Abstract
To assess the impact of CR on survival in multiple myeloma. Retrospective evaluation of response and survival among 758 consecutive patients with multiple myeloma treated at a single center, of whom 395 patients received intensive therapy supported by autologous stem cells within the first year. Survival times were calculated after 1 and 2 years from the start of chemotherapy. On the basis of the response status after a 2-year landmark, the subsequent median survival was 9.7 years for patients with CR, 4.4 years for those with PR and 2.7 years for patients with NR (P < 0.001). Longer survival was attributed in part to intensive therapy that converted the myeloma of 67% of patients with NR to PR or CR, and induced CR in 26% of patients with PR. Intensive therapy did not prolong survival for patients with CR after primary therapy. For patients with multiple myeloma, Cox regression analyses showed that CR was the dominant prognostic factor for long survival, followed by stage I disease, PR and intensive treatment as independent factors. A cure fraction of 2% was identified for nine patients who have remained in CR > 10 years.
Keywords: multiple myeloma, prognostic factors, auto-SCT, complete response
Introduction
In recent years, there have been major advances in the treatment of patients with multiple myeloma, which are attributed primarily to new agents and their combinations, and the increased use of intensive therapy supported by autologous stem cells.1–5 Although longer survival has been the ultimate goal of better treatment, earlier end points have included the frequencies and durations of PR and CR. There is agreement that high-dose treatment induces a higher frequency of CR and prolongs the median survival by approximately 1 year in comparison with that achieved by continuing standard therapies.1,2 Although CR has identified patients who are more likely to live longer, the duration of survival gain and the function of transplantation remain controversial. Whether further intensive therapy benefits patients already in CR is not clear. This report describes a retrospective review of outcomes among a large number of newly diagnosed patients who received chemotherapy alone or chemotherapy followed by intensive therapy within the first year.
Patients and methods
Patients and treatment
Between 1987 and 2007, 758 consecutive newly diagnosed patients received primary chemotherapy with an intermittent, high-dose, dexamethasone-based regimen (for example, dexamethasone alone, VAD, dexamethasone and melphalan or combinations with thalidomide, IFN and so on in accordance with sequential protocols).3–5 Table 1 summarizes the clinical features revealing younger age and more frequent anemia among those who received high-dose, melphalan-based intensive therapy supported by autologous stem cells (HDT). As we focused on the impact of CR on survival, all patients were combined in the analysis of results before HDT. Patients older than 65 years were excluded to assess the outcomes among those with consistent eligibility for intensive treatment supported by autologous stem cells. Only patients with measurable serum myeloma protein of ≥ 0.2 g per 100 ml and/or Bence Jones protein of > 50 mg/day were included so that all showed signs of measurable disease; patients with lower myeloma protein or nonsecretory multiple myeloma were excluded.
Table 1.
Clinical features of 758 patients with untreated myeloma
| Transplanted | Non- transplanted |
P-value | |
|---|---|---|---|
| Number of patients | 395 | 363 | |
| Median values | |||
| Age | 52 (20–65) | 56 (22–65) | < 0.01 |
| Hb (g per 100 ml) | 10.4 | 11.1 | 0.01 |
| B2M (mg/l) | 3.8 | 3.6 | NS |
| Creatinine > 2.0 mg per 100 ml (%) | 18 | 17 | NS |
| ISS stage8 (%) | |||
| I | 40 | 42 | NS |
| II | 27 | 30 | NS |
| III | 32 | 28 | NS |
| Myeloma protein type (%) | |||
| IgG/IgA/IgD | 80 | 78 | NS |
| BJP only | 20 | 22 | NS |
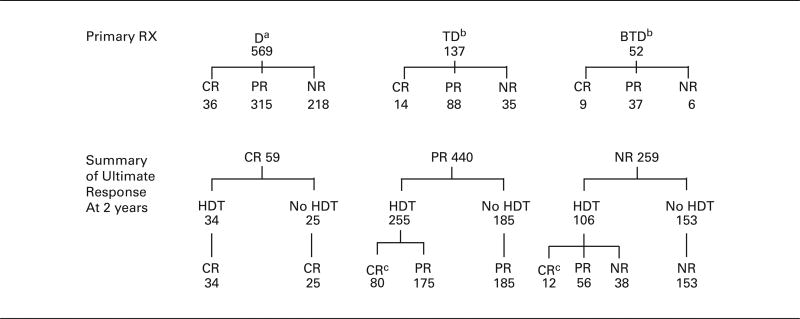
HDT was given within 1 year to 395 patients, representing 52% of the study group. The median time between initial chemotherapy and HDT was 5.5 months (range 2–11.9 months). Patients received one of five intensive programs before auto-SCT as described earlier.6,7 As the clinical features and outcomes were similar for patients who received different HDT or sources of stem cells, all patients were combined in the analysis of transplant-supported therapy. The 363 patients not intensified were limited primarily to those who refused such treatment or those who had socioeconomic factors that prevented such therapy (for example, absence or denial of insurance coverage); six patients were excluded from the analysis because of medical contraindications to intensive treatment. Approximately 15% of patients received later or repeat intensive treatment with autologous or allogeneic support (analyzed in the non-transplant group), but the effect of these programs on long-term outcomes was considered minor, as will be described. Table 2 provides the flow pattern of treatments after primary dexamethasone (D), thalidomide-dexamethasone or bortezomib-thalidomide-dexamethasone revealing serial increases of response rates (62, 74 and 88%), primary CR rates (6, 10 and 17%), frequencies of HDT (43, 79 and 79%) and eventual CR rates (16, 29 and 39%), respectively. As these data commenced from 1987, evaluable, abnormal cytogenetics were available in less than 20% of patients so that a meaningful analysis of this feature was not possible. Written permission for this retrospective review was provided by our Institutional Review Board, in accordance with an assurance approved by the Department of Health and Human Services.
Table 2.
Flow pattern of treatment for 758 previously untreated patients
Dexamethasone alone, or with IFN, melphalan or VAD. A total of 306 patients received later TD or BTD with disease resistance or relapse, including 123 patients who had not received HDT.
TD = thalidomide, dexamethasone. BTD = bortezomib, thalidomide, dexamethasone. All patients received TD or BTD upon relapse, including 40 patients who had not received HDT.
Twenty-two patients had achieved CR after 1 year (19 with earlier PR and 3 with earlier NR).
Clinical response
Response to treatment was rated according to the EBMT criteria.9 Partial response (PR) was defined as reduction of serum myeloma protein by > 50%, of Bence Jones protein by > 90%, and of marrow plasmacytosis to less than 5%; CR required the disappearance of serum myeloma protein by immunofixation on two consecutive studies for at least 2 months. As studies became available, CR for patients with only Bence Jones protein required negative urine immunofixation and normal ratio of serum-κ/λ light chains. The duration of CR was measured from onset to the earliest sign of relapse (for example, the reappearance of myeloma protein on immunofixation).
Statistical methods
All patients were assessed for pretreatment stage, response to primary and intensive therapies, and survival. The distribution of OS times was estimated using the Kaplan–Meier method10 and differences between groups were compared using the log-rank test.11 With primary therapy, PR occurred after a median of 1 month and CR after a median of 3 months; HDT was given after a median of 5.5 months. Consequently, to avoid the potential bias of guaranteed survival, landmark analysis was used to compare survival times for different groups of patients.12 For the landmark analysis at 1 year, patients with at least 12 months of follow-up were included, and survival times after 12 months of treatment were compared among the response groups defined at 12 months. Cox proportional hazard regression models were fitted for multivariate analysis.13 All 83 patients who died and 9 patients who were censored within 12 months were excluded from the survival analyses from a landmark of 1 year; these consisted of 63 patients with NR, 27 patients with PR, and 2 patients with CR; 20 of these patients had received HDT with treatment-related deaths in 11 patients. The median follow-up of currently living patients has been 6.2 years.
Results
Response
The response rate with primary chemotherapy for all 758 patients was 66%, including 8% with CR. Results were similar for patients with or without subsequent HDT. There was no relationship between age, gender, ISS Stage, Hb, serum albumin or protein type and the likelihood of response.
Among 395 patients who received HDT within 12 months, treatment-related deaths occurred in 11 patients (3%). Among 98 patients with primary resistant disease (NR), as defined by status at a landmark of 1 year, CR was achieved in 9 (9%) and PR in 54 (55%); among 245 patients with myeloma in PR, CR was achieved in 25%; all 32 patients with disease in CR remained in CR. The median time to conversion of NR or PR to CR after HDT was 2.4 months (range 0.2–9.5 months), and the median duration of CR was similar regardless of the pathway followed in reaching CR (2.7–4.6 years) (P = 0.31). Thus, HDT improved the response status of 67% of patients with primary resistant myeloma and of 25% of those in PR. After 1 year, there was conversion of PR to CR in 19 patients and of NR to CR in three patients rated as PR or NR for the primary analysis after 1 year, but as CR for the upgraded analysis after 2 years; all of these patients had continued on primary therapy with dose and/or schedule adjustments until they received HDT after a median of 7 months because of delayed insurance approval. There was no relationship between age, gender, Hb or protein type and the likelihood of change in response status.
Survival
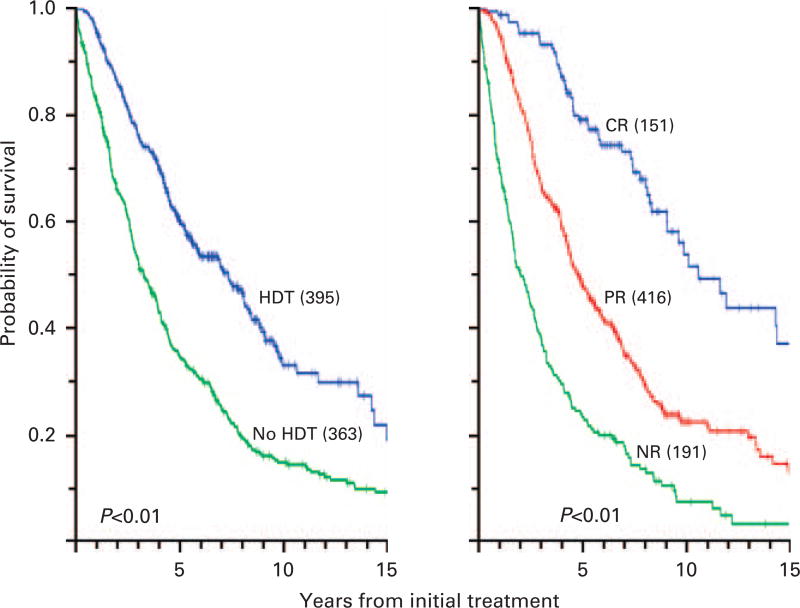
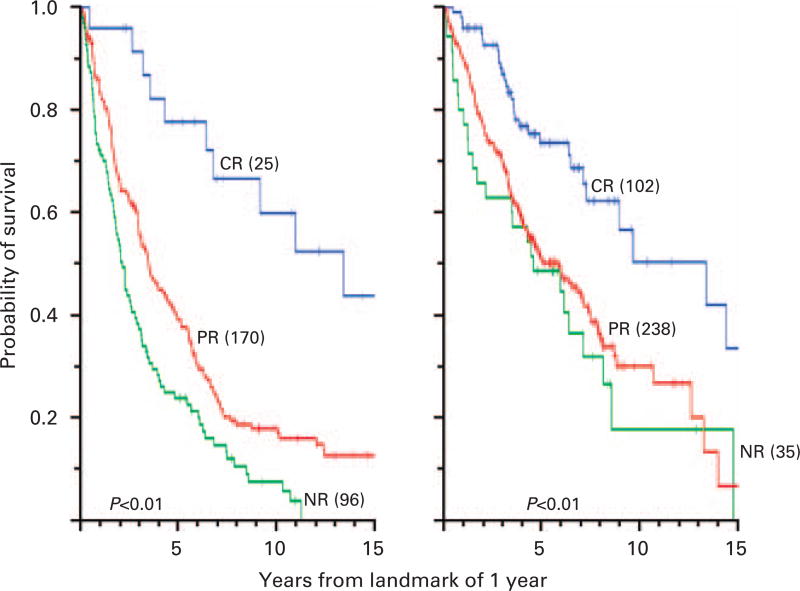
Figure 1 depicts survival analyses from the onset of primary treatment for patients who received chemotherapy alone or chemotherapy followed by HDT, and for groups of patients based on their best response status after 2 years; Figure 2 shows survival analyses at a landmark of 1 year for the same groups, thus excluding 92 patients who died or were censored within the first year; after HDT and based on the landmark of 1 year, there was more frequent CR (27 vs 9%), less frequent NR (9 vs 33%) and longer survival with CR than with PR or NR (< 0.001).
Figure 1.
(Left panel) Survival from primary treatment for all patients who received or did not receive HDT within the first year. (Right panel) Survival from primary treatment for all patients with myeloma in CR, PR or NR as best outcome.
Figure 2.
(Left panel) OS by landmark analysis for 291 patients who received chemotherapy alone based on response status at 12 months. The percentages of CR, PR and NR defined at 12 months were 9, 58 and 33%, respectively. Among 25 patients with CR, initial therapy had been dexamethasone (D) in 19, thalidomide-dexamethasone (TD) in 3, and bortezomib-thalidomide-dexamethasone (BTD) in 3; among 170 patients with PR, initial therapy had been D in 154, TD in 11 and BTD in 5. (Right panel) OS of 375 patients who received chemotherapy followed by HDT within 1 year based on response status at 12 months. The percentages of CR, PR and NR defined at 12 months were 27, 64 and 9%, respectively. Among 102 patients in CR, initial therapy had been D in 50, TD in 35 and BTD in 17; among 238 patients with PR, initial therapy had been D in 152, TD in 65 and BTD in 21.
There were 4 patients with NR and 17 patients with PR to primary treatment who received HDT between 12 and 24 months. Myeloma status did not improve in any of these patients for whom median survival after a landmark of 1 year was 3.3 years, similar to that of 2.8 years for similar patients who had not received HDT between 12 and 24 months. These findings support our analysis of these 21 patients among those who had not received HDT and are consistent with the progressive resistance to HDT with time as described earlier.7
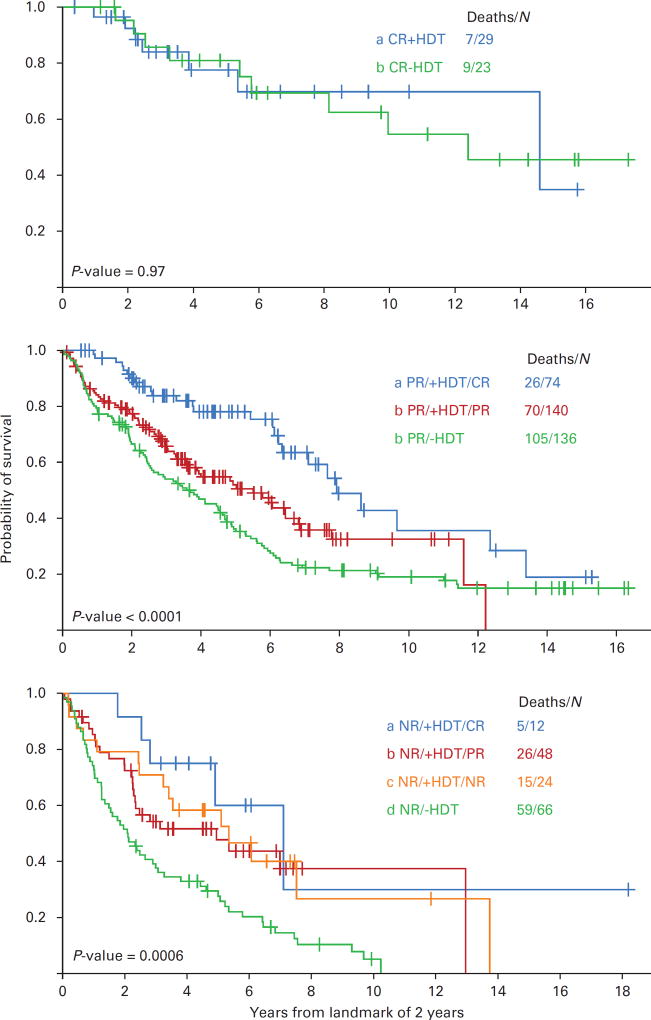
We correlated further response to intensive therapy with survival for groups of patients who had been treated for disease in NR, PR or CR. On account of the upgraded response for 22 patients with PR or NR converted to CR after 12 months, a landmark of 2 years was assessed in Figure 3. On the basis of response status at 2 years, the subsequent median survival was 9.7 years for patients with CR, 4.4 years for those with PR, and 2.7 years for patients with NR (< 0.001). Twenty-nine patients with myeloma in CR after primary therapy received intensive treatment; the median survival of 14.6 years at a 2-year landmark was similar to that of 12.4 years for 23 patients with disease in CR after only primary therapy (Figure 3, top panel). Among 214 patients who received HDT for disease in PR, survival evaluated at a 2-year landmark was significantly longer for patients in CR (median 7.9 years) than for those who remained in PR (median 5.5 years) (P < 0.01) and for those who had not received HDT (median 3.7 years) (Figure 3, middle panel). Among patients with NR evaluated at 2 years, survival times were similar for 12 patients with disease converted to CR after HDT (median 7.1 years), for 48 patients with disease reduced to PR (median 4.9 years) and for 24 patients with persistent NR (median 5.3 years) (P = 0.38), all significantly longer than that of 2.1 years for those who had not received HDT (P < 0.01) (Figure 3, lower panel). Survival at a 2-year landmark was similar for all groups who achieved CR by the different pathways (P = 0.39).
Figure 3.
(Top panel) OS by landmark analysis of 2 years for patients who achieved CR after chemotherapy alone or after added HDT. Among 23 patients with CR without HDT, initial therapy had been dexamethasone (D) in 18, thalidomide-dexamethasone (TD) in 3 and bortezomib-thalidomide-dexamethasone (BTD) in 2; among 29 patients with intensive therapy of CR, initial therapy had been D in 14, TD in 10 and BTD in 5. (Middle panel) OS by landmark analysis of 2 years for 350 patients with PR who received HDT. Among 74 patients with CR, initial therapy had been D in 46, TD in 21 and BTD in 7; among 140 patients with PR, initial therapy had been D in 81, TD in 48 and BTD in 11; among 136 patients who achieved PR after chemotherapy alone, initial treatment had been D in 125, TD in 10 and BTD in 1. (Lower panel) OS by landmark analysis of 2 years for 150 patients with NR who received HDT. Among 12 patients with CR, initial therapy had been D in 8, and TD in 4; among 48 patients with PR, initial therapy had been D in 32, TD in 13 and BTD in 3; among 24 patients who remained NR despite HDT, initial therapy had been D in 18 and TD in 6; for 66 patients who remained NR after chemotherapy alone, initial treatment had been D in 64 and TD in 2.
Among 62 patients who lived longer than 10 years after primary therapy, CR had occurred in 39%, PR in 50% and NR in 11% by a landmark of 2 years, so that CR was not a prerequisite for long survival. For all 151 patients with CR, two died of complications of HDT < 1 year, and eight died between 1 and 3 years (seven with relapsed disease and one from another disease). The projected survival for patients in CR after primary thalidomide-dexamethasone was similar to that for patients after D, but remains too early to assess for those treated with bortezomib-thalidomide-dexamethasone. After a 1-year landmark, survival was similar for patients with CR < 2 years and for those with PR (median 4.3 and 4.1 years), but was significantly longer for patients with CR > 2 years (median 14 years) (P < 0.01). Among 467 patients with primary treatment between 1987 and 1998, nine patients currently remain in CR for longer than 10 years (2%), of whom seven patients received HDT, six were staged as ISS I, and five patients achieved CR after primary therapy.
Multivariate analysis
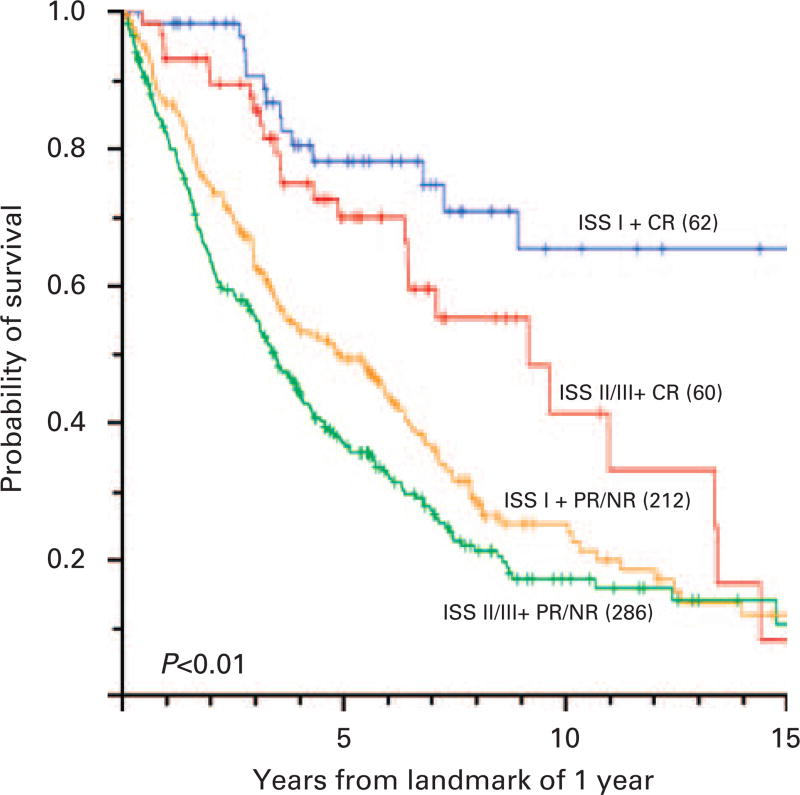
Cox proportional hazard models at a landmark time of 1 year were used to assess the effect of prognostic factors on OS As summarized in Table 3, the presence of stage I disease, occurrence of CR or PR, and HDT were associated independently with longer survival. When patients were grouped by ISS stage and occurrence of CR, the median survival of 62 patients with ISS stage I and CR was approximately 15 years (Figure 4).
Table 3.
Multivariate analysis of maximum likelihood estimates for survival after 1 year
| Hazard ratio | 95% CI | P-value | |
|---|---|---|---|
| CR vs NR | 0.30 | 0.21–0.44 | < 0.001 |
| PR vs NR | 0.69 | 0.54–0.87 | < 0.01 |
| Stage I vs II/III | 0.68 | 0.55–0.84 | < 0.001 |
| HDT vs no HDT | 0.71 | 0.56–0.91 | < 0.01 |
Abbreviation: CI = confidence interval.
Figure 4.
OS of patients by a landmark analysis of 1 year by ISS stage and eventual response status at 1 year. The percentages of patients with ISS I + CR, ISS II/III + CR, ISS I + PR/NR or ISS II/III + PR/NR were 10, 10, 34 and 46%, respectively.
Discussion
Early intensive therapy supported by autologous stem cells has become a standard program for most patients with multiple myeloma. Randomized and consecutive trials have shown a higher frequency of CR and longer survival after such treatment in comparison with control patients who continued conventional therapy without intensification.1,2,14,15 Previous studies had shown that conversion of NR or PR to CR was associated with a longer lifespan.6,7 In an attempt to define more clearly the impact of CR on survival, we conducted a retrospective analysis of 395 consecutive patients with multiple myeloma who received early HDT. Expanding on our previous reports that were limited to fewer patients less than 60 years old and with advanced disease, we included all patients up to the age of 65 years, including those with only Bence Jones protein. With follow-up for as long as 20 years, we focused on the impact of CR on survival and on the function of HDT in achieving or consolidating CR. To avoid potential bias from the guaranteed survival of patients with responsive disease, all survival times were compared by landmark analyses. Multivariate analyses were conducted to define the most important prognostic factors, but the absence of consistent studies of cytogenetics was a shortcoming.
Sixty-eight percent of patients who died within the first year had primary resistant disease. As HDT converted the myeloma of two-thirds of patients with NR to PR or CR, for whom lifespan was prolonged, such treatment offered a better chance to survive the first year and live for several more years. Thus, early HDT should be considered for all patients with NR to prevent disabling complications, death within the first year, and a chance for longer survival.16–18 Among patients who received HDT while in PR, one-fourth achieved CR with longer survival, so that HDT was useful for many of these patients.
Early intensification of disease already in CR was not associated with longer survival in comparison with the onset of CR with chemotherapy alone. Even though the pretreatment features were comparable and the follow-up long, the number of patients in both groups was small; a prospective randomized study on the value of HDT for patients in CR after primary therapy is necessary to clarify this question. The long survival of patients in CR regardless of HDT suggested no further reduction of residual disease with HDT, showing results similar to those described in the French clinical trial of single vs tandem transplant.19 In that study, there was no improvement of survival after second intensive treatment for those with > 90% reduction of myeloma, in contrast to the benefit observed for those with less. As HDT did not prolong survival for patients in CR, as also described by Dingli et al.,20 one explanation is that there was little further reduction of residual tumor cells because they were more resistant to current programs of intensification. When one considers the long survival usually observed with CR after primary treatment, especially when CR duration exceeds 2 years, further intensive treatment for many patients in CR may not be justified, especially older patients or those with a limited prognosis from other disorders. Similar conclusions were reached by Dingli et al.20 Nevertheless, early-onset of CR was an important prognostic factor in patients with multiple myeloma regardless of whether they received subsequent HDT.21,22 As HDT was an independent factor for longer survival on multivariate analyses, with CR and PR as more dominant factors, HDT had a favorable impact even without major change in disease status, perhaps by a combination of further modest cytoreduction and inhibition of later plasma cell growth.23 Such an effect may have contributed to longer survival for some patients with stable PR who may have resumed a previous smoldering myeloma status.
With older treatments that rarely used HDT or newer drugs, the response rates were less, CR was rare, and the median survival was shorter.3,4 Not surprisingly, correlations between the degree of myeloma reduction and survival were less evident than other prognostic factors, such as time to tumor progression.24 With effective new agents, more frequent intensive therapies and more frequent CR, the relation of CR to longer survival has become more evident, along with the heterogeneity of CR.6,7,19–25 More sensitive probes of gene rearrangement or genomics may detect the residual myeloma of patients destined for early relapse. Our findings and those of Barlogie et al.25 indicated that at least 2 years of CR was a prerequisite for long survival. We also identified nine patients who have remained in sustained CR > 10 years (representing 2% of those with long follow-up), who appeared to be ‘cured’ of myeloma, a group resembling those with prolonged EFS described by Barlogie et al.26
Acknowledgments
We are indebted to Monica Miller for typing this paper. This study was supported in part by grants from the National Cancer Institute, CA16672 and 5U01HL69334-08.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Child J, Morgan G, Davies F, Owen R, Bell S, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Alexanian R, Barlogie B, Tucker S. VAD-based regimens as primary treatment for multiple myeloma. Am J Hematol. 1990;33:86–89. doi: 10.1002/ajh.2830330203. [DOI] [PubMed] [Google Scholar]
- 4.Alexanian R, Dimopoulos M, Delasalle K, Barlogie B. Primary dexamethasone treatment of multiple myeloma. Blood. 1992;80:887–890. [PubMed] [Google Scholar]
- 5.Weber D, Rankin K, Gavino M, Delasalle K, Alexanian R. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol. 2003;21:16–19. doi: 10.1200/JCO.2003.03.139. [DOI] [PubMed] [Google Scholar]
- 6.Alexanian R, Weber D, Giralt S, Dimopoulos M, Delasalle K, Smith T, et al. Impact of complete remission with intensive therapy in patients with responsive multiple myeloma. Bone Marrow Transplant. 2001;27:1037–1043. doi: 10.1038/sj.bmt.1703035. [DOI] [PubMed] [Google Scholar]
- 7.Alexanian R, Weber D, Delasalle K, Handy B, Champlin R, Giralt S. Clinical outcomes with intensive therapy for patients with primary resistant multiple myeloma. Bone Marrow Transplant. 2004;34:229–234. doi: 10.1038/sj.bmt.1704562. [DOI] [PubMed] [Google Scholar]
- 8.Greipp PR, San Miguel J, Durie BG, Crowly J, Barlogie B, Bladé J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 9.Blade J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 11.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 12.Anderson J, Cain K, Gelber R. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 13.Cox D. Regression models and life tables. J Royal Stat Soc. 1972;34:187–220. [Google Scholar]
- 14.Desikan R, Barlogie B, Sawyer J, Ayers D, Tricot G, Badros A, et al. Results of high-dose therapy for 1000 patients with multiple myeloma: durable complete remissions and superior survival in the absence of chromosome 13 abnormalities. Blood. 2000;95:4008–4010. [PubMed] [Google Scholar]
- 15.Fermand JP, Katsahian S, Divine M, Leblond V, Dreyfus F, Macro M, et al. High-dose therapy and autologous blood stem cell transplantation compared with conventional treatment in myeloma patients aged 55–65 years: long-term results of a randomized control trail from the Group Myeloma-Autogreffe. J Clin Oncol. 2005;23:9227–9233. doi: 10.1200/JCO.2005.03.0551. [DOI] [PubMed] [Google Scholar]
- 16.Vesole D, Crowley S, Catchatourian R, Stiff P, Johnson D, Cromer J, et al. High-dose melphalan with autotransplantation for refractory multiple myeloma. J Clin Oncol. 1999;17:2173–2179. doi: 10.1200/JCO.1999.17.7.2173. [DOI] [PubMed] [Google Scholar]
- 17.Singhal S, Powles R, Sirohi B, Treleaven J, Kulkarni S, Mehta J. Response to induction chemotherapy is not essential to obtain survival benefit from high-dose melphalan and autotransplantation in myeloma. Bone Marrow Transplant. 2002;30:673–679. doi: 10.1038/sj.bmt.1703717. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Lacy MQ, Dispenzieri A, Rajkumar S, Fonseca R, Geyer S, et al. High-dose therapy and autologous stem cell transplantation for multiple myeloma poorly responsive to initial therapy. Haematologica. 2004;34:161–167. doi: 10.1038/sj.bmt.1704545. [DOI] [PubMed] [Google Scholar]
- 19.Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet J, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 20.Dingli D, Pacheco J, Nowakowski G, Kumar S, Dispenzieri A, Hayman S, et al. Relationship between depth of response and outcome in multiple myeloma. J Clin Oncol. 2007;25:4933–4937. doi: 10.1200/JCO.2007.11.7879. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Kim K, Cheong J, Min Y, Suh C, Kim H, et al. Complete remission status before autologous stem cell transplantation is an important prognostic factor in patients with multiple myeloma undergoing upfront single autologous transplantation. Biol Blood Marrow Transplant. 2009;15:463–470. doi: 10.1016/j.bbmt.2008.12.512. [DOI] [PubMed] [Google Scholar]
- 22.Lahuerta J, Mateos M, Martinez-Lopez J, Rosiñol L, Sureda A, de la Rubia J, et al. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26:5775–5782. doi: 10.1200/JCO.2008.17.9721. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S, Dingli D, Dispenzieri A, Lacy M, Hayman S, Buadi F, et al. Impact of additional cytoreduction following autologous SCT in multiple myeloma. Bone Marrow Transplant. 2008;42:259–264. doi: 10.1038/bmt.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durie B, Jacobson J, Barlogie B, Crowley J. Magnitude of response with myeloma frontline therapy does not predict outcomes. J Clin Oncol. 2004;22:1857–1863. doi: 10.1200/JCO.2004.05.111. [DOI] [PubMed] [Google Scholar]
- 25.Barlogie B, Anaissie E, Haessler J, van Rhee F, Pineda-Roman M, Hollmig K, et al. Complete remission sustained for 3 years from treatment initiation is a powerful surrogate marker for extended survival in multiple myeloma. Cancer. 2008;113:355–359. doi: 10.1002/cncr.23546. [DOI] [PubMed] [Google Scholar]
- 26.Barlogie B, Tricot G, van Rhee F, Angtuaco E, Walker R, Epstein J, et al. Long-term outcome results of the first tandem autotransplant trial for multiple myeloma. Br J Haemat. 2006;135:158–164. doi: 10.1111/j.1365-2141.2006.06271.x. [DOI] [PubMed] [Google Scholar]