Abstract
Annexin A7 (ANXA7) is a suppressor of tumorigenesis and metastasis in prostate cancer. Activated ANXA7 GTPase promotes prostate cancer cell apoptosis. However, the role and underlying mechanism of ANXA7 GTPase in prostate cancer metastasis have not been established. RKIP is a metastatic suppressor and downregulated in prostate cancer metastases. The binding of RKIP and its target proteins could inhibit the activation of its interactive partners. However, the effect of RKIP on ANXA7 GTPase activation is not clear. Here, we report that activation of ANXA7 GTPase by a small molecule SEC ((S)-ethyl 1-(3-(4-chlorophenoxy)-2-hydroxypropyl)-3-(4-methoxyphenyl)-1H-pyrazole-5-carboxylate) effectively inhibited prostate cancer metastasis. Mechanistically, activated ANXA7 promoted AMPK phosphorylation, leading to decreased mTORC1 activity, suppressed STAT3 nuclear translocation, and downregulation of pro-metastatic genes, including CCL2, APLN, and IL6ST. Conversely, RKIP interacted with ANXA7 and impaired activation of ANXA7 GTPase by SEC and its downstream signaling pathway. Notably, SEC treatment suppressed metastasis of prostate cancer cells in in vivo orthotopic analysis. Together, our findings provide a novel insight into how metastasis of prostate cancer with low RKIP expression is suppressed by SEC-induced activation of ANXA7 GTPase via the AMPK/mTORC1/STAT3 signaling pathway.
Keywords: ANXA7 GTPase, AMPK, RKIP, metastasis, prostate cancer
1. Introduction
Cancer metastasis is the major cause of cancer mortality, accounting for approximately 90% of deaths. Once cancer cells leave and spread beyond their primary site, metastatic cancers are highly incurable and fatal, whereas most primary tumors are manageable or curable[1]. Prostate cancer is the most frequently diagnosed cancer in men with an estimated 180,000 new cases in U.S. in 2016. Five-year relative survival rate of localized and regional prostate cancer reaches approximately 99%; however, only about 27% of metastatic prostate cancer patients survive in 5 years[2]. Cancer metastasis is a complicated progress that involves four essential steps: detachment, migration, invasion and adhesion[3]. Whereas our understanding of the biology behind mechanisms of androgen receptor pathway activation, tumor-microenvironment interaction, and anti-tumor immunotherapy has advanced therapies for metastatic prostate cancer[4], a better understanding of molecular and cellular mechanisms of suppressor gene and associated signaling pathway in prostate cancer metastasis is critical and in great need to identify new therapeutic targets and agents.
Annexin A7 (ANXA7), a member of annexin superfamily, is a Ca2+- and phospholipid-binding protein and possesses GTPase activity[5, 6]. The ANXA7 gene is located on human chromosome 10q21, where multiple potential tumor suppressor genes exist. Homozygous ANXA7 (−/−) knockout mice showed a lethal phenotype at embryonic day 10[7]. Heterozygous ANXA7 (+/−) knockout mice have a high frequency of spontaneous tumors (about 20–50%) in multiple organs, including liver, prostate, endometrium, salivery gland and thymus[8]. Moreover, tumor metastasis was observed in 42% of the mutant mice developing spontaneous tumor[8]. These findings suggest that ANXA7 may play an important role in inhibiting tumorigenesis and metastasis. Compared with that in normal and healthy prostate tissue, ANXA7 expression is reduced by approximately 50% in primary prostate tumors and even more downregulated in metastatic prostate cancers[7, 9]. However, the mechanism of ANXA7 GTPase in the regulation of metastasis has not been established. Our recent work has demonstrated that activation of ANXA7 GTPase by a small molecule SEC ((S)-ethyl 1-(3-(4-chlorophenoxy)-2-hydroxypropyl)-3-(4-methoxyphenyl)-1H-pyrazole-5-carboxylate) induces prostate cancer cell apoptosis[10]. We wondered whether activated ANXA7 GTPase could inhibit prostate cancer metastasis.
Raf kinase inhibitory protein (RKIP) has been associated with natural anti-metastasis property in multiple tumor types[11]. The metastatic suppression role of RKIP was initially identified in prostate cancer[12]. RKIP expression is low in metastatic prostate cancer cell lines PC3 and DU145, while RKIP is relatively high expressed in non-invasive prostate cancer cell line LNCaP[13]. Analysis of clinical samples showed that metastases of human prostate cancer exhibited dramatically decreased RKIP level compared with benign prostate hyperplasia and localized prostate cancer samples[12, 13]. It has been proposed that low RKIP expression is a good prognostic marker for the pathogenesis of human prostate cancer[14].
Accumulating evidence suggests that RKIP associates with GTP-binding proteins[15]. RKIP associates with Raf-1 and inhibits the Raf/MEK/ERK/Myc signaling, leading to the inhibition of Snail and TET activity and decreased pro-metastatic gene expression[16]. RKIP interferes with NF-κB signaling by the interaction with several upstream kinases TAK1, NIK and IKK, contributing to metastasis inhibition[17]. The binding of RKIP and its target proteins could inhibit the interactive proteins activation by their activators, leading to the inversion of related signaling and the inhibition of tumor metastasis[18]. In addition, it was reported that nonylphenol induced the upregulation of RKIP in testicular Sertoli cells, accompanied with decreased ANXA7 protein level[19]. However, the role and mechanism of RKIP in the regulation of ANXA7 signaling pathway remains unknown so far. We seek to determine whether RKIP binds to ANXA7 and antagonizes the activation of ANXA7 GTPase by SEC.
In the current study, we used metastatic prostate cancer cell PC3 as well as non-metastatic LNCaP, and constructed HEK293T RKIP−/− cell line, together with HEK293T RKIP+/+ cell line as the cell model, to study the role and mechanism of ANXA7 GTPase in prostate cancer metastasis. Our substantial evidence suggests that SEC treatment increases AMPK phosphorylation via activating ANXA7 GTPase, and that activated AMPK inhibits STAT3 nuclear translocation via the mTORC1/STAT3 pathway, leading to downregulation of pro-metastatic genes and inhibition of prostate cancer metastasis. Notably, RKIP interacts with and compromises the inhibitory effect of SEC on prostate cancer metastasis. Together, our findings indicate that activation of ANXA7 by a small molecule SEC antagonizes prostate cancer metastasis with low RKIP expression via the AMPK/mTORC1/STAT3 signaling pathway, providing a novel insight into how prostate cancer metastasis is suppressed by a small molecule SEC via previously uncharacterized but essential interplay between the two metastasis suppressors ANXA7 and RKIP.
2. Materials and methods
2.1 Antibodies
Antibodies to RKIP (CST#5291), AMPKα (CST#5832), phospho-AMPKα (T172) (CST#8208), p70S6K (CST#2708), phospho-p70S6K (CST#9208), 4EBP1 (CST#9644), phospho-4EBP1 (CST#9459), STAT3 (CST#9132), phospho-STAT3 (Tyr705) (CST#9131) and phospho-STAT3 (Ser727) (CST#9134) were purchased from Cell Signaling Technology. Antibodies to E-Cadherin (20874-1-AP) and vimentin (10366-1-AP) were obtained from Proteintech Group. Anti-ANXA7 antibody (sc-17815) used for co-immunoprecipitation and anti-p-Thr antibody (sc-5267) were obtained from Santa Cruz Biotechnology. Anti-ANXA7 antibody (A4475 MSDS) used for western blot and antibody to β-actin (A1978 MSDS) were purchased from Sigma. Horseradish peroxidase-conjugated secondary antibodies for Western blot were from Santa Cruze Biotechnology. Secondary antibody for immunofluorescence was donkey anti-rabbit IgG Alexa Fluor-488 (CA21206s, Invitrogen).
2.2 Cell culture
Prostate cancer-derived metastatic PC3 and non-metastatic LNCaP cell lines were grown in RPMI Medium 1640 (Gibco, 3180-022) with 10% fetal bovine serum (FBS; Hyclone, SV30087.02). HEK293T cell lines carrying RKIP knockout (RKIP−/−) and wild-type RKIP expression (RKIP+/+) were originally constructed by SiDanSai Biotechnology Company. HEK293T cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, 12800-058) supplemented with 10% FBS. All cell lines were maintained in a humidified incubator at 5% CO2 and 37 °C.
2.3 Wound healing assay
Cells were seeded into 6-well culture plates and grown to confluence. Scratch wounds were made into the cell monolayer by use of a white pipette tip. Before replacement of conditioned media, cells were rinsed twice with phosphate buffered saline (PBS) to remove all non-adherent cells. Cell migration to wound surface was then monitored over time at 12 h intervals. The distances of the migrating cells from the wound edge to the migrated point were quantified in three biological replicates.
2.4 Plasmid and overexpression
The plasmids mCherry-ANXA7-wt, mCherry-ANXA7-mt1 (T275A), -mt2 (T286A) and -mt3 (T286D) were constructed as described previously[20]. Full-length cDNA of human RKIP was subcloned into pCMV6 plasmid to produce the pCMV6-RKIP construct. PC3 or HEK293T KO cells were transfected with the plasmids using Lipofectamine 2000 (Invitrogen, 11668-019) as described by the manufacturer’s instructions.
2.5 RNA isolation and PCR
Total cell RNA was extracted using Trizol reagent (Invitrogen, USA). RNA (1000 ng) was reverse-transcribed into cDNA by M-MLV reverse transcriptase (Promega, USA). Semiquantitative RT-PCR was conducted by use of mastermix (MegaMix, Cambio). Quantitative real-time PCR (qPCR) was performed by use of the SYBR Green PCR master Mix (Takara, DRR041A). qPCR product amplification was monitored by SYBR fluorescence. The expression of target genes was normalized to β-actin standard curve in each tested cDNA sample. Primers for β-actin were sense 5′ -GAAGTGTGACGTGGACATCC-3, and anti-sense 5′ -CCGATCCACACGGAGTA CTT-3. The sequences of primers for microarray confirmation are shown in Supplementary Table S1.
2.6 Western blot
Western blot was performed as we described previously. Briefly, cell lysates were applied to 15 % SDS-polyacrylamide gel and electroblotted onto poly-vinylidene difluoride membranes. Subsequently, membranes were blocked for 1 h at room temperature (RT) and incubated with primary antibodies over night at 4 °C. After three washing in PBST, membranes were probed with peroxidase-conjugated secondary antibodies at RT for 1 h. Protein signals were detected using an enhanced chemiluminescence detection kit (Thermo Fisher, 34080). Protein levels were quantified using Image J and relative normalized to loading controls.
2.7 Co-immunoprecipitation (co-IP)
Cells were harvested and lysed with IP buffer (Beyotime, P0013). Incubation with protein A/G agarose beads (Beyotime, P2012) at 4°C for 1 h was performed to pre-clear cell lysates. Supernatant was collected after centrifuging, and was incubated with rabbit polyclonal anti-ANXA7 antibody or normal rabbit control IgG added with protein A/G agarose beads overnight at 4°C. After washing with IP buffer three times, bound proteins were eluted in the 2×SDS loading buffer by boiling beads. The immunoprecipitated proteins were subjected to western blot analysis.
2.8 Immunofluorescence assay
4% paraformaldehyde (w/v) was used to fix treated cells for 30 min at RT. Then cells were incubated with normal donkey serum (1:30) for 20 min and followed by primary antibody (1:100) overnight at 4°C. After rewarming, cells were washed with PBS for 3 times and subsequently incubated with secondary antibody (1:200) for 1 h at 37°C. Laser scanning confocal microscopy (Leica, Wetzlar, Germany) was used to detect fluorescence.
2.9 In vivo metastasis assay
Luciferase-labeled PC-3M-Luc cells (2×106 per 50 µl sterile HBSS−/−) were orthotopically inoculated into the prostates of 8-week-old nude mice. Four days after implantation, mice were divided randomly into 3 groups, with 5 in each group. Group 1 was control group injected with dimethyl sulfoxide diluted in PBS. Group 2 and 3 were SEC-treated groups that received intraperitoneal injections of 3 mg/kg/day or 18 mg/kg/day SEC for 3 weeks. Body weight was monitored bi-weekly. Bioluminescence imaging was observed by IVIS 100 Imaging System to detect metastasis. Luminescent images were analyzed by use of TrueQuant software. The care and use of mice were performed according to the Institutional Animal Care and Use Committee (IACUC) guidelines at Shandong University.
2.10 Statistical analysis
GraphPad Prism software (version 5.0) was utilized to perform statistical analysis. Data were analyzed by one-way ANOVA and presented as mean±SEM. P values of less than 0.05 were taken as significant differences. Statistical calculations were derived from as least three independent replicates.
3. Results
3.1 SEC inhibited migration in HEK293T RKIP−/− cells
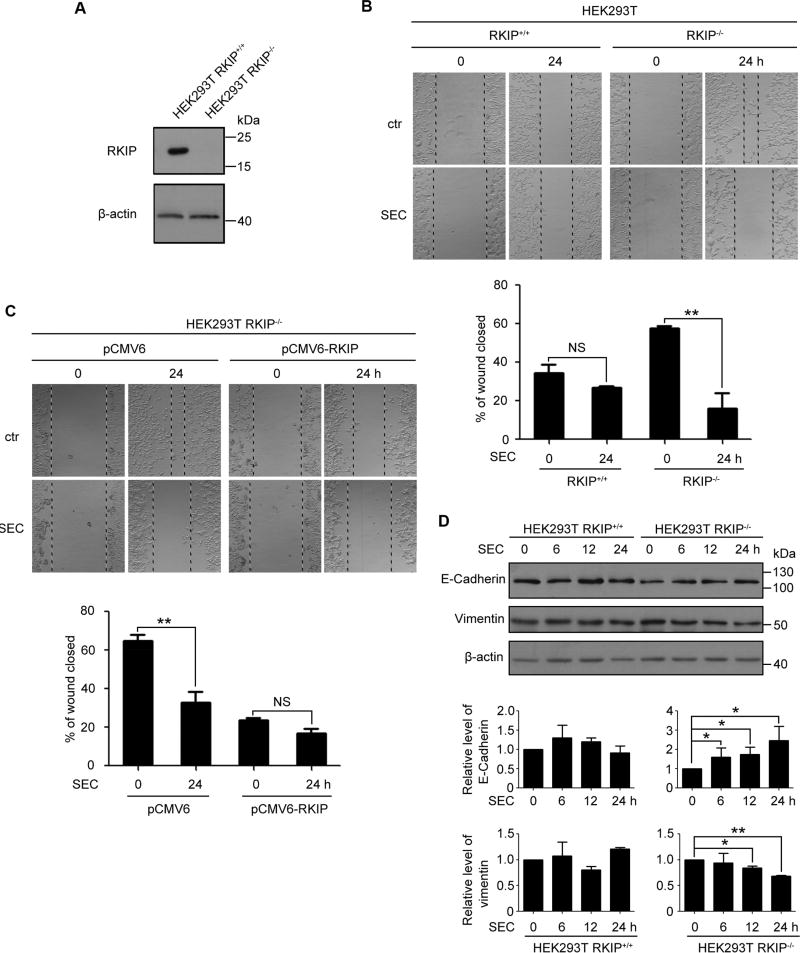
It is well established that RKIP has an anti-metastatic property. To get an in-depth understanding of underlying mechanism, we constructed HEK293T cell lines carrying RKIP knockout (RKIP−/−) and wild-type RKIP expression (RKIP+/+) (Fig. 1A). RKIP-null HEK293T cells showed higher migration ability than wild-type RKIP expressing cells (Fig. 1B). The small molecule SEC dramatically suppressed HEK293T RKIP−/− cell migration while had no effect on HEK293T RKIP+/+ cells (Fig. 1B). Moreover, SEC further increased RKIP level in HEK293T RKIP+/+ cells, and had no effect on HEK293T RKIP−/− cells (Fig. S1A). Restoration of RKIP expression in RKIP-null HEK293T cells by transfection with pCMV6-RKIP decreased the migration ability, meanwhile the effect of SEC was blocked, as compared with the empty vector-transfected cells (Fig. 1C, Fig. S2).
Fig. 1. SEC inhibited the cell migration of HEK 293T RKIP−/− cells.
(A) RKIP protein level in HEK293T RKIP+/+ and RKIP−/− cells. (B) A scratch on HEK293T RKIP+/+ and RKIP−/− cells was made, followed by incubation with SEC (20 µM) for 24 h. Relative wound closure was quantified by measuring the width of the wounds. (C) A scratch was made on HEK293T RKIP−/− cells transfected with pCMV6 empty vector and pCMV6-RKIP plasmid for 24 h, then treated with 20 µM SEC for 24 h. The width of the wounds was measured and relative wound closure was quantified. (D) HEK293T RKIP+/+ and RKIP−/− cells were treated with 20 µM SEC for 6, 12 and 24 h. The protein level of epithelial marker E-Cadherin and mesenchymal marker Vimentin was examined by western blot. Data are mean ± SEM; * p < 0.05, ** p < 0.01, NS p > 0.05, n = 3.
Epithelial-mesenchymal transition (EMT) is critical for the acquisition of migratory property[21]. Western blot analysis revealed that SEC suppressed EMT in HEK293T RKIP−/− cells as the downregualtion of mesenchymal marker vimentin and the upregulation of epithelial marker E-cadherin (Fig. 1D). Moreover, SEC had no effect on EMT process in HEK293T RKIP+/+ cells (Fig. 1D). Therefore, these observations indicate that SEC effectively inhibited cell migration of HEK293T cells with aberrant RKIP expression.
3.2 SEC inhibited migration in PC3 prostate cancer cells
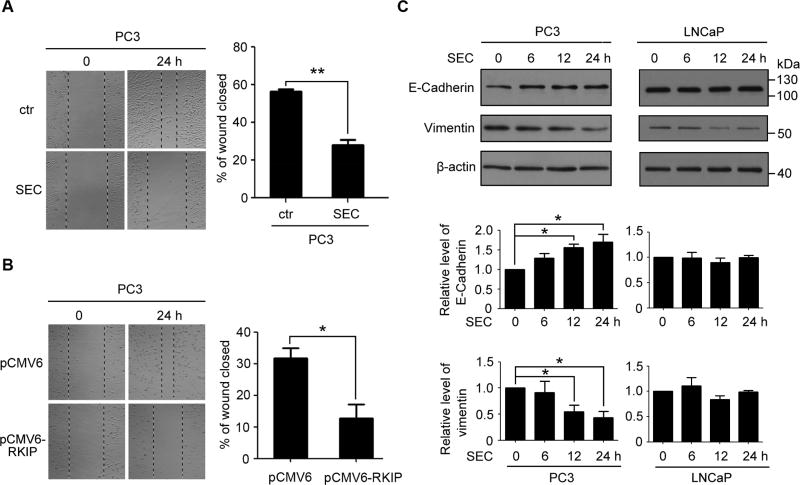
Inspired by the interesting results observed in HEK293T RKIP+/+ and RKIP−/− cells, we wondered the effect of SEC on cancer metastasis. PC3 prostate cancer cell is high metastatic with low RKIP level[22]. Would healing assay showed that the small molecule SEC significantly inhibited PC3 prostate cancer cell migration (Fig. 2A). Meanwhile, SEC had no effect on RKIP expression in PC3 cells (Fig. S1B). Consistent with previous studies showing that RKIP is a metastatic suppressor of prostate cancer[14, 23], overexpression of RKIP in PC3 cells with pCMV6-RKIP transfection suppressed PC3 migration (Fig. 2B). Moreover, SEC treatment decreased vimentin level and increased E-cadherin in PC3 cells (Fig. 2C). In addition, LNCaP prostate cancer cell line is non-invasive with relatively high expressed RKIP. SEC did not affect the levels of EMT markers in LNCaP cells (Fig. 2C). The results indicate that SEC inhibited RKIP low expressing prostate cancer metastasis.
Fig. 2. SEC inhibited PC3 cell migration.
(A) PC3 cells were treated with 20 µM SEC for 24 h after making a scratch. Then the width of the wounds was measured and relative wound closure was quantified. (B) Overexpression of RKIP in PC3 cells inhibited cell migration. A scratch was made on PC3 cells transfected with pCMV6 empty vector or pCMV6-RKIP plasmid for 24 h. The width of the wounds was measured and relative wound closure was quantified. (C) PC3 and LNCaP cells were incubated with 20 µM SEC for 6, 12 and 24 h. Western blot analysis of the protein level of epithelial marker E-Cadherin and mesenchymal marker Vimentin. Data are mean ± SEM; * p < 0.05, ** p < 0.01, n = 3.
3.3 SEC downregulated pro-metastatic gene expression
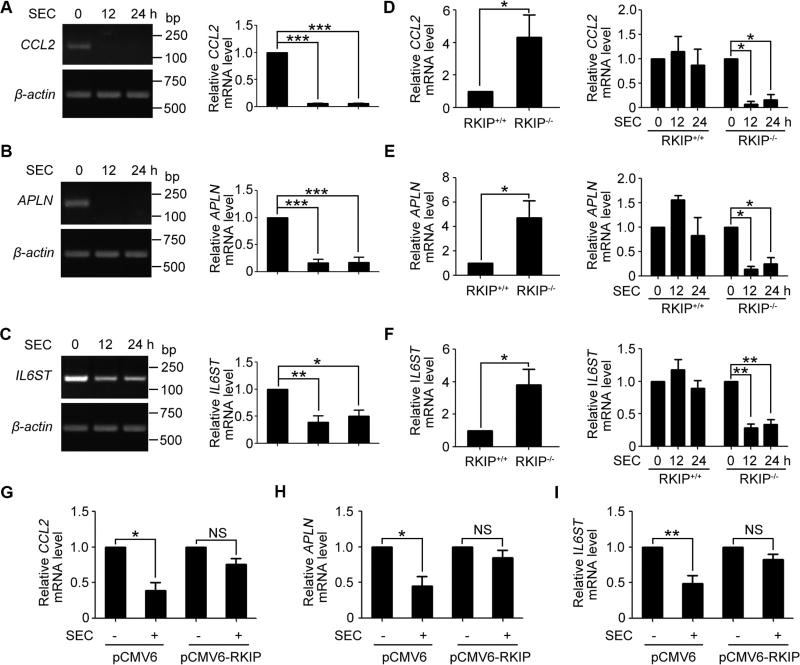
Our previous study has verified that SEC increased pro-apoptotic gene expression[10]. Further analysis of the microarray data, we found that most of the downregulated genes (e.g., CCL2, APLN, IL6ST, RPS6KB1 and EIF4H, Table 1) by SEC treatment are associated with tumor metastasis. CCL2 is a potent recruiter of monocytes to metastasizing tumor cells and is well known to facilitate efficient lung metastasis[24, 25]. APLN is reported to promote migration in multiple cancer types, including lung cancer, hepatocellular carcinoma and prostate cancer[26]. IL6ST triggers pancreatic cancer metastasis by facilitating endothelial adhesion and transendothelial migration[27, 28]. Notably, SEC treatment in PC3 cells indeed decreased the mRNA levels of CCL2, APLN and IL6ST (Fig. 3, A to C), with negligible effect on RPS6KB1 and EIF4H transcription (Fig. S3, A and B). RKIP-null HEK293T cells, with enhanced migration ability, have higher expression of CCL2, APLN and IL6ST than wild-type RKIP expressing cells (Fig. 3, D to F). Consistent with migration-inhibitory effect of SEC on RKIP-null cells, SEC significantly reduced the three metastasis-promoting gene expression in HEK293T RKIP−/− cells, while had little effect on HEK293T RKIP+/+ cells (Fig. 3, D to F). Restoration of RKIP expression in HEK293T RKIP−/− cells resulted in impairment on the inhibitory effect of SEC on mRNA expression of pro-metastasis genes CCL2, APLN and IL6ST (Fig. 3, G to I). These findings indicate that SEC treatment suppresses migration by downregulating metastasis-promoting genes, including CCL2, APLN and IL6ST, when RKIP is aberrantly expressed.
Table 1.
Microarray analysis shows the five most downregulated pro-metastatic genes.
| Gene symbol | Fold change (SEC VS ctr) | Gene name |
|---|---|---|
| RPS6KB1 | 0.0782 | Ribosomal protein S6 kinase beta-1 |
| EIF4H | 0.11965 | Eukaryotic translation initiation factor 4H |
| CCL2 | 0.1383 | Small inducible cytokine A2 precursor |
| APLN | 0.1764 | Apelin, AGTRL1 ligand |
| IL6ST | 0.1806 | Interleukin-6 signal transducer |
Fig. 3. Inhibition of pro-metastatic gene expression by SEC in PC3 and HEK293T RKIP−/− cells.
(A to C) RT-PCR analysis of mRNA levels of CCL2, APLN and IL6ST in PC3 cells treated with 20 µM SEC for 12 h and 24 h. (D to F) qPCR analysis of mRNA levels of CCL2, APLN and IL6ST in HEK293T RKIP+/+ and RKIP−/− cells. Then HEK293T RKIP+/+ and RKIP−/− cells were incubated with 20 µM SEC for 12 h and 24 h, followed by qPCR analysis of mRNA levels of CCL2, APLN and IL6ST. (G to I) qPCR analysis of mRNA levels of CCL2, APLN and IL6ST treated with 20 µM SEC for 24 h in HEK293T RKIP−/− cells transfected with pCMV6 empty vector and pCMV6-RKIP plasmid. Data are mean ± SEM; * p < 0.05, ** p < 0.01, *** p < 0.001, NS p > 0.05, n = 3.
3.4 ANXA7 promoted AMPK phosphorylation by exerting GTPase activity
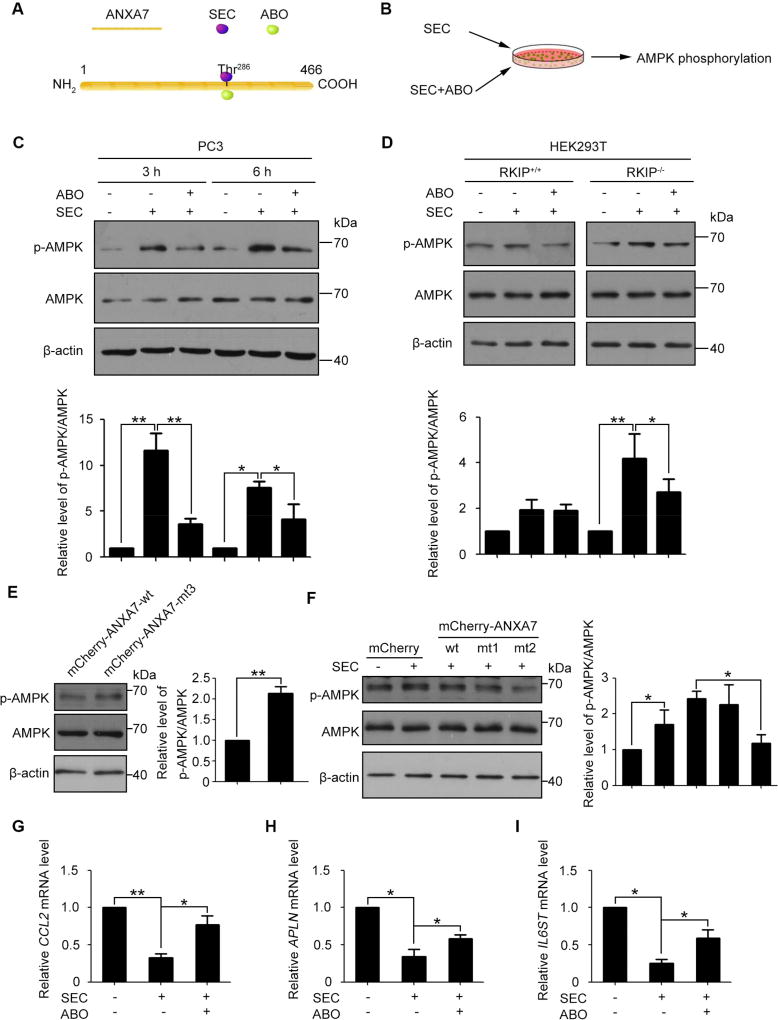
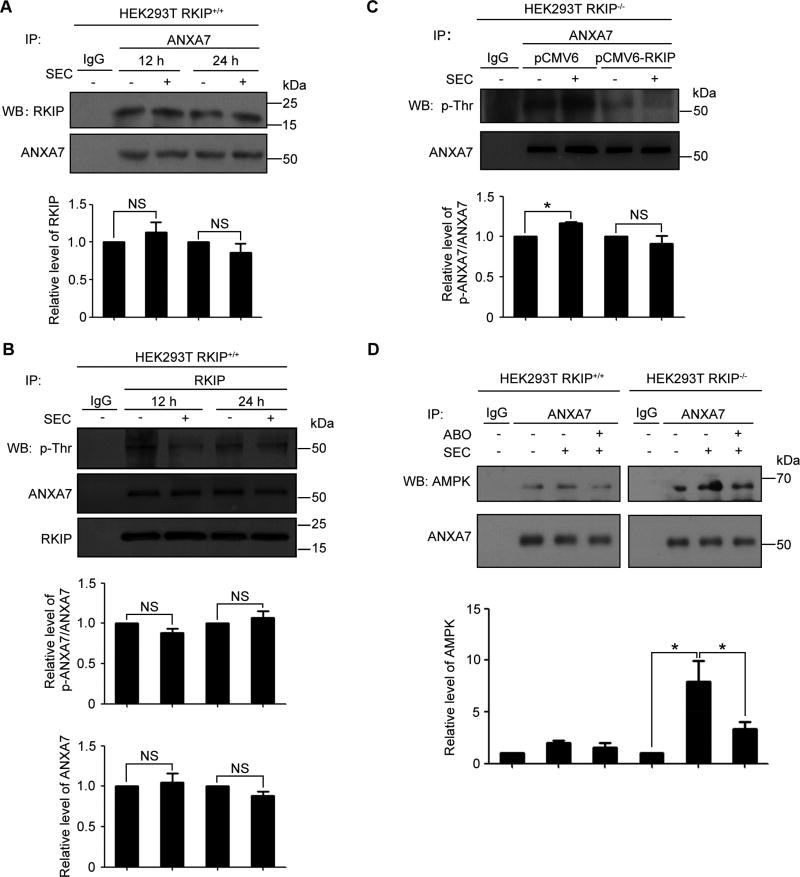
Studies have demonstrated that CCL2, APLN and IL6ST are downstream targets of STAT3[29, 30]. AMPK phosphorylation has a negative effect on STAT3 activation[31]. In our previous study we have demonstrated that SEC activated and ABO inhibited ANXA7 GTPase activity and that ANXA7 GTPase positively regulated Y1494 phosphorylation of its interactive protein ITGB4[10, 20]. ANXA7 Thr286 is the common site that both SEC and ABO bind to (Fig. 4A). Based on these studies, we reason that SEC may increase AMPK phosphorylation by activating ANXA7 GTPase and further impede STAT3 target gene expression. To explore the possibility, we examined the effect of SEC on AMPK phosphorylation in PC3, HEK293T RKIP−/− and RKIP+/+ cells with or without ABO (Fig. 4B). With ANXA7 GTPase activated by SEC and inhibited by ABO, the level of ANXA7 phosphorylation was elevated with SEC treatment and was decreased by ABO in PC3 and HEK293T RKIP−/− cells, while both SEC and ABO were ineffective in HEK293T RKIP+/+ cells (Fig. S4, A and B). Notably, SEC significantly increased AMPK phosphorylation in PC3 cells, which was inverted by ANXA7 GTPase specific inhibitor ABO (Fig. 4C), indicating that activated ANXA7 with enhanced phosphorylation level promotes AMPK phosphorylation. Similar result was observed in HEK293T RKIP−/− cells. In contrast, SEC did not affect AMPK phosphorylation in RKIP+/+ HEK293T cells (Fig. 4D).
Fig. 4. ANXA7 GTPase activity promoted AMPK phosphorylation in PC3 and HEK293T RKIP−/− cells.
(A) Schematic of the binding site of SEC and ABO to ANXA7. (B) AMPK phosphorylation analysis in PC3, HEK293T RKIP+/+ and RKIP−/− cells treated with SEC with or without ABO. (C) Western blot analysis of phosphorylated AMPK and total AMPK in PC3 cells treated with 20 µM SEC for 3 h and 6 h with or without ABO (50 µM). (D) Western blot analysis of phosphorylated AMPK and total AMPK in HEK293T RKIP+/+ and RKIP−/− cells treated with 20 µM SEC for 3 h with or without ABO (50 µM). (E) Western blot analysis of phosphorylated AMPK and total AMPK in HEK293T RKIP−/− cells transfected with expression vectors mCherry-ANXA7-wt and mCherry-ANXA7-mt3 (T286D) for 48 h. (F) Western blot analysis of phosphorylated AMPK and total AMPK in HEK293T RKIP−/− cells transfected with expression vectors mCherry, mCherry-ANXA7-wt, -mt1 (T275A) and -mt2 (T286A) for 48 h, then treated with 20 µM SEC for 3 h. (G to I) qPCR analysis of mRNA levels of CCL2, APLN and IL6ST treated with 20 µM SEC for 24 h with or without ABO (50 µM) in PC3 cells. Data are mean ± SEM; * p < 0.05, ** p < 0.01, n = 3.
To further confirm the role of ANXA7 GTPase activity in the regulation of AMPK phosphorylation, we generated a dominant active mutant mCherry-ANXA7-mt3 (T286D). Overexpression of mCherry-ANXA7-mt3 (T286D) dramatically increased AMPK phosphorylation compared with mCherry-ANXA7-wt -transfected cells (Fig. 4E, Fig. S5). Moreover, the level of SEC-induced AMPK phosphorylation was decreased in cells transfected with mCherry-ANXA7-mt2 (T286A), but not mCherry-ANXA7-mt1 (T275A), in comparison with cells transfected with mCherry-ANXA7-wt (Fig. 4F, Fig. S5). Consistent with suppressed AMPK phosphorylation by ABO, the inhibitory effect of SEC on pro-metastatic gene expression was compromised with the presence of ABO (Fig. 4, G to I). EMT transition was examined in PC3 cells with overexpression of wild-type ANXA7 and dominant active mutant (mCherry-ANXA7-mt3) and negative mutant (mCherry-ANXA7-mt2). The results showed that compared with cells transfected with mCherry-ANXA7-wt, E-cadherin level was increased and vimentin was decreased in mCherry-ANXA7-mt3-transfected cells, which were compromised in cells transfected with mCherry-ANXA7-mt2 (Fig. S6).
3.5 RKIP inhibited ANXA7 signaling as an interactive partner of ANXA7
It has been reported that RKIP can interact with GTP-binding proteins[15]. The above results exhibited that SEC was ineffective in RKIP-carrying HEK293T cells. To explore whether RKIP was involved in SEC-induced ANXA7 activation and subsequent AMPK phosphorylation, the RKIP-ANXA7 association was examined in HEK293T RKIP+/+ cells. Co-immunoprecipitation assays with anti-ANXA7 antibodies confirmed that RKIP can bind to ANXA7 and that the RKIP-ANXA7 association was not affected by SEC treatment (Fig. 5A). Reciprocal co-immunoprecipitation assays with anti-RKIP antibodies further validated the RKIP-ANXA7 interaction. Furthermore, the level of ANXA7 and its phosphorylation were not affected by SEC treatment (Fig. 5B). To elucidate the effect of RKIP-ANXA7 association on SEC-induced ANXA7 activation, we restored RKIP expression in HEK293T RKIP−/− cells by transfection with pCMV6-RKIP and examined ANXA7 phosphorylation. The result revealed that SEC significantly triggered ANXA7 phosphorylation in RKIP-null HEK293T cells, while SEC was dispensable for ANXA7 phosphorylation in cells transfected with RKIP (Fig. 5C). In addition, HEK293T RKIP+/+ cells were transfected with mCherry-ANXA7-wt, -mt2 (T286A) and mt3 (T286D) to detect the binding of RKIP to the ANXA7 active and negative mutants. We found that there is no significant difference among wild-type ANXA7 and its mutants (i.e., T286A and T286D) (Fig. S7). This observation indicates that the active site Thr286 within ANXA7 is dispensable for the interaction between ANXA7 and RKIP. It has been reported that RKIP’s binding to the subdomains I and II within Raf-1 inhibited Raf-1 phosphorylation induced by PAK and Src, blocked the binding of Raf-1 to MEK and interfered with MAPK/ERK pathway[16, 32, 33]. Therefore, we speculate that RKIP may interact with one or multi-fragments outside of the active site of ANXA7, which interferes the binding of SEC to ANXA7 and leads to the failure of ANXA7 activation.
Fig. 5. The affinity of RKIP and ANXA7 blocked the enhanced binding of ANXA7 and AMPK induced by SEC.
(A) Western blot analysis of co-immunoprecipitation of RKIP with anti-ANXA7 antibodies in HEK293T RKIP+/+ cells treated with 20 µM SEC for 12 h and 24 h and quantification of RKIP level. (B) Western blot analysis of reciprocal co-immunoprecipitation of ANXA7 with anti-RKIP antibodies in HEK293T RKIP+/+ cells treated with 20 µM SEC for 12 h and 24 h. ANXA7 and its phosphorylation level were quantified. (C) Western blot analysis of co-immunoprecipitation of ANXA7 and its phosphorylation in HEK293T RKIP−/− cells transfected with expression vectors pCMV6 and pCMV6-RKIP for 24 h, then treated with 20 µM SEC for 3 h. ANXA7 and its phosphorylation level were quantified. (D) Western blot analysis of co-immunoprecipitation of AMPK with ANXA7 antibody in HEK293T RKIP+/+ and RKIP−/− cells treated with 20 µM SEC with or without ABO for 3 h and quantification of AMPK level. Data are mean ± SEM; * p < 0.05, NS p > 0.05, n = 3.
Next, we explored the effect of RKIP on the binding of ANXA7 and AMPK using HEK293T RKIP+/+ and RKIP−/− cell lines. There was an association between RKIP and ANXA7 under basal conditions in both cell lines. In HEK293T RKIP−/− cells, SEC enhanced the binding of ANXA7 and AMPK, which was inverted by ABO. In contrast, the ANXA7-AMPK association was not affected by SEC or ABO treatment in HEK293T RKIP+/+ cells (Fig. 5D). These observations suggest that RKIP can bind to ANXA7 and inhibit the interaction of ANXA7 and AMPK enhanced by SEC.
3.6 Activated AMPK inhibited STAT3 activation by mTORC1 suppression
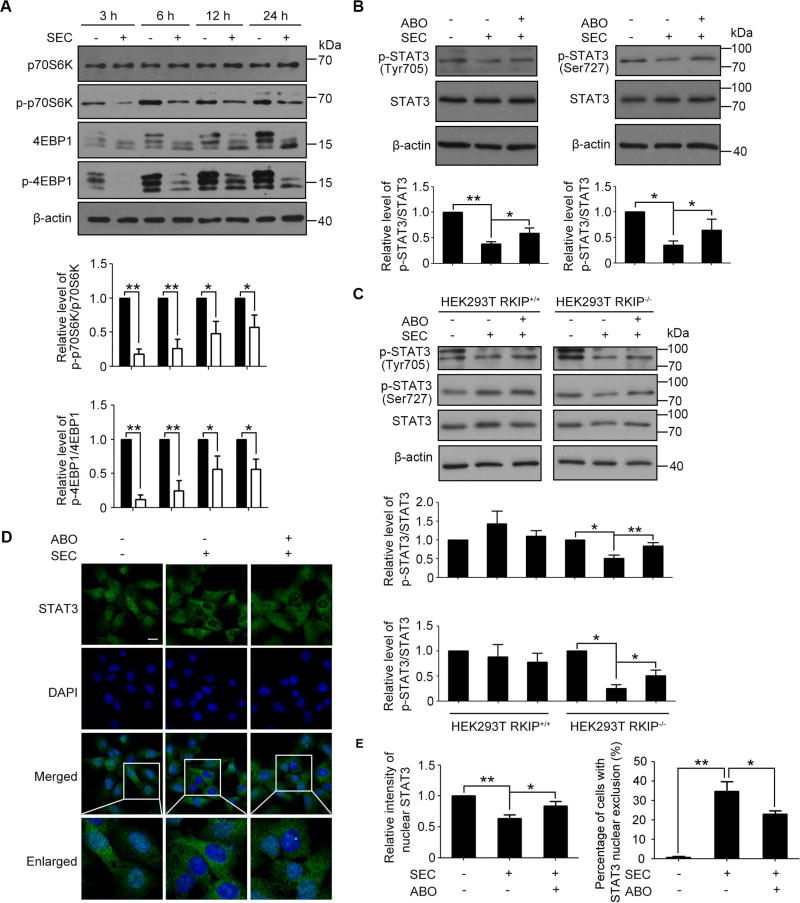
We next analyzed the STAT3 signaling affected by activated AMPK. It has been reported that AMPK inhibited mTORC1 through phosphorylating tuberous sclerosis complex (TSC2)[34]. Moreover, mTOR is responsible for STAT3 phosphorylation at Tyr705 and Ser727[35–37]. Because phosphorylation of STAT3 at Tyr705 and Ser727 induces its nuclear translocation and transcriptional activation, we hypothesize that SEC-induced AMPK activation may inhibit mTORC1 and further contribute to reduced STAT3 activity by decreasing STAT3 Tyr705 and Ser727 phosphorylation. To test this hypothesis, we first analyzed the mTORC1 activity affected by SEC. We found that SEC dramatically suppressed the phosphorylation of p70S6K and 4EBP1, two substrates of mTORC1 (Fig. 6A). Consistently, the phosphorylation of STAT3 at Tyr705 and Ser727 was inhibited with SEC treatment in PC3 cells, which was inverted by ABO (Fig. 6B). In contrast, SEC did not affect STAT3 phosphorylation regardless the presence of ABO in LNCaP cells with high RKIP expression (Fig. S8). By detecting ANXA7 expression at both the mRNA and protein levels in PC3 and LNCaP prostate cancer cells, we found that ANXA7 is relatively high expressed in LNCaP compared with that in PC3 (Fig. S9, A and B). Similarly, SEC strongly decreased STAT3 Tyr705 and Ser727 phosphorylation in HEK293T RKIP−/− cells in an ABO-sensitive manner, while SEC is ineffective in HEK293T RKIP+/+ cells (Fig. 6C). In addition, inhibition of STAT3 activity was further evaluated by imaging its nuclear localization. Immunofluorescence analysis showed that STAT3 was reduced in nucleus when treated with SEC, and this observation was inverted by ABO treatment (Fig. 6, D and E). Taken together, these data suggest that SEC suppressed pro-metastatic gene expression by the AMPK/mTORC1/STAT3 signaling pathway.
Fig. 6. SEC inhibited the phosphorylation and nuclear translocation of STAT3 by mTORC1 in PC3 and HEK293T RKIP−/− cells.
(A) Western blot analysis of phosphorylated p70S6K and total p70S6K, phosphorylated 4EBP1 and total 4EBP1 in PC3 cells treated with 20 µM SEC for the indicated time. (B and C) Western blot analysis of phosphorylated STAT3 at Ser727 and Tyr705 and total STAT3 in PC3 cells (B) and HEK293T RKIP+/+ and RKIP−/− cells (C) treated with 20 µM SEC for 24 h with or without ABO (50 µM). (D) Immunofluorescence analysis of localization of STAT3 in PC3 cells treated with 20 µM SEC for 12 h with or without ABO (50 µM). (E) Statistic analysis showed the intensity of nuclear STAT3 and the ratio of STAT3 nuclear exclusion. Bar, 16 µm. Data are mean ± SEM; * p < 0.05, ** p < 0.01, n = 3.
3.7 SEC suppressed metastasis in PC-3M-Luc orthotopic implantation nude mice model
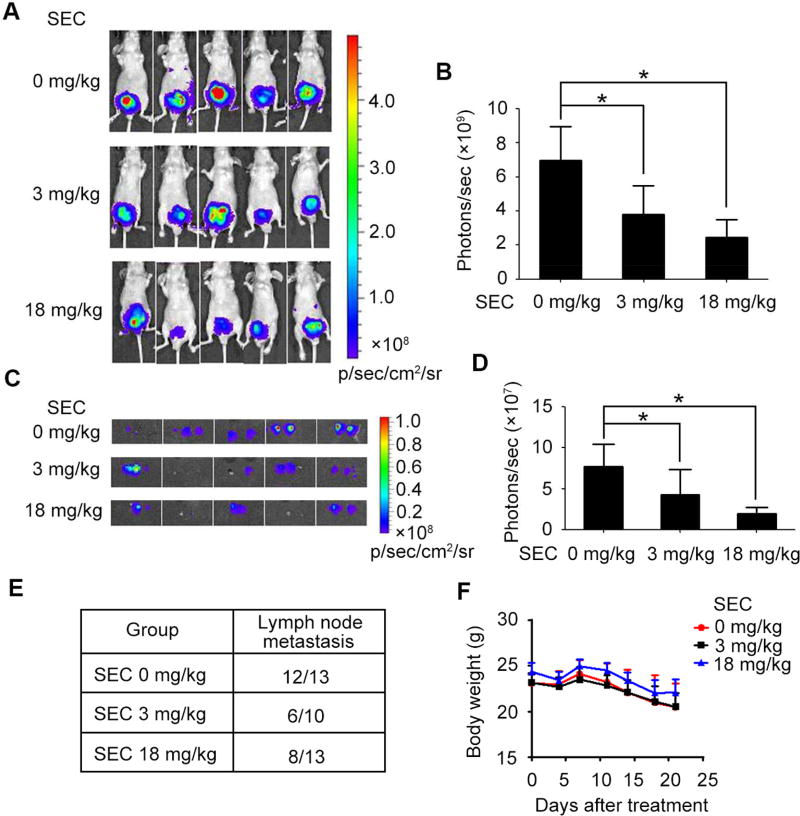
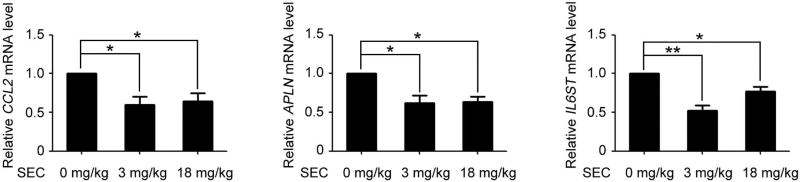
Next, we evaluated in vivo efficacy of SEC by monitoring orthotopic prostate cancer metastasis in mice. Mice receiving orthotopic inoculation of PC-3M-Luc were injected with SEC (3 mg/kg/day or 18 mg/kg/day) for 3 weeks. Importantly, SEC decreased the bioluminescent signals expressed as photon counts (Fig. 7, A and B). The lymph node is a frequent metastatic site for prostate cancer[38]. Then, aortic lymph nodes were isolated and followed by bioluminescence in vitro imaging. PC-3M-Luc cells were able to spread to distant organs, as indicated by the presence of lymph node metastasis. Notably, SEC compromised bioluminescent signals of metastatic lymph nodes and reduced the number of aortic lymph node with prostate cancer metastasis (Fig. 7, C to E). Meanwhile, SEC treatment did not affect the body weight of the mice (Fig. 7F). Consistently, SEC injection indeed decreased the mRNA level of CCL2, APLN and IL6ST in implanted tumors (Fig. 8). These in vivo observations indicate that SEC suppresses the lymph node metastatic capacities of PC-3M-Luc cells in the nude mice model.
Fig. 7. SEC suppressed metastasis of prostate cancer cells in vivo.
(A) Bioluminescence imaging of mice with orthotopic inoculation of PC-3M-Luc injected with SEC for 3 weeks. (B) The bioluminescent signals expressed as photon counts were quantified. (C) Bioluminescence imaging of aortic lymph node. (D) Photon counts of lymph node with prostate cancer metastasis were quantified. (E) Table summarized the results of lymph node metastasis detected in mice. (F) The effect of SEC injection on mice bodyweight. Scale bar represents the color intensity map for bioluminescence signals. Data are mean ± SEM; * p < 0.05, n = 5.
Fig. 8. SEC decreased pro-metastatic gene expression in vivo.
qPCR analysis of mRNA levels of CCL2, APLN and IL6ST in tumors of PC-3M-Luc orthotopic implantation nude mice with or without SEC infection. Data are mean ± SEM; * p < 0.05, ** p < 0.01, n = 5.
4. Discussion
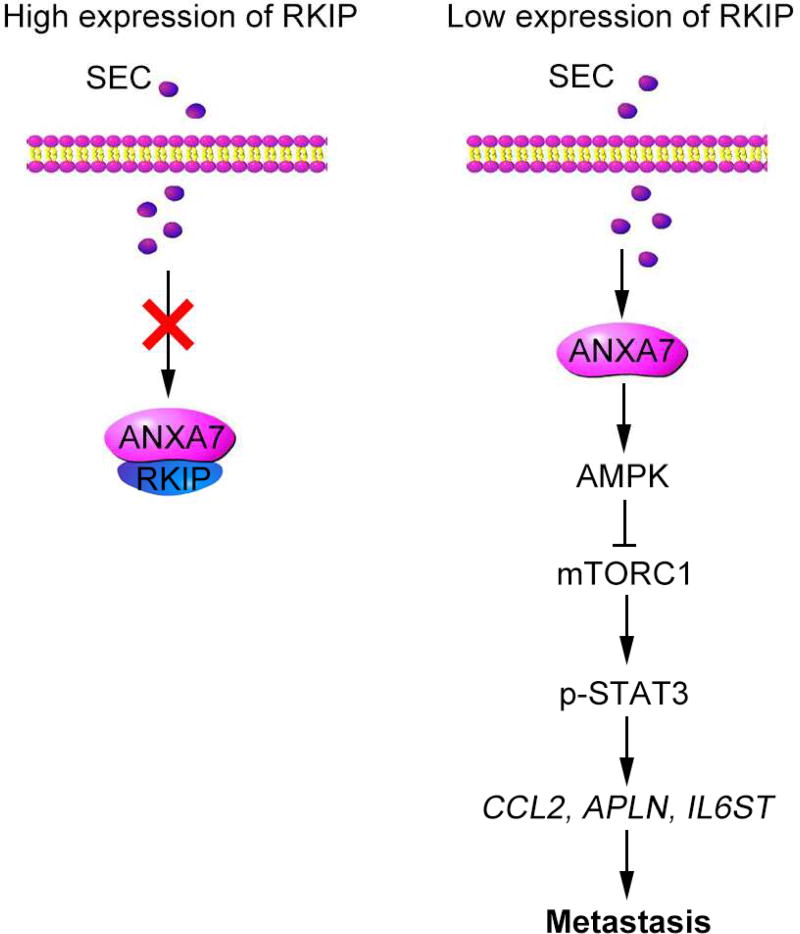
Directly modulating specific target proteins and signaling pathways with small-molecule drugs or agonistic antibodies is widely used for cancer metastasis therapies[39–41]. ANXA7 acts as a metastasis suppressor in prostate cancer. However, the mechanism of ANXA7 on inhibiting prostate cancer metastasis is not clear. In the current study, we revealed for the first time that activation of ANXA7 GTPase by SEC increases AMPK phosphorylation to suppress mTORC1/STAT3 signal pathway and to compromise the expression of pro-metastatic genes—CCL2, APLN and IL6ST, leading to the inhibition of prostate cancer metastasis. The binding of RKIP to ANXA7 is the critical point that blocked SEC-induced ANXA7 GTPase activation and its downstream signaling pathway (Fig. 9).
Fig. 9. A working model of how activation of ANXA7 GTPase by a small molecule SEC antagonizes prostate cancer metastasis.
In HEK293T cells with wide-type RKIP expression, RKIP bound to ANXA7 and blocked SEC-induced ANXA7 GTPase activation. In contrast, SEC increased ANXA7 GTPase activity in PC3 cells with low RKIP expression and RKIP-null HEK293T cells. Activated ANXA7 promoted AMPK phosphorylation and suppressed pro-metastatic gene expression via mTORC1/STAT3 pathway, leading to metastasis inhibition.
AMPK is an evolutionally conserved energy sensor across various species from yeast to mammal[42, 43]. AMPK could recede disordered cellular metabolism in cancers and inhibited tumorigenesis and development by controlling proliferation, autophagy and migration[44, 45]. It has been reported that AMPK activators, such as antroquinonol, metformin and A-769662, could suppress the deterioration of prostate cancer, breast cancer, ovarian cancer and lymphoma[46]. Metformin targets c-MYC oncogene to prevent prostate cancer and is also able to decrease the risk of certain cancers induced by type II diabetes cancer[47, 48] Several GTPases are able to regulate AMPK activity, including the activation of AMPK by Rab13 and Rheb[49, 50], and the inhibition of AMPK by SPAG-1[51]. Our previous work demonstrated that ANXA7 GTPase could regulate its interactive protein phosphorylation[10]. Herein, we focused on the effect of ANXA7 on AMPK phosphorylation. Co-immunoprecipitation assay showed that SEC enhanced the binding of ANXA7 to AMPK and increased AMPK phosphorylation. To further investigate the effect of ANXA7 GTPase on AMPK phosphorylation, we constructed mCherry-ANXA7-mt3 (T286D), a dominant-active mutant of ANXA7 GTPase. Compared with mCherry-ANXA7-wt transfection, overexpression of mCherry-ANXA7-mt3 (T286D) increased AMPK phosphorylation. These results indicate that SEC enhanced ANXA7 GTPase activity, which subsequently activated AMPK. In addition to the intended target, compounds are possible to bind to off-target molecules, thereby causing beneficial or side effects[52, 53]. Whether the off-target effect of SEC enhances or attenuates SEC efficiency needs further investigation.
AMPK is a serine/threonine protein kinase and suppressed mTORC1 activity by promoting the phosphorylation of TSC2 and raptor[54–56]. AMPK/mTOR signaling-mediated anoikis resistance and autophagy induction participated in the metastasis regulation of various cancers[57, 58]. Here, we discovered that SEC downregulated the expression of CCL2, APLN and IL6ST, which play a critical role in promoting cancer metastasis[24–28]. In addition, CCL2, APLN and IL6ST are STAT3 target genes and the expression of the three pro-metastatic genes is controlled by STAT3[29, 30]. STAT3 functions at the downstream of mTORC1. The phosphorylation of STAT3 at Ser727 and Tyr705 triggers STAT3 dimerization, induces STAT3 nuclear translocation and promotes the binding of STAT3 to its target gene promoters[35, 36, 59]. Our current study elucidated that SEC inhibited mTORC1 activity, decreased the phosphorylation of STAT3, and blocked STAT3 nuclear translocation. Therefore, our new findings suggest that SEC activates ANXA7 GTPase and decreases the expression of CCL2, APLN and IL6ST, consequently leading to the inhibition of metastasis. In addition, ITGB4 located on membrane could bind to PI3K and activate AKT/mTOR signaling, which promotes glioma metastasis[60]. Our previous study has demonstrated that SEC could induce ITGB4 nuclear translocation. Therefore, we reason that ITGB4 nuclear translocation may inhibit mTOR activity and suppress metastasis.
It is interesting that SEC significantly inhibited cell migration and EMT in PC3 cells expressing a low RKIP level and HEK293T RKIP−/− cells, while had no effect on HEK293T RKIP+/+ cells. These observations can be interpreted that the RKIP-ANXA7 association may block SEC-induced ANXA7 GTPase activation. It is well known that RKIP could reverse signaling pathways by binding to its target proteins: (a) The RKIP-Raf-1 association inhibited Raf-1 phosphorylation induced by PAK and Src, and blocked the MAPK pathway; (b) RKIP interfered NF-κB signaling by binding to IKK, TAK1 and NIK; and (c) the interaction between RKIP and GRK2 inhibited the binding of GRK with its receptor substrate and contributed to the activation of GPCR signal pathway[18, 58, 61]. Here, we focused on the effect of RKIP on AMPK/mTORC1/STAT3 pathway mediated by ANXA7 GTPase. Our results revealed that RKIP binds to ANXA7 in HEK293T RKIP+/+ cells, which blocks the enhanced binding of ANXA7 to AMPK with SEC treatment and results in the inhibitory effect on the downstream signaling pathway. In contrary, the activation of ANXA7 GTPase by SEC activates AMPK/mTORC1/STAT3 pathway successfully in PC3 cells expressing a low RKIP level and HEK293T RKIP−/− cells. Our findings indicate that the RKIP-ANXA7 association is the key point that inhibits SEC-induced ANXA7 GTPase activation and further blocks AMPK/mTORC1/STAT3 signaling pathway. More work is needed to determine the explicit mechanism by which RKIP inhibits the activation of ANXA7 GTPase induced by SEC.
In conclusion, this study revealed that ANXA7 inhibited prostate cancer metastasis by exerting GTPase activity. Activated ANXA7 GTPase by SEC enhanced the binding of ANXA7 to AMPK and increased AMPK phosphorylation, which led to the inhibition of mTORC1/STAT3 signals and the downregualtion of pro-metastatic genes. The affinity of RKIP to ANXA7 inhibited SEC-induced ANXA7 GTPase activation and blocked its downstream pathway. Our findings are significant because a small molecule SEC promotes ANXA7 GTPase activity to suppress prostate cancer migration and metastasis with low RKIP expression, opening new avenues for future clinical studies targeting this novel ANXA7-RKIP-mediated signaling pathway.
Supplementary Material
SEC-activated ANXA7 GTPase inhibits prostate cancer metastasis by AMPK/mTORC1/STAT3 signaling pathway.
RKIP-ANXA7 interaction compromises SEC-induced ANXA7 activation and blocks its downstream signaling pathway.
ANXA7 functionally interplays with RKIP in prostate cancer metastasis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 91313303, 31570834, 81502948, 31270877), the Major Project of Science and Technology of Shandong Province (NO. 2015ZDJS04001) and Shandong Excellent Young Scientist Award Fund (No. BS2015YY031, BS2013SW001,). The Yan lab was supported in part by funds provided by University of North Carolinat at Charlotte and grants from the NIH/NIGMS (R15GM101571 and R15GM114713).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ward C, Meehan J, Mullen P, Supuran C, Dixon JM, Thomas JS, Winum JY, Lambin P, Dubois L, Pavathaneni NK, Jarman EJ, Renshaw L, Um IH, Kay C, Harrison DJ, Kunkler IH, Langdon SP. Evaluation of carbonic anhydrase IX as a therapeutic target for inhibition of breast cancer invasion and metastasis using a series of in vitro breast cancer models. Oncotarget. 2015;6:24856–24870. doi: 10.18632/oncotarget.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Guan X. Cancer metastases: challenges and opportunities. Acta pharmaceutica Sinica. B. 2015;5:402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JC, Eisenberger MA. Advances in the Treatment of Metastatic Prostate Cancer. Mayo Clin Proc. 2015;90:1719–1733. doi: 10.1016/j.mayocp.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Guo C, Liu S, Greenaway F, Sun MZ. Potential role of annexin A7 in cancers. Clinica chimica acta; international journal of clinical chemistry. 2013;423:83–89. doi: 10.1016/j.cca.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Caohuy H, Pollard HB. Protein kinase C and guanosine triphosphate combine to potentiate calcium-dependent membrane fusion driven by annexin 7. The Journal of biological chemistry. 2002;277:25217–25225. doi: 10.1074/jbc.M202452200. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava M, Bubendorf L, Srikantan V, Fossom L, Nolan L, Glasman M, Leighton X, Fehrle W, Pittaluga S, Raffeld M, Koivisto P, Willi N, Gasser TC, Kononen J, Sauter G, Kallioniemi OP, Srivastava S, Pollard HB. ANX7, a candidate tumor suppressor gene for prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4575–4580. doi: 10.1073/pnas.071055798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava M, Montagna C, Leighton X, Glasman M, Naga S, Eidelman O, Ried T, Pollard HB. Haploinsufficiency of Anx7 tumor suppressor gene and consequent genomic instability promotes tumorigenesis in the Anx7(+/−) mouse. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14287–14292. doi: 10.1073/pnas.2235927100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivastava M, Torosyan Y, Raffeld M, Eidelman O, Pollard HB, Bubendorf L. ANXA7 expression represents hormone-relevant tumor suppression in different cancers. International journal of cancer. 2007;121:2628–2636. doi: 10.1002/ijc.23008. [DOI] [PubMed] [Google Scholar]
- 10.Liu SY, Ge D, Chen LN, Zhao J, Su L, Zhang SL, Miao JY, Zhao BX. A small molecule induces integrin beta4 nuclear translocation and apoptosis selectively in cancer cells with high expression of integrin beta4. Oncotarget. 2016;7:16282–16296. doi: 10.18632/oncotarget.7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamiman K, Keller JM, Mizokami A, Zhang J, Keller ET. Survey of Raf kinase inhibitor protein (RKIP) in multiple cancer types. Critical reviews in oncogenesis. 2014;19:455–468. doi: 10.1615/critrevoncog.2014011987. [DOI] [PubMed] [Google Scholar]
- 12.Fu Z, Smith PC, Zhang L, Rubin MA, Dunn RL, Yao Z, Keller ET. Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. Journal of the National Cancer Institute. 2003;95:878–889. doi: 10.1093/jnci/95.12.878. [DOI] [PubMed] [Google Scholar]
- 13.Beach S, Tang H, Park S, Dhillon AS, Keller ET, Kolch W, Yeung KC. Snail is a repressor of RKIP transcription in metastatic prostate cancer cells. Oncogene. 2008;27:2243–2248. doi: 10.1038/sj.onc.1210860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Z, Kitagawa Y, Shen R, Shah R, Mehra R, Rhodes D, Keller PJ, Mizokami A, Dunn R, Chinnaiyan AM, Yao Z, Keller ET. Metastasis suppressor gene Raf kinase inhibitor protein (RKIP) is a novel prognostic marker in prostate cancer. The Prostate. 2006;66:248–256. doi: 10.1002/pros.20319. [DOI] [PubMed] [Google Scholar]
- 15.Bucquoy S, Jolles P, Schoentgen F. Relationships between molecular interactions (nucleotides, lipids and proteins) and structural features of the bovine brain 21-kDa protein. Eur J Biochem. 1994;225:1203–1210. doi: 10.1111/j.1432-1033.1994.1203b.x. [DOI] [PubMed] [Google Scholar]
- 16.Yesilkanal AE, Rosner MR. Raf kinase inhibitory protein (RKIP) as a metastasis suppressor: regulation of signaling networks in cancer. Critical reviews in oncogenesis. 2014;19:447–454. doi: 10.1615/critrevoncog.2014012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su L, Du H, Dong X, Zhang X, Lou Z. Raf kinase inhibitor protein regulates oxygen-glucose deprivation-induced PC12 cells apoptosis through the NF-kappaB and ERK pathways. Journal of clinical biochemistry and nutrition. 2016;59:86–92. doi: 10.3164/jcbn.15-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajkumar K, Nichita A, Anoor PK, Raju S, Singh SS, Burgula S. Understanding perspectives of signalling mechanisms regulating PEBP1 function. Cell biochemistry and function. 2016;34:394–403. doi: 10.1002/cbf.3198. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Wang F, Gong Y, Li D, Sha J, Huang X, Han X. Proteomic analysis of changes induced by nonylphenol in Sprague-Dawley rat Sertoli cells. Chemical research in toxicology. 2009;22:668–675. doi: 10.1021/tx800406z. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Liu N, Wang S, Wang L, Zhao J, Su L, Zhang Y, Zhang S, Xu Z, Zhao B, Miao J. Identification of a small molecule targeting annexin A7. Biochimica et biophysica acta. 2013;1833:2092–2099. doi: 10.1016/j.bbamcr.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Marcucci F, Stassi G, De Maria R. Epithelial-mesenchymal transition: a new target in anticancer drug discovery, Nature reviews. Drug discovery. 2016;15:311–325. doi: 10.1038/nrd.2015.13. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt J, Noble A, Otsuka M, Berry P, Maitland NJ, Rumsby MG. Phorbol ester stimulates ethanolamine release from the metastatic basal prostate cancer cell line PC3 but not from prostate epithelial cell lines LNCaP and P4E6. British journal of cancer. 2014;111:1646–1656. doi: 10.1038/bjc.2014.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escara-Wilke J, Yeung K, Keller ET. Raf kinase inhibitor protein (RKIP) in cancer. Cancer Metastasis Rev. 2012;31:615–620. doi: 10.1007/s10555-012-9365-9. [DOI] [PubMed] [Google Scholar]
- 24.Roth I, Campbell H, Rubio C, Vennin C, Wilson M, Wiles A, Williams G, Woolley A, Timpson P, Berridge MV, Fleming N, Baird M, Braithwaite AW. The Delta133p53 isoform and its mouse analogue Delta122p53 promote invasion and metastasis involving pro-inflammatory molecules interleukin-6 and CCL2. Oncogene. 2016;35:4981–4989. doi: 10.1038/onc.2016.45. [DOI] [PubMed] [Google Scholar]
- 25.Hauselmann I, Roblek M, Protsyuk D, Huck V, Knopfova L, Grassle S, Bauer AT, Schneider SW, Borsig L. Monocyte Induction of E-Selectin-Mediated Endothelial Activation Releases VE-Cadherin Junctions to Promote Tumor Cell Extravasation in the Metastasis Cascade. Cancer research. 2016;76:5302–5312. doi: 10.1158/0008-5472.CAN-16-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Lv SY, Ye W, Zhang L. Apelin/APJ system and cancer. Clinica chimica acta; international journal of clinical chemistry. 2016;457:112–116. doi: 10.1016/j.cca.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Huang C, Li N, Li Z, Chang A, Chen Y, Zhao T, Li Y, Wang X, Zhang W, Wang Z, Luo L, Shi J, Yang S, Ren H, Hao J. Tumour-derived Interleukin 35 promotes pancreatic ductal adenocarcinoma cell extravasation and metastasis by inducing ICAM1 expression. Nature communications. 2017;8:14035. doi: 10.1038/ncomms14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X, Cao Y, Xiao H, Li C, Lin J. Bazedoxifene as a Novel GP130 Inhibitor for Pancreatic Cancer Therapy. Molecular cancer therapeutics. 2016;15:2609–2619. doi: 10.1158/1535-7163.MCT-15-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abroun S, Saki N, Ahmadvand M, Asghari F, Salari F, Rahim F. STATs: An Old Story, Yet Mesmerizing. Cell J. 2015;17:395–411. doi: 10.22074/cellj.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han S, Wang G, Qi X, Englander EW, Greeley GH., Jr Involvement of a Stat3 binding site in inflammation-induced enteric apelin expression. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1068–1078. doi: 10.1152/ajpgi.90493.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu X, Yan Q, Zhang Z, Du G, Wan X. Acrp30 inhibits leptin-induced metastasis by downregulating the JAK/STAT3 pathway via AMPK activation in aggressive SPEC-2 endometrial cancer cells. Oncology reports. 2012;27:1488–1496. doi: 10.3892/or.2012.1670. [DOI] [PubMed] [Google Scholar]
- 32.Trakul N, Menard RE, Schade GR, Qian Z, Rosner MR. Raf kinase inhibitory protein regulates Raf-1 but not B-Raf kinase activation. The Journal of biological chemistry. 2005;280:24931–24940. doi: 10.1074/jbc.M413929200. [DOI] [PubMed] [Google Scholar]
- 33.Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, Sedivy JM, Kolch W. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature. 1999;401:173–177. doi: 10.1038/43686. [DOI] [PubMed] [Google Scholar]
- 34.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 35.Ai D, Jiang H, Westerterp M, Murphy AJ, Wang M, Ganda A, Abramowicz S, Welch C, Almazan F, Zhu Y, Miller YI, Tall AR. Disruption of mammalian target of rapamycin complex 1 in macrophages decreases chemokine gene expression and atherosclerosis. Circ Res. 2014;114:1576–1584. doi: 10.1161/CIRCRESAHA.114.302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lashinger LM, Malone LM, Brown GW, Daniels EA, Goldberg JA, Otto G, Fischer SM, Hursting SD. Rapamycin partially mimics the anticancer effects of calorie restriction in a murine model of pancreatic cancer. Cancer Prev Res (Phila) 2011;4:1041–1051. doi: 10.1158/1940-6207.CAPR-11-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol. 2000;10:47–50. doi: 10.1016/s0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]
- 38.Gao X, Li LY, Rassler J, Pang J, Chen MK, Liu WP, Chen Z, Ren SC, Zhou FJ, Xie KJ, Zhou X, Qian HJ, Bai XZ, Liu JM, Yang JG, He D, Shao CK, Su ZL, Wang J, Qiu JG, Ling L. Prospective Study of CRMP4 Promoter Methylation in Prostate Biopsies as a Predictor For Lymph Node Metastases. Journal of the National Cancer Institute. 2017;109 doi: 10.1093/jnci/djw282. [DOI] [PubMed] [Google Scholar]
- 39.Yap TA, Smith AD, Ferraldeschi R, Al-Lazikani B, Workman P, de Bono JS. Drug discovery in advanced prostate cancer: translating biology into therapy, Nature reviews. Drug discovery. 2016;15:699–718. doi: 10.1038/nrd.2016.120. [DOI] [PubMed] [Google Scholar]
- 40.Yin Y, Shen WH. PTEN: a new guardian of the genome. Oncogene. 2008;27:5443–5453. doi: 10.1038/onc.2008.241. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Hou X, Shao C, Li J, Cheng JX, Kuang S, Ahmad N, Ratliff T, Liu X. Plk1 inhibition enhances the efficacy of androgen signaling blockade in castration-resistant prostate cancer. Cancer research. 2014;74:6635–6647. doi: 10.1158/0008-5472.CAN-14-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan AS, Frigo DE. A spatiotemporal hypothesis for the regulation, role, and targeting of AMPK in prostate cancer, Nature reviews. Urology. 2017;14:164–180. doi: 10.1038/nrurol.2016.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carling D, Mayer FV, Sanders MJ, Gamblin SJ. AMP-activated protein kinase: nature's energy sensor. Nature chemical biology. 2011;7:512–518. doi: 10.1038/nchembio.610. [DOI] [PubMed] [Google Scholar]
- 44.Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer letters. 2015;356:156–164. doi: 10.1016/j.canlet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, Mamer OA, Avizonis D, DeBerardinis RJ, Siegel PM, Jones RG. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell metabolism. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010;6:457–470. doi: 10.2217/fon.09.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rehman G, Shehzad A, Khan AL, Hamayun M. Role of AMP-activated protein kinase in cancer therapy. Archiv der Pharmazie. 2014;347:457–468. doi: 10.1002/ardp.201300402. [DOI] [PubMed] [Google Scholar]
- 48.Akinyeke T, Matsumura S, Wang X, Wu Y, Schalfer ED, Saxena A, Yan W, Logan SK, Li X. Metformin targets c-MYC oncogene to prevent prostate cancer. Carcinogenesis. 2013;34:2823–2832. doi: 10.1093/carcin/bgt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L, Dai F, Cui L, Zhou B, Guo Y. Up-regulation of the active form of small GTPase Rab13 promotes macroautophagy in vascular endothelial cells. Biochimica et biophysica acta. 2017;1864:613–624. doi: 10.1016/j.bbamcr.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Hong X, Wang J, Yin Y, Zhang Y, Zhou Y, Piao HL, Liang Z, Zhang L, Li G, Xu G, Kwiatkowski DJ, Liu Y. Inhibition of MAPK pathway is essential for suppressing Rheb-Y35N driven tumor growth. Oncogene. 2017;36:756–765. doi: 10.1038/onc.2016.246. [DOI] [PubMed] [Google Scholar]
- 51.Huang C, Wu D, Khan FA, Jiao X, Guan K, Huo L. The GTPase SPAG-1 orchestrates meiotic program by dictating meiotic resumption and cytoskeleton architecture in mouse oocytes. Molecular biology of the cell. 2016;27:1776–1785. doi: 10.1091/mbc.E16-02-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ostrowska M, Adamski P, Kozinski M, Navarese EP, Fabiszak T, Grzesk G, Paciorek P, Kubica J. Off-target effects of glycoprotein IIb/IIIa receptor inhibitors. Cardiology journal. 2014;21:458–464. doi: 10.5603/CJ.a2014.0020. [DOI] [PubMed] [Google Scholar]
- 53.Galloway TJ, Wirth LJ, Colevas AD, Gilbert J, Bauman JE, Saba NF, Raben D, Mehra R, Ma AW, Atoyan R, Wang J, Burtness B, Jimeno A. A Phase I Study of CUDC-101, a Multitarget Inhibitor of HDACs, EGFR, and HER2, in Combination with Chemoradiation in Patients with Head and Neck Squamous Cell Carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:1566–1573. doi: 10.1158/1078-0432.CCR-14-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W, Pan Y, Wang S, Liu Y, Chen G, Zhou L, Ni W, Wang A, Lu Y. Cryptotanshinone activates AMPK-TSC2 axis leading to inhibition of mTORC1 signaling in cancer cells. BMC cancer. 2017;17:34. doi: 10.1186/s12885-016-3038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howell JJ, Hellberg K, Turner M, Talbott G, Kolar MJ, Ross DS, Hoxhaj G, Saghatelian A, Shaw RJ, Manning BD. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell metabolism. 2017;25:463–471. doi: 10.1016/j.cmet.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ng TL, Leprivier G, Robertson MD, Chow C, Martin MJ, Laderoute KR, Davicioni E, Triche TJ, Sorensen PH. The AMPK stress response pathway mediates anoikis resistance through inhibition of mTOR and suppression of protein synthesis. Cell death and differentiation. 2012;19:501–510. doi: 10.1038/cdd.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farooqi AA, Li Y, Sarkar FH. The biological complexity of RKIP signaling in human cancers. Experimental & molecular medicine. 2015;47:e185. doi: 10.1038/emm.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang F, Zhang W, Li D, Zhan Q. Gadd45a suppresses tumor angiogenesis via inhibition of the mTOR/STAT3 protein pathway. The Journal of biological chemistry. 2013;288:6552–6560. doi: 10.1074/jbc.M112.418335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu Y, Ylivinkka I, Chen P, Li L, Hautaniemi S, Nyman TA, Keski-Oja J, Hyytiainen M. Netrin-4 promotes glioblastoma cell proliferation through integrin beta4 signaling. Neoplasia. 2012;14:219–227. doi: 10.1593/neo.111396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lorenz K, Schmid E, Deiss K. RKIP: a governor of intracellular signaling. Critical reviews in oncogenesis. 2014;19:489–496. doi: 10.1615/critrevoncog.2014011923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.