Abstract
The aim of the present study was to develop a water-soluble biomarker for the detection of breast cancer using quantum dots (QDs) conjugated to Ki-67, a nuclear protein associated with the cell cycle. Ki-67 is also a marker of cell proliferation, with expression levels categorizing good and poor prognosis in invasive breast cancer. Ki-67 is a clinically used biomarker for breast cancer diagnosis, treatment and prognosis. Owing to the optical and chemical advantages of QDs, QD-based nanotechnology may aid the construction of a biomedical imaging platform for the study of cancer cell behavior. In the present study, a biomarker was prepared by employing the water-soluble CuInS2/ZnS QDs conjugated to an anti-Ki-67 monoclonal antibody to detect Ki-67 expression in breast cancer. The QDs, which were hydrophobic and coated with octadecylamine, were encapsulated with an amphiphilic biocompatible centipede-like polymer, and then conjugated to anti-Ki-67 monoclonal antibodies (QD-Ki-67 probes). The QD-Ki-67 probes retained the original optical properties of the unadorned QDs and did not exhibit distinct toxic side effects in in vitro cytotoxicity experiments. Therefore, this CuInS2/ZnS QD-labeled bioprobe, with a high quantum yield and low cytotoxicity, is a promising candidate for bioimaging and may be used as a cell label.
Keywords: quantum dots, bioconjugation, cancer diagnosis, breast cancer, Ki-67
Introduction
Breast cancer is the most frequent carcinoma in humans with the second-highest mortality rate among women (1). The initiation and progression of breast cancer is a complicated multi-stage process in which certain factors interact to disrupt normal cell growth and division. In addition to the conventional prognostic indicators, including clinical stage, tumor size and lymph node metastasis, other biological indicators are being used to assess the prognosis of patients with breast cancer, guiding clinical treatment (2).
Ki-67 is a nuclear antigen, identified by Gerdes et al (3) as a nuclear proliferative marker present in all stages of the cell cycle, occurring maximally in M phase and absent from G0 (4,5). Ki-67 has been demonstrated to be present in the early stage of the breast cancer in previous studies (5–7). Furthermore, Ki-67, acknowledged as a prognostic marker in breast cancer, is commonly used to predict the magnitude of chemotherapeutic benefits in clinical practice (8).
The rapid development of immunohistochemistry (IHC), has led to it becoming a major supplementary tool for diagnosis and research in a clinicopathological environment. Since the underlying technology of IHC encompasses antigen-antibody interactions, a high-sensitivity and -specificity antibody, in addition to an imaging system are the key factors of the technique. The development of semiconductor quantum dots (QDs) will lead to the creation of an interdisciplinary field comprising bioassays and bioimaging technologies (9–11). This may also result in substantial advantages over the use of conventional antibody-based IHC assays, which rely on organic fluorophores or fluorescent proteins. QDs are able to emit adjustable light (broad excitation spectrum and narrow emission spectrum) that may be dyed with a variety of fluorescent dyes simultaneously under a single excitation wavelength (12). QDs may also be combined with different target materials. QDs coupled to materials with similar imaging and treatment function produce diverse biological functional probes that may be concurrently used for tumor molecular imaging and targeted therapy (13–15). Previous studies have assessed the feasibility of using QDs in cancer diagnosis, molecular classification, treatment and prognosis, and have established a broad prospect in basic and clinical cancer research (16,17).
In the present study, a novel class of poly(aspartate)-Na-graft-poly(ethylene glycol)-dodecylamine (PASP-Na-g-PEG-DDA) was synthesized, which was used to convert the hydrophobic octadecylamine-coated QD molecules into hydrophilic forms through surface modification and then chemically conjugate them to Ki-67 antibodies (QD-Ki-67 probes). Furthermore, the applicability of the newly synthesized QD-Ki-67 probes was tested in various breast cancer cell lines by comparing the light stability between the QD-Ki-67 probes and organic dyes. The in vitro cytotoxicity of the QD-Ki-67 probes was also assessed.
Materials and methods
Materials
CuInS2/ZnS hydrophilic QDs (λex=605 nm) were purchased from Ocean Nanotech LLC (Springdale, AZ, USA). Water-soluble QDs (QDs encapsulated with PASP-Na-g-PEG-DDA) were supplied by the Alan G. MacDiarmid Institute of Jilin University (Changchun, China). The SP6 clone mouse anti-human Ki-67 monoclonal antibody (cat. no. RMA-0542) was obtained from Fuzhou Maixin Biotech Co., Ltd. (Fuzhou, China). Immunoglobulin G (IgG) (from goat serum), bovine serum albumin (BSA), dimethylsulfoxide (DMSO) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) were procured from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany); DAPI was acquired from Roche Applied Science (Penzberg, Germany); MTT was procured from Beyotime Institute of Biotechnology (Nantong, China); Bisphenol A (BPA), HCl and NaOH were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Human breast cancer cell line MDA-MB-231 and a normal human mammary microvascular endothelial cell (HMMEC) line were acquired from the China-Japan Union Hospital, Jilin University (Changchun, China). The synthesis of QDs was described previously (18–20).
Bioconjugation of CuInS2/ZnS QDs with anti-Ki-67
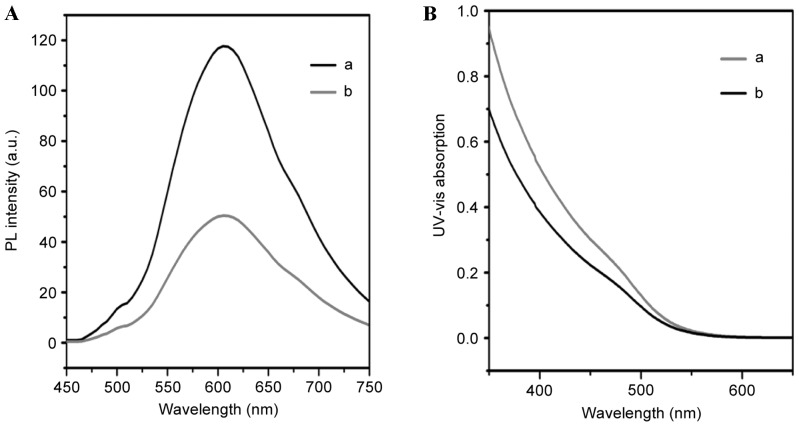
A total of 500 µl antibody (0.1 mg/l SP6 clone mouse anti-human Ki-67 monoclonal antibody was incubated with 500 µl EDC and sulfo-N-hydroxysulfosuccinimide (0.1 mmol/l) for 15 min at room temperature. This concoction was mixed with 500 µl water-soluble QDs (50 mg) suspended in PBS (pH 7.4). The reaction mixture was incubated at 4°C for 24 h. The antibody-coupled QDs were washed with PBS to remove the excess antibody, producing QD-Ki-67 probes. The formation of QD-Ki-67 was varied by photoluminescence (PL) and UV. The characteristic peak of QDs appeared following Ki-67 antibody conjugation in and the shape of the peak was retained during the reaction process. The UV-Vis absorption spectrum of the QD-Ki-67 revealed the characteristic peaks of the QDs.
Cell culture and fixation
Human breast cancer MDA-MB-231 cells and HMMECs (control) were incubated in 24-well plates (6×104 cells/well), for adherence, in high-glucose Dulbecco's modified Eagle's medium (Shanghai Solarbio Bioscience & Technology Co., Ltd, Shanghai, China) or RPMI-1640 medium (Shanghai Solarbio Bioscience & Technology Co., Ltd.), respectively, supplemented with 10% fetal bovine serum (cat. no. 10100147; Gibco; Thermo Fisher Scientific, Inc.) and 1% (v/v) penicillin/streptomycin. The two cell lines were incubated in 5% CO2 at 37°C. Subsequently, the cells were fixed with 4% formaldehyde for 20 min at 37°C and blocked with 2% (w/v) BSA for 30 min at 37°C, followed by permeabilization with 0.3% Triton X-100 for 10 min at room temperature. Cells were washed three times with PBS for 5 min each. Following washing, the samples were incubated with the QD-Ki-67 complex at 25°C for 1 h.
Imaging of labeled cells
Following fixation and blocking, the two cell samples were incubated in a confocal Petri dish (37°C, 5% CO2) for 24 h to allow for adherence to the wells. Subsequently, 100 µl QD-Ki-67 probes (100 µg/ml) was added to the dish, which was incubated at 37°C for 1 h. Concurrently, control cells were treated similarly, but with mock-conjugated QDs (0.2 mg/ml). The cells were washed three times with PBS to prevent the non-specific binding of QD-Ki-67 probes or QDs. Finally, 1 µg/ml DAPI was used for nuclear staining at 37°C for 15 min and images were captured using a laser-scanning confocal microscope with excitation wavelength of 480 nm at room temperature. The cell imaging analysis was performed using FV10-ASW 3.1 Viewer software (Olympus Corporation, Tokyo, Japan).
Photostability comparison
Following fixing and blocking, the MDA-MB-231 cells were incubated with mouse anti-human Ki-67 monoclonal antibody (dilution, 1:1,000) at 37°C for 1 h, followed by incubation with IgG as a secondary antibody (dilution, 1:500) at 37°C for 30 min. Alternatively, the fixed and blocked MDA-MB-231 cells were co-incubated with QD-Ki-67 probes for QD-based IHC detection. The cell nuclei were counterstained with DAPI (1 µg/ml) at 37°C for 15 min. The fluorescence intensity of QDs was monitored and images were captured using a laser-scanning confocal microscope camera at 1-min intervals for 10 min at the excitation wavelength of 480 nm.
Cytotoxicity analysis of CuInS2/ZnS QDs
The cytotoxicity of the CuInS2/ZnS QDs was elucidated using the MTT assay. Briefly, HMMECs and MDA-MB-231 cells were cultured in 96-well plates as aforementioned to achieve a count of 1,000 cells/well. Next, the corresponding medium was replenished with a range of QD-Ki-67 probe concentrations (0.1, 0.2, 0.3 and 0.4 mg/ml). After 24, 48, and 72 h, 10 µl MTT solution (5 mg/ml) was mixed in each well prior to incubation for an additional 4 h. The reaction was quenched by removing the MTT-containing culture medium and adding 100 µl DMSO. The plate was agitated for ~10 min and the optical density (OD) was estimated at 490 nm on an ELx800 Absorbance Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA).
Statistical analysis
All quantitative values are expressed as the mean ± standard error of the mean. Statistical analyses were performed with SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA); the different groups were compared by one-factor analysis of variance. P<0.05 was considered to indicate a statistically significant difference.
Results
Structural characterization of QD-Ki-67
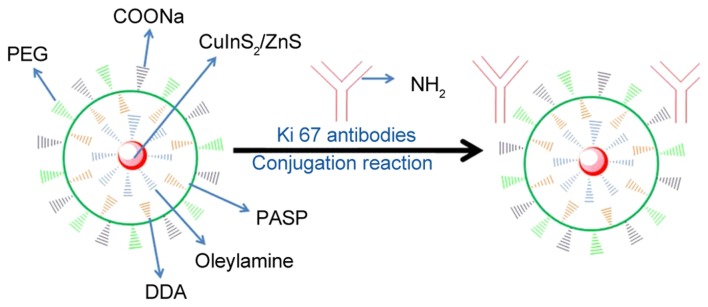
Fig. 1 presents a schematic diagram of QD-Ki-67 conjugation. Fig. 2 presents the PL and UV confirmation of the establishment of QD-Ki-67 probes.
Figure 1.
Structural representation of the QDs conjugated to anti-Ki-67 antibodies. The free COOH groups of the water-soluble QDs chemically conjugated with the free NH2 group of Ki-67 monoclonal antibodies. QD, quantum dot; PASP, poly(aspartate); DDA, dodecylamine; PEG, poly(ethylene glycol).
Figure 2.
(A) PL characteristics of QDs and the shape of the peak are retained following their bioconjugation; (B) The ultraviolet-visible absorption spectrum of QD-Ki-67 probes is similar to that of QDs. The results indicated that the interaction with the anti-Ki-67 antibodies did not affect the functionality of the QD nanocrystals. a represents QDs and b represents QD-Ki-67. QD, quantum dot. PL, photoluminescence; a.u., absorbance units.
Cell imaging
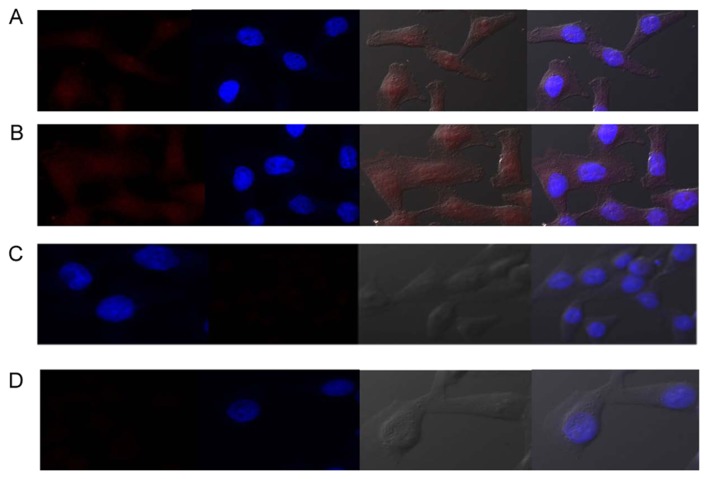
Fig. 3 presents the fluorescent images of MD-MBA-231 cells and HMMECs labeled with QD-Ki-67 probes and mock-conjugated with Qdot Blue indicates the nuclear counterstaining with DAPI.
Figure 3.
Confocal microscopic images of (A) MD-MBA-231 cells with QD-Ki-67 probes, (B) MD-MBA-231 cells with QD-Ki-67 probes with mock-conjugated QDs, (C) MD-MBA-231 cells with PBS as a blank control and (D) human epithelial mammary microvascular endothelial cells with QD-Ki-67 probes. Cell nuclei were counterstained with DAPI (blue). QD, quantum dot.
The properties of superior water-solubility, excitation-dependent emission and high fluorescence quantum yield render QD-Ki-67 probes as an attractive alternative for live cell imaging. To substantiate the ability of the probe to combine with Ki-67, MDA-MB-231 cells (Ki-67-high) and HMMECs (Ki-67-low) cell lines were incubated with QD-Ki-67 probes, and the results were obtained using laser-scanning confocal microscopy. All the images were captured under identical parameters. Fig. 3A indicates that, using QD-Ki-67 probes, the fluorescence signal is homogeneously concentrated in the nucleus, and the cytoplasmic labeling is minimal in MDA-MB-231 cells. Conversely, mock-conjugated QDs did not demonstrate a homogeneous concentration in the nucleus (Fig. 3B). Furthermore, no fluorescence signal was detected in MDA-MB-231 cells (Fig. 3C) or HMMECs (Fig. 3D) for the blank controls.
QD-Ki-67 probes that entered into the MDA-MB-231 cells displayed multi-colored PL, whereas the shape and viability of cells were not altered substantially, thereby indicating successful cell labeling with QD-Ki-67 probes. The present study indicated that the Ki-67 monoclonal antibody that was conjugated with the quantum dots maintained the ability to form Ki-67-specific antigen-antibody complexes. The QD-Ki-67 probes were able to specifically bind to Ki-67 in the MDA-MB-231 cell nuclei in vitro. Consecutively, the non-specific binding was minimal. Therefore, this particular characteristic indicated the ability of QD-Ki-67 probes to detect of Ki-67 in breast cancer cells.
In vitro cytotoxicity
The toxicity of QDs which contain metal components such as cadmium ions is a serious concern and several groups have attempted to identify the primary origins in order to resolve the associated challenges (21). Certain studies reported that the coating on the surface of QDs has a substantial function in its emerging toxicity harbored in the physiological system (22–25). Evaluating the viability of the cells is the most common quantitative assay for assessing the toxicity of any biological material (26,27). In the present study, an MTT assay was employed for assessing the QD toxicity.
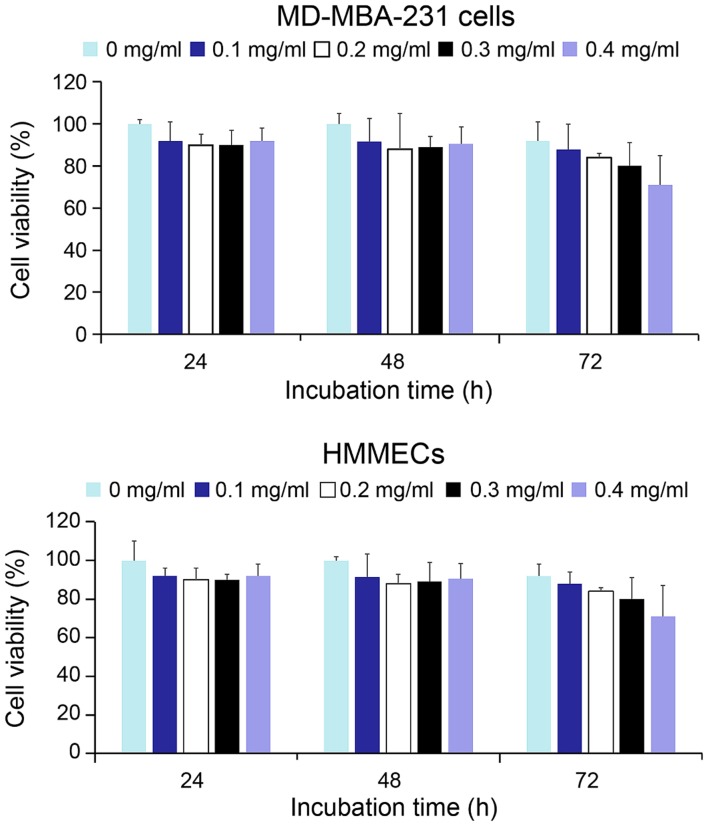
As presented in Fig. 4, the viability of MD-MBA-231 cells and HMMECs treated with a broad range of concentrations of QD-Ki-67 probes, persisted in >88% of cells 48 h following treatment. However, at 72 h after treatment, the viability of HMMECs began to decline at a concentration of 0.4 mg/ml QD-Ki-67 probe, the highest concentration. In a previous study by Cho et al (28), 3-mercaptopropionic acid-modified CdSe/ZnS QDs were demonstrated to be nontoxic to MCF-7 cells (28). However, this result was attained from a short experimental period ranging between 12 and 24 h. Furthermore, the model system used by Cho et al (28) was cancer cells, which may exhibit prolonged viability. The results of the present study were consistent with those from Qi et al (29), which hypothesized that, over an extended period, the QDs in the cells ended up in the lysosome and the degraded QDs subsequently released Cd2+ from the particle surface of the QDs into the cell. The results of the present study demonstrated that QD-Ki-67 probes exerted slight toxic effects on living cells in vitro within 48 h.
Figure 4.
Relative cell viability of MD-MBA-231 cells and HMMECs treated with different concentrations of quantum dots-Ki-67 probe (0.1, 0.2, 0.3 and 0.4 mg/ml) at 24, 48 and 72 h after treatment. HMMECs, human mammary microvascular endothelial cells.
Discussion
QDs have enormous potential in breast cancer research. In the present study, QD-Ki-67 probes entered the MDA-MB-231 cells, and displayed bright and colorful PL, with no corresponding change in the shape and viability of cells. Ki-67 is frequently measured as a static marker of proliferative activity and by making multiple measurements during treatment, making it a possible dynamic intermediate marker of treatment efficiency. Previous studies have demonstrated the prognostic value of Ki-67 in breast cancer (5,30). To evaluate the routine use and value of Ki-67 as a prognostic marker, the results of analysis of a large population-based cohort of a cancer registry indicated that Ki-67 expression is an independent prognostic parameter for disease-free and overall survival (31). QD-based fluorescent imaging techniques may quantitatively and simultaneously detect the expression of Ki-67 in breast cancer in situ, and sensitively assess the prognostic values of Ki-67. Jerjees et al (31) compared the Ki-67 labeling index (Ki-67-LI) and human epidermal growth factor 2 (HER-2) expression levels to assess their effect on the biological behavior of luminal breast cancer cells and prognosis of patients with luminal breast cancer, and identified that Ki-67-LI and HER-2 were associated with high proliferation and poor prognosis in estrogen receptor (ER)-positive breast cancer.
When QDs are used in in vitro or in vivo experiments, they are present in complex biological environments that may directly or indirectly affect the colloidal and optical stability of the QD formulation (32–34). Thus, the aqueous colloidal stability of QDs is of concern for use in long-term in vivo imaging applications, including for tumor detection and labeling. The present study monitored the PL intensity of the prepared QD formulations in various cell culture media for >3 days. The change in the PL intensity is used as an indicator for evaluating the overall colloidal stability of the QD formulations. Common factors that may affect the PL intensity of QD dispersion include pH, ions and oxygen concentration (25–27). The present study optimized the experimental conditions and observed that QD-Ki-67 probes exhibited substantial water colloid stability. The results of the present study indicate that the QD-Ki-67 probes represent a promising alternative to the traditional cellular labeling agents for the imaging of cells. The in vitro or in vivo use of QDs has an impact on the physiological environment, and as a result may directly or indirectly affect the properties of the formulation, such as colloidal and optical stability. Thus, the aqueous colloidal stability of QDs necessitates intensive investigation for usage in the long-term in vivo applications, including in tumor detection and labeling. The present study scrutinized the PL intensity of previous formulations of QDs in different cell culture media over a period of 72 h. The modified PL intensity aids in the evaluation of the overall colloidal stability of the QDs. Factors including pH, ions and oxygen levels may affect the PL intensity of QD dispersion (24–26). In the present study, the experimental parameters were optimized and the high water colloid stability of QD-Ki-67 probes was observed. QD-Ki-67 probes appear to be promising substitutes for conventional labeling agents for cell imaging. However, QD toxicity is of grave concern. In comparison with the existing organic fluorescent dyes, including Alexa Fluor 488 that was used in the present study, QD-ER probes exhibited excellent optical properties, with high light stability and high fluorescence quantum yields in particular. The antibody-conjugated QD probes also exhibited no evident cytotoxicity in live cells. These advantages indicated that the novel QD-ER probes described in the present study have the potential for use in in vivo imaging of cancer tumors; they may be used for cellular imaging and long-term in vivo observation of tumors.
In conclusion, in the present study a novel water-soluble compound, QD-Ki-67, was prepared which specifically combined with the Ki-67 antigen. Furthermore, the efficiency of detectability of the Ki-67 antigen in MD-MBA-231 cells and HMMECs was analyzed. Owing to their low cytotoxicity and adequate biocompatibility, QD-Ki-67 probes were able to permeabilize MD-MBA-231 cells and HMMECs with only slight changes to the shape and viability of the cells. Therefore, this novel water-soluble QD probe exhibits substantial promise for future use in vitro for the diagnosis of breast cancer.
Acknowledgements
The study was financially supported by The Jilin Province Key Fund (grant no. 20150204081SF) and The Jilin Province Development and Reform Commission (grant no. 2015Y035-02).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Soerjomataram I, Louwman MW, Ribot JG, Roukema JA, Coebergh JW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat. 2008;107:309–330. doi: 10.1007/s10549-007-9556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 4.Lopez F, Belloc F, Lacombe F, Dumain P, Reiffers J, Bernard P, Boisseau MR. Modalities of synthesis of Ki-67 antigen during the stimulation of lymphocytes. Cytometry. 1991;12:42–49. doi: 10.1002/cyto.990120107. [DOI] [PubMed] [Google Scholar]
- 5.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: Prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 6.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto S, Ibusuki M, Yamamoto Y, Fu P, Fujiwara S, Murakami K, Iwase H. Clinical relevance of Ki67 gene expression analysis using formalin-fixed paraffin-embedded breast cancer specimens. Breast Cancer. 2013;20:262–270. doi: 10.1007/s12282-012-0332-7. [DOI] [PubMed] [Google Scholar]
- 8.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Panel members: Strategies for subtypes-dealing with the diversity of breast cancer: Highlights of the St gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alivisatos P. The use of nanocrystals in biological detection. Nat Biotechnol. 2004;22:47–52. doi: 10.1038/nbt927. [DOI] [PubMed] [Google Scholar]
- 10.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alivisatos A, Gu W, Larabell C. Quantum dots as cellular probes. 2005;7:55–76. doi: 10.1146/annurev.bioeng.7.060804.100432. [DOI] [PubMed] [Google Scholar]
- 12.Zrazhevskiy P, Sena M, Gao X. Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chem Soc Rev. 2010;39:4326–4354. doi: 10.1039/b915139g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bentolila L, Ebenstein Y, Weiss S. Quantum dots for in vivo small-animal imaging. J Nucl Med. 2009;50:493–496. doi: 10.2967/jnumed.108.053561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He X, Gao J, Gambhir SS, Cheng Z. Near-infrared fluorescent nanoprobes for cancer molecular imaging: Status and challenges. Trends Mol Med. 2010;16:574–583. doi: 10.1016/j.molmed.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilderbrand SA, Weissleder R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr Opin Chem Biol. 2010;14:71–79. doi: 10.1016/j.cbpa.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Byers RJ, Hitchman ER. Quantum dots brighten biological imaging. Prog Histochem Cytochem. 2011;45:201–237. doi: 10.1016/j.proghi.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Chen L. Quantum dots, lighting up the research and development of nanomedicine. Nanomedicine. 2011;7:385–402. doi: 10.1016/j.nano.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Li Y, Sun X, Lv Y, Chen L, Wang J. Preparation and biological characterization of pH-responsive PASP-g-PEG-DDA-Hyd-ADR. New J Chem. 2013;37:1623–1629. doi: 10.1039/c3nj41155a. [DOI] [Google Scholar]
- 19.Sun T, Li K, Li Y, Li C, Zhao W, Chen L, Chang Y. Optimizing conditions for encapsulation of QDs by varying PEG chain density of amphiphilic centipede-like copolymer coating and exploration of QDs probes for tumor cell targeting and tracking. New J Chem. 2012;36:2383–2391. doi: 10.1039/c2nj40312a. [DOI] [Google Scholar]
- 20.Sun X, Li Y, Huang H, Yang B, Wang Y. Synthesis and application of a targeting diagnosis system via quantum dots coated by amphiphilic polymer for the detection of liver cancer cells. Luminescence. 2014;29:831–836. doi: 10.1002/bio.2629. [DOI] [PubMed] [Google Scholar]
- 21.Hardman R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu WW, Chang E, Drezek R, Colvin VL. Water-soluble quantum dots for biomedical applications. Biochem Biophys Res Commun. 2006;348:781–786. doi: 10.1016/j.bbrc.2006.07.160. [DOI] [PubMed] [Google Scholar]
- 23.Hoshino A, Fujioka K, Oku T, Suga M, Sasaki YF, Ohta T, Yasuhara M, Suzuki K, Yamamota K. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett. 2004;4:2163–2169. doi: 10.1021/nl048715d. [DOI] [Google Scholar]
- 24.Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Surface coatings determine cytotoxicity and irritation potential of quantum dot nanoparticles in epidermal keratinocytes. J Invest Dermatol. 2007;127:143–153. doi: 10.1038/sj.jid.5700508. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Ji K, Kim J, Park C, Lim KH, Yoon TH, Choi K. Acute toxicity of two CdSe/ZnSe quantum dots with different surface coating in Daphnia magna under various light conditions. Environ Toxicol. 2010;25:593–600. doi: 10.1002/tox.20520. [DOI] [PubMed] [Google Scholar]
- 26.Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull. 1996;19:1518–1520. doi: 10.1248/bpb.19.1518. [DOI] [PubMed] [Google Scholar]
- 27.Tominaga H, Ishiyama M, Ohseto F, Sasamoto K, Hamamoto T, Suzuki K, Watanabe M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal Commun. 1999;36:47–50. doi: 10.1039/a809656b. [DOI] [Google Scholar]
- 28.Cho SJ, Maysinger D, Jain M, Röder B, Hackbarth S, Winnik F. Long-term exposure to CdTe quantum dots causes functional impairments in live cells. Langmuir. 2007;23:1974–1980. doi: 10.1021/la060093j. [DOI] [PubMed] [Google Scholar]
- 29.Qi Q, Liu X, Li S, Joshi HC, Ye K. Synergistic suppression of noscapine and conventional chemotherapeutics on human glioblastoma cell growth. Acta Pharmacol Sin. 2013;34:930–938. doi: 10.1038/aps.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, Ortmann O. Ki-67 is a prognostic parameter in breast cancer patients: Results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139:539–552. doi: 10.1007/s10549-013-2560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jerjees DA, Alabdullah M, Green AR, Alshareeda A, Macmillan RD, Ellis IO, Rakha EA. Prognostic and biological significance of proliferation and HER2 expression in the luminal class of breast cancer. Breast Cancer Res Treat. 2014;145:317–330. doi: 10.1007/s10549-014-2941-7. [DOI] [PubMed] [Google Scholar]
- 32.Hines DA, Kamat PV. Recent advances in quantum dot surface chemistry. ACS Appl Mater Interfaces. 2014;6:3041–3057. doi: 10.1021/am405196u. [DOI] [PubMed] [Google Scholar]
- 33.Durisic N, Godin AG, Walters D, Grütter P, Wiseman PW, Heyes CD. Probing the ‘dark’ fraction of core-shell quantum dots by ensemble and single particle pH-dependent spectroscopy. ACS Nano. 2011;5:9062–9073. doi: 10.1021/nn203272p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boldt K, Bruns OT, Gaponik N, Eychmüller A. Comparative examination of the stability of semiconductor quantum dots in various biochemical buffers. J Phys Chem B. 2006;110:1959–1963. doi: 10.1021/jp056371p. [DOI] [PubMed] [Google Scholar]