Abstract
Aim
Inhibitors of nerve growth factor (NGF) reduce pain in several chronic pain indications. NGF signals through tyrosine kinase receptors of the tropomyosin‐related kinase (Trk) family and the unrelated p75 receptor. PF‐06273340 is a small molecule inhibitor of Trks A, B and C that reduces pain in nonclinical models, and the present study aimed to investigate the pharmacodynamics of this first‐in‐class molecule in humans.
Methods
A randomized, double‐blind, single‐dose, placebo‐ and active‐controlled five‐period crossover study was conducted in healthy human subjects (NCT02260947). Subjects received five treatments: PF‐06273340 50 mg, PF‐06273340 400 mg, pregabalin 300 mg, ibuprofen 600 mg and placebo. The five primary endpoints were the pain detection threshold for the thermal pain tests and the pain tolerance threshold for the cold pressor, electrical stair and pressure pain tests. The trial had predefined decision rules based on 95% confidence that the PF‐06273340 effect was better than that of placebo.
Results
Twenty subjects entered the study, with 18 completing all five periods. The high dose of PF‐06273340 met the decision rules on the ultraviolet (UV) B skin thermal pain endpoint [least squares (LS) mean vs. placebo: 1.13, 95% confidence interval: 0.64–1.61], but not on the other four primary endpoints. The low dose did not meet the decision criteria for any of the five primary endpoints. Pregabalin (cold pressor and electrical stair tests) and ibuprofen (UVB thermal pain) showed significant analgesic effects on expected endpoints.
Conclusions
The study demonstrated, for the first time, the translation of nonclinical effects into man in an inflammatory pain analgesic pharmacodynamic endpoint using a pan‐Trk inhibitor.
Keywords: pain, pharmacodynamics, phase I (drug development)
What is Already Known about this Subject
Inhibitors of nerve growth factor (NGF) have been shown to reduce pain in several chronic pain indications.
Inhibitors of Trks A, B and C demonstrate pain reductions in a preclinical setting.
What this Study Adds
This was the first demonstration of analgesic efficacy in humans using a pan‐Trk inhibitor.
The study confirmed the usefulness of the human evoked pain models to profile novel pain therapeutics in early clinical development.
Introduction
Nerve growth factor (NGF) is a key mediator of chronic pain. Administration of NGF to animals or to human subjects causes pain 1, and studies with anti‐NGF scavenging monoclonal antibodies such as tanezumab have demonstrated efficacy in phase III trials in several chronic pain indications 2, 3. NGF is a member of the neurotrophin family that signals through both tyrosine kinase receptors of the tropomyosin‐related kinase (Trk) family and the unrelated p75 receptor. The neurotrophins comprise NGF, which signals preferentially through TrkA, brain‐derived neurotrophic factor (BDNF) and neurotrophin 4 (NT‐4), which signal through TrkB, and neurotrophin 3 (NT‐3), which signals through TrkC. The neurotrophins are equipotent at the p75 receptor. NGF signalling through TrkA is known to induce both acute and chronic regulation of pain signalling, through phosphorylation‐dependent regulation of ion channels involved in pain transmission and upregulation of pain‐related genes, respectively 4. BDNF was also found to be implicated in nonclinical pain signalling and was found to be upregulated in clinical pain states in human subjects 5.
PF‐06273340 is a peripherally restricted small molecule inhibitor of Trks A, B and C, the structural formula of which has been published previously 6. It is equipotent at the three Trk receptors but is otherwise broadly selective. PF‐06273340 and other molecules in this class reverse chronic pain in nonclinical models where there has been some sensitization, such as ultraviolet (UV) B sensitization to the skin 6 or carrageenan irritation of the joint 7. To date, there are no data on whether these analgesic effects of small molecule pan‐Trk inhibitors translate to human subjects.
In the current study, the analgesic effects of PF‐06273340 were assessed using a battery of human evoked pain models. These models have been shown to provide robust evidence of analgesia in healthy human subjects using a number of positive controls assessed against different pain stimuli and endpoints 8, 9. By assessing PF‐06273340 using this methodology, we intended to establish whether the nonclinical data demonstrating efficacy in the UVB sensitization model would translate to human subjects. The inclusion of other pain models in the study provided a comparison with nonsensitized pain states. Furthermore, this would be the first demonstration of analgesic efficacy for this novel class of compounds in a small, easy‐to‐recruit trial prior to investing in larger patient studies.
Methods
Subjects and study design
The study was a double‐blind, double‐dummy, single‐dose, randomized, placebo‐controlled, five‐period crossover study (NCT02260947). PF‐06273340 was the drug under investigation, and ibuprofen and pregabalin were used as positive controls. The study was conducted at a single site at the Centre for Human Drug Research in Leiden, the Netherlands. It was approved by the Medical Ethics Committee of Stichting Beoordeling Ethiek Biomedisch Onderzoek (Assen, the Netherlands), and was conducted in accordance with the Dutch Act on Medical Research Involving Human Subjects (WMO) and in compliance with all International Conference on Harmonization Good Clinical Practice (ICH‐GCP) guidelines and the Declaration of Helsinki. Each subject provided written informed consent before any screening procedures were performed.
Approximately 20 healthy male subjects, between 18 and 55 years of age, were to attend the clinic on seven separate occasions (screening, periods 1–5 and at follow‐up) to examine the effects of PF‐06273340 on evoked pain endpoints. Periods 1–5 were spaced apart by at least 7 days, which, based on the half‐lives of the single doses of treatments, would give sufficient time to allow for the washout of pharmacokinetic (PK) and potential pharmacodynamic (PD) effects.
Study drugs
The study included placebo, ibuprofen and pregabalin (positive controls) and two dose levels of PF‐06273340. Subjects were randomized to one of 10 sequences that consisted of two Williams 5 × 5 designs that were balanced for first‐order carry‐over. At each investigational period, subjects received a total of four tablets and one capsule: two tablets of 200 mg PF‐06273340/placebo, one tablet of 50 mg PF‐06273340/placebo, one tablet of 600 mg ibuprofen/placebo and one capsule of 300 mg pregabalin/placebo. The doses of PF‐06273340 selected were 50 mg and 400 mg, given as single doses. These doses were justified based on safety margins elucidated in toxicology studies and on clinical toleration and safety data from phase I single and multiple ascending dose studies (https://clinicaltrials.gov/ct2/results?term=PF‐06273340) in healthy young and elderly subjects. The top dose of 400 mg PF‐06273340 was close to the maximum dose given previously to human subjects, whereas the lower dose of 50 mg PF‐06372865 allowed examination of the bottom end of the predicted pharmacologically active range.
Doses of 600 mg ibuprofen and 300 mg pregabalin had been used as positive controls in previous human evoked pain model studies 8, 9. These doses had been found to be well tolerated and within the labelled dose range for ibuprofen and pregabalin in the European Union (EU).
PK assessments
During all study periods, blood samples (3 ml) to provide a minimum of 1.5 ml plasma for PK analysis of PF‐06273340, pregabalin and ibuprofen were collected at predose and at 0.5, 1, 2, 3, 4, 5, 6, 8 and 10 h after study drug administration. Plasma samples were analysed for PF‐06273340, pregabalin and ibuprofen concentrations at WuXi AppTec (Shanghai, P.R. China) using validated analytical assays. Plasma PF‐06273340 PK parameters [maximum observed plasma concentration (Cmax), area under the plasma concentration–time curve from time 0 to the time of last quantifiable concentration (AUClast) and time to reach Cmax (Tmax)] were calculated for each subject using noncompartmental analysis of plasma concentration–time data. Plasma ibuprofen and plasma pregabalin concentrations were listed and summarized descriptively (results not shown).
PD assessments
Pain detection and tolerance thresholds were measured using a battery of human pain models that assess a range of modalities of pain using previously described methodology 8, 9. Briefly, the pain models included thermode, electrical stimulation, mechanical pain and cold pressor, which were all performed sequentially at predose (twice) and at 0.5, 1, 2, 3, 4, 6, 8 and 10 h after study drug administration in each period. Thermal (heat) pain was determined on normal skin and on UVB‐exposed skin. Pain intensity was measured continuously during each test using an electronic visual analogue scale (eVAS) ranging from 0 (no pain) to 100 (most intense pain tolerable). The pain detection threshold (PDT) and pain tolerance threshold (PTT) were of primary interest (see Supplementary data for more details).
Ibuprofen and pregabalin were included as active controls, based on previous studies 8, 9; ibuprofen had shown effects on the UVB heat PDT endpoint and pregabalin had shown effects on the cold pressor PTT, pressure pain PTT, electrical stair PTT (pre‐cold pressor) and normal heat PDT endpoints.
Statistical analysis
A mixed‐effects model was fitted to each endpoint, using data collected during the first 6 h post‐treatment. Absolute values were analysed for PDT endpoints and loge transformed values for PTT endpoints as the latter had been shown to have skewed distributions in previous studies 8, 9. The fixed effects included in the model were baseline, period, time, treatment and treatment‐by‐time interaction, with baseline as covariate. Subject was fitted as a random effect. Baseline was included as two separate variables – the average baseline for the subject, and the deviation of each period baseline from the average baseline for each subject 10. The Kenward–Roger approximation was used for estimating degrees of freedom for the model parameters. The primary analysis included all subjects randomized into the study.
The least squares (LS) means, together with 90% confidence intervals (CIs), were obtained for each treatment, averaged across time points that covered the peak exposure for each treatment. Based on the known human PK, the average across the first 4 h was obtained from the mixed‐effects model for PF‐06273340 and ibuprofen, whereas the average across the first 6 h was obtained for pregabalin. The averages over both the first 4 h and 6 h were obtained for placebo. Differences between treatments and placebo were therefore made using the appropriate average (i.e. ibuprofen was compared with the placebo 4 h average, whereas pregabalin was compared with the placebo 6 h average). Differences to placebo are presented as absolute differences for PDT endpoints and ratios for PTT endpoints, together with corresponding 90% CIs.
As a sensitivity analysis to the primary analysis, a mixed‐effects model was fitted for the maximum (over 4 h or 6 h post‐treatment, where appropriate) change from baseline for each primary endpoint. The fixed effects included in the model were baseline, period and treatment. Baseline was similarly included as two separate variables. Subject was fitted as a random effect. Additional sensitivity analyses were conducted that applied the primary analysis models to only subjects who completed all five treatment periods or applied the models to all subjects but included all time points (i.e. up to and including the 10 h measurement).
Sample size
Decision rules were prespecified to quantify what was required in the primary objective of the study. The criteria were based on a Bayesian interpretation of the results, assuming a non‐informative prior. The criterion used for each endpoint was having at least 95% confidence that the effect of either dose of PF‐06273340 was greater than that of placebo. This is equivalent to a one‐sided test for statistical significance using an alpha of 0.05. No adjustment was made for multiplicity as this was an early‐phase clinical study designed to explore the PD of PF‐06273340 and, as such, no stringent requirement to control the type 1 error rate was required for internal decision making.
The sample size was based on the mean effect over the first 4 h after dosing (i.e. average of 0.5, 1, 2, 3 and 4 h time points) for the five primary endpoints: cold pressor PTT; pressure pain PTT; electrical stair PTT; normal heat PDT; and UVB heat PDT. The primary comparison was of either dose of PF‐06273340 against placebo. A conservative estimate of within‐subject standard deviation was derived from two previous methodology studies 8, 9, yielding estimates of 0.25, 0.21, 0.16, 1.79 and 1.63 for the cold pressor PTT, pressure pain PTT, electrical stair PTT, normal heat PDT and UVB heat PDT endpoints, respectively. A sample size of 20 subjects was selected to ensure balance in the design, and gave at least 80% power to detect differences of 0.20, 0.17, 0.13, 1.42 and 1.30 for the five primary endpoints listed previously.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 11, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 12.
Results
Subject disposition
Of the 20 subjects who were randomized, 18 completed the study (Figure 1). Two subjects discontinued from the study: one owing to failure to meet inclusion/exclusion criteria (electrocardiogram abnormalities), who did not proceed to period 5 (missed the PF‐06273340 50 mg dose); and the other subject owing to no longer being willing to participate in the study, having received only treatments assigned to periods 1 and 3 (only treated with PF‐06273340 50 mg and ibuprofen 600 mg, respectively). All 20 subjects were male, with a mean age (standard deviation) of 26.0 (6.9) years and body mass index of 23.7 (2.5) kg m–2 (Table 1). Fifteen subjects reported ethnicity as ‘white’, with the five remaining as ‘other’. All 20 subjects were dosed with ibuprofen 600 mg, and 19 subjects were dosed with placebo, PF‐06273340 50 mg, PF‐06273340 400 mg or pregabalin 300 mg.
Figure 1.
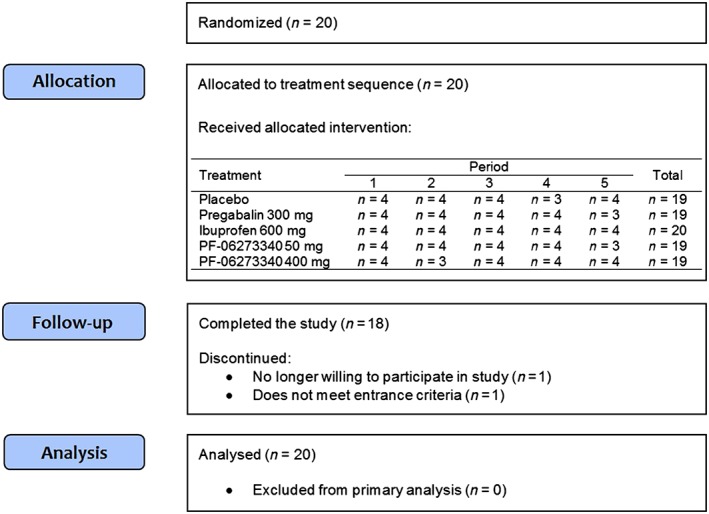
Disposition of subjects
Table 1.
Summary of demographic and baseline characteristics
| All subjects | |
|---|---|
| Number of subjects | 20 |
| Gender: | |
| Male | 20 |
| Female | 0 |
| Age (years): | |
| <25 | 11 |
| 25–44 | 9 |
| >45 | 0 |
| Mean (SD) | 26.0 (6.9) |
| Range | 18–43 |
| Race: | |
| White | 15 |
| Other | 5 |
| Weight (kg): | |
| Mean (SD) | 77.1 (8.1) |
| Range | 62.7–95.4 |
| Body mass index (kg m–2): | |
| Mean (SD) | 23.7 (2.5) |
| Range | 18.2–29.7 |
| Height (cm): | |
| Mean (SD) | 180.4 (5.5) |
| Range | 169.8–190.2 |
SD, standard deviation
PD
A summary of the results for the primary analyses are presented in Table 2 and Figure 2. PF‐06273340 400 mg met the decision criteria for the UVB heat PDT endpoint, as shown by a statistically significant increase over placebo of 1.13 units (90% CI 0.64, 1.61). There were no statistically significant effects of PF‐06273340 50 mg relative to placebo on any of the five primary endpoints. Ibuprofen showed a statistically significant effect on the UVB heat PDT endpoint compared with placebo, with an increase of 1.39 units (90% CI 0.91, 1.87). Pregabalin 300 mg had statistically significant effects over placebo on the cold pressor PTT (effect vs. placebo = 1.22, 90% CI 1.11, 1.34) and the electrical stair PTT (effect vs. placebo = 1.09, 90% CI 1.01, 1.19). The time course profiles of the five treatments across the five primary endpoints are presented in Figure 3. Sensitivity analyses gave similar results (data not shown).
Table 2.
Summary of results for the primary analyses
| Endpoint | Placebo (n = 19; 0–4 h) Mean (SD)/GM (%CV) | Placebo (n = 19; 0–6 h) Mean (SD)/GM (%CV) | Pregabalin 300 mg (n = 19; 0–6 h) | Ibuprofen 600 mg (n = 20; 0–4 h) | PF‐06273340 50 mg (n = 19; 0–4 h) | PF‐06273340 400 mg (n = 19; 0–4 h) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD)/GM (%CV) | LS mean effect vs. placebo (90% CI) | Mean (SD)/GM (%CV) | LS mean effect vs. placebo (90% CI) | Mean (SD)/GM (%CV) | LS mean effect vs. placebo (90% CI) | Mean (SD)/ GM (%CV) | LS mean effect vs. placebo (90% CI) | |||
| Cold pressor PTT | 28.57 (72%) | 28.33 (72%) | 34.07 (72%) | 1.22 (1.11, 1.34) | 28.58 (71%) | 1.05 (0.95, 1.15) | 28.60 (76%) | 0.98 (0.89, 1.08) | 27.69 (76%) | 1.02 (0.93, 1.12) |
| Electrical stair PTT | 23.97 (68%) | 24.20 (68%) | 25.47 (67%) | 1.09 (1.01, 1.19) | 25.96 (67%) | 1.03 (0.95, 1.11) | 24.30 (69%) | 0.97 (0.90, 1.05) | 23.10 (73%) | 0.94 (0.87, 1.02) |
| Pressure pain PTT | 51.99 (67%) | 51.98 (67%) | 58.54 (64%) | 1.07 (0.99, 1.15) | 57.51 (66%) | 1.06 (0.99, 1.14) | 53.02 (67%) | 1.01 (0.93, 1.08) | 55.93 (66%) | 1.05 (0.98, 1.13) |
| Normal heat PDT | 46.73 (2.29) | 46.79 (2.23) | 47.46 (2.09) | 0.35 (−0.11, 0.81) | 46.27 (3.42) | −0.13 (−0.57, 0.31) | 46.68 (2.99) | 0.19 (−0.26, 0.64) | 46.87 (2.59) | 0.16 (−0.29, 0.60) |
| UVB heat PDT | 40.59 (2.49) | 40.59 (2.51) | 41.37 (2.41) | 0.47 (−0.02, 0.95) | 42.12 (2.98) | 1.39 (0.91, 1.87) | 41.21 (2.71) | 0.27 (−0.22, 0.76) | 41.52 (2.60) | 1.13 (0.64, 1.61) |
PTT endpoints were analysed on the log scale, so results are presented as geometric means with %CVs for individual treatment results, and back‐transformed LS mean ratios and 95% CIs for treatment comparisons. CI, confidence interval; CV, coefficient of variation; GM, geometric mean; LS mean, least squares mean; PDT, pain detection threshold; PTT, pain tolerance threshold; SD, standard deviation; UVB, ultraviolet B
Bold text indicates that the effect over placebo met the predefined decision criterion
Figure 2.
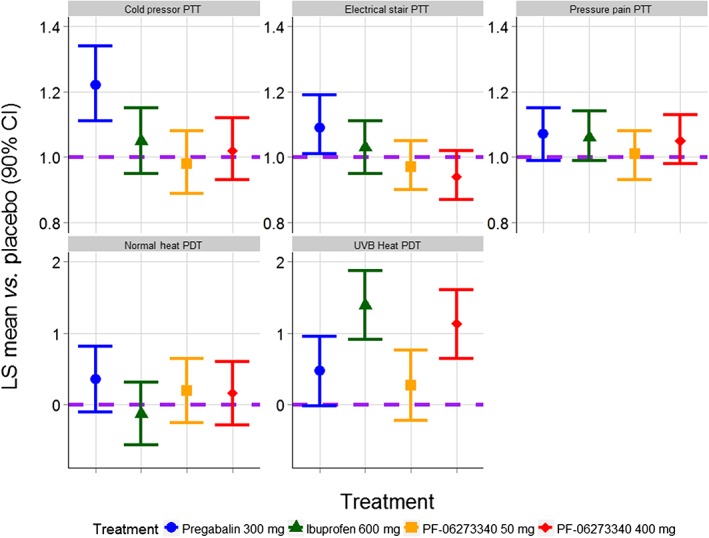
Primary analysis results. The comparisons of PF‐06273340 vs. placebo, and ibuprofen vs. placebo were made with LS means averaged over 4 h. The comparison of pregabalin vs. placebo was made with LS means averaged over 6 h. The purple horizontal dashed line represents no effect over placebo. PTT endpoints are presented on the fold‐change to placebo scale, whereas PDT endpoints are presented on the absolute difference to placebo scale. CI, confidence interval; LS mean, least squares mean; PDT, pain detection threshold; PTT, pain tolerance threshold; UVB, ultraviolet B
Figure 3.
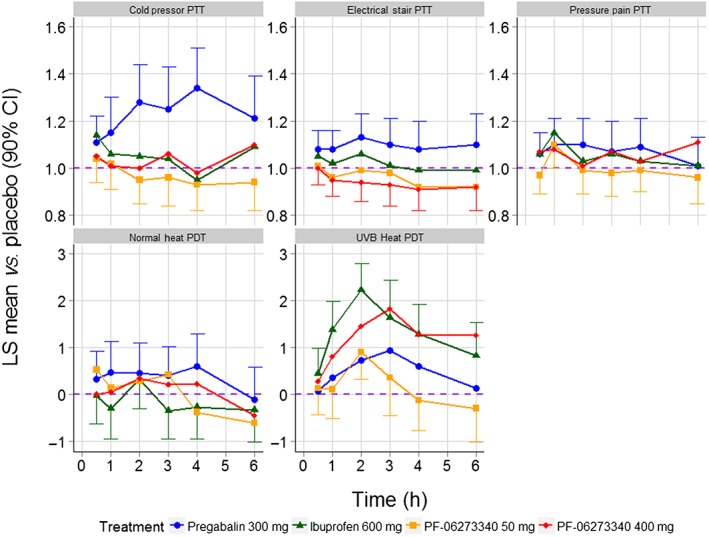
Time course of treatment effects across the five primary endpoints. The purple horizontal dashed line represents no effect over placebo. PTT endpoints are presented on the fold‐change to placebo scale, whereas PDT endpoints are presented on the absolute difference to placebo scale. CI, confidence interval; LS mean, least squares mean; PDT, pain detection threshold; PTT, pain tolerance threshold; UVB, ultraviolet B
Safety
Single doses of PF‐06273340 400 mg or PF‐06273340 50 mg administered to healthy male subjects were generally safe and well tolerated in the present study. There were no serious adverse events or other clinically significant adverse events reported.
The most frequently reported all causality treatment‐related adverse events were dizziness (16 subjects: four subjects in the PF‐06273340 400 mg group, two subjects each in the PF‐06273340 50 mg and placebo groups, and eight subjects in the pregabalin group), somnolence (13 subjects: two subjects each in the PF‐06273340 50 mg and ibuprofen groups, one subjects each in the PF‐06273340 400 mg and placebo groups, and seven subjects in the pregabalin group) and fatigue (11 subjects: two subjects each in the PF‐06273340 400 mg and pregabalin groups, three subjects each in the PF‐06273340 50 mg and ibuprofen groups, and one subject in the placebo group). All treatment‐related adverse events were mild in severity, except in one subject in the PF‐06273340 50 mg treatment group, who had upper abdominal pain which was moderate in severity and considered by the investigator to be treatment related.
PK
The median plasma PF‐06273340 concentration–time profile is presented in Figure 4 and PK parameters are summarized descriptively in Table 3.
Figure 4.
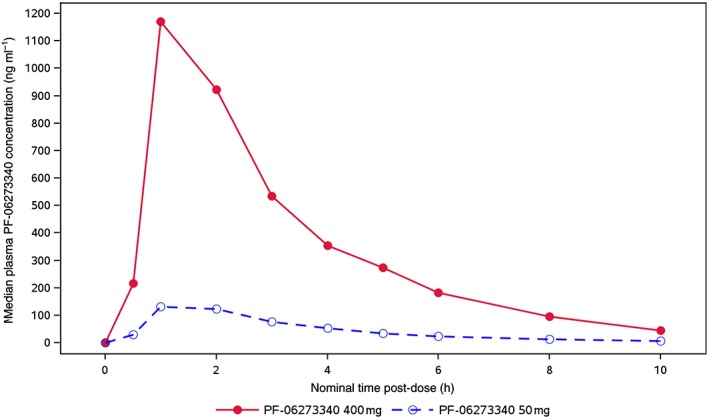
Median plasma PF‐06273340 concentration–time profiles
Table 3.
Descriptive summary of plasma PF‐06273340 pharmacokinetic parameter values
| Parameter (units) | Parameter summary statistics a by PF‐06273340 treatment | |
|---|---|---|
| 50 mg | 400 mg | |
| N | 19 | 19 |
| AUC last (ng•h ml –1 ) | 483.5 (42) | 3630 (43) |
| C max (ng ml –1 ) | 150.4 (57) | 1396 (56) |
| T max (h) | 1.08 (1.00–3.00) | 1.00 (0.500–2.00) |
AUClast, area under the plasma concentration–time curve from time 0 to the time of last quantifiable concentration; Cmax, maximum observed plasma concentration; %CV, percentage coefficient of variation; Tmax, time to reach Cmax
Geometric mean (geometric %CV) for all except: median (range) for Tmax.
PF‐06273340 was rapidly absorbed following oral administration, with a mean Tmax of 1 h for both treatments. Cmax and AUClast appeared to increase proportionally, with doses from 50 mg to 400 mg, and between‐subject variability in plasma PF‐06273340 exposure based on the geometric percentage coefficient of variation for Cmax and AUClast ranged from 42% to 57%.
Discussion
This was the first study to test a novel candidate analgesic agent targeting the NGF pathway using a panel of human evoked pain models. Previous studies had demonstrated that these models provided a reproducible method to assess the effects of analgesic drugs on a battery of evoked pain assessments in healthy human subjects, with consistent results obtained from the ibuprofen and pregabalin positive controls 8, 9. The current study confirmed previous results showing a significant effect of ibuprofen on the UVB heat PDT assessment, and of pregabalin on the cold pressor test. Pregabalin also demonstrated a modest effect in the electrical stair PTT which achieved statistical significance; this agent had demonstrated an effect at this endpoint in some but not all previous studies 8, 9. Overall, the results for the positive controls (ibuprofen and pregabalin) confirmed the validity of this methodology for detecting reproducible analgesic signals in healthy human subjects.
The 400 mg dose of pan‐Trk inhibitor PF‐06273340 significantly reduced the hyperalgesia seen in the UVB heat PDT assessment but did not have an effect on any other endpoint. This is similar to the pattern seen with ibuprofen and is in agreement with the expected biology of the mechanism of action of this molecule. NGF is upregulated in experimental models of inflammation, including UVB sensitization 13, 14, and anti‐NGF monoclonal antibodies and Trk inhibitors (including PF‐06273340) have shown efficacy in nonclinical models of inflammatory pain, e.g. involving the use of complete Freund's adjuvant, carrageenan and UVB radiation 6, 15. NGF has direct and indirect actions in inflammatory pain (reviewed by Mantyh et al. 15). Administration of NGF leads to binding at TrkA on immune cells (including mast cells) and the subsequent release of inflammatory mediators which contribute to the sensitization of nociceptors. In addition, NGF binding to TrkA on sensory nerve fibres elicits signalling cascades which result in the trafficking of nociceptors to the cell surface and their sensitization by phosphorylation. One of the receptors that contributes to increased signalling in this manner is the heat‐sensitive ion channel transient receptor potential cation channel subfamily V member 1 (TRPV1), which is likely to be an important component of the UV‐induced hyperalgesia in man 16; inhibition of TRPV1 signalling may be one component of PF‐06273340 efficacy in this model. The indirect effects of NGF involve the retrograde transport of NGF–TrkA complexes to the nucleus, where the transcription of nociceptors and peptides involved in pain signalling are upregulated. In vitro data have shown that this transport is inhibited by PF‐06273340 (Bilsland, personal communication), and this may also contribute to the effect on UVB seen in the present study, although the longer timescale of this process implies that it may be more important in chronic pain states.
Given the proven efficacy of anti‐NGF monoclonal antibodies in nonclinical species and in human clinical studies, it is tempting to ascribe the efficacy of PF‐06273340 to a blockade of TrkA signalling. However, a role for BDNF (which signals through TrkB) cannot be discounted as it has been shown to play a role in hyperalgesia and pain in some nonclinical systems, and has been implicated in some human biology studies in visceral and neuropathic pain states 5. The effects of NT‐3 signalling through TrkC, and NT‐4 signalling through TrkB are not as well defined 17 but published data do not suggest a major role in nociception. One conclusion that can be drawn from the effects of PF‐06273340 is that blockade of signalling through p75 (which is spared by PF‐06273340 but not by anti‐NGF monoclonal antibodies) is not required for an analgesic effect in man.
The effect size of the 400 mg dose of PF‐06273340 vs. placebo on the UVB endpoint was similar to that of ibuprofen; however, the lower (50 mg) dose of PF‐06273340 did not have a significant effect. The median exposure of PF‐06273340 at the top dose achieved was ~ 30 × IC50 at Cmax and dropped to ~9 × IC50 at the end of the assessment period (4 h), whereas the lower dose achieved was ~ 4 × IC50 at Cmax and dropped to ~1 × IC50 at 4 h. The differential efficacy of these two doses implies that, at least for this endpoint, an exposure that achieves a multiple of IC50 throughout the assessment period is required for an acute PD effect in inflammatory pain. This conclusion is consistent with the prediction from a systems pharmacology model of the NGF pathway 18 utilizing PF‐06273340 data. Further studies are needed to determine how these PD effects in a healthy volunteer study relate to the exposures needed in patients with a chronic pain condition.
The current study demonstrated, for the first time, that a pan‐Trk molecule can reduce hyperalgesia in human subjects. The observed effect in the presensitized UVB assessment is consistent with the observed efficacy of anti‐NGF monoclonal antibodies in chronic pain states with an inflammatory component, such as osteoarthritis (OA). We did not see a significant impact on the cold pressor or electrical stair tests, where pregabalin was shown to be effective. This may indicate that the pan‐Trk mechanism will be less effective in neuropathic pain states where pregabalin has proven efficacy; however, there is uncertainty regarding the translation of studies in healthy human subjects to those with chronic pain conditions, and it should be noted that the anti‐NGF monoclonal antibodies previously showed efficacy in neuropathic pain 19, albeit at higher doses and exposures than in the inflammatory pain state of OA. Another uncertainty is how to interpret the effect size seen with the top dose of PF‐06273340, which was similar to that observed with ibuprofen. We regard the primary role of the human evoked pain models as being an early demonstration of PD for novel molecules, and to provide some guidance as to which pain states might be selected for future clinical studies. Optimism that pan‐Trk inhibitors may be more efficacious that nonsteroidal anti‐inflammatory drugs such as ibuprofen comes from the data with tanezumab showing superior efficacy to naproxen in OA 2. Given that the methodology in the present study was limited by the use of single doses in a healthy subject population, the translation of effect sizes to long‐term dosing in chronic pain states is uncertain, and will need to take account of physiological responses such as changes to nocioceptors and signalling pathways brought about by chronic stimulation 20.
A concern of the anti‐NGF monoclonal antibodies is the increased risk of rapidly progressing OA (RPOA), which for tanezumab monotherapy ranges from 0 events per 1000 patient‐years at a dose of 2.5 mg, to 11 events per 1000 patient‐years at a dose of 10 mg 21. Small molecule pan‐Trk inhibitors have the potential advantages of greater flexibility in dosing, and that once dosing is stopped the drug will be rapidly eliminated compared with the much slower clearance of a humanized monoclonal antibody. Whether the risk of RPOA is reduced by small molecule pan‐Trk inhibitors is unknown. Given the low frequency of RPOA in subjects who received anti‐NGF monoclonal antibodies, an assessment of this risk must await larger clinical trials, unless a predictive nonclinical model of RPOA becomes available. To date, we have seen no safety concerns for joint damage in our phase I programme.
In summary, the current study confirms the usefulness of the human evoked pain models to profile novel pain therapeutics in early clinical development. The pan‐Trk inhibitor PF‐06273340 demonstrated a significant effect in the UVB heat PDT assessment, providing good evidence of a translation of nonclinical effects into man. This human pain model is easy to execute, as a result of the small sample size of healthy subjects needed, and we believe that it provides a powerful method for demonstrating an analgesic effect for novel pain medications.
Competing Interests
The trial was sponsored by Pfizer. P.L., D.G., K.G., P.D. and R.B. are or were employees of Pfizer at the time of this research and may own stock in the company. P.S., G.A., J.H. and G.J.G. are or were employees of CHDR at the time of this research and report no conflicts of interest. There are no other known conflicts of interest to declare.
The authors thank the subjects who participated in this trial.
Supporting information
Data S1 Supplementary Information
Loudon, P. , Siebenga, P. , Gorman, D. , Gore, K. , Dua, P. , van Amerongen, G. , Hay, J. L. , Groeneveld, G. J. , and Butt, R. P. (2018) Demonstration of an anti‐hyperalgesic effect of a novel pan‐Trk inhibitor PF‐06273340 in a battery of human evoked pain models. Br J Clin Pharmacol, 84: 301–309. doi: 10.1111/bcp.13448.
References
- 1. Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. NGF induces non‐inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain 2010; 148: 407–413. [DOI] [PubMed] [Google Scholar]
- 2. Ekman EF, Gimbel JS, Bello AE, Smith MD, Keller DS, Annis KM, et al Efficacy and safety of intravenous tanezumab for the symptomatic treatment of osteoarthritis: 2 randomized controlled trials versus naproxen. J Rheumatol 2014; 41: 2249–2259. [DOI] [PubMed] [Google Scholar]
- 3. Gimbel JS, Kivitz AJ, Bramson C, Nemeth MA, Keller DS, Brown MT, et al Long‐term safety and effectiveness of tanezumab as treatment for chronic low back pain. Pain 2014; 155: 1793–1801. [DOI] [PubMed] [Google Scholar]
- 4. Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 2006; 29: 507–538. [DOI] [PubMed] [Google Scholar]
- 5. Nijs J, Meeus M, Versijpt J, Moens M, Bos I, Knaepen K, et al Brain‐derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: a new therapeutic target? Expert Opin Ther Targets 2015; 19: 565–576. [DOI] [PubMed] [Google Scholar]
- 6. Skerrat S, Andrews MD, Bagal SK, Bilsland J, Brown D, Bungay PJ, et al The discovery of a potent, selective and peripherally restricted pan‐Trk inhibitor (PF‐06273340) for the treatment of pain. J Med Chem 2016; 59: 10084–10099. [DOI] [PubMed] [Google Scholar]
- 7. Ashraf S, Bouhana KS, Pheneger J, Andrews SW, Walsh DA. Selective inhibition of tropomyosin‐receptor‐kinase A (TrkA) reduces pain and joint damage in two rat models of inflammatory arthritis. Arthritis Res Ther 2016; 18: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hay JL, Okkerse P, van Amerongen G, de Kam ML, Groeneveld GJ. The use of a battery of pain models to detect analgesic properties of compounds: a two‐part, four‐way, randomised, placebo‐controlled, crossover study. Clin Ther 2015; 37: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hay JL, Okkerse P, van Amerongen G, Groeneveld GJ. Determining pain detection and tolerance thresholds using an integrated, multi‐modal pain task battery. J Vis Exp 2016; 110: e53800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kenward MG, Roger JH. The use of baseline covariates in crossover studies. Biostatistics 2010; 11: 1–17. [DOI] [PubMed] [Google Scholar]
- 11. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 2015; 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saadé NE, Nasr IW, Massaad CA, Safieh‐Garabedian B, Jabbur SJ, Kanaan SA. Modulation of ultraviolet‐induced hyperalgesia and cytokine upregulation by interleukins 10 and 13. Br J Pharmacol 2000; 131: 1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weinkauf B, Rukwied R, Quiding H, Dahllund L, Johansson P, Schmelz M. Local gene expression changes after UV‐irradiation of human skin. PloS One 2012; 7: e39411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of nerve growth factor‐TrkA signaling and the relief of pain. Anesthesiology 2011; 115: 189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002; 36: 57–68. [DOI] [PubMed] [Google Scholar]
- 17. Khan N, Smith MT. Neurotrophins and neuropathic pain: role in pathobiology. Molecules 2015; 20: 10657–10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benson N, Matsuura T, Smirnov S, Demin O, Jones HM, Dua P, et al Systems pharmacology of the nerve growth factor pathway: use of a systems biology model for the identification of key drug targets using sensitivity analysis and the integration of physiology and pharmacology. Interface Focus 2013; 3: 20120071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bramson C, Herrmann DN, Carey W, Keller D, Brown MT, West CR, et al Exploring the role of tanezumab as a novel treatment for the relief of neuropathic pain. Pain Med 2015; 16: 1163–1176. [DOI] [PubMed] [Google Scholar]
- 20. Turk DC, Wilson HD, Cahana A. Treatment of chronic non‐cancer pain. Lancet 2011; 377: 2226–2235. [DOI] [PubMed] [Google Scholar]
- 21. Hochberg MC, Tive LA, Abramson SB, Vignon E, Verburg KM, West CR, et al When is osteonecrosis not osteonecrosis?: adjudication of reported serious adverse joint events in the tanezumab clinical development program. Arthritis Rheumatol 2016; 68: 382–391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supplementary Information


