Abstract
Previous studies have assessed nucleobindin 2 (NUCB2) expression in multiple urological cancer cell lines and detected its effect on renal cancer cell apoptosis. Additionally, certain reports have indicated a novel function of NUCB2 in promoting invasion in renal cancer. The levels of NUCB2 expression in different tumor cell lines were detected by quantitative reverse transcription polymerase chain reaction (RT-qPCR). Human NUCB2 and β-actin (ACTB) cDNA plasmids were inserted into lentivirus plasmids, which were then transfected into 786-O cells. Western blotting and RT-qPCR were then performed to determine the gene expression in NUCB2-knocked down cells. Apoptosis was also examined by flow cytometry subsequent to successful transfection. Finally, a transwell invasion assay was performed to investigate the effects on invasive abilities in renal cancer cells. The RT-qPCR results demonstrated a high expression of NUCB2 in 786-O, ACHN and LNCaP cells, and there was particularly high expression in renal cancer 786-O cells. Following successful transfection, downregulation of NUCB2 facilitated renal carcinoma cell apoptosis, as demonstrated by an increased apoptosis rate in the lenti-NUCB2-KD 786-O cells (13.72±0.84 vs. 3.32±0.10; lenti-NUCB2-KD group vs. negative control). Notably, a significant decreased invasion rate was observed in the NUCB2 knocked-down cells compared with negative control, suggesting an invasion-promoting effect of NUCB2. These results suggested a novel function of NUCB2 in the process of development and invasion in renal cell carcinoma. NUCB2 may be an important prognostic factor and target in the diagnosis and treatment of human renal cancer.
Keywords: nucleobindin 2, renal cell carcinoma, invasion, apoptosis, urological cancer
Introduction
Renal cell carcinoma (RCC) is the most common malignant neoplasms of the kidney (1). RCC represents 3–4% of adult solid tumors globally, and metastasis is detected in ~30% of patients during initial diagnosis due to the lack of symptoms typical of the disease at the early stage (1). Additionally, the 5-year survival of the patients with metastatic RCC has been identified to be <10% (2). The major therapeutic tool for RCC is surgery, though targeted therapy may be opted for in cases where surgery does not prove effective. Therefore, attention has been focused on the diagnosis and targeted treatment of RCC. As previously described, RCC development is induced by metabolic factors (3). Genes, including Von Hippel-Lindau tumor suppressor, MET, folliculin, fumarate hydratase, SDH, tuberous sclerosis (TSC) 1 and TSC2, are typically mutated in RCC, all of which have fundamental roles in the regulation of metabolic processes (3). This suggests that metabolic disorders involved in energy and nutrient sensing are key factors in the development of RCC, offering novel targets for tumor diagnosis and treatment. Therefore, metabolic factors, which underlie RCC development, may provide novel targets for screening and developing more effective therapeutic strategies.
Nucleobindin 2 (NUCB2), as a metabolic factor, is a neuropeptide that serves important roles in regulating food intake and energy homeostasis (4). The protein, NUCB2/nesfatin-1 was identified by Oh et al (4) in 2006 as a satiety molecule expressed in the hypothalamus. The NUCB2 gene is located on chromosome 11, which consists of 14 exons spanning 54,785 nucleotides and codes a precursor 396-amino-acid protein NUCB2 (5,6). NUCB2 was first reported to be expressed in the arcuate nucleus, lateral hypothalamus, paraventricular nucleus and supraoptic nucleus of the hypothalamus (4,5). Following this, a number of studies have suggested that the primary peripheral source of NUCB2 was originated from stomach mucosa, and NUCB2 was also peripherally expressed in pancreas and fat tissues (7,8). In the hypothalamus, NUCB2 inhibits food intake and promotes body metabolism (4). As previously described, it has been report that the fasting plasma NUCB2 levels were significantly decreased in patients with type 2 diabetes compared with healthy or type 1 diabetes subjects (9). Additionally, plasma NUCB2 appears to regulate glucose and fatty acid metabolism (10,11). NUCB2 also inhibits the apoptosis of pancreatic cells, which tends to accelerate the development of metabolic disorders (12). In previous years, an increasing number of studies have focused on the association between NUCB2 and carcinoma development. Suzuki et al (13) suggested that NUCB2, as a tumor promoter, served an important role in the process of development and metastasis in breast carcinomas. Additionally, an association between NUCB2 expression and biochemical recurrence and progression of prostatic cancer has been reported (6,14,15). In a recent study, clear cell RCC tissues were observed to express NUCB2 protein, which served a critical function in tumorigenesis and tumor development (16).
However, there is a lack of studies investigating the effects of NUCB2 protein on the invasion and apoptosis rates of tumors, particularly in RCC. In the present study, NUCB2 expression was first analyzed in various urological carcinoma cell lines, and an in vitro study investigating the effect of NUCB2 on RCC invasion and development was designed.
Materials and methods
Cell culture
All of the cell lines (293, 786-O, ACHN, PC-3 and LNCaP) were purchased from the American Type Culture Collection (Manassas, VA, USA) and maintained at −80°C with Cell Culture Freezing medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA). All the cell lines were cultured and maintained in RPMI-1640 medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 10% fetal bovine serum (Thermo Fisher Scientific, Inc.) and 50 µg/ml streptomycin. All cells were cultured at 37°C in an atmosphere of 5% CO2 under saturated humidity. The 786-O cells were divided into three groups: i) non-transfected control cells (NT group); ii) β-actin (ACTB)-transfected cells (lenti-NC cells) and iii) NUCB2-knocked down 786-O cells (lenti-NUCB2-KD cells).
Western blotting
The cells were lysed in ice-cold standard RIPA lysis buffer (Beyotime Institute of Biotechnology, Haimen, China), and the protein concentration was determined using a bicinchoninic protein assay kit. In total, 50 µg of each protein was loaded per lane and separated using SDS-PAGE with a 10% gel and then transferred onto polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA). Following blocking with 5% bovine serum albumin (Solarbio, Beijing, China) for 1 h at 37°C, primary antibodies against NUCB2 (1 µg/ml; cat. no. AF5949; R&D Systems, Inc., Minneapolis, MN, USA) and GAPDH (1:3,000; cat. no. 8884; Cell Signaling Technology, Inc., Danvers, MA, USA) were used. The secondary antibodies were peroxidase-conjugated immunoglobulin G (rabbit anti-sheep, ZDR-5308; sheep anti-rabbit, ZDR-5306; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) and were used at a dilution of 1:8,000. The intensity of bands was analyzed using Quantity One software (version 4.6.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA). Three replicates were performed.
Quantitative reverse transcription polymerase chain reaction (RT-qPCR)
The total RNA was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol. Following this, 20 µl RNase-free water was added into the RNA, and ultraviolet analysis was used to measure the RNA content. RNA was then subjected to RT to obtain cDNA for qPCR analysis. The sequences of primers are summarized in Table I, and the human NUCB2 and ACTB primers were designed according to the sequences listed. Subsequent to the synthesis of the probes, RT-qPCR was performed according to the manufacturer's protocol of the PrimeScript™ RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China). The qPCR was performed with the PCR mix containing 2X SYBR Green mix (Takara Biotechnology Co., Ltd.). The Cq value was automatically recorded using the PCR machine. Thermal cycler PCR conditions consisted of heating samples to 95°C for 2 min; 40 cycles of 95°C for 15 sec, 60°C for 20 sec, and 72°C for 20 sec; and 72°C for 30 min. The relative expression levels of NUCB2 mRNA were calculated using Quantity One software (version 4.6.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA) using the 2−ΔΔCq method: ΔΔCq = (Cqtarget gene - Cqreference gene)test group - (Cqtarget gene - Cqreference gene)blank control group (17). Three replicates were performed and the HEK-293 cell line was used as the control group.
Table I.
List of primer sequences for polymerase chain reaction.
| Target | Primer sequence (5′-3′) | Size of product (bp) |
|---|---|---|
| h ACTB-F2 | GCACCCAGCACAATGAAGA | 254 |
| h ACTB-R2 | AATAAAGCCATGCCAATCTCA | |
| h NUCB2-F1 | AAAAGGCAAGAAGTAGGAAGGTT | 124 |
| h NUCB2-R1 | CAGGATTCAGGTGGTTTAGGTG |
ACTB, β-actin; NUCB2, nucleobindin 2; F, forward; R, reverse.
Lentivirus infection
5′-GAGGACCACTGCTACAGTA-3′, 5′-TTTAGTAACACCATGTGAG-3′ and 5′-GCTCAGAATGGAATATCAT-3′, (lenti-NUCB2-KD1, lenti-NUCB2-KD2 and lenti-NUCB2-KD3, respectively) were selected to be knocked down in the present study. A β-actin (ACTB) cDNA plasmid was used as a negative control, and the lenti-NC cells were treated as the negative control group. Human NUCB2 and ACTB cDNA plasmids were transfected with ViraPower packaging mix using Lipofectamine 2000 reagent (Thermo Fisher Scientific, Inc.) into 786-O cells for 48 h to generate the lentivirus according to the manufacturer's protocol. The viral supernatant was then collected and used to infect the 786-O renal cells. Additional analysis was performed 72 h post-transfection. Following this procedure, qPCR and western blotting were used for the analysis of gene expression.
Apoptosis analysis
Subsequent to successful transfection, the cells were collected by centrifugation (1,000 × g) for 5 min from the supernatant at room temperature. Following trypsinization and washing with PBS twice, the collected cells were mixed with 400 µl Annexin V-fluorescein isothiocyanate binding buffer (Beyotime Institute of Biotechnology). Subsequently, 5 µl adenomatous polyposis coli and propidium iodide were added and mixed. The cell mixture was incubated for 30 min at 20°C and detected by flow cytometry with fluorescence-activated cell sorting analysis following the use of FACS Calibur (BD Biosciences, San Jose, CA, USA). Apoptotic rate was calculated as follows: Apoptotic rate = apoptotic cells/(apoptotic cells + normal cells). Lenti-NUCB2-KD3 was compared with the 786-O cell line and the lenti-NC cell line.
Transwell invasion assay
Matrigel invasion chambers (Corning Incorporated, Corning, NY, USA) were used for the invasion assay. The cells were added to the upper chamber at a density of 5.0×105 cells/ml. A total of 0.1 ml cell suspension liquid was added and 0.6 ml complete medium (RPMI-1640) was added to the bottom of the chambers. Subsequent to observation at 24–30 h following treatment, the supernatant was removed from the chamber, and the upper layer of the filters was wiped with cotton swabs. The cells that had infiltrated were fixed with 4% paraformaldehyde solution and stained with 1% crystal violet for 30 min at 37°C. Images of 5 different fields were captured using an Olympus fluorescence microscope (magnification, ×100; Olympus CKX41; Olympus Corporation, Tokyo, Japan) equipped with a camera and the number of invading cells was counted. The control and negative control were the same as aforementioned in the present study.
Statistical analysis
All data are presented as the mean ± standard error of the mean. Statistically significant differences between groups were assessed using one-way analysis of variance followed by the LSD test. All statistical analysis was performed using a commercially available statistical package (SPSS 17.0; SPSS Inc., Chicago, IL, USA). P≤0.05 was considered to indicate a statistically significant difference.
Results
Analysis of NUCB2 gene expression
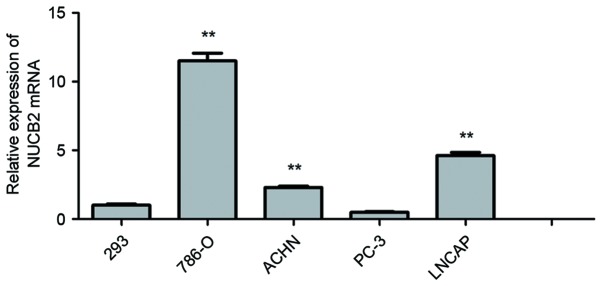
In the 5 cell lines, the expression of NUCB2 in 786-O (11.50±0.57; P<0.01), ACHN (2.30±0.10; P<0.01) and LNCaP cell lines (4.62±1.08; P<0.01) was observed to be significantly increased compared with HEK-293 cells (1.00±0.09). Among the RCC cell lines, the expression of NUCB2 in 786-O and ACHN cells was increased most significantly. NUCB2 expression was also increased in LNCaP cells compared with 293 cells, while there was a low expression of NUCB2 in PC3 cells compared with HEK-293 cells (0.50±0.03 vs. 1.00±0.09; P=0.23). These data are presented in Fig. 1.
Figure 1.
Polymerase chain reaction analysis of NUCB2 in various urological tumor cells. There was a significant increased expression of NUCB2 in 786-O (11.50±0.57; P<0.01), ACHN (2.30±0.10; P<0.01) and LNCaP cells (4.62±1.08; P<0.01) compared with the HEK-293 cells. The y-axis indicates the relative levels of mRNA (2−ΔΔCq). Data are presented as the mean ± standard error. **P<0.01. NUCB2, nucleobindin 2.
Construction of NUCB2 lentiviral vector and expression analysis
Subsequent to the construction of the NUCB2 lentiviral vector according to the protocol aforementioned in the present study, the levels of NUCB2 mRNA expression were detected by qPCR in the normal 786-O cells, negative control and lenti-NUCB2-KD group. Significant differences were observed in the lenti-NUCB2-KD group compared with the 786-O cell (P<0.01) and negative control group (P<0.01). The present study used 3 different gene segments for knockdown: i) 5′-GAGGACCACTGCTACAGTA-3′, ii) 5′-TTTAGTAACACCATGTGAG-3′ and iii) 5′-GCTCAGAATGGAATATCAT-3′. NUCB2 mRNA expression was significantly decreased at all three knockdown groups, while the decrease in the third group was the most marked (Fig. 2A). Therefore, in subsequent analyses, the third gene fragment, 5′-GCTCAGAATGGAATATCAT-3′, was used. Compared with the negative control, NUCB2 protein expression was significantly inhibited in the lenti-NUCB2-kD3 group (P<0.01) as determined by western blotting (Fig. 2B).
Figure 2.
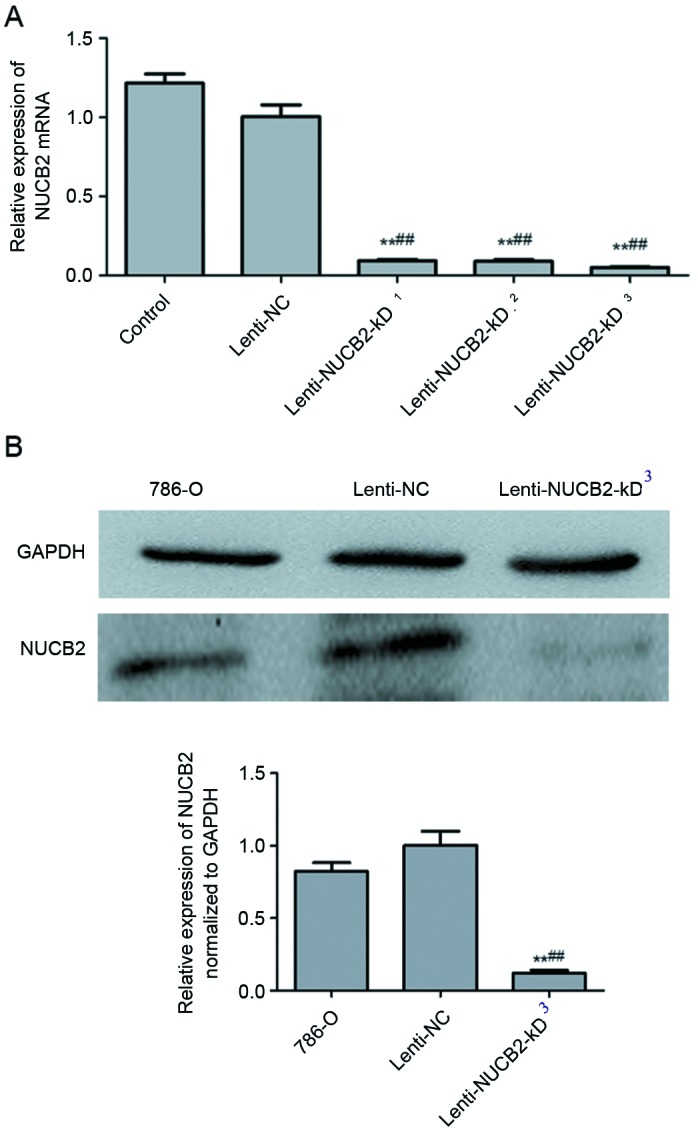
NUCB2 expression in NUCB2-knocked down cells. (A) There was a significant decreased expression of NUCB2 in lenti-NUCB2-KD cells compared with the negative control in the 3 different knockdown groups (0.094±0.08, 0.092±0.11 and 0.051±0.004 vs. 1.01±0.075; lenti-NUCB2-KD1, lenti-NUCB2-KD2 and lenti-NUCB2-KD3 vs. the lenti-NC, respectively; all P<0.01). The y-axis indicates the relative mRNA levels (2−ΔΔCq). (B) Western blotting analysis of NUCB2 expression. The y-axis indicates the relative expression of NUCB2 normalized to GAPDH. Data are presented as the mean ± standard error. The control was the 786-O cell line. **P<0.01 vs. control cells; ##P<0.01 vs. lenti-NC group. NUCB2, nucleobindin 2; NC, negative control.
Downregulation of NUCB2 facilitated apoptosis of renal carcinoma cells
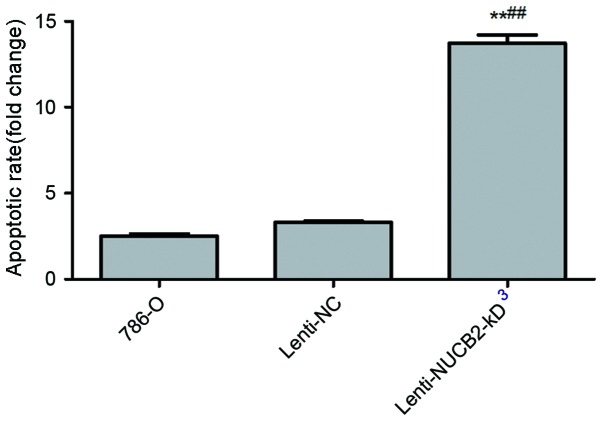
As indicated in Fig. 3, it was observed that the NUCB2 appeared to inhibit apoptosis of 786-O cells. The results indicated that downregulation of NUCB2 resulted in an increase in the rate of apoptosis compared with the negative control from 3.32±0.10 to 13.72±0.84% (P<0.01), as determined by flow cytometry.
Figure 3.
Effect of NUCB2 expression on the rate of apoptosis in 786-O cells. The rate of apoptosis was significantly increased in the lenti-NUCB2-KD group compared with the negative control group (13.72±0.84 vs. 3.32±0.10%, respectively; P<0.01). The y-axis indicates fold changes in apoptotic rate. Data are presented as the mean ± standard error. **P<0.01 vs. control; ##P<0.01 vs. lenti-NC group. NUCB2, nucleobindin 2; NC, negative control.
Invasive and migratory abilities of transfected 786-O cells
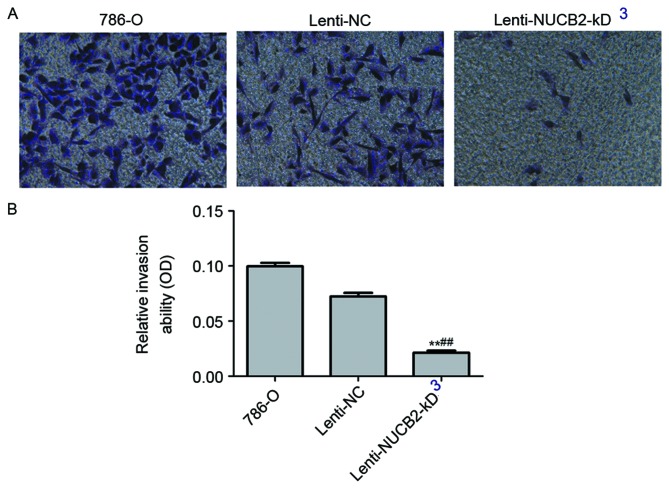
Downregulation of NUCB2 resulted in a significant decreased invasive ability in 786-O cells as determined by Transwell migration assay (lenti-NUCB2-KD group vs. negative control group; 0.02±0.002 vs. 0.07±0.003; P<0.01). Representative images were captured following transfection, and the cells were stained using crystal violet (Fig. 4).
Figure 4.
NUCB2 promotes invasion of 786-O cells. (A) Flow cytometric analysis of tumor cell invasion (magnification, ×100). (B) The relative invasion rate was significantly decreased in the lenti-NUCB2-KD cells compared with the negative control group (0.02±0.002 vs. 0.07±0.003; P<0.01). The y-axis indicates the relative invasion ability. Data are presented as the mean ± standard error. **P<0.01 vs. control; ##P<0.01 vs. lenti-NC group. NUCB2, nucleobindin 2; OD, optical density; NC, negative control.
Discussion
RCC is the most common malignant tumor in human kidney cancer (2,18). However at present, the etiology of RCC has not been completely expounded (2). RCC is a heterogeneous and multifactorial disease with clinical outcomes that are difficult to predict. An improved understanding of RCC etiology is essential for the efficient management and early diagnosis, which is of great concern for its prognosis (2,3). It has been suggested that RCC is a heterogeneous genetic disease with variable biological and clinical features (19). Therefore, a number of studies have focused on the identification of the diagnostic markers to predict the prognosis of this multifaceted disease process and have identified a number of genes, which are involved in the regulation of metabolic disorders. It has been suggested that all of these genes are important for the development of RCC (3,20,21).
To the best of our knowledge, the present study is the first to investigate the novel function of NUCB2 in RCC development and invasion. The main results consist of three points. NUCB2 expression was detected in various cell lines of urinary carcinoma, which may provide a basis for further study of the mechanism of action of NUCB2 within urologic tumors. Additionally, to the best our knowledge, the present study first observed that the downregulation of NUCB2 leads to apoptosis of renal cancer cells (786-O cell line) in vitro, which indicated the proliferative effect of NUCB2 on renal carcinoma (16). Finally, the present study identified a novel function of NUCB2, in that it contributes to renal cancer invasion as demonstrated by results from the transwell assay.
As a metabolism-regulated peptide, NUCB2 has been widely studied in the field of glucose and fatty acid metabolism, particularly in the pancreas, fat tissue and hypothalamus (4). In recent years, increased attention has focused on the metabolic pathways regulating tumor development. Numerous neuropeptides have been discussed due to their tumor-promoting effects (18). NUCB2 was suggested to promote the proliferation of various cells, including adipocytes and pancreatic cells (12,22,23). In addition, it has been suggested that NUCB2 is highly expressed in the cancer of breast, stomach, ovarian epithelium, prostate and kidneys, according to serological identification and expression analysis (6,13–16,24). In breast carcinoma, it has been suggested that NUCB2 may be upregulated by estrogen and serve an important role in increased proliferative, migratory and invasive properties in MCF-7 and SK-BR-3 breast cancer cells (13). It has also been suggested in gastric carcinoma that NUCB2 is a candidate biomarker gene for cancer, which may be identified as a novel potential target for immunotherapeutic treatment (25). Furthermore, a study investigating ovarian epithelial carcinoma in 2013 by Xu et al (24) revealed a notable function of NUCB2/nesfatin-1, where it prevented proliferation in the human ovarian epithelial carcinoma HO-8910 cell line through apoptosis signaling, via the mechanistic target of rapamycin-RhoA/RhoA kinase signaling pathway, which serves an important role in cancer cell proliferation and invasion. In a clinical study of prostate cancer, it was demonstrated that high NUCB2 expression was associated with a shorter biochemical recurrence-free survival time compared with low NUCB2 expression, indicating a potential target for ameliorating the diagnosis, prognosis and treatment methods of prostate cancer (6,14,15). Recently, overexpression of NUCB2 in clear cell RCC was observed to contribute to malignant clinical pathology and a poor prognosis in renal cancer, indicating that it may be a novel biomarker (16). Based on a previous publication by the present authors (10), in the present study NUCB2 expression in ACHN, 786-O, PC-3 and DU 145 cells was verified, and a significant high expression was demonstrated in renal carcinoma 786-O cells. Therefore, the NUCB2 gene was knocked down in the 786-O cells in order to study its function. The results in Fig. 2 indicated that the levels of mRNA and protein were significantly decreased in the lenti-NUCB2-KD group compared with the control group. Furthermore, this effect was examined in a subsequent in vitro study, which indicated that lenti-NUCB2 KD upregulated the apoptotic rate of the renal carcinoma cells, indicating a proliferation-promoting function of NUCB2 in renal carcinoma cells. Therefore, gene production appears to be involved in tumorigenesis and development of RCC. Notably, a novel function was observed in invasion analysis. Invasion was significantly decreased in NUCB2-knocked down cells and a 0.21-fold decrease was identified in the lenti-NUCB2-KD renal cancer cells compared with the lenti-NC cells. Therefore, this suggested that NUCB2 contributed to renal carcinoma invasion. These results contributed to the hypothesis that neuro-hormonal factors serve an important role in tumorigenesis and invasion.
One limitation of the present study was that only the proliferation and invasion of NUCB2-down regulated cells was examined. The specific signaling pathway requires additional clarification, according to the cytological results of the present study. In conclusion, the results of the present study suggest a novel and important function for NUCB2 in cancer progression, particularly in the process of development and invasion in renal cell carcinoma. Therefore, NUCB2 should be considered as a potent prognostic factor and target in the diagnosis and treatment of human RCC.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 81172450), the Key Disciplines Group Construction Project of Pudong Health Bureau of Shanghai (grant no. PWZxq2014-11) and The Program for Outstanding Medical Academic Leader.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Massari F, Ciccarese C, Santoni M, Brunelli M, Piva F, Modena A, Bimbatti D, Fantinel E, Santini D, Cheng L, et al. Metabolic alterations in renal cell carcinoma. Cancer Treat Rev. 2015;41:767–776. doi: 10.1016/j.ctrv.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 5.Cowley MA, Grove KL. To be or NUCB2, is nesfatin the answer? Cell Metab. 2006;4:421–422. doi: 10.1016/j.cmet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Qi C, Wang A, Yao B, Li L, Wang Y, Xu Y. Prognostication of prostate cancer based on NUCB2 protein assessment: NUCB2 in prostate cancer. J Exp Clin Cancer Res. 2013;32:77. doi: 10.1186/1756-9966-32-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao X, Liu XM, Zhou LH. Recent progress in research on the distribution and function of NUCB2/nesfatin-1 in peripheral tissues. Endocr J. 2013;60:1021–1027. doi: 10.1507/endocrj.EJ13-0236. [DOI] [PubMed] [Google Scholar]
- 8.García-Galiano D, Navarro VM, Gaytan F, Tena-Sempere M. Expanding roles of NUCB2/nesfatin-1 in neuroendocrine regulation. J Mol Endocrinol. 2010;45:281–290. doi: 10.1677/JME-10-0059. [DOI] [PubMed] [Google Scholar]
- 9.Li QC, Wang HY, Chen X, Guan HZ, Jiang ZY. Fasting plasma levels of nesfatin-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient-related fluctuation of nesfatin-1 level in normal humans. Regul Pept. 2010;159:72–77. doi: 10.1016/j.regpep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Dong J, Xu H, Xu H, Wang PF, Cai GJ, Song HF, Wang CC, Dong ZT, Ju YJ, Jiang ZY. Nesfatin-1 stimulates fatty-acid oxidation by activating AMP-activated protein kinase in STZ-induced type 2 diabetic mice. PLoS One. 2013;8:e83397. doi: 10.1371/journal.pone.0083397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su Y, Zhang J, Tang Y, Bi F, Liu JN. The novel function of nesfatin-1: Anti-hyperglycemia. Biochem Biophys Res Commun. 2010;391:1039–1042. doi: 10.1016/j.bbrc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez R, Reingold BK, Gao X, Gaidhu MP, Tsushima RG, Unniappan S. Nesfatin-1 exerts a direct, glucose-dependent insulinotropic action on mouse islet β- and MIN6 cells. J Endocrinol. 2011;208:R9–R16. doi: 10.1530/JOE-10-0492. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki S, Takagi K, Miki Y, Onodera Y, Akahira J, Ebata A, Ishida T, Watanabe M, Sasano H, Suzuki T. Nucleobindin 2 in human breast carcinoma as a potent prognostic factor. Cancer Sci. 2012;103:136–143. doi: 10.1111/j.1349-7006.2011.02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Qi C, Li L, Luo F, Xu Y. Clinical significance of NUCB2 mRNA expression in prostate cancer. J Exp Clin Cancer Res. 2013;32:56. doi: 10.1186/1756-9966-32-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Qi C, Wang A, Li L, Xu Y. High expression of nucleobindin 2 mRNA: An independent prognostic factor for overall survival of patients with prostate cancer. Tumour Biol. 2014;35:2025–2028. doi: 10.1007/s13277-013-1268-z. [DOI] [PubMed] [Google Scholar]
- 16.Qi C, Ma H, Zhang HT, Gao JD, Xu Y. Nucleobindin 2 expression is an independent prognostic factor for clear cell renal cell carcinoma. Histopathology. 2015;66:650–657. doi: 10.1111/his.12587. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 19.Muriel L, ópez C, Esteban E, Berros JP, Pardo P, Astudillo A, Izquierdo M, Crespo G, Sanmamed M, Fonseca PJ, Martínez-Camblor P. Prognostic factors in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2012;10:262–270. doi: 10.1016/j.clgc.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Chen K, Zeng J, Xiao H, Huang C, Hu J, Yao W, Yu G, Xiao W, Xu H, Ye Z. Regulation of glucose metabolism by p62/SQSTM1 through HIF1α. J Cell Sci. 2016;129:817–830. doi: 10.1242/jcs.178756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aboudehen K, Kim MS, Mitsche M, Garland K, Anderson N, Noureddine L, Pontoglio M, Patel V, Xie Y, DeBose-Boyd R, Igarashi P. Transcription factor hepatocyte nuclear factor-1β regulates renal cholesterol metabolism. J Am Soc Nephrol. 2016;27:2408–2421. doi: 10.1681/ASN.2015060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramanjaneya M, Tan BK, Rucinski M, Kawan M, Hu J, Kaur J, Patel VH, Malendowicz LK, Komarowska H, Lehnert H, Randeva HS. Nesfatin-1 inhibits proliferation and enhances apoptosis of human adrenocortical H295R cells. J Endocrinol. 2015;226:1–11. doi: 10.1530/JOE-14-0496. [DOI] [PubMed] [Google Scholar]
- 23.Dai H, Li X, He T, Wang Y, Wang Z, Wang S, Xing M, Sun W, Ding H. Decreased plasma nesfatin-1 levels in patients with acute myocardial infarction. Peptides. 2013;46:167–171. doi: 10.1016/j.peptides.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, Pang X, Dong M, Wen F, Zhang Y. Nesfatin-1 inhibits ovarian epithelial carcinoma cell proliferation in vitro. Biochem Biophys Res Commun. 2013;440:467–472. doi: 10.1016/j.bbrc.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Line A, Stengrēvics A, Slucka Z, Li G, Jankevics E, Rees RC. Serological identification and expression analysis of gastric cancer-associated genes. Br J Cancer. 2002;86:1824–1830. doi: 10.1038/sj.bjc.6600321. [DOI] [PMC free article] [PubMed] [Google Scholar]