Abstract
Genipin, a natural compound derived from the fruit of Gardenia jasminoides, possesses numerous biological properties. The aim of the present study was to investigate the anticancer effects of genipin in human bladder cancer. T24 and 5637 bladder cancer cells were treated with different concentrations of genipin (0–200 µM) and tested for cell viability, colony formation, cell cycle progression and apoptosis. A xenograft model of bladder cancer was established to determine the anticancer effect of genipin in vivo. The involvement of the phosphoinositide-3 kinase (PI3K)/Akt pathway in the action of genipin was examined. Genipin treatment significantly inhibited the viability and clonogenic growth of bladder cancer cells and inhibited the growth of T24 xenograft tumors, compared with vehicle controls (P<0.05). Genipin-treated cells exhibited a cell cycle arrest at the G0/G1-phase, which was accompanied by a deregulation of numerous cell cycle regulators. Genipin-treated cells demonstrated a significant increase in the percentage of apoptotic cells, loss of mitochondrial membrane potential, Bax translocation to the mitochondria and the release of cytochrome c to the cytosol. Additionally, genipin treatment significantly (P<0.05) reduced the phosphorylation levels of PI3K and Akt in bladder cancer cells. Importantly, genipin-mediated anticancer effects were reversed by the overexpression of constitutively active Akt. In conclusion, to the best of our knowledge, the present study demonstrates for the first time the growth inhibitory effects of genipin in bladder cancer cells, and indicates its potential as a natural anticancer agent for bladder cancer.
Keywords: apoptosis, bladder cancer, cell cycle arrest, growth, natural compound
Introduction
Bladder cancer is the most common malignancy of the urinary tract and the 14th leading cause of cancer-associated mortality worldwide (1,2). In Western countries, bladder cancer ranks the 4th most frequently diagnosed cancer (3). Multidisciplinary treatment approaches, including surgery, chemotherapy and radiation are available for bladder cancer. Despite advances in diagnosis and treatment, the prognosis, particularly for muscle-invasive disease, remains poor, with a 5-year overall survival of 30–50% (4,5). Therefore, the development of novel effective therapeutic methods for bladder cancer is of clinical significance.
The phosphoinositide-3 kinase/protein kinase B (PI3K/Akt) pathway has a pivotal role in tumor growth and development (6). Depletion of ubiquitin-conjugating enzyme E2T was found to inhibit the proliferation and invasion of osteosarcoma cells (7). It has been reported that activation of PI3K/Akt signaling contributes to Derlin-1-mediated malignant phenotype in muscle invasive bladder cancer cells (8). Inhibition of PI3K/Akt signaling accounts for the induction of death in bladder cancer cells by combination treatment with rapamycin and resveratrol (9). These studies suggest that the PI3K/Akt pathway is an important target for anticancer treatment.
Genipin, a natural compound derived from the fruit of Gardenia jasminoides, has demonstrated various biological properties including anti-inflammatory (10), anti-oxidative (11), anti-diabetic (12), anti-thrombotic (13), and neuroprotective (14) activities. Previous studies indicate that genipin also exhibits a broad range of anticancer activities in different types of human cancer cells (15,16). For instance, genipin has been suggested to suppress hepatocellular carcinoma metastasis through the inhibition of matrix metalloproteinase-2 activity (15). However, few studies have explored the biological activity of genipin in bladder cancer.
The present study aimed to investigate the effects of genipin on cell growth, cell cycle progression and apoptosis in human bladder cancer. Considering the importance of the PI3K/Akt pathway in tumor progression (6,7), it was also investigated whether this signaling pathway is involved in the action of genipin.
Materials and methods
Antibodies
The primary antibodies used in western blot analysis were as follows: Rabbit anti-cyclin D1 (catalog no. 2922), rabbit anti-cyclin-dependent kinase (CDK)2 (catalog no. 2546), rabbit anti-CDK4 (catalog no. 12790), mouse anti-cytochrome c (catalog no. 12963), rabbit anti-phospho-PI3K (catalog no. 4228), rabbit anti-PI3K (catalog no. 4257), mouse anti-phospho-Akt (catalog no. 12694), and mouse anti-Akt (catalog no. 2920) (all from Cell Signaling Technology, Inc., Danvers, MA, USA), mouse anti-cyclin-dependent kinase inhibitor 1 (p21; catalog no. sc-136020), rabbit anti-cyclin-dependent kinase inhibitor 1B (p27; catalog no. sc-528), mouse anti-β-actin (catalog no. sc-81178), mouse anti-B-cell lymphoma 2-like protein 4 (Bax; catalog no. sc-20067) (all from Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and rabbit anti-Heat shock protein 60 (catalog no. ab46798; Abcam, Cambridge, UK). Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology, Inc.
Cell culture and genipin treatment
The human bladder cancer T24 and 5637 cell lines and the immortalized normal human uroepithelial SV-HUC-1 cell line were purchased from the Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). They were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 µg/ml) (all from Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified 5% CO2 atmosphere. Cells were treated with genipin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), which was dissolved in 0.1% dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA). DMSO-treated cells were used as a vehicle control.
Cell transfection
A constitutively active Akt construct (Myr-Akt plasmid) and empty vector were purchased from AddGene (Cambridge, MA, USA). Cells were transfected with 0.3 µg Myr-Akt or vector using Lipofectamine 2000® (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. At 24 h post-transfection, cells were exposed to genipin for additional 48 h prior to cell viability and apoptosis analysis. For each condition, three replicates were performed.
Cell viability assay
Cells were plated in 96-well plates at a density of 2×104 cells/well and incubated with different concentrations (10, 30, 60, 100, and 300 µM) of genipin for 48 h. DMSO-treated cells were used as a control. Each assay was performed in quadruplicate. Cell viability was measured using the MTT assay. Briefly, cells were added with MTT (0.5 mg/ml; Sigma-Aldrich; Merck KGaA) and allowed to incubate for 4 h at 37°C. Following removal of MTT, the resulting formazan was dissolved in DMSO. Absorbance was measured at 570 nm using a microplate reader (xMark; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Clonogenic growth assay
T24 and 5637 cells (5×103 cells/well) were seeded in triplicate onto 6-well plates and treated with 30 or 60 µM of genipin or DMSO (vehicle control) on the following day. DMEM containing genipin was renewed every 3 days. Following 10 days of culture, cells were stained with 0.5% crystal violet (Sigma-Aldrich; Merck KGaA). Colonies (>50 cells) were counted in 10 independent microscopic fields (magnification, ×40) using an inverted microscope.
Animal experiments
A total of 12 athymic male BALB/c (nu/nu) mice, 4 to 6 weeks old and weighing 16–20 g, were obtained from the Animal Center of the Chinese Academy of Medical Science (Beijing, China). The mice were housed 4 per cage at 22°C and 80% humidity in a 12-h light/dark cycle with access to food and water ad libitum. Mice were injected subcutaneously in the left flank with 4×106 T24 cells. When xenograft tumors reached a diameter of ~5 mm, the mice were divided into 3 groups (n=4 for each group) and DMSO (vehicle control) or genipin (20 and 50 mg/kg) was administered intraperitoneally three times/week for 4 weeks. The tumors were measured every week with microcalipers. Tumor volume was calculated using the following equation: Tumor volume (mm3) = 1/2 × (tumor length) × (tumor width)2. Mice were sacrificed 5 weeks after cell injection and tumors were resected and weighted. Animal experiments were approved by the Institutional Animal Care and Use Committee of Chinese Academy of Medical Science. Animal care and treatment were performed in accordance with the international guidelines for laboratory animals use (https://www.apa.org/science/leadership/care/guidelines.aspx).
Cell cycle and apoptosis analysis
Cell cycle distribution was determined by flow cytometry following staining with propidium iodide (PI). Briefly, subsequent to 30 or 60 µg of genipin or DMSO (vehicle control) for 48 h at 37°C, T24 and 5637 cells were collected and fixed in 70% ethanol overnight at 4°C. The cells were resuspended in staining solution containing 0.05 mg/ml PI and 7 U/ml RNase A (Sigma-Aldrich; Merck KGaA) for 30 min at 37°C. Stained cells were analyzed by a flow cytometer (FACSCanto II; BD Biosciences, San Jose, CA, USA) with FlowJo software version 7.6.1 (TreeStar, Inc., Ashland, OR, USA). Cell apoptosis was examined using the Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit (Beyotime Institute of Biotechnology, Haimen, China), as per the manufacturer's instructions. Following incubation with Annexin V-FITC and PI, T24 and 5637 cells were analyzed by flow cytometry using FlowJo software version 7.6.1. Each assay was performed in triplicate.
Preparation of protein samples
For preparation of the whole cellular lysates, cells were suspended in radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Inc.) containing protease inhibitors on ice for 15 min. For extraction of mitochondrial and cytosolic fractions, a commercial mitochondria/cytosol fractionation kit (Beyotime Biotechnology, Inc.) was used. Briefly, cells were suspended in ice-cold cytosolic separation buffer containing protease inhibitors for 15 min and centrifuged at 800 × g for 5 min at 4°C. The supernatant was centrifuged at 12,000 × g for 30 min at 4°C to obtain the cytosolic fraction. The pellet was resuspended in mitochondrial separation buffer for 30 min and then centrifuged at 10,000 × g for 10 min at 4°C to obtain the mitochondrial fraction. Total protein concentrations were determined using a Pierce Bicinchoninic Acid Protein Assay kit (Thermo Fisher Scientific, Inc.).
Western blot analysis
Whole and fractionated cell lysates (50 µg total protein per lane) were mixed with loading buffer and subjected to 12% SDS-PAGE and transferred onto polyvinylidene fluoride membranes. Membranes were blocked with 5% fat-free milk in TBS containing 0.05% Tween-20 for 1 h at room temperature and incubated with primary antibodies overnight at 4°C. All primary antibodies were diluted to 1:500. Subsequent to washing three times (10 min for each time), the membranes were incubated with HRP-conjugated secondary antibodies (dilution at 1:3,000) for 1 h. Membranes were developed with enhanced chemiluminescence reagents (Amersham Biosciences; GE Healthcare, Chicago, IL, USA). Densitometric analysis of protein signals was conducted with Quantity One software version 4.6.2 (Bio-Rad Laboratories). Each assay was performed in triplicate.
Assessment of mitochondrial membrane potential (Δψm)
Measurement of Δψm was performed using the fluorescent probe JC-1, as previously described (17). At low Δψm, JC-1 exists primarily in a monomeric form and produces green fluorescence. At high Δψm, JC-1 aggregates in intact mitochondria and yields red fluorescence. In brief, T24 and 5637 cells were incubated for 30 min at 37°C with 2 µM JC-1 (Molecular Probes, Inc.; Thermo Fisher Scientific, Inc.). Cells were collected and analyzed by the FACSCanto II flow cytometer using FlowJo software version 7.6.1. Results are expressed as the red/green fluorescence intensity ratio; whose reduction indicates loss of Δψm.
Statistical analysis
Data are presented as the mean ± standard deviation, and were analyzed using one-way analysis of variance followed by pair-wise comparisons with Tukey's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Genipin exerts growth suppressive effects on bladder cancer cells
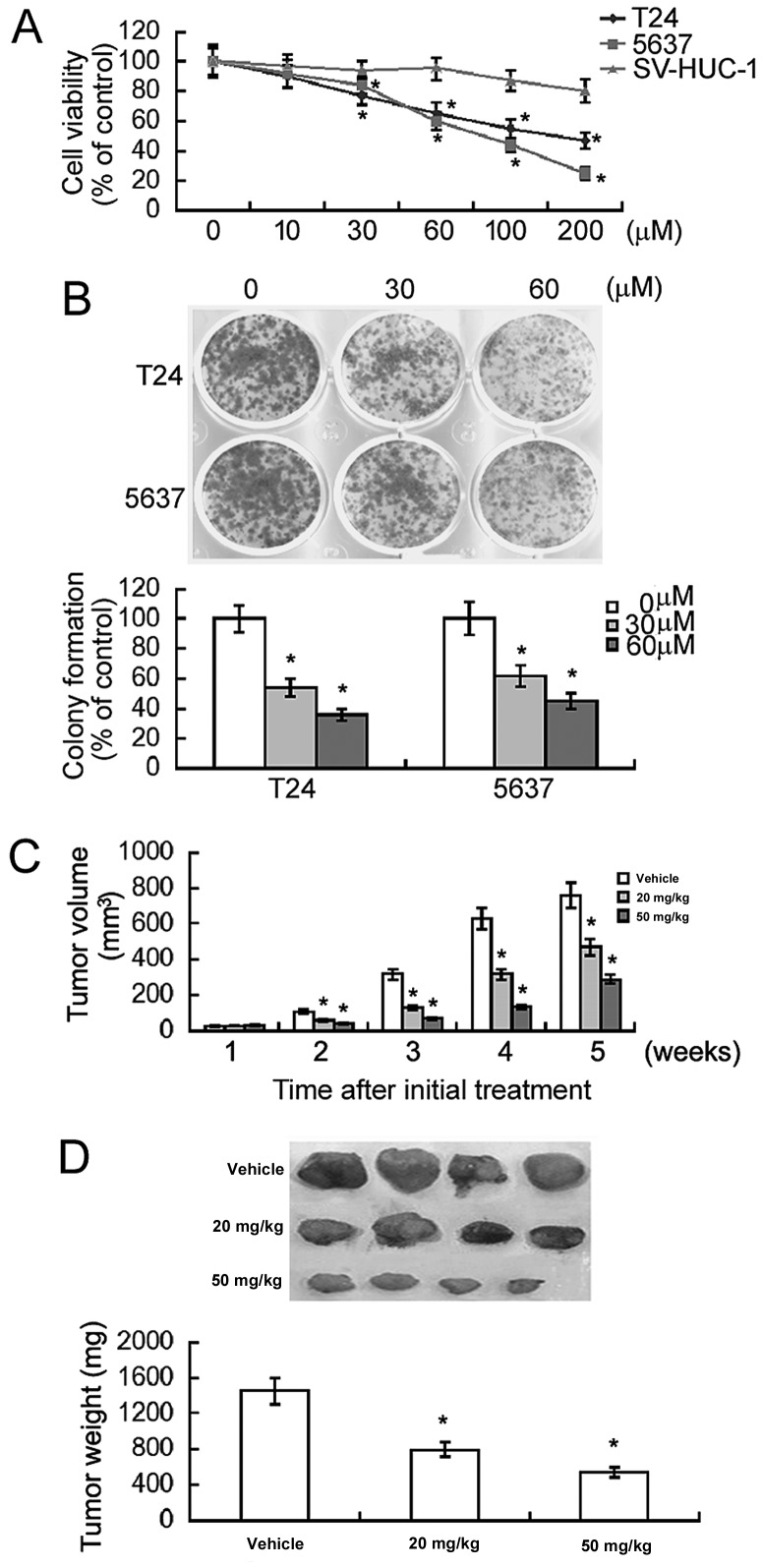
Genipin treatment for 48 h resulted in a concentration-dependent inhibition of the viability of T24 and 5637 bladder cancer cells, compared with the vehicle control (Fig. 1A). In contrast, ≤60 µM genipin did not exhibit a significant effect on the viability of non-malignant uroepithelial SV-HUC-1 cells. These results suggested that a low concentration of genipin was selectively cytotoxic to bladder cancer cells. In the following experiments, genipin was used at a final concentration of 30 or 60 µM unless indicated otherwise.
Figure 1.
Genipin exerts growth suppressive effects against bladder cancer cells. (A) MTT assay was performed to measure the viability of cells exposed to different concentrations (0–200 µM) of genipin for 48 h. (B) The clonogenic growth of cells with or without genipin treatment (30 or 60 µM) was examined following 10 days of culture. Magnification, ×40. (C and D) Mice were injected subcutaneously with T24 cells. When xenograft tumors reached a diameter of ~5 mm, dimethyl sulfoxide (vehicle control) or genipin (20 and 50 mg/kg) was administered intraperitoneally to the mice. (C) Tumor volume was determined every week up to 5 weeks following initial genipin treatment. (D) At the end of the experiment, animals were sacrificed and tumors were excised and weighed. *P<0.05 vs. vehicle-treated cells.
Next, the effect of genipin on clonogenic growth of bladder cancer cells was determined. As demonstrated in Fig. 1B, exposure to genipin significantly decreased the colony formation in T24 and 5637 cells by 2–3-fold. The in vivo antitumor effect of genipin on the growth of T24 xenograft tumors was also examined in nude mice. Compared to the DMSO-treated group, genipin treatment significantly inhibited the growth of T24 xenograft tumors (P<0.05; Fig. 1C). Genipin at the dose of 50 mg/kg caused significantly greater tumor suppression compared with 20 mg/kg genipin. At the end of the experiment, the tumor weight was reduced by >60% in the genipin (50 mg/kg)-treated group (Fig. 1D). These results confirmed the growth suppressive effects of genipin in bladder cancer.
Genipin induces G0/G1 cell cycle arrest in bladder cancer cells
Flow cytometric analysis revealed that genipin-treated T24 and 5,637 cells exhibited a marked increase in the percentage of cells in the G0/G1 phase and a concomitant reduction in the percentage of S phase cells (Fig. 2A). The percentage of cells in the G2/M phase was comparable between genipin-treated and DMSO-treated cells. Western blot analysis demonstrated that genipin treatment significantly reduced the expression levels of cyclin D1, CDK2 and CDK4 in T24 and 5,637 cells (Fig. 2B). By contrast, the levels of the CDK inhibitors (p21 and p27) were significantly raised in response to genipin exposure.
Figure 2.
Genipin induces G0/G1 cell cycle arrest in bladder cancer cells. (A) Flow cytometry analysis of propidium iodide-stained cells following genipin treatment for 48 h. Representative histograms of cell cycle distribution profiles are presented in the top panels. Bottom panels demonstrate quantification of the percentage of cells in each cell cycle phase. (B) Western blot analysis of indicated proteins in cells with or without genipin (30 or 60 µM). Bar graphs demonstrate densitometric analysis of western blots from three independent experiments. *P<0.05 vs. vehicle-treated cells. CDK, cyclin-dependent kinase; p21, cyclin-dependent kinase inhibitor 1; p27, rabbit anti-cyclin-dependent kinase inhibitor 1B.
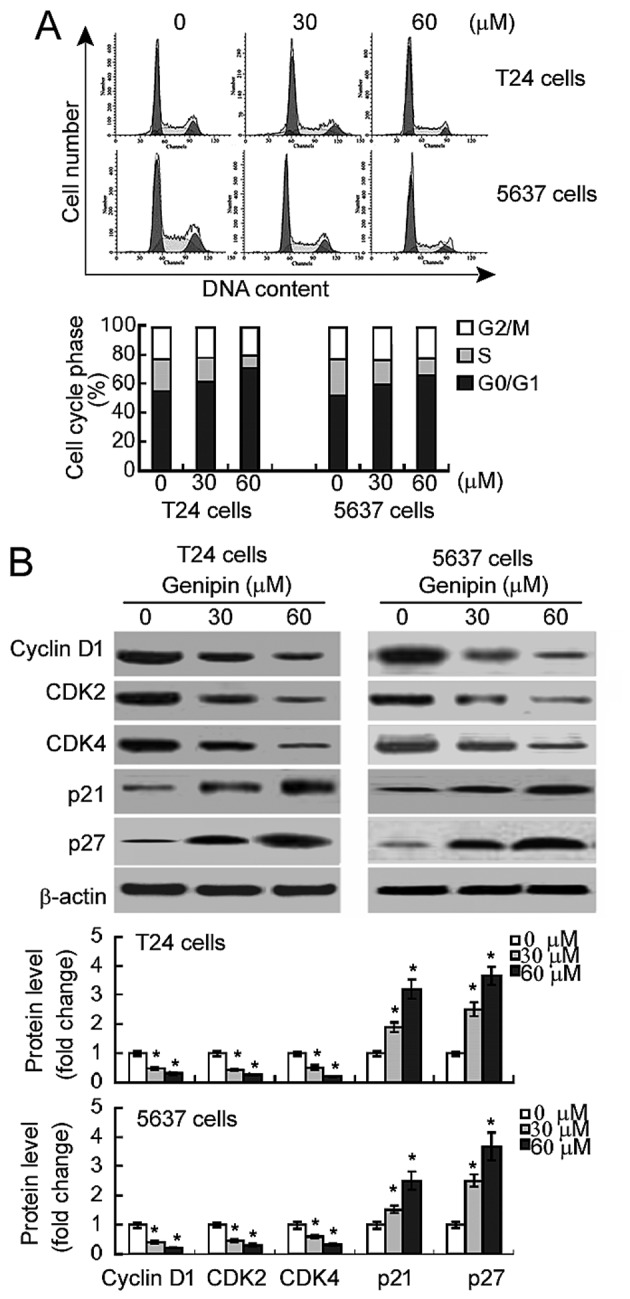
Genipin promotes apoptosis in bladder cancer cells via the mitochondrial pathway
Annexin V/PI staining analysis demonstrated that genipin treatment resulted in a significant increase in the percentage of Annexin V-positive apoptotic cells, compared with DMSO treatment (P<0.05; Fig. 3A). Subsequent to treatment with 60 µM genipin, the percentage of apoptotic cells increased from 4.5±0.9 to 34.6±2.1% in T24 cells and from 3.9±0.8 to 28.5±2.4% in 5637 cells. To test the involvement of the mitochondrial pathway in genipin-mediated apoptosis, Δψm, Bax translocation into mitochondria and the release of mitochondrial cytochrome c were measured. As demonstrated in Fig. 3B, there was 70–80% loss in Δψm in the cells treated with 60 µM genipin, compared with DMSO-treated controls (P<0.05). Additionally, genipin-treated cells exhibited increased Bax and decreased cytochrome c expression in the mitochondrial fractions, compared with control cells (P<0.05; Fig. 3C). Additionally, there was decreased Bax and increased cytochrome c expression in the cytoplasmic fractions of genipin-treated cells (Fig. 3C). These results suggest that genipin-induced apoptosis involves mitochondrial damage and cytochrome c release.
Figure 3.
Genipin induces apoptosis of bladder cancer cells via the mitochondrial apoptotic cascade. (A) Flow cytometry analysis of apoptosis of bladder cancer cells with or without genipin treatment for 48 h following staining with Annexin V and PI. Representative dot plots of apoptosis are presented in top panels. Bar graphs represent quantification of apoptosis from three independent experiments. (B) Measurement of Δψm was performed by flow cytometry using JC-1 staining. Results are expressed as percentage of control values (assigned 100%). (C) Western blot analysis of indicated proteins following genipin treatment (30 or 60 µM) for 48 h. Bar graphs indicate densitometric analysis of western blots from three independent experiments. *P<0.05 vs. vehicle-treated cells. PI, propidium iodide; HSP60, heat shock protein 60; Bax, B-cell-2-like protein 4.
Inactivation of PI3K/Akt signaling mediates genipin-induced anticancer effects
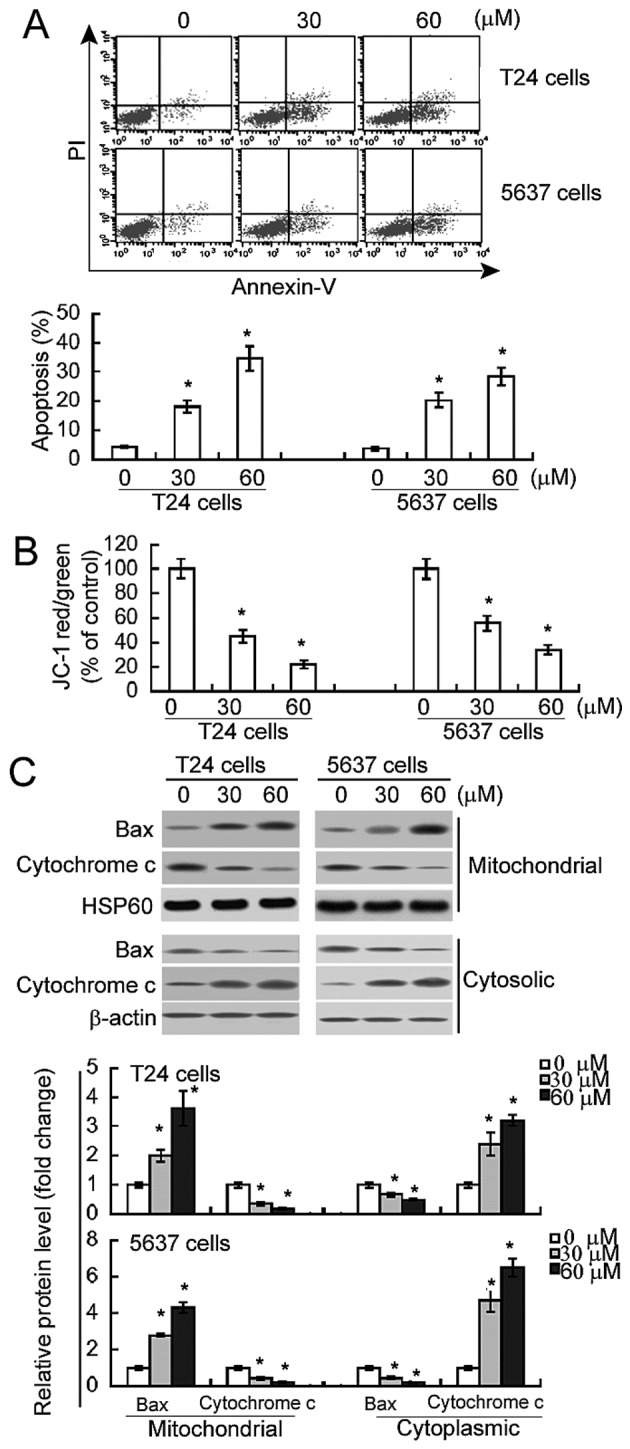
Finally, the potential involvement of PI3K/Akt signaling in the anticancer activity of genipin was examined. Western blot analysis suggested that genipin treatment significantly (P<0.05) reduced the phosphorylation levels of PI3K and Akt in T24 and 5637 cells, compared to DMSO-treated cells (Fig. 4A). Of note, overexpression of constitutively active Akt significantly (P<0.05) increased the viability (Fig. 4B) and inhibited apoptotic response (Fig. 4C) in genipin-treated T24 and 5,637 cells.
Figure 4.

Inactivation of PI3K/Akt signaling mediates genipin-induced anticancer effects. (A) Western blot analysis of the phosphorylation of PI3K and Akt in bladder cancer cells with or without genipin (30 or 60 µM). Bar graphs indicate densitometric analysis of western blots from three independent experiments. *P<0.05 vs. vehicle-treated cells. (B and C) Bladder cancer cells were transfected with active Akt-expressing plasmid or empty vector prior to exposure to 60 µM genipin for 48 h. (B) MTT assay was performed to test cell viability. (C) Flow cytometry analysis of apoptosis following Annexin V/propidium iodide staining. Bar graphs present data from three independent experiments. *P<0.05 for indicated comparisons. PI3K, phosphoinositide-3 kinase; Akt, protein kinase B; pPI3K, phosphorylated PI3K; pAkt, phosphorylated Akt.
Discussion
Natural phytochemicals have attracted increasing attention in cancer therapy due to their broad anticancer activity and low cytotoxicity in normal cells (14,18). Genipin has demonstrated anticancer properties in different types of cancers such as hepatocellular carcinoma (15) and colon cancer cells (16). The data of the present study indicated that this natural compound also exerted anticancer effects against bladder cancer cells. Genipin treatment significantly inhibited the viability and clonogenic growth of bladder cancer cells in vitro. Notably, a low concentration of genipin (≤60 µM) was selectively cytotoxic to bladder cancer cells, but not non-malignant uroepithelial cells. A previous study also indicates the low cytotoxicity of genipin to normal cells (19). In vivo studies additionally confirmed the inhibitory effect of genipin on the growth of bladder cancer xenografts. Therefore, genipin may represent a promising chemotherapeutic agent for bladder cancer.
As reduced cell growth often occurs as a result of cell cycle arrest, the effect of genipin on cell cycle progression of bladder cancer cells was next examined. It was identified that genipin treatment induced a G0/G1 cell cycle arrest in T24 and 5,637 cells, as evidenced by an increase in the percentage of G0/G1-phase cells and a reduction in the percentage of S-phase cells. In agreement with these results, Cao et al (20) suggested that genipin induces cell cycle arrest at the G1 phase in HeLa human cervical carcinoma cells. Genipin has also been identified to induce G2/M-phase cell cycle arrest in AGS human gastric cancer cells (21) and human leukemia K562 cells (22). These results suggest that the growth suppressive activity of genipin is not cell cycle-specific. The data of the present study also suggested that genipin-mediated cell cycle arrest may be ascribed to a reduction of cyclin D1, CDK2 and CDK4 expression, particularly the upregulation of p21 and p27. Increased p21 and p27 levels have been identified to interfere with the formation of CDKs/cyclin complexes and prevent the cell cycle progression to S phase (23).
In addition to induction of cell cycle arrest, genipin demonstrated the ability to cause apoptosis in bladder cancer cells. In addition, genipin treatment resulted in mitochondrial depolarization and the release of mitochondrial cytochrome c into the cytosol. The pro-apoptotic protein Bax has been documented to translocate from the cytosol to the mitochondria in response to apoptotic stimuli, resulting in loss of Δψm and release of cytochrome c (24). In the present study, it was observed that genipin-treated bladder cancer cells exhibited increased Bax levels in mitochondrial fractions and decreased Bax amounts in cytoplasmic fractions. Overall, genipin induced apoptosis in bladder cancer cells through Bax-mediated cytochrome c release from the mitochondria. Similarly, induction of a mitochondrial apoptotic cascade has been described in non-small cell lung cancer H1299 cells following genipin treatment (25).
Inactivation of the PI3K/Akt pathway in genipin-treated bladder cancer cells was also observed in the present study, as evidenced by reduced phosphorylation levels of PI3K and Akt. The PI3K/Akt pathway is involved in the regulation of cancer cell growth and survival, and has been suggested as an important anticancer target (6). Qin et al (26) demonstrated that (−)-epigallocatechin-3-gallate, a component of green tea, promotes apoptosis in T24 bladder cancer cells through the inhibition of the PI3K/Akt pathway. Kim et al (27) revealed that naproxen [(S)-6-methoxy-α-methyl-2-naphthaleneacetic acid] induces cell-cycle arrest and apoptosis in bladder cancer cells through the inhibition of PI3K activity. To confirm the role of the PI3K/Akt pathway in mediating the action of genipin, constitutively active Akt was overexpressed in bladder cancer cells prior to genipin exposure. Notably, it was observed that overexpression of constitutively active Akt reversed the effects of genipin on bladder cancer cell viability and apoptosis. Therefore, it may be suggested that the genipin-induced anticancer effects against bladder cancer cells are likely mediated through the inhibition of PI3K/Akt signaling.
In summary, genipin exerts growth-suppressive effects against bladder cancer cells via the induction of a G0/G1 cell cycle arrest and mitochondrial apoptosis. These anticancer effects of genipin are largely ascribed to inhibition of the PI3K/Akt pathway. Genipin may represent a potential anticancer agent for bladder cancer.
References
- 1.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, Lotan Y. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Mahdavifar N, Ghoncheh M, Pakzad R, Momenimovahed Z, Salehiniya H. Epidemiology, incidence and mortality of bladder cancer and their relationship with the development index in the world. Asian Pac J Cancer Prev. 2016;17:381–386. doi: 10.7314/APJCP.2016.17.1.381. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Zeng XT, Ruan XL, Wang XH, Guo Y, Yang ZH. Simultaneous transurethral resection of bladder cancer and prostate may reduce recurrence rates: A systematic review and meta-analysis. Exp Ther Med. 2012;4:685–692. doi: 10.3892/etm.2012.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinall RL, Tepper CG, Ripoll AA, Gandour-Edwards RF, Durbin-Johnson BP, Yap SA, Ghosh PM, deVere White RW. Decreased expression of let-7c is associated with non-response of muscle-invasive bladder cancer patients to neoadjuvant chemotherapy. Genes Cancer. 2016;7:86–97. doi: 10.18632/genesandcancer.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cirone P, Andresen CJ, Eswaraka JR, Lappin PB, Bagi CM. Patient-derived xenografts reveal limits to PI3K/mTOR-and MEK-mediated inhibition of bladder cancer. Cancer Chemother Pharmacol. 2014;73:525–538. doi: 10.1007/s00280-014-2376-1. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Leng H, Chen H, Wang L, Jiang N, Huo X, Yu B. Knockdown of UBE2T inhibits osteosarcoma cell proliferation, migration, and invasion by suppressing the PI3K/Akt signaling pathway. Oncol Res. 2016;24:361–369. doi: 10.3727/096504016X14685034103310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Q, Fu L, Zhao Y, Tan S, Wang E. Derlin-1 overexpression confers poor prognosis in muscle invasive bladder cancer and contributes to chemoresistance and invasion through PI3K/AKT and ERK/MMP signaling. Oncotarget. 2017;8:17059–17069. doi: 10.18632/oncotarget.15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alayev A, Salamon RS, Schwartz NS, Berman AY, Wiener SL, Holz MK. Combination of rapamycin and resveratrol for treatment of bladder cancer. J Cell Physiol. 2017;232:436–446. doi: 10.1002/jcp.25443. [DOI] [PubMed] [Google Scholar]
- 10.Nam KN, Choi YS, Jung HJ, Park GH, Park JM, Moon SK, Cho KH, Kang C, Kang I, Oh MS, Lee EH. Genipin inhibits the inflammatory response of rat brain microglial cells. Int Immunopharmacol. 2010;10:493–499. doi: 10.1016/j.intimp.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Wang GF, Wu SY, Rao JJ, Lü L, Xu W, Pang JX, Liu ZQ, Wu SG, Zhang JJ. Genipin inhibits endothelial exocytosis via nitric oxide in cultured human umbilical vein endothelial cells. Acta Pharmacol Sin. 2009;30:589–596. doi: 10.1038/aps.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang CY, Parton LE, Ye CP, Krauss S, Shen R, Lin CT, Porco JA, Jr, Lowell BB. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006;3:417–427. doi: 10.1016/j.cmet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, Kondo K, Ikeda Y, Umemura K. Antithrombotic effect of geniposide and genipin in the mouse thrombosis model. Planta Med. 2001;67:807–810. doi: 10.1055/s-2001-18842. [DOI] [PubMed] [Google Scholar]
- 14.Hughes RH, Silva VA, Ahmed I, Shreiber DI, Morrison B., III Neuroprotection by genipin against reactive oxygen and reactive nitrogen species-mediated injury in organotypic hippocampal slice cultures. Brain Res. 2014;1543:308–314. doi: 10.1016/j.brainres.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Wang N, Zhu M, Tsao SW, Man K, Zhang Z, Feng Y. Up-regulation of TIMP-1 by genipin inhibits MMP-2 activities and suppresses the metastatic potential of human hepatocellular carcinoma. PLoS One. 2012;7:e46318. doi: 10.1371/journal.pone.0046318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, MoYung KC, Zhao YJ, Poon K. A mechanism for the temporal potentiation of genipin to the cytotoxicity of cisplatin in colon cancer cells. Int J Med Sci. 2016;13:507–516. doi: 10.7150/ijms.15449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perelman A, Wachtel C, Cohen M, Haupt S, Shapiro H, Tzur A. JC-1: Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012;3:e430. doi: 10.1038/cddis.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh AN, Baruah MM, Sharma N. Structure Based docking studies towards exploring potential anti-androgen activity of selected phytochemicals against Prostate Cancer. Sci Rep. 2017;7:1955. doi: 10.1038/s41598-017-02023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koo HJ, Song YS, Kim HJ, Lee YH, Hong SM, Kim SJ, Kim BC, Jin C, Lim CJ, Park EH. Antiinflammatory effects of genipin, an active principle of gardenia. Eur J Pharmacol. 2004;495:201–208. doi: 10.1016/j.ejphar.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 20.Cao H, Feng Q, Xu W, Li X, Kang Z, Ren Y, Du L. Genipin induced apoptosis associated with activation of the c-Jun NH2-terminal kinase and p53 protein in HeLa cells. Biol Pharm Bull. 2010;33:1343–1348. doi: 10.1248/bpb.33.1343. [DOI] [PubMed] [Google Scholar]
- 21.Ko H, Kim JM, Kim SJ, Shim SH, Ha CH, Chang HI. Induction of apoptosis by genipin inhibits cell proliferation in AGS human gastric cancer cells via Egr1/p21 signaling pathway. Bioorg Med Chem Lett. 2015;25:4191–4196. doi: 10.1016/j.bmcl.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Feng Q, Cao HL, Xu W, Li XR, Ren YQ, Du LF. Apoptosis induced by genipin in human leukemia K562 cells: Involvement of c-Jun N-terminal kinase in G2/M arrest. Acta Pharmacol Sin. 2011;32:519–527. doi: 10.1038/aps.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coqueret O. New roles for p21 and p27 cell-cycle inhibitors: A function for each cell compartment? Trends Cell Biol. 2003;13:65–70. doi: 10.1016/S0962-8924(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 24.Pastorino JG, Chen ST, Tafani M, Snyder JW, Farber JL. The overexpression of bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem. 1998;273:7770–7775. doi: 10.1074/jbc.273.13.7770. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Yao J, Luo Y, Han Y, Wang Z, Du L. P38 MAP kinase mediates apoptosis after genipin treatment in non-small-cell lung cancer H1299 cells via a mitochondrial apoptotic cascade. J Pharmacol Sci. 2013;121:272–281. doi: 10.1254/jphs.12234FP. [DOI] [PubMed] [Google Scholar]
- 26.Qin J, Xie LP, Zheng XY, Wang YB, Bai Y, Shen HF, Li LC, Dahiya R. A component of green tea, (−)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. Biochem Biophys Res Commun. 2007;354:852–857. doi: 10.1016/j.bbrc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Kim MS, Kim JE, Lim DY, Huang Z, Chen H, Langfald A, Lubet RA, Grubbs CJ, Dong Z, Bode AM. Naproxen induces cell-cycle arrest and apoptosis in human urinary bladder cancer cell lines and chemically induced cancers by targeting PI3K. Cancer Prev Res (Phila) 2014;7:236–245. doi: 10.1158/1940-6207.CAPR-13-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]