Abstract
BACKGROUND
Comorbid executive dysfunction in autism spectrum disorder (ASD) is a barrier to adaptive functioning, despite remittance of core social-communication symptoms. Network models of ASD address core symptoms but not comorbid executive dysfunction. Following recent demonstrations in healthy adults that, with increasing executive demands, hubs embedded within frontoparietal-insular control networks interact with a more diverse set of networks, we hypothesized that the capability of hubs to do so is perturbed in ASD and predicts executive behavior.
METHODS
Seventy-five 7- to 13-year-old children with ASD (n = 35) and age- and IQ-matched typically developing control subjects (n = 40) completed both a resting-state and a selective attention task functional magnetic resonance imaging session. We assessed changes in the participation coefficient, a graph theory metric indexing hubness, of 264 brain regions comprising 12 functional networks between the two sessions. Parent reported executive impairment in everyday life was measured using the Behavior Rating Inventory of Executive Function.
RESULTS
The participation coefficient of the frontoparietal-insular cortex, including core nodes of the frontoparietal control and salience networks, significantly increased in typically developing children but not in children with ASD during the task relative to rest. Change in frontoparietal-insular participation coefficient predicted Behavior Rating Inventory of Executive Function scores indexing the ability to attend to task-oriented output, plan and organize, and sustain working memory.
CONCLUSIONS
Our results suggest that executive impairments in ASD emerge from a failure of frontoparietal-insular control regions to function as adaptive and integrative hubs in the brain’s functional network architecture. Our results also demonstrate the utility of examining dynamic network function for elucidating potential biomarkers for disorders with comorbid executive dysfunction.
Keywords: Autism, fMRI, Frontoparietal, Graph theory, Hubs, Networks
Functional network–level investigations of autism spectrum disorder (ASD) pathophysiology have focused primarily on social cognition, despite pervasive impairment in another domain, executive function (EF), the goal-oriented control of cognition. Comorbid EF impairment is observed in 41% to 78% of cases (1), increases with age (2,3), and persists despite amelioration of ASD symptoms (4). This impairment in ASD spans component processes of EF (e.g., inhibition, shifting, working memory, planning/organization) (5), moderates defining ASD symptoms (6), and predicts worse adaptive functioning (7,8) as well as quality of life (3). Current hypotheses of ASD, which posit pathophysiology of network-level dysfunction, target core social-communicative symptoms (9), but leave open our understanding of comorbid executive dysfunction.
EF is supported by frontoparietal and salience/cingulo-opercular functional networks anchored in the prefrontal cortex (PFC), collectively termed control networks (10). A long-standing theory posits that PFC enables adaptive goal-oriented behaviors by integrating information from distributed cortical regions (11). Indeed, functional connectivity (FC) of PFC control regions increases with a diverse array of brain regions (i.e., those belonging to other networks) during tasks evoking EF processes (12,13). Select nodes within control networks, often identified as hubs, are posited to enable these widespread interactions by integrating information from multiple brain networks (14,15). Dysfunction of control network hubs is a candidate mechanism for EF impairment in ASD, as pervasive EF deficits could result from an inability of hubs to interact widely with other networks or serve as convergence zones (16) when it is necessary to adapt to new behavioral demands.
While a growing body of work has established disruption of large-scale functional networks in ASD (16–18), the integrity of integrative processing within the PFC remains unexamined. Findings from studies using task (17,18) and task-free (i.e., resting-state) experimental designs (19–23) reveal atypical FC in children and adults with ASD, including both weaker and stronger FC relative to typically developing (TD) control subjects (24). Contrasting task states requiring EF relative to rest, atypical FC changes were observed between a subset of brain network nodes (25) and among voxelwise long-distance connections (26) in children with ASD. While this evidence suggests a possible maladaptive response of brain networks to task demands in ASD, whether hubs within PFC are instrumental in that response is not known. Here we tested the hypothesis that EF impairment in ASD results from the frontoparietal control network failing to adaptively integrate information from throughout the brain.
We examined changes in hubness across the entire brain between two cognitive states in children with ASD and their TD peers. In the brain, hubness can be quantified using participation coefficient (PC), a graph theoretical measure capturing the diversity of a brain region’s FC with all other networks (27). Often hubs are thought of as individual nodes, however, groups of nodes comprising functional networks, such as the frontoparietal network (28), can collectively carry out an integrative function. For this reason, we examined network-level PC by averaging the PC of all nodes within a given network in addition to examining the PC of individual nodes.
We manipulated EF demands across two cognitive states: resting state, signifying an absence of EF demands, and a selective attention task requiring monitoring a target shape in the context of distractors, signifying the presence of EF demands. Contrasting the resting state and task state allows examining adaptation to EF demands, which may manifest as changing FC patterns between hub regions and the rest of the brain (28). Specifically, we predicted that the PC of control networks, both at the network level and the nodes contained within, would increase in the task state in TD but would increase less so in children with ASD. Further, we predicted that these changes in PC would predict EF abilities. We examined EF manifested in stable behavioral characteristics (termed trait level) instead of a performance measure because EF impairments are multidimensional in ASD and EF tasks generally only capture a single dimension of EF (29). Furthermore, the performance of children with ASD in structured settings may not be ecologically representative of their ability to engage and disengage behavior in a goal-oriented manner in daily life (30). For these reasons, we utilized the Behavior Rating Inventory of Executive Function (BRIEF) (31), a commonly used parent-report questionnaire used in clinical settings that is also sensitive to normal EF variability.
METHODS AND MATERIALS
Participant Demographics
Seventy-five participants 7 to 13 years old (35 with a diagnosis of ASD and 40 TD children) participated in the study after complying with consenting guidelines of the Georgetown University and Children’s National Medical Center Institutional Review Boards. A final sample of 23 children with ASD was retained after applying strict criteria for head motion to both resting-state and task functional magnetic resonance imaging (fMRI) data (see criteria below). A group of 23 TD children matched for age, IQ, and head motion were selected. Children with ASD were recruited through the Center for ASD at Children’s National Medical Center, and TD children were recruited from the Washington, DC, area. This sample partially overlaps with (26). See Table 1 for demographic information.
Table 1.
Participant Demographics
| Age (years) |
Full- Scale IQ |
BRIEF- MI |
BRIEF- BRI |
ADOS- Social |
ADOS- Communication |
ADOS-Restricted/ Repetitive Interests |
ADI-Social | ADI-Restricted/ Repetitive Interests |
ADI- Communication |
|
|---|---|---|---|---|---|---|---|---|---|---|
| TD | 11.33 ± 0.33 | 119.59 ± 2.76 | 46.75 ± 1.81 | 44.30 ± 1.22 | — | — | — | — | — | — |
| ASD | 11.18 ± 0.34 | 120.43 ± 2.87 | 66.09 ± 2.61 | 63.77 ± 2.86 | 7.14 ± 3.52 (2–14) | 3.14 ± 1.59 (1–7) | 1.74 ± 1.67 (0–5) | 20.85 ± 4.87 (13–28) | 4.80 ± 1.91 (1–9) | 15.95 ± 4.60 (7–24) |
Values are mean ± SD (range).
ADI, Autism Diagnostic Interview; ADOS, Autism Diagnostic Observation Schedule, Module 3; ASD, autism spectrum disorder; BRI, Behavioral Regulation Index; BRIEF, Behavior Rating Inventory of Executive Function; MI, Metacognition Index; TD, typically developing.
Exclusion criteria included 1) full-scale IQ below 80 as measured by the Wechsler Intelligence Scale for Children or Wechsler Abbreviated Scale of Intelligence, 2) other neurological diagnosis (e.g., epilepsy) based on parent report, 3) psychiatric diagnosis based on Child and Adolescent Symptom Inventory-4R (32) for control children, and 4) contra-indications for MRI. Five children with ASD were prescribed stimulant medication, which was withheld for at least 24 hours before fMRI data acquisition; no other children were medicated.
ASD classification followed diagnosis by author LK and staff based on DSM-IV-TR criteria and was confirmed with the Autism Diagnostic Interview-Revised (33) and the Autism Diagnostic Observation Schedule, Module 3 (34) following the criteria established by the National Institute of Child Health and Human Development/National Institute of Deafness and Other Communication Disorders Collaborative Programs for Excellence in Autism. These criteria require that the child meet the Autism Diagnostic Interview-Revised cutoff for autism in the social domain and at least one other domain (communication and/or repetitive behaviors and restricted interests), or meet Autism Diagnostic Observation Schedule, Module 3 cutoff for the combined social and communication score.
Behavior
A parent completed the BRIEF (31), providing two composite scores for each child, the Metacognition Index (MI) and Behavioral Regulation Index (BRI). The MI (initiate, working-memory, plan/organize, organization of materials, and monitor subscales) indexes the child’s ability to attend to task-oriented output, plan and organize problem-solving approaches, and sustain working memory, whereas the BRI (inhibit, shift, and emotional control subscales) indexes the ability to modulate emotion and behavior through inhibitory control and shifting between task sets in everyday behavior. Higher scores index worse abilities. Group differences were assessed using independent sample t tests.
fMRI Task
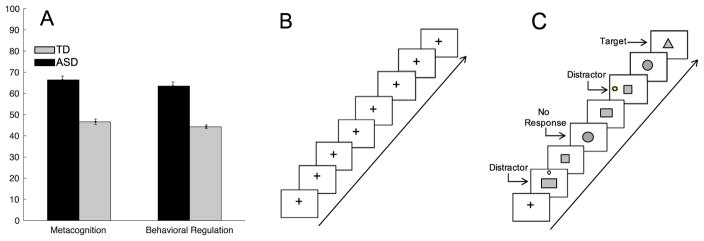
Each participant completed a resting state followed by a task fMRI run [task modified from (35)], requiring goal-oriented monitoring for task-relevant information. For the resting state, participants were told to relax, stay awake with eyes on a central fixation cross. For the task, participants monitored a central serial presentation of shapes (circle, rectangle, square, triangle) in random order and were instructed to press a right handheld button in response to a designated target shape (triangle). Target shapes appeared on 25% of the trials, leaving the remaining 75% of trials without any motor response. A subset (50%) of these trials included a flashing distractor appearing in the periphery (colorful symbols of variable frequency) that was irrelevant to the ongoing central task. The remaining 25% of the trials did not contain a distractor. Thus, the total number of trials was 168, with 25% with motor response and no distractor, 50% with a distractor, and 25% with no distractor; in addition, 56 fixation trials were included for purposes of jittering. Trial order was optimized for event-related design using OPTSEQ2 (36).
fMRI Acquisition
Functional echo-planar images were acquired on a Siemens Trio 3T (Siemens, Erlangen, Germany) with parameters: 3-mm isotropic resolution (3.0 × 3.0 × 2.5 mm), repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, field of view = 192 × 192 mm. The resting-state and cognitive task runs consisted of 156 and 172 whole-brain images, respectively. The first four images were removed from each run to allow for signal stabilization.
Image Preprocessing
Using SPM8 (Wellcome Department of Cognitive Neurology, London, United Kingdom) implemented in MATLAB version 2016A (The MathWorks, Inc., Natick, MA), images were slice-time corrected and corrected for translational and rotational motion by realigning to the first image of the session for each run. Images were then normalized to Montreal Neurological Institute space using a standard echo-planar imaging template, and smoothed using an 8-mm full width at half maximum Gaussian kernel. Participants with >0.5 mean framewise displacement in head motion during either fMRI run (prior to scrubbing) were excluded. Across participants, the average volumes scrubbed during the resting state were 7.5 ± 9.3 (range: 0–32 volumes) and during the task were 7.5 ± 10.1 (range: 0–44 volumes). The mean framewise displacement during the resting state was 0.19 ± 0.10 mm (range: 0.07–0.42 mm) and during the cognitive task was 0.16 ± 0.08 mm (range: 0.07–0.43 mm). Importantly, during both the resting state and task state, the groups did not differ on mean framewise displacement or number of volumes scrubbed (all ps > .09; see the Supplement for details of motion control procedures).
Regions of Interest and Brain Network Partitions
For unbiased FC analyses, we selected an independently defined set of 264 regions of interest (ROIs), each with a 6-mm radius sphere centered on a putative functional area (37) (see Figure 1A). Each ROI belongs to one of 12 functional networks (37). The groups did not significantly differ in how well this independently defined network partition aligned with their own individually defined network structures (fit quantified using normalized variation of information, see the Supplement). Although these ROIs are commonly used, there are other independent ROIs and network partitions available, and there is variability in how networks are defined in each.
Figure 1.
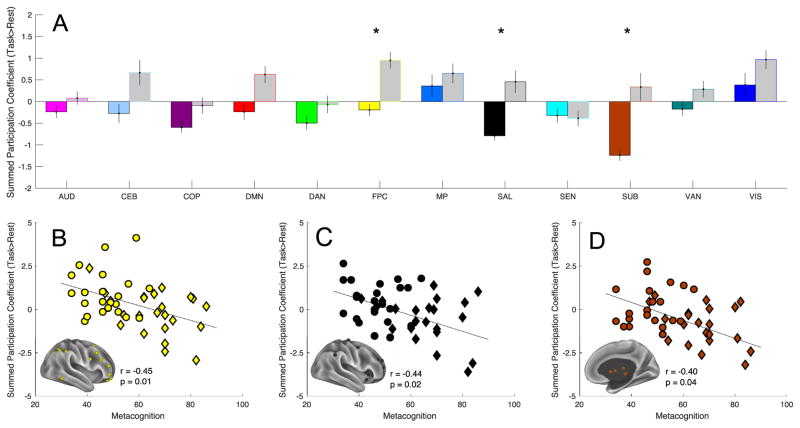
Regions of interest and network assignments (A). Typically developing children demonstrated significant task-induced increase (task > rest) in the participation coefficient of the frontoparietal control (FPC) network (B). Autism spectrum disorder children demonstrated significant task-induced decrease (task < rest) in the participation coefficient of the subcortical (SUB) network (C). *Denotes Holm-Bonferroni pcorrected < .05. Color-filled bars indicate resting state, gray-filled bars indicate task state. AUD, auditory; CEB, cerebellar; COP, cingulo-opercular; DAN, dorsal attention network; DMN, default mode network; MP, medial parietal; SAL, salience; SEN, sensorimotor; VAN, ventral attention network; VIS, visual.
FC Analysis
Using custom scripts implemented in MATLAB, data were subjected to simultaneous bandpass filtering and nuisance variable regression followed by scrubbing procedures recommended by Hallquist et al. (38) and Power et al. (39), respectively. Specifically, a bandpass filter was applied to restrict signal variation to frequencies between 0.01 and 0.1 Hz. Nuisance regressors included motion (six motion parameters and their temporal derivatives), mean signal time courses from white matter and cerebrospinal fluid masks for both resting and task runs, and an additional trial condition regressor (for the task). Volumes with framewise displacement greater than 0.5 mm were identified and excluded. Following nuisance regression and scrubbing, the correlation between each ROI with all other ROIs was computed for each run. The resulting 264 × 264 × 2 matrix for each subject represented the FC between every ROI during each state.
Graph Theory Analysis
For each subject, PC values (27), for the resting and task state, separately, were calculated for each of the 264 ROIs using the Brain Connectivity Toolbox (https://sites.google.com/site/bctnet/) and the independently defined brain network partitions described above. It is common practice to put graphs into sparse (edge densities of a few percent or less) and binary form. To avoid selecting a single absolute correlation (r) threshold, this analysis was repeated 16 times using binarized matrices, each with a unique proportional threshold (top 20% to 5% of connections, in 1% steps, resulting in inclusion of positive correlations only). Proportional thresholds, in contrast to absolute thresholds, ensure that each participant’s graphs have a similar number of edges. While proportional thresholds can introduce biases when the overall FC strength between groups significantly differs (40), we did not observe such a difference in our data (ASD [mean = 0.37], TD [mean = 0.36]; t44 = 0.70, p = .48).
PC values were summed across these thresholds, resulting in a single metric, as done by previous work identifying brain network hubs (14). For each of the 12 networks, a summed PC value was calculated by averaging the summed PC of all ROIs within that network, providing a network-level PC value, as done in (28). The PC is a measure of cross-network connection diversity. Brain regions with higher PC values participate in many brain networks and those with lower values participate in fewer networks. In the equation below, M is the total set of networks and Ki (m) is the number of connections between node i and all nodes in network m.
We conducted three analyses. First, to assess whether EF demands affected network hubness, we compared the PC of all 12 networks between resting and task runs with paired t tests, separately in ASD and TD groups. Second, to assess whether these changes in network-level PC differed between TD children and children with ASD, we computed a difference score (TaskPC – RestPC) for every network and compared the groups on this metric using independent sample t tests. Third, to assess whether changes in network hubness predicted EF, we computed Pearson correlations between difference scores and BRIEF indices across the entire sample. Reported p values reflect Holm-Bonferroni correction (41,42). To parse how nodes contributed to network-level findings, we repeated these analyses separately for each ROI in networks showing significant differences. These were repeated using an alternative approach that increased graph sparseness (top 10% to 2% of connections, in 1% steps) and removed local connections (those <20 mm in euclidean space). Findings reported in the main text were unchanged (Supplement). Nonparametric permutation tests were used as well (Supplement).
RESULTS
Group Differences in EF
Comparison of BRIEF t scores revealed that, as expected (28), children with ASD scored significantly higher relative to TD children on both the MI (t44 = −6.36, p < .0001) and the BRI (t44= −6.35, p < .0001) indices, indicative of worse EF (see Figure 2). Subsequent analyses were limited to these composite scores to reduce the number of comparisons, but for completeness differences between groups on each individual subscale of the BRIEF are listed in the Supplement. While accuracy on the selective attention was high in both groups, it was significantly lower in ASD (mean = 98.70 ± 0.02%) than TD (mean = 99.800 ± 0.006%) children (t44= 2.09, p < .04). There was a trend of longer reaction times in the ASD group (mean = 560.00 ± 0.02 seconds) versus the TD group (mean = 520.000 ± 0.006 seconds; t44 = 1.81, p < .08).
Figure 2.
Worse scores on Behavior Rating Inventory of Executive Function indices of metacognition and behavioral regulation. Gray bar indicates typically developing (TD) group, black bar indicates autism spectrum disorder (ASD) group (A). Illustration of resting-state scan (B) and conditions in selective attention task (C).
Task-Induced Differences in Hubness Within Groups
At the network level, hubness of the frontoparietal control network (t22 = −3.04, pcorrected = .03) significantly increased in the task state relative to the resting state in TD children (see Figure 1B). No network demonstrated this pattern in the ASD group; however, hubness of the subcortical network (t22 = −3.71, pcorrected = .007) significantly decreased in the task state relative to the resting state (see Figure 1C). Thus, as EF demands increased, the frontoparietal control network in TD children interacted with a more diverse array of functional networks, whereas the subcortical network in children with ASD reduced its diversity of interaction with other networks.
Group Differences in Task-Induced Hubness
Group comparison of network-level difference scores revealed that task-induced hubness was greater in TD children than in children with ASD for the frontoparietal control (t44 = −3.07, pcorrected = .03), salience (t44 = −2.98, pcorrected = .04), and subcortical (t44 = −3.18, pcorrected = .04) networks (see Figure 3A). No network showed greater hubness change in ASD than TD children did.
Figure 3.
Task-induced change in summed participation coefficient hubness of frontoparietal (yellow), salience (SAL) (black), subcortical (SUB) (brown) networks significantly differed between autism spectrum disorder children and typically developing children (A). *Denotes Bonferroni-Holm corrected. Gray-filled bars indicate the typically developing group, color-filled bars indicate the autism spectrum disorder group. Correlations between the change of these networks: panel (B) is frontoparietal control (FPC), panel (C) is salience, and panel (D) is subcortical—with Metacognition Index of the Behavior Rating Inventory of Executive Function. Autism spectrum disorder and typically developing subjects are plotted as diamonds and circles, respectively. AUD, auditory; CEB, cerebellar; COP, cingulo-opercular; DAN, dorsal attention network; DMN, default mode network; MP, medial parietal; SEN, sensorimotor; VAN, ventral attention network; VIS, visual.
Correlation Between Task-Induced Hubness and BRIEF
Across the entire sample, increased hubness of the frontoparietal, salience, and subcortical networks predicted better MI score. Difference scores for the frontoparietal control (r44= −.45, pcorrected = .01), salience (r44= −.44, pcorrected = .02), and subcortical (r44= −.40, pcorrected = .04) networks negatively correlated with MI scores (see Figure 3B–D). Correlation with BRI scores did not survive correction for multiple comparisons for any network.
Node-Level Task-Induced Hubness Within Groups
Post hoc examination of individual nodes within the frontoparietal control, salience, and subcortical networks revealed that within the TD group the hubness of frontal and parietal nodes significantly increased (see Figure 4A). In contrast, within the ASD group the hubness of anterior insular and subcortical ROIs significantly decreased (see Figure 4B). No significant change in hubness in any other direction was observed within either group.
Figure 4.
Node-level analyses. Nodes showing significant task-induced increases (task > rest) in hubness in the typically developing group (A). Nodes showing significant task-induced decreases (task < rest) in hubness within the autism spectrum disorder group; no nodes showed task-induced increase (B). Nodes showing group differences in task-induced change in hubness (typically developing > autism spectrum disorder) (C). Nodes with significant correlations (all ps < .05) between summed participation coefficient (task > rest) and scores on the Metacognition Index of the Behavior Rating Inventory of Executive Function (D).
Node-Level Task-Induced Hubness Group Differences
Group comparison of difference scores revealed greater TD hubness change (TD > ASD) in the lateral PFC, parietal, and anterior aspects of the cinguloinsular cortex (see Figure 4C). No region demonstrated the opposite pattern (ASD > TD).
Node-Level Correlation Between Task-Induced Hubness and BRIEF
We examined correlations between MI scores and the individual frontoparietal control, salience, and subcortical nodes. This analysis revealed significant negative correlations between hubness changes of the lateral PFC, parietal, and cinguloinsular regions with the MI (Figure 4D). However, only nodes in the left anterolateral PFC and right anterior insular cortex, collectively representing core nodes of frontoparietal and salience networks, survived correction for multiple comparisons.
DISCUSSION
Our results revealed that executive dysfunction in ASD relates to a failure to increase the diversity of frontoparietal, salience, and subcortical FC with cognitive demands. Hubness, indexed by PC, which measures the diversity of a region’s functional connections, was measured at the network and node levels in both cognitive states. Relative to the resting state, hubness of the frontoparietal-insular cortex, core nodes of the frontoparietal control and salience networks, significantly increased in TD children, but not in children with ASD during a task with EF demands, monitoring a target in the presence of distractors. In contrast, hubness of subcortical nodes significantly decreased in children with ASD but not in TD children. These differences were confirmed by direct group comparison. Behaviorally, greater executive dysfunction was evident in children with ASD relative to TD children on both the MI and the BRI indices of the BRIEF. Relationships with task-induced hubness survived multiple correction only with MI, such that children with greater task-induced increases in hubness of frontoparietal, salience, and subcortical networks, particularly the left anterolateral PFC and right anterior insular nodes, had everyday behavior that was indicative of better working memory and ability to plan, initiate, and monitor activities.
While interpreting these findings, the following points must be kept in mind. First, head motion is common in clinical and pediatric populations (43) and can confound FC estimates (39). We implemented a simultaneous bandpass filtering and nuisance regression strategy (38), which controls for the inadvertent reintroduction of nuisance signals by the bandpass filter, and removed high-motion time points (39). In addition, we found that head motion did not significantly differ between groups nor did it relate to changes in PC. Although these steps do not allow us to rule out the contribution of head motion entirely, it is unlikely that our findings are related to head motion alone (see the Supplement). Second, the application of functional brain network topology, as defined in the healthy adult brain (37), to the developing or atypical brain may be imprecise, albeit in hitherto unknown ways. We assessed this by quantifying the distance between the independent network partition used in our primary analyses and each subject’s own individually defined network structure using normalized variation of information, a graph theory metric that quantifies the amount of information lost or gained between a partition and another. The groups did not significantly differ in either state. Thus, it is unlikely that observed group differences are related to one group’s network structure being better aligned with this independent partition (see the Supplement). Third, our aim was to contrast cognitive states with EF demands absent (resting state) and present (selective attention task). State order was fixed to avoid known task after-effects on the subsequent resting state (44). The simplicity of the task allows for maximizing accuracy while still evoking a goal-oriented state requiring subjects to monitor and ignore relevant and irrelevant stimuli, respectively. A more cognitively demanding EF task would likely produce the observed pattern of results, perhaps stronger rather than qualitatively different. This prediction can be tested by future work. Fourth, we selected PC as the metric of hubness, as it is sensitive to the diversity of a given region’s FC, as opposed to other hub metrics, such as degree, which identify nodes that have strong FC (i.e., total number of connections) without consideration to whether those connections are within or outside of that node’s own network. Thus, PC is well suited for testing our hypotheses, considering that the integration of information across many different networks is thought to be a hallmark of optimal EF abilities (11,12,45). It is noteworthy that we calculated PC using networks defined at one specific spatial scale and it is unknown whether our findings hold when defining networks at other spatial scales (46). Fifth, our repeated-measures design required two scans with minimal motion, thereby reducing sample size substantially. Thus, replication of these findings is important.
The present study extends current understanding of the functional neuropathology of ASD by highlighting the importance of distributed and dynamic network function. Previous work has focused on select brain networks ascribed functions aligned with core symptoms of the disorder (e.g., default mode network: social cognition), and reported whether isolated patterns of abnormal connectivity, either increased or decreased, relate to social symptomatology (21,47). Other studies have examined strength of FC patterns within a single network or how a graph metric differs in ASD during the resting state (48–50). In contrast, we sought to capture interactions between a given brain region and many different brain networks and their change with cognitive demand. Recent investigations of FC modulation by cognitive state reflected in effective connectivity between the core default mode and frontoparietal network nodes (25) and brainwide voxelwise distant FC of frontoparietal regions (26) revealed weak or atypical changes, respectively, in children with ASD and also observed associations with EF symptoms. The present study extends this line of work by providing a potential maladaptive mechanism—a failure to modulate the hubness of frontoparietal-insular control regions in response to EF demands in children with ASD. Although our results cannot adjudicate underlying pathophysiological mechanisms (e.g., inhibitory and excitatory imbalance), they emphasize the importance of considering dynamic FC in models of ASD pathophysiology.
Changes in frontoparietal-insular hubness were most strongly associated with MI scores. The MI indexes a child’s parent reported ability to plan, initiate, and monitor activities in daily life, whereas the BRI indexes the ability to modulate emotions and behavior through appropriate inhibitory control. BRI subscales (i.e., emotional control, shift) also showed association, albeit that it did not survive correction for multiple comparisons. Association with MI may have been more robust because the selective attention task was most demanding of monitoring and sustained attention rather than inhibitory or set-shifting abilities. It remains to be seen whether the present results would also be observed with a task that was more demanding of inhibition/shifting. An alternate reason for the stronger MI association may relate to heterogeneity of EF dysfunction among ASD cohorts such that this particular group of children with ASD may have a wider range of MI than BRI scores, affording the opportunity to detect a correlation more reliably with the MI than with the BRI. Standard deviations of subscales (Supplemental Table S2) are slightly larger for three of the MI subscales than are those for the BRI. Association of functional network characteristics with ecological assessment of EF is highly promising in the development of biomarkers for ASD. EF comorbidity in ASD is well recognized in light of its incorporation in the current DSM-V ASD diagnostic assessment of ASD. Furthermore, the persistence of EF difficulties despite alleviation of ASD symptoms (4) highlights the importance of developing interventions focused on EF (51). Indexing the hubness of key neurocognitive networks using graph theory metrics such as PC might be useful in tracking intervention-related changes in the brain.
PFC abnormalities in ASD are well known, dating back to reports by Damasio and Maurer (52) describing the behavioral similarities between individuals with ASD and patients with frontal lesions. Later, Courchesne and Pierce (53) proposed that a failure of context-dependent integration between the PFC and the rest of the brain could be central to cognitive impairments in ASD. The present study is compatible with these theoretical models but makes a novel contribution by providing a mechanistic account of how executive deficits in this population might emerge from a failure of frontoparietal-insular control regions to function as adaptive and integrative hubs in the brain’s functional network architecture. Importantly, this framework can be applied in other psychiatric conditions characterized by deficits in EF such as attention-deficit/hyperactivity disorder (54) and schizophrenia (55), both of which show topological alterations of large-scale functional networks examined in a single cognitive state. A lack of modulation of frontoparietal-insular hubness in response to increased cognitive demand might not be unique to ASD but rather common across conditions with comorbid EF. Testing this possibility can provide a biomarker for executive dysfunction, which will refine diagnosis and spur development of targeted neurostimulation therapies.
Supplementary Material
Acknowledgments
This work was supported by Grant Nos. MH0849661 (to CJV) and HD040677-07 to Children’s National Medical Center from the National Institutes of Health.
Footnotes
DISCLOSURES
LK receives financial compensation for use of the Behavior Rating Inventory of Executive Function. All other authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.bpsc.2017.03.008.
References
- 1.Murray MJ. Attention-deficit/hyperactivity disorder in the context of autism spectrum disorders. Curr Psychiatry Rep. 2010;12:382–388. doi: 10.1007/s11920-010-0145-3. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal M, Wallace GL, Lawson R, Wills MC, Dixon E, Yerys BE, Kenworthy L. Impairments in real-world executive function increase from childhood to adolescence in autism spectrum disorders. Neuropsychology. 2013;27:13–18. doi: 10.1037/a0031299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sikora DM, Vora P, Coury DL, Rosenberg D. Attention-deficit/hyperactivity disorder symptoms, adaptive functioning, and quality of life in children with autism spectrum disorder. Pediatrics. 2012;130(suppl 2):S91–S97. doi: 10.1542/peds.2012-0900G. [DOI] [PubMed] [Google Scholar]
- 4.Troyb E, Rosenthal M, Eigsti IM, Kelley E, Tyson K, Orinstein A, et al. Executive functioning in individuals with a history of ASDs who have achieved optimal outcomes. Child Neuropsychol. 2014;20:378–397. doi: 10.1080/09297049.2013.799644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granader Y, Wallace GL, Hardy KK, Yerys BE, Lawson RA, Rosenthal M, et al. Characterizing the factor structure of parent reported executive function in autism spectrum disorders: The impact of cognitive inflexibility. J Autism Dev Disord. 2014;44:3056–3062. doi: 10.1007/s10803-014-2169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yerys BE, Wallace GL, Sokoloff JL, Shook DA, James JD, Kenworthy L. Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Res. 2009;2:322–333. doi: 10.1002/aur.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szatmari P, Bartolucci G, Bremner R. Asperger’s syndrome and autism: comparison of early history and outcome. Dev Med Child Neurol. 1989;31:709–720. doi: 10.1111/j.1469-8749.1989.tb04066.x. [DOI] [PubMed] [Google Scholar]
- 8.Pugliese CE, Anthony LG, Strang JF, Dudley J, Wallace GL, Naiman DQ, Kenworthy L. Longitudinal examination of adaptive behavior in autism spectrum disorders: Influence of executive function. J Autism Dev Disord. 2015;46:467–477. doi: 10.1007/s10803-015-2584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ecker C, Bookheimer SY, Murphy DG. Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. Lancet Neurol. 2015;14:1121–1134. doi: 10.1016/S1474-4422(15)00050-2. [DOI] [PubMed] [Google Scholar]
- 10.Power JD, Petersen SE. Control-related systems in the human brain. Curr Opin Neurobiol. 2013;23:223–228. doi: 10.1016/j.conb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 12.Spielberg JM, Miller GA, Heller W, Banich MT. Flexible brain network reconfiguration supporting inhibitory control. Proc Natl Acad Sci U S A. 2015;112:10020–10025. doi: 10.1073/pnas.1500048112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun U, Schäfer A, Walter H, Erk S, Rmanczuk-Seiferth N, Haddad L, et al. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci U S A. 2015;112:11678–11683. doi: 10.1073/pnas.1422487112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013;79:798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertolero MA, Yeo BT, D’Esposito M. The modular and integrative functional architecture of the human brain. Proc Natl Acad Sci U S A. 2015;112:E6798–E6807. doi: 10.1073/pnas.1510619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci. 2013;17:683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: Visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damarla SR, Keller TA, Kana RK, Cherkassky VL, Williams DL, Minshew NJ, Just MA. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism Res. 2010;3:273–279. doi: 10.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Supekar K, Uddin LQ, Khouzam A, Phillips J, Gallard WD, Kenworthy LE, et al. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 2013;5:738–747. doi: 10.1016/j.celrep.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, et al. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013;70:869–879. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch CJ, Uddin LQ, Supekar K, Khouzam A, Phillips J, Menon V. Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biol Psychiatry. 2013;74:212–219. doi: 10.1016/j.biopsych.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- 23.Keown CL, Shih P, Nair A, Peterson N, Mulvey ME, Müller RA. Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell Rep. 2013;5:567–572. doi: 10.1016/j.celrep.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011;21:2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uddin LQ, Supekar K, Lynch CJ, Cheng KM, Odriozola P, Barth ME, et al. Brain state differentiation and behavioral inflexibility in autism. Cereb Cortex. 2015;25:4740–4747. doi: 10.1093/cercor/bhu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.You X, Norr M, Murphy E, Kuschner ES, Bal E, Gaillard WD, et al. Atypical modulation of distant functional connectivity by cognitive state in children with autism spectrum disorders. Front Hum Neurosci. 2013;7:482. doi: 10.3389/fnhum.2013.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guimera R, Nunes Amaral LA. Functional cartography of complex metabolic networks. Nature. 2005;433:895–900. doi: 10.1038/nature03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16:1348–1355. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenworthy L, Yerys BE, Gutermuth Anthony L, Wallace GL. Understanding executive control in autism spectrum disorders in the lab and in the real world. Neuropsychol Rev. 2008;18:320–338. doi: 10.1007/s11065-008-9077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovaas OI. Behavioral treatment and normal educational and intellectual functioning in young autistic children. J Consult Clin Psychol. 1987;55:3–9. doi: 10.1037//0022-006x.55.1.3. [DOI] [PubMed] [Google Scholar]
- 31.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychol. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- 32.Lavigne JV, Cromley T, Sprafkin J, Gadow KD. The Child and Adolescent Symptom Inventory-Progress Monitor: A brief Diagnostic and Statistical Manual of Mental Disorders, 4th edition-referenced parent-report scale for children and adolescents. J Child Adolesc Psychopharmacol. 2009;19:241–252. doi: 10.1089/cap.2008.052. [DOI] [PubMed] [Google Scholar]
- 33.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 34.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 35.Zink CF, et al. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23:8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Power JD, Cohen Al, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: Spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Heuvel M, de Lange SC, Zalesky A, Seguin C, Yeo BT, Schmidt R. Proportional thresholding in resting-state fMRI functional connectivity networks and consequences for patient-control connectome studies: Issues and recommendations. Neuroimage. 2017;152:437–449. doi: 10.1016/j.neuroimage.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: The Bonferroni vs Holm methods. Am J Public Health. 1996;86:726–728. doi: 10.2105/ajph.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eichstaedt KE, Kovatch K, Maroof DA. A less conservative method to adjust for familywise error rate in neuropsychological research: The Holm’s sequential Bonferroni procedure. Neurorehabilitation. 2013;32:693–696. doi: 10.3233/NRE-130893. [DOI] [PubMed] [Google Scholar]
- 43.Yerys BE, Jankowski KF, Shook D, Rosenberger LR, Barnes KA, Berl MM, et al. The fMRI success rate of children and adolescents: Typical development, epilepsy, attention deficit/hyperactivity disorder, and autism spectrum disorders. Hum Brain Mapp. 2009;30:3426–3435. doi: 10.1002/hbm.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon EM, Breeden AL, Bean SE, Vaidya CJ. Working memory-related changes in functional connectivity persist beyond task disengagement. Hum Brain Mapp. 2014;35:1004–1017. doi: 10.1002/hbm.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang X, Zou Q, He Y, Yang Y. Topologically reorganized connectivity architecture of default-mode, executive-control, and salience networks across working memory task loads. Cereb Cortex. 2016;26:1501–1511. doi: 10.1093/cercor/bhu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Betzel RF, Bassett DS. Multi-scale brain networks [published online ahead of print 11 Nov] Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudie JD, Brown JA, Beck-Pancer D, Hernandez LM, Dennis EL, Thompson PM, et al. Altered functional and structural brain network organization in autism. Neuroimage Clin. 2012;2:79–94. doi: 10.1016/j.nicl.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itahashi T, Yamada T, Watanabe H, Nakamura M, Jimbo D, Shioda S, et al. Altered network topologies and hub organization in adults with autism: A resting-state fMRI study. PLoS One. 2014;9:e94115. doi: 10.1371/journal.pone.0094115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yerys BE, Gordon EM, Abrams DN, Satterthwaite TD, Weinblatt R, Jankowski KF, et al. Default mode network segregation and social deficits in autism spectrum disorder: Evidence from non-medicated children. Neuroimage Clin. 2015;9:223–232. doi: 10.1016/j.nicl.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kenworthy L, Anthony LG, Naiman DQ, Cannon L, Wills MC, Luong-Tran C, et al. Randomized controlled effectiveness trial of executive function intervention for children on the autism spectrum. J Child Psychol Psychiatry. 2014;55:374–383. doi: 10.1111/jcpp.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Damasio AR, Maurer RG. A neurological model for childhood autism. Arch Neurol. 1978;35:777–786. doi: 10.1001/archneur.1978.00500360001001. [DOI] [PubMed] [Google Scholar]
- 53.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Cao M, Shu N, Cao Q, Wang Y, He Y. Imaging functional and structural brain connectomics in attention-deficit/hyperactivity disorder. Mol Neurobiol. 2014;50:1111–1123. doi: 10.1007/s12035-014-8685-x. [DOI] [PubMed] [Google Scholar]
- 55.van den Heuvel MP, Sporns O, Collin G, Sheewe T, Mandl RC, Cahn W, et al. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70:783–792. doi: 10.1001/jamapsychiatry.2013.1328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.