SUMMARY
N6-methyladenosine (m6A) affects multiple aspects of mRNA metabolism and regulates developmental transitions by promoting mRNA decay. Little is known about the role of m6A in the adult mammalian nervous system. Here we report that sciatic nerve lesion elevates levels of m6A-tagged transcripts encoding many regeneration-associated genes and protein translation machinery components in the adult mouse dorsal root ganglion (DRG). Single-base resolution m6A-CLIP mapping further reveals a dynamic m6A landscape in the adult DRG upon injury. Loss of either m6A methyltransferase complex component Mettl14, or m6A-binding protein Ythdf1, globally attenuates injury-induced protein translation in adult DRGs and reduces functional axon regeneration in the peripheral nervous system in vivo. Furthermore, Pten deletion-induced axon regeneration of retinal ganglion neurons in the adult central nervous system is attenuated upon Mettl14 knockdown. Our study reveals a critical epitranscriptomic mechanism in promoting injury-induced protein synthesis and axon regeneration in the adult mammalian nervous system.
ETOC
N6-methyladenosine (m6A) occurs in many mRNAs. Weng et al. uncovered an epitranscriptomic mechanism wherein axonal injury elevates m6A levels and signaling to promote protein translation, including regeneration-associated genes, which is essential for functional axon regeneration of peripheral sensory neurons.
INTRODUCTION
Studies in the past few years have revealed various dynamic modifications of mRNA, including N6-methyladenosine (m6A), N1-methyladenosine (m1A), 5-methylcytosine (m5C), and pseudouridine (ψ) (Gilbert et al., 2016; Li et al., 2016; Zhao et al., 2017a). Among these modifications, m6A is the most abundant internal modification of mRNA in eukaryotic cells (Desrosiers et al., 1975). m6A sites are present in over 25% of human transcripts, with enrichment in long exons, and near transcription start sites and stop codons (Dominissini et al., 2012; Ke et al., 2015; Meyer et al., 2012). Almost every gene produces both methylated and unmethylated transcripts, highlighting the highly complex and heterogeneous nature of transcriptomes (Molinie et al., 2016). So far, m6A profiling analyses have been performed mostly with cell lines and bulk tissues due to the requirement of a substantial amount of input mRNA (Li et al., 2016). In part due to this technical limitation, the m6A landscape and its temporal and spatial dynamics in specific regions of the mammalian nervous system in vivo remain largely unknown.
In mammals, m6A is installed by a methyltransferase complex consisting of Mettl3, Mettl14, and other components, and can be removed by demethylases Fto and Alkbh5 (Wang et al., 2017; Zhao et al., 2017a). Recent studies have implicated m6A in regulating mRNA processing in the nucleus, and translation and decay in the cytoplasm (Zhao et al., 2017a). These different functions of m6A modifications are believed to be mediated by diverse m6A-binding proteins, such as YT521-B homology domain family (YTHDF) proteins (Zhao et al., 2017a). For example, in vitro studies in cell lines have suggested that m6A promotes protein translation efficacy via YTHDF1 and YTHDF3, and promotes mRNA decay via YTHDF2 (Li et al., 2017a; Lin et al., 2016; Meyer et al., 2015; Shi et al., 2017; Wang et al., 2015; Zhou et al., 2015). Functionally, m6A regulates self-renewal and differentiation of mouse embryonic stem cells and glioblastoma stem cells in vitro by promoting mRNA decay (Batista et al., 2014; Cui et al., 2017; Geula et al., 2015; Wang et al., 2014). During development, m6A regulates sex determination and neuronal functions by modulating mRNA splicing in Drosophila (Haussmann et al., 2016; Lence et al., 2016) and maternal-to-zygotic transition via Ythdf2-mediated maternal mRNA clearance in Zebrafish (Zhao et al., 2017b). More recent in vivo studies of embryonic mouse development have revealed deficits in stem cell self-renewal and differentiation in the blood and nervous systems (Li et al., 2017b; Yoon et al., 2017; Zhang et al., 2017). These studies have established critical roles for m6A-dependent mRNA decay in regulating developmental transitions (Zhao et al., 2017a). The role of m6A in the adult mammalian nervous system under physiological and pathological conditions remains largely unexplored.
Sensory neurons in the adult mouse dorsal root ganglion (DRG) exhibit robust axon regeneration in the peripheral nervous system (PNS) through a process involving de novo gene transcription and protein synthesis of regeneration-association genes (RAGs) (Costigan et al., 2002; Moore and Goldberg, 2011; Smith and Skene, 1997). Axon regeneration can also be induced in the adult central nervous system (CNS), for example, by Pten deletion in retinal ganglion neurons and corticospinal neurons (Liu et al., 2010; Park et al., 2008). Previous studies have identified transcriptional mechanisms that promote intrinsic axon growth capacity (Liu et al., 2011; Moore and Goldberg, 2011; Tedeschi and Bradke, 2016). More recently, epigenetic mechanisms, including both histone acetylation (Cho et al., 2013; Finelli et al., 2013; Gaub et al., 2011; Puttagunta et al., 2014) and DNA methylation (Weng et al., 2017), have been shown to promote transcriptional activation of multiple RAGs, and are required for robust axon regeneration of adult mouse DRG neurons upon peripheral nerve injury (Weng et al., 2013; Weng et al., 2016). The discovery of widespread m6A modification and its potential roles in regulating RNA metabolism (Gilbert et al., 2016; Li et al., 2016; Zhao et al., 2017a) raises the question of whether an epitranscriptomic mechanism may contribute to axon regeneration in the adult mammalian nervous system. Here we investigated the potential role and mechanism of m6A methylation in regulating injury responses and axon regeneration in the adult mouse PNS and CNS.
RESULTS
Peripheral Axon Injury Elevates m6A-tagged Transcript Levels
Using a mouse line that specifically labels DRG neurons (Kim et al., 2016) and glutamine synthetase as a marker for surrounding satellite glia, we found that m6A was present mostly in neurons within the adult DRGs (Figure S1A). Furthermore, peripheral sciatic nerve lesion (SNL) elevated m6A levels in adult DRG neurons, reaching a peak around days 1 to 3 (D1-3) and then gradually returning back to the basal level (Figure S1A–C). We next performed genome-wide profiling of m6A-tagged mRNA in the adult DRG under naïve and SNL D1 conditions. To overcome limited mRNA input from L4/L5 DRGs of adult mice, we adapted a m6A-seq method using the SMART2-seq technology, which has recently been used to linearly amplify transcripts for single-cell RNA-seq (Picelli et al., 2014) (named m6A-SMART-seq; Figure S1D–E). Since the same gene could produce both m6A-tagged and untagged transcripts (Molinie et al., 2016), we applied a statistical approach to identify genes for which a substantial proportion of total transcripts was m6A-tagged (Figure S1F and Table S1). The majority of genes with substantial m6A-tagging was shared between naïve and SNL D1 conditions (Figure 1A). Interestingly, 129 of 304 known RAGs (Chandran et al., 2016) exhibit significant m6A-tagging at SNL D1 (Figure 1B; P < 4.550e-07; hypergeometric test).
Figure 1. SNL upregulates levels of m6A-tagged mRNAs encoding RAGs and protein translation machinery in adult DRGs in vivo.
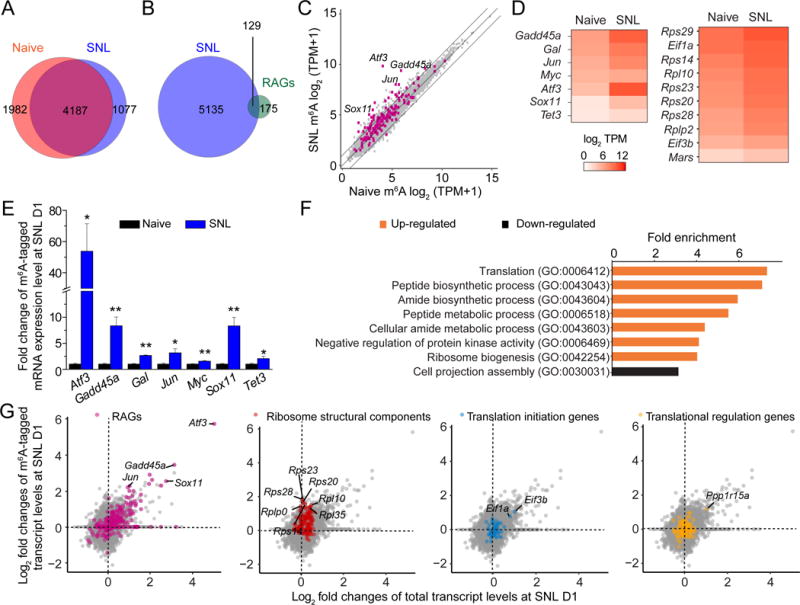
(A) Venn diagram of m6A-tagged transcripts identified by m6A-SMART-seq in adult mouse DRGs under naïve and SNL D1 conditions.
(B) Venn diagram of all m6A-tagged genes at SNL D1 and known RAGs.
(C) Scatter plot of expression levels of m6A-tagged transcripts under naïve and SNL D1 conditions. Lines indicate 2 fold differences and RAGs are indicated by magenta dots.
(D) Heatmap diagrams of the m6A transcript levels under naïve and SNL D1 conditions for a select group of RAGs and genes related to protein translation functions.
(E) m6A-MeRIP Q-PCR validation of differential m6A transcript levels under naïve and SNL D1 conditions for selected RAGs. Values are normalized to the naïve condition and represent mean ± SEM (n = 3 experimental replications from 6 animals; *P < 0.05; **P < 0.01; t-test).
(F) GO enrichment analyses of the top 400 genes with increased m6A-tagged transcript levels (orange) and the top 400 genes with decreased m6A-tagged transcript levels (black) at SNL D1.
(G) Scatter plots of log2 fold changes of m6A-tagged and total transcript levels between naïve and SNL D1 conditions. Subsets of genes are labeled with different colors in the same plot: RAGs (magenta), ribosomal subunit-related genes (red), translation initiation-related genes (blue), and translation regulation-related genes (yellow).
See also Figure S1.
We next performed a quantitative comparison of m6A-tagged mRNA levels between naïve and SNL D1 conditions. A total of 182 m6A-tagged genes were substantially upregulated, while few were downregulated (fold change ≥ 2; Figure 1C and Table S2). Therefore, consistent with m6A immunostaining results (Figure S1A–C), peripheral nerve injury mostly elevates m6A-tagged transcript levels. Notably, 30 RAGs, including Atf3 (Fagoe et al., 2015; Seijffers et al., 2007), Sox11 (Jankowski et al., 2009), Gadd45a (Befort et al., 2003), and Tet3 (Weng et al., 2017), exhibited increased levels of m6A-tagged transcripts at SNL D1 (Figure 1C–D). We validated our m6A-SMART-seq results for a select group of RAGs using m6A-MeRIP Q-PCR analysis of independent biological samples (Figure 1E and Table S3).
We further performed nonbiased Gene Ontology (GO) analysis for upregulated m6A-tagged transcripts. Notably, the most enriched biological term was translation, followed by metabolism-related process (Figure 1F). For example, many transcripts encoding ribosomal subunit proteins, such as Rps14, Rps20, Rps23, Rps28 and Rps29, and eukaryotic initiation factors, such as Eif1a and Eif3b, exhibited elevated levels of m6A-tagged transcripts at SNL D1 (Figure 1D).
For a given gene, an increase in the m6A-tagged transcript level could be due to elevated total transcript levels without changes in the proportion of tagged transcripts, or increased tagging with or without affecting the total transcript level. Therefore, we compared fold changes in m6A-tagged and total transcript levels between naïve and SNL D1 conditions. The majority of RAGs, such as Atf3, Sox11, and Jun, exhibited a correlated increase in both m6A-tagged and total transcript levels upon SNL, whereas most ribosomal subunit genes with increased m6A-tagged transcript levels did not alter their total transcript levels (Figure 1G). Taken together, these quantitative analyses reveal that peripheral nerve injury mostly up-regulates m6A levels in DRGs with an enrichment of transcripts related to RAGs and protein translation machinery, involving both transcription activation-coupled m6A methylation and an increased proportion of m6A-tagged transcript levels without transcription upregulation.
Single-base m6A Mapping Reveals a Dynamic m6A Landscape in Response to Injury
While our m6A-SMART-seq approach can quantify the amount of m6A-tagged transcripts, it does not identify the location of m6A sites within transcripts. We next performed m6A-CLIP-seq, which provides single-base resolution mapping of m6A across the transcriptome (Linder et al., 2015). Similarly, we adapted the SMART2-seq technology to overcome the small amount of mRNA input from L4/L5 DRGs (named m6A-CLIP-SMART-seq; Figure S2A–B and Table S4). Under both naïve and SNL D1 conditions, we identified m6A sites enriched in exons and near transcription start sites and stop codons across transcriptomes (Figure S2C–D), which is similar to previous findings from cell lines (Linder et al., 2015).
Consistent with our m6A-SMART-seq results (Figure 1A), m6A-CLIP-SMART-seq showed that the majority of m6A-tagged transcripts was shared between naïve and SNL D1 conditions (Figure 2A). Notably, there were dynamic changes in m6A sites (Figure 2B). Some transcripts exhibited a gain and/or loss of m6A sites across the 5′ UTR, coding regions, and 3′ UTR, whereas other transcripts displayed region-specific changes (Figure 2B and Table S5). Multiple RAGs, such as Atf3 and Tet3, gained new m6A sites upon SNL (Figure 2C). Notably, transcripts encoding retrograde injury signaling molecules, such as Vimentin (Vim) (Perlson et al., 2005), exhibited dynamic m6A sites upon SNL (Figure 2C). In general, RAG transcripts exhibited a larger gain in m6A sites compared to non-RAG transcripts and new sites were located mostly in coding regions, whereas ribosomal subunit-related genes exhibited a similar gain in m6A sites as other genes (Figure 2D). Across the transcriptome, GO analysis showed that transcripts with newly added m6A sites were enriched for axonal regulation, whereas transcripts with a loss of m6A sites were enriched for presynaptic functions of neurons (Figure 2E).
Figure 2. SNL modifies the m6A landscape of transcriptomes of adult mouse DRGs in vivo.
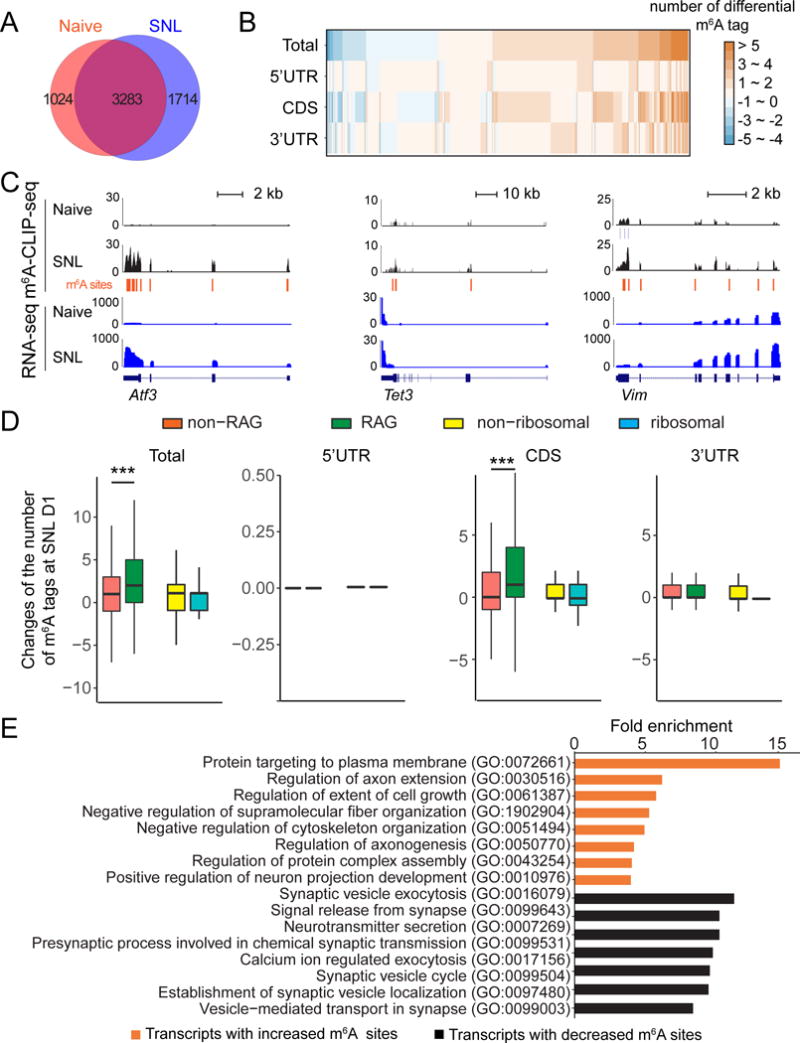
(A) Venn diagram of m6A tagged-transcripts identified by m6A-CLIP-SMART-seq in adult mouse DRGs under naïve and SNL D1 conditions.
(B) Dynamic changes of m6A sites in transcripts from adult DRGs at SNL D1. Cn8ghanges of m6A sites are plotted for the whole transcript (total) and in different sub-transcript regions (5′ UTR, CDS, and 3′ UTR). CDS: coding sequence region.
(C) m6A-CLIP-SMART-seq examples for multiple RAGs. Shown are sample tracks for both m6A-CLIP-seq (top panels) and RNA-seq (bottom panels). CLIP unique tag coverage is shown in black, and m6A sites are indicated with vertical lines.
(D) Comparison of dynamic m6A sites between RAGs and non-RAGs, and between transcripts encoding ribosomal subunit-related and non-ribosomal subunit-related proteins, in different transcript regions (total, 5′ UTR, CDS, and 3′ UTR) under naïve and SNL D1 conditions. Values represent mean differential m6A tag numbers (n = 154 RAGs and 5,867 non RAGs; n = 55 ribosomal subunit-related and 5,966 non-ribosomal subunit-related genes; ***P < 0.001; one-way ANOVA with Tukey’s post hoc test).
(E) GO enrichment analyses of transcripts with differential m6A sites at SNL D1.
See also Figure S2.
We next cross-compared m6A-seq and m6A-CLIP-seq datasets. While many RAGs exhibited increased m6A-tagged transcripts and gained new m6A sites, most transcripts encoding protein translation machinery components showed increased m6A-tagged transcript levels, but not new m6A sites (Figure S2E). Together, our quantitative and single-base m6A mapping reveals a dynamic landscape of mRNA methylation in adult DRGs in response to injury.
Mettl14 Regulates Injury-induced de novo Protein Synthesis
To determine the function of m6A in the adult DRG, we examined conditional knockout mice of Mettl14 (Yoon et al., 2017), a core subunit of the mammalian m6A methyltransferase complex (Wang et al., 2017). We deleted Mettl14 specifically in post-mitotic neurons in vivo using the Syn1-Cre; Mettl14f/f (cKO) model. We confirmed Mettl14 deletion in adult DRGs at the protein level by Western blot (Figure S3A). Quantitative dot blot analysis showed largely diminished m6A levels in purified mRNA from cKO DRGs compared to wildtype (WT) littermates (Figure 3A).
Figure 3. Mettl14 deletion attenuates SNL-induced global protein translation and ATF3 protein expression in the adult DRG.
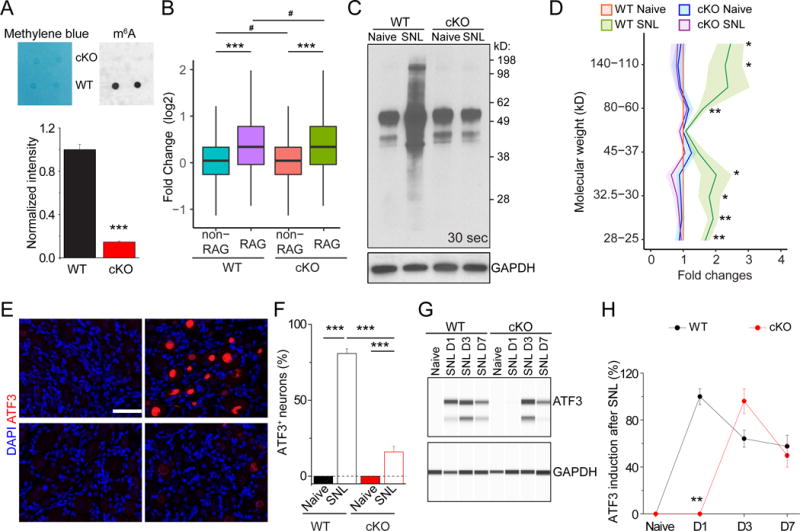
(A) m6A dot-blot showing diminished m6A levels in mRNA from DRGs of adult Syn-Cre;Mettl14 cKO mice. Methylene blue was used to assess the equal loading of mRNA. Representative images (top panel) and quantification (bottom panel) are shown. Values represent mean ± SEM (n = 2 animals per group; ***P < 0.001; two-way ANOVA).
(B) Box plots depicting the fold changes of the gene expression level between RAGs and non-RAGs after injury in WT and Mettl14 cKO DRGs. Each box shows the first quartile, median, and third quartile (***P < 0.001; #P > 0.05; one-way ANOVA with Tukey’s post hoc test).
(C-D) SUnSET analysis of new protein synthesis in adult L4/5 DRGs of WT and Mettl14 cKO mice. De novo synthesized proteins were pulse-chase labeled for one hour after injection of puromycin at SNL D1. Western blot of DRG lysates were performed for different conditions. GAPDH was used as the loading control. Representative images (C) and quantification (D) are shown. Values are normalized to the WT naïve condition and plots represent ranges of mean ± SEM (n = 4 animals; **P < 0.01; *P < 0.05; two-way ANOVA). See Figure S3E for images from different exposures of the same Western blot example.
(E-F) Assessment of ATF3 induction in WT and Mettl14 cKO DRGs at SNL D1. Sample images of ATF3 immunostaining (E) and quantification (F) are shown. Scale bars: 50 μm. Values represent mean ± SEM (n = 4 animals; ***P < 0.001; two-way ANOVA).
(G-H) Time-course analysis of ATF3 induction in WT and Mettl14 cKO adult DRGs. Immunoassay of DRG protein lysates were performed by capillary electrophoresis. GAPDH was used as the loading control. Sample images of blots (G) and quantification (H) are shown. Values represent mean ± SEM (n = 3 animals; **P < 0.01; two-way ANOVA).
See also Figure S3.
m6A methylation has been implicated in regulating both mRNA decay and protein translation of tagged transcripts (Zhao et al., 2017a). To examine the potential impact of m6A on total mRNA levels, we performed RNA-seq analysis of adult DRGs from WT and Mettl14 cKO mice under both naïve and SNL D1 conditions (Table S6). We found very similar gene expression profiles between WT and cKO DRGs, under both naïve and injury conditions (Figure S3B). For RAGs, we also observed similar induction in WT and cKO DRGs (Figure 3B). Therefore, the impact of m6A methylation on total transcript levels appears to be minimal under our experimental conditions.
We next examined the effect of Mettl14 deletion on protein translation in the adult DRG. We employed the SUnSET assay in vivo to label nascent proteins with puromycin (Goodman et al., 2011; Schmidt et al., 2009) (Figure S3C). Analysis of WT adult DRGs showed a global increase of new protein synthesis at SNL D1 (Figure 3C–D and S3D), indicating that peripheral nerve lesion promotes protein translation in the cell body as part of the injury response. In Mettl14 cKO DRGs, SNL-induced protein synthesis was significantly reduced globally compared to WT DRGs, whereas the basal level under the naïve condition was similar to WT (Figure 3C–D and S3D). To validate our result using an independent approach, we examined Atf3, one of the most robustly induced genes by SNL, which has been shown to enhance peripheral nerve regeneration by increasing the intrinsic growth competence of adult DRG neurons (Fagoe et al., 2015; Seijffers et al., 2007). The Atf3 mRNA was also induced in Mettl14 cKO DRGs, although at a lower level compared to WT at SNL D1 (Figure S3E). We confirmed the loss of m6A methylation in Atf3 mRNA in Mettl14 cKO DRGs (Figure S3F). Immunostaining showed little ATF3 protein expression under the naïve condition, in contrast to robust induction at SNL D1 in WT adult DRGs (Figure 3E–F). This induction was drastically reduced in Mettl14 cKO DRGs at SNL D1 (Figure 3E–F). Using quantitative Western blot analysis, further time course analysis showed a delayed induction of ATF3 protein in Mettl14 cKO DRGs (Figure 3G–H). Together, these results indicate that Mettl14-mediated m6A methylation is critical for SNL-induced protein translation in adult DRGs in vivo, which is known to promote axon regeneration of mature mammalian neurons (Abe et al., 2010).
Mettl14 is Required for Robust DRG Neuron Axon Regeneration and Behavioral Recovery
We next directly examined the functional role of Mettl14 on axon regeneration of DRG neurons after injury. We first used an in vitro neurite outgrowth assay with primary neurons from adult mouse DRGs (Chen et al., 2016). Cultures were infected with AAV2 to express the shRNA against Mettl14 (Wang et al., 2014), followed by re-plating to mimic axotomy. We found that expression of shRNA-Mettl14 reduced the length of the longest neuronal process of each neuron compared to expression of shRNA-control, indicating an important role of Mettl14 in axon regeneration of DRG neurons (Figure S4A–B).
We next assessed the in vivo role of Mettl14 in functional axon regeneration of adult DRG neurons after SNL. To avoid potential complications of Mettl14 deletion on DRG neuronal development and maturation in the Syn1-Cre;Mettl14f/f cKO model, we instead infected L4/L5 DRGs in adult Mettl14f/f mice via targeted intrathecal injection of AAV2/9 expressing Cre (Weng et al., 2017). This approach leads to infection of over 70% of all neurons, but not surrounding satellite glia, in L4/5 DRGs (Figure S4C) (Weng et al., 2017). Regenerating sensory axons were identified by SCG10 immunostaining at SNL D3 (Shin et al., 2014). We found that extension of SCG10+ axons was substantially decreased in AAV-Cre;Mettl14 cKO mice compared to WT littermates (Figure 4AB). Similar results were obtained from the Syn1-Cre;Mettl14f/f cKO model (Figure S4D–E). We observed minimal cleaved-caspase 3 expression in the adult DRG upon SNL, indicating that cell death is not a major factor under these conditions (Figure S4F).
Regenerating axons of sciatic nerves extend to the epidermis and start to re-innervate the skin of the hind paw around 2-3 weeks after injury (Weng et al., 2017). Analysis of skin biopsies showed no PGP9.5+ sensory axon innervation to the epidermis of the hind paw at SNL D7, indicating effective degeneration of pre-existing mature axons of both WT and AAV-Cre;Mettl14 cKO DRG neurons (Figure S4G). At SNL D21, innervation to all three epidermal zones by regenerating axons in adult AAV-Cre;Mettl14 cKO mice was significantly reduced compared to those in WT mice, but no difference was observed under the naïve condition (Figure 4C–D).
Figure 4. Mettl14 deletion attenuates functional axon regeneration of adult DRG neurons in vivo.
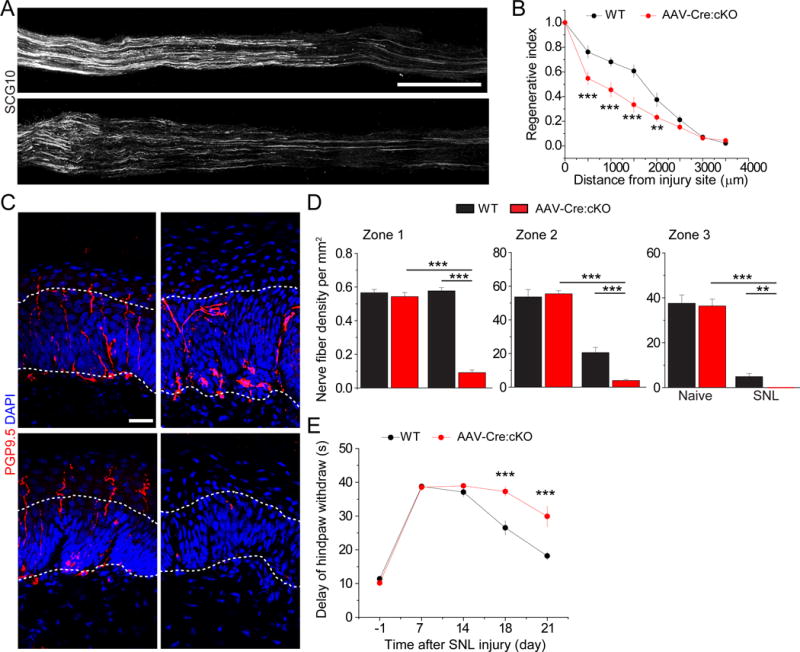
(A-B) Analysis of regeneration of sensory axons by SCG10 immunostaining at SNL D3 in adult WT and Mettl14f/f mice upon intrathecal injection of AAV2/9 to express Cre. Sample images of regenerating sensory axons identified by SCG10 (A; scale bar: 1 mm) and quantification (B) are shown. SCG10 immunofluorescence intensity was measured at different distal distances and normalized to that at 1 mm before the lesion site as the regenerative index. Values represent mean ± SEM (n = 8 animals for WT and 10 animals for AAV-Cre;Mettl14 cKO; ***P < 0.001; **P < 0.01; two-way ANOVA).
(C-D) Assay for re-innervation of the hindpaw epidermal area by regenerating sensory axons. Sample images of cross sections of hindpaw glabrous skin of WT and AAV-Cre;Mettl14 cKO mice immunostained with the pan neuronal marker PGP9.5 are shown (C). The dotted line indicates the border between dermis and epidermis. Scale bar: 20 μm. Also shown are quantifications of the number of intra-epidermal nerve fibers in a 1 mm segment of different epidermal areas (D). Values represent mean ± SEM (n = 5 animals per group; ***P < 0.001; **P < 0.01; two-way ANOVA).
(E) Assessment of thermal sensory recovery after SNL in WT and AAV-Cre;Mettl14 cKO mice. Values represent mean ± SEM (n = 10 animals per group; ***P < 0.001; two-way ANOVA).
See also Figure S4.
To further assess the functional outcome on axon regeneration, we performed a behavioral test to quantify the latency of heat-evoked hind paw withdrawal (Wright et al., 2014). Both WT and AAV-Cre;Mettl14 cKO animals exhibited similar response latencies to a radiant thermal stimulus at SNL D1 and D7 (Figure 4E). Starting from SNL D18, the withdrawal latency gradually recovered in the WT group, but only minimally recovered in Mettl14 cKO animals (Figure 4E). Together, these results indicate an essential role of m6A mRNA methylation in functional sensory axon regeneration of adult DRG neurons in vivo.
YTHDF1 is Required for SNL-induced Global Protein Synthesis and Robust Axon Regeneration of DRG Neurons
To further support our model that m6A signaling promotes global protein synthesis and axon regeneration upon injury and to investigate the downstream mechanism, we examined the KO mice of Ythdf1 (Figure S5A), an m6A reader that has been implicated in promoting protein translation efficacy of m6A-tagged transcripts in cell lines (Shi et al., 2017; Wang et al., 2015). Q-PCR analysis showed similar induction of RAGs at mRNA levels in WT and Ythdf1 KO adult DRGs (Figure S5B). In contrast, the SUnSET assay revealed a marked reduction of SNL-induced global de novo protein synthesis in adult DRGs of Ythdf1 KO mice (Figure 5A–B and S5C). Similar to Mettl14 deletion, the extension of regenerating SCG10+ axons was substantially reduced in Ythdf1 KO mice compared to WT mice at SNL D3 (Figure 5C–D). We observed minimal cleaved-caspase 3 expression in the adult DRG in WT and Ythdf1 cKO mice upon SNL (Figure S5D). Together, these results further support our model and identify YTHDF1 as a key player in promoting injury-induced protein translation and axon regeneration of adult DRGs in vivo.
Figure 5. YTHDF1 is required for injury-induced global de novo protein synthesis and robust axon regeneration of adult DRG neurons.
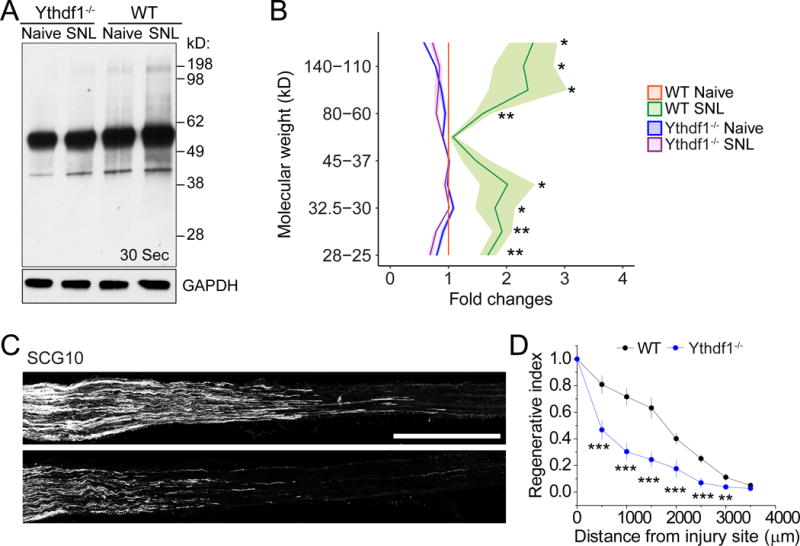
(A-B) SUnSET analysis of new protein synthesis in adult L4/5 DRGs of WT and Ythdf1 KO mice. De novo synthesized proteins were pulse-chase labeled for one hour after injection of puromycin at SNL D1. Western blot of DRG lysates were performed for different conditions. GAPDH was used as the loading control. Representative images (A) and quantification (B) are shown. Values are normalized to WT naïve conditions and plots represent ranges of mean ± SEM (n = 3 for WT and Ythdf1 KO each; ***P < 0.01; **P < 0.01; two-way ANOVA). See Figure S5C for images from different exposures of the same Western blot example.
(C-D) Analysis of regeneration of sensory axons by SCG10 immunostaining at SNL D3 in adult WT and Ythdf1 KO mice. Sample images of regenerating sensory axons identified by SCG10 (C; scale bar: 1 mm) and quantification (D) are shown. SCG10 immunofluorescence intensity was measured at different distal distances and normalized to the level 1 mm before the lesion site as the regenerative index. Values represent mean ± SEM (n = 7 animals for WT and 6 animals for Ythdf1 KO mice; ***P < 0.001; **P < 0.01; two-way ANOVA).
See also Figure S5.
Mettl14 is Required for Pten Deletion-induced Robust Axon Regeneration of Adult Retinal Ganglion Neurons
Finally, we assessed whether m6A signaling is also involved in axon regeneration in the adult CNS. We employed the model of Pten deletion-induced axon regeneration of retinal ganglion cells (RGCs) in adult mice (Park et al., 2008). We co-expressed Cre and shRNA-Mettl14 in adult RGCs by AAV, followed by axotomy, axonal labeling and analysis in Pten cKO or WT mice (Park et al., 2008). Expression of shRNA-Mettl14 alone did not lead to any axon regeneration of RGCs (Figure S6A), but markedly attenuated Pten deletion-induced regeneration compared to shRNA-control (Figure 6A–B). The ratio of phospho-S6+ RGCs remained the same between expression of shRNA-control and shRNA-Mettl14 in Pten cKO mice (Figure 6C–D), suggesting that blockage of axon regeneration is not likely to be due to the inactivation of mTOR signaling. Notably, there was a 35.1% reduction in the number of Tuj1+ RGCs upon Mettl14 knockdown in the Pten cKO mice, but not in the WT mice (Figure 6D and S6B–C), suggesting that Mettl14 is also involved in Pten deletion-induced survival of RGCs. There was a larger decrease in the number of regenerating axons at all distances examined (53.3% reduction on average; Figure 6B), indicating that the survival effect alone could not explain the impact of Mettl14 knockdown on axon regeneration in Pten cKO mice. Previous studies have shown that survival rates varied dramatically among neuronal subtypes in the adult retina, with SMI32+ alpha-RGCs (αRGCs) surviving preferentially and accounting for nearly all axon regeneration following Pten deletion (Duan et al., 2015). We found that the percentage of αRGCs among surviving Tuj1+ RGCs was not affected by Mettl14 knockdown in Pten cKO mice (Figure S6D). Together, these result suggest that Mettl14 promotes both survival and axonal extension of Pten−/− RGCs after injury in the adult CNS.
Figure 6. Mettl14 is required for robust Pten deletion-induced axonal regeneration of retinal ganglion neurons in the adult mouse CNS.
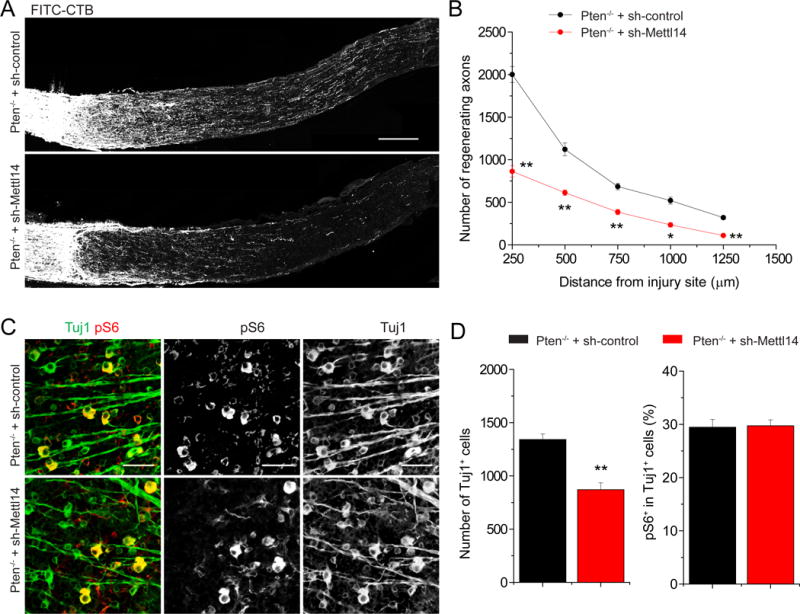
Adult Ptenf/f mice were co-injected with AAV-Cre and AAV-shRNA-control or AAV-shRNA-Mettl14. Optic nerve was crushed 4 weeks after AAV injection and RGC axons were traced by fluorescence conjugated cholera toxin B (FITC-CTB) 2 weeks later. Shown are sample images of sections of optic nerve containing FITC-CTB-labeled axons (A; scale bar: 200 μm) and quantification of numbers of regenerating axons at different distances from the injury site (B). Values represent mean + SEM (n = 5 animals per group; ** P < 0.01; *P < 0.05; ANOVA followed by Fisher’s LSD). Also shown are sample images of whole-mount retina with Tuj1 (green) and pS6 (red) immunostaining (C; scale bar: 50 μm) and quantification of densities of Tuj1+ RGCs and percentages of Tuj1+ RGCs expressing pS6. Values represent mean + SEM (n = 5 animals per each group; ** P < 0.01; Student’s t test).
See also Figure S6.
DISCUSSION
Our study reveals a critical role of m6A epitranscriptomic regulation in injury responses and functional axon regeneration in the adult mammalian nervous system in vivo. De novo gene transcription and protein translation are known to be required for robust axon regeneration of adult neurons upon injury and previous studies have identified important roles of transcriptional and epigenetic mechanisms, including both histone and DNA modifications (Cho and Cavalli, 2014; Trakhtenberg and Goldberg, 2012; Weng et al., 2016; Wong and Zou, 2014). Our study reveals another layer of regulation and suggests a model wherein PNS injury elevates methylated mRNA transcripts, including RAGs, which are then subjected to enhanced protein translation for effective axon regeneration. The finding that some epigenetic regulators, such as Tet3 and Gadd45 (Guo et al., 2011; Yao et al., 2016), are m6A-tagged, suggests a potential interaction between epigenetic and epitranscriptomic pathways. Our initial study also suggests a similar role of m6A epitranscriptomic regulation of induced axon regeneration in the adult mammalian CNS.
Mechanistically, our study provides in vivo evidence for a critical role of m6A in promoting protein translation in the mammalian system. Different from previous findings on the in vivo role of m6A-dependent promotion of mRNA decay in regulating embryonic development (Li et al., 2017b; Yoon et al., 2017; Zhang et al., 2017; Zhao et al., 2017b), the impact of m6A methylation on total mRNA levels appears to be minimal in adult mouse DRGs under both basal and injury conditions. Instead, m6A plays a critical role in peripheral nerve injury-induced global protein translation in adult mouse DRGs in vivo via YTHDF1. De novo protein synthesis is known to be critical for axon regeneration in the adult mammalian PNS and CNS (Belin et al., 2015; Cho et al., 2015; Donnelly et al., 2013; Jung et al., 2012; Rishal and Fainzilber, 2014; Song and Poo, 2001; van Niekerk et al., 2016). We have identified three classes of transcripts with substantial m6A-tagging. First, many transcripts encoding RAGs exhibit elevated m6A levels and new m6A sites upon SNL. Second, some retrograde injury signaling molecules exhibit m6A-tagging and increased m6A sites upon SNL. This result raises the possibility that m6A-tagging may promote local protein translation to enhance retrograde signaling upon injury, which is known to be required for robust axonal regeneration of DRG neurons (Rishal and Fainzilber, 2014). Third, many transcripts encoding the molecular machinery for protein translation, including both ribosomal subunits and initial complex components, are themselves m6A-tagged. Therefore, injury may promote global protein translation by augmenting the general translation machinery. Together, this injury-induced reconfiguration of the epitranscriptome may prioritize mechanisms to synthesize critical factors and rapidly turn on the regenerative program. How the specificity of dynamic m6A modification arises is an important question for future investigation. Our detailed analysis of ATF3 in Mett14 cKO mice showed a delayed induction at the protein level (Figure 3G–H), yet functional axon regeneration did not recover (Figure 4E). It is likely that injury induces a coordinated response with a cascade of de novo gene expression and protein synthesis. We thus propose a model wherein m6A methylation is critical for the coordination of new protein synthesis in injury responses, deficits of which lead to defective axon regeneration and functional recovery.
Typically, MeRIP-seq and HITS-CLIP-seq have been used for m6A or protein-RNA interaction profiling. One major technical limitation of these methods is that the library preparation normally requires a large amount of starting material and the process involves multiple tedious steps. To overcome limitations imposed on the quantity of source material imposed by the small number of axotomized DRGs, we developed new methods for library construction to quantify m6A-tagged transcript levels and identify m6A locations. Our approach not only utilizes a template-switching mechanism to avoid effects of ligation bias, but also substantially increases the sensitivity and shortens the processing time. These techniques will allow analyses of epitranscriptomes, including m6A, m1A and potentially other mRNA modifications, in a tissue-specific manner.
m6A levels increase over development in the mouse nervous system (Meyer et al., 2012) and can be dynamically regulated via active demethylation (Jia et al., 2011; Zheng et al., 2013). Recent studies of the m6A demethylase Fto have revealed its critical roles in regulating adult neurogenesis (Li et al., 2017c), memory formation and consolidation (Walters et al., 2017; Widagdo et al., 2016), and local axonal protein translation (Yu et al., 2017). Together with these studies, our findings suggest that m6A mRNA methylation may play a broader role in normal physiology and responses to pathological stimuli in the adult mammalian nervous system.
STAR METHODS
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-Puromycin | Millipore | MABE343; RRID:AB_2566826 |
| Rabbit anti-ATF3 | Santa Cruz | sc-188; RRID:AB_2258513 |
| Rabbit anti-PGP9.5 | AbD Serotec | 7863-0504; RRID:AB_2210505 |
| Rabbit anti-SCG10 | Novus Biologicals | NBP1-49461; RRID:AB_10011569 |
| Rabbit anti-m6A | Synaptic systems | 202003; RRID:AB_2279214 |
| Rabbit anti-Mettl14 | Proteintech | 26158-1-AP; N/A |
| Rabbit anti-cleaved caspase 3 | Invitrogen | 9H19L2; RRID:AB_2532293 |
| Rabbit anti-GAPDH | Abcam | Ab9485; RRID:AB_307275 |
| Mouse anti-GAPDH | EMD Millipore | AB2302; RRID:AB_10615768 |
| Rabbit anti-YTHDF1 | Proteintech | 17479-1-AP; RRID:AB_2217473 |
| Rabbit anti-pS6 | Cell Signaling | 4858; RRID:AB_916156 |
| Mouse anti-SMI32 | BioLegend | 801701; RRID:AB_2564642 |
| Mouse anti-Tuj1 | BioLegend | 801202; RRID:AB_10063408 |
| Mouse anti-Glutamine Synthetase | Santa Cruz | sc-74430; RRID:AB_1127501 |
| Cy2–, Cy3– or Cy5–conjugated secondary antibodies | Jackson ImmunoResearch | 705-225-147;
RRID:AB_2307341 711-165-152; RRID:AB_2307443 715-175-150; RRID:AB_2340819 |
| HRP-conjugated goat anti-mouse IgG | Santa Cruz | sc-2031; RRID:AB_631737 |
| HRP-conjugated goat anti-rabbit IgG | Santa Cruz | sc-2004; RRID:AB_631746 |
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| TRIzol | Thermo Fisher | 15596018 |
| Formaldehyde | Sigma-Aldrich | F8775-25ML |
| N6-Methyladenosine | Sigma-Aldrich | M2780 |
| Puromycin Dihydrochloride | Sigma-Aldrich | P8833-25MG |
| Cholera Toxin B Subunit, FITC Conjugate | Sigma-Aldrich | C1655-5MG |
| Fluoro-Max fluorescent beads | Invitrogen | B0100 |
| Critical Commercial Assays | ||
| Collagenase | Invitrogen | 17100017 |
| Zamboni’s fixative | Newcomer Supply | 1459 |
| RNA fragmentation reagents | Thermo Fisher | AM8740 |
| RNA clean and concentrator | Zymo Research | R1017 |
| SMARTScribe reverse transcriptase | Clontech | 639537 |
| SuperSignal West Dura Extended Duration Substrate | Thermo Fisher | 34075 |
| Protein A Dynabeads | Thermo Fisher | 10002D |
| Fast SYBR Green Master Mix | ABI | 4385612 |
| FastAP | Thermo Fisher | EF0651 |
| E. coli Poly(A) Polymerase | NEB | M0276S |
| Proteinase K | Thermo Fisher | 25530049 |
| SUPERase In RNase Inhibitor | Thermo Fisher | AM2696 |
| mMessage mMachine T7 Ultra kit | ThermoFisher | AM1345 |
| Dynabeads Oligo (dT)25 | ThermoFisher | 61006 |
| SMARTScribe reverse transcriptase | Clontech | 639537 |
| Advantage 2 Polymerase Mix | Clontech | 639201 |
| KAPA HiFi PCR Kits | Kapa Biosystems | KK2502 |
| Agencourt AMPure XP | Beckman Coulter | A63880 |
| EZ-Tn5 Transposase | Epicentre | TNP92110 |
| Rabbit IgG | Cell Signaling | 2729 |
| Deposited Data | ||
| Raw and analyzed data | This paper | GSE106423 |
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| Mouse: Adult C57Bl6/J | Charles River | N/A |
| Mouse: Mettl14fl/fl C57Bl6/J | Yoon et al., 2017 | N/A |
| Mouse: Ythdf1−/− C57Bl6/J | C.H., in preparation | N/A |
| Mouse: Ptenf/f mice C57Bl6/J | Park et al., 2008 | N/A |
| Mouse: Pirt-GCaMP3 C57Bl6/J | Weng et al., 2017 | N/A |
| Mouse: Synapsin1-Cre | Charles River | N/A |
| Recombinant DNA | ||
| AAV2/9 virus | Guo et al., 2011b | N/A |
| AAV2 virus | Weng et al. 2017 | N/A |
| AAV2/9 GFP-Cre virus | UPenn Vector Core | V1656 |
| Sequence-Based Reagents | ||
| qPCR primers | This paper | See Table S5 |
| Control shRNA: CCTAAGGTTAAGTCGCCCTC | Wang, Y. et al. 2014 | N/A |
| Mettl14 shRNA: GCATTGGTGCTGTGTTAAATA | Wany, Y. et al. 2014 | N/A |
| Software and Algorithms | ||
| NeuronJ | Meijering et al., 2004 | RRID:SCR_002074; https://imagescience.org/meijering/software/neuronj/ |
| Image J | NIH | RRID:SCR_003070; https://imagej.nih.gov/ij/ |
| STAR | Dobin, A., 2013 | RRID:SCR_005622; https://github.com/alexdobin/STAR |
| CIMS | Zhang, C., 2011 | https://github.com/chaolinzhanglab/ctk/tree/v1.0.3 |
| Trimmomatic | Bolger, A.M., 2014 | RRID:SCR_011848; http://www.usadellab.org/cms/?page=trimmomatic |
| Samtools | Li, H., 2009 | RRID:SCR_002105;http://samtools.sourceforge.net/ |
| Bedtools | Quinlan, A.Q., 2010 | RRID:SCR_006646; http://bedtools.readthedocs.io/en/latest/ |
| Other | ||
| 30 gauge syringe | Hamilton | |
| Ultra-fine hemostatic forceps | F.S.T. | 13021-12 |
| Radiant heat light source (model 33 Analgesia Meter) | IITC/Life Science Instruments | |
| Confocal Microscope | Zeiss | |
| Applied Biosystems 7500 | ThermoFisher | |
| Dumont number 5 forceps | Roboz | |
| Hybond-N+ membrane | GE Healthcare | RPN2020N |
| UV stratalinker 1800 | Stratagene | |
| 10% Mini-PROTEAN® TGX™ Precast Protein Gels | Bio-rad | 4561033 |
| Trans-Blot® Turbo™ Mini PVDF Transfer Packs | Bio-rad | 1704156 |
| Trans-Blot® Turbo™ Transfer Starter System | Bio-rad | 1704155 |
| Dounce tissue grinder set | Sigma | D9938-1SET |
| Bioruptor plus | Diagenode | |
TABLE WITH EXAMPLES FOR AUTHOR REFERENCE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-Snail | Cell Signaling Technology | Cat#3879S; RRID: AB_2255011 |
| Mouse monoclonal anti-Tubulin (clone DM1A) | Sigma-Aldrich | Cat#T9026; RRID: AB_477593 |
| Rabbit polyclonal anti-BMAL1 | This paper | N/A |
| Biological Samples | ||
| Healthy adult BA9 brain tissue | University of Maryland Brain & Tissue Bank; http://medschool.umaryland.edu/btbank/ | Cat#UMB1455 |
| Human hippocampal brain blocks | New York Brain Bank | http://nybb.hs.columbia.edu/ |
| Patient-derived xenografts (PDX) | Children’s Oncology Group Cell Culture and Xenograft Repository | http://cogcell.org/ |
| Chemicals, Peptides, and Recombinant Proteins | ||
| MK-2206 AKT inhibitor | Selleck Chemicals | S1078; CAS: 1032350-13-2 |
| SB-505124 | Sigma-Aldrich | S4696; CAS: 694433-59-5 (free base) |
| Picrotoxin | Sigma-Aldrich | P1675; CAS: 124-87-8 |
| Human TGF-β | R&D | 240-B; GenPept: P01137 |
| Activated S6K1 | Millipore | Cat#14-486 |
| GST-BMAL1 | Novus | Cat#H00000406-P01 |
| Critical Commercial Assays | ||
| EasyTag EXPRESS 35S Protein Labeling Kit | Perkin-Elmer | NEG772014MC |
| CaspaseGlo 3/7 | Promega | G8090 |
| TruSeq ChIP Sample Prep Kit | Illumina | IP-202-1012 |
| Deposited Data | ||
| Raw and analyzed data | This paper | GEO: GSE63473 |
| B-RAF RBD (apo) structure | This paper | PDB: 5J17 |
| Human reference genome NCBI build 37, GRCh37 | Genome Reference Consortium | http://www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/human/ |
| Experimental Models: Cell Lines | ||
| Hamster: CHO cells | ATCC | CRL-11268 |
| D. melanogaster: Cell line S2: S2-DRSC | Laboratory of Norbert Perrimon | FlyBase: FBtc0000181 |
| Human: Passage 40 H9 ES cells | MSKCC stem cell core facility | N/A |
| Human: HUES 8 hESC line (NIH approval number NIHhESC-09-0021) | HSCI iPS Core | hES Cell Line: HUES-8 |
| Experimental Models: Organisms/Strains | ||
| Streptococcus pyogenes: M1 serotype strain: strain SF370; M1 GAS | ATCC | ATCC:700294 |
| C. elegans: Strain BC4011: srl-1(s2500) II; dpy-18(e364) III; unc-46(e177)rol-3(s1040) V. | Caenorhabditis Genetics Center | WB Strain: BC4011; WormBase: WBVar00241916 |
| D. melanogaster: RNAi of Sxl: y[1] sc[*] v[1]; P{TRiP.HMS00609}attP2 | Bloomington Drosophila Stock Center | BDSC:34393; FlyBase: FBtp0064874 |
| S. cerevisiae: Strain background: W303 | ATCC | ATTC: 208353 |
| Mouse: R6/2: B6CBA-Tg(HDexon1)62Gpb/3J | The Jackson Laboratory | JAX: 006494 |
| Mouse: OXTRfl/fl: B6.129(SJL)-Oxtrtm1.1Wsy/J | The Jackson Laboratory | RRID: IMSR_JAX:008471 |
| Zebrafish: Tg(Shha:GFP)t10: t10Tg | Neumann and Nuesslein-Volhard, 2000 | ZFIN: ZDB-GENO-060207-1 |
| Arabidopsis: 35S::PIF4-YFP, BZR1-CFP | Wang et al., 2012 | N/A |
| Arabidopsis: JYB1021.2: pS24(AT5G58010)::cS24:GFP(-G):NOS #1 | NASC | NASC ID: N70450 |
| Recombinant DNA | ||
| pLVX-Tight-Puro (TetOn) | Clonetech | Cat#632162 |
| Plasmid: GFP-Nito | This paper | N/A |
| cDNA GH111110 | Drosophila Genomics Resource Center | DGRC:5666; FlyBase:FBcl0130415 |
| AAV2/1-hsyn-GCaMP6-WPRE | Chen et al., 2013 | N/A |
| Mouse raptor: pLKO mouse shRNA 1 raptor | Thoreen et al., 2009 | Addgene Plasmid #21339 |
| Sequence-Based Reagents | ||
| siRNA targeting sequence: PIP5K I alpha #1: ACACAGUACUCAGUUGAUA | This paper | N/A |
| Primers for XX, see Table SX | This paper | N/A |
| Primer: GFP/YFP/CFP Forward: GCACGACTTCTTCAAGTCCGCCATGCC | This paper | N/A |
| Morpholino: MO-pax2a GGTCTGCTTTGCAGTGAATATCCAT | Gene Tools | ZFIN: ZDB-MRPHLNO-061106-5 |
| ACTB (hs01060665_g1) | Life Technologies | Cat#4331182 |
| RNA sequence: hnRNPA1_ligand: UAGGGACUUAGGGUUCUCUCUAGGGACUUAGGGUUCUCUCUAGGGA | This paper | N/A |
| Software and Algorithms | ||
| Bowtie2 | Langmead and Salzberg, 2012 | http://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| Samtools | Li et al., 2009 | http://samtools.sourceforge.net/ |
| Other | ||
| Sequence data, analyses, and resources related to the ultra-deep sequencing of the AML31 tumor, relapse, and matched normal. | This paper | http://aml31.genome.wustl.edu |
| Resource website for the AML31 publication | This paper | https://github.com/chrisamiller/aml31SuppSite |
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact Guo-li Ming (gming@pennmedicine.upenn.edu). There are no restrictions on any data or materials presented in this paper.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
All animal procedures used in this study were performed in accordance with the protocol approved by the Institutional Animal Care and Use Committee of Johns Hopkins University School of Medicine, University of Pennsylvania School of Medicine, and The Hong Kong University of Science and Technology. Six mouse lines were used for this study: C57Bl6/J mice, Pirt-GCaMP3 mice (Kim et al., 2016), Mettl14f/f mice (Yoon et al., 2017); Syn1-Cre mice (JAX 003966), Ythdf1−/− mice (manuscript in preparation), and Ptenf/f mice (Bonaguidi et al., 2011). Adult mice (6-8 weeks) were used. Housing and husbandry conditions followed standard settings. Experimental and control mice were male littermates housed together before the experiment.
METHOD DETAILS
AAV constructs
The recombinant AAV2/9 vectors for Cre and GFP were from the UPenn Vector Core. AAV2 for shRNA-control and shRNA-Mettl14 were constructed and prepared in house.
Animal surgery
Intrathecal injection of AAV2/9 was performed in adult 6-8 weeks old male mice as previously described (Weng et al., 2017). Briefly, 3 μl of viral solution was injected into the cerebrospinal fluid between vertebrae L5 and L6 using a 30 gauge Hamilton syringe slowly over 2 min followed an additional 2-minute wait to allow the fluid to diffuse. Following the injection, the mice were left undisturbed for 3 weeks for recovery and for complete dissemination of the virus.
For sciatic nerve lesion (SNL), mice were anesthetized and a small incision was made on the skin at the mid-thigh level. The sciatic nerve was exposed after opening the fascial plane between the gluteus superficialis and biceps femoris muscles. The nerve was carefully freed from surrounding connective tissue and then crushed for 15 s at 3 clicks of ultra-fine hemostatic forceps (F.S.T. 13021-12). The crush site was labeled by Fluoro-Max dyed blue aqueous fluorescent particles (ThermoFisher; B0100; Figure S4D). Skin was then closed with two suture clips. For the sham surgery (the naïve conditions), the sciatic nerve in the contralateral side was exposed and mobilized but left uninjured. For the thermal withdrawal test and skin biopsy experiments, the saphenous nerve was ligated and transected above the knee region after sciatic nerve crush, so that the hindpaw epidermis could only be innervated by regenerating sciatic nerve axons.
For optic nerve injury, the procedure was performed as previously described (Park et al., 2008). Briefly, individual AAV-shRNA-control or AAV-shRNA-Mettl14 was mixed with AAV-Cre and intravitreally injected to the left eye of adult WT or Ptenf/f mice. Two weeks after viral injection, the left optic nerve was exposed intraorbitally and crushed with jeweler’s forceps (Dumont number 5; Roboz) for 5 s, approximately 1 mm behind the optic disc. To visualize regenerating axons, RGC axons in the optic nerve were anterogradely labeled by 1 μl of cholera toxin β subunit (CTB; 2 μg/μl; Invitrogen) 12 days after injury. Animals were fixed by 4% PFA 2 days after CTB injection in the eye. Quantification of regenerating axons was also performed according the previously described method (Park et al., 2008).
DRG cultures and neurite outgrowth assay
DRG primary culture was performed as previously described (Chen et al., 2016). Briefly, mice were anesthetized and perfused with sterile PBS. L4-L6 DRGs were dissected, washed in cold HBSS medium and then digested in 0.1% collagenase (Invitrogen; 17100017) in HBSS for 90 min. After triturated into single cell suspension, DRG neurons were precipitated at room temperature for 20 min and plated on a coated culture dish. Cultures were infected with AAV co-expressing Cre and different shRNAs (See KEY RESOURCE TABLE). For re-plating, DRG neurons cultured with AAV for 10 days were gently flushed, resuspended and replated on a coated culture dish. The replated neurons were cultured for another one day and fixed for Tuj1 staining. The average lengths of longest neurites from each DRG neurons were measured and quantified by ImageJ.
m6A-SMART-seq
mRNA from total RNA of adult mouse DRGs was purified with Dynabeads Oligo (dT)25 (ThermoFisher; 61006). Five μg of anti-m6A polyclonal antibody (Synaptic Systems; 202003) was conjugated to Dynabeads Protein A (ThermoFisher; 10001D) overnight at 4°C. A total of 150 ng of mRNA was then incubated with the antibody/beads in 1× IP buffer (10 mM Tris-HCL, 150 mM NaCl, and 0.1% (vol/vol) Igepal CA-630), supplemented with 200 U SUPERase In RNase Inhibitor (ThermoFisher; AM2696), for 2 hr at 4°C. After incu bation, the beads were washed 3 times with IP buffer and the m6A RNA was eluted twice with 6.7 mM N6-Methyladenosine (Sigma-Aldrich; M2780) in 1× IP buffer. The eluted RNA was extracted with Trizol reagent (ThermoFisher; 15596018) and recovered by RNA Clean and Concentrator-5 spin columns (Zymo; R1015). The equivalent amount of input and m6A-IPed RNA were prepared for library generation using the SMART-seq protocol as described (Picelli et al., 2014). Two biological replicates of naïve and SNL conditions were sequenced using Illumina NextSeq 500.
m6A-CLIP-SMART-seq
A total of 150 ng mRNA was first fragmented to ~ 100 nt by RNA Fragmentation Reagent (ThermoFisher; AM8740) at 70°C for 8 min. After the reaction was stopped by 100 mM EDTA, the RNA mixtures were then directly diluted to 450 μl CLIP buffer (150 mM NaCl, 0.1% NP-40, 10 mM Tris-HCl (pH 7.4)) with 5 g anti-m6A polyclonal antibody (Synaptic Systems; 202003) and incubated at 4 °C for 2 hr. Anti-m6A antibody - RNA interactions were stabilized with UV crosslinking (254 nm, 150 mJ/cm2) twice in a Stratalinker (Agilent). Antibody-RNA complexes were then precipitated with 50 l Protein A/G beads (Thermo Scientific) for 2 hr at 4 °C, followed by two stringent washes (50 mM Tris, pH 7.4, 1 M NaCl, 1 mM EDTA, 1% NP-40, 0.1% SDS) and two CLIP buffer washes. After dephosphorylation with 10 U FastAP (ThermoFisher; EF0652) at 10 min at room temperature and polyadenylation with 5U E. coli Poly(A) Polymerase (NEB, M0276S) for 15 min at room temperature on beads, the RNA was then eluted by treatment with proteinase K (Thermo Scientific; 25530049) at 37°C for 1 hr. The eluted RNA was extracted with Trizol reagent (ThermoFisher; 15596018) and recovered by RNA Clean and Concentrator-5 spin columns (Zymo; R1015). The m6A CLIP library was prepared using a modified SMART-seq2 protocol without tagmentation. Briefly, RNA was first primed with customized dT primer (GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG T30VN) and incubated with RT master mix containing customized TSO primer (TCGTCGGCAGCGTCAGATGTGTATAAGAGACArGrGrG) at 42°C for 1.5 hr. Libraries were PCR amplified for 1 9 cycles, size selected via BluePippin and sequenced on Illumina NextSeq 500.
RNA-seq
Total RNA of L4/L5 DRGs was isolated from WT and Syn1-Cre;Mettl14f/f cKO mice, extracted with Trizol reagent (ThermoFisher; 15596018) and recovered by RNA Clean and Concentrator-5 spin columns (Zymo; R1015). The RNAseq library was prepared using the Smart-seq2 protocol. The distribution of fragment sizes was verified. Libraries of three biological replicates under different conditions were uniquely barcoded, pooled at equimolar concentrations, and sequenced on Illumina NextSeq 500 (Su et al., 2017).
Analysis m6A-SMART-Seq and RNA-seq
Adapters were trimmed from original reads using Trimmomatic and low-quality reads were removed. The remaining reads were then mapped to the mouse genome (mm10) using STAR aligner (Dobin et al., 2013). To measure the relative m6A level per gene, the ratio of m6A IP/ Input was first calculated. The Z scores were then obtained by comparing the ratios (m6A IP/ Input) to the mean of the group to reflect the relative m6A level per gene on a transcriptome-wide scale (Batista et al., 2014). GAPDH is barely methylated (validated by m6A-MeRIP Q-PCR) with Z score < 0. We set Z score > 0 as a threshold to obtain genes with modest to high m6A levels. Shared m6A-tagged genes in two biological replicates were identified as high confidence m6A-tagged transcripts for downstream analysis. The gene list of RAGs was obtained from the magenta module in the previous study (Chandran et al., 2016). For the comparison of m6A transcriptomes between naïve and SNL D1 conditions, m6A-tagged transcript levels were presented as mean TPM (Mean transcripts per kilobase million). The top 400 differentially m6A-tagged genes were uploaded to the Panther Classification System for a statistical overrepresentation test.
Analysis of m6A-CLIP-SMART-seq
Adapters, and the first three nucleotides of the sequencing read (derived from the TSO oligo) were removed by Trimmomatic. The remaining reads (> 20 nt) were then mapped to the mouse genome (mm10) using STAR aligner (–outFilterMultimapNmax 1 –outFilterMismatchNoverLmax 0.08 –alignEndsType EndToEnd). After removal of PCR duplicates, uniquely mapped reads were used for CIMS analysis similar to previous studies (Linder et al., 2015; Moore et al., 2014; Zhang and Darnell, 2011). All mutations were considered as signals. The number of overlapping unique tags (k) and the number of tags with mutations (m) at the position were determined using the CIMS algorithm. The m/k was restricted to between 1% and 50% to reduce noise and remove SNP and mis-mapping artifacts. Only m6A residues in DRACH consensus sequence were considered for the downstream analysis. The list of genes with differential m6A-tag numbers (gain > 3 or lost > 2) was uploaded to the Panther Classification System for a statistical overrepresentation test.
m6A-MeRIP Q-PCR
The m6A-modified control spike-in RNA (eGFP, 0.7 kb) was synthesized by in vitro transcription using the mMessage mMachine T7 Ultra kit (ThermoFisher; AM1345). A total of 150 ng of input mRNA was mixed with 10 pg of control spike-in RNA and subjected to m6A-IP as described above. Immunoprecipitated RNA was purified and reverse-transcribed with Oligo (dT) primer using SMARTScribe Reverse Transcriptase (Clontech; 639537). Indicated genes were analyzed by Q-PCR using Fast SYBR Green Master Mix (ThermoFisher; 4385612) and normalized to the spike-in eGFP RNA levels. Relative fold change was calculated as the ratio of normalized transcript levels between naïve and SNL D1 conditions. Primer sequences are listed in Table S3.
m6A dot blot analysis
mRNA was harvested from homogenized WT and Mettl14 cKO DRGs using Dynabeads mRNA Direct Purification Kit (Ambion; 61011). Three biological replicates were pooled for each sample to ensure sufficient concentration of mRNA. Duplicates of 100 ng mRNA per 1 μl were applied to an Amersham Hybond-N+ membrane (GE Healthcare) as previously described (Yoon et al., 2017). UV crosslinking of RNA to the membrane was performed by running the auto-crosslink program twice in Stratalinker 2400. The membrane was then washed in PBST three times and blocked with 5% skim milk in PBST for 2 hr. After an additional PBST wash, primary anti-m6A antibody (Synaptic Systems; 212B11) at 1:1000 dilution was applied for 2 hr incubation at room temperature. After 3 washes in PBST, the membrane was incubated in HRP-conjugated anti-mouse IgG secondary antibody for 2 hr at room temperature, then washed again 3 times in PBST. Finally, the signal was visualized using SuperSignal West Dura Extended Duration Substrate (Thermo Scientific; 34075). To confirm equal mRNA loading, the same membrane was stained with 0.02% methylene blue in 0.3 M sodium acetate (pH 5.2). Quantified m6A levels were normalized to the amount of mRNA loaded.
Immunohistology, imaging and analysis
Immunohistology was performed as described previously (Weng et al., 2017). Briefly, samples were collected from perfused animals, post-fixed overnight in 4% PFA in PBS, and cryoprotected in 30% sucrose (wt/vol) for 24 hr at 4°C. Samples were sec tioned to 20 m and mounted onto slides. Primary antibody was applied at 4°C overnight. Seco ndary antibody was applied for 2 hr at room temperature. The following primary antibodies were used in this study: rabbit anti-m6A (Synaptic Systems; 212B11; 1:2000), rabbit anti-ATF3 (Santa Cruz; sc-188; 1:500), rabbit anti-PGP9.5 (AbD Serotec; 7863-0504; 1:800), rabbit anti-SCG10 (Novus Biologicals; NBP1-49461, 1: 2000), and anti-cleaved (active) form of caspase 3 (Invitrogen; 9H19L2; 1:500). Secondary antibodies corresponding to the primary antibody species were Cy2–, Cy3– or Cy5 conjugated (Jackson ImmunoResearch; 1:500). The images were acquired by confocal microscopy (Zeiss 710) and analyzed with ImageJ software (National Institutes of Health).
Quantification of the proportion of ATF3+ neurons was determined by counting and scoring at least 200 neurons/mouse as ATF3+ or ATF3− (Weng et al., 2017). A cell was scored as ATF3+ if there was any fluorescence above the threshold set in ATF3− cells under the naïve conditions. Sections were randomly chosen from cross-sectioned L4/L5 DRGs.
Measurement of newly synthesized protein
WT and Syn1-Cre;Mettl14f/f (cKO) mice at 8-10 weeks of age were injected with puromycin (10 mg/kg, intraperitoneal, i.p.) at SNL D1. After 1 hr labeling, L4/L5 naïve or injured DRGs were collected and processed for Western blot as previously described (Weng et al., 2017). Briefly, protein samples were separated by 10% Mini-PROTEAN TGX™ Precast Protein Gels (Bio-rad) and transferred to PVDF membrane using the transblot turbo system (Biorad) following manufacturer’s instructions. The membrane was incubated in blocking buffer (5% non-fat dry milk and 0.1% Tween 20 in TBS) for 1 hr at room temperature and then in mouse anti-puromycin antibody (MABE343; Millipore; 1:1000) at 4°C overnight. The blots were washed and incubated in HRP-conjugated goat anti-mouse IgG (Santa Cruz; sc-2031; 1:5000) at room temperature for 1 hr. Membranes were stripped and re-blotted with rabbit anti-GAPDH antibodies (Abcam; ab9485; 1:2000) as the loading control. Newly synthesized protein was quantified by measuring signal intensities at different size ranges from 198 to 15 KDa. Signals were quantified using ImageJ and data were normalized to that of the WT naïve conditions in the same blots.
Capillary electrophoresis immunoassay
The time course of the expression of ATF3 protein was determined by the capillary electrophoresis immunoassay using the Simple Western system as described previously. In brief, L4/L5 naïve or injured DRGs were collected 1, 3, and 7 days post-SNL. Tissues were homogenized in CelLytic M (Sigma; C2978) containing a protease inhibitor cocktail (Sigma; 4693159001). The lysate protein concentration was determined using the Pierce BCA protein assay kit (Thermo Scientific; 23227). Equal amounts of DRG lysates (1 μg) were mixed with Simple Western reagents and loaded to each capillary. The primary antibodies were diluted with antibody diluent (ProteinSimple) at 1:50 for ant-ATF3 (Santa Cruz; sc-188), 1:50 for anti-Mettl14 (Proteintech; 26158-1-AP), and 1:50 for anti-GAPDH (Abcam; ab9485).
Western blot analysis
L4/L5 injured or naive DRGs were rapidly dissected and extracted protein samples were run on 10% Mini-PROTEAN TGX Precast Protein Gels (Bio-rad) and transferred to PVDF membrane. The membrane was blocked overnight in 5% dry milk at 4°C with rocking. Rabbit anti-YTHDF1 antibody (Proteintech; 1:1000) was applied overnight at 4°C followed by HRP-conjugated anti-rabbit IgG antibody (Santa Cruz; 1:10000). Protein loading was verified by mouse anti-GAPDH (EMD Millipore; AB2302).
In vivo DRG axon regeneration assay
To measure regeneration of the sciatic nerve, samples were collected at SNL D3 and prepared as described above. Samples were sectioned longitudinally at 30 m and stained with SCG10 (Novus Biologicals, NBP1-49461). An SCG10 intensity plot was created using average intensities calculated across 10 μm non-overlapping regions and normalized. Intensity was measured by Image J as previously described (Di Maio et al., 2011; Shin et al., 2012; Weng et al., 2017)
Punch biopsies of glabrous footpad skin from hind paws were collected for the quantification of nerve re-innervation (see Figure S2J in (Weng et al., 2017)). The biopsy was prepared and post-fixed in Zamboni’s fixative. Samples were mounted on gelatin-coated slides and stained with rabbit anti-PGP9.5 which visualizes nerve fibers. To quantify regeneration, nerve fiber density was counted across 3 zones (defined in Figure 2F in (Weng et al., 2017)).
Behavioral analysis
The thermal withdrawal behavioral test was performed following a previously established protocol (Wright et al., 2014). Briefly, the mice were placed on a glass surface with a consistent temperature of 30°C. The plantar surface of the hindpaw was hea ted using a focused, radiant heat light source (model 33 Analgesia Meter; IITC/Life Science Instruments, Woodland Hills, CA, USA). A timer linked to the light source was used to measure the paw-withdrawal latency. Only quick hind paw movements away from the stimulus were considered to be a withdrawal response, and seven individual measurements were repeated for each paw (Weng et al., 2017).
Optic nerve regeneration quantifications
Quantification was performed as previously described (Park et al., 2008). For RGC regenerating axon quantification, the number of axons at different distances from the injury site was estimated by the following formula: Σad=πr2× [average axon numbers per mm/t], where r is equal to half the width of the nerve at the counting site, the average number of axons per millimeter is equal to the average of (axon number)/(nerve width) in four sections of one optic nerve, and t is equal to the section thickness (8 μm). For RGC survival and pS6+ RGCs quantification in the whole mount retina preparation, twelve images (three from each quarter, covering from inner to outer retina) of each retina were captured under a confocal microscope. Tuj1+ or pS6+ RGCs were quantified in a blinded fashion. Quantification of SMI32+ RGCs was performed from images of Tuj1 and SMI32 immunostaining on retina sections.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data in figure panels reflect several independent experiments performed on different days.
The number of animals used for experiments is listed in the figure legends. An estimate of variation within each group of data is indicated using standard error of the mean (SEM). We performed two-way ANOVA tests for assessing the significance of differences between two treatments based the data properties or as indicated in the figure legends.
DATA AND SOFTWARE AVAILABILITY
The access number for the data for m6A-SMART-seq, m6A-SMART-CLIP-seq, and RNA-seq reported in this study is NCBI GEO: GSE106423.
Supplementary Material
Table S1. Dataset from m6A-SMART-seq analysis of adult mouse DRGs under naïve and SNL D1 conditions, related to Figures 1 and S2. (See Excel file)
Table S2. Gene list with significantly different levels of m6A-tagged transcripts between naïve and SNL D1 conditions, related to Figures 1 and S2. (See Excel file)
Table S3. List of primer sequences used in the current study, related to Figures 1, S3, and S5. (See Excel file)
Table S4. Dataset from m6A-CLIP-SMART-seq analysis of adult mouse DRGs under naïve and SNL D1 conditions, related to Figures 2 and S2. (See Excel file)
Table S5. List with locations of dynamic m6A sites between naïve and SNL D1 conditions, related to Figures 2 and S2. (See Excel file)
Table S6. Dataset from RNA-seq analysis of WT and Syn1-Cre;Mettl14f/f cKO DRGs under naïve and SNL D1 conditions, related to Figure 3 and S3. (See Excel file)
HIGHLIGHTS.
PNS nerve injury elevates m6A-tagged mRNA encoding RAGs and translational machinery
PNS nerve injury induces dynamic changes in the m6A landscape of adult DRGs
m6A tagging promotes injury-induced global de novo protein synthesis in adult DRGs
m6A signaling is required for robust axon regeneration in adult PNS and CNS
One sentence summary.
m6A methylation promotes axon regeneration\
Acknowledgments
We thank members of Ming and Song laboratories, and The Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (AMRF) investigators for discussion, J. Schnoll and K. Christian for comments, and Y. Cai, L. Liu, and D. Johnson for technical support. This work was supported by grants from AMRF (to G-l.M.), National Institutes of Health (P01NS097206 and RM1HG008935 to H.S., J.P., and C.H., R37NS047344 to H.S., and R35NS097370 to G-l.M.), Hong Kong Research Grants Council (16103315 and 16149316 to K.L.), and National Natural Science Foundation of China (81671214 to K.L.). C.H. is an HHMI investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS: Y.L.W. led the project and was involved in all aspects of the study. Xu W., and K.L. performed in vitro neurite outgrowth of DRG neurons and optic nerve injury studies. R.A. performed surgical procedures and data quantification for DRG studies. T.X., P.J. and H.W. helped with some of the bioinformatics analyses, J.C., C.V., Xinyuan W., Z.H.S.W., J.J., Q.D., and W.Z. contributed to other data collection. L.C.D., X.Z. and C.H. provided Mettl14f/f mice. Yuanyuan L., Yajing L., B.S., X.Z., and C.H. provided Ythdf1−/− mice. Y.L.W., H.S. and G-l.M. designed the project, analyzed the data and wrote the paper. All authors helped prepare the manuscript.
COMPETING FINANCIAL INTERESTS: The authors declare no competing financial interests.
References
- Abe N, Borson SH, Gambello MJ, Wang F, Cavalli V. Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. J Biol Chem. 2010;285:28034–28043. doi: 10.1074/jbc.M110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista PJ, Molinie B, Wang J, Qu K, Zhang J, Li L, Bouley DM, Lujan E, Haddad B, Daneshvar K, et al. m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707–719. doi: 10.1016/j.stem.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Befort K, Karchewski L, Lanoue C, Woolf CJ. Selective up-regulation of the growth arrest DNA damage-inducible gene Gadd45 alpha in sensory and motor neurons after peripheral nerve injury. The European journal of neuroscience. 2003;18:911–922. doi: 10.1046/j.1460-9568.2003.02827.x. [DOI] [PubMed] [Google Scholar]
- Belin S, Nawabi H, Wang C, Tang S, Latremoliere A, Warren P, Schorle H, Uncu C, Woolf CJ, He Z, et al. Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomics. Neuron. 2015;86:1000–1014. doi: 10.1016/j.neuron.2015.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran V, Coppola G, Nawabi H, Omura T, Versano R, Huebner EA, Zhang A, Costigan M, Yekkirala A, Barrett L, et al. A Systems-Level Analysis of the Peripheral Nerve Intrinsic Axonal Growth Program. Neuron. 2016;89:956–970. doi: 10.1016/j.neuron.2016.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Lu N, Ding Y, Wang Y, Chan LT, Wang X, Gao X, Jiang S, Liu K. Rapamycin-Resistant mTOR Activity Is Required for Sensory Axon Regeneration Induced by a Conditioning Lesion. eNeuro. 2016;3 doi: 10.1523/ENEURO.0358-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Cavalli V. HDAC signaling in neuronal development and axon regeneration. Curr Opin Neurobiol. 2014;27:118–126. doi: 10.1016/j.conb.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Park D, Cavalli V. Filamin A is required in injured axons for HDAC5 activity and axon regeneration. J Biol Chem. 2015;290:22759–22770. doi: 10.1074/jbc.M115.638445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Sloutsky R, Naegle KM, Cavalli V. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 2013;155:894–908. doi: 10.1016/j.cell.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun G, Lu Z, Huang Y, Yang CG, et al. m6A RNA Methylation Regulates the Self-Renewal and Tumorigenesis of Glioblastoma Stem Cells. Cell Rep. 2017;18:2622–2634. doi: 10.1016/j.celrep.2017.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers RC, Friderici KH, Rottman FM. Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5′ terminus. Biochemistry. 1975;14:4367–4374. doi: 10.1021/bi00691a004. [DOI] [PubMed] [Google Scholar]
- Di Maio A, Skuba A, Himes BT, Bhagat SL, Hyun JK, Tessler A, Bishop D, Son YJ. In vivo imaging of dorsal root regeneration: rapid immobilization and presynaptic differentiation at the CNS/PNS border. J Neurosci. 2011;31:4569–4582. doi: 10.1523/JNEUROSCI.4638-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- Donnelly CJ, Park M, Spillane M, Yoo S, Pacheco A, Gomes C, Vuppalanchi D, McDonald M, Kim HH, Merianda TT, et al. Axonally synthesized beta-actin and GAP-43 proteins support distinct modes of axonal growth. J Neurosci. 2013;33:3311–3322. doi: 10.1523/JNEUROSCI.1722-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Qiao M, Bei F, Kim IJ, He Z, Sanes JR. Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mTOR signaling. Neuron. 2015;85:1244–1256. doi: 10.1016/j.neuron.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagoe ND, Attwell CL, Kouwenhoven D, Verhaagen J, Mason MR. Overexpression of ATF3 or the combination of ATF3, c-Jun, STAT3 and Smad1 promotes regeneration of the central axon branch of sensory neurons but without synergistic effects. Human molecular genetics. 2015;24:6788–6800. doi: 10.1093/hmg/ddv383. [DOI] [PubMed] [Google Scholar]
- Finelli MJ, Wong JK, Zou H. Epigenetic regulation of sensory axon regeneration after spinal cord injury. J Neurosci. 2013;33:19664–19676. doi: 10.1523/JNEUROSCI.0589-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaub P, Joshi Y, Wuttke A, Naumann U, Schnichels S, Heiduschka P, Di Giovanni S. The histone acetyltransferase p300 promotes intrinsic axonal regeneration. Brain. 2011;134:2134–2148. doi: 10.1093/brain/awr142. [DOI] [PubMed] [Google Scholar]
- Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, Hershkovitz V, Peer E, Mor N, Manor YS, et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science. 2015;347:1002–1006. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- Gilbert WV, Bell TA, Schaening C. Messenger RNA modifications: Form, distribution, and function. Science. 2016;352:1408–1412. doi: 10.1126/science.aad8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CA, Mabrey DM, Frey JW, Miu MH, Schmidt EK, Pierre P, Hornberger TA. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:1028–1039. doi: 10.1096/fj.10-168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Su Y, Zhong C, Ming GL, Song H. Emerging roles of TET proteins and 5-hydroxymethylcytosines in active DNA demethylation and beyond. Cell cycle. 2011;10:2662–2668. doi: 10.4161/cc.10.16.17093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann IU, Bodi Z, Sanchez-Moran E, Mongan NP, Archer N, Fray RG, Soller M. m6A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature. 2016;540:301–304. doi: 10.1038/nature20577. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, McIlwrath SL, Jing X, Cornuet PK, Salerno KM, Koerber HR, Albers KM. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 2009;1256:43–54. doi: 10.1016/j.brainres.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci. 2012;13:308–324. doi: 10.1038/nrn3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, Haripal B, Zucker-Scharff I, Moore MJ, Park CY, et al. A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev. 2015;29:2037–2053. doi: 10.1101/gad.269415.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Anderson M, Park K, Zheng Q, Agarwal A, Gong C, Saijilafu, Young L, He S, LaVinka PC, et al. Coupled Activation of Primary Sensory Neurons Contributes to Chronic Pain. Neuron. 2016;91:1085–1096. doi: 10.1016/j.neuron.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lence T, Akhtar J, Bayer M, Schmid K, Spindler L, Ho CH, Kreim N, Andrade-Navarro MA, Poeck B, Helm M, et al. m6A modulates neuronal functions and sex determination in Drosophila. Nature. 2016;540:242–247. doi: 10.1038/nature20568. [DOI] [PubMed] [Google Scholar]
- Li A, Chen YS, Ping XL, Yang X, Xiao W, Yang Y, Sun HY, Zhu Q, Baidya P, Wang X, et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell research. 2017a;27:444–447. doi: 10.1038/cr.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HB, Tong J, Zhu S, Batista PJ, Duffy EE, Zhao J, Bailis W, Cao G, Kroehling L, Chen Y, et al. m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017b;548:338–342. doi: 10.1038/nature23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zang L, Zhang F, Chen J, Shen H, Shu L, Liang F, Feng C, Chen D, Tao H, et al. Fat mass and obesity-associated (FTO) protein regulates adult neurogenesis. Human molecular genetics. 2017c doi: 10.1093/hmg/ddx128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xiong X, Yi C. Epitranscriptome sequencing technologies: decoding RNA modifications. Nat Methods. 2016;14:23–31. doi: 10.1038/nmeth.4110. [DOI] [PubMed] [Google Scholar]
- Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells. Mol Cell. 2016;62:335–345. doi: 10.1016/j.molcel.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods. 2015;12:767–772. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annual review of neuroscience. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, Jaffrey SR. 5′ UTR m(6)A Promotes Cap-Independent Translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinie B, Wang J, Lim KS, Hillebrand R, Lu ZX, Van Wittenberghe N, Howard BD, Daneshvar K, Mullen AC, Dedon P, et al. m(6)A-LAIC-seq reveals the census and complexity of the m(6)A epitranscriptome. Nat Methods. 2016;13:692–698. doi: 10.1038/nmeth.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DL, Goldberg JL. Multiple transcription factor families regulate axon growth and regeneration. Developmental neurobiology. 2011;71:1186–1211. doi: 10.1002/dneu.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ, Zhang C, Gantman EC, Mele A, Darnell JC, Darnell RB. Mapping Argonaute and conventional RNA-binding protein interactions with RNA at single-nucleotide resolution using HITS-CLIP and CIMS analysis. Nature protocols. 2014;9:263–293. doi: 10.1038/nprot.2014.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Picelli S, Faridani OR, Bjorklund AK, Winberg G, Sagasser S, Sandberg R. Full-length RNA-seq from single cells using Smart-seq2. Nature protocols. 2014;9:171–181. doi: 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
- Puttagunta R, Tedeschi A, Soria MG, Hervera A, Lindner R, Rathore KI, Gaub P, Joshi Y, Nguyen T, Schmandke A, et al. PCAF-dependent epigenetic changes promote axonal regeneration in the central nervous system. Nature communications. 2014;5:3527. doi: 10.1038/ncomms4527. [DOI] [PubMed] [Google Scholar]
- Rishal I, Fainzilber M. Axon-soma communication in neuronal injury. Nat Rev Neurosci. 2014;15:32–42. doi: 10.1038/nrn3609. [DOI] [PubMed] [Google Scholar]
- Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nature methods. 2009;6:275–277. doi: 10.1038/nmeth.1314. [DOI] [PubMed] [Google Scholar]
- Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27:7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell research. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron. 2012;74:1015–1022. doi: 10.1016/j.neuron.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, Geisler S, DiAntonio A. Dynamic regulation of SCG10 in regenerating axons after injury. Exp Neurol. 2014;252:1–11. doi: 10.1016/j.expneurol.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Skene JH. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci. 1997;17:646–658. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Poo M. The cell biology of neuronal navigation. Nature cell biology. 2001;3:E81–88. doi: 10.1038/35060164. [DOI] [PubMed] [Google Scholar]
- Su Y, Shin J, Zhong C, Wang S, Roychowdhury P, Lim J, Kim D, Ming GL, Song H. Neuronal activity modifies the chromatin accessibility landscape in the adult brain. Nat Neurosci. 2017;20:476–483. doi: 10.1038/nn.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi A, Bradke F. Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr Opin Neurobiol. 2016;42:118–127. doi: 10.1016/j.conb.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Trakhtenberg EF, Goldberg JL. Epigenetic regulation of axon and dendrite growth. Front Mol Neurosci. 2012;5:24. doi: 10.3389/fnmol.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niekerk EA, Tuszynski MH, Lu P, Dulin JN. Molecular and Cellular Mechanisms of Axonal Regeneration After Spinal Cord Injury. Mol Cell Proteomics. 2016;15:394–408. doi: 10.1074/mcp.R115.053751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BJ, Mercaldo V, Gillon CJ, Yip M, Neve RL, Boyce FM, Frankland PW, Josselyn SA. The Role of The RNA Demethylase FTO (Fat Mass and Obesity-Associated) and mRNA Methylation in Hippocampal Memory Formation. Neuropsychopharmacology. 2017;42:1502–1510. doi: 10.1038/npp.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Huang J, Zou T, Yin P. Human m6A writers: Two subunits, two roles. RNA Biol. 2017;14:300–304. doi: 10.1080/15476286.2017.1282025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nature cell biology. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng YL, An R, Cassin J, Joseph J, Mi R, Wang C, Zhong C, Jin SG, Pfeifer GP, Bellacosa A, et al. An Intrinsic Epigenetic Barrier for Functional Axon Regeneration. Neuron. 2017;94:337–346. e336. doi: 10.1016/j.neuron.2017.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng YL, An R, Shin J, Song H, Ming GL. DNA modifications and neurological disorders. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2013;10:556–567. doi: 10.1007/s13311-013-0223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng YL, Joseph J, An R, Song H, Ming GL. Epigenetic regulation of axonal regenerative capacity. Epigenomics. 2016;8:1429–1442. doi: 10.2217/epi-2016-0058. [DOI] [PubMed] [Google Scholar]
- Widagdo J, Zhao QY, Kempen MJ, Tan MC, Ratnu VS, Wei W, Leighton L, Spadaro PA, Edson J, Anggono V, et al. Experience-Dependent Accumulation of N6-Methyladenosine in the Prefrontal Cortex Is Associated with Memory Processes in Mice. J Neurosci. 2016;36:6771–6777. doi: 10.1523/JNEUROSCI.4053-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JK, Zou H. Reshaping the chromatin landscape after spinal cord injury. Front Biol (Beijing) 2014;9:356–366. doi: 10.1007/s11515-014-1329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MC, Mi R, Connor E, Reed N, Vyas A, Alspalter M, Coppola G, Geschwind DH, Brushart TM, Hoke A. Novel roles for osteopontin and clusterin in peripheral motor and sensory axon regeneration. J Neurosci. 2014;34:1689–1700. doi: 10.1523/JNEUROSCI.3822-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao B, Christian KM, He C, Jin P, Ming GL, Song H. Epigenetic mechanisms in neurogenesis. Nat Rev Neurosci. 2016;17:537–549. doi: 10.1038/nrn.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KJ, Ringeling FR, Vissers C, Jacob F, Pokrass M, Jimenez-Cyrus D, Su Y, Kim NS, Zhu Y, Zheng L, et al. Temporal Control of Mammalian Cortical Neurogenesis by m(6)A Methylation. Cell. 2017;171:877–889. e817. doi: 10.1016/j.cell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Chen M, Huang H, Zhu J, Song H, Zhu J, Park J, Ji SJ. Dynamic m6A modification regulates local translation of mRNA in axons. Nucleic Acids Res. 2017 doi: 10.1093/nar/gkx1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Chen Y, Sun B, Wang L, Yang Y, Ma D, Lv J, Heng J, Ding Y, Xue Y, et al. m6A modulates haematopoietic stem and progenitor cell specification. Nature. 2017;549:273–276. doi: 10.1038/nature23883. [DOI] [PubMed] [Google Scholar]
- Zhang C, Darnell RB. Mapping in vivo protein-RNA interactions at single-nucleotide resolution from HITS-CLIP data. Nature biotechnology. 2011;29:607–614. doi: 10.1038/nbt.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nature reviews Molecular cell biology. 2017a;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BS, Wang X, Beadell AV, Lu Z, Shi H, Kuuspalu A, Ho RK, He C. m6A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature. 2017b;542:475–478. doi: 10.1038/nature21355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vagbo CB, Shi Y, Wang WL, Song SH, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015;526:591–594. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Dataset from m6A-SMART-seq analysis of adult mouse DRGs under naïve and SNL D1 conditions, related to Figures 1 and S2. (See Excel file)
Table S2. Gene list with significantly different levels of m6A-tagged transcripts between naïve and SNL D1 conditions, related to Figures 1 and S2. (See Excel file)
Table S3. List of primer sequences used in the current study, related to Figures 1, S3, and S5. (See Excel file)
Table S4. Dataset from m6A-CLIP-SMART-seq analysis of adult mouse DRGs under naïve and SNL D1 conditions, related to Figures 2 and S2. (See Excel file)
Table S5. List with locations of dynamic m6A sites between naïve and SNL D1 conditions, related to Figures 2 and S2. (See Excel file)
Table S6. Dataset from RNA-seq analysis of WT and Syn1-Cre;Mettl14f/f cKO DRGs under naïve and SNL D1 conditions, related to Figure 3 and S3. (See Excel file)


