Abstract
BACKGROUND
Neoadjuvant locoregional therapies (LRT) have been widely used to reduce tumor burden or downstage hepatocellular carcinoma (HCC) prior to orthotopic liver transplantation (OLT). We examined the impact of LRT response on HCC recurrence after OLT.
STUDY DESIGN
We performed a retrospective study of 384 HCC patients treated by OLT. Tumor necrosis was determined by pathologic evaluation. The vascular and lymphatic vessels were localized by immunofluorescence (IF) staining in formalin-fixed, paraffin-embedded tissue; expressions of VEGFR-2 and VEGFR-3 were analyzed by Western Blot. Plasma VEGF-A and VEGF-C levels of a consecutive cohort of 171 HCC patients were detected by ELISA.
RESULTS
Of the 384 HCC patients, 268 had undergone pre-transplant neoadjuvant LRT. Patients with no tumor necrosis (n=58, 5.2% recurrence) or complete tumor necrosis (n=70, 6.1% recurrence) had significantly lower 5-year recurrence rates than those with partial tumor necrosis (n=140, 22.6% recurrence, p<0.001). Lymphatic metastases were significantly more numerous in patients with partial tumor necrosis than those without tumor necrosis after OLT (p<0.001). With immunofluorescent of peritumor zone, lymphatics were visualized around partially-necrotic tumors, but not around tumors without necrosis. Plasma levels of VEGF-A and VEGF-C were significantly elevated in patients with evidence of tumor necrosis (n=102) compared to those without necrosis (n=69; p<0.001). By Western blot, VEGFR-2 and VEGFR-3 expression in the peritumoral tissue associated with partially necrotic tumors was significantly higher than in peritumoral tissue of no necrosis tumors (n=3/group, p<0.020 and 0.006, respectively).
CONCLUSION
LRT-induced or spontaneous partially necrotic HCC were associated with an increased risk of lymphatic metastases compared with tumors with no or complete tumor necrosis. Anti-lymphangiogenic agents with neoadjuvant LRT may decrease the pattern of lymphatic metastasis after OLT.
INTRODUCTION
The incidence of hepatocellular carcinoma (HCC) in the United States is rapidly increasing, from approximately 10,000 cases per year in the 1980s to a projected incidence of 34,000 cases per year by 2019.1 Orthotopic liver transplantation (OLT) is the optimal treatment option for HCC in cirrhosis because of the removal of the “field defect” of the cirrhotic liver, and establishment of normal hepatic synthetic function.2 However, only patients presenting with early-stage HCC and cirrhosis are currently recognized as appropriate candidates for OLT.2 Organ allocation by the United Network for Organ Sharing (UNOS) for HCC is based on the Milan criteria under the model for end-stage liver disease (MELD); since 2002, only patients with stage II tumors receive automatic exception points. Selected by these criteria, liver transplant results for HCC are similar to those of chronic liver disease without malignancy. Prolonged waiting times due to the shortage of donor organs may increase the risk of disease progression.3 Neoadjuvant locoregional therapies (LRT) such as transarterial chemoembolization (TACE), transarterial radioembolization (TARE), and radiofrequency ablation (RFA) have been used to prevent tumor progression for early-stage patients or to down-stage potential candidates.3,4
The effect of LRT on the outcome of transplantation for HCC has been an area of active investigation. The employment of preoperative LRT using either TACE, TARE, RFA, or some combination has been variable among transplant centers. Several studies have shown remarkable antitumor activity with TACE, but no long-term oncologic benefits were observed.2,5,6 Therefore in principle, it was recognized that downstaging could have served as an additional selection tool for tumors with more favorable biology and a better prognosis, which can be assessed by response to LRT.7,8 It was also demonstrated that continued use of TACE while on the wait list for OLT should be considered as long as the patient and the lesions were suitable for retreatment; the wait time before OLT appeared to be related to survival and recurrence after OLT, which could reflect the presence of more aggressive tumor biology in patients prematurely undergoing transplantation.9 However, some randomized controlled trials demonstrated that a small portion of selected patients benefited from TACE.10,11 TACE has been reported to be more effective in terms of histologic tumor necrosis when performed for tumors between 3 and 5 cm in diameter12; both single versus multiple tumor nodules and tumor nodules larger than 3 cm versus smaller ones were more likely to show complete or partial necrosis versus no necrosis.9 Theoretically, the necrosis and blood flow reduction resulting from LRT could limit the dissemination of tumor cells. Thus, LRT may provide a beneficial effect beyond prevention of tumor progression. Some have suggested that there are upper limits in tumor size and number beyond which downstaging was not likely to be successful and the outcome might be significantly worse as well.13 Our previous study and reports from other groups have shown that long-term outcomes of OLT in patients downstaged to meet Milan criteria for the purpose of transplantation were similar to those of stage II recipients.3 Intention-to-treat analysis demonstrated that excellent long-term prognosis after successful downstaging of HCC to within T2 criteria was associated with a low risk of HCC recurrence and excellent post-transplant survival, comparable to those meeting T2 criteria without downstaging. However, the dropout rate for downstaging was significantly higher than that of the T2 group.13 To examine the impact of neoadjuvant LRT on HCC recurrence after liver transplantation at our institution, we performed a retrospective study on patients who underwent liver transplantation for HCC.
METHODS
Study Conduct
The research protocol was approved by the Institutional Review Board of Washington University School of Medicine, St. Louis and used a prospectively maintained clinical database of patients after liver transplantation. Data consisting of demographics, clinical characteristics, locoregional therapies prior liver transplantation, pathology findings, tumor status, tumor recurrence, and outcomes were obtained for all recipients of liver transplantation for HCC from January 1, 1989 to December 31, 2014.
Local Regional Therapies
LRT was not routinely performed at our institution before 1998. Since then LRT, predominately TACE has been used in patients with stage II HCC as diagnosed on cross-sectional imaging. LRT was performed by experienced interventional radiologists in a standardized fashion utilizing either a femoral or brachial approach. Patients with significant hepatic dysfunction were not considered appropriate candidates for TACE. Superior mesenteric angiography was performed to evaluate portal vein status and evaluate for the presence of anatomic variation. Celiac angiography was performed with a selection of the tumor-bearing artery. A mixture of chemotherapeutic agent (mitomycin and cisplatin) and ethiodized oil, followed by embolization with absorbable gelatin sponge was used most commonly for TACE. MRI follow-up was performed 4–6 weeks after TACE to assess for treatment response. While on the wait list, the patients were followed every three months with interval imaging studies (usually with MRI or CT) to assess for new or progressive disease. A bone scan was also performed to rule out evidence of metastatic disease. If a complete response (no remaining tumor enhancement) was observed, interval surveillance was scheduled for three months. If there was evidence of residual disease, repeat TACE was performed. For tumors outside of Milan criteria, the patient received repeated TACE in an attempt to downstage within the Milan criteria. For neoadjuvant LRT, a small number of patients were treated with radiofrequency ablation (RFA), Y90, percutaneous ethanol ablation (PEA), or a combination of the above procedures.
Orthotopic Liver Transplantation and Follow-Up of HCC Recurrence
All transplant candidates were evaluated by a multidisciplinary transplantation team, and only patients with stage II tumors and these who had been down-staged to be stage II tumors received regional review board approval for liver transplantation after 2005. The standard piggy-back technique without the use of venovenous bypass has been used since 1995 at our center. The standard immunosuppression is a 3-drug regimen with tacrolimus, mycophenolic acid, and a short steroid taper. HCC was confirmed in the 81.5% patients by liver explant pathology; in the remainder, there was evidence of prior but ablated tumor (18.5%). Additionally, the number and sizes of all tumors, as well as extent of tumor necrosis were recorded by a pathologist. Tumor necrosis was recorded as no necrosis, partial necrosis or complete necrosis for this study. Incidental HCC was defined as the diagnosis of HCC not detected on preoperative imaging. Post-transplantation surveillance every 6 months for 5 years with alpha-fetoprotein and MRI was the routine. The recurrence of HCC was diagnosed by either biopsy or radiological examinations. The size, number, and location of recurrent HCCs were recorded.
H and E and Immunofluorescence Staining
Formalin-fixed sections were taken from 1–3mm transversely cut gross explants; special attention was given by prosectors to the location of image-detected, and LRT-tumors. Tumors that showed any evidence grossly of necrosis were submitted entirely to best analyze percent necrosis, otherwise, only representative sections of tumor were submitted to document a diagnosis. As well, sections of the right and left lobes and hilar structures were taken per protocol for explant liver hepatectomy. FFPE 5 µm sections were routinely processed for H and E and special stains analyses, per explant protocol. Findings were reported and recorded in the liver transplant database, from which information was drawn for this study. In this study, patients with pathological evidence of tumor necrosis and viable tumor were defined with partial tumor necrosis, including patients with single or multiple tumors.
For immunofluorescence staining, blocks were chosen that included tumor and peritumoral liver tissue for patients who had undergone LRT and transplantation. Antigen retrieval was in Diva Decloaker buffer (DV2004MX) for 45 minutes, blocked with goat serum, and antibodies optimized with appropriate controls. Subsequently, the slides were blocked with goat serum and were subjected to the following primary antibodies overnight at 4°C: rabbit anti-CD31 (CST, 3528S) for all hepatic blood vessels, and mouse anti- lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) (Abcam, Ab10278) for lymphatic vessels. After incubation with secondary antibodies, the slides were counterstained with 4, 6-diamidino-2-phenylindole (DAPI) and analyzed under the fluorescence microscope. Negative controls were prepared by replacing the primary antibodies with PBS. The slides were assessed under a light and fluorescence microscope equipped with an internal digital camera (Zeiss Observer.Z1). Results were recorded as positive or negative of anti-LYVE/CD31 double staining (20×).
Enzyme-linked immunosorbent assay (ELISA) and Western Blot Assays
To study the expression of VEGF-A and VEGF-C in HCC patients with or without tumor necrosis, the ideal way is to use blood samples from the same cohort of transplant patients. However, in many cases blood samples were not available due to the time frame of this study. Thus, we used blood samples from another consecutive cohort of HCC patients (n=171) in our center. These samples were drawn from 1 week to 3 months after LRT. The ELISA assays were performed according to the manufacturer’s protocol to detect VEGF-A (Thermo Fisher, Cat. KHG0112) and VEGF-C (Thermo Fisher, Cat. EHVEGFC) levels in plasma. Finally, whole protein was extracted from peritumor zone of the FFPE samples using the Qproteome FFPE Tissue Kit (Cat. 37623) according to the manufacturer’s instructions. Protein concentrations of supernatants were measured using the Qubit protein assay kit (Thermo Fisher, Ref. Q33211). Prepared homogenates were distributed in aliquots and stored at −80°C until use. For each sample, 50 micrograms of lysate of the total protein were loaded in a well of 4–12% Nu-PAGE Bis-Tris (Invitrogen) gel and subjected to 2 hours of electrophoresis at 80V. Then, proteins on the gels were transferred to membranes in a semidry apparatus at 30 V for 1.5 hours. The membranes were blocked in 5% milk, and incubated with rabbit anti-VEGFR-2 mAb (CST, Cat. 2479S) and rabbit anti-VEGFR-3 mAb (CST, Cat. 3408S) overnight at 4°C. The secondary antibodies were Horseradish peroxidase conjugated goat anti-rabbit (CST, Cat. 7074S) and goat anti-mouse immunoglobulins (CST, Cat. 7076S) diluted at 1:1000. Membranes were developed with an ECL Kit (CST, Cat. 6883S) at different time points.
Statistical Analysis
Results were expressed as mean ± SD or median with range. One-way ANOVA with post hoc testing using Tukey HSD was used to compare continuous variables. Overall survival and recurrence-free survival were analyzed using the Kaplan-Meier estimator and were statistically determined using the log-rank test. Categorical variables were compared using Fisher’s exact test. The Cox proportional hazard was applied to ascertain variables that had significant correlations with survival and tumor recurrence. For all comparisons, statistical significance was defined as a two-tailed p-value less than 0.05.
RESULTS
Patients
During the study period, 384 transplanted patients were identified with radiographically or pathologically documented HCC; 268 had undergone LRT pre-transplant, and 116 had not. The demographics of age, race, etiology, body mass index (BMI), alpha-fetoprotein (AFP) levels before transplantation, type of neoadjuvant pretreatments, the model for end-stage liver disease (MELD) scores, and tumor details in the explanted livers were extracted from the database. The mean recipient age was 57.8±7.9 years; 79.7% (n=306) of patients were male, 82% (n=315) were Caucasians, 9.9% (n=39) were African Americans, and the remaining 31 were of varied races and ethnicities. All patients were cirrhotic. The primary etiology of HCC was hepatitis C (63.8%, n=245), alcohol (9.4%, n=36), non-alcoholic steatohepatitis (14.6%, n=56), hepatitis B (6.0%, n=23), and others (6.2%, n=24). The mean BMI was 28.5±5.1, and the mean AFP level prior to transplantation was 101.1±539.1 ng/mL (normal<9.9ng/mL). The mean MELD score prior to transplantation was 15.6±8.3. Two hundred sixty-eight patients received neoadjuvant LRT: TACE, 66.1% (n=254) ; RFA: 2.3% (n=9), Y90, 1.0% patients (n=4) and PEA, 0.3% patients (n=1). One hundred sixteen patients did not receive neoadjuvant LRT for a number of reasons: 4.2% (n=16) were transplanted prior to 1998 when LRT use was not common; 18.8% (n=72) HCC was found incidentally in the explanted livers and in 6.5% of patients (n=28), were not deemed candidate for LRT due to severe compromise in hepatic function.
By pathologic examination of all explanted livers, the mean tumor number found was 1.9±1.4 (range 1 – 15) with a mean tumor diameter of 2.4±1.5cm (range 0.2cm – 15.8cm). 91.9% of recipients (n=353) were within Milan criteria by pathologic evaluation. 4.2% of hepatectomy specimens (n=16) showed macrovascular invasion by tumor (noted on gross examination) and 12.5% (n=48) had microscopic finding of lymphovascular space invasion (LVSI). 27.9% of the tumors (n=107) were well differentiated, 39.1% (n=150) were moderately differentiated, and 7.3% (n=28) were poorly differentiated. In 25.8% (n=99) of patients, tumor differentiation was not evaluable mainly due to the lack of viable tumor. The overall average percent of tumor necrosis in all patients was 47.0%±42.6 (range 0–100%). 41.1% of patients (n=158) had no tumor necrosis, 40.4% patients (n=155) had partial tumor necrosis, and 18.5% of patients (n=71) had complete tumor necrosis (Table 1). The average tumor necrosis in the explants with neoadjuvant LRT was 56.2%±40.9% (range 0–100%), while average tumor necrosis in explants without neoadjuvant LRT was 5.4%±16.1% (range 0–75%).
Table 1.
Demographics of Hepatocellular Carcinoma Patients Treated by Orthotopic Liver Transplantation.
| Variable | Hepatocellular carcinoma patients treated with orthotopic liver transplantation, (N=384) |
|---|---|
| Age, y, mean ± SD | 57.8±7.9 |
| Male sex, n (%) | 306 (79.7) |
| Race, n (%) | |
| Caucasian | 315 (82.0) |
| African American | 38 (9.9) |
| Other | 31 (8.1) |
| Etiology, n (%) | |
| Hepatitis C | 245 (63.8) |
| Alcoholic | 36 (9.4) |
| Nonalcoholic steatohepatitis | 56 (14.6) |
| Hepatitis B | 23 (6.0) |
| Other | 24 (6.2) |
| BMI, kg/m2, mean ± SD | 28.5±5.1 |
| Alpha-fetoprotein before orthotopic liver transplantation,ng/mL, mean ± SD | 101.1±539.1 |
| Neoadjuvant locoregionaltherapy, n (%) | |
| Transarterial chemoembolization | 254 (66.1) |
| Radiofrequency ablation | 9 (2.3) |
| Y90 | 4 (1.0) |
| Palmitoylethanolamide | 1 (0.3) |
| Non-neoadjuvant locoregionaltherapy | 116 (30.3) |
| MELD score | 15.6±8.3 |
| Explanted tumors | |
| No. of tumors, mean ± SD | 1.9±1.4 |
| Nodule size, cm, mean ± SD | 2.4±1.5 |
| Milan criteria within, n (%) | 353 (91.9) |
| Macrovascular invasion, n (%) | 16 (4.2) |
| Differentiation, n (%) | |
| Not applicable | 99 (25.8) |
| Well | 107 (27.9) |
| Moderate | 150 (39.1) |
| Poor | 28 (7.3) |
| Microscopic lymphovascularspace invasion, n (%) | 49 (12.8) |
| Necrosis, n (%) | |
| Non-necrosis | 158 (41.1) |
| Partial necrosis | 155 (40.4) |
| Complete necrosis | 71 (18.5) |
MELD, Model for End-Stage Liver Disease.
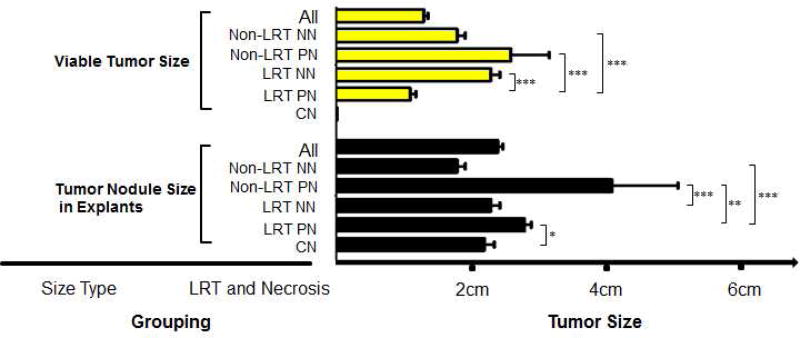
To evaluate the response to LRT with transplant outcomes, we stratified tumor treatment and response into the following five subgroups: non-LRT and non-necrosis (NN), non-LRT and partial necrosis (PN), LRT and NN, LRT and PN, and complete necrosis (CN, all had LRT except one case). The tumor sizes measured by pathological examination in each subgroup are summarized in Figure 1. The figure shows a significant difference in the viable tumor size measured pathologically among the five subgroups (p<0.001); the viable tumor size of the LRT PN subgroup was significantly smaller than that of the other subgroups (all p<0.001, respectively). The tumor size of non-LRT PN subgroup was larger than all other subgroups (p≤0.006, respectively). The non-LRT PN subgroup had a significantly higher HCC recurrence rate than that in the LRT PN subgroup (p=0.013).
Figure 1.
Viable tumor size and nodule size in the explants. The significance of difference was examined by post-hoc test (Tukey HSD), and p<0.05 was considered as significant (*p<0.05; **p≤0.01; ***p ≤0.001). NN, non-necrosis; PN, partial necrosis; CN, complete necrosis.
Risk Factors of Tumor Recurrence after OLT
No significant association was detected between tumor recurrence and age, sex, race and ethnicity, BMI, AFP, disease etiology, LRT number, or MELD score by univariate or multivariate analyses (Table 2). Multifocal HCCs with more than three tumors were associated with higher recurrence risk (p=0.031, Hazard ratio (HR) =3.26). Univariate analysis showed that tumor size larger than 3cm was significantly associated with tumor recurrence, but this effect was marginally significant in multivariate analysis (p<0.001, HR=3.15 and 0.043, HR=1.99, respectively). Patients with macrovascular invasion and LVSI had higher rates of tumor recurrence after OLT (p=0.04, HR=4.56 and p=0.032, HR=2.49, respectively).
Table 2.
Univariate and Multivariate Analyses of Risk Factors Associated with Hepatocellular Carcinoma Recurrence after Orthotopic Liver Transplantation
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value |
HR | 95% CI | p Value |
|
| Age, y, ≤58 vs >58 | 1.08 | 0.73 to 1.56 | 0.745 | |||
| Sex, male vs female | 1.48 | 0.89 to 2.50 | 0.129 | |||
| Race, non-Caucasian vs Caucasian | 0.89 | 0.46 to 1.69 | 0.703 | |||
| BMI, kg/m2, ≤28 vs >28 | 0.86 | 0.59 to 1.25 | 0.433 | |||
| AFP before OLT, ng/mL (≤500 vs >500) | 1.46 | 0.67 to 3.12 | 0.342 | |||
| Etiology, non-viral vs viral | 1.29 | 0.84 to 1.98 | 0.255 | |||
| LRT, no vs yes | 1.02 | 0.69 to 1.49 | 0.933 | |||
| LRT, single vs multiple | 0.84 | 0.48 to 1.47 | 0.560 | |||
| MELD score, ≤20 vs >20 | 1.45 | 0.88 to 2.38 | 0.142 | |||
| Explanted tumor | ||||||
| No. of tumors, ≤3 vs >3 | 5.94 | 2.44 to 14.4 | 0.001 | 3.26 | 1.28 to 8.27 | 0.031 |
| Tumor size, ≤3 cm vs >3 cm | 3.15 | 1.56 to 6.37 | 0.001 | 1.99 | 0.96 to 4.16 | 0.043 |
| Macrovascular invasion, no vs yes | 7.95 | 3.24 to 19.4 | 0.006 | 4.56 | 1.64 to 12.7 | 0.004 |
| Microscopic LVSI, no vs yes | 3.64 | 1.72 to 7.68 | 0.001 | 2.49 | 1.08 to 5.78 | 0.032 |
| Non-partial necrosis vs partialnecrosis | 5.21 | 2.41 to 11.3 | 0.001 | 4.79 | 2.19 to 10.5 | 0.001 |
Cox regression model, p<0.05 was considered significant.
AFP, alpha-fetoprotein; HR, hazard ratio; LRT, locoregional therapy; MELD, Model for End-Stage Liver Disease; OLT, orthotopic liver transplantation
Recurrence Free Survival Stratified by LRT and Tumor Necrosis in Explants
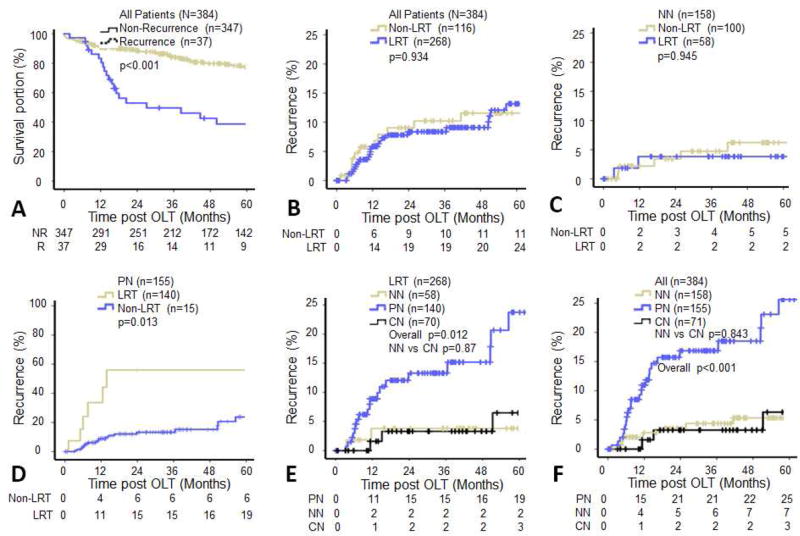
The 1-, 3-, and 5-year survival rates of all HCC transplant recipients were 90.1%, 80.7%, and 73.4%; the 1-, 3-, and 5-year recurrence rates were 5.6%, 8.6%, and 11.6%, respectively. The overall survival of patients with tumor recurrence was significantly lower than that of patients without recurrence (p<0.001, Figure 2A). Log-rank analysis failed to demonstrate a significant difference in recurrence rates between the LRT and non-LRT groups (p=0.934, Figure 2B), and between non-LRT non-necrosis (NN) and LRT NN groups (p=0.945, Figure 2C). However, the recurrence rates of the non-LRT partial necrosis (PN) group was remarkably higher than that of the LRT PN group (p=0.013, Figure 2D). Furthermore, as shown in Figure 2E among the LRT subgroups, the recurrence rate of the PN group was significantly higher than that of the NN and complete necrosis (CN) subgroups (p=0.012). As shown in Figure 2F, patients with non-necrosis (5.2% recurrence) and complete tumor necrosis (6.1% recurrence) had significantly lower 5-year recurrence rates than those with partial necrosis (22.6% recurrence, p<0.001).
Figure 2.
Kaplan-Meier analysis of hepatocellular carcinoma (HCC) recurrence after orthotopic liver transplantation. (A) The survival rate was significantly decreased in patients with HCC recurrence. (B–F) Recurrence risks were stratified by locoregional therapies and tumor necrosis. Log Rank Test, p<0.05 was considered as significant. NN, non-necrosis; PN, partial necrosis; CN, complete necrosis.
Tumor Necrosis and HCC Recurrence Pattern after OLT
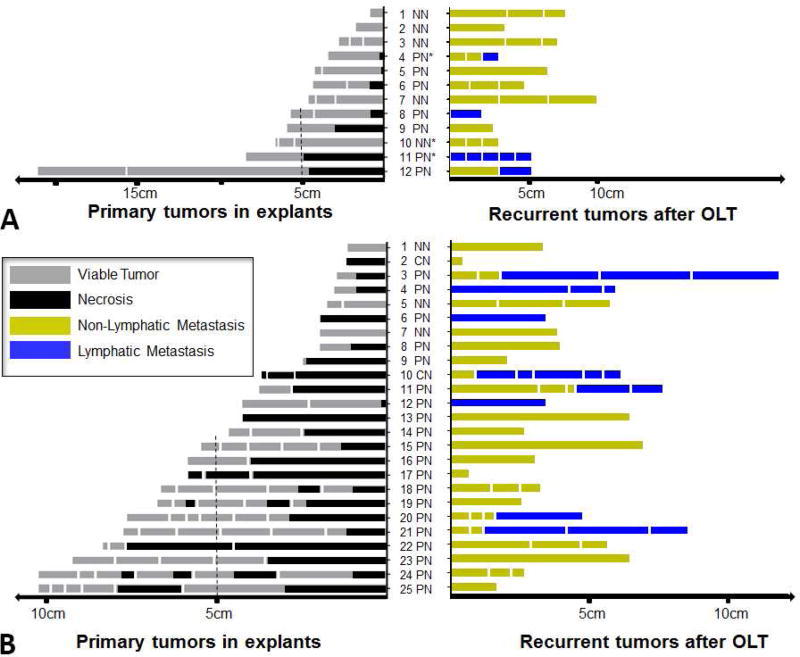
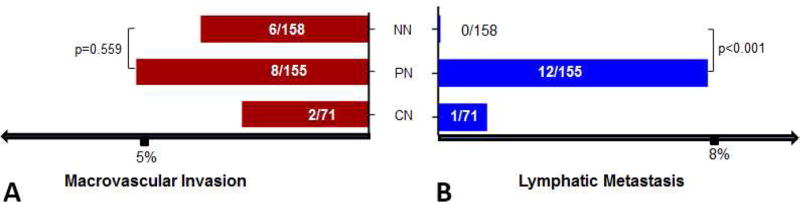
To investigate the association of tumor necrosis in the explants and HCC recurrence pattern after OLT, we examined the tumor number and size, necrosis percentage, and neoadjuvant LRT as well as the number, size, and type of recurrent tumors (n=37). When the tumors were within Milan criteria, the number of recurrent lymph node metastases were significantly higher in the LRT group as compared to those not receiving neoadjuvant LRT (p=0.047, 1/17 versus 15/31). Interestingly as shown in Figure 3A and 3B, we noted that all tumor recurrence with lymph node metastases had a certain degree of tumor necrosis as seen in the explants (12/12), whereas patients without any tumor necrosis had no lymph node metastases (0/8). The rate of lymph node metastasis in these patients with partial tumor necrosis was significantly higher than those without tumor necrosis (p<0.001, Figure 4).
Figure 3.
Tumors and locoregional therapy (LRT) response of recurrent patients. (A) Non-LRT. (B) LRT. The left panels show the tumor number, tumor size, and proportion of tumor necrosis on explants; the right panels present the corresponding tumor number, tumor size, and type of recurrence after orthotopic liver transplantation, without or with neoadjuvant LRT. OLT, orthotopic liver transplantation; NN, non-necrosis; PN, partial necrosis; CN, complete necrosis.
Figure 4.
(A) No significant difference of macrovesicular invasion between NN and PN subgroup. (B) There was significant increase of lymphatic metastasis in PN than that in NN subgroup (p<0.001). NN, non-necrosis; PN, partial necrosis; CN, complete necrosis.
VEGF and its receptors were upregulated in HCC patients with tumor necrosis
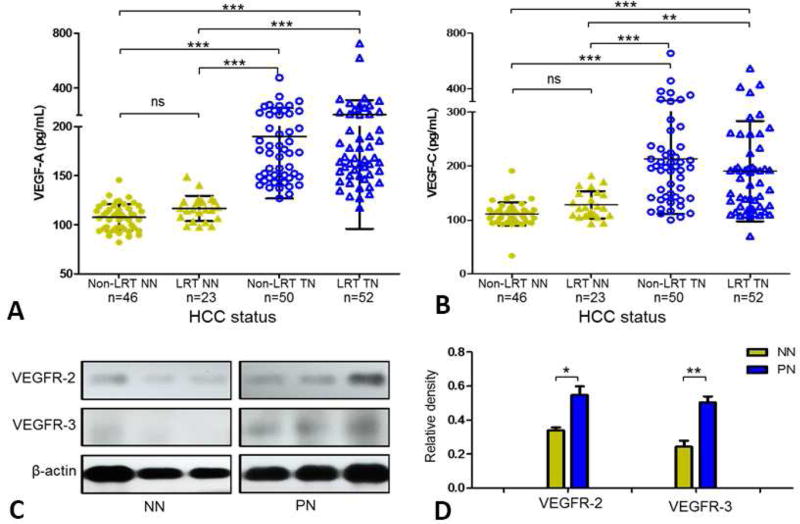
We measured the plasma levels of growth factors of VEGF-A and VEGF-C among a consecutive cohort of 171 HCC patients and the blood samples of patients with LRT were drawn from week 1 to month 3 post-LRT. The patients were grouped into four subgroups according to the status of tumor necrosis and LRT treatment. As shown in Figure 5A and 5B, the plasma levels of VEGF-A and VEGF-C in patients with tumor necrosis were significantly higher than that in those without tumor necrosis, regardless of whether the necrosis was LRT-induced or spontaneous (p<0.001 or p<0.005, respectively). Figure 5C presents the relative expression of VEGFR-2 and VEGFR-3 in the peritumor zone of FFPE tissue from the study cohort of HCC patients, and image analysis (Figure 5D) indicated that the VEGF receptors were upregulated in the peritumor zone of HCC patients with tumor necrosis (p=0.020 and 0.006, respectively).
Figure 5.
(A and B) Plasma vascular endothelial growth factor (VEGF)-A and VEGF-C levels of hepatocellular carcinoma patients (N=171) with or without tumor necrosis (Post Hoc-Tukey test, p<0.05 was considered as significant). (C) Representative Western blot and (D) densitometry (normalized by β-actin) analysis show the expression of vascular endothelial growth factor receptor (VEGFR)-2 and VEGFR-3 in peritumor formalin-fixed, paraffin-embedded tissue (Student’s test, p<0.05 was considered significant). HCC, hepatocellular carcinoma; NN, non-necrosis; LRT, locoregional therapy; PN, partial necrosis; TN, tumor necrosis; NS, not significant.
Localization of lymphatic and vascular vessels in HCC
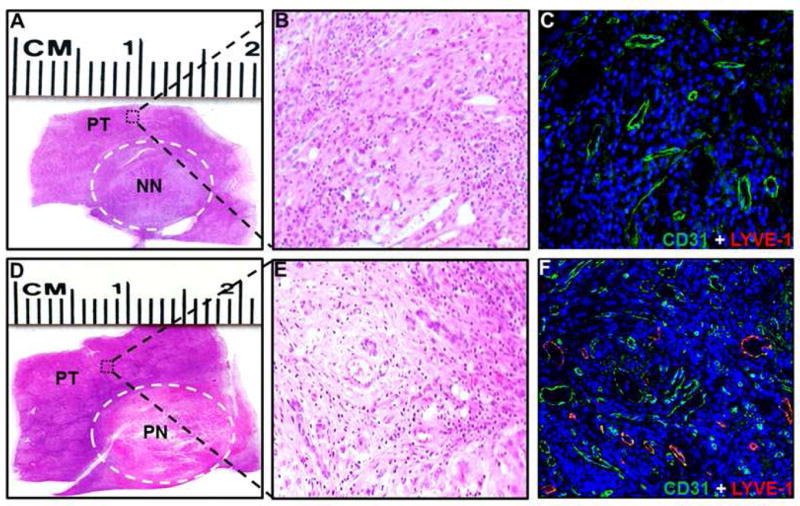
To visualize the lymphatic vessels in the peritumor area from cases with or without necrosis, we performed histopathologic and molecular characterizations of representative explanted HCCs. As shown in Figure 6, H and E staining was used to differentiate the tumor and peritumor zone as well as the necrotic area of the tumors (6A, 6B, 6D, and 6E); Immunofluorescence staining for markers of blood vessels (CD31) and lymphatic vessels (LYVE-1) revealed a higher density of lymphatic vessels in peritumor area of HCC patients with necrosis either after treatment with LRT or spontaneously necrosed than that in the patients without tumor necrosis (6C and 6F).
Figure 6.
Representative sections of hepatocellular carcinoma with (A) non-necrosis and (D) partial necrosis. (A and D) Microscopic figures (20×, hematoxylin and eosin stained) of peritumor areas (B) with or (E) without tumor necrosis. (C and F) Double Immunofluorescence staining of the sections for the blood vessels (CD31) and lymphatic vessels (LYVE-1). As illustrated, there are fewer CD31 and LYVE-1 structures in the peritumoral tissue adjacent to tumor without necrosis than in tissue from tumor with necrosis. NN, non-necrosis; PN, partial necrosis; PT, peritumor.
DISCUSSION
Liver transplantation in properly selected patients achieves very good outcomes for patients with hepatocellular carcinoma (HCC) with recurrence rates of 10–20%. Patients with down-staged tumors by LRT have comparable prognosis3,13, although it is controversial whether this is just due to better patient selection. Despite the ability of LRT to induce tumor necrosis, we observed that this was not necessarily correlated with a survival benefit. We therefore examined various clinicopathologic factors such as the extent of tumor necrosis in the explants and the pattern of tumor recurrence associated with LRT-treated tumors. Over a 26-year period at our institution, the overall recurrence rate for HCC after liver transplantation 11.6%. Univariate and multivariate analyses continued to emphasize the importance of tumor size, tumor number, macrovasculature invasion, and partial tumor necrosis. All of these have been documented in the literature.2, 14, 15 Stratified by LRT and tumor necrosis, we found that the recurrence rate in patients with complete tumor necrosis was around 5%-similar to that of stage I HCC patients. The partial necrosis subgroup demonstrated a relatively higher recurrence rate of 22%, although the size of viable tumors was significantly smaller than that of the non-necrosis subgroup, suggesting a possible link between tumor partial necrosis and recurrence after liver transplantation. Previously, Ravaioli et al.15 reported that partial tumor necrosis was an independent risk factor for tumor recurrence after OLT, and Terzi et al.9 and Schaudt et al.16 did not find significant decreases in recurrence after histopathologically favorable responses to TACE. Biologically, one would expect a continuum of effect between no-necrosis, partial necrosis and complete necrosis. A possible explanation to the paradoxical finding in our study is that the upregulation of growth factors during necrosis could facilitate progression of the remnant tumor cells in the partial necrosis subgroup. In contrast, the patients with non-necrosis had less level of growth factors to promote tumor progression, while patients with complete necrosis may have had elevated growth factors, but no viable tumor to progress. The dominant pattern of HCC metastasis is considered to primarily occur through hematogenous routes2,17, while the lymphatic system is involved in advanced stages.18–20 To explore the association between tumor necrosis and recurrence after liver transplantation, we examined various clinical variables such as pre-treatment status, explant tumor size and number, lymph node metastases, and the sizes of recurrent and metastatic lesions. Unexpectedly, we observed that a significant number of recurrent tumors which had lymph node metastases were tumors within Milan criteria that received neoadjuvant LRT in the initial native liver. Furthermore, all patients who developed tumor recurrence with lymph node metastases had some degree of tumor necrosis in the explanted liver specimen. Lymph node metastases appeared to be associated with tumor necrosis regardless of the cause of necrosis, whether spontaneous in larger tumors or induced by LRT.
We were also seeking biological mechanisms to provide an explanation for these findings. HCC derives approximately 80% to 85% of its blood supply from the hepatic artery while normal hepatic parenchyma receives 60% of blood supply from the portal vein, and 40%from the hepatic artery.21 The development of more than 90% HCC necrosis upon pathological analysis was associated with avid lesion enhancement and the presence of a feeding vessel larger than 0.9 mm in diameter on the pre-TACE visceral angiogram.22 Vascular endothelial growth factor A (VEGF-A) production can be induced in cells that are hypoxic. When cells are deficient in oxygen, hypoxia-inducible factor (HIF) a transcription factor that stimulates the release of VEGF-A is produced.23 When TACE is not totally effective, it may induce a significant neoangiogenesis reaction, as suggested by an increase in VEGF and fibroblast growth factor (FGF) following treatment.24 The remnants of liver tissue may lead to recurrence of HCC due to an increase in proliferative activity after TACE.25 VEGF, FGF, microvascular density, and proliferative activity have all been demonstrated to be increased in the viable tumor after TACE as well.26 Therefore the benefits and risks of LRT have to be cautiously weighed since on the one hand it can significantly disrupt tumor vasculature, on the other hand it can induce numerous factors that promote neoangiogenesis and tumor metastasis. However, no significant difference of macrovesicular invasion was found between the patients with or without tumor necrosis in the studies of Ravaioli et al. and ours, suggesting there may be some mechanisms beyond neoangiogenesis-related tumor spread. Ravaioli et al. found that a lower expression of the adhesion molecule E-cadherin in the cases with partial necrosis could lead tumor cells to be less attached to the surrounding extracellular matrix and thus be easily dislodged into the blood stream.15 However, this hypothesis may not fully explain our findings of the increased lymph node metastases in the partial necrosis subgroup.
In the present study, we found the significant earlier lymphatic metastases in patients with neoadjuvant LRT may be one of the mechanisms accounting for the absence of long-term oncological benefit of LRT even if significant tumor necrosis has been achieved. In one report, Miyayama et al found that hepatic lymphatic vessels were directly visualized by serial computed tomography (CT) scan in about 3% of HCC patients (N=255) after TACE treatment.27 The authors speculated that this change was induced by physical force of embolic materials and communication between vascular and lymphatic system, however there was no lymphatic metastasis observed after 1 year follow-up leaving the clinical significance of these findings unknown.27 In our study, the average time of lymph node metastasis was 7.2 months after OLT suggesting different mechanisms of lymphatic metastasis other than physical force may be involved. Indeed, there is strong evidence in the literature that the proteolytic processing during necrosis can upregulate the expression of VEGF-C and VEGF-D28–33, both of which are strongly related to tumor-associated lymphangiogenesis and lymphatic metastasis.34,35
Lymphatic capillaries consist of a single thin layer of endothelial cells lacking tight junctions and the basement membrane of these vessels is discontinuous. Since they are not ensheathed by pericytes or smooth muscle cells, these features may facilitate the entry of cells into the lymphatic system.34 Moreover, the lymphaticovenous communication has been demonstrated under some pathological circumstances, for instance in cirrhosis.35 Today, it is recognized that the lymphatic vasculature has both passive and active roles in cancer metastasis.36 Intratumoral lymphatics and lymphangiogenesis have been detected in head and neck cancer, thyroid carcinoma, and melanoma, and may contribute to lymph node metastases.34, 37, 38 Peritumoral lymphatics located immediately adjacent to tumors or in the peritumoral stroma, which can be dilated or enlarged, are known to be associated with human tumors.39–41 Moreover, the lymphatic enlargement that occurs through lymphatic hyperplasia has been observed around primary tumors, which was thought to favor entry of tumor cells into the lymphatic vasculature.42, 43
Based on the above factors, anti-lymphangiogenesis could be a promising strategy to prevent tumor cell dissemination. One potential therapeutic candidate, Sorafenib, can abrogate tumor growth and VEGFR-1, VEGFR-2, VEGFR-3, and PDGFR-β via inhibiting molecular components of the Raf-MEK-ERK signaling pathway.44 However, previous studies have demonstrated conflicting results when Sorafenib had been combined with TACE to treat HCC and these differences could be due to the variable time of initiating the treatment45–47, indicating that further basic and translational clinical studies are required to explore this topic.
One obvious limitation of our study is the absence of randomized prospective studies investigating tumor recurrence in this setting. Also, the current and prior studies have taken place over an extended time interval. Additionally, given increased possibility of lymphatic metastasis in patients with tumor necrosis, the role of selective regional lymphadenectomy may be performed among the patients with radiological indication of possible tumor necrosis prior to OLT and could provide further information for the treatment of HCC patients after OLT. It is also difficult to determine association between change of VEGF levels and other biological characteristics such as tumor tolerance to hypoxia and the mixed response pattern of tumors to neoadjuvant LRT.
CONCLUSION
In summary, we found that patients with complete tumor necrosis after neoadjuvant LRT had very low HCC recurrence rates after OLT. LRT-induced and/or spontaneous partial necrosis of HCC was associated with an increased risk of lymphatic nodes metastases. Anti-lymphangiogenesis together with LRT prior to OLT may help confine tumor cells in the native, explanted liver.
Acknowledgments
We appreciate the contribution from Drs. Zhengyan Zhang, Jianluo Jia, Deepak K Nayak, and Gundumi A Upadhya. Thanks to the Digestive Diseases Research Core Centers (DDRCC) at Washington University for sharing equipment and facilities. Thanks to the trainees and attendings of the Liver/GI Pathology Service for adhering to the demanding explant protocol.
Support: This work was funded by the Barnes-Jewish Hospital Foundation Project Award, Transplant Research Support.
ABBREVIATIONS
- HCC
Hepatocellular Carcinoma
- LRT
Locoregional Therapies
- TACE
Transarterial Chemoembolization
- OLT
Orthotopic Liver Transplantation
- NN
Non-Necrosis
- PN
Partial necrosis
- CN
Complete Necrosis
- N
Number of Group
- n
Number of Subgroup
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at the Western Surgical Association 124th Scientific Session, Coronado, CA, November 2016.
AUTHOR CONTRIBUTIONS
Study conception and design: Xu, Lin, Chapman, Doyle, Fowler, Saad,
Acquisition of data: Vachharajani, Xu, Zhang, Upadhya, Nayak, Jia,
Analysis and interpretation of data: Xu, Chapman, Lin,
Drafting of manuscript: Xu, Wang, Banan
Critical revision: Chapman, Lin, Brunt,
References
- 1.Wong JB, McQuillan GM, McHutchison JG, et al. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health. 2000;90:1562–9. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 3.Chapman WC, Majella Doyle MB, Stuart JE, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617–25. doi: 10.1097/SLA.0b013e31818a07d4. [DOI] [PubMed] [Google Scholar]
- 4.Yao FY, Kinkhabwala M, LaBerge JM, et al. The impact of pre-operative loco-regional therapy on outcome after liver transplantation for hepatocellular carcinoma. Am J Transplant. 2005;5:795–804. doi: 10.1111/j.1600-6143.2005.00750.x. [DOI] [PubMed] [Google Scholar]
- 5.Figueras J, Jaurrieta E, Valls C, et al. Survival after liver transplantation in cirrhotic patients with and without hepatocellular carcinoma: a comparative study. Hepatology. 1997;25:1485–9. doi: 10.1002/hep.510250629. [DOI] [PubMed] [Google Scholar]
- 6.Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–701. doi: 10.1097/00000658-199712000-00006. discussion 701-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravaioli M, Grazi GL, Piscaglia F, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547–57. doi: 10.1111/j.1600-6143.2008.02409.x. [DOI] [PubMed] [Google Scholar]
- 8.Volk ML, Vijan S, Marrero JA. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant. 2008;8:839–46. doi: 10.1111/j.1600-6143.2007.02138.x. [DOI] [PubMed] [Google Scholar]
- 9.Terzi E, Ray Kim W, Sanchez W, et al. Impact of multiple transarterial chemoembolization treatments on hepatocellular carcinoma for patients awaiting liver transplantation. Liver Transpl. 2015;21:248–57. doi: 10.1002/lt.24041. [DOI] [PubMed] [Google Scholar]
- 10.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–88. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 11.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 12.Golfieri R, Cappelli A, Cucchetti A, et al. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology. 2011;53:1580–9. doi: 10.1002/hep.24246. [DOI] [PubMed] [Google Scholar]
- 13.Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: Long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61:1968–77. doi: 10.1002/hep.27752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 15.Ravaioli M, Grazi GL, Ercolani G, et al. Partial necrosis on hepatocellular carcinoma nodules facilitates tumor recurrence after liver transplantation. Transplantation. 2004;78:1780–6. doi: 10.1097/01.tp.0000145892.97114.ee. [DOI] [PubMed] [Google Scholar]
- 16.Schaudt A, Kriener S, Schwarz W, et al. Role of transarterial chemoembolization for hepatocellular carcinoma before liver transplantation with special consideration of tumor necrosis. Clin Transplant. 2009;23(Suppl 21):61–7. doi: 10.1111/j.1399-0012.2009.01111.x. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Bruix J, Fuster J, et al. Liver transplantation for small hepatocellular carcinoma: the tumor-node-metastasis classification does not have prognostic power. Hepatology. 1998;27:1572–7. doi: 10.1002/hep.510270616. [DOI] [PubMed] [Google Scholar]
- 18.Katyal S, Oliver JH, 3rd, Peterson MS, et al. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 19.Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781–7. doi: 10.1111/j.1440-1746.2005.03919.x. [DOI] [PubMed] [Google Scholar]
- 20.Uka K, Aikata H, Takaki S, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414–20. doi: 10.3748/wjg.v13.i3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose DM, Chapman WC, Brockenbrough AT, et al. Transcatheter arterial chemoembolization as primary treatment for hepatocellular carcinoma. Am J Surg. 1999;177:405–10. doi: 10.1016/s0002-9610(99)00069-0. [DOI] [PubMed] [Google Scholar]
- 22.Kwan SW, Fidelman N, Ma E, et al. Imaging predictors of the response to transarterial chemoembolization in patients with hepatocellular carcinoma: a radiological-pathological correlation. Liver Transpl. 2012;18:727–36. doi: 10.1002/lt.23413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes K, Roberts OL, Thomas AM, et al. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19:2003–12. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–21. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Hu DY, Chu Q, et al. Cell apoptosis and regeneration of hepatocellular carcinoma after transarterial chemoembolization. World J Gastroenterol. 2004;10:1876–80. doi: 10.3748/wjg.v10.i13.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Feng GS, Zheng CS, et al. Influence of transarterial chemoembolization on angiogenesis and expression of vascular endothelial growth factor and basic fibroblast growth factor in rat with Walker-256 transplanted hepatoma: an experimental study. World J Gastroenterol. 2003;9:2445–9. doi: 10.3748/wjg.v9.i11.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyayama S, Matsui O, Yamashiro M, et al. Visualization of hepatic lymphatic vessels during transcatheter arterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2007;18:1111–7. doi: 10.1016/j.jvir.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Joukov V, Sorsa T, Kumar V, et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. Embo j. 1997;16:3898–911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegfried G, Basak A, Cromlish JA, et al. The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. J Clin Invest. 2003;111:1723–32. doi: 10.1172/JCI17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stacker SA, Stenvers K, Caesar C, et al. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J Biol Chem. 1999;274:32127–36. doi: 10.1074/jbc.274.45.32127. [DOI] [PubMed] [Google Scholar]
- 31.Baldwin ME, Roufail S, Halford MM, et al. Multiple forms of mouse vascular endothelial growth factor-D are generated by RNA splicing and proteolysis. J Biol Chem. 2001;276:44307–14. doi: 10.1074/jbc.M106188200. [DOI] [PubMed] [Google Scholar]
- 32.McColl BK, Baldwin ME, Roufail S, et al. Plasmin activates the lymphangiogenic growth factors VEGF-C and VEGF-D. J Exp Med. 2003;198:863–8. doi: 10.1084/jem.20030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McColl BK, Paavonen K, Karnezis T, et al. Proprotein convertases promote processing of VEGF-D, a critical step for binding the angiogenic receptor VEGFR-2. Faseb j. 2007;21:1088–98. doi: 10.1096/fj.06-7060com. [DOI] [PubMed] [Google Scholar]
- 34.Achen MG, McColl BK, Stacker SA. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell. 2005;7:121–7. doi: 10.1016/j.ccr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Threefoot SA, Kossover MF. Lymphaticovenous communications in man. Arch Intern Med. 1966;117:213–23. [PubMed] [Google Scholar]
- 36.Stacker SA, Williams SP, Karnezis T, et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159–72. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 37.Hall FT, Freeman JL, Asa SL, et al. Intratumoral lymphatics and lymph node metastases in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129:716–9. doi: 10.1001/archotol.129.7.716. [DOI] [PubMed] [Google Scholar]
- 38.Straume O, Jackson DG, Akslen LA. Independent prognostic impact of lymphatic vessel density and presence of low-grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res. 2003;9:250–6. [PubMed] [Google Scholar]
- 39.Kirkin V, Mazitschek R, Krishnan J, et al. Characterization of indolinones which preferentially inhibit VEGF-C- and VEGF-D-induced activation of VEGFR-3 rather than VEGFR-2. Eur J Biochem. 2001;268:5530–40. doi: 10.1046/j.1432-1033.2001.02476.x. [DOI] [PubMed] [Google Scholar]
- 40.Dadras SS, Paul T, Bertoncini J, et al. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162:1951–60. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bono P, Wasenius VM, Heikkila P, et al. High LYVE-1-positive lymphatic vessel numbers are associated with poor outcome in breast cancer. Clin Cancer Res. 2004;10:7144–9. doi: 10.1158/1078-0432.CCR-03-0826. [DOI] [PubMed] [Google Scholar]
- 42.Skobe M, Hawighorst T, Jackson DG, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–8. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 43.Stacker SA, Caesar C, Baldwin ME, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–91. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 44.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 45.Pawlik TM, Reyes DK, Cosgrove D, et al. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29:3960–7. doi: 10.1200/JCO.2011.37.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffmann K, Ganten T, Gotthardtp D, et al. Impact of neo-adjuvant Sorafenib treatment on liver transplantation in HCC patients - a prospective, randomized, double-blind, phase III trial. BMC Cancer. 2015;15:392. doi: 10.1186/s12885-015-1373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–27. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]