Abstract
Pregnant and breastfeeding women have been rendered therapeutic orphans as they have been historically excluded from clinical trials. Labelling for most approved drugs does not provide information about safety and efficacy during pregnancy. This lack of data is mainly due to ethico‐legal challenges that have remained entrenched in the post‐diethylstilbestrol and thalidomide era, and that have led to pregnancy being viewed in the clinical trial setting primarily through a pharmacovigilance lens. Policy considerations that encourage and/or require the inclusion of pregnant or lactating women in clinical trials may address the current lack of available information. However, there are additional pragmatic strategies, such the employment of pharmacometric tools and the introduction of innovative clinical trial designs, which could improve knowledge about the safety and efficacy of medication use during pregnancy and lactation. This paper provides a broad overview of the pharmacoepidemiology of drugs used during pregnancy and lactation, and offers recommendations for regulators and researchers in academia and industry to increase the available pharmacokinetic and ‐dynamic understanding of medication use in pregnancy.
Keywords: breastfeeding, clinical pharmacology, drug utilization, obstetric, pregnancy
Antecedents and pharmacoepidemiology
Prior to the 1940s, little information was available about the use of drugs in pregnant women. Discovery of birth defects resulting from fetal exposure to certain drugs between the 1940s and beginning of the 1970s led to new awareness of the teratogenic potential of prescription drugs. For example, diethylstilbestrol (DES), taken by pregnant women during the 1940s, 1950s and 1960s to protect against miscarriages, was identified as the cause of male and female reproductive tract anomalies, and a rare vaginal clear‐cell carcinoma in women exposed to it in utero 1. Likewise, in the 1960s, thalidomide prescribed in pregnant women around the world as a treatment for nausea and vomiting during the first weeks of pregnancy led to multiple birth defects – mainly phocomelia 2. Curiously, thalidomide's human embryopathy was not evident through the required animal testing, and was reported only in a chicken model.
Major lessons were learnt from the thalidomide tragedy. As a result, the US Food and Drug Administration (FDA) released new regulations regarding approval of drugs for use in pregnant and lactating women. In addition to reproductive and fertility data, and animal studies in two different species 3, the FDA approved in 1977 the Guideline ‘General Considerations for the Clinical Evaluation of Drugs’ 4, which excluded women of childbearing age from participating in phase I and early phase II clinical trials. Additionally, in 1979, the FDA introduced a pregnancy category labelling system 5 which was expected to provide guidance to prescribers on the availability of nonclinical and clinical information to inform about the safe and efficacious use in pregnant women. This system classified the drugs into five categories (A, B, C, D and X), based on known or suspected reproductive toxicity and the quantity and quality of the information available to address that risk.
Policy and legislative changes
The exclusion of premenopausal women from any clinical research since 1977 inadvertently led to biased knowledge towards male participants, limiting data in women. At present, the variability in drug response and disease progression between men and women has been documented 6, 7, necessitating clinical studies/trials that will explore the efficacy and safety of drugs during pregnancy and lactation. This study of drugs in pregnant and breastfeeding women should not only be limited to the possible fetal/neonatal risk, but also the understanding of the differences in the pharmacokinetics (PK) of the drugs during those two periods.
The need to address the lack of available clinical data led the National Institutes of Health (NIH), in 1993, to recommend the inclusion of pregnant women in clinical trials 8 (Figure 1). In the same year, the FDA also acknowledged the need for clinical data regarding the use of drugs in women, and published the ‘Guideline for the Study and Evaluation of Gender Differences in the Clinical Evaluation of Drugs’ 4. Soon thereafter (1994), the Office of Women's Health (OWH) was created within the FDA to facilitate these efforts further. One of the main missions of this office was to promote research initiatives to understand gender differences and health conditions exclusive to women 9.
Figure 1.
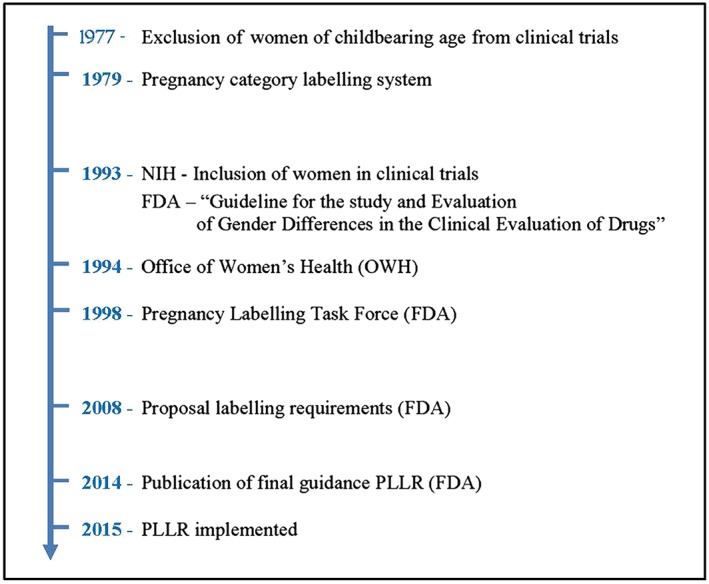
Evolution of regulations related to pregnancy drug development over the time. FDA, US Food and Drug administration; NIH, National Institutes for Health; PLLR, pregnancy and lactation labelling rule
Finally, in 1997, the FDA began to reassess and improve the current pregnancy category labelling system 10. That effort resulted in the establishment, in 1998, of a Pregnancy Labelling Taskforce. In 2008, the FDA issued a proposal to revise the requirements of the labelling of drugs and biological products for pregnancy and lactation 11. The final guideline, called the ‘Pregnancy and Lactation Labelling Rule’, or PLLR, was published in December 2014 and became effective on 30 June 2015 12. This rule replaces the old letter‐based categories with a more descriptive report about the risks of using a drug during pregnancy and lactation, and requires provision of human and animal studies data along with clinical considerations that should help healthcare providers better to understand the available information. The guideline also includes two new subsections; one provides information about the use of drugs during breastfeeding, and with the other emphasizes the need for pregnancy testing, and contraception, and alerts about possible infertility related to the drug. The new PLLR also requires that sponsors of drugs include a list of current pregnancy exposure registries for the drug.
Present situation and trends over time
Currently, most medications do not include labelling information about their use during pregnancy and lactation. Although the absolute number of human PK trials has increased over time (particularly from the mid‐1980s to mid‐1990s), the proportion of those involving pregnant women has remained relatively constant and only represents 1.29% of the total registered trials from the 1960s to 2013. A closer look at these data reveal that among all PK trials in pregnant women published since 2008, only 68% involved medications given chronically, and of these, a large proportion (29%) were for human immunodeficiency virus (HIV) treatment and prevention of vertical transmission 13. In addition, a review of recently approved medications by the FDA between 2000 and 2010 showed that 97.7% of them had insufficient information to determine teratogenic risk 14, and for 73.3% of them there were no available data supporting their use in pregnant women 15.
In cases where there is information available, data are scarce for most approved drugs 16. A recent work by Anderson et al. 17 identified 53 case reports and 16 studies on adverse drug reactions in breastfed infants from 2002 to 2004. Serious adverse drug effects appear to be rare and mostly affecting breastfed infants under 2 months of age (79%). Central nervous system drugs, including opioids, antidepressants, anticonvulsants, antipsychotic agents, lithium and sedatives, were associated with 70% of the adverse reactions.
Nonetheless, although the use of drugs during pregnancy and lactation should be limited, sometimes it is compulsory, in the setting of acute or chronic disease, both for pregnancy‐related (e.g. gestational diabetes and pre‐eclampsia) and nonpregnancy‐related conditions (e.g. epilepsy, depression, asthma, hypertension, cancer or HIV). It is estimated that pre‐existing medical conditions complicate 25% of pregnancies, as a result of advanced maternal age at the time of conception (due to effective birth control, advances in assisted reproductive technologies, women's pursuit of higher education and career advancement) and medical advancements prolonging lives in medical conditions which in the past would have been fatal (e.g. cystic fibrosis, childhood cancers and transplantation) 18. As a result, the overall number and type of medications used (both prescription and over‐the‐counter) during pregnancy have increased over the past three decades 19. In the United States, from 1976 to 2008, the overall use of medications during pregnancy increased by 68%, while the use of medicines during the first trimester rose from 1.6 to 2.6 (60%) 19. More recently, between 2006 and 2008, over 80% of pregnant women reported taking at least one medication during the first trimester, and 90% at any time during pregnancy 19.
Additionally, the benefits of breastfeeding have been widely accepted, and the American Academy of Pediatrics (AAP) recommends its exclusive use in the first 6 months, followed by its continuation during the first year of life 20. In 2012, the US Centres for Disease Control and Prevention reported that more than 74.6% of women breastfeed in early postpartum, and that the rate drops to 47.2% at 6 months, and to 25.5% at 12 months 21. The use of medications during this period is also significant. Surveys in different countries show that 90–99% of women take at least one drug in the postpartum period 22, and up to 5% of them take medications to treat chronic conditions (e.g. epilepsy, thyroid disorders, diabetes, hypertension and psychiatric conditions) 23, 24. Since initiating and maintaining breastfeeding is crucial in the immediate postpartum setting, it is important to ensure that sufficient information exists about the pharmacokinetics and safety profiles of medications taken by the mother.
In summary, while it is clear that many women will need and use medications during pregnancy and breastfeeding, there is both a quantitative and qualitative scarcity of available clinical pharmacology data to inform on the appropriate use of these drugs in pregnant and lactating women. Recognizing pregnant and lactating women as special populations is essential to obtain information about the safety and efficacy of medications, which is of paramount importance.
Why consider pregnant and breastfeeding women as special populations?
Physiological considerations
Pregnancy and the puerperium are associated with significant physiological changes that can alter the PK of drugs 25, and potentially change both their efficacy and safety. The absorption, distribution and clearance (metabolism and excretion) of various medications are altered during pregnancy, affecting their PK. These changes occur throughout pregnancy, and revert to the nonpregnant state several weeks after delivery. Table 1 summarizes the main physiological changes of pregnancy and their effects on drugs’ absorption, distribution, metabolism and elimination (ADME) processes.
Table 1.
Physiological and pharmacokinetic changes to drugs during pregnancy
| Pharmacokinetic process | Physiological changes during pregnancy | Pharmacokinetic modifications |
|---|---|---|
| Absorption | ↓ Gastric acidity | Altered absorption |
| ↓ Gastric emptying | Delayed absorption | |
| ↓ Gastrointestinal motility | ↓ C max | |
| ↑ Blood flow in skin, mucous membranes and muscles | ↑ Bioavailability IM and external administration | |
| Distribution | ↑ Total body water | ↑ Vd of hydrophilic and lipophilic drugs |
| ↓ Protein concentration | ↑ Free fraction of drugs | |
| Metabolism | ↑ or ↓ enzymatic activity | Altered hepatic clearance |
| Cholestasis | ↓ Biliary elimination | |
| Renal excretion | ↑ Renal blood flow and GFR ↓ Tubular reabsorption | ↑ Renal clearance |
C max, maximum concentration; GFR, glomerular filtration rate; IM, intramuscular; Vd, volume of distribution
The greatest variability in drug absorption is seen when drugs are administered orally. Several physiological factors occurring during pregnancy can affect the rate and extent of absorption. The absorption of medications is affected by a decrease in gastric acidity, delayed gastric emptying and a prolonged small‐bowel transit time 26, 27. Nausea and vomiting, frequent in early pregnancy, can also decrease the amount of drug available for absorption following oral administration; in these cases, patients should be advised to take medications when nausea is minimal. Similarly, the volume of distribution (Vd) of a drug depends on several factors, such as body composition, tissue binding and plasma protein binding 27. During pregnancy, there is an increase in total body water, and extracellular volume increases by around 25%, which results in an increase in the Vd of most medications, especially hydrophilic ones. Additionally, the decrease in serum albumin concentrations observed in pregnant women causes drugs that are highly bound to protein to show an increase in the active fraction of the drug (free fraction) 28.
Changes in the elimination of drugs are also likely to occur. Hepatic drug‐metabolizing enzymes can also be influenced by hormonal changes occurring during pregnancy, with an enhancement in the activity of some [cytochrome P450 (CYP) 34A, CYP2C9 and CYP2D6] 29 and a decrease in others (CYP1A2) 30. These genetic factors can have a significant influence on the PK of drugs, leading to substantial variability in their metabolism. The use of pharmacogenomics to guide drug treatment decisions has the potential to improve the maternal therapeutic response and decrease adverse effects, including potential risks to the newborn. However, there are few pharmacogenomic studies in pregnancy and during breastfeeding, indicating the need for further research. In addition, the increase in renal clearance occurring in pregnancy can significantly increase the elimination rate of drugs removed by the kidneys, leading to shorter half‐lives (e.g. digoxin, aminoglycosides and antiepileptic drugs), thus requiring higher doses or shorter intervals of administration 27.
Additionally, other physiological changes occur during labour which can modify the PK behaviour of several drugs 31. Some of the drugs most used during labour include oxytocin for labour augmentation, antibiotics for the prevention of neonatal infections, and pain relief medications (e.g. narcotics). Despite the regular use of these drugs during labour, the influence of labour on the PK of these drugs has been scarcely studied 32, 33. PK studies during labour deal not only with ethical issues, but also with practical difficulties.
Fetal and neonatal exposure
The use of drugs during pregnancy and lactation is also associated with a potential fetal/neonatal risk.
When a drug is administered to a pregnant woman, there should also be a concern about the potential toxicity to the fetus. The placenta is the interface between mother and fetus. Placental drug transfer is dependent on the physical properties of the placenta and on the pharmacological properties of the drug (i.e. in general, lipophilic drugs cross the placenta rapidly). Additionally, the human placenta also disposes of metabolic enzymes and active membrane transporters. The expression of membrane transporters varies with advancing gestation and in pathological pregnancies (e.g. preterm and pre‐eclampsia) 34. Finally, although the fetal liver can metabolize drugs to some extent, it is not expected to account for the total clearance of a drug. Therefore, drug exposure should be limited to necessary treatment, as in some cases it can result in fetal adverse events, such as those associated with antiepileptic drugs 35 and antibiotics 36. However, limited data about fetal drug exposure are available, and they are limited to case reports and pharmacoepidemiology studies 37.
Many drugs taken while breastfeeding are secreted into breast milk. The ability of a drug to be secreted into breast milk is dependent on its physicochemical characteristics, such as molecular size, lipid solubility, plasma protein‐binding affinity and its degree of ionization, and does not necessarily correlate with toxicity 38.
Challenges of clinical trials in pregnancy and lactation
Pregnant women and children are considered as ‘therapeutic orphans’, with similar challenges with respect to drug development. Over the last 20 years, however, active advocacy to address paediatric needs has led to the introduction of regulatory policy in both the United States and Europe which is now changing the mindset of drug developers, regulators and academicians that children are better protected through research, not from research 39. Nonetheless, these barriers are still present for pregnant and lactating women.
Pregnant women are routinely excluded from phase I and II clinical drug development trials. There is a hesitation from clinicians and researchers on enrolling pregnant women in studies due to ethical concerns and possible harm to the developing fetus. Possible litigation discourages pharmaceutical industries to involve them; their inclusion requires long‐term follow‐up to assess fetal and newborn outcomes; and the revenue benefit is relatively small in relation to the investment. Additionally, the presence of operational and recruitment‐related challenges, as well a lack of codified regulations to drive the development of drugs for pregnant and lactating women, are some of the reasons that could explain the low enrolment rate of pregnant women in clinical trials. These complexities have reinforced the absence of clinical studies to evaluate the safe and efficacious use of drugs during lactation and pregnancy.
Physiologic changes occurring throughout pregnancy and during the postpartum period contribute to the difficulty in design and performance of studies in pregnant women. Therefore, pregnancy should not be considered as a dichotomous covariate. This is of particular importance for clinical trials designed to assess the efficacy of drugs used to treat chronic conditions. In this scenario, clinical trials should be designed to include prepregnancy (for baseline comparison), all three trimesters, and the postpartum period. This type of design can allow a patient to serve as their own control, to account for likely interindividual variability 40. Nonetheless, these designs may be complicated and lead to limited enrolment of subjects or a restricted number of samples or sampling occasions. The employment of pharmacometric tools could help to solve this problem.
Steps that can be taken to expand obstetric clinical pharmacology knowledge
Pharmacometrics in pregnancy and lactation
Because of ethical and practical restrictions, conventional PK studies are difficult to perform in pregnancy and during lactation. Pharmacometric approaches, such as population PK (PPK) and simulation, or physiologically based PK (PBPK), can be useful for study design and PK analysis, and can be used to quantify the effects of pregnancy and relate measured exposure to clinical outcomes.
PPK models may use heterogeneous sparse sampling data, especially suitable for these populations where often only sparse data are available. They allow simultaneous estimation of PPK parameters and quantification of the percentage of inter‐ and intraindividual variability, as well accounting for the influence of potential covariates that influence PK parameters. If samples are collected from cord blood and/or amniotic fluid, drug transfer to the fetus can be characterized. Simulation approaches can provide probability measures of drug exposure levels of the drug, and thus can be valuable to establish potential dose adjustments and inform on more practical clinical study designs.
There are already several examples of PPK studies in pregnancy in the literature 41, 42, 43, although these data are limited in breastfeeding 44. PPK modelling and simulation were used to evaluate and optimize amoxicillin prophylaxis dose recommendations in pregnancy 41, as well to assess the PK of glyburide (an oral hypoglycaemic agent), and to establish doses in pregnant women that would match glyburide exposure to that in nonpregnant women 42. The application of PBPK in pregnancy integrates maternal physiology and metabolizing enzyme changes to predict the disposition of drugs. These mechanistic methods are able to predict the PK of drugs based on their properties and some physiological information. Given the complexity of PBPK models, they require rich experimental data, but with the possibility to use in silico prediction tools alternatively to obtain the required physiochemical parameters.
Although the use of PBPK modelling has been mainly limited to assess toxicokinetic and risk effects in mother and fetus 45, 46, 47 for several years, its applications in PK are increasing 48, 49, 50, 51, 52, 53. PBPK modelling supports that PK is altered as a result of pregnancy, and has been demonstrated to be a useful tool for evaluating different dosing regimens.
Prospective pregnancy registers
There is evidence for a lack of availability of preapproval data for medications used in pregnancy and lactation, once prescription drugs are introduced into the market. As a result, postmarketing monitoring has become critical for the detection and evaluation of any kind of adverse events. The FDA Adverse Events Reporting System (FAERS) is a database, available to any consumer or healthcare provider for reporting adverse events or medication errors. Its mission is to support the FDA postmarketing safety programme for drugs. However, this passive mechanism for providing information on adverse drug effects has some important limitations: consumers and healthcare providers may not be aware of the existence of such registers; reporting is voluntary, so the prevalence of certain adverse effects is not well understood; and data quality can be incomplete and inconsistent.
To reduce these limitations, postmarketing pregnancy registries are used increasingly. They allow for the monitoring of drug‐related adverse reactions. Unlike other postmarketing strategies, such as spontaneous reporting, pregnancy registries are prospective studies in which pregnant women are enrolled and followed throughout their pregnancy. In 2002, with the aim of standardizing the characteristics of these registers, the FDA published a guidance that promoted the establishment of written study protocols a priori 54. Some examples of these registers are the North American Antiepileptic Drug (NAAED) Pregnancy Registry (http://www.aedpregnancyregistry.org/), the Transplant Pregnancy Registry International (TPRI) (http://ntpr.giftoflifeinstitute.org/) and the National Pregnancy Registry for Antidepressants (NPRAD) (https://womensmentalhealth.org/clinical‐and‐research‐programs/pregnancyregistry/antidepressants/). To advocate their availability and to enhance public knowledge, the FDA maintains an updated list of pregnancy registers on its website (http://www.fda.gov/scienceresearch/specialtopics/womenshealthresearch/ucm251314.htm). As of June 2017, there were 108 pregnancy registers on the list, including more than 30 different medication groups, including those used to treat asthma, autoimmune disease, cancer, epilepsy, HIV infection and transplant rejection.
Although pregnancy registries could avoid the ethical and logistical pitfalls of randomized controlled trials, they still have drawbacks. A lack of awareness can lead to deficiencies in participation, thus leading to a false prevalence of outcomes. Pregnancy registers can reflect a bias in the patients included, with a preference for those with greater disease severity, and as reporter burden is high, this can lead to incomplete data capture on exposure and outcomes. Further, registers have a high rate of patient loss to follow‐up, and there are often no controlled internal comparator groups, thus leading to an inconsistency in comparison with external data sources. For a registry to be successful over its lifecycle, it requires continuous commitment to enrolment, recruitment and retention, in addition to prespecification of a similar comparator group, to understand the implications of its findings.
Collaborative research networks
The establishment of large collaborative research networks of clinical trial sites can help to reduce the low consent rates for the participation of pregnant women in clinical studies, but may also provide essential infrastructure for research, and may facilitate a multidisciplinary approach to improving the understanding of obstetric PK and pharmacodynamics.
For that purpose, the National Institute of Child Health and Human Development (NICHD) sponsored two clinical research networks: the Maternal–Fetal Medicine Units (MFMU) Network and the Obstetric Pharmacology Research Units (OPRU) Network. The MFMU Network was established in 1986 to investigate issues in clinical obstetrics, particularly with respect to preterm birth, but also to medical problems during pregnancy. A workshop on the use of drugs in pregnancy 55, held by the NICHD in September 2000, framed the needs for the OPRU Network. In 2003, a Network consisting of four academic sites established in the USA, changing to specialized centres in 2015. The mission of these networks is to improve the safety and effective use of drugs in women during pregnancy and lactation. This Network promotes and facilitates multidisciplinary research. This structure has already generated important findings, providing safety and PK data regarding the use of several drugs during pregnancy, such as oral hypoglycaemic agents for the treatment of gestational diabetes 56, pravastatin for the prevention of pre‐eclampsia, indomethacin for preterm labour 57, 58, immunosuppressive therapies 59 and chemotherapeutic agents 60.
Big data in obstetric research
Given the difficulty of including pregnant women in clinical trials, the use of big data may provide some insights into obstetric clinical research 61. Big data in clinical research usually refers to the information collected through electronic medical systems. These data come from daily routine clinical practice, without any modification, thus being a reflection of current clinical practice 62. Several studies have shown a good correlation between observational studies and clinical trials 63, 64. However, the use of big data implies the need to perform internal or external validation to confirm their reproducibility and generalization.
Dissemination of knowledge
The study by Stockmann et al. 65 examined the characteristics of obstetric studies registered within http://clinicaltrials.gov from 2007 to 2012, demonstrating that these studies represented less than 10% of the total number registered. The authors also found that more than 80% of obstetric studies registered from 2007 to 2012 incorporated elements of high‐quality trial design (e.g. randomization and safety/efficacy endpoints).
However, an important aspect observed by Stockmann et al. was the low publication rate of completed obstetric studies registered in http://clinicaltrials.gov. Specifically, for studies completed more than 2 years ago, only 11% reported results and less than 7% had been published. In a domain in which there is a need for available information about safe and effective therapies, this low report and publication rate is of concern. Dissemination of all available obstetric clinical pharmacology knowledge is fundamental to creating an area of expertise that is able to increase the available clinical data supporting the use of drugs in pregnant and lactating women.
This latter fact reinforces the need to establish multidisciplinary research teams that lead the expertise in obstetric clinical pharmacology. The establishment of collaborative research teams that work together to elucidate all the current inconsistencies in obstetric drug development would set up the basis for an obstetric clinical pharmacology knowledge database.
Conclusions and future directions
The use of medications during pregnancy and lactation is common, and is expected to rise over time. However, for most approved drugs there are no data on their efficacy and safety for use in pregnant and breastfeeding women. This raises concerns both for pregnant women and the healthcare providers who care for them, leading to significant uncertainty for prescribers. As a result, the same dosages labelled for use in nonpregnant women are often used for pregnant women, with little consideration for the PK changes that occur during pregnancy.
Pregnant women have been excluded systematically from clinical trials over the last few decades. The lack of regulations to drive or incentive research in pregnant and lactating women, and the challenges associated with this subpopulation, have all contributed to an absence of generation of clinical data in this population. Having recognized the need to include pregnant women in clinical trials, several other initiatives have already been established to address this problem (e.g. a new system of labelling of drugs and biological products, the establishment of collaborative research networks and postmarketing registries). Nonetheless, more is needed to drive the development of safe and efficacious treatment.
The development of obstetric research needs rigorous legislative actions that provide the regulatory framework for the study of medication use in pregnancy and lactation. Obstetric research calls for a structure that provides what the Best Pharmaceuticals for Children Act (BPCA) and Pediatric Research Equity Act (PREA) did for paediatric clinical research 39. Specifically, legislative actions should allow the implementation of several incentives for drug development in pregnant and breastfeeding women, as well as the motivation for major networks to include a specific percentage of both subpopulations in all their studies.
The inclusion of pregnant women in clinical trials is challenging to both the design and operational aspects of a clinical study. The design should consider pregnancy not as a dichotomous but a continuous variable, capturing all the physiological changes occurring during pregnancy. However, researchers have an opportunity to improve the incorporation of pharmacometric approaches, as they are a tool which could be of great utility for the pragmatic design and analysis of data in pregnant and lactating women.
Although some recent initiatives have already been introduced, it is too soon to understand how they will affect clinical practice. Indeed, it will take some years to accumulate a meaningful quantity of information. In the meantime, it is essential to establish well‐trained researchers and clinicians specialized in obstetric clinical pharmacology, and to incorporate more pragmatic research practice to generate meaningful information for use in drug development programmes, in order to address the needs of pregnant and lactating women. The combination of these initiatives will allow the expansion of obstetric clinical research and the transfer of knowledge on the safe and effective use of medicines in pregnancy and lactation.
Competing Interests
C.B.‐R. is a full‐time employee of, and stockholder in, Novartis Pharmaceuticals Corporation. K.T. is program officer for the Obstetric‐Fetal Pharmacology Research Center (OPRC), for which M.M.C. is an investigator at UTMB. The authors have no other competing interests or conflicts of interest to declare.
Illamola, S. M. , Bucci‐Rechtweg, C. , Costantine, M. M. , Tsilou, E. , Sherwin, C. M. , and Zajicek, A. (2018) Inclusion of pregnant and breastfeeding women in research – efforts and initiatives. Br J Clin Pharmacol, 84: 215–222. doi: 10.1111/bcp.13438.
References
- 1. Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, et al Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med 2011; 365: 1304–1314. [DOI] [PubMed] [Google Scholar]
- 2. Vargesson N. Thalidomide‐induced teratogenesis: history and mechanisms. Birth Defects Res C Embryo Today 2015; 105: 140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doering PL, Boothby LA, Cheok M. Review of pregnancy labeling of prescription drugs: is the current system adequate to inform of risks? Am J Obstet Gynecol 2002; 187: 333–339. [DOI] [PubMed] [Google Scholar]
- 4. US Food and Drug Administration . Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs; notice. Fed Regist 1993; 58: 39406–39416. [PubMed] [Google Scholar]
- 5. US Food and Drug Administration . Labeling and prescription drug advertising: content and format for labeling for human prescription drugs. Fed Regist 1979; 44: 37434–37467. [Google Scholar]
- 6. El‐Eraky H, Thomas SH. Effects of sex on the pharmacokinetic and pharmacodynamic properties of quinidine. Br J Clin Pharmacol 2003; 56: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenblatt DJ, Harmatz JS, von Moltke LL, Wright CE, Durol ALB, Harrel‐Joseph LM, et al Comparative kinetics and response to the benzodiazepine agonists triazolam and zolpidem: evaluation of sex‐dependent differences. J Pharmacol Exp Ther 2000; 293: 435–443. [PubMed] [Google Scholar]
- 8. National Institutes of Health Revitalization Act of 1993. Science and Technology. Public Law 1993; 103–43.
- 9. US Food and Drug Administration . Women's health research [online]. Office of Women's Health (OWH), 2011. Available at http://www.fda.gov/ScienceResearch/SpecialTopics/WomensHealthResearch (Last accessed 1 November 2014).
- 10. US Food and Drug Administration . Content and format of labeling for human prescription drugs; pregnancy labeling; public hearing. Fed Regist 1997; 62: 41061–41063. [PubMed] [Google Scholar]
- 11. US Food and Drug Administration . Requirements on content and format of labeling for human prescription drug and biological products. Proposed rule. Fed Regist 2006; 71: 3921–3997. [PubMed] [Google Scholar]
- 12. US Food and Drug Administration . Content and format for labeling for human prescription drugs; amendment of effective date for certain biological products – Food and Drug Administration. Final rule. Fed Regist 1981; 46 (15 pt 1): 7271–7273. [PubMed] [Google Scholar]
- 13. McCormack SA, Best BM. Obstetric pharmacokinetic dosing studies are urgently needed. Front Pediatr 2014; 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adam MP, Polifka JE, Friedman JM. Evolving knowledge of the teratogenicity of medications in human pregnancy. Am J Med Genet C Semin Med Genet 2011; 157c: 175–182. [DOI] [PubMed] [Google Scholar]
- 15. Friedman JM, Polifka PJ. TERIS. Micromedex Reproductive Risk Information System (REPRORISK). Englewood, CO: Thomson MICROMEDEX, 2011. [Google Scholar]
- 16. Ito S. Drug therapy: drug therapy for breast‐feeding women. N Engl J Med 2000; 343: 118–126. [DOI] [PubMed] [Google Scholar]
- 17. Anderson PO, Manoguerra AS, Valdes V. A review of adverse reactions in infants from medications in breastmilk. Clin Pediatr 2016; 55: 236–244. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention . Use of medication in pregnancy [online]. Available at http://www.cdc.gov/pregnancy/meds/treatingfortwo/data.html (last accessed 18 August 2016).
- 19. Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernandez‐Diaz S, et al Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol 2011; 205: 51.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eidelman AI, Schanler RJ, Johnston M, Landers S, Noble L, Szucs K, et al Breastfeeding and the use of human milk. Pediatrics 2012; 129: E827–E841. [DOI] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention . Breastfeeding report card – United States, 2012 [online]. Available at http://www.cdc.gov/breastfeeding/pdf/2012BreastfeedingReportCard.pdf (last accessed August 2012).
- 22. Matheson I. Drugs taken by mothers in the puerperium. Br Med J 1985; 290: 1588–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McNamara PJ, Abbassi M. Neonatal exposure to drugs in breast milk. Pharm Res 2004; 21: 555–566. [DOI] [PubMed] [Google Scholar]
- 24. Saha MR, Ryan K, Amir LH. Postpartum women's use of medicines and breastfeeding practices: a systematic review. Int Breastfeed J 2015; 10: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ke AB, Rostami‐Hodjegan A, Zhao P, Unadkat JD. Pharmacometrics in pregnancy: an unmet need. Annu Rev Pharmacol 2014; 54: 53–69. [DOI] [PubMed] [Google Scholar]
- 26. Loebstein R, Lalkin A, Koren G. Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet 1997; 33: 328–343. [DOI] [PubMed] [Google Scholar]
- 27. Cunningham FG, Leveno KJ, Bloom SL, Spong CY, Dashe JS, Hoffman BL, et al Maternal Physiology, 24th edn) edn. NewYork, NY: McGraw‐Hill, 2013. [Google Scholar]
- 28. Notarianni LJ. Plasma protein binding of drugs in pregnancy and in neonates. Clin Pharmacokinet 1990; 18: 20–36. [DOI] [PubMed] [Google Scholar]
- 29. Ryu RJ, Eyal S, Easterling TR, Caritis SN, Venkataraman R, Hankins G, et al Pharmacokinetics of metoprolol during pregnancy and lactation. J Clin Pharmacol 2016; 56: 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu T, Campbell SC, Stockmann C, Tak C, Schoen K, Clark EAS, et al Pregnancy‐induced changes in the pharmacokinetics of caffeine and its metabolites. J Clin Pharmacol 2016; 56: 590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nation RL. Drug kinetics in childbirth. Clin Pharmacokinet 1980; 5: 340–364. [DOI] [PubMed] [Google Scholar]
- 32. Muller AE, Dorr PJ, Mouton JW, De Jongh J, Oostvogel PM, Steegers EA, et al The influence of labour on the pharmacokinetics of intravenously administered amoxicillin in pregnant women. Br J Clin Pharmacol 2008; 66: 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kokki M, Franco MG, Raatikainen K, Valitalo P, Sankilampi U, Heinonen S, et al Intravenous oxycodone for pain relief in the first stage of labour – maternal pharmacokinetics and neonatal exposure. Basic Clin Pharmacol Toxicol 2012; 111: 182–188. [DOI] [PubMed] [Google Scholar]
- 34. Iqbal M, Audette MC, Petropoulos S, Gibb W, Matthews SG. Placental drug transporters and their role in fetal protection. Placenta 2012; 33: 137–142. [DOI] [PubMed] [Google Scholar]
- 35. Wlodarczyk BJ, Palacios AM, George TM, Finnell RH. Antiepileptic drugs and pregnancy outcomes. Am J Med Genet A 2012; 158A: 2071–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muanda FT, Sheehy O, Berard A. Use of antibiotics during pregnancy and the risk of major congenital malformations: a population based cohort study. Br J Clin Pharmacol 2017; 83: 2557–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. US Food and Drug Administration . Reviewer guidance evaluating the risks of drug exposure in human pregnancies [online]. Available at https://www.fda.gov/downloads/Drugs/Guidances/ucm071645.pdf (last accessed April 2005).
- 38. Rowe H, Baker T, Hale TW. Maternal medication, drug use, and breastfeeding. Pediatr Clin North Am 2013; 60: 275. [DOI] [PubMed] [Google Scholar]
- 39. Gonzalez D, Boggess KA, Cohen‐Wolkowiez M. Lessons learned in pediatric clinical research to evaluate safe and effective use of drugs in pregnancy. Obstet Gynecol 2015; 125: 953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. US Food and Drug Administration . Guidance for industry: pharmacokinetics in pregnancy – study design, data analysis, and impact on dosing and labeling [online]. Available at http://www.fda.gov/downloads/Drugs/Guidances/ucm072133.pdf (last accessed October 2004).
- 41. Andrew MA, Easterling TR, Carr DB, Shen D, Buchanan ML, Rutherford T, et al Amoxicillin pharmacokinetics in pregnant women: modeling and simulations of dosage strategies. Clin Pharmacol Ther 2007; 81: 547–556. [DOI] [PubMed] [Google Scholar]
- 42. Hebert MF, Ma X, Naraharisetti SB, Krudys KM, Umans JG, Hankins GD, et al Obstetric-Fetal Pharmacology Research Unit Network. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009; 85: 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pillai VC, Han KL, Beigi RH, Hankins GD, Clark S, Hebert MF, et al Population pharmacokinetics of oseltamivir in non‐pregnant and pregnant women. Br J Clin Pharmacol 2015; 80: 1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Panchaud A, Garcia‐Bournissen F, Csajka C, Kristensen JH, Taddio A, Ilett KF, et al Prediction of infant drug exposure through breastfeeding: population PK modeling and simulation of fluoxetine exposure. Clin Pharmacol Ther 2011; 89: 830–836. [DOI] [PubMed] [Google Scholar]
- 45. Luecke RH, Wosilait WD, Pearce BA, Young JF. A computer model and program for xenobiotic disposition during pregnancy. Comput Methods Programs Biomed 1997; 53: 201–224. [DOI] [PubMed] [Google Scholar]
- 46. Clewell HJ, Gearhart JM, Gentry PR, Covington TR, VanLandingham CB, Crump KS, et al Evaluation of the uncertainty in an oral reference dose for methylmercury due to interindividual variability in pharmacokinetics. Risk Anal 1999; 19: 547–558. [DOI] [PubMed] [Google Scholar]
- 47. Corley RA, Mast TJ, Carney EW, Rogers JM, Daston GP. Evaluation of physiologically based models of pregnancy and lactation for their application in children's health risk assessments. Crit Rev Toxicol 2003; 33: 137–211. [DOI] [PubMed] [Google Scholar]
- 48. Andrew MA, Hebert MF, Vicini P. Physiologically based pharmacokinetic model of midazolam disposition during pregnancy. Conf Proc IEEE Eng Med Biol Soc 2008; 2008: 5454–5457. [DOI] [PubMed] [Google Scholar]
- 49. Ke AB, Nallani SC, Zhao P, Rostami‐Hodjegan A, Unadkat JD. Expansion of a PBPK model to predict disposition in pregnant women of drugs cleared via multiple CYP enzymes, including CYP2B6, CYP2C9 and CYP2C19. Br J Clin Pharmacol 2014; 77: 554–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xia BF, Heimbach T, Gollen R, Nanavati C, He HD. A simplified PBPK modeling approach for prediction of pharmacokinetics of four primarily renally excreted and CYP3A metabolized compounds during pregnancy. AAPS J 2013; 15: 1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lu GH, Abduljalil K, Jamei M, Johnson TN, Rostami‐Hodjegan A. A pregnancy physiologically based pharmacokinetic (p‐PBPK) model for disposition of drugs metabolized by CYP1A2, CYP2D6 and CYP3A4. Br J Clin Pharmacol 2012; 74: 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ke AB, Nallani SC, Zhao P, Rostami‐Hodjegan A, Isoherranen N, Unadkat JD. A physiologically based pharmacokinetic model to predict disposition of CYP2D6 and CYP1A2 metabolized drugs in pregnant women. Drug Metab Dispos 2013; 41: 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ke AB, Nallani SC, Zhao P, Rostami‐Hodjegan A, Unadkat JD. A PBPK model to predict disposition of CYP3A‐metabolized drugs in pregnant women: verification and discerning the site of CYP3A induction. CPT Pharmacometrics Syst Pharmacol 2012; 1: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. US Food and Drug Administration . Guidance for industry: establishing pregnancy exposure registries [online]. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071639.pdf (Last accessed 2 January 2003).
- 55. Giacoia G. Introduction. Semin Perinatol 2001; 25: 115–119. [Google Scholar]
- 56. Eyal S, Easterling TR, Carr D, Umans JG, Miodovnik M, Hankins GDV, et al Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos 2010; 38: 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Costantine MM, Cleary K, Hebert MF, Ahmed MS, Brown LM, Ren ZX, et al Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high‐risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 2016; 214: 720.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rytting E, Nanovskaya TN, Wang XM, Vernikovskaya DI, Clark SM, Cochran M, et al Pharmacokinetics of indomethacin in pregnancy. Clin Pharmacokinet 2014; 53: 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zheng SM, Easterling TR, Hays K, Umans JG, Miodovnik M, Clark S, et al Tacrolimus placental transfer at delivery and neonatal exposure through breast milk. Br J Clin Pharmacol 2013; 76: 988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hays KE, Ryu RJ, Swisher EM, Reed E, McManus T, Rybeck B, et al Duration of cisplatin excretion in breast milk. J Hum Lact 2013; 29: 469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schneeweiss S. Learning from big health care data. N Engl J Med 2014; 370: 2161–2163. [DOI] [PubMed] [Google Scholar]
- 62. Albert RK. ‘Lies, damned lies …’ and observational studies in comparative effectiveness research. Am J Respir Crit Care Med 2013; 187: 1173–1177. [DOI] [PubMed] [Google Scholar]
- 63. Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev 2014; MR000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ioannidis JP, Haidich AB, Pappa M, Pantazis N, Kokori SI, Tektonidou MG, et al Comparison of evidence of treatment effects in randomized and nonrandomized studies. JAMA 2001; 286: 821–830. [DOI] [PubMed] [Google Scholar]
- 65. Stockmann C, Sherwin CMT, Koren G, Campbell SC, Constance JE, Linakis M, et al Characteristics and publication patterns of obstetric studies registered in ClinicalTrials. gov. J Clin Pharmacol 2014; 54: 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]


