Abstract
Aims
Oral systemic corticosteroids have been used to induce remission in patients with active inflammatory bowel disease (IBD) for over 50 years; however, the wide array of adverse events (AEs) associated with these drugs prompted the development of steroid compounds with targeted delivery and low systemic bioavailability. This study assessed corticosteroids' comparative harm using network meta‐analysis.
Methods
We searched PubMed, Scopus, Embase, the Cochrane Library, clinical trial registries, regulatory authorities' websites and major conference proceedings, through March 2017. Randomized controlled trials that recruited adult IBD patients and compared oral systemic corticosteroids (prednisone/prednisolone) or compounds/formulations with low systemic bioavailability (budesonide, budesonide MMX, and beclomethasone dipropionate) with placebo, or against each other, were considered eligible for inclusion. Two reviewers independently extracted study data and outcomes, and rated each trial's risk‐of‐bias.
Results
We identified and synthesized evidence from 31 trials including 5689 IBD patients. Budesonide MMX was associated with significantly fewer corticosteroid‐related AEs than oral systemic corticosteroids [odds ratio (OR): 0.25, 95% confidence interval (CI): 0.13–0.49] and beclomethasone (OR: 0.35, 95% CI: 0.13–1.00), but not significantly fewer AEs than budesonide (OR: 0.64, 95% CI: 0.37–1.11); it performed equally good with placebo. By contrast, the occurrence of serious AEs, and treatment discontinuations due to AEs, did not differ between the comparator treatments.
Conclusions
Budesonide MMX is associated with fewer corticosteroid‐related AEs than its comparator steroid treatments for adult IBD patients. Further high‐quality research is warranted to illuminate the steroid drugs' comparative safety profiles.
Keywords: Crohn's disease, glucocorticosteroids, inflammatory bowel disease, network meta‐analysis, systematic review, ulcerative colitis
Introduction
Crohn's disease (CD) and ulcerative colitis (UC) are chronic inflammatory bowel diseases (IBDs) with a remitting and relapsing clinical course and unclear aetiology. They require long‐lasting treatment targeting both flare‐up periods and maintenance of remission 1, 2. A variety of therapeutic agents are currently available in IBD clinical practice, including glucocorticosteroids.
Oral systemic steroids (prednisone, prednisolone) have been used to induce remission in patients with active IBD for over 50 years due to their potent anti‐inflammatory effects 3. However, the plethora of adverse effects associated with these drugs due to their systemic metabolism, including ophthalmic (cataracts and glaucoma), skin, metabolic (from an altered fat distribution to diabetes mellitus), gastrointestinal, musculoskeletal (from osteopenia to osteoporosis) and central nervous system effects, as well as hypertension, adrenal suppression and opportunistic infections 4, 5, has prompted the development of less toxic steroid compounds. Currently, topically acting oral steroids (budesonide and beclomethasone dipropionate), which are characterized by a high topical anti‐inflammatory activity and a low systemic bioavailability, represent an important weapon in the IBD armamentarium 6. Moreover, a novel oral formulation of budesonide, which uses the Multi‐Matrix System (MMX) technology for delivering drugs to the colon (budesonide MMX), has been developed 7.
Given the widespread use of the different steroids in IBD clinical practice, structured evidence on comparative safety of systemic and topically acting (low‐bioavailability) steroids would be very useful for patients and clinicians. To address this issue, we conducted a systematic review of randomized controlled trials (RCTs) evaluating corticosteroid use in IBD adults. We assessed their comparative harm using the methodology of network meta‐analysis, also known as multiple‐treatments meta‐analysis or mixed‐treatment comparison 8, 9, 10, which allows a unified and coherent synthesis of data from RCTs for simultaneous comparison of multiple interventions, while respecting randomization. We aimed to provide a clinically useful summary of the existing evidence to assist physicians in the decision‐making process.
Methods
Protocol and registration
Our study protocol 11 is registered with the International Prospective Register of Systematic Reviews (PROSPERO – http://www.crd.york.ac.uk/prospero). The current systematic review was performed in accordance with the Cochrane Handbook 12, the ISPOR network meta‐analysis guidance 13, 14, and the PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions 15.
Data sources and search strategy
We systematically searched the PubMed, Scopus and Embase bibliographic databases from their inception to 31 March 2017 (date of final search). Search algorithms included the following terms: prednisone, prednisolone, budesonide or beclomethasone, combined with Crohn's disease, ulcerative colitis or inflammatory bowel disease. The search was limited to clinical trials. Language restrictions were not imposed.
We also searched the Cochrane Library for any relevant RCT included in the IBD Group Specialized Trials Register, and for any systematic review that addressed a similar question; the WHO International Clinical Trials Registry Platform, and the http://clinicaltrials.gov to ensure identification of all trials; and major international conference proceedings (European Crohn's and Colitis Organisation, 2011–2016; Digestive Disease Week, 2011–2016; and the United European Gastroenterology Week, 2011–2016).
Two authors (S.B. and T.L.) independently reviewed titles and abstracts; the full texts of the selected articles were critically examined for eligibility; and their reference lists (and those of relevant systematic reviews and meta‐analyses in the discipline) were searched to identify further eligible trials. Finally, we conducted supplemental searches of regulatory authorities' websites (www.fda.gov, www.ema.europa.eu and www.tga.gov.au) to identify drug assessment reports including data of completed but unpublished studies.
Selection criteria
Parallel‐group RCTs having enrolled adult patients (>90% of participants over the age of 18 years) with IBD (either CD or UC), and comparing systemic corticosteroid drugs (prednisone, prednisolone) or compounds/formulations with low systemic bioavailability (budesonide, budesonide MMX, beclomethasone dipropionate) with placebo, or against each other (i.e. head‐to‐head trials), were eligible for inclusion. Studies evaluating multi‐interventional therapies, in which the effect of the steroid treatment could be separated from that of other treatments, were also considered eligible.
If the results of a study were reported at multiple time‐points, we included those corresponding to the longest duration, provided it remained a randomized trial and fully reported the outcomes of interest. Studies were excluded if they were observational; examined topical corticosteroids (e.g. suppositories, enemas, foam preparations) or drugs not commonly used in practice (e.g. betamethasone phosphate, fluticasone propionate); did not investigate patients with CD or UC (i.e. trials in lymphocytic colitis were excluded); evaluated different dosages of one drug (i.e. dose‐comparison studies) without another active drug or placebo as a control arm; did not report (or provided insufficient data for) the outcomes of interest; or were conducted in paediatric populations. No restrictions were applied by drug dose, or length of follow‐up.
Data extraction and quality assessment
Two reviewers (S.B. and T.L.) independently abstracted the following data from each study in a form: first author, journal and year of publication, study design and duration, number of participants, underlying condition, patient characteristics (age, sex and concomitant treatments), interventions (drug and dosage), and number of patients with events reported for the intervention and control group. Study results posted at http://clinicaltrials.gov were also checked. Any discrepancies were resolved via consensus referring back to the original article.
Different doses were treated as the same intervention (i.e. they define a single node in the network).
The estimated measures of effect were the odds ratios (ORs) for the following adverse outcomes:
number of treatment discontinuations or withdrawals from the study due to adverse events (AEs);
number of patients with any (one or more) serious AEs (SAEs), which are defined as any untoward medical occurrence that results in death, requires hospital admission or prolongation of existing hospital stay, causes persistent or significant disability/incapacity, or is life threatening 16;
number of patients with corticosteroid‐related AEs (i.e. the occurrence of one or more of the following symptoms: moon face, buffalo hump, acne, hirsutism, purple skin striae, easy bruising, ankle swelling, hair loss, mood swings, depression, sleep changes and insomnia).
Analysis was based on the total number of randomly assigned patients (intention‐to‐treat principle), wherever trial reporting allowed this (if not applicable, then all evaluable patients).
We assessed risk of bias (RoB) in included studies using the Cochrane Collaboration's tool 17, 18.
For methodological details see Appendix (Risk of bias assessment: Methods).
Data synthesis and analysis
We conducted the network meta‐analysis within a frequentist framework in STATA (Stata Corp., College Station, TX, USA) using the network suite 19 and other network‐related commands 20, 21.
Data were analysed in two formats: In the augmented format, all treatments were compared with a reference group (placebo), and studies without the reference arm were augmented with an artificial placebo group including a small amount of data 19, 22, 23. In the standard format, each study had its own reference group. Results using the two formats were almost identical. Unless stated otherwise, results are from analyses on data in the augmented format. When no events occurred in one group of a trial, we used a continuity correction inversely proportional to the relative size of the opposite group. In particular, the continuity correction for the treatment group was 1/(R + 1), where R is the ratio of control group to treatment group sizes. Similarly, the continuity correction for the control group was R/(R + 1). This approach outperforms the use of a constant continuity correction of 0.5 in settings of sparse event data and imbalanced study groups 24. Trials reporting zero‐event data for both study groups were not included in the analyses.
Multivariate random‐effects meta‐analyses were fit to model the intervention effects across studies using consistency and inconsistency models 19, 22, 23, 25, 26. The off‐diagonal cells of the league tables contain odds ratios and 95% confidence intervals from all pairwise comparisons in network meta‐analyses. The contribution of direct evidence to the mixed estimates and the entire network was also calculated and plotted 20, 27. Probabilities of each treatment being at a specific order, mean ranks of treatments, and surface under the cumulative ranking area (SUCRA) values 19, 20, 28, were estimated running 10 000 replications. SUCRA is used to provide a hierarchy of treatments for each outcome. The larger the SUCRA value, the better (i.e. the safer) the treatment.
To assess the comparative safety profiles distinguishing short‐term from long‐term side effects, subgroup analyses were also performed according to study duration; a cut‐off of 12 weeks was used.
Heterogeneity (within each comparison) was estimated through the restricted maximum likelihood approach, and was assumed to be constant across treatment contrasts (common τ2) 19, 23. Predictive intervals that reflect the extent of heterogeneity in network meta‐analytic effect estimates, and in which future relative treatment effects are expected to lie, were estimated and plotted 20, 21. Cochran Q test for heterogeneity, I2 and τ2 for all direct comparisons were computed. The magnitude of τ2 estimated in every direct synthesis of evidence was compared to quantiles of empirical distributions found in meta‐analyses 27, 29.
For the inconsistency models, the design‐by‐treatment interaction approach was used 23, 25, 30. Inconsistency terms were modelled as fixed parameters. Global Wald tests for inconsistency were performed that jointly examine the inconsistency parameters 23, 25. Inconsistency was also explored by node‐splitting using the symmetrical option 25, 31, and by calculating the inconsistency factor between direct and indirect evidence in all closed loops (triangular and quadratic) in the networks 20, 27, 32. Inconsistency factor is the ratio of the direct and indirect ORs in each loop.
Selective outcome reporting or publication bias was assessed by inspecting funnel plots appropriately adjusted for the inclusion of studies that compare different pairs of treatments 20, 21, 33. Comparisons were consistently defined across studies focusing on active drugs vs. placebo. In the absence of small‐study effects, the plot should be symmetric around the zero line.
Results
Search results
After removal of duplicates, the database search yielded 1552 literature citations (Figure 1, flow chart). Records clearly not eligible or irrelevant to the topic were excluded. We retrieved 97 publications for detailed evaluation. The full text was read, and the bibliographies were checked. We initially identified 27 RCTs eligible for inclusion in the Network 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59. Four additional eligible studies were identified in regulatory authorities' drug assessment reports 60, 61, for a total of 31 trials (Table 1).
Figure 1.
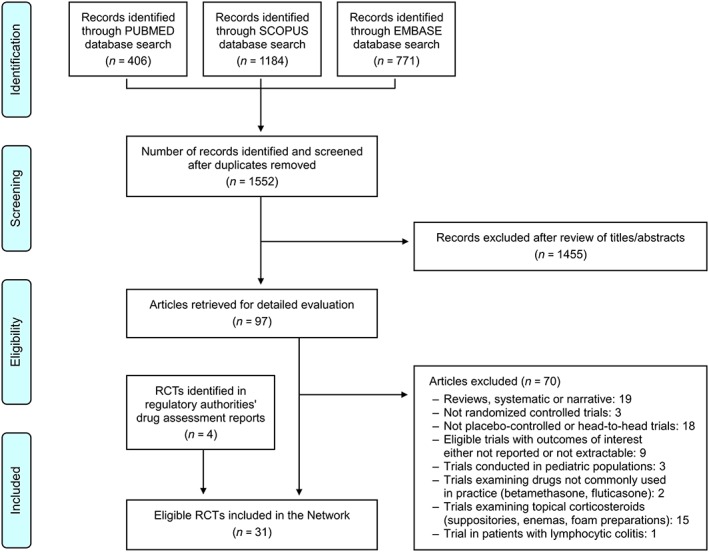
Summary of the evidence search and selection process (flow chart). RCTs, randomized controlled trials
Table 1.
Characteristics of the randomized controlled trials included in the network
| Study [reference] | Condition | Study groups, intervention parameters, patients randomized |
Duration
(weeks) |
Mean age
(years) |
Women
(%) |
|---|---|---|---|---|---|
| Bar‐Meir et al., 1998 34 | CD | Budesonide 9 mg day–1 (n = 100) | 8 | 32.8 | 48.3 |
| Prednisone 40 mg day–1 tapered gradually to 5 mg day–1 (n = 101) | |||||
| Campieri et al., 1997 35 | CD |
Budesonide 9 mg day–1 tapered to 6 mg day–1 after 8 weeks, and to 3 mg day–1 after a further 2 weeks (n = 119) |
12 | 36.7 | 59.3 |
|
Prednisolone 40 mg day–1 tapered to 30 mg day–1 after 2 weeks, and then continuously throughout the study, reaching 5 mg day–1 after 9 weeks (n = 58) | |||||
| Cortot et al., 2001 36 | CD | Budesonide 6 mg day–1 (n = 60) | 13 | 33.5 | 59.0 |
| Placebo (n = 60) | |||||
| Ewe et al., 1999 37 | CD | Budesonide 3 mg day–1 (n = 43) | 52 | 34.0 | 55.4 |
| Placebo (n = 40) | |||||
| Ferguson et al., 1998 38 | CD | Budesonide 6 mg day–1, or 3 mg day–1 (n = 48) | 52 | 35.9 | 54.7 |
| Placebo (n = 27) | |||||
| Greenberg et al., 1996 39 | CD | Budesonide 6 mg day–1, or 3 mg day–1 (n = 69) | 52 | 35.6 | 60.0 |
| Placebo (n = 36) | |||||
| Greenberg et al., 1994 40 | CD | Budesonide 15 mg day–1, 9 mg day–1, or 3 mg day–1 (n = 192) | 8 | NR | 62.4 |
| Placebo (n = 66) | |||||
| Gross et al., 1998 41 | CD | Budesonide 3 mg day–1 (n = 84) | 52 | 32.0 | 59.2 |
| Placebo (n = 95) | |||||
| Gross et al., 1996 42 | CD | Budesonide 9 mg day–1 (n = 34) | 8 | 31.0 | 58.2 |
| M‐Prednisolone 48 mg day–1 tapered gradually to 8 mg day–1 (n = 33) | |||||
| Hanauer et al., 2005 43 | CD | Budesonide 6 mg day–1 (n = 55) | 52 | 40.4 | 62.7 |
| Placebo (n = 55) | |||||
| Hellers et al., 1999 44 | CD | Budesonide 6 mg day–1 (n = 63) | 52 | 35.0 | 51.9 |
| Placebo (n = 67) | |||||
| Löfberg, Danielsson et al., 1996 45 | UC | Budesonide 10 mg day–1 tapered gradually to 4 mg day–1 (n = 34) | 9 | 33.5 | 41.7 |
| Prednisolone 40 mg day–1 tapered gradually to 5 mg day–1 (n = 38) | |||||
| Löfberg, Rutgeerts et al., 1996 46 | CD | Budesonide 6 mg day–1, or 3 mg day–1 (n = 63) | 52 | 35.0 | 60.0 |
| Placebo (n = 27) | |||||
| Malchow et al., 1984 (ECCDS) 47 | CD |
M‐Prednisolone 48 mg day–1 tapered gradually to 12 mg day–1 (active disease), or 8 mg day–1 (quiescent disease) (n = 113) |
104 | 29.9 | 54.7 |
| Placebo (n = 110) | |||||
| Malchow et al., 1984 (ECCDS) 47 | CD |
M‐Prednisolone 48 mg day–1 tapered gradually to 12 mg day–1 (active disease), or 8 mg day–1 (quiescent disease) + sulfasalazine 3 g day–1 (n = 112) |
104 | 30.9 | 54.6 |
| Placebo + sulfasalazine 3 g day–1 (n = 117) | |||||
| Prantera et al., 2011 48 | CD |
Beclomethasone dipropionate 15 mg day–1 for 2 weeks, and then 10 mg day–1 for 22 weeks (n = 37) |
24 | 42.4 | 53.4 |
| Placebo (n = 36) | |||||
| Rizzello et al., 2002 49 | UC | Beclomethasone dipropionate 5 mg day–1 +5‐ASA 3.2 g day–1 (n = 58) | 4 | 43.9 | 29.4 |
| Placebo +5‐ASA 3.2 g day–1 (n = 61) | |||||
| Rutgeerts et al., 1994 50 | CD | Budesonide 9 mg day–1 for 8 weeks, and then 6 mg day–1 for 2 weeks (n = 88) | 10 | 35.5 | 61.9 |
|
Prednisolone 40 mg day–1 for 2 weeks, then gradually reduced to 5 mg day–1 (n = 88) | |||||
| Sandborn et al., 2012 (CORE I) 51 | UC | Budesonide MMX 9 mg day–1, or 6 mg day–1 (n = 255) | 8 | 42.2 | 44.1 |
| Placebo (n = 128) | |||||
| Schoon et al., 2005 52 | CD | Budesonide 9 mg day–1; dose could be adjusted by clinicians (n = 138) | 104 | 36.9 | 51.3 |
| Prednisolone 40 mg day–1; dose could be adjusted by clinicians (n = 134) | |||||
|
Singleton et al., 1979
(NCCDS: part I, phase 1) 53 |
CD |
Prednisone 0.25–0.75 mg kg–1 daily; maximum daily dose was 60 mg (n = 85) |
17 | 32.7 | 50.6 |
| Placebo (n = 77) | |||||
|
Singleton et al., 1979
(NCCDS: part II) 53 |
CD |
Prednisone 0.25 mg kg–1 daily; maximum daily dose was 20 mg (n = 61) |
104 | 32.0 | 47.5 |
| Placebo (n = 101) | |||||
| Suzuki et al., 2013 54 | CD | Budesonide 15 mg day–1, or 9 mg day–1 (n = 51) | 8 | 36.5 | 28.6 |
| Placebo (n = 26) | |||||
| Travis et al., 2014 (CORE II) 55 | UC | Budesonide MMX 9 mg day–1, or 6 mg day–1 (n = 254) | 8 | 38.0 | 45.2 |
| Budesonide 9 mg day–1 (n = 126) | |||||
| Placebo (n = 128) | |||||
| Tremaine et al., 2002 56 | CD | Budesonide 9 mg day–1 (n = 159) | 8 | 39.4 | 64.0 |
| Placebo (n = 41) | |||||
| Tursi et al., 2006 57 | CD | Budesonide 9 mg day–1 (n = 15) | 8 | 33.4 | 56.7 |
| Beclomethasone dipropionate 10 mg day–1 (n = 15) | |||||
| Van Assche et al., 2015 58 | UC |
Beclomethasone dipropionate 5 mg day–1 for 4 weeks, followed by 5 mg every other day for the further 4 weeks (n = 137) |
8 | NR | 40.4 |
|
Prednisone 40 mg day–1 for 2 weeks, tapered of 10 mg every 2 weeks during the 8‐week study period (n = 145) | |||||
| Rubin et al., 2017 59 | UC | Budesonide MMX 9 mg day–1 as add‐on to existing 5‐ASA therapy (n = 255) | 8 | 44.5 | 46.0 |
| Placebo as add‐on to existing 5‐ASA therapy (n = 255) | |||||
| BUC‐16/CDA 60 | CD | Budesonide 18 mg day–1, 9 mg day–1, or 3 mg day–1, followed by a dose reduction period of 2 weeks (n = 307) | 10 | NR | NR |
| Placebo (n = 102) | |||||
| CB‐01‐02/05 & CRO‐03‐53‐period 1 61 | UC | Budesonide MMX 9 mg day–1, or 3 mg day–1 (n = 50) | 4–8 | NR | NR |
| Placebo (n = 35) | |||||
| CB‐01‐02/04 61 | UC | Budesonide MMX 6 mg day–1 (n = 62) | 52 | NR | NR |
| Placebo (n = 60) |
CD, Crohn's disease; UC, ulcerative colitis
A total of 5689 adults were randomized (CD, n = 3608; UC, n = 2081). Mean age of participants ranged between 30 and 44 years, females between 40% and 55%, and follow‐up times from 1 to 24 months. The total period of observation was over 40 000 person‐months (7 months per patient, on average) with a high number of AEs; 378 patients discontinued treatment or withdrew from study due to AEs, 210 patients had one or more SAEs, and 1061 patients had one or more corticosteroid‐related AEs. Publication dates ranged from 1979 to 2017. A summary of trials' characteristics is given in Table 1.
The RoB assessment indicated low RoB in two studies (6%), which had short duration and reported high rates of complete follow‐up without other threats to validity. 21 trials (68%) were rated as high‐risk, while RoB was unclear for the remaining eight (26%). For details see Appendix (Risk of bias assessment: Results). The quality assessment items (per trial) are shown in Figure 2.
Figure 2.
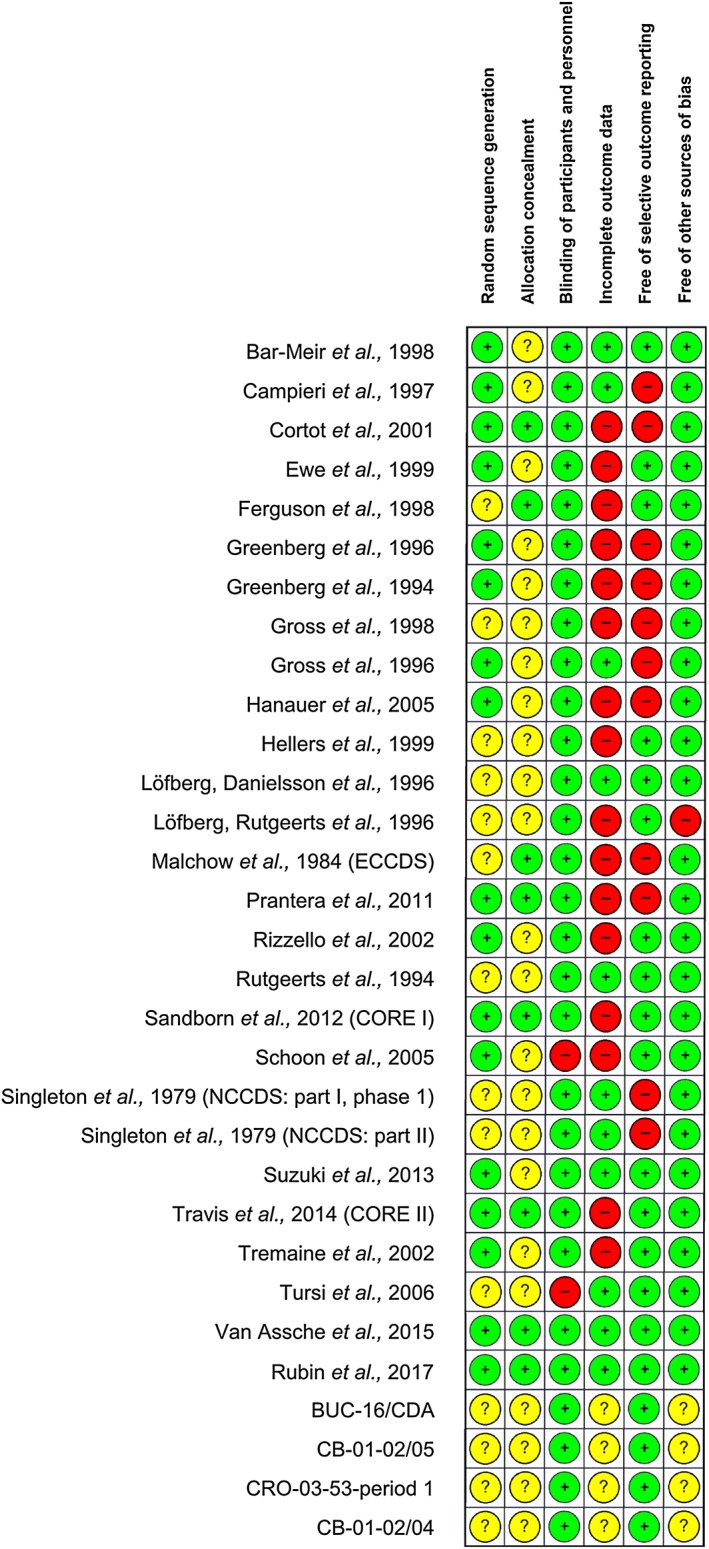
Risk of bias assessment for the studies included in the network. Green (+), low risk of bias; yellow (?), unclear risk of bias; red (–), high risk of bias
Results of network meta‐analyses
Treatment discontinuations or withdrawals from the studies due to AEs
Twenty‐seven RCTs 34, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 54, 55, 56, 58, 59, 60, 61 comparing budesonide to placebo (n = 13), budesonide MMX to placebo (n = 5), prednisone/prednisolone to placebo (n = 2), beclomethasone dipropionate to placebo (n = 2), budesonide to budesonide MMX (n = 1), prednisone/prednisolone to budesonide (n = 5), and prednisone/prednisolone to beclomethasone dipropionate (n = 1) were analysed (Table 1, and Figure 3A).
Figure 3.
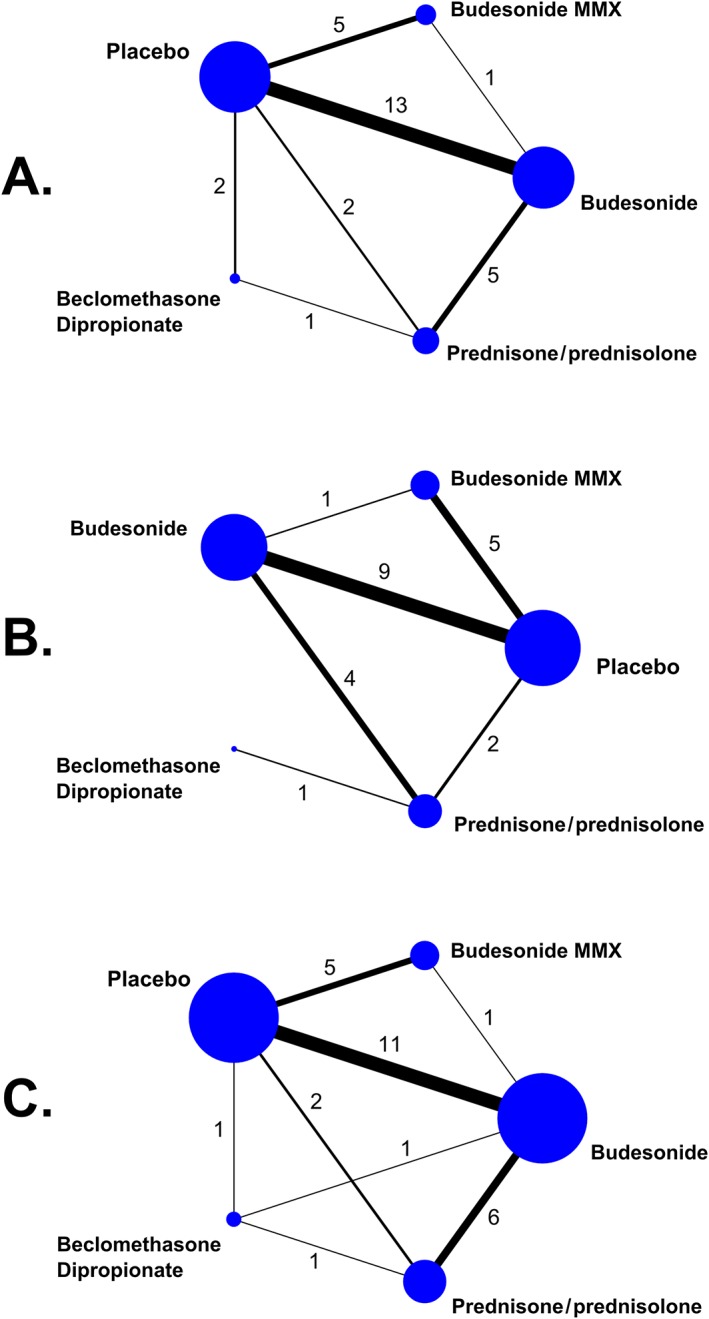
Network geometry. The nodes represent the individual drugs; lines represent direct comparisons using clinical trials; thickness of lines represents the number of available clinical trials, also represented by the numbers. (A) Treatment discontinuation or withdrawal from the study due to adverse events. (B) Serious adverse events. (C) Corticosteroid‐related adverse events
A total of 5158 patients had been randomized to placebo (n = 1572), budesonide (n = 1714), budesonide MMX (n = 876), prednisone/prednisolone (n = 764) and beclomethasone dipropionate (n = 232). Of them, 378 patients (7.3%) discontinued treatment or withdrew from the studies due to AEs.
In the network meta‐analysis, none of the comparisons among the competing treatments was statistically significant (Table 2A). By contrast, the SUCRA values, the mean ranks and the estimated probabilities of each treatment being the best demonstrated a trend favouring beclomethasone dipropionate (Table 3A).
Table 2.
Assessment of comparative safety of systemic and low‐bioavailability corticosteroids in inflammatory bowel diseases
| A. Treatment discontinuations or withdrawals from the study due to adverse events | ||||
|---|---|---|---|---|
| PBO | ||||
| 1.07 (0.73–1.55) | B‐MMX | |||
| 0.93 (0.64–1.35) | 0.87 (0.54–1.41) | BUD | ||
| 1.88 (0.67–5.26) | 1.76 (0.59–5.24) | 2.03 (0.70–5.90) | BDP | |
| 1.03 (0.53–1.98) | 0.96 (0.46–2.02) | 1.11 (0.57–2.15) | 0.55 (0.19–1.56) | PRED |
| B. Serious adverse events | ||||
|---|---|---|---|---|
| PBO | ||||
| 0.73 (0.36–1.50) | B‐MMX | |||
| 0.94 (0.56–1.59) | 1.29 (0.54–3.06) | BUD | ||
| 0.30 (0.01–8.29) | 0.42 (0.01–12.1) | 0.32 (0.01–8.46) | BDP | |
| 1.01 (0.53–1.94) | 1.38 (0.53–3.57) | 1.07 (0.69–1.65) | 3.32 (0.13–84.9) | PRED |
| C. Corticosteroid‐related adverse events | ||||
|---|---|---|---|---|
| PBO | ||||
| 1.02 (0.64–1.64) | B‐MMX | |||
| 0.65 (0.47–0.92) | 0.64 (0.37–1.11) | BUD | ||
| 0.36 (0.14–0.93) | 0.35 (0.13–1.00) | 0.55 (0.23–1.35) | BDP | |
| 0.26 (0.16–0.42) | 0.25 (0.13–0.49) | 0.39 (0.27–0.57) | 0.71 (0.31–1.66) | PRED |
The column‐defining treatment is compared with the row‐defining treatment. The effect estimates in the cells are odds ratios (with 95% confidence intervals) from network meta‐analysis. Because the outcomes are negative, ORs lower than 1.0 favour the treatment in the left upper square. Statistically significant results are shown in bold.
BDP, beclomethasone dipropionate; BUD, budesonide; B‐MMX, budesonide MMX; IBD, inflammatory bowel disease; PBO, placebo; PRED, prednisone/prednisolone.
Table 3.
Assessment of comparative safety of systemic and low‐bioavailability corticosteroids in inflammatory bowel diseases
| SUCRA value (%) | Probability best (%) | Mean rank | |
|---|---|---|---|
| A. Treatment discontinuations or withdrawals from the study due to adverse events | |||
| PBO | 40.2 | 2.3 | 3.4 |
| B‐MMX | 51.4 | 9.8 | 2.9 |
| BUD | 27.6 | 2.3 | 3.9 |
| BDP | 87.4 | 77.7 | 1.5 |
| PRED | 43.5 | 7.9 | 3.3 |
| B. Serious adverse events | |||
| PBO | 66.1 | 27.7 | 2.4 |
| B‐MMX | 35.7 | 10.1 | 3.6 |
| BUD | 56.7 | 12.0 | 2.7 |
| BDP | 25.5 | 20.6 | 4.0 |
| PRED | 66.0 | 29.6 | 2.4 |
| C. Corticosteroid‐related adverse events | |||
| PBO | 85.8 | 44.4 | 1.6 |
| B‐MMX | 86.6 | 54.2 | 1.5 |
| BUD | 48.9 | 0.2 | 3.0 |
| BDP | 23.1 | 1.3 | 4.1 |
| PRED | 5.6 | 0.0 | 4.8 |
Herein we present the SUCRA values providing the hierarchy of the competing treatments, the estimated probabilities of each treatment being the best, and the mean ranking of each treatment using 10 000 draws.
BDP, beclomethasone dipropionate; BUD, budesonide; B‐MMX, budesonide MMX; IBD, inflammatory bowel disease; PBO, placebo; PRED, prednisone/prednisolone.
SAEs
Twenty RCTs 34, 37, 38, 41, 44, 45, 46, 50, 51, 52, 53, 54, 55, 56, 58, 59, 60, 61 comparing budesonide to placebo (n = 9), budesonide MMX to placebo (n = 5), prednisone/prednisolone to placebo (n = 2), budesonide to budesonide MMX (n = 1), prednisone/prednisolone to budesonide (n = 4), and prednisone/prednisolone to beclomethasone dipropionate (n = 1), were incorporated in the analysis (Table 1, and Figure 3B).
In total, 4178 patients had been randomized to placebo (n = 1209), budesonide (n = 1304), budesonide MMX (n = 876), prednisone/prednisolone (n = 652) and beclomethasone dipropionate (n = 137). Overall, 210 patients (5.0%) suffered SAEs.
In network meta‐analysis, none of the comparisons among the treatments was statistically significant (Table 2B). Similarly, the corresponding SUCRA values, the estimated probabilities of each treatment being the best, as well as the comparative treatment ranks, were inconclusive (Table 3B).
Corticosteroid‐related AEs
Twenty‐six RCTs 34, 35, 37, 38, 39, 40, 42, 43, 44, 45, 46, 48, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61 comparing budesonide to placebo (n = 11), budesonide MMX to placebo (n = 5), prednisone/prednisolone to placebo (n = 2), beclomethasone to placebo (n = 1), budesonide to budesonide MMX (n = 1), budesonide to prednisone/prednisolone (n = 6), beclomethasone to prednisone/prednisolone (n = 1), and budesonide to beclomethasone (n = 1) were analysed (Table 1, and Figure 3C).
A total of 4819 patients had been randomized to placebo (n = 1307), budesonide (n = 1704), budesonide MMX (n = 876), prednisone/prednisolone (n = 743) and beclomethasone dipropionate (n = 189). Of them, 1061 patients (22.0%) suffered corticosteroid‐related AEs.
In the network meta‐analysis, budesonide MMX was associated with statistically significantly fewer corticosteroid‐related AEs than prednisone/prednisolone (OR: 0.25, 95% CI: 0.13–0.49) and beclomethasone dipropionate (OR: 0.35, 95% CI: 0.13–1.00), but not significantly fewer corticosteroid‐related AEs than budesonide (OR: 0.64, 95% CI: 0.37–1.11); it performed equally good with placebo (Table 2C). All other treatments were significantly less safe than placebo (Table 2C), and budesonide was better than prednisone/prednisolone (OR: 0.39, 95% CI: 0.27–0.57).
In agreement, the SUCRA values providing the hierarchy of the treatments, the estimated probabilities of each treatment being the best, and the ranking of the treatments with regards to the occurrence of corticosteroid‐related AEs, demonstrated budesonide MMX ranking at the top among its comparator treatments (Table 3C).
Assessment of publication bias, homogeneity and consistency of the models
The inspection of funnel plots appropriately adjusted for inclusion of studies comparing different treatments against placebo (Figure S1) suggested a low probability of publication bias or selective outcome reporting, for any of the models.
The conventional statistics (Cochran Q, I2, τ2) calculated for all direct comparisons, and the estimated and plotted predictive intervals reflecting the extent of heterogeneity in network meta‐analytic estimates, indicated low to moderate heterogeneity for all outcomes (data not shown). This was confirmed by the overall network heterogeneity variance (τ2) estimates (Table 4).
Table 4.
Networks' assessment for homogeneity and consistency
| Outcome | Heterogeneity variance | Global Wald test for inconsistency |
|---|---|---|
| Drug discontinuations or withdrawals due to AEs | τ2 = 0.21 | P‐value = 0.14 |
| Serious AEs | τ2 < 0.01 | P‐value = 0.17 |
| Corticosteroid‐related AEs | τ2 = 0.30 | P‐value = 0.80 |
AEs, adverse events.
Finally, there was no evidence of substantial inconsistency when explored either by node splitting, or by calculating the difference between direct and indirect evidence in all closed loops in the networks (data not shown). The global Wald tests for inconsistency were not significant (Table 4).
Nevertheless, given the moderate number of studies included in the analyses, relevant inconsistency or heterogeneity between trials cannot be ruled out.
Additional analyses
To assess the comparative safety profiles distinguishing the short‐term from the long‐term side effects, we also performed subgroup analyses using a cut‐off of 12 weeks for separating studies of short duration (up to 12 weeks) 34, 35, 40, 42, 45, 49, 50, 51, 54, 55, 56, 57, 58, 59, 60, 61 from those of long duration (over 12 weeks) 36, 37, 38, 39, 41, 43, 44, 46, 47, 48, 52, 53, 61 (Table 1).
Again, the occurrence of SAEs, and the occurrence of treatment discontinuations due to AEs, did not differ between the comparator treatments, both in the short and long term. Regarding the corticosteroid‐related AEs, the SUCRA values providing the hierarchy of treatments, the estimated probabilities of each treatment being the best, and the ranking of the treatments, demonstrated budesonide MMX ranking at the top among comparator treatments, both in the short and long term. Moreover, the direction of the pairwise effect estimates was in agreement with the overall analysis; however, the statistical power was rather low, and few comparisons reached statistical significance.
The results of subgroup analyses are detailed in the Appendix (short‐term group: Tables S1, S2, S3; long‐term group: Tables S4, S5, S6).
Discussion
In this systematic review and network meta‐analysis, we included safety data from 31 trials comparing oral systemic steroid drugs (prednisone, prednisolone) or steroids with low systemic bioavailability (budesonide, budesonide MMX, beclomethasone) with placebo, or against each other. We found that budesonide MMX is associated with significantly fewer corticosteroid‐related AEs than oral systemic steroids and beclomethasone, but not significantly fewer events than budesonide; it performs equally good with placebo. By contrast, the occurrence of serious AEs, and treatment discontinuations due to AEs, were not shown to be different between the comparator steroid treatments.
Previous works 62, 63, 64, 65, 66, 67, 68, 69, 70 have examined the corticosteroid treatments' safety profiles in a conventional, pairwise manner. Our network meta‐analysis includes not only the results of direct comparisons but also incorporates indirect comparisons, such as for budesonide MMX vs. oral systemic steroids or budesonide MMX vs. beclomethasone dipropionate, which have never been compared head‐to‐head. Thus, our study uses a much broader base of research data and provides new comparative evidence that can be appropriately integrated into relevant clinical practice guidelines.
Our work has merits: a rigorous and extensive search was conducted to retrieve all eligible studies, data were extracted by two independent reviewers with any disagreements checked and resolved, studies were analysed on an intention‐to‐treat basis, and appropriate frequentist methodology was employed to synthesize the evidence, also accounting for sparse data and imbalanced study groups. Finally, there was no evidence of substantial inconsistency, heterogeneity between studies was low to moderate and the probability of selective outcome reporting or publication bias was low.
Nevertheless, the strengths of this systematic review should be weighed against some limitations. First, the majority of the trials included in our meta‐analysis were judged to be at high or unclear RoB, as assessed with the Cochrane's Collaboration tool; this is a fact of concern because the quality of the current analysis may be limited by the quality of primary data. Second, many studies were registration trials for regulatory purposes; as such, they have enrolled selective IBD populations (e.g. elderly and high‐risk patients are under‐represented). This is a limitation that might compromise external validity. Moreover, we did not evaluate the comparator treatments in terms of efficacy and cost, which are key considerations in the clinical decision‐making process. Finally, the additional limitations of network meta‐analysis should be acknowledged 71. In a network meta‐analysis of RCTs, the value of randomization does not hold across studies. Hence, a network meta‐analysis of RCTs is a form of observational evidence: results and conclusions may be undermined if extensive clinical and/or methodological heterogeneity is present. For example, differences among the trials regarding treatment history, ascertainment bias arising from frequency of follow‐up visits, or the geographic regions where the studies were conducted (reflecting differences between patient populations) might act as effect modifiers. For all these reasons, it remains important that further high‐quality research (head‐to‐head trials, real‐world studies, pharmacoeconomic evaluations) is conducted in IBD to confirm and extend the current evidence, and illuminate the steroid treatments' comparative profiles.
Conclusion
Our meta‐analysis synthesized data from a large number of RCTs and brings new evidence into the field with practical implications. Budesonide MMX has an advantage over oral systemic steroids and beclomethasone dipropionate for corticosteroid‐related AEs (non‐serious and not leading to drug withdrawal), and possibly a slight unconfirmed advantage over standard budesonide for these AEs.
This knowledge together with other important considerations, such as treatments' comparative efficacy and cost, will assist patients and physicians to make evidence‐based decisions that align with their values, preferences, and tolerance of risks and benefits.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 72, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 73.
Competing Interests
Dr Gionata Fiorino has served as a consultant and advisory board member for MSD, AbbVie, Takeda, Pfizer, Mundipharma, Nikkiso, Otsuka. Prof Laurent Peyrin‐Biroulet has received consulting fees from Abbvie, Amgen, Biogaran, Biogen, Boerhinger‐Ingelheim, Bristol–Myers Squibb, Celgene, Celltrion, Ferring, Forward Pharma, Genentech, H.A.C. Pharma, Hospira, Index Pharmaceuticals, Janssen, Lycera, Merck, Lilly, Mitsubishi, Norgine, Pfizer, Pharmacosmos, Pilège, Samsung Bioepis, Sandoz, Takeda, Therakos, Tillots, UCB Pharma and Vifor, and lecture fees from Abbvie, Ferring, H.A.C. Pharma, Janssen, Merck, Mitsubishi, Norgine, Takeda, Therakos, Tillots and Vifor. Prof Silvio Danese has served as a speaker, a consultant and an advisory board member for Abbvie, Allergan, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Hospira, Johnson & Johnson, Merck, MSD, Takeda, Mundipharma, Pfizer, Sandoz, Tigenix, UCB Pharma and Vifor. All other authors have no conflicts of interest to declare.
Contributors
Guarantor of the article: Dr Stefanos Bonovas.
Conception and design: All authors; Literature search and data collection: S.B., T.L. Statistical analysis: S.B., G.N; Data interpretation: All authors; Drafting of the manuscript: S.B., G.N; Critical revision of the manuscript for important intellectual content: All authors; Final approval of the version to be published: All authors; Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: All authors.
Supporting information
Figure S1 Funnel plots adjusted for inclusion of trials comparing different treatments against placebo
Table S1 Safety assessment of systemic and low‐bioavailability steroids in inflammatory bowel disease: short‐term group
Table S2 Safety assessment of systemic and low‐bioavailability steroids in inflammatory bowel disease: short‐term group
Table S3 Networks' assessment for homogeneity and consistency: short‐term group
Table S4 Safety assessment of systemic and low‐bioavailability steroids in inflammatory bowel disease: long‐term group
Table S5 Safety assessment of systemic and low‐bioavailability steroids in inflammatory bowel disease: long‐term group
Table S6 Networks' assessment for homogeneity and consistency: long‐term group
Bonovas, S. , Nikolopoulos, G. K. , Lytras, T. , Fiorino, G. , Peyrin‐Biroulet, L. , and Danese, S. (2018) Comparative safety of systemic and low‐bioavailability steroids in inflammatory bowel disease: Systematic review and network meta‐analysis. Br J Clin Pharmacol, 84: 239–251. doi: 10.1111/bcp.13456.
References
- 1. Jewell DP. Crohn's disease In: Oxford Textbook of Medicine, 4th edn, eds Warrell DA, Cox TM, Firth JD, Benz EJ. Oxford: Oxford University Press, 2005. [Google Scholar]
- 2. Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011; 365: 1713–1725. [DOI] [PubMed] [Google Scholar]
- 3. Nunes T, Barreiro‐de Acosta M, Marin‐Jiménez I, Nos P, Sans M. Oral locally active steroids in inflammatory bowel disease. J Crohns Colitis 2013; 7: 183–191. [DOI] [PubMed] [Google Scholar]
- 4. De Cassan C, Fiorino G, Danese S. Second‐generation corticosteroids for the treatment of Crohn's disease and ulcerative colitis: more effective and less side effects? Dig Dis 2012; 30: 368–375. [DOI] [PubMed] [Google Scholar]
- 5. Rutgeerts PJ. Review article: the limitations of corticosteroid therapy in Crohn's disease. Aliment Pharmacol Ther 2001; 15: 1515–1525. [DOI] [PubMed] [Google Scholar]
- 6. Fasci Spurio F, Aratari A, Margagnoni G, Clemente V, Moretti A, Papi C. Low bioavailability and traditional systemic steroids in IBD: can the former take over the latter? J Gastrointestin Liver Dis 2013; 22: 65–71. [PubMed] [Google Scholar]
- 7. Danese S, Siegel CA, Peyrin‐Biroulet L. Review article: integrating budesonide‐MMX into treatment algorithms for mild‐to‐moderate ulcerative colitis. Aliment Pharmacol Ther 2014; 39: 1095–1103. [DOI] [PubMed] [Google Scholar]
- 8. Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta‐analysis. BMJ 2013; 346: f2914. [DOI] [PubMed] [Google Scholar]
- 9. Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005; 331: 897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004; 23: 3105–3124. [DOI] [PubMed] [Google Scholar]
- 11. Bonovas S, Nikolopoulos G, Lytras T, Fiorino G, Peyrin‐Biroulet L, Danese S. Comparative safety of systemic and low‐bioavailability corticosteroids in inflammatory bowel disease: a systematic review and network meta‐analysis of randomized controlled trials. PROSPERO 2016: CRD42016038694. Available from http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016038694 [DOI] [PMC free article] [PubMed]
- 12. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration; 2011, version 5.0.1.
- 13. Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, et al Interpreting indirect treatment comparisons and network meta‐analysis for health‐care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health 2011; 14: 417–428. [DOI] [PubMed] [Google Scholar]
- 14. Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC, et al Conducting indirect‐treatment‐comparison and network‐meta‐analysis studies: report of the ISPOR task force on indirect treatment comparisons good research practices: part 2. Value Health 2011; 14: 429–437. [DOI] [PubMed] [Google Scholar]
- 15. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–784. [DOI] [PubMed] [Google Scholar]
- 16. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000; 356: 1255–1259. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bonovas S, Lytras T, Nikolopoulos G. On the criteria used for assessing the risk of bias in randomized trials included in systematic reviews and meta‐analyses addressing adverse effects. Eur J Epidemiol 2015; 30: 249–250. [DOI] [PubMed] [Google Scholar]
- 19. White IR. Network meta‐analysis. Stata J 2015; 15: 951–985. [Google Scholar]
- 20. Chaimani A, Salanti G. Visualizing assumptions and results in network meta‐analysis: the network graphs package. Stata J 2015; 15: 905–950. [Google Scholar]
- 21. Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS One 2013; 8: e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. White IR. Multivariate random‐effects meta‐regression: updates to mvmeta. Stata J 2011; 11: 255–270. [Google Scholar]
- 23. White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Res Synth Methods 2012; 3: 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Stat Med 2004; 23: 1351–1375. [DOI] [PubMed] [Google Scholar]
- 25. White IR. Software updates: st0156 2: multivariate random‐effects meta‐analysis. Stata J 2015; 15: 1185–1186. [Google Scholar]
- 26. White IR. Multivariate random‐effects meta‐analysis. Stata J 2009; 9: 40–56. [Google Scholar]
- 27. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JPT. Evaluating the quality of evidence from a network meta‐analysis. PLoS One 2014; 9: e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. J Clin Epidemiol 2011; 64: 163–171. [DOI] [PubMed] [Google Scholar]
- 29. Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane database of systematic reviews. Int J Epidemiol 2012; 41: 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Res Synth Methods 2012; 3: 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Stat Med 2010; 29: 932–944. [DOI] [PubMed] [Google Scholar]
- 32. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. J Clin Epidemiol 1997; 50: 683–691. [DOI] [PubMed] [Google Scholar]
- 33. Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Res Synth Methods 2012; 3: 161–176. [DOI] [PubMed] [Google Scholar]
- 34. Bar‐Meir S, Chowers Y, Lavy A, Abramovitch D, Sternberg A, Leichtmann G, et al Budesonide versus prednisone in the treatment of active Crohn's disease. The Israeli Budesonide Study Group. Gastroenterology 1998; 115: 835–840. [DOI] [PubMed] [Google Scholar]
- 35. Campieri M, Ferguson A, Doe W, Persson T, Nilsson LG. Oral budesonide is as effective as oral prednisolone in active Crohn's disease. The Global Budesonide Study Group. Gut 1997; 41: 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cortot A, Colombel JF, Rutgeerts P, Lauritsen K, Malchow H, Hämling J, et al Switch from systemic steroids to budesonide in steroid dependent patients with inactive Crohn's disease. Gut 2001; 48: 186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ewe K, Böttger T, Buhr HJ, Ecker KW, Otto HF. Low‐dose budesonide treatment for prevention of postoperative recurrence of Crohn's disease: a multicentre randomized placebo‐controlled trial. German budesonide study group. Eur J Gastroenterol Hepatol 1999; 11: 277–282. [DOI] [PubMed] [Google Scholar]
- 38. Ferguson A, Campieri M, Doe W, Persson T, Nygård G. Oral budesonide as maintenance therapy in Crohn's disease–results of a 12‐month study. Global budesonide study group. Aliment Pharmacol Ther 1998; 12: 175–183. [DOI] [PubMed] [Google Scholar]
- 39. Greenberg GR, Feagan BG, Martin F, Sutherland LR, Thomson AB, Williams CN, et al Oral budesonide as maintenance treatment for Crohn's disease: a placebo‐controlled, dose‐ranging study. Canadian inflammatory bowel disease study group. Gastroenterology 1996; 110: 45–51. [DOI] [PubMed] [Google Scholar]
- 40. Greenberg GR, Feagan BG, Martin F, Sutherland LR, Thomson AB, Williams CN, et al Oral budesonide for active Crohn's disease. Canadian inflammatory bowel disease study group. N Engl J Med 1994; 331: 836–841. [DOI] [PubMed] [Google Scholar]
- 41. Gross V, Andus T, Ecker KW, Raedler A, Loeschke K, Plauth M, et al Low dose oral pH modified release budesonide for maintenance of steroid induced remission in Crohn's disease. The Budesonide Study Group. Gut 1998; 42: 493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gross V, Andus T, Caesar I, Bischoff SC, Lochs H, Tromm A, et al Oral pH‐modified release budesonide versus 6‐methylprednisolone in active Crohn's disease. German/Austrian budesonide study group. Eur J Gastroenterol Hepatol 1996; 8: 905–909. [PubMed] [Google Scholar]
- 43. Hanauer S, Sandborn WJ, Persson A, Persson T. Budesonide as maintenance treatment in Crohn's disease: a placebo‐controlled trial. Aliment Pharmacol Ther 2005; 21: 363–371. [DOI] [PubMed] [Google Scholar]
- 44. Hellers G, Cortot A, Jewell D, Leijonmarck CE, Löfberg R, Malchow H, et al Oral budesonide for prevention of postsurgical recurrence in Crohn's disease. The IOIBD Budesonide Study Group. Gastroenterology 1999; 116: 294–300. [DOI] [PubMed] [Google Scholar]
- 45. Löfberg R, Danielsson A, Suhr O, Nilsson A, Schiöler R, Nyberg A, et al Oral budesonide versus prednisolone in patients with active extensive and left‐sided ulcerative colitis. Gastroenterology 1996; 110: 1713–1718. [DOI] [PubMed] [Google Scholar]
- 46. Löfberg R, Rutgeerts P, Malchow H, Lamers C, Danielsson A, Olaison G, et al Budesonide prolongs time to relapse in ileal and ileocaecal Crohn's disease. A placebo‐controlled one year study. Gut 1996; 39: 82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Malchow H, Ewe K, Brandes JW, Goebell H, Ehms H, Sommer H, et al European cooperative Crohn's disease study (ECCDS): results of drug treatment. Gastroenterology 1984; 86: 249–266. [PubMed] [Google Scholar]
- 48. Prantera C, Rizzi M, Cottone M, Casa A, Annese V, Sturniolo GC, et al Beclomethasone dipropionate in Crohn's ileitis: a randomised, double‐blind trial. Dig Liver Dis 2011; 43: 459–464. [DOI] [PubMed] [Google Scholar]
- 49. Rizzello F, Gionchetti P, D'Arienzo A, Manguso F, Di Matteo G, Annese V, et al Oral beclometasone dipropionate in the treatment of active ulcerative colitis: a double‐blind placebo‐controlled study. Aliment Pharmacol Ther 2002; 16: 1109–1116. [DOI] [PubMed] [Google Scholar]
- 50. Rutgeerts P, Löfberg R, Malchow H, Lamers C, Olaison G, Jewell D, et al A comparison of budesonide with prednisolone for active Crohn's disease. N Engl J Med 1994; 331: 842–845. [DOI] [PubMed] [Google Scholar]
- 51. Sandborn WJ, Travis S, Moro L, Jones R, Gautille T, Bagin R, et al Once‐daily budesonide MMX® extended‐release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology 2012; 143: 1218–1226, e1‐2. [DOI] [PubMed] [Google Scholar]
- 52. Schoon EJ, Bollani S, Mills PR, Israeli E, Felsenberg D, Ljunghall S, et al Bone mineral density in relation to efficacy and side effects of budesonide and prednisolone in Crohn's disease. Clin Gastroenterol Hepatol 2005; 3: 113–121. [DOI] [PubMed] [Google Scholar]
- 53. Singleton JW, Law DH, Kelley ML Jr, Mekhjian HS, Sturdevant RA. National Cooperative Crohn's disease study: adverse reactions to study drugs. Gastroenterology 1979; 77 (4 Pt 2): 870–882. [PubMed] [Google Scholar]
- 54. Suzuki Y, Motoya S, Takazoe M, Kosaka T, Date M, Nii M, et al Efficacy and tolerability of oral budesonide in Japanese patients with active Crohn's disease: a multicentre, double‐blind, randomized, parallel‐group phase II study. J Crohns Colitis 2013; 7: 239–247. [DOI] [PubMed] [Google Scholar]
- 55. Travis SP, Danese S, Kupcinskas L, Alexeeva O, D'Haens G, Gibson PR, et al Once‐daily budesonide MMX in active, mild‐to‐moderate ulcerative colitis: results from the randomised CORE II study. Gut 2014; 63: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tremaine WJ, Hanauer SB, Katz S, Winston BD, Levine JG, Persson T, et al Budesonide CIR capsules (once or twice daily divided‐dose) in active Crohn's disease: a randomized placebo‐controlled study in the United States. Am J Gastroenterol 2002; 97: 1748–1754. [DOI] [PubMed] [Google Scholar]
- 57. Tursi A, Giorgetti GM, Brandimarte G, Elisei W, Aiello F. Beclomethasone dipropionate for the treatment of mild‐to‐moderate Crohn's disease: an open‐label, budesonide‐controlled, randomized study. Med Sci Monit 2006; 12: PI29–PI32. [PubMed] [Google Scholar]
- 58. Van Assche G, Manguso F, Zibellini M, Cabriada Nuño JL, Goldis A, Tkachenko E, et al Oral prolonged release beclomethasone dipropionate and prednisone in the treatment of active ulcerative colitis: results from a double‐blind, randomized, parallel group study. Am J Gastroenterol 2015; 110: 708–715. [DOI] [PubMed] [Google Scholar]
- 59. Rubin DT, Cohen RD, Sandborn WJ, Lichtenstein GR, Axler J, Riddell RH, et al Budesonide multi‐matrix is efficacious for mesalamine‐refractory, mild to moderate ulcerative colitis: a randomized, placebo‐controlled trial. J Crohns Colitis 2017; 11: 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Australian Government, Department of Health and Aging, Therapeutic Goods Administration . Australian public assessment report for budesonide; October 2012. Available from https://www.tga.gov.au/sites/default/files/auspar‐budesonide‐121022‐1.pdf
- 61. U.S. Food and Drug Administration, Center for Drug Evaluation and Research . Drug approval package for Uceris (budesonide) 9 mg tablets, Medical Review; January 2013. Available from http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203634Orig1s000MedR.pdf
- 62. Sherlock ME, MacDonald JK, Griffiths AM, Steinhart AH, Seow CH. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 2015; (10): CD007698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lichtenstein GR, Travis S, Danese S, D'Haens G, Moro L, Jones R, et al Budesonide MMX for the induction of remission of mild to moderate ulcerative colitis: a pooled safety analysis. J Crohns Colitis 2015; 9: 738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rezaie A, Kuenzig ME, Benchimol EI, Griffiths AM, Otley AR, Steinhart AH, et al Budesonide for induction of remission in Crohn's disease. Cochrane Database Syst Rev 2015; 6: CD000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kuenzig ME, Rezaie A, Seow CH, Otley AR, Steinhart AH, Griffiths AM, et al Budesonide for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev 2014; (8): CD002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ford AC, Bernstein CN, Khan KJ, Abreu MT, Marshall JK, Talley NJ, et al Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta‐analysis. Am J Gastroenterol 2011; 106: 590–599. [DOI] [PubMed] [Google Scholar]
- 67. Doherty G, Bennett G, Patil S, Cheifetz A, Moss AC. Interventions for prevention of post‐operative recurrence of Crohn's disease. Cochrane Database Syst Rev 2009; (4): CD006873. [DOI] [PubMed] [Google Scholar]
- 68. Lichtenstein GR, Bengtsson B, Hapten‐White L, Rutgeerts P. Oral budesonide for maintenance of remission of Crohn's disease: a pooled safety analysis. Aliment Pharmacol Ther 2009; 29: 643–653. [DOI] [PubMed] [Google Scholar]
- 69. Steinhart AH, Ewe K, Griffiths AM, Modigliani R, Thomsen OO. Corticosteroids for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev 2003; (4): CD000301. [DOI] [PubMed] [Google Scholar]
- 70. Papi C, Luchetti R, Gili L, Montanti S, Koch M, Capurso L. Budesonide in the treatment of Crohn's disease: a meta‐analysis. Aliment Pharmacol Ther 2000; 14: 1419–1428. [DOI] [PubMed] [Google Scholar]
- 71. Bonovas S, Moja L, Danese S. In the presence of conceptual heterogeneity, results of network meta‐analysis comparing therapies in Crohn's disease need to be interpreted with caution. Gastroenterology 2015; 148: 1483–1484. [DOI] [PubMed] [Google Scholar]
- 72. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE, et al The concise guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 2015; 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Funnel plots adjusted for inclusion of trials comparing different treatments against placebo
Table S1 Safety assessment of systemic and low‐bioavailability steroids in inflammatory bowel disease: short‐term group
Table S2 Safety assessment of systemic and low‐bioavailability steroids in inflammatory bowel disease: short‐term group
Table S3 Networks' assessment for homogeneity and consistency: short‐term group
Table S4 Safety assessment of systemic and low‐bioavailability steroids in inflammatory bowel disease: long‐term group
Table S5 Safety assessment of systemic and low‐bioavailability steroids in inflammatory bowel disease: long‐term group
Table S6 Networks' assessment for homogeneity and consistency: long‐term group


