Abstract
In primary hyperparathyroidism (PHPT), bone loss results from the resorptive effects of excess parathyroid hormone (PTH). Under physiological conditions, PTH has actions that are more targeted to homeostasis and to bone accrual. The predominant action of PTH, either catabolic, anabolic or homeostatic, can be understood in molecular and pharmacokinetic terms. When administered intermittently, PTH increases bone mass, but when present continuously and in excess (e.g. PHPT), bone loss ensues. This dual effect of PTH depends not only on the dosing regimen, continuous or intermittent, but also on how the PTH molecule interacts with various states of its receptor (PTH/PTHrP receptor) influencing downstream signalling pathways differentially. Altering the amino‐terminal end of PTH or PTHrP could emphasize the state of the receptor that is linked to an osteoanabolic outcome. This concept led to the development of a PTHrP analogue that interacts preferentially with the transiently linked state of the receptor, emphasizing an osteoanabolic effect. However, designing PTH or PTHrP analogues with prolonged state of binding to the receptor would be expected to be linked to a homeostatic action associated with the tonic secretory state of the parathyroid glands that is advantageous in treating hypoparathyroidism. Ideally, further development of a drug delivery system that mimics the physiological tonic, circadian, and pulsatile profile of PTH would be optimal. This review discusses basic, translational and clinical studies that may well lead to newer approaches to the treatment of osteoporosis as well as to different PTH molecules that could become more advantageous in treating hypoparathyroidism.
Keywords: abaloparatide, clinical pharmacology, hypoparathyroidism, osteoporosis, parathyroid hormone, primary hyperparathyroidism, PTH, PTHrP
Introduction
Parathyroid hormone (PTH), a peptide hormone that is secreted from the parathyroid glands, regulates calcium homeostasis. The peptide is first formed as a precursor hormone with an additional primary presequence of 25 amino acids and a prosequence of six amino acids. These pre‐ and prosequences are proteolytically cleaved before PTH is secreted as a full‐length 84‐amino acid polypeptide. The PTH gene is located at the short arm of chromosome 11 and the genetic regulation of PTH production and secretion is mediated principally by the calcium sensing receptor, 1,25 dihydroxyvitamin D 1, 2. Physiologically, in response to low extracellular calcium, reduced binding of ionic calcium to the calcium sensing receptor stimulates PTH synthesis and release. There are three servomechanisms that restore the serum calcium: (i) stimulation of renal tubular calcium reabsorption; (ii) increased osteoclastic bone resorption; and (iii) increased renal production of 1,25‐dihydroxyvitamin D by stimulating the renal 1α hydroxylase, thus converting 25 hydroxyvitamin D to the active vitamin D metabolite. 1,25 Dihydroxyvitamin D facilitates gastrointestinal absorption of calcium. This latter effect is an indirect effect of PTH, while the first two actions are directly attributable to the actions of PTH on its target tissues. These three mechanisms help to restore blood calcium to normal levels. PTH also increases urinary phosphate excretion by decreasing phosphate reabsorption from the proximal renal tubule via decrease expression of the type 2a sodium‐phosphate cotransporter 3. The physiologically mirror image of these actions is seen with increased calcium, 1,25 dihydroxyvitamin D and, to a lesser extent, phosphate exerting a negative feedback to decrease PTH production and secretion.
Under physiological conditions, PTH influences bone remodelling by increasing osteoblast proliferation, decreasing osteoblast apoptosis and converting quiescent lining cells into osteoblasts. PTH also increases osteoclast activity by enhancing receptor activator of nuclear factor‐κB ligand (RANKL) production from osteoblasts. More recently, it has been suggested that osteocytes can directly influence osteoclastic activity via PTH induced RANKL expression 4. Depending upon the balance of PTH's actions to stimulate osteoblast, osteocyte or osteoclast activities, the net effect of PTH can be either an anabolic one or a catabolic one 5.
The tonic, continuous secretion of PTH is modulated by a circadian rhythm in which there is an early morning peak and a late morning trough followed by a smaller peak in the afternoon 6. Superimposed upon this tonic and circadian dynamic are small amplitude pulses that can occur as often as 10–20 min with a frequency of 6–7 pulses h–1 7. Pulsatile PTH secretion accounts for about 25% of the total amount of PTH secreted 8. Sharper, larger bursts of PTH secretion, thought to be stochastic, have also been described 9. Hypocalcaemia increases the frequency and amplitude of these pulses, whereas hypercalcaemia and 1,25 dihydroxyvitamin D reduces their frequency and amplitude 10.
Circulating PTH is rapidly metabolized by renal and hepatic tissue endopeptidases and excreted into the urine by glomerular filtration. Systemic levels are therefore determined, to a great extent, by the production rate of the hormone 11. The amino‐ and carboxy‐terminal fragments of PTH have different half‐lives. The estimated half‐life of amino‐terminal fragments is short (a few minutes) whereas the carboxy‐terminal fragments is long (hours) 12. PTH has been administered as continuous intravenous infusion which indeed demonstrated a very short half‐life. In humans, the estimated half‐life of PTH (1–34) is about 10 min. 13 PTH is available pharmaceutically as rhPTH (1–84) and as rhPTH (1–34). Both are administered as subcutaneous injections, and the half‐life of both forms are 2.5 and 1 h, respectively. These differences in elimination kinetics are probably due to different absorption kinetics from the subcutaneous reservoir rather than metabolism of these peptides 14, 15.
The first 34 amino‐terminal residues of PTH, PTH (1–34) demonstrates biological responses that are indistinguishable from the full‐length peptide 16. The amino‐terminal portion of PTH is required to bind and activate its receptor. PTH binds to the PTH/PTHrP receptor type 1, a family B G protein‐coupled receptor. This receptor is able to exist in at least 2 distinct conformation states (R0 and RG) that differ in their signalling response. Ligands that bind selectively to the RG state result in a shorter signalling response whereas ligands that bind selectively to the R0 state result in prolonged signalling response (Figure 1) 17, 18. This downstream cAMP signalling response is considered to be the primary signalling cascade that mediates the effect of PTH receptor. The receptor can modulate a ligand's biological activity depending of the preferential binding to either state 17. Since most PTHs have some affinity for both states of the PTH/PTHrP receptor, the behaviour of a given PTH vis à vis duration of effect is determined by the ratio of binding affinities to each of these receptor states (Figure 2).
Figure 1.
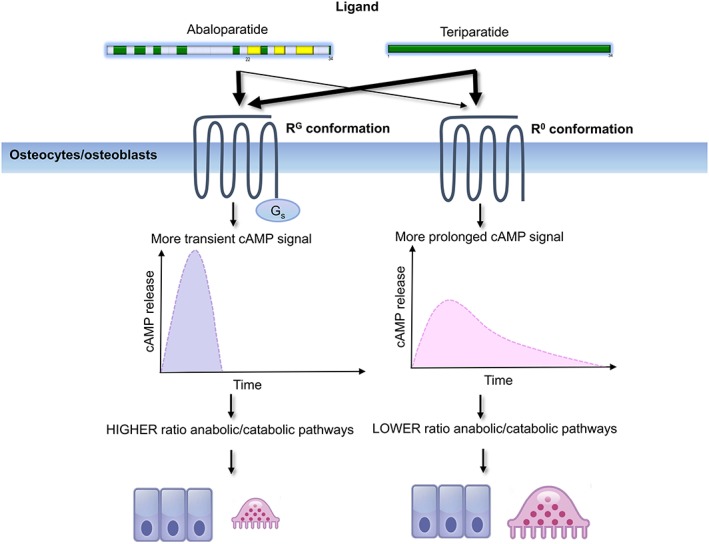
Binding selectivity for the different conformational states of the parathyroid hormone/PTHrP receptor leads to differential effects on downstream signalling. The preferential binding of abaloparatide for the RG state elicits a transient cyclic AMP signalling response favouring a more anabolic effect whereas teriparatide has a preferential binding for the R0 state and portends towards a more prolonged cyclic AMP signalling response and a more pronounced catabolic effect
Figure 2.
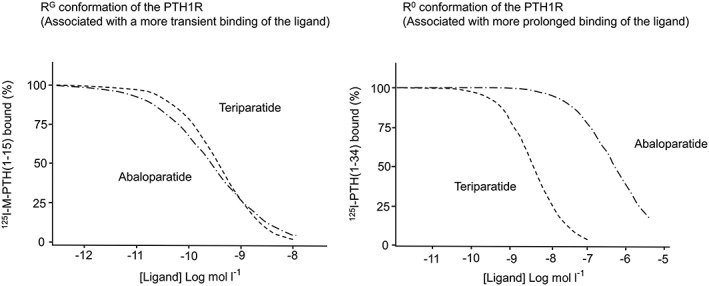
The two panels show the relative binding affinities of abaloparatide and teriparatide to two distinct parathyroid hormone (PTH)/PTHrP conformations (RG and R0). 125I–M‐PTH (1–15) is the competitive tracer radioligand that binds selectively to the RG conformation while 125I–PTH (1–34) is the competitive tracer radioligand that binds selectively to the R0 conformation (adapted from 104)
The PTH/PTHrP receptor not only mediates the biological action of PTH but also PTH‐related peptide (PTHrP). The receptor is therefore present in the classical sites of PTH action such as the bone and kidney but also in various other tissues not involved in the regulation of calcium homeostasis 19. The presence of PTH/PTHrP receptor in developing tissues that effectuate the physiological role of PTHrP indicates its role in growth and development. PTHrP is a longer peptide than PTH with a linear sequence that is 141 amino acids long. There is close sequence homology with PTH in the amino‐terminal first 13 amino acids. Eight residues are identical 20. Naturally occurring PTHrP has important physiological roles that include but are not limited to mammary gland development, lactation, tooth eruption, regulation of keratinocytes differentiation, vascular and uterine smooth muscle regulation, and placental calcium transport to the fetus. Its physiological role also extends to bone, contributing to the growth and development of the skeleton. In the skeleton, both PTHrP and PTH exert their distinct effects through binding and activation of a common PTH/PTHrP type 1 receptor in osteoblasts. While both peptides have the ability to stimulate bone formation, PTH principally functions as a circulating endocrine response system, while PTHrP is believed to be primarily a paracrine regulator.
Pathophysiological effects of PTH – clinical manifestations of PTH excess or deficiency
The effects of PTH on skeletal remodelling can be appreciated by reviewing the effect of excess PTH or deficient PTH on the skeleton. To this point, the natural histories of both primary hyperparathyroidism (PHPT) and hypoparathyroidism, and the effect of treatment of both diseases on the skeleton give us insights into the dual anabolic and catabolic sequelae of PTH.
PHPT and the skeleton
The old adage of stones, bones, abdominal groans and psychic moans described the classic manifestations of symptomatic PHPT 21, 22. Classical radiological features of skeletal involvement, such as osteitis fibrosa cystica, are rarely seen in countries where biochemical screening tests are commonly performed. Even in these countries, however, skeletal manifestations can be readily detected by bone mineral densitometry. Bone loss is thought to be due to the resorptive effects of PTH which is present in excess in these individuals.
Bone mineral density
Conventionally, it has been thought that continuous PTH secretion selectively affects cortical bone more than trabecular bone. Longitudinal studies describing the progression of skeletal disease in the natural history of PHPT using bone mineral densitometry have shown that cortical bone is predominantly affected with relative preservation in the trabecular bone. The 15‐year study of Rubin et al. 23 showed that the distal radius, a site comprising predominantly cortical bone, decreased the most by 35% over 15 years while the femoral neck, which contains a relative even admixture of cortical and trabecular bone, decrease to a smaller extent, by 10%, while the lumbar spine, which is primarily trabecular bone, remained stable for 15 years. This densitometric study highlights the vulnerability of cortical bone to the catabolic effects of continuous PTH secretion in a disease state such as PHPT. Of note, the decline in the densitometric profile of cortical bone occurs primarily after 10 years of follow up. For the first 10 years of this observational study, bone mineral density (BMD) remained stable at all skeletal sites 24. Cure of PHPT by successful parathyroidectomy leads to an improvement of trabecular bone first (e.g. lumbar spine), followed later by equivalent gains in the hip and forearm regions 23.
Bone histomorphometry
Histomorphometric studies confirm the apparent preferential negative effect of excessive PTH on cortical bone, gleaned first from the densitometric studies. Detailed analyses of iliac crest bone biopsies by histomorphometry provide evidence for increased cortical porosity as well as cortical thinning. In contrast, trabecular bone mass and microarchitecture are typically preserved as described by structural indices such as trabecular bone volume, trabecular number, connectivity and separation. These findings suggest a protective effect of PTH on trabecular bone 25, 26, 27, 28, 29, 30. Bone histomorphometry offers the advantage of allowing study of the dynamic characteristics of bone formation as well as static parameters. Dempster et al. 28 showed that the adjusted apposition rate and active formation period is higher in PHPT than in normal postmenopausal women. These observations were among the first to suggest that, even in a disorder characterized mainly by bone loss, there was nevertheless evidence for some anabolic action of PTH in the trabecular compartment of bone. While this is undoubtedly the case, it should be pointed out that histomorphometric studies classically involve the iliac crest, a site that has never been clearly shown to be representative of weight‐bearing sites. Such observations led to noninvasive imaging studies of skeletal sites that are traditionally more representative sites such as the lumbar spine and peripheral skeleton.
Bone turnover markers
In patients with PHPT, markers of bone formation and bone resorption correlate with bone histomorphometry 31. Both bone formation markers and resorption markers are elevated, reflecting a state of increased bone turnover that is characteristic of PHPT 32. In mild asymptomatic disease, bone turnover markers (BTMs) may not be frankly elevated but rather, near the upper limit of normal 33. Following curative therapy, both bone formation and resorption markers decrease significantly from preoperative values and are within the normal range within the 1st year postparathyroidectomy 34, 35, 36. Although both bone formation and resorption markers decline post parathyroidectomy, there is a suggestion that the pattern of decline may differ between the two with the decline of bone resorption markers preceding that of bone formation markers 36, 37.
Trabecular bone score
Trabecular bone score (TBS) is a semiquantitative textural analysis of the dual‐energy X‐ray absorptiometry image that is associated qualitatively with skeletal microstructure. It contributes to fracture risk independent of the BMD. TBS values are low in patients with PHPT even when areal BMD of the lumbar spine is seemingly normal. Silva et al. showed that TBS identified more patients with abnormal trabecular bone than lumbar spine BMD in a cohort of postmenopausal women with PHPT 38. Romagnoli et al. showed that patients with PHPT had TBS values significantly lower than matched controls and that TBS could identify those with vertebral fractures 39. These findings support the proposition that in PHPT, trabecular bone is not normal and contributes to fracture risk. In this regard, the work of Khosla et al. 40 and Vignali et al. 41, in which vertebral risk was higher in PHPT than normal controls is substantiated by these observations. Longitudinal studies with TBS are limited but in a small cohort of 10 subjects, there was no change after a 2‐year conservative follow‐up period and an improvement in 20 surgically treated patients 42.
High‐resolution peripheral quantitative computed tomography
More detailed quantitative microstructural information in PHPT is available by using high‐resolution peripheral quantitative computed tomography (HRpQCT). HRpQCT is limited to measurements of the ultradistal radius and a comparable tibial site. Its advantage is that the voxel resolution of 80 μm, or more recently, with the second‐generation instrument, 62 μm, permits quantification of microstructural elements of the cortical and trabecular compartments. By HRpQCT, it is clearly apparent that the detrimental effects of PTH excess are not limited to cortical bone but also extend to trabecular bone 43, 44 (Figure 3). At both the radius and the tibia, there are decreased volumetric densities in both the cortical and trabecular compartment, thinner cortices and more widely spaced and heterogeneously distributed trabeculae as well as a reduced plate‐like trabeculae and decreased plate/rod configuration, all of which indicate bone of poorer quality at those sites 44. After successful parathyroidectomy, improvements in cortical and trabecular volumetric BMD as well as trabecular microarchitecture with increased trabecular number and decreased trabecular separation were evident 45.
Figure 3.
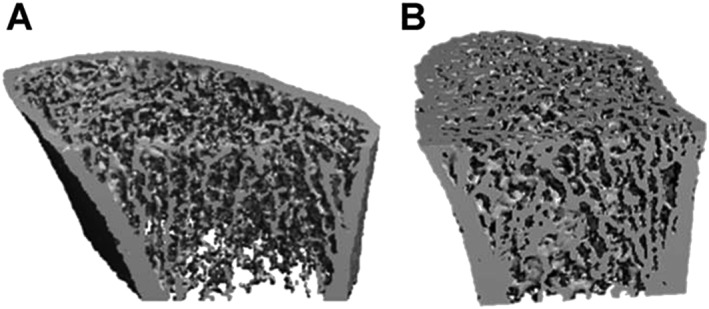
Representative using high‐resolution peripheral quantitative computed tomography images of the distal radius of a patient with primary hyperparayhyroidism (A) and a healthy subject (B) 44
Hypoparathyroidism and the skeleton
In hypoparathyroidism, decreased levels of PTH results in the inability to maintain calcium and phosphate homeostasis and thus hypocalcaemia and hyperphosphataemia follows. The effect of low calcium on different tissues results in typical symptoms of neuromuscular irritability, cognitive dysfunction, renal and cardiac manifestations. The lack of PTH hinders renal calcium resorption and thus hypercalciuria ensues, only to be aggravated by needs for large amounts of supplemental calcium and calcitriol. This renal pathophysiology and the therapeutic use of large amounts of calcium and active vitamin D lead to nephrolithiasis, nephrocalcinosis and eventual renal impairment as well as systemic calcification. The effects of deficient PTH on bone, stand in stark contrast to the effects of excess PTH on the skeleton. These effects relate importantly to the profound reduction in bone turnover that is characteristic of untreated hypoparathyroidism.
BMD
Virtually all studies show higher BMD at all skeletal sites 46, 47, 48, 49, 50, 51, 52, 53. Rubin et al., for example, showed that in hypoparathyroidism, BMD is greater at all sites compared to age‐ and sex‐matched reference values. BMD Z‐scores were +2.2 at the lumbar spine, +1.3 at the femoral neck, +1.1 at the total hip and +0.64 at the distal 1/3 radius 48. Treatment with PTH either by teriparatide [PTH (1–34)] 54, 55, 56, 57 or PTH (1–84) 52, 58, 59 generally shows an increase or unchanged BMD at the lumbar spine and hip, but a small decrease at the distal 1/3 radius. Further work by Cusano et al., in a 4‐year longitudinal study, showed that the small decline in the distal 1/3 radius site was, at the end of the study period, not different from baseline 59. It is of interest that these observations seemed, initially, to be paradoxical in that higher than normal bone density in the context of absent PTH was being further augmented at the lumbar spine and hip by PTH replacement therapy. However, these results do recall a key observation that when PTH is administered in relatively low dose and intermittently to normal subjects, an osteoanabolic effect is seen. This basic premise of therapy of PTH in osteoporosis may be unwittingly recapitulated in these studies of hypoparathyroidism. Consistent with this formulation, when PTH was administered at a fixed dose of 100 μg day–1 for a short 6‐month period, there were small declines at the lumbar spine and hip 52. In this study, there was an appreciable incidence of hypercalcaemia, suggesting that the doses used were beyond that associated with an osteoanabolic effect of PTH. Gafni et al. also showed in a small cohort followed for 20–60 months, a small effect of PTH (1–34) to reduce BMD at the distal 1/3 radius and the lumbar spine 57.
Bone histomorphometry
Dynamic skeletal indices confirm the remarkably low turnover state in hypoparathyroidism. There is both a reduction in bone formation rate as well as reduction in bone resorption rate. Static indices show increased bone mass in both the trabecular and cortical compartment 48. Trabecular number is increased and trabecular separation is decreased 56. Therapy with PTH(1–84) stimulates bone remodelling and further increases trabecular bone volume, trabecular number and reduces separation while increasing cortical porosity and maintaining cortical width 56.
BTMs
Consistent with bone histomorphometry, decreased PTH levels are associated with decreased BTMs in the lower half of the normal range 48. Both bone resorption and bone formation markers are lower in hypoparathyroidism than in age‐matched controls 60. With treatment, the low bone turnover state is reversed with increases in both bone formation and resorption markers (see section below for further description).
TBS
In a study comparing hypoparathyroid patients treated for 2 years to patients with PHPT postparathyroidectomy followed‐up for 2 years, baseline TBS was normal (> 1.35) in the hypoparathyroid patients (1.44 ± 0.12) but partially degraded in the hyperparathyroid patients (1.28 ± 0.13). Hypoparathyroid patients who were treated showed a significant increase in TBS at 18 months compared to baseline. TBS also remained significantly higher compared to treated PHPT patients who became euparathyroid throughout the study 61.
HRpQCT
Consistent with increased BMD at all skeletal sites, HRpQCT of the radius showed an increase in both volumetric BMD in the trabecular and cortical compartments compared to controls 47. HRpQCT also revealed increased cortical volumetric density associated with a decrease in cortical porosity but also a thinning of cortical bone (contrary to histomorphometry findings). The trabecular compartment showed more varied results. Trabeculae were more numerous and closer together (consistent with histomorphometric data) but the trabeculae were thinner and no difference in trabecular volumetric BMD was noted 62. In short, replacement with PTH is associated with increase trabecular volumetric BMD and decrease cortical volumetric BMD. This observation is consistent with the hypothesis that parathyroid hormone helps to regulate the distribution of cortical and trabecular compartments of bone. Supporting this hypothesis, Sikjaer et al. showed that 6 months of replacement PTH improved volumetric BMD at the lumbar spine but was associated with a decline in the total hip and a trend towards decline in the femoral neck 63.
Pharmacological effects of PTH – use in osteoporosis and hypoparathyroidism
Use of PTH in osteoporosis
In contrast to the effect of continuous PTH secretion on the skeleton, when PTH is administered subcutaneously and intermittently, an osteoanabolic effect is seen. This potential of PTH to rebuild the skeleton provided the rationale for its therapeutic development.
Duration of ligand exposure – intermittent vs. continuous PTH
We now appreciate that the dosing regimen influences which of PTH's dual pathways will predominate. Dobnig et al. studied the effect of different dosing regimens in rodents with daily subcutaneous injection or infusion, 1, 2 or 6 h day–1 vs. a continuous 24‐h infusion regimen 64. By histomorphometry, osteoblast number and bone formation rate were increased under conditions of daily intermittent subcutaneous injections or 1 h day–1 infusion. Longer, more continuous infusion regimens were associated with skeletal changes resembling patients with chronic PHPT. These seminal observations helped to confirm the idea that osteoanabolic effects of PTH can be appreciated primarily in the setting of brief and intermittent exposure. The registration phase 3 trial, that led to the approval of teriparatide [PTH (1–34)], tested two doses against placebo in a double blinded, randomized study. Neer et al. 65 showed that once daily injections of 20 μg PTH (1–34) was able to reduce vertebral and nonvertebral fracture incidence by 65% and 53% respectively. BMD values at the lumbar spine and femoral neck were increased by 9% and 3% respectively but not at the radius. There were, however, small declines at the radial site with the higher 40 μg dose 65. The 20 μg dose was developed further as the therapeutic dose because it was associated with less hypercalcemia and no reduction in radial BMD, while providing the same fracture protection as the 40 μg dose. The concept of a smaller dose given more frequently has also been supported by animal studies. Riond found that the same total PTH (1–34) dose given in a smaller but more frequent dosing regimen showed a better anabolic effect 66.
Effect on BTMs
Studies using PTH (1–34) for osteoporosis therapy consistently show an initial rapid and large increase in bone formation markers that is followed by a subsequent increase in bone resorption markers 67, 68, 69 (Figure 4). The kinetic profile of BTMs where bone formation precedes bone resorption is supported by early changes seen with quadruple tetracycline labelled bone histomorphometry. Modelling‐based bone accrual contributes initially to the effect of PTH (1–34) on bone formation. Modelling‐based bone formation is not coupled to bone resorption and typically occurs in the quiescent surfaces of the bone. Modelling‐based bone formation can be differentiated from remodelling‐based bone formation by the absence of scalloping beneath the surface of the newly formed bone. It can be inferred that smooth surfaces represent bone formation over unresorbed surfaces (modelling) whereas scalloped surfaces indicate a coupling of bone resorption by osteoclast (remodelling). Lindsay et al. showed that 30% of bone formation in PTH (1–34)‐treated patients can be attributed to bone modelling as compared to controls in whom bone formation was exclusively remodelling based 70. From these observations, it is also important to recognize that the majority of bone accrual under the influence of PTH is remodelling‐based.
Figure 4.
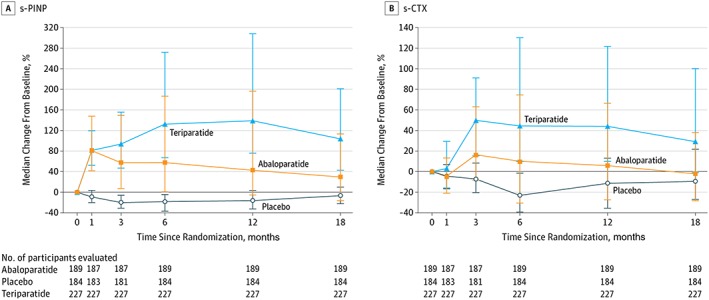
Median change from baseline in the serum bone formation (s‐PINP) and resorption (s‐CTX) marker over time during placebo, teriparatide and abaloparatide treatment of postmenopausal women with osteoporosis (n = 184 placebo, n = 189 abaloparatide, and n = 227 teriparatide participants) 107
Is PTH effective when given at even lower doses on a weekly basis?
Fujita et al. showed in a phase 2 study that weekly injection of teriparatide (about 30 and 60 μg – equivalent to approximately 4.3 and 8.6 μg day–1) for 12 months in a cohort of Japanese women increased lumbar spine BMD significantly 71. In the subsequent phase 3 study (TOWER) by the same group, a weekly dose of 60 μg teriparatide was compared to placebo in an elderly Japanese cohort with osteoporosis and prevalent vertebral fracture 72. The primary endpoint of new vertebral fracture incidence was reduced by 80% compared to placebo. However, there were no differences in nonvertebral fractures between the two groups. Densitometric gains of 6.4% at the lumbar spine, 3.0% at the total hip and 2.3% at the femoral neck compared to placebo were seen. BTMs followed a course that was markedly different from once‐daily regimens 67, 68. The bone formation marker, P1NP, increased moderately but fell to levels below the placebo group by 24 months. Similarly, the bone resorption marker, urine NTX, fell to levels below the placebo group from the 4th week to the end of study. Weekly injections of PTH (1–84) showed a somewhat similar pattern in that bone formation markers increased without a corresponding increase in bone resorption markers 73. Analyses of the microstructure of the cortex of the tibia by micro‐ computed tomography or bone histomorphometry in rabbits show that the same total dose administered daily increased cortical porosity but not if administered weekly over 1 month. Newly deposited endosteal bone collagen fibres were well organized in the weekly regimen suggesting lamellar bone formation was normal but haphazard in the daily regimen 74. However, the mere demonstration of an increase in cortical porosity does not imply poorer bone quality, particularly since there are concomitant increases in periosteal and endosteal bone formation. Hansen et al. showed, for example, that despite increased cortical porosity, bone strength as evaluated by finite element analysis at the distal radius and tibia from postmenopausal women was preserved with teriparatide 75. If validated in other populations, weekly dosing of PTH is a cost‐effective and innovative approach.
Use of PTH in hypoparathyroidism
Designing PTH or PTHrP analogues that display a more prolonged state of binding to the PTH/PTHrP receptor would be expected to approximate the tonic secretory state of PTH. Both PTH (1–84) and PTH (1–34) are formulated as subcutaneous injections and both have been used to replace the deficient or absent parathyroid hormone in patients with hypoparathyroidism. In this disease, however, the goal is the opposite of the goal in the treatment of osteoporosis. In hypoparathyroidism, it is desirable to take advantage of the longer‐lived configuration of the PTH‐PTH/PTHrP receptor interaction, not the shorter one. In this setting, the more prolonged action of PTH would be beneficial because the tonic secretory state of PTH as secreted by normal parathyroid glands would be more closely mimicked. Therefore, PTH (1–84), with its extended bioavailable pharmacokinetic profile compared with PTH (1–34) would be advantageous. PTH (1–84) has been approved in the USA for the management of hypoparathyroidism since January 2015. It received approval by the EMA in Europe in April 2017 76.
The difference in pharmacokinetics between intact PTH (1–84) and PTH (1–34) is further illustrated by the clinical experience with both molecules in the treatment of hypoparathyroidism. As anticipated by the foregoing discussion, PTH (1–34) requires at least twice daily injections and sometimes three injections per day to achieve adequate control whereas PTH (1–84) can be used successfully with a once‐daily dosing regimen 77. Therapy with PTH replacement reduces the need for calcium and calcitriol. Mannstadt et al. showed that 53% of patients who received PTH (1–84) replacement were able to reduce their oral calcium and active vitamin D by 50% while maintaining stable and normal calcium levels at 24 weeks of therapy 78. There seems to be a suggestion that more physiological delivery of PTH may reduce urinary calcium excretion. Winer et al. showed that continuous delivery of PTH (1–34) via pump was able to reduce urinary calcium by 59% when compared to twice daily injections of PTH (1–34), which seems to suggest that a more physiological approach is beneficial 79.
Fox et al. compared four routes and sites of administration of PTH (1–84) 80. Intravenously administered PTH (1–84) was cleared rapidly and provided the shortest calcium response, clearly not an attractive course for a disease such as hypoparathyroidism. Subcutaneous routes were compared between the thigh and the abdomen. The thigh sites provide for slower absorption than the abdomen and therefore a more prolonged calcaemic response. Subcutaneous thigh injection results in a biphasic profile with an initial peak 15 min postinjection representing rather direct delivery of the drug into the circulation and a second peak after 2 h 63 indicating a slower uptake mechanism via the lymphatic system. PTH levels return to baseline after 12 h and calcium levels returned to baseline after 24 h. For this reason, thigh injections are recommended for the treatment of hypoparathyroidism with PTH (1–84).
Effects on BTMs
Treatment with either PTH (1–34) or PTH (1–84) reverses the low bone turnover state in hypoparathyroidism. In studies of PTH (1–34), intermittent replacement increase BTMs above the normal range. Winer et al. showed that BTMs remained persistently elevated in longitudinal studies of up to 3 years with twice daily regime with a peak at 2–2.5 years with a suggestion of a taper at 3 years 54. There was a suggestion of a smaller increase in BTMs in the twice a day regimen compared to the once a day regimen although both bone formation and resorption markers were above the normal range 77. Additionally, continuous administration of PTH (1–34) via pump delivery was able to keep both bone formation and resorption markers within normal range as opposed to twice daily regimen, which remained above the normal range 79. In studies of PTH (1–84), once a day injection of PTH (1–84) of up to 6 years, also resulted in an increase in BTMs. BTMs peaked at 1 year on therapy and then a decrease but remaining above baseline values by 6 years of therapy. At the peak, the extent of this increase in bone formation marker was three‐fold higher than baseline as compared to bone resorption marker which was two‐fold higher than baseline 81. Similarly, more frequent regimens of PTH (1–84) either twice daily injections or continuous administration via pump is expected to reverse the low bone turnover state, possibly keeping BTMs within the normal range and correspondingly without inducing bone remodelling excessively.
Systems pharmacology of PTH
The preclinical and clinical pharmacokinetics (PK) and pharmacodynamics (PD) of PTH have been described for most forms of PTH such as PTH (1–84) 15, 63, 80, 82, 83, PTH (1–34) 77, 84, 85, 86 and PTHrP (1–36) 87, during various modes of administration such as intravenous, subcutaneous, transdermal, continuous and intermittent with various dose intervals in healthy volunteers 84, and in patients with osteoporosis and in patients with hypoparathyroidism 15, 88. In addition, some PTH‐releasing compounds have been studied 89. In many of these studies all earlier‐mentioned PD parameters were assessed, such as BTMs, ionized and total calcium in serum and urine, phosphate in serum and urine, 25OHD, 1,25 dihydroxyvitamin D, urinary cAMP, as well as other hormones and factors that play a role in bone such as RANKL and OPG 90, 91, FGF‐23 92 and sclerostin 93. Most data linking changes in BMD with changes in minerals, hormones and BTM in osteoporosis come from studies that did not include a PK component 68, 92, 94, 95, 96. From this pool of data, mathematical PK/PD models have been iteratively developed over several years, with increasing complexity but also an increasing ability to describe the PK of PTH and its analogues as well as the effects of treatment in time. In 2010, Peterson and Riggs integrated three previously published models that were all able to quantify some aspects of bone biology and related physiology 97; an intricate general mathematical model that describes systemic calcium homeostasis 98; a quantitative cellular model describing the linking of osteoclasts and osteoblasts 99; and an intracellular control mechanism that describes the differential responses to PTH 100. The latter model qualitatively describes the relative activities of the intracellular signalling pathways involving Runx2, Bcl‐2 and CREB transcription factors (Runx2‐Bcl‐2‐CREB system), which has been purported to influence the differential effects of continuous and intermittently administered PTH on catabolic and anabolic bone changes, respectively. A schematic of the physiological system model to describe calcium homeostasis and bone remodelling, which is really a system of 28 differential equations, is depicted in Figure 5. While some values were estimated from actual patient data, other values of the many parameters of this highly complex mathematical model were derived from the preclinical and clinical literature. In addition, while complex, this modelling exercise might not entirely reflect our current understanding of bone physiology and calcium homeostasis. Despite these potential flaws, the model adequately describes BTMs, hormone, calcium and phosphate levels as well as BMD during hyperparathyroidism and hypoparathyroidism, as well as during other conditions such as renal impairment. Moreover, it describes the effects of various drugs such as bisphosphonates, cathepsin K inhibitors, the RANKL‐antibody denosumab (for which this model was originally developed), the sclerostin antibody romosozumab, as well as PTH remarkably well. In all these settings, this model, to some extent, is able to predict the effect of various dose regimens, the simultaneous transient and continuous effect on BTMs and BMD, respectively as observed during romosozumab treatment, as well as the rebound effect in terms of BTM and rapid loss in BMD after stopping of treatment of oestrogens, cathepsin K inhibitors and denosumab 101.
Figure 5.
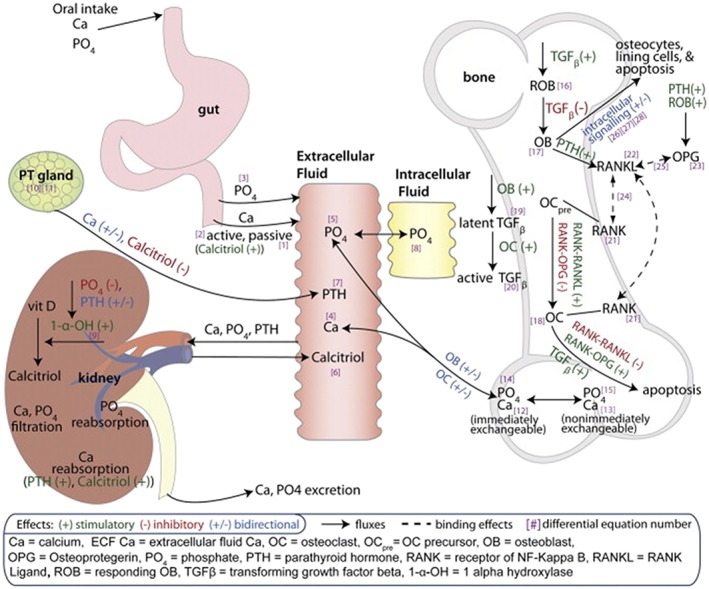
Schematic of physiological system model to describe calcium homeostasis and bone remodelling 97
Figures 6, 7, 8, 9 illustrate nicely the ability of the model to describe the various effects of hypo‐ and hyperparathyroidism as well as the effects of intermittent subcutaneous PTH (1–34). The model does not adequately address the transient effects on BTM of intermittent PTH treatment, neither in osteoporosis nor in hypoparathyroidism. In addition, the model does not seem to include all (patho‐)physiological aspects potentially relevant for PTH, such as the different conformation states of the PTH/PTHrP type 1 receptor. Like any PK/PD model, this highly complex model for bone physiology and calcium homeostasis might therefore also be limited in its ability to predict some future developments related to PTH but not yet captured by the model, such as the clinical development of abaloparatide. Interestingly, in 2005, Potter et al. described a mathematical model for PTH receptor kinetics, focusing on the receptor's response to PTH dosing to discern bone formation responses from bone resorption 102. In essence, this model is based on the concept of receptor desensitization with the active and inactive receptor species explained as G protein coupled and uncoupled receptors, respectively. While it is not known to us if this model has been incorporated yet into other pharmacological models, it might be useful in support of further development of PTH‐based compounds.
Figure 6.
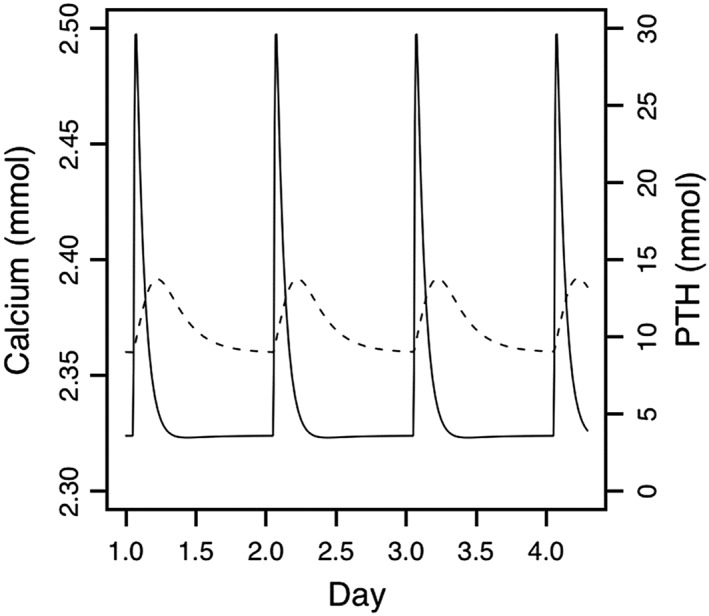
Daily steady‐state changes in plasma calcium (dashed line, left axis) and parathyroid hormone (PTH; solid line, right axis) predicted by the model after 1 month of once‐daily PTH 1–34 administration (20 μg teriparatide) 97
Figure 7.
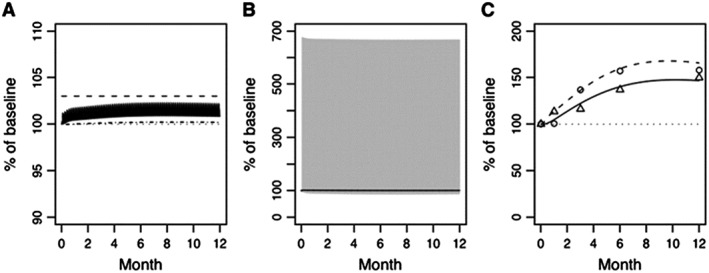
Percent of baseline (%) following once‐daily PTH 1–34 (20 μg teriparatide) administration for (A) plasma calcium (solid line) and phosphate (dot dash line), (B) plasma PTH (solid grey line) and calcitriol (solid line), and (C) bone‐related osteoclast (dashed line) and osteoblast (solid line). The solid bands in panels (A) and (B) represent the peak‐to‐trough fluctuations in plasma calcium (A) and PTH (B), respectively. Circles (O) and triangles (Δ) represent observed urine N‐telopeptide and bone‐specific alkaline phosphatase (C), respectively 97
Figure 8.
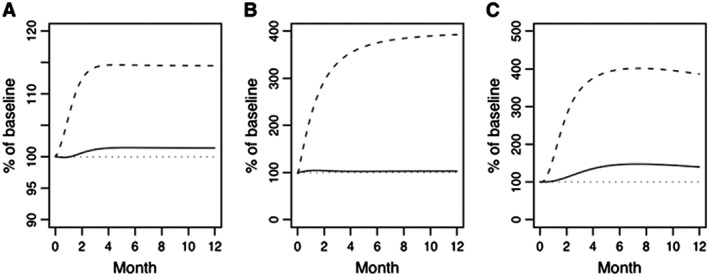
Primary hyperparathyroidism instituted in the model as a progressive increase in endogenous parathyroid gland production of parathyroid hormone (PTH) to affect an approximate 3‐fold increase in plasma PTH leading to percent of baseline (%) values for (A) plasma calcium (dashed line) and phosphate (solid line), (B) plasma PTH (dashed line) and calcitriol (solid line), and (C) bone‐related osteoclast (dashed line) and osteoblast (solid line) function. A horizontal reference (dotted line) is included on each figure at the baseline value of 100% 97
Figure 9.
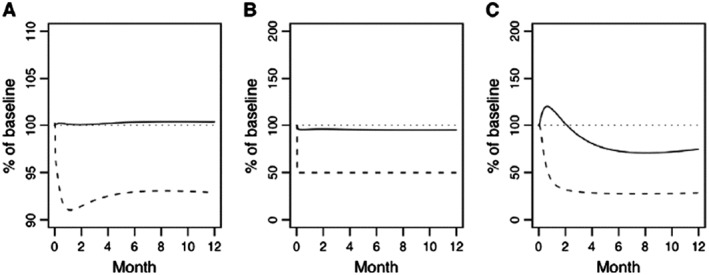
Primary hypoparathyroidism instituted in the model as an immediate 50% lowering of endogenous parathyroid gland production of parathyroid hormone (PTH) leading to changes in percent of baseline (%) for (A) plasma calcium (dashed line) and phosphate (solid line), (B) plasma PTH (dashed line) and calcitriol (solid line), and (C) bone‐related osteoclast (dashed line) and osteoblast (solid line) function. A horizontal reference (dotted line) is included on each figure at the baseline value of 100% 97
New developments
New developments in the area of PTH include new analogues of PTH such as abaloparatide, and new modes of administration of PTH such as transdermal and oral formulations and the use of pump delivery systems.
Abaloparatide
Abaloparatide was approved on 28 April 2017, by the Food and Drug Administration for therapy of postmenopausal women with osteoporosis at high risk for fracture 103. Abaloparatide is identical to PTHrP through the first 22 residues but, thereafter, several substituent amino acids render this molecule very different in terms of its interactions at the receptor level. By virtue of the different primary sequences, abaloparatide's interaction with the PTH/PTHrP‐1 receptor is indeed more fleeting than PTHrP or teriparatide. Abaloparatide binds with lower affinity to the R0 conformation state of the PTH/PTHrP‐1 receptor but similar affinity to the RG conformation state than teriparatide or PTHrP (Figures 1 and 2). The preferential binding of abaloparatide for the RG state elicits only a transient cyclic AMP signalling response and makes it ideally set up for the transient interaction associated with greater anabolic potential 104. The greater anabolic effect of abaloparatide, explained by this receptor mechanism, permits a dosing regimen that is four‐fold higher than teriparatide while maintaining its anabolic properties without an associated increase in adverse effects, such as hypercalcemia.
The PK profile of abaloparatide and teriparatide are similar. A single dose of subcutaneously administered abaloparatide (80 μg) has a half‐life of 2.3 h, peaks at about 45 min and remains in the circulation for about 7 h 105. Besides greater selectivity, another distinguishing property, that has clinical relevance, is that abaloparatide does not require refrigeration.
In the dose‐finding phase 2 trial, daily subcutaneous injections with 20, 40 and 80 μg of abaloparatide were compared with 20 μg of teriparatide for up to 24 weeks 106. There was a linear dose response to gains in BMD at the lumbar spine with increasing dose of abaloparatide. Both the 40 and 80 μg doses were effective at increasing lumbar spine BMD when compared to placebo (5.2 and 6.7% vs. 1.6%) with only the 80 μg dose showing similar efficacy when compared with 20 μg of teriparatide. At the hip, only the 80 μg dose was effective at increasing the femoral neck or total hip BMD when compared against placebo. At this dose, the change in femoral neck BMD was not different between abaloparatide and teriparatide but the increase in total hip BMD was higher in abaloparatide compared to teriparatide. Of note, teriparatide did not significantly increase hip BMD at 24 weeks in this short study. The effect on BTMs was also different between the two anabolic agents. There was a linear dose response with bone formation markers with abaloparatide. The increase in formation markers was similar between 40 and 80 μg doses and 20 μg teriparatide. In contrast, and as expected, abaloparatide's effect on bone resorption markers was smaller than teriparatide.
The 80 μg abaloparatide dose was thus chosen for the definitive phase 3 double blinded, randomized controlled clinical trial (ACTIVE) 107. The 80 μg dose of abaloparatide was compared with placebo and an open label comparison with teriparatide over 18 months. The primary end point was new vertebral fractures. The study was underpowered to appreciate the differences in the primary end point between abaloparatide and teriparatide although both appeared similarly effective in reducing new vertebral fractures by 86 and 80% respectively compared with placebo. Abaloparatide reduced nonvertebral fractures by 43% compared with the 28% reduction in the teriparatide group, the latter of which did not reach statistical significance. If one takes into account the nonvertebral fracture efficacy from the original teriparatide phase 3 study, the reduction in nonvertebral fracture with teriparatide was only appreciable after 9–12 months of use 65, 107. In contrast, fracture efficacy with abaloparatide was evident earlier as compared to the time course from the report of Neer et al. for teriparatide 65.
Abaloparatide increased femoral neck BMD by 3.6%, total hip BMD by 4.2% and lumbar spine BMD by 11.2% by 18 months. Abaloparatide demonstrated significantly greater BMD gains at the hip compared to teriparatide but the gains at the lumbar spine were similar. The effect on BTMs was different between the two ligands (Figure 4). Increases in bone formation markers with abaloparatide were smaller from 3 months to end of study and a transient and smaller increase in bone resorption markers from 3 months to 12 months was observed compared with teriparatide. Abaloparatide demonstrated less hypercalcaemia than the teriparatide group (3.42% vs. 6.1% respectively). The less prominent change in BTMs was mirrored by findings of lower eroded surface in the substudy evaluating the effect of abaloparatide on bone histomorphometry 108. This is consistent with a smaller effect on parameters of bone resorption.
Pegylated PTH
Modifying PTH or PTHrP or combining them with other moieties has the potential of exploiting differential interactions with the PTH/PTHrP‐1 receptor. Depending upon the intent of such alterations, greater therapeutic benefits could be applicable to either osteoporosis or hypoparathyroidism. Attaching a polyethylene glycol chain to the PTH (1–34) polypeptide can extend its pharmacodynamics and pharmacokinetic properties by increasing its molecular size and delaying its renal clearance. Pegylated PTH can be detected in the circulation for at least 24 h compared to the 4‐h circulatory life of PTH (1–34) 109. This is a profile that would be attractive for the treatment of hypoparathyroidism.
Cys25hPTH (1–34)
The residues from 25–34 of the native PTH hormone form the primary domain for receptor binding. Discovery of a rare cause of hypoparathyroidism due to a substitution of the 25th residue of the native PTH from arginine to cysteine with a high bone mass phenotype led to a study of once a day injections of Cys25hPTH (1–34) in ovariectomized mice. It confirmed a briefer interaction with the receptor with a lower binding affinity and shorter cAMP generation and a greater anabolic effect on both trabecular and cortical bone. This PTH analogue may confer RG selectivity but would be hampered by its relatively weak biological activity 110. It would be attractive in the management of osteoporosis.
Hybrid analogue – PTH (1–14)/PTHrP (15–36) hybrid ligand
After PTH binds to the PTH/PTHrP‐1 receptor, the ligand bound receptor is internalized. This process requires the phosphorylation of the C‐terminus region of PTH. It was found that modifying the C‐terminal portion increased binding affinity to the R0 conformation and prolonged the signalling response 111. Joining the 15–36 region of the PTHrP to the 1–14 sequence of the PTH fragment prolonged calcemic effects for >24 h even though the molecular hybrid had a half‐life of only 8 min.
Pump delivery systems incorporating the tonic, circadian and pulsatile secretory dynamics
Extending the concept of prolonged in vivo ‘life’ of PTH in hypoparathyroidism, Winer et al. used continuous administration of PTH (1–34) via a subcutaneous pump delivery system in hypoparathyroidism. In that comparative study with a twice daily injection group, pump administration was associated with a marked and sustained reduction in urinary calcium excretion 79. Further development of drug delivery system using native ligand or analogues that would mimic the normal secretory physiology of PTH with tonic, circadian and pulsatile secretory dynamics would be ideal as a therapy for hypoparathyroidism.
Oral
As peptides, both PTH (1–84) and PTH (1–34) have very poor oral bioavailability. Thus, technologies that enhances the gastrointestinal absorption and/or retards the breakdown of these peptides may make possible orally administered therapeutics in the future. PTH (1–34) attached to a carrier molecule (5‐CNAC) 112 or enteric coated formulation of PTH (1–31) 113 are promising developments in osteoporosis treatment. In preclinical studies, a promising orally administered non‐peptidyl PTHR1 agonist has been shown to restore calcium longer than PTH (1–84) or PTH (1–34) in parathyroidectomized rodents thereby exhibiting durable calcium homeostatic activity 114.
Transdermal
Cosman et al. 115 showed that with a transdermal route of administration, various doses of PTH (1–34) were effective in increasing BMD in postmenopausal women with osteoporosis. The transdermal microneedle patch was able to deliver PTH (1–34) in a rapid pulse with a shorter half‐life as compared to the injectable form. The increase in total hip BMD was thus greater with the 40 μg patch than the 20 μg injections of teriparatide after 6 months of therapy 115. Although short term, this study suggests that the transdermal microneedle patch is a convenient and effective delivery system. Recent reports have also suggested promising development of a transdermal abaloparatide 116.
Conclusions
Remarkable progress has been made since the emergence of PTH as an osteoanabolic therapeutic agent. Not only has the discovery of PTH's therapeutic use broadened the options for treatment of osteoporosis, but new paradigms for treatment of hypoparathyroidism, in which the deficient hormone is replaced, are now possible. Further characterization of the molecular mechanisms that form the basis for the way PTH ligands interact with the PTH/PTHrP receptor has refined its pharmacological use in treatment of more common disorders such as osteoporosis as well as rare diseases such as hypoparathyroidism. With the growing wealth of knowledge arising from the physiological, pathophysiological and pharmacological actions of PTH, development of new PTH analogues that modulate a predominant catabolic, anabolic or homeostatic function is likely to translate into novel therapies for these metabolic bone diseases.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 117, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 118.
Competing interests
There are no competing interests to declare.
The authors gratefully acknowledge the expert input of Teresita Bellido for Figure 1 .
Tay, D. , Cremers, S. , and Bilezikian, J. P. (2018) Optimal dosing and delivery of parathyroid hormone and its analogues for osteoporosis and hypoparathyroidism – translating the pharmacology. Br J Clin Pharmacol, 84: 252–267. doi: 10.1111/bcp.13455.
References
- 1. Kumar R, Thompson JR. The regulation of parathyroid hormone secretion and synthesis. J Am Soc Nephrol 2011; 22: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Demay MB, Kiernan MS, DeLuca HF, Kronenberg HM. Sequences in the human parathyroid hormone gene that bind the 1,25‐dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25‐dihydroxyvitamin D3. Proc Natl Acad Sci U S A 1992; 89: 8097–8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kempson SA, Lotscher M, Kaissling B, Biber J, Murer H, Levi M. Parathyroid hormone action on phosphate transporter mRNA and protein in rat renal proximal tubules. Am J Physiol 1995; 268 (4 Pt 2): F784–F791. [DOI] [PubMed] [Google Scholar]
- 4. Bonewald LF. The amazing osteocyte. J Bone Miner Res 2011; 26: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silva BC, Kousteni S. Chapter 8 – cellular actions of PTH: osteoblasts, osteoclasts, and osteocytes A2. In: Bilezikian In: The Parathyroids, Third edn, ed JP San Diego: Academic Press, 2015; 127–137. [Google Scholar]
- 6. el‐Hajj Fuleihan G, Klerman EB, Brown EN, Choe Y, Brown EM, Czeisler CA. The parathyroid hormone circadian rhythm is truly endogenous –a general clinical research center study. J Clin Endocrinol Metab 1997; 82: 281–286. [DOI] [PubMed] [Google Scholar]
- 7. Schmitt CP, Schaefer F, Bruch A, Veldhuis JD, Schmidt‐Gayk H, Stein G, et al Control of pulsatile and tonic parathyroid hormone secretion by ionized calcium. J Clin Endocrinol Metab 1996; 81: 4236–4243. [DOI] [PubMed] [Google Scholar]
- 8. Samuels MH, Veldhuis J, Cawley C, Urban RJ, Luther M, Bauer R, et al Pulsatile secretion of parathyroid hormone in normal young subjects: assessment by deconvolution analysis. J Clin Endocrinol Metab 1993; 77: 399–403. [DOI] [PubMed] [Google Scholar]
- 9. Harms HM, Kaptaina U, Külpmann WR, Brabant G, Hesch RD. Pulse amplitude and frequency modulation of parathyroid hormone in plasma. J Clin Endocrinol Metab 1989; 69: 843–851. [DOI] [PubMed] [Google Scholar]
- 10. Chiavistelli S, Giustina A, Mazziotti G. Parathyroid hormone pulsatility: physiological and clinical aspects. Bone Res 2015; 3: 14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bringhurst FR, Stern AM, Yotts M, Mizrahi N, Segre GV, Potts JT Jr. Peripheral metabolism of PTH: fate of biologically active amino terminus in vivo . Am J Physiol 1988; 255: E886–E893. [DOI] [PubMed] [Google Scholar]
- 12. Davies C, Demeure MJ, St John A, Edis AJ. Study of intact (1‐84) parathyroid hormone secretion in patients undergoing parathyroidectomy. World J Surg 1990; 14: 355–359 discussion 60. [DOI] [PubMed] [Google Scholar]
- 13. Fraher LJ, Klein K, Marier R, Freeman D, Hendy GN, Goltzman D, et al Comparison of the pharmacokinetics of parenteral parathyroid hormone‐(1‐34) [PTH‐(1‐34)] and PTH‐related peptide‐(1‐34) in healthy young humans. J Clin Endocrinol Metab 1995; 80: 60–64. [DOI] [PubMed] [Google Scholar]
- 14. Satterwhite J, Heathman M, Miller PD, Marin F, Glass EV, Dobnig H. Pharmacokinetics of teriparatide (rhPTH[1‐34]) and calcium pharmacodynamics in postmenopausal women with osteoporosis. Calcif Tissue Int 2010; 87: 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clarke BL, Kay Berg J, Fox J, Cyran JA, Lagast H. Pharmacokinetics and pharmacodynamics of subcutaneous recombinant parathyroid hormone (1‐84) in patients with hypoparathyroidism: an open‐label, single‐dose, phase I study. Clin Ther 2014; 36: 722–736. [DOI] [PubMed] [Google Scholar]
- 16. Potts JT Jr, Tregear GW, Keutmann HT, Niall HD, Sauer R, Deftos LJ, et al Synthesis of a biologically active N‐terminal tetratriacontapeptide of parathyroid hormone. Proc Natl Acad Sci U S A 1971; 68: 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dean T, Vilardaga JP, Potts JJT, Gardella TJ. Altered selectivity of parathyroid hormone (PTH) and PTH‐related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol Endocrinol 2008; 22: 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okazaki M, Ferrandon S, Vilardaga JP, Bouxsein ML, Potts JT, Gardella TJ. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc Natl Acad Sci 2008; 105: 16525–16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ureña P, Kong XF, Abou‐Samra AB, Jüppner H, Kronenberg HM, Potts JJT, et al Parathyroid hormone (PTH)/PTH‐related peptide receptor messenger ribonucleic acids are widely distributed in rat tissues. Endocrinology 1993; 133: 617–623. [DOI] [PubMed] [Google Scholar]
- 20. Martin TJ. Parathyroid hormone‐related protein, its regulation of cartilage and bone development, and role in treating bone diseases. Physiol Rev 2016; 96: 831–871. [DOI] [PubMed] [Google Scholar]
- 21. St. Goar WT. Gastrointestinal symptoms as a clue to the diagnosis of primary hyperparathyroidism: a review of 45 cases. Ann Intern Med 1957; 46: 102–118. [DOI] [PubMed] [Google Scholar]
- 22. Mieher WC Jr, Thibaudeau Y, Frame B. Primary hyperparathyroidism: a diagnostic challenge. Arch Intern Med 1961; 107: 361–371. [DOI] [PubMed] [Google Scholar]
- 23. Rubin MR, Bilezikian JP, McMahon DJ, Jacobs T, Shane E, Siris E, et al The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab 2008; 93: 3462–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10‐year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med 1999; 341: 1249–1255. [DOI] [PubMed] [Google Scholar]
- 25. Silverberg SJ, Shane E, de la Cruz L, Dempster DW, Feldman F, Seldin D, et al Skeletal disease in primary hyperparathyroidism. J Bone Miner Res 1989; 4: 283–291. [DOI] [PubMed] [Google Scholar]
- 26. Parisien M, Silverberg SJ, Shane E, de la Cruz L, Lindsay R, Bilezikian JP, et al The histomorphometry of bone in primary hyperparathyroidism: preservation of cancellous bone structure. J Clin Endocrinol Metab 1990; 70: 930–938. [DOI] [PubMed] [Google Scholar]
- 27. Parisien M, Mellish RW, Silverberg SJ, Shane E, Lindsay R, Bilezikian JP, et al Maintenance of cancellous bone connectivity in primary hyperparathyroidism: trabecular strut analysis. J Bone Miner Res 1992; 7: 913–919. [DOI] [PubMed] [Google Scholar]
- 28. Dempster DW, Parisien M, Silverberg SJ, Liang XG, Schnitzer M, Shen V, et al On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab 1999; 84: 1562–1566. [DOI] [PubMed] [Google Scholar]
- 29. Uchiyama T, Tanizawa T, Ito A, Endo N, Takahashi HE. Microstructure of the trabecula and cortex of iliac bone in primary hyperparathyroidism patients determined using histomorphometry and node‐strut analysis. J Bone Miner Metab 1999; 17: 283–288. [DOI] [PubMed] [Google Scholar]
- 30. Dempster DW, Muller R, Zhou H, Kohler T, Shane E, Parisien M, et al Preserved three‐dimensional cancellous bone structure in mild primary hyperparathyroidism. Bone 2007; 41: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eriksen EF, Charles P, Melsen F, Mosekilde L, Risteli L, Risteli J. Serum markers of type I collagen formation and degradation in metabolic bone disease: correlation with bone histomorphometry. J Bone Miner Res 1993; 8: 127–132. [DOI] [PubMed] [Google Scholar]
- 32. Guo CY, Thomas WE, al‐Dehaimi AW, Assiri AM, Eastell R. Longitudinal changes in bone mineral density and bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab 1996; 81: 3487–3491. [DOI] [PubMed] [Google Scholar]
- 33. Silverberg SJ, Gartenberg F, Jacobs TP, Shane E, Siris E, Staron RB, et al Longitudinal measurements of bone density and biochemical indices in untreated primary hyperparathyroidism. J Clin Endocrinol Metab 1995; 80: 723–728. [DOI] [PubMed] [Google Scholar]
- 34. Tamura Y, Araki A, Chiba Y, Mori S, Hosoi T, Horiuchi T. Remarkable increase in lumbar spine bone mineral density and amelioration in biochemical markers of bone turnover after parathyroidectomy in elderly patients with primary hyperparathyroidism: a 5‐year follow‐up study. J Bone Miner Metab 2007; 25: 226–231. [DOI] [PubMed] [Google Scholar]
- 35. Thorsen K, Kristoffersson AO, Lorentzon RP. Changes in bone mass and serum markers of bone metabolism after parathyroidectomy. Surgery 1997; 122: 882–887. [DOI] [PubMed] [Google Scholar]
- 36. Christiansen P, Steiniche T, Brixen K, Hessov I, Melsen F, Heickendorff L, et al Primary hyperparathyroidism: short‐term changes in bone remodeling and bone mineral density following parathyroidectomy. Bone 1999; 25: 237–244. [DOI] [PubMed] [Google Scholar]
- 37. Seibel MJ, Gartenberg F, Silverberg SJ, Ratcliffe A, Robins SP, Bilezikian JP. Urinary hydroxypyridinium cross‐links of collagen in primary hyperparathyroidism. J Clin Endocrinol Metab 1992; 74: 481–486. [DOI] [PubMed] [Google Scholar]
- 38. Silva BC, Boutroy S, Zhang C, McMahon DJ, Zhou B, Wang J, et al Trabecular bone score (TBS)‐‐a novel method to evaluate bone microarchitectural texture in patients with primary hyperparathyroidism. J Clin Endocrinol Metab 2013; 98: 1963–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Romagnoli E, Cipriani C, Nofroni I, Castro C, Angelozzi M, Scarpiello A, et al "Trabecular bone score" (TBS): an indirect measure of bone micro‐architecture in postmenopausal patients with primary hyperparathyroidism. Bone 2013; 53: 154–159. [DOI] [PubMed] [Google Scholar]
- 40. Khosla S, Melton LJ 3rd, Wermers RA, Crowson CS, O'Fallon W, Riggs B. Primary hyperparathyroidism and the risk of fracture: a population‐based study. J Bone Miner Res 1999; 14: 1700–1707. [DOI] [PubMed] [Google Scholar]
- 41. Vignali E, Viccica G, Diacinti D, Cetani F, Cianferotti L, Ambrogini E, et al Morphometric vertebral fractures in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab 2009; 94: 2306–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eller‐Vainicher C, Filopanti M, Palmieri S, Ulivieri FM, Morelli V, Zhukouskaya VV, et al Bone quality, as measured by trabecular bone score, in patients with primary hyperparathyroidism. Eur J Endocrinol 2013; 169: 155–162. [DOI] [PubMed] [Google Scholar]
- 43. Hansen S, Beck Jensen JE, Rasmussen L, Hauge EM, Brixen K. Effects on bone geometry, density, and microarchitecture in the distal radius but not the tibia in women with primary hyperparathyroidism: a case‐control study using HR‐pQCT. J Bone Miner Res 2010; 25: 1941–1947. [DOI] [PubMed] [Google Scholar]
- 44. Stein EM, Silva BC, Boutroy S, Zhou B, Wang J, Udesky J, et al Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J Bone Miner Res 2013; 28: 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hansen S, Hauge EM, Rasmussen L, Jensen J‐EB, Brixen K. Parathyroidectomy improves bone geometry and microarchitecture in female patients with primary hyperparathyroidism: a one‐year prospective controlled study using high‐resolution peripheral quantitative computed tomography. J Bone Miner Res 2012; 27: 1150–1158. [DOI] [PubMed] [Google Scholar]
- 46. Mitchell DM, Regan S, Cooley MR, Lauter KB, Vrla MC, Becker CB, et al Long‐term follow‐up of patients with hypoparathyroidism. J Clin Endocrinol Metab 2012; 97: 4507–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen Q, Kaji H, Iu MF, Nomura R, Sowa H, Yamauchi M, et al Effects of an excess and a deficiency of endogenous parathyroid hormone on volumetric bone mineral density and bone geometry determined by peripheral quantitative computed tomography in female subjects. J Clin Endocrinol Metab 2003; 88: 4655–4658. [DOI] [PubMed] [Google Scholar]
- 48. Rubin MR, Dempster DW, Zhou H, Shane E, Nickolas T, Sliney J Jr, et al Dynamic and structural properties of the skeleton in hypoparathyroidism. J Bone Miner Res 2008; 23: 2018–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abugassa S, Nordenstrom J, Eriksson S, Sjoden G. Bone mineral density in patients with chronic hypoparathyroidism. J Clin Endocrinol Metab 1993; 76: 1617–1621. [DOI] [PubMed] [Google Scholar]
- 50. Chan FK, Tiu SC, Choi KL, Choi CH, Kong AP, Shek CC. Increased bone mineral density in patients with chronic hypoparathyroidism. J Clin Endocrinol Metab 2003; 88: 3155–3159. [DOI] [PubMed] [Google Scholar]
- 51. Laway BA, Goswami R, Singh N, Gupta N, Seith A. Pattern of bone mineral density in patients with sporadic idiopathic hypoparathyroidism. Clin Endocrinol (Oxf) 2006; 64: 405–409. [DOI] [PubMed] [Google Scholar]
- 52. Sikjaer T, Rejnmark L, Rolighed L, Heickendorff L, Mosekilde L. The effect of adding PTH(1‐84) to conventional treatment of hypoparathyroidism: a randomized, placebo‐controlled study. J Bone Miner Res 2011; 26: 2358–2370. [DOI] [PubMed] [Google Scholar]
- 53. Takamura Y, Miyauchi A, Yabuta T, Kihara M, Ito Y, Miya A. Attenuation of postmenopausal bone loss in patients with transient hypoparathyroidism after total thyroidectomy. World J Surg 2013; 37: 2860–2865. [DOI] [PubMed] [Google Scholar]
- 54. Winer KK, Ko CW, Reynolds JC, Dowdy K, Keil M, Peterson D, et al Long‐term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone‐(1‐34) versus calcitriol and calcium. J Clin Endocrinol Metab 2003; 88: 4214–4220. [DOI] [PubMed] [Google Scholar]
- 55. Winer KK, Sinaii N, Reynolds J, Peterson D, Dowdy K, Cutler GB Jr. Long‐term treatment of 12 children with chronic hypoparathyroidism: a randomized trial comparing synthetic human parathyroid hormone 1‐34 versus calcitriol and calcium. J Clin Endocrinol Metab 2010; 95: 2680–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gafni RI, Brahim JS, Andreopoulou P, Bhattacharyya N, Kelly MH, Brillante BA, et al Daily parathyroid hormone 1‐34 replacement therapy for hypoparathyroidism induces marked changes in bone turnover and structure. J Bone Miner Res 2012; 27: 1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gafni RI, Guthrie LC, Kelly MH, Brillante BA, Christie CM, Reynolds JC, et al Transient increased calcium and calcitriol requirements after discontinuation of human synthetic parathyroid hormone 1‐34 (hPTH 1‐34) replacement therapy in hypoparathyroidism. J Bone Miner Res 2015; 30: 2112–2118. [DOI] [PubMed] [Google Scholar]
- 58. Rubin MR, Sliney J Jr, McMahon DJ, Silverberg SJ, Bilezikian JP. Therapy of hypoparathyroidism with intact parathyroid hormone. Osteoporos Int 2010; 21: 1927–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cusano NE, Rubin MR, McMahon DJ, Zhang C, Ives R, Tulley A, et al Therapy of hypoparathyroidism with PTH(1‐84): a prospective four‐year investigation of efficacy and safety. J Clin Endocrinol Metab 2013; 98: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fujiyama K, Kiriyama T, Ito M, Nakata K, Yamashita S, Yokoyama N, et al Attenuation of postmenopausal high turnover bone loss in patients with hypoparathyroidism. J Clin Endocrinol Metab 1995; 80: 2135–2138. [DOI] [PubMed] [Google Scholar]
- 61. Cipriani C, Abraham A, Silva BC, Cusano NE, Rubin MR, McMahon DJ, et al Skeletal changes after restoration of the euparathyroid state in patients with hypoparathyroidism and primary hyperparathyroidism. Endocrine 2017; 55: 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cusano NE, Nishiyama KK, Zhang C, Rubin MR, Boutroy S, McMahon DJ, et al Noninvasive assessment of skeletal microstructure and estimated bone strength in hypoparathyroidism. J Bone Miner Res 2016; 31: 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sikjaer T, Amstrup AK, Rolighed L, Kjaer SG, Mosekilde L, Rejnmark L. PTH(1‐84) replacement therapy in hypoparathyroidism: a randomized controlled trial on pharmacokinetic and dynamic effects after 6 months of treatment. J Bone Miner Res 2013; 28: 2232–2243. [DOI] [PubMed] [Google Scholar]
- 64. Dobnig H, Turner RT. The effects of programmed administration of human parathyroid hormone fragment (1‐34) on bone histomorphometry and serum chemistry in rats. Endocrinology 1997; 138: 4607–4612. [DOI] [PubMed] [Google Scholar]
- 65. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al Effect of parathyroid hormone (1‐34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344: 1434–1441. [DOI] [PubMed] [Google Scholar]
- 66. Riond JL. Modulation of the anabolic effect of synthetic human parathyroid hormone fragment‐(I‐34) in the bone of growing rats by variations in the dosage regimen. Clin Sci (Lond) 1993; 85: 223–228. [DOI] [PubMed] [Google Scholar]
- 67. Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez‐Perez A, et al The effect of teriparatide [human parathyroid hormone (1‐34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 2003; 18: 9–17. [DOI] [PubMed] [Google Scholar]
- 68. Chen P, Satterwhite JH, Licata AA, Lewiecki EM, Sipos AA, Misurski DM, et al Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res 2005; 20: 962–970. [DOI] [PubMed] [Google Scholar]
- 69. Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, et al The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 2003; 349: 1207–1215. [DOI] [PubMed] [Google Scholar]
- 70. Lindsay R, Cosman F, Zhou H, Bostrom MP, Shen VW, Cruz JD, et al A novel tetracycline labeling schedule for longitudinal evaluation of the short‐term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res 2006; 21: 366–373. [DOI] [PubMed] [Google Scholar]
- 71. Fujita T, Inoue T, Morii H, Morita R, Norimatsu H, Orimo H, et al Effect of an intermittent weekly dose of human parathyroid hormone (1‐34) on osteoporosis: a randomized double‐masked prospective study using three dose levels. Osteoporos Int 1999; 9: 296–306. [DOI] [PubMed] [Google Scholar]
- 72. Nakamura T, Sugimoto T, Nakano T, Kishimoto H, Ito M, Fukunaga M, et al Randomized Teriparatide [human parathyroid hormone (PTH) 1‐34] once‐weekly efficacy research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab 2012; 97: 3097–3106. [DOI] [PubMed] [Google Scholar]
- 73. Black DM, Bouxsein ML, Palermo L, McGowan JA, Newitt DC, Rosen E, et al Randomized trial of once‐weekly parathyroid hormone (1‐84) on bone mineral density and remodeling. J Clin Endocrinol Metab 2008; 93: 2166–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yamane H, Takakura A, Shimadzu Y, Kodama T, Lee JW, Isogai Y, et al Acute development of cortical porosity and endosteal naive bone formation from the daily but not weekly short‐term administration of PTH in rabbit. PLoS One 2017; 12: e0175329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hansen S, Hauge EM, Beck Jensen J‐E, Brixen K. Differing effects of PTH 1–34, PTH 1–84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18‐month open‐labeled observational study using HR‐pQCT. J Bone Miner Res 2013; 28: 736–745. [DOI] [PubMed] [Google Scholar]
- 76. European Medicines Agency website . Available at https://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003861/human_med_002093.jsp&mid=wc0b01ac058001d124 (last accessed 16 November 2017).
- 77. Winer KK, Yanovski JA, Sarani B, Cutler GB Jr. A randomized, cross‐over trial of once‐daily versus twice‐daily parathyroid hormone 1‐34 in treatment of hypoparathyroidism. J Clin Endocrinol Metab 1998; 83: 3480–3486. [DOI] [PubMed] [Google Scholar]
- 78. Mannstadt M, Clarke BL, Vokes T, Brandi ML, Ranganath L, Fraser WD, et al Efficacy and safety of recombinant human parathyroid hormone (1‐84) in hypoparathyroidism (REPLACE): a double‐blind, placebo‐controlled, randomised, phase 3 study. Lancet Diabetes Endocrinol 2013; 1: 275–283. [DOI] [PubMed] [Google Scholar]
- 79. Winer KK, Zhang B, Shrader JA, Peterson D, Smith M, Albert PS, et al Synthetic human parathyroid hormone 1‐34 replacement therapy: a randomized crossover trial comparing pump versus injections in the treatment of chronic hypoparathyroidism. J Clin Endocrinol Metab 2012; 97: 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fox J, Wells D, Garceau RJ. Relationships between pharmacokinetic profile of human PTH(1‐84) and serum calcium response in postmenopausal women following four different methods of administration. (Abstract MO0173). 33rd Annual Meeting of the American Society of Bone and Mineral Research 2011.
- 81. Rubin MR, Cusano NE, Fan W‐W, Delgado Y, Zhang C, Costa AG, et al Therapy of hypoparathyroidism with PTH(1–84): a prospective six year investigation of efficacy and safety. J Clin Endocrinol Metab 2016; 101: 2742–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li Q, Qiao J, Deng J, Zeng T, Zhou P, Li W. Safety, tolerability and pharmacokinetic study of recombinant human parathyroid hormone [rhPTH (1‐84)] in Chinese healthy volunteers. J Huazhong Univ Sci Technolog Med Sci 2009; 29: 431–434. [DOI] [PubMed] [Google Scholar]
- 83. Schwietert HR, Groen EW, Sollie FA, Jonkman JH. Single‐dose subcutaneous administration of recombinant human parathyroid hormone [rhPTH(1‐84)] in healthy postmenopausal volunteers. Clin Pharmacol Ther 1997; 61: 360–376. [DOI] [PubMed] [Google Scholar]
- 84. Liu Y, Yang C, Li Z, Zhou J, Lv Y, Zhang Y, et al Safety, tolerability, pharmacokinetics, and pharmacodynamics of recombinant human parathyroid hormone (1‐34) in healthy Chinese subjects. Clin Ther 2014; 36: 940–952. [DOI] [PubMed] [Google Scholar]
- 85. Winer KK, Yanovski JA, Cutler GB Jr. Synthetic human parathyroid hormone 1‐34 vs calcitriol and calcium in the treatment of hypoparathyroidism. JAMA 1996; 276: 631–636. [PubMed] [Google Scholar]
- 86. Winer KK, Sinaii N, Peterson D, Sainz B Jr, Cutler GB Jr. Effects of once versus twice‐daily parathyroid hormone 1‐34 therapy in children with hypoparathyroidism. J Clin Endocrinol Metab 2008; 93: 3389–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Henry JG, Mitnick M, Dann PR, Stewart AF. Parathyroid hormone‐related protein‐(1–36) is biologically active when administered subcutaneously to Humans1. J Clin Endocrinol Metab 1997; 82: 900–906. [DOI] [PubMed] [Google Scholar]
- 88. Winer KK, Fulton K, Albert P, Cutler GB. Effects of pump versus twice‐daily injection delivery of synthetic parathyroid hormone 1‐34 in children with severe congenital hypoparathyroidism. J Pediatr 2014; 165: 556–63.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. John MR, Harfst E, Loeffler J, Belleli R, Mason J, Bruin GJ, et al AXT914 a novel, orally‐active parathyroid hormone‐releasing drug in two early studies of healthy volunteers and postmenopausal women. Bone 2014; 64: 204–210. [DOI] [PubMed] [Google Scholar]
- 90. Drake MT, Srinivasan B, Modder UI, Ng AC, Undale AH, Roforth MM, et al Effects of intermittent parathyroid hormone treatment on osteoprogenitor cells in postmenopausal women. Bone 2011; 49: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Anastasilakis AD, Goulis DG, Polyzos SA, Gerou S, Pavlidou V, Koukoulis G, et al Acute changes in serum osteoprotegerin and receptor activator for nuclear factor‐kappaB ligand levels in women with established osteoporosis treated with teriparatide. Eur J Endocrinol 2008; 158: 411–415. [DOI] [PubMed] [Google Scholar]
- 92. Sridharan M, Cheung J, Moore AE, Frost ML, Fraser WD, Fogelman I, et al Circulating fibroblast growth factor‐23 increases following intermittent parathyroid hormone (1‐34) in postmenopausal osteoporosis: association with biomarker of bone formation. Calcif Tissue Int 2010; 87: 398–405. [DOI] [PubMed] [Google Scholar]
- 93. Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, et al Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 2010; 95: 5056–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Burshell AL, Möricke R, Correa‐Rotter R, Chen P, Warner MR, Dalsky GP, et al Correlations between biochemical markers of bone turnover and bone density responses in patients with glucocorticoid‐induced osteoporosis treated with teriparatide or alendronate. Bone 2010; 46: 935–939. [DOI] [PubMed] [Google Scholar]
- 95. Blumsohn A, Marin F, Nickelsen T, Brixen K, Sigurdsson G, González de la Vera J, et al Early changes in biochemical markers of bone turnover and their relationship with bone mineral density changes after 24 months of treatment with teriparatide. Osteoporos Int 2011; 22: 1935–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bauer DC, Garnero P, Bilezikian JP, Greenspan SL, Ensrud KE, Rosen CJ, et al Short‐term changes in bone turnover markers and bone mineral density response to parathyroid hormone in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 2006; 91: 1370–1375. [DOI] [PubMed] [Google Scholar]
- 97. Peterson MC, Riggs MM. A physiologically based mathematical model of integrated calcium homeostasis and bone remodeling. Bone 2010; 46: 49–63. [DOI] [PubMed] [Google Scholar]
- 98. Raposo JF, Sobrinho LG, Ferreira HG. A minimal mathematical model of calcium homeostasis. J Clin Endocrinol Metab 2002; 87: 4330–4340. [DOI] [PubMed] [Google Scholar]
- 99. Lemaire V, Tobin FL, Greller LD, Cho CR, Suva LJ. Modeling the interactions between osteoblast and osteoclast activities in bone remodeling. J Theor Biol 2004; 229: 293–309. [DOI] [PubMed] [Google Scholar]
- 100. Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, et al Proteasomal degradation of Runx2 shortens parathyroid hormone‐induced anti‐apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem 2003; 278: 50259–50272. [DOI] [PubMed] [Google Scholar]
- 101. Yu EW, Kumbhani R, Siwila‐Sackman E, DeLelys M, Preffer FI, Leder BZ, et al Teriparatide (PTH 1‐34) treatment increases peripheral hematopoietic stem cells in postmenopausal women. J Bone Miner Res 2014; 29: 1380–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Potter LK, Greller LD, Cho CR, Nuttall ME, Stroup GB, Suva LJ, et al Response to continuous and pulsatile PTH dosing: a mathematical model for parathyroid hormone receptor kinetics. Bone 2005; 37: 159–169. [DOI] [PubMed] [Google Scholar]
- 103. FDA/CEDR resources page . Food and Drug Administration web site. Available at https%3A%2F%2Fwww.accessdata.fda.gov%2Fscripts%2Fcder%2Fdaf%2Findex.cfm%3Fevent%3Doverview.process%26amp%3BApplNo%3D208743 (last accessed 1 May 2017).
- 104. Hattersley G, Dean T, Corbin BA, Bahar H, Gardella TJ. Binding selectivity of Abaloparatide for PTH‐Type‐1‐receptor conformations and effects on downstream signaling. Endocrinology 2016; 157: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Obaidi M, Chavira RE, Reinbolt L, Offman E, McKay E, O'Dea LL . Pharmacokinetics and Pharmacokinetics and pharmacodynamic of subcutaneously (SC) administered doses of BA058, a bone mass density restoring agent in healthy postmenopausal women. AAPS 2010. (abstract); W5385.
- 106. Leder BZ, O'Dea LS, Zanchetta JR, Kumar P, Banks K, McKay K, et al Effects of abaloparatide, a human parathyroid hormone‐related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 2015; 100: 697–706. [DOI] [PubMed] [Google Scholar]
- 107. Miller PD, Hattersley G, Riis B, et al Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 2016; 316: 722–733. [DOI] [PubMed] [Google Scholar]
- 108. Moreira CA, Fitzpatrick LA, Wang Y, Recker RR. Effects of abaloparatide‐SC (BA058) on bone histology and histomorphometry: the ACTIVE phase 3 trial. Bone 2017; 97: 314–319. [DOI] [PubMed] [Google Scholar]
- 109. Guo J, Khatri A, Maeda A, Potts JT Jr, Juppner H, Gardella TJ. Prolonged pharmacokinetic and pharmacodynamic actions of a pegylated parathyroid hormone (1‐34) peptide fragment. J Bone Miner Res 2017; 32: 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bae CH, Kang M, Park CY, Park BM, Zhang D, Nam HJ, et al A novel human PTH analog [Cys25]hPTH(1–34) restores bone mass in ovariectomized mice. J Clin Endocrinol Metab 2016; 101: 3700–3708. [DOI] [PubMed] [Google Scholar]
- 111. Maeda A, Okazaki M, Baron DM, Dean T, Khatri A, Mahon M, et al Critical role of parathyroid hormone (PTH) receptor‐1 phosphorylation in regulating acute responses to PTH. Proc Natl Acad Sci 2013; 110: 5864–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hämmerle SP, Mindeholm L, Launonen A, Kiese B, Loeffler R, Harfst E, et al The single dose pharmacokinetic profile of a novel oral human parathyroid hormone formulation in healthy postmenopausal women. Bone 2012; 50: 965–973. [DOI] [PubMed] [Google Scholar]
- 113. Henriksen K, Andersen JR, Riis BJ, Mehta N, Tavakkol R, Alexandersen P, et al Evaluation of the efficacy, safety and pharmacokinetic profile of oral recombinant human parathyroid hormone [rhPTH(1–31)NH2] in postmenopausal women with osteoporosis. Bone 2013; 53: 160–166. [DOI] [PubMed] [Google Scholar]
- 114. Tamura T, Noda H, Joyashiki E, Hoshino M, Watanabe T, Kinosaki M, et al Identification of an orally active small‐molecule PTHR1 agonist for the treatment of hypoparathyroidism. Nat Commun 2016; 7: 13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cosman F, Lane NE, Bolognese MA, Zanchetta JR, Garcia‐Hernandez PA, Sees K, et al Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J Clin Endocrinol Metab 2010; 95: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jamal Saeh DP, Hamad E, Moseman J, Wirtanen D, Brown K, Dick L, et al Clinical development of an optimized abaloparatide transdermal patch. J Bone Miner Res 2016; 31 (Suppl 1). Available at http://www.asbmr.org/education/AbstractDetail?aid=435753d1-2dcf-47f5-afb6-ac0b156d1ec8 (last accessed 16 November 2017). [Google Scholar]
- 117. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44 (D1): D1054–D1D68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al The concise guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 2015; 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]


