ T cell immune response. | Rapid tumor growth and persistence. | 72, 73 |
| Greater efficacy of chemotherapeutics compared to mice at Ta of 20-22°C. | |||
|
| |||
| Food intake | (see Figure 2b) | Increased as compared to mice at Ta of 30°C (see Figure 2b) | |
Although there is a tendency to consider the thermoneutral zone as a fixed entity, it is a highly variable parameter that differs across species and is affected by their life history. The classic studies by Scholander et al. demonstrated that the thermoneutral zone of a particular mammal reflected its adaptations to its natural habitat23. For example, arctic mammals have a much larger thermoneutral zone and exhibit a shallower rise in their metabolic rate in colder environments because they are more insulated (Figure 1b). The converse is observed for equatorial mammals, who are better adapted for heat dissipation, exhibit a rightward shift in their thermoneutral zone, and have a steeper rise in their metabolic rate at colder temperatures (Figure 1c). These plots, which relate changes in metabolic rate (energy expenditure) to environmental temperature, are now referred to as Scholander plots13,23. If one hypothetically extends the lines that relate metabolic rate to environmental temperature (dashed lines in Figures 1a-c), they intersect the x-axis at the defended core temperature of the animal. This occurs because when ambient is equal to the core temperature, no additional energy is required (BMR is zero) to defend the core temperature10,12.
In addition to these interspecies differences, a large number of parameters can affect the size of the thermoneutral zone and cold tolerance within a given species. For example, age (neonates and young mice have higher thermoneutral zones), muscle mass (basal metabolism and heat production are proportional to muscle mass), locomotor activity (exercise increases heat production to lower the thermoneutral zone), pregnancy (fetal metabolism increases heat production), lactation (milk production generates heat because it is energy intensive), and insulation (higher insulation blunts the rise in metabolic rate at lower temperatures) can dynamically modulate the thermoneutral zone and the organism’s susceptibility to environmental cold5,10-13. As pointed out by Nedergaard and Cannon13, this variance in the thermoneutral zone provides an explanation for the observed differences in the cold tolerance of some mutant animals, such as those lacking hair, fur or subdermal fat24-28, suggesting that its empirical determination is necessary to understand how genetic mutations in mice affect physiology and disease susceptibility. Below we discuss recent studies that demonstrate how housing Ta affects the cardiovascular physiology, onset and progression of metabolic diseases, and host immune responses to pathogens and tumors.
Cardiovascular physiology
Cardiac output, which is a product of stroke volume (amount of blood ejected from left ventricle per contraction) and heart rate, is a major determinant of oxygen delivery to tissues29. In both mice and humans, when oxygen demand increases, such as during exercise, there is a proportionate increase in cardiac output, which is primarily driven by an increase in heart rate, to meet tissue demands for oxygen. The opposite occurs during the rest phase, such as during sleeping, when oxygen demand, cardiac output, and heart rate all fall. Thus, it is not surprising that housing Ta, which is a major driver of oxygen consumption (Figure 2a), has a profound effect on the cardiovascular physiology of mice. For example, the resting heart rate of mice is normally thought to be around ~550-600 beats/min. This is indeed the case for mice housed at 20-22°C, but when housing Ta is 30°C, their resting heart rate is ~300 beats/min30-32. This increase in heart rate at Ta of 20°C is driven by the sympathetic nervous system to meet the metabolic demands of thermogenesis12. Congruent with this, both parameters revert to their basal levels in real time when the housing Ta for mice is changed from 20°C to 30°C30,33 (Figure 2a). A similar change is observed in the mean arterial blood pressure, which goes from 75 mmHg at Ta of 30°C to 105 mmHg at Ta of 20°C30. It is worthwhile noting that these Ta-driven changes in metabolic rate, heart rate, and mean arterial blood pressure are not subtle, but rather large, especially in context of human diseases, where much smaller changes in these physiologic parameters have been linked to progression of obesity and cardiovascular disease34-38.
Energy balance and adiposity
Because energy expenditure decreases by ~50% in thermoneutral mice (Figure 2a), it should not be surprising that the metabolic phenotypes of obesity and adiposity are highly dependent on the housing Ta of mice. The first example of this emerged from studies on UCP1, a protein required for uncoupled respiration and non-shivering thermogenesis12,39,40. The initial studies with Ucp1-/- mice, which had been conducted under thermal stress conditions (Ta 20-22°C), failed to demonstrate a role for UCP1 in diet-induced thermogenesis and obesity40,41. However, when the same mice were housed at thermoneutrality, Ucp1-/- mice exhibited increased metabolic efficiency, resulting in increased adiposity and obesity42. This switching of metabolic phenotypes between thermal stress and thermoneutral conditions is not limited to Ucp1-/- mice. One pertinent example comes from studies of thyroid hormone on metabolism. In humans, hyperthyroidism is associated with a hypermetabolic state that is characterized by heat-intolerance and fat loss, whereas hypothyroidism lowers metabolic rate to promote cold-intolerance and obesity. The surprise came when investigators initially examined the metabolic phenotype of mice lacking type 2 deiodinase, an enzyme required for conversion of the prohormone thyroid hormone T4 to active thyroid hormone T3. Unlike hypothyroid humans, mice lacking type 2 deiodinase did not develop metabolic dysfunction when they were housed at Ta of 22°C. However, this discrepancy was resolved when control and type 2 deiodinase knockout mice were housed at thermoneutrality (Ta=30°C), which led to increased adiposity, hepatic steatosis, and glucose intolerance in type 2 deiodinase knockout mice43. These authors concluded that housing at Ta of 22°C resulted in increased adrenergic activity to brown adipose tissue, which compensated for the loss of type 2 deiodinase activity and T3 production. Together, these findings suggest that chronic housing of mice under thermal stress conditions can mask the functions of genes that participate in energy balance and metabolic homeostasis.
Inflammation and metabolic diseases
Chronic low grade inflammation has been suggested to contribute to the progression of metabolic and degenerative disorders, including obesity, type 2 diabetes, coronary artery disease, neurodegenerative disorders, and cancers44-47. For metabolic disorders, mechanistic studies in mice have suggested that the recruitment and inflammatory activation of macrophages plays an important role in initiation and progression of diet-induced atherosclerosis and obesity-associated insulin resistance48-56. However, it should be noted that while many initial studies linked obesity-induced inflammation to insulin resistance, recent studies in mice and humans demonstrate that insulin resistance often precedes the onset of inflammation in adipose tissue57-59. In light of this, it has been suggested that inflammation in metabolic tissues might participate in the maintenance rather than initiation of insulin resistance60,61. Despite this controversy, it is not known whether these results from murine studies are translatable to humans because nearly all of these studies have been performed in thermally stressed mice, whereas humans mostly live in their thermal comfort or neutral zone.
To investigate whether thermoneutral housing modulates the expression of metabolic disease, Tian et al. studied the onset of metabolic inflammation in C57BL/6J mice housed at Ta of 22°C or 30°C that were fed regular chow or high fat diet62. They found that thermoneutral housing accelerated the onset of metabolic inflammation in the white and brown adipose tissues, which was observed as early as 3 weeks after initiation of high fat diet. However, this accelerated increase in metabolic inflammation was not associated with worsening of insulin resistance, glucose tolerance, or impairment in insulin signaling in white adipose tissue or liver, suggesting that metabolic inflammation can be uncoupled from obesity-induced insulin resistance in thermoneutral mice. Because modern humans do not experience chronic cold stress, studies in thermoneutral mice might be useful for elucidating the inflammation-independent mechanisms by which obesity contributes to pathogenesis of insulin resistance and type 2 diabetes in humans. In addition, thermoneutral mice might be an appropriate model to study the physiologic functions of macrophages in adipose tissue remodeling and fibrosis, processes that are observed during diet-induced obesity63,64.
Atherosclerosis, a leading cause of coronary artery and cerebrovascular diseases, is characterized by accumulation of cholesterol-rich plaques in the subendothelial space of vessel walls50,65. Since the dominant cholesterol carrier in mice is high density lipoprotein (HDL)66,67, wild type C57BL/6J mice are resistant to the development of atherosclerosis, necessitating the use of knockout animals to model atherosclerosis68. Using Apoe-/- and Ldlr-/- mice, it has been demonstrated that hypercholesterolemia results in the entrapment and modification of lipoproteins in the subendothelial space, which initiates the recruitment of inflammatory monocytes50,69. These recruited monocytes subsequently differentiate into macrophages, phagocytize modified-lipoproteins, and undergo inflammatory activation to give rise to fatty streaks, which over time evolve into the characteristic lesions of atherosclerotic plaques50-52,69. Based on these mechanistic studies in mice, it has been hypothesized that atherosclerosis is an inflammatory disease, which might be amenable to treatment with anti-inflammatory therapies65. Although evidence in support of this hypothesis is strong, nearly all of it comes from studies performed in thermally stressed mice.
Because thermoneutral housing accelerated the onset of metabolic inflammation during obesity62, two recent studies asked whether it might potentiate vascular inflammation and atherosclerosis62,70. Using the Apoe-/- mice, Tian et al. found that thermoneutral housing enhanced the development of atherogenic lesions in the aortas of mice fed the western diet62. This increase in atherogenesis was associated with inflammatory changes in and around the vessel wall, as evidenced by increased infiltration of vessel wall and perivascular fat by inflammatory macrophages and dendritic cells. A similar increase in lesion area was observed by Giles et al., who found that Apoe-/- mice housed at thermoneutrality had larger atherosclerotic lesions in their aortic roots70. These investigators also tested whether thermoneutral housing might induce atherogenesis in C57BL/6J mice, which are normally resistant to development of atherosclerosis. Albeit smaller, C57BL/6J mice housed at Ta of 30°C had evidence of inflammatory atherogenic lesions in their aortic roots, which was not observed in mice housed Ta of 23°C. Moreover, C57BL/6J mice fed a western diet at thermoneutrality developed hypercholesterolemia with a substantial fraction of their total cholesterol in low density lipoprotein (LDL), a lipoprotein profile that is similar to that of humans. It is important to note that, in these two studies, the increased propensity of mice to develop atherogenic plaques at Ta of 30°C occurred despite improvements in hemodynamic parameters (heart rate and blood pressure), suggesting that thermoneutral housing of Apoe-/- mice might be a useful preclinical model for testing the efficacy of anti-inflammatory therapies.
Infection and cancer
Innate and adaptive immunity protect the host against pathogens and tumors71,72. Studies dating back to 1940’s indicate that the housing Ta of mice has a profound effect on host immune responses to infections. For example, Moragues and Pinkerton noted that weather-dependent changes in ambient housing temperature affected the survival of mice during experimental typhus73. As the temperature in the laboratory became cooler (29.4–36.6°C in summer to 18.3–22.8°C in winter), mortality rose from 9 to 100%. Although these were anecdotal observations, others have reported a similar decline in host immunity against bacterial (Salmonella typhimurium, Staphylococcus aureus, Klebsiella pneumonia, and Rickettsia typhi), viral (influenza virus, herpes simplex virus, and rabies virus), and protozoal (Trypanosoma cruzi) infections at cooler housing temperatures73-80. The effects of the cold housing environment are not limited to host responses to pathogenic infections, because dramatic reorganization of the gut microbial communities has been reported in cold-acclimated mice. For example, acclimation to environmental cold (housing of mice at 6°C for 31 days) requires increased energy uptake to support thermogenesis81. This increased demand for energy in cold acclimated mice is met by the remodeling of small intestines to increase their absorptive surface area, a response that is partly mediated by the gut microbiome. Although these studies have examined how acclimation to environmental cold alters the microbial communities residing in the gut, it is likely that the gut microbiome of thermoneutral mice, who exhibit decreased thermogenesis and food intake, is quite different from those raised under thermally stressed (20-22°C) conditions. Thus, in the future, it will be important to systematically study how thermoneutral housing affects microbial communities at barrier sites and its impact on host immunity during pathogenic infections.
It is well known that mice and humans vary in their sensitivity and responsiveness to bacterial products, such as lipopolysaccharide (LPS). For example, humans are highly sensitive to LPS and become febrile upon its administration, whereas mice are quite resistant to LPS and exhibit a paradoxical hypothermic response. These differences in responsiveness to LPS has led some to suggest that mice are a poor model for studying LPS-mediated sepsis in humans82,83. However, it is worthwhile noting the ability of the mouse to mount a fever or become hypothermic is dependent on the housing Ta84. For example, when mice are housed at thermoneutral Ta of 31°C, intravenous injection with LPS results in fever. In contrast, when mice are housed at subneutral temperature 26°C, intravenous administration of LPS causes transient hypothermia. These observations thus suggest that fever, which is an evolutionarily conserved response to microbial infections in fish, reptiles, and humans85, can be effectively modeled in mice when they are housed within their thermoneutral zone.
Innate and adaptive immune cells not only participate in tumor surveillance and antitumor immunity, but can also support tumor growth46,47,72. For example, tumor-associated macrophages facilitate the growth and metastasis of primary tumors, whereas antitumor immunity is primarily provided by CD8+ T helper cells and natural killer (NK) cells. However, our current understanding of how these innate and adaptive immune cells participate in tumor growth, surveillance, and antitumor immunity is based on studies performed in thermally stressed animals, which might not accurately reflect the functions of host immunity in tumorigenesis. In support of this, a recent study by Kokolus and colleagues found that growth of syngeneic and carcinogen-induced tumors was significantly delayed in mice housed at Ta of 30-31°C compared with those housed at 22-23°C86. This delay in tumor growth was dependent on the adaptive immune system because it was not observed in immunodeficient SCID and NUDE mice. Furthermore, antibody-mediated depletion experiments revealed that tumors of thermoneutral mice were infiltrated by CD8+ T helper cells, which were required for antitumor immunity. In subsequent experiments, it was found that the efficacy of anti-tumor therapies was also enhanced in thermoneutral mice87, suggesting that thermoneutral housing might improve preclinical assessment of novel antitumor therapies.
Concluding remarks
Although the mouse has emerged as a preferred model system for studying human diseases, the extant literature is littered with studies that have failed to provide mechanistic insights into human biology. This failure to translate preclinical studies in mice to therapeutics in humans often causes many to say that mice are a poor model for studying human diseases. However, as discussed here, the failure to translate these preclinical studies might stem from our anthropocentric approach to designing experiments in mice. For example, one parameter that is ubiquitously overlooked is the ambient temperature of the vivarium where mice are housed. Rather than reflecting the thermal preference of mice, it reflects our thermal preference, causing mice to be chronically housed under thermal stress. Mice adapt to this cold stress (20-22°C) just fine, but it requires activation of thermogenesis to defend their core temperature. This is not an insignificant adaptation, as evidenced by the doubling of energy expenditure and heart rate. The human equivalent of these changes in mouse physiology would be strenuous exercise, which even the elite athletes among us could not endure forever. Thus, this simple oversight on our part has a profound effect on mouse physiology and might limit our capacity to model human diseases.
Using examples from cardiovascular physiology, energy homeostasis, metabolic disorders, infections, and cancers, we have discussed how chronic thermal stress alters the basal physiology of mice and limits their capacity to adapt to other environmental challenges. However, these studies likely represent just the tip of the iceberg, because what we consider to be the “basal state” is likely representative of a “stressed state.” For example, it is widely appreciated that C57BL/6J male mice are aggressive and prone to fighting, which is true when they are housed at the normal vivarium temperature of 20-22°C. However, when these same animals are housed at thermoneutrality (30°C), their aggressive behaviors disappear (personal observations). From an anthropocentric viewpoint, chronically living ~8-10°C below ones’ thermal comfort or neutral zone (from 22°C (~72°F) to 12°C (~54°F)) would cause most humans to become agitated, which is precisely what is observed in mice. Thus, our understanding of behaviors and their underlying neural circuits in mice are likely more representative of a “stressed” rather than a “basal” state. This is likely to be true for other organ systems, such as pulmonary (higher breathing rate at 22°C), digestive (increased food intake and gut motility at 22°C), and endocrine (increased activation of hypothalamic-pituitary-adrenal axis and thyroid hormone axis at 22°C), which will collectively impair our ability to model human homeostasis and diseases in mice. Therefore, given the influence housing Ta exerts on mouse physiologic and pathophysiologic responses, it behooves us to pay attention to this simple environmental variable, which can be easily corrected by warming the mouse.
It should be noted that differences in a number of other physiologic and metabolic parameters might also limit our ability to model diseases in mice. For instance, the major cholesterol carrier in mice is HDL, whereas humans primarily transport cholesterol in LDL67. This difference in cholesterol profile makes mice naturally resistant to development of atherosclerosis50,52. Though not reviewed here, differences in circadian rhythms of mice and humans might significantly impact our understanding of biological processes. While mice are nocturnal, humans are diurnal88,89. Thus, studies performed on mice during their rest phase (daytime) might not accurately model human physiology during our active phase. Since nearly every physiological parameter is under circadian control89, this often-overlooked housing parameter likely has a huge effect on the direct translation of preclinical studies to humans.
Key Points.
Mice and humans prefer to live within their thermal neutral or comfort zone, respectively, at which they expend the least amount of energy to defend their core temperature.
The normal housing temperature (Ta=20-22°C) is within the thermal comfort zone of clothed humans, but below the thermoneutral zone (Ta=30°C) of mice. Housing of mice below their thermoneutral zone results in activation of thermogenesis to defend their core temperature.
Mice housed at Ta=20-22°C expend twice as much energy as those at Ta=30°C, which results is profound changes in their metabolic, cardiovascular, and immune responses.
Housing of mice at thermoneutrality might allow for more predictive modeling of human physiology, diseases, and therapeutics.
Box 1. Glossary of thermal physiology terms.
Ambient temperature (Ta): the temperature of the environment in which the animal lives.
Core temperature: the temperature of deep body organs is considered core temperature, because it exhibits minimal fluctuation at different ambient temperatures. Rectal temperature is routinely used as a close proxy of core temperature.
Basal metabolic rate: energy expended by an animal during the post-absorptive rest state in its thermoneutral environment. This is during the day for mice (rest phase for nocturnal animals) and night for humans.
Resting metabolic rate: energy expended by an animal during the post-absorptive rest state at a given Ta that is outside its thermoneutral zone. As depicted in Figure 1, the resting metabolic rate varies with Ta.
Thermoneutral zone: the range of ambient temperatures at which sufficient heat is generated by basal metabolism to maintain core temperature within a specified range. Deviations from thermoneutral zone require energy intensive programs of thermogenesis or evaporative cooling to defend the core temperature. Thermoneutral zone is not a fixed entity; it differs across species and changes with their life history.
Thermal comfort zone: the range of ambient temperatures, and associated humidity and air movement, at which clothed humans express satisfaction with their thermal environment.
Acknowledgments
We thank members of the Chawla laboratory for discussions, and A. Loh for comments on the manuscript. Work in authors’ laboratories was supported by NIH grants DK094641, DK101064, and P30DK098722 (A.C.), and K.G. has been supported by a postdoctoral fellowship from the Hillblom Foundation.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Lovegrove BG. The evolution of endothermy in Cenozoic mammals: a plesiomorphic-apomorphic continuum. Biol Rev Camb Philos Soc. 2012;87:128–162. doi: 10.1111/j.1469-185X.2011.00188.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruben J. The evolution of endothermy in mammals and birds: from physiology to fossils. Annu Rev Physiol. 1995;57:69–95. doi: 10.1146/annurev.ph.57.030195.000441. [DOI] [PubMed] [Google Scholar]
- 3.Bennett AF, Ruben JA. Endothermy and activity in vertebrates. Science. 1979;206:649–654. doi: 10.1126/science.493968. [DOI] [PubMed] [Google Scholar]
- 4.Portner HO. Climate variability and the energetic pathways of evolution: the origin of endothermy in mammals and birds. Physiological and biochemical zoology : PBZ. 2004;77:959–981. doi: 10.1086/423742. [DOI] [PubMed] [Google Scholar]
- 5.Richards S. Temperature regulation. Wykeham Publications; 1973. [Google Scholar]
- 6.Wu CW, Storey KB. Life in the cold: links between mammalian hibernation and longevity. Biomol Concepts. 2016;7:41–52. doi: 10.1515/bmc-2015-0032. [DOI] [PubMed] [Google Scholar]
- 7.Bouma HR, et al. Induction of torpor: mimicking natural metabolic suppression for biomedical applications. Journal of cellular physiology. 2012;227:1285–1290. doi: 10.1002/jcp.22850. [DOI] [PubMed] [Google Scholar]
- 8.Maloney SK, Fuller A, Mitchell D, Gordon C, Overton JM. Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda) 2014;29:413–420. doi: 10.1152/physiol.00029.2014. [DOI] [PubMed] [Google Scholar]
- 9.Karp CL. Unstressing intemperate models: how cold stress undermines mouse modeling. The Journal of experimental medicine. 2012;209:1069–1074. doi: 10.1084/jem.20120988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon CJ. Thermal physiology of laboratory mice: Defining thermoneutrality. Journal of Thermal Biology. 2012;37:654–685. [Google Scholar]
- 11.Gordon CJ. Temperature regulation in laboratory rodents. Cambridge University Press; 1993. [Google Scholar]
- 12.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. The Journal of experimental biology. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 13.Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 14.David JM, Chatziioannou AF, Taschereau R, Wang H, Stout DB. The hidden cost of housing practices: using noninvasive imaging to quantify the metabolic demands of chronic cold stress of laboratory mice. Comp Med. 2013;63:386–391. [PMC free article] [PubMed] [Google Scholar]
- 15.David JM, Knowles S, Lamkin DM, Stout DB. Individually ventilated cages impose cold stress on laboratory mice: a source of systemic experimental variability. J Am Assoc Lab Anim Sci. 2013;52:738–744. [PMC free article] [PubMed] [Google Scholar]
- 16.Lodhi IJ, Semenkovich CF. Why we should put clothes on mice. Cell Metab. 2009;9:111–112. doi: 10.1016/j.cmet.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Hill RW, Muhich TE, Humphries MM. City-scale expansion of human thermoregulatory costs. PLoS One. 2013;8:e76238. doi: 10.1371/journal.pone.0076238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erikson H, Krog J, Andersen KL, Scholander PF. The critical temperature in naked man. Acta Physiol Scand. 1956;37:35–39. doi: 10.1111/j.1748-1716.1956.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 19.Scholander PF, Andersen KL, Krog J, Lorentzen FV, Steen J. Critical temperature in Lapps. J Appl Physiol. 1957;10:231–234. doi: 10.1152/jappl.1957.10.2.231. [DOI] [PubMed] [Google Scholar]
- 20.Scholander PF, Hammel HT, Andersen KL, Loyning Y. Metabolic acclimation to cold in man. J Appl Physiol. 1958;12:1–8. doi: 10.1152/jappl.1958.12.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Wilkerson JE, Raven PB, Horvath SM. Critical temperature of unacclimatized male Caucasians. J Appl Physiol. 1972;33:451–455. doi: 10.1152/jappl.1972.33.4.451. [DOI] [PubMed] [Google Scholar]
- 22.Qiu Y, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scholander PF, Hock R, Walters V, Irving L. Adaptation to cold in arctic and tropical mammals and birds in relation to body temperature, insulation, and basal metabolic rate. The Biological bulletin. 1950;99:259–271. doi: 10.2307/1538742. [DOI] [PubMed] [Google Scholar]
- 24.Sampath H, et al. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J Biol Chem. 2009;284:19961–19973. doi: 10.1074/jbc.M109.014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirata M, et al. Genetic defect in phospholipase Cdelta1 protects mice from obesity by regulating thermogenesis and adipogenesis. Diabetes. 2011;60:1926–1937. doi: 10.2337/db10-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura Y, et al. Phospholipase Cdelta1 is required for skin stem cell lineage commitment. The EMBO journal. 2003;22:2981–2991. doi: 10.1093/emboj/cdg30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narvaez CJ, Matthews D, Broun E, Chan M, Welsh J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology. 2009;150:651–661. doi: 10.1210/en.2008-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li YC, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall JE. Guyton and Hall Textbook of Medical Physiology. 12. Saunders; 2010. [Google Scholar]
- 30.Swoap SJ, et al. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. American journal of physiology Heart and circulatory physiology. 2008;294:H1581–1588. doi: 10.1152/ajpheart.01000.2007. [DOI] [PubMed] [Google Scholar]
- 31.Swoap SJ, Overton JM, Garber G. Effect of ambient temperature on cardiovascular parameters in rats and mice: a comparative approach. Am J Physiol Regul Integr Comp Physiol. 2004;287:R391–396. doi: 10.1152/ajpregu.00731.2003. [DOI] [PubMed] [Google Scholar]
- 32.Williams TD, Chambers JB, Henderson RP, Rashotte ME, Overton JM. Cardiovascular responses to caloric restriction and thermoneutrality in C57BL/6J mice. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1459–1467. doi: 10.1152/ajpregu.00612.2001. [DOI] [PubMed] [Google Scholar]
- 33.Kaiyala KJ, et al. Acutely decreased thermoregulatory energy expenditure or decreased activity energy expenditure both acutely reduce food intake in mice. PLoS One. 2012;7:e41473. doi: 10.1371/journal.pone.0041473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Claussnitzer M, et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. The New England journal of medicine. 2015;373:895–907. doi: 10.1056/NEJMoa1502214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smemo S, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507:371–375. doi: 10.1038/nature13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson PW, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 37.Lawes CM, Vander Hoorn S, Rodgers A International Society of, H. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 38.Kannel WB, Wolf PA. Framingham Study insights on the hazards of elevated blood pressure. JAMA. 2008;300:2545–2547. doi: 10.1001/jama.2008.759. [DOI] [PubMed] [Google Scholar]
- 39.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 40.Enerback S, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, et al. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest. 2003;111:399–407. doi: 10.1172/JCI15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 43.Castillo M, et al. Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes. 2011;60:1082–1089. doi: 10.2337/db10-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 45.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 46.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 47.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 51.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 54.Olefsky J, Glass C. Macrophages, Inflammation, and Insulin Resistance. Annu Rev Physiol. 2010;72:1–28. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 55.Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kraakman MJ, et al. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metab. 2015;21:403–416. doi: 10.1016/j.cmet.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Chen M, Liu B, Thompson CH, Wittert GA, Heilbronn LK. Acute Overfeeding Does Not Alter Liver or Adipose Tissue-Derived Cytokines in Healthy Humans. Ann Nutr Metab. 2016;69:165–170. doi: 10.1159/000452678. [DOI] [PubMed] [Google Scholar]
- 59.Tam CS, et al. Short-term overfeeding may induce peripheral insulin resistance without altering subcutaneous adipose tissue macrophages in humans. Diabetes. 2010;59:2164–2170. doi: 10.2337/db10-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee YS, et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60:2474–2483. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian XY, et al. Thermoneutral Housing Accelerates Metabolic Inflammation to Potentiate Atherosclerosis but Not Insulin Resistance. Cell Metab. 2016;23:165–178. doi: 10.1016/j.cmet.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest. 2017;127:74–82. doi: 10.1172/JCI88883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gordon SM, et al. A comparison of the mouse and human lipoproteome: suitability of the mouse model for studies of human lipoproteins. J Proteome Res. 2015;14:2686–2695. doi: 10.1021/acs.jproteome.5b00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ishikawa M, et al. Comparison of circulating lipid profiles between fasting humans and three animal species used in preclinical studies: mice, rats and rabbits. Lipids Health Dis. 2015;14:104. doi: 10.1186/s12944-015-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee YT, et al. Mouse models of atherosclerosis: a historical perspective and recent advances. Lipids Health Dis. 2017;16:12. doi: 10.1186/s12944-016-0402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ross R. Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 70.Giles DA, et al. Modulation of ambient temperature promotes inflammation and initiates atherosclerosis in wild type C57BL/6 mice. Mol Metab. 2016;5:1121–1130. doi: 10.1016/j.molmet.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Medzhitov R, Janeway C., Jr Innate immunity. The New England journal of medicine. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 72.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moragues V, Pinkerton H. Variation in Morbidity and Mortality of Murine Typhus Infection in Mice with Changes in the Environmental Temperature. The Journal of experimental medicine. 1944;79:41–43. doi: 10.1084/jem.79.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miraglia GJ, Berry LJ. Enhancement of Salmonellosis and Emergence of Secondary Infection in Mice Exposed to Cold. Journal of bacteriology. 1962;84:1173–1180. doi: 10.1128/jb.84.6.1173-1180.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Previte JJ, Berry LJ. The effect of environmental temperature on the host-parasite relationship in mice. The Journal of infectious diseases. 1962;110:201–209. doi: 10.1093/infdis/110.3.201. [DOI] [PubMed] [Google Scholar]
- 76.Underwood GE, Baker CA, Weed SD. Protective effect of elevated temperature on mice infected with Coe virus. J Immunol. 1966;96:1006–1012. [PubMed] [Google Scholar]
- 77.Amrein YU. Effects of environmental temperature on Trypanosoma cruzi infection in mice. The Journal of parasitology. 1967;53:1160. [PubMed] [Google Scholar]
- 78.Baetjer AM. Role of environmental temperature and humidity in susceptibility to disease. Archives of environmental health. 1968;16:565–570. doi: 10.1080/00039896.1968.10665104. [DOI] [PubMed] [Google Scholar]
- 79.Won WD, Ross H. Relationship of low temperature to mouse resistance to infection with Klebsiella pneumoniae. Aerospace medicine. 1971;42:642–645. [PubMed] [Google Scholar]
- 80.Bell JF, Moore GJ. Effects of high ambient temperature on various stages of rabies virus infection in mice. Infection and immunity. 1974;10:510–515. doi: 10.1128/iai.10.3.510-515.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chevalier C, et al. Gut Microbiota Orchestrates Energy Homeostasis during Cold. Cell. 2015;163:1360–1374. doi: 10.1016/j.cell.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 82.Seok J, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2015;112:1167–1172. doi: 10.1073/pnas.1401965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1244–1252. doi: 10.1152/ajpregu.00370.2005. [DOI] [PubMed] [Google Scholar]
- 85.Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15:335–349. doi: 10.1038/nri3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kokolus KM, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci U S A. 2013;110:20176–20181. doi: 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eng JW, et al. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through beta2-adrenergic receptor activation. Nature communications. 2015;6:6426. doi: 10.1038/ncomms7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Longo VD, Panda S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016;23:1048–1059. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science. 2016;354:994–999. doi: 10.1126/science.aah4965. [DOI] [PubMed] [Google Scholar]


